Abstract
Lysine is the second most limiting amino acid after methionine and is considered the most limiting amino acid for growth in poultry. Lysine requirement for broiler chickens has changed over the years. Leptin and adiponectin represent 2 adipokines that mediate metabolism by eliciting satiety effects whereas ghrelin peptide hormone influences appetite. We hypothesize that this affects growth performance of chicks. This study evaluates the effect of varying dietary lysine homeostasis on performance of broiler chickens through satiety- and appetite-mediating hormones. In 3 replications, 270 one-day-old chicks were reared for 8 wk feeding on diets comprising 0.85, 1.14, and 1.42% lysine during the starter period and 0.75, 1.00, and 1.25% lysine during the grower period. These concentrations of lysine represent 75% (low lysine), 100% (control), and 125% (high lysine) of National Research Council recommendation for broiler chickens. Feed and water were provided for ad libitum consumption. At 8 wk of age, liver, pancreas, brain, and hypothalamus tissues were collected from 18 birds randomly selected from each treatment, snap frozen in liquid nitrogen, and stored at −80°C until use. Total RNA was extracted, and cDNA was synthesized for quantitative real-time PCR assays. Low lysine concentration caused slow growth and high mortality. There was significant upregulation of ghrelin in the hypothalamus and pancreas, and leptin and adiponectin in the hypothalamus and liver, and downregulation of ghrelin in the intestines. At low lysine concentrations, adiponectin was not expressed in both pancreas and intestines. High lysine concentration exhibited increased growth, upregulation of ghrelin in the liver, and downregulation of ghrelin in the intestines, and both adiponectin and leptin in the liver. The expression of ghrelin was negatively correlated with the expression of adiponectin and leptin (P < 0.05) in the liver, hypothalamus, and pancreas. Expression of leptin was positively correlated with adiponectin in the hypothalamus and liver (P < 0.05), exhibiting satiety effects when the concentrations of lysine were low.
Key words: lysine, gene expression, broiler chicken, food regulation and hypothalamus
Introduction
Lysine is one of the most vital amino acids required in poultry nutrition. The main role of lysine is to participate in protein synthesis. Lysine is required for growth and maintenance, and it is considered a reference amino acid in the ideal protein diets (Wijtten et al., 2004). Lysine interacts with other amino acids such as threonine (Kidd et al., 1997) through its metabolic pathways (Struys and Jakobs, 2010) as it contributes to protein utilization in animal feeding (Toride, 2000). Its availability and digestibility must therefore be put into account when formulating dietary lysine concentrations. Lysine has been characterized as one of the amino acids that cannot be synthesized by mammals and hence is an indispensable amino acid in broiler chickens (Tome and Bos, 2007). It is well known that protein, lysine, and their interaction is considered an important factor that affects performance and carcass quality of growing chicks, and so, dietary requirement of protein is a requirement for the lysine contained in the protein (Nasr and Kheiri, 2011). Lysine has been known to be the second-limiting amino acid after methionine. Lysine is therefore among the amino acids that are vital in poultry production. One of the most important areas in poultry production is to improve feed conversion and digestibility of feed provided to the birds to maximize performance. Researchers continue to contribute towards this course. Lysine is one component of nutrition where research has been done extensively. However, very little has been studied to link the digestibility of various feed components to assimilation and metabolism of various products of digestion (Boling and Firman, 1998). Controlling the amino acid imbalance is of great importance if its metabolism is to be improved (Boling and Firman, 1998). Many researchers have explored the dietary requirement of lysine in broiler chickens and egg-laying birds to determine the concentrations of lysine required for maximum carcass production and eggs. Given the cost of feeding poultry and the need to maximize production with minimal input of nutrients, there is urgent need to formulate diets based on response to individual nutrient intake.
Regulation of food intake has been shown to be controlled by peptide hormones such as leptin and ghrelin. Researchers have not been able to confirm the production of leptin hormone in chickens, but avian genomes contain a highly conserved leptin receptor (Adachi et al., 2008, Prokop et al., 2014), which is associated with nutrient sensing and signaling. More recently, Seroussi et al. (2016) identified leptin genes in chicken and duck genomes, resolving a long-lasting controversy on the existence of leptin genes in this species. The leptin receptor gene, which has been cloned and sequenced for chickens (Horev et al., 2000, Ohkubo et al., 2000, Ohkubo et al., 2007, Liu et al., 2007), mediates physiological functions of leptin. Lysine also plays a role in fatty acid metabolism. Peptide hormones such as adiponectin which is involved in adipogenesis pathway may be hypothesized to play a role in energy homeostasis and food intake when concentrations of lysine vary in feed. Studies by Denbow et al. (2000) on administration of recombinant chicken leptin proteins to birds showed reduced feed intake in some trials. Similar studies conducted by Bungo et al. (1999) showed that administration of mouse leptin did not reduce food intake in the chicken.
The mechanisms surrounding lysine metabolism and food regulation have not been fully explored to maximize its efficient utilization in poultry feeding. The pathways of lysine metabolism and regulation of food intake through neuroendocrine peptides must therefore be explored fully. As such, metabolism and homeostasis of lysine is of great importance. The main objective of this study was to evaluate the effect of dietary lysine homeostasis on performance of broiler chickens and correlate performance of these birds with the genes regulating nutrient utilization.
Materials and methods
This research was reviewed and approved by the Institutional Animal Care and Use Committee. The feeding trial and laboratory assays were conducted in 2017 and 2018.
Management of Experimental Birds
A total of 270 one-day-old broiler chicks were obtained from Aviagen (Huntsville, AL) and assigned to 18 floor pens where they were raised at Frank A. Young Poultry Research Facility (Tennessee State University, Nashville, TN) up to 8 weeks of age (WOA). The birds were randomly assigned to 3 dietary treatments and raised in floor pens, each housing a total of 15 birds. The housing temperature was maintained at 32.2°C for the first wk and was gradually reduced by 2.8°C weekly to a steady temperature of 23.8°C. At 5–8 WOA, no supplemental heating was provided to the birds and a constant room temperature was maintained at 21°C. Ventilation within the growing pens was kept by thermostatically controlled exhaust fans for all birds. Feed was provided in mash form, and both feed and water were provided for ad libitum consumption. BW and feed consumption (FC) were measured weekly, and BW gain (BWG) and feed conversion ratio (FCR) were also calculated weekly. Feed and water troughs were cleaned weekly. Mortality was monitored and recorded daily.
Dietary Treatments
Dietary treatments were arranged as 3 treatments x 6 replications x 15 birds per replication. Birds were randomly assigned to 3 dietary lysine treatments comprising distinct levels for the starter and grower periods. The diets were replicated 6 times. The lysine concentrations were 0.855, 1.14, and 1.42% for the starter period and 0.75, 1.00, and 1.25% for the grower period. These diets were 75, 100, and 125% of National Research Council (NRC)–recommended lysine requirement for broiler chickens. The control lysine diets were 1.14 and 1.00% for the starter and grower periods, respectively. The starter diets were isocaloric (3,100 kcal ME/kg) and isonitrogenous (23% CP) and were fed from 0–4 WOA. The grower diets were also isocaloric (3,200 kcal ME/kg) and isonitrogenous (22% CP), and were fed from 5–8 WOA (Table 1). All experimental diets containing the various concentrations of supplemental lysine were mixed at the Frank A. Young Poultry Research Facility.
Table 1.
Composition of experimental diets in starter (0–4 WOA) and grower period (5–8 WOA).
| Age (weeks) |
0–4 |
5–8 |
||||
|---|---|---|---|---|---|---|
| Lysine concentration (%) | 0.85 | 1.14 | 1.425 | 0.75 | 1.00 | 1.08 |
| Ingredients | % | |||||
| Corn, yellow # 2 (8% CP) | 50.61 | 49.22 | 50.33 | 61.67 | 62.02 | 53.14 |
| Soybean meal (48% CP) | 14.10 | 30.92 | 31.20 | 13.50 | 27.12 | 34.90 |
| Corn gluten meal (60% CP) | 17.60 | 6.00 | 6.00 | 17.80 | 0.00 | 0.00 |
| Wheat middlings | 10.36 | 4.40 | 2.67 | 0.00 | 0.00 | 0.00 |
| Alfalfa meal (17% CP) | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Poultry blended fat | 2.30 | 4.62 | 4.62 | 2.10 | 5.50 | 6.80 |
| Dicalcium phosphate (18% P, 22% Ca) | 1.74 | 1.74 | 1.74 | 1.75 | 1.75 | 1.74 |
| Limestone flour (38.8% Ca) | 1.45 | 1.45 | 1.45 | 1.25 | 1.40 | 1.45 |
| Salt | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 |
| Vitamin–mineral premix1 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| DL-methionine (98%)2 | 0.03 | 0.01 | 0.16 | 0.01 | 0.16 | 0.13 |
| L-Arg,% | 0.20 | 0.10 | 0.28 | 0.23 | 0.29 | 0.20 |
| L-Thr,% | 0.00 | 0.00 | 0.00 | 0.12 | 0.15 | 0.00 |
| L-Ile,% | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| L-Val,% | 0.00 | 0.00 | 0.00 | 0.02 | 0.02 | 0.00 |
| L-Lys HCl,% | 0.06 | 0.00 | 0.36 | 0.00 | 0.04 | 0.09 |
| Calculated levels | ||||||
| ME (kcal/kg diet) | 3,100 | 3,100 | 3,100 | 3,200 | 3,200 | 3,200 |
| Crude protein | 23 | 23 | 23 | 22 | 22 | 22 |
| L-Lys | 0.85 | 1.14 | 1.42 | 0.75 | 1.00 | 1.08 |
| Analyzed levels | ||||||
| ME, kcal/kg | 3,100 | 3,100 | 3,100 | 3,200 | 3,200 | 3,200 |
| CP, % | 23 | 23 | 23 | 22 | 22 | 22 |
Abbreviation: WOA, weeks of age.
Provided per kg of diet: retinyl acetate, 3,500 IU; cholecalciferol, 1,000 ICU; DL-α-tocopheryl acetate, 4.5 IU; menadione sodium bisulfate complex, 2.8 mg; vitamin B12, 5.0 mg; riboflavin, 2.5 mg; pantothenic acid, 4.0 mg; niacin, 15.0 mg; choline, 172 mg; folic acid, 230 mg; ethoxyquin, 56.7 mg; manganese, 65 mg; iodine, 1 mg; iron, 54.8 mg; copper, 6 mg; zinc, 55 mg; and selenium, 0.3 mg.
Degussa Corporation, Kennesaw, GA.
Growth Performance
Growth performance was evaluated based on the calculations of BWG and FCR. BW of each broiler bird and FC from each replicate was measured weekly. The amount of feed given to each replicate was recorded. The remainder of the feed was deducted from the aggregated amount of feed appropriated throughout the week. The weekly FCR were calculated by dividing the FC by the average BWG.
Tissue Sample Collection and RNA Extraction
At 8 WOA, a total of 54 birds (20%), 18 birds from each of the 3 dietary treatments, were randomly selected and sacrificed via cervical dislocation. Tissue samples were excised from the liver, pancreas, brain, and hypothalamus. The samples were snap frozen in liquid nitrogen and stored at −80°C until use. RNA was extracted using TRIzol Reagent (Thermo Fisher Scientific, Waltham, MA) according to the manufacturer's protocol. The RNA was dissolved in RNase-free water. The sample concentration and purity were determined using the NanoDrop Spectrophotometer (NanoDrop Technologies, Wilmington, DE) by taking the optical density at 260 nm and 280 nm. RNA with A260/A280 ratio above 1.7 was retained (Chomczynski and Mackey, 1995).
Primer Design, Reverse Transcription, and Quantitative Real-Time PCR
Nucleotide sequences for genes of interest were obtained from the National Center for Biotechnology Information's Primer3 Software (Massachusetts Institute of Technology's Whitehead Institute for Biomedical Research, Cambridge, MA) as shown in Table 2. The primers were synthesized by Eurofins Genomics (Louisville, KY). Reverse transcription was performed under standard conditions with High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific). Real-time PCR assays were performed according to QiaGen's QuantiTect SYBR Green PCR Kit (QIAGEN Incorporated, Valencia, CA) on ABI PRISM 7000 Sequence Detection System (Foster City, CA). Real-time PCR assays were programmed at the following cycling parameters: initial denaturation at 95°C for 15 min; followed by 40 cycles of denaturation at 94°C for 15 s and primer annealing at 55 or 58°C for 30 s; and final extension at 72°C for 30 s. For quality control, a dissociation curve was added. Data are presented as threshold cycle relative to an internal control glyceraldehyde-3-phosphate dehydrogenase. Real-time PCR products were visualized via 2% agarose gel electrophoresis.
Table 2.
Sequences of primers used in the amplification of leptin, ghrelin, adiponectin receptors, and GAPDH of chicken broilers fed diets containing varying concentrations of lysine from hatch to 8 WOA.
| Receptor and accession No | Primer sequence1 | Amplicon length (bp) | Product length | Tm2 (°C) |
|---|---|---|---|---|
| Leptin NM_204323.1 |
Forward: 5′ CACTCGCTGGGAACACTTGA 3′ | 20 | 116 | 56.0 |
| Reverse: 5′ TTCAGCAGCCCATCGTTTCT3′ | 20 | 116 | 56.0 | |
| Ghrelin NM_204394.1 |
Forward: 5′ TGCCTTTTCACGTAGGACGA 3′ | 20 | 118 | 54.0 |
| Reverse: 5′ GCGCTCAGGTAGAAGAGGAC 3′ | 20 | 118 | 54.0 | |
| Adiponectin AY786316.1 |
Forward: 5′ TGATCCCCAGGTCTACAAGG 3′ | 20 | 235 | 55.0 |
| Reverse: 5′ TGCTGCTGTCGTAGTGGTTC 3′ | 20 | 235 | 55.0 | |
| GAPDH3 | Forward: 5′ AGAACATCATCCCAGCGTCC 3′ | 20 | 133 | 56.0 |
| NM_204305.1 | Reverse: 5′ CGGCAGGTCAGGTCAACAAC 3′ | 20 | 133 | 56.0 |
Abbreviations: GAPDH, glyceraldehyde-3-phosphate dehydrogenase; WOA, weeks of age.
Chicken primers for leptin, ghrelin, and adiponectin receptors.
Tm represents the optimized primer annealing temperature.
The GADPH was used as internal controls.
Statistical Analysis
Growth performance data were subjected to ANOVA using the GLM of the SAS software (SAS Institute, 2011) as completely randomized design with dietary treatments as main effects. All variables were analyzed as repeated measurements. Percentage and gene expression data were transformed into log form before analysis. The data were then backtransformed for tabulation and discussion. The statistical model used is Yijk = μ + Ti + Rij + gijk, where Yijk = response variables from each individual replication; μ = the overall mean; Ti = the effect of dietary treatment (lysine); Rij = the interexperimental unit (replications) error term; and gijk = the intraexperimental unit error term. Differences in mortality among dietary treatments were analyzed using the chi-square method. Least significant difference comparisons were made between treatment means for main effects having a significant P-value. Data are presented as means ± the standard error. Significance denotes P ≤ 0.05. Fold change was calculated using the comparative CT method discussed by Schmittgen and Livak (Nature Protocols, 2008). Fold change equals 2−ΔΔCT, where −ΔΔCT = [CT gene of interest - CT internal control sample A] – [CT gene of interest - CT internal control sample B].
Results
BW Gain
The mean BWG values of male and female broilers fed diets varying in lysine concentrations from hatch to 8 WOA are presented in Table 3. Different concentrations of supplemental lysine showed significant differences (P < 0.05) on BWG of broiler chickens during the starter and grower periods. The differences in BW were noticeable starting from second wk. BWG was significantly higher (P < 0.05) in broilers that were fed a diet with high lysine concentration when compared with the control and low lysine concentrations from 0–8 WOA (Table 3). For example, in the sixth wk, increasing lysine concentration from 75 to 100% in the male birds increased their BW by 73%, whereas increasing lysine concentration from 100 to 125% increased birds' BW by 33.7%. Increasing lysine concentration for female birds from 75 to 100% in the fifth wk increased the BW by 57%, whereas increasing lysine concentration from 100 to 125% increased the BW by 17.4%. There was a significant increase in mean BW with increasing lysine concentration. The mean BWG of male broilers was higher than that of female broilers in each ration across the entire period of 8 weeks.
Table 3.
Mean body weight gain of broiler chickens fed diets containing varying concentrations of lysine from hatch to 8 WOA.
| Weeks of age | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Lysine (%) |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
Total | |
| Starter | Grower | g body weight gain/week | |||||||||
| M | 0.85 | 0.75 | 58c | 99c | 170c | 259c | 319c | 343c | 287b | 445c | 1,983c |
| 1.14 | 1.00 | 86b | 185b | 325b | 517b | 486b | 595b | 739a | 557b | 3,493b | |
| 1.42 | 1.25 | 119a | 255a | 368a | 552a | 685a | 796a | 672a | 699a | 4,147a | |
| SEM | 2.4 | 6.5 | 9.7 | 11.8 | 15.6 | 24.1 | 40.7 | 18.0 | 128.7 | ||
| F | 0.85 | 0.75 | 58c | 94c | 176b | 251c | 296c | 333b | 275c | 333c | 1,820c |
| 1.14 | 1.00 | 83b | 172b | 325a | 407b | 465b | 538a | 333b | 541a | 2,868b | |
| 1.42 | 1.25 | 94a | 227a | 322a | 471a | 546a | 529a | 557a | 467b | 3,216a | |
| SEM | 3.1 | 5.9 | 9.2 | 13.2 | 15.6 | 23.5 | 15.4 | 17.9 | 103.8 | ||
a–cMeans within sex and column with no common superscripts differ significantly (P < 0.05).
Abbreviations: M, male; F, female; WOA, weeks of age.
Feed Consumption
The mean FC values of male and female broilers fed diets varying in lysine concentrations from hatch to 8 WOA are presented in Table 4. The FC was significantly different (P < 0.05) across the 8-wk study period, with broilers that were fed 125% of supplemental lysine showing a higher FC compared with birds feeding on 75 and 100% supplemental lysine. Male broilers had significantly higher FC than the female broilers for the entire period of 8 weeks. Increasing lysing concentration from 75 to 125% significantly (P < 0.05) increased the FC. For example, in week 6, the FC increased by 24.6 and 65.5% in male birds, whereas in female birds, the increment was 17 and 64.2% in the control and 125% of supplemental lysine diet, respectively.
Table 4.
Feed consumption of broiler chickens fed diets containing varying concentrations of lysine from hatch to 8 WOA.
| Weeks of age | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Lysine% |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
Total | |
| Starter | Grower | g feed/week | |||||||||
| M | 0.85 | 0.75 | 80c | 198c | 274c | 476c | 583c | 666c | 671c | 912b | 3,860c |
| 1.14 | 1.00 | 117b | 257b | 473b | 605b | 883b | 830b | 1,633a | 1,479a | 6,277b | |
| 1.42 | 1.25 | 137a | 319a | 510a | 776a | 1,069a | 1,427a | 1,410b | 1,484a | 7,132a | |
| SEM | 1.5 | 0.6 | 5.3 | 10.6 | 4.3 | 6.5 | 32.8 | 11.1 | 72.6 | ||
| F | 0.85 | 0.75 | 80c | 162c | 289b | 433c | 600c | 659c | 698c | 805c | 3,727c |
| 1.14 | 1.00 | 117b | 247b | 460a | 624b | 847b | 771b | 1,235a | 1,204b | 5,506b | |
| 1.42 | 1.25 | 126a | 302a | 457a | 661a | 969a | 1,266a | 1,174b | 1,241a | 6,197a | |
| SEM | 0.8 | 4.5 | 7.1 | 4.9 | 11.6 | 12.4 | 4.2 | 7.24 | 52.6 | ||
a–cMeans within sex and column with no common superscripts differ significantly (P < 0.05).
Abbreviations: F, female; M, male; WOA, weeks of age.
Feed Conversion Ratio
The mean FCR of male and female broilers fed diets varying in lysine concentrations from hatch to 8 WOA are presented in Table 5. The FCR of male broilers fed the varying dietary concentrations of lysine were significantly different (P < 0.05) in all the weeks except for week 7. The female broilers' FCR were significantly different (P < 0.05) among the lysine concentrations from 2–8 WOA. Increasing dietary lysine concentrations from 75 to 125% of NRC recommendation decreased the FCR significantly (P < 0.05) in both sexes. For example, at 2 WOA, increasing lysine concentration from the 75 to 125% of NRC-recommended levels decreased the FCR by 30.9%, whereas increasing lysine concentration from 25 to 100% of NRC recommendation decreased the FCR by 14.3% in male broilers. On the other hand, the FCR of females decreased by 20% when dietary lysine concentration was increased from 75 to 125% of NRC recommendation. However, increasing lysine concentration from 75 to 100% of NRC-recommended levels decreased the FCR by 8.8% at 2 WOA. Overall, the male broilers exhibited better FCR which were significantly lower (P < 0.05) than those of female broilers in all the 3 lysine concentrations evaluated. These observations are supported by the higher BWG of the birds that were feeding on the control (100%) and 125% of NRC-recommended supplemental lysine and also the negative correlations between BWG and FCR.
Table 5.
Feed conversion ratio of broiler chickens fed diets containing varying concentrations of lysine from hatch to 8 WOA.
| Weeks of age | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Lysine% |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
Average | |
| Starter | Grower | g feed/g body weight gain/week | |||||||||
| M | 0.85 | 0.75 | 1.45a | 2.13a | 1.74a | 1.97a | 1.94a | 2.03a | 2.40 | 2.20b | 1.98a |
| 1.14 | 1.00 | 1.39a | 1.47b | 1.51b | 1.20c | 1.84a | 1.37c | 2.26 | 2.74a | 1.75b | |
| 1.42 | 125 | 1.15b | 1.26b | 1.41b | 1.41b | 1.58b | 1.82b | 2.31 | 2.19b | 1.63b | |
| SEM | 0.05 | 0.09 | 0.07 | 0.07 | 0.08 | 0.07 | 0.13 | 0.12 | 0.09 | ||
| F | 0.85 | 0.75 | 1.42 | 1.85a | 1.75a | 1.81a | 2.10a | 2.05b | 2.53b | 2.5ab | 2.00a |
| 1.14 | 1.00 | 1.47 | 1.48b | 1.42a | 1.56b | 1.84b | 1.51c | 3.40a | 2.30b | 1.87b | |
| 1.42 | 1.25 | 1.40 | 1.35b | 1.46b | 1.45b | 1.86ab | 2.56a | 2.14c | 2.73a | 1.87b | |
| SEM | 0.06 | 0.07 | 0.07 | 0.06 | 0.09 | 0.12 | 0.09 | 0.11 | 0.08 | ||
a–cMeans within sex and column with no common superscripts differ significantly (P < 0.05).
Abbreviations: F, female; M, male; WOA, weeks of age.
Lysine Consumption
The mean lysine consumption (LC) of male and female broilers fed diets varying in lysine concentrations from hatch to 8 WOA are presented in Table 6. Lysine consumption increased with increase in dietary concentration of lysine and the mean differences in LC among the lysine concentrations were significantly different (P < 0.05) from each other in both sexes as expected. Lysine consumption for male broilers fed on control and 125% lysine diets was 70 and 173% higher than that of birds fed diets containing the low lysine concentration, respectively, at 4 WOA. At 8 WOA, male broilers feeding on the control and 125% supplemental lysine diets consumed 117 and 172% more lysine than those that fed the low-lysine diets. The same trend of LC was observed in female broilers. There was, however, a higher LC for male broilers compared with the female broilers in the starter and the grower periods.
Table 6.
Lysine consumption of broiler chickens fed diets containing varying concentrations of lysine from hatch to 8 WOA.
| Weeks of age | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Lysine% |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
Total | |
| Starter | Grower | g Lysine/week | |||||||||
| M | 0.85 | 0.75 | 0.68c | 1.69c | 2.33c | 4.05c | 4.95c | 5.66c | 5.70c | 7.76c | 32.81c |
| 1.14 | 1.00 | 1.33b | 2.92b | 5.39b | 6.90b | 10.07b | 9.46b | 18.62b | 16.87b | 71.56b | |
| 1.42 | 1.25 | 1.95a | 4.52a | 7.25a | 11.02a | 15.18a | 20.26a | 20.03a | 21.07a | 101.3a | |
| SEM | 0.37 | 0.82 | 1.43 | 2.02 | 2.95 | 4.37 | 4.56 | 3.93 | 19.83 | ||
| F | 0.85 | 0.75 | 0.68c | 1.38c | 2.46c | 3.68c | 5.10c | 5.60c | 5.93c | 6.84c | 31.68c |
| 1.14 | 1.00 | 1.38b | 2.82b | 5.25b | 7.12b | 9.66b | 8.79b | 14.08b | 13.73b | 62.77b | |
| 1.42 | 1.25 | 1.81a | 4.29a | 6.49a | 9.39a | 13.76a | 17.98a | 16.68a | 17.62a | 87.99a | |
| SEM | 0.33 | 0.84 | 1.19 | 1.66 | 2.5 | 3.71 | 3.24 | 3.15 | 16.28 | ||
a–cMeans within sex and column with no common superscripts differ significantly (P < 0.05).
Abbreviations: F, female; M, male; WOA, weeks of age.
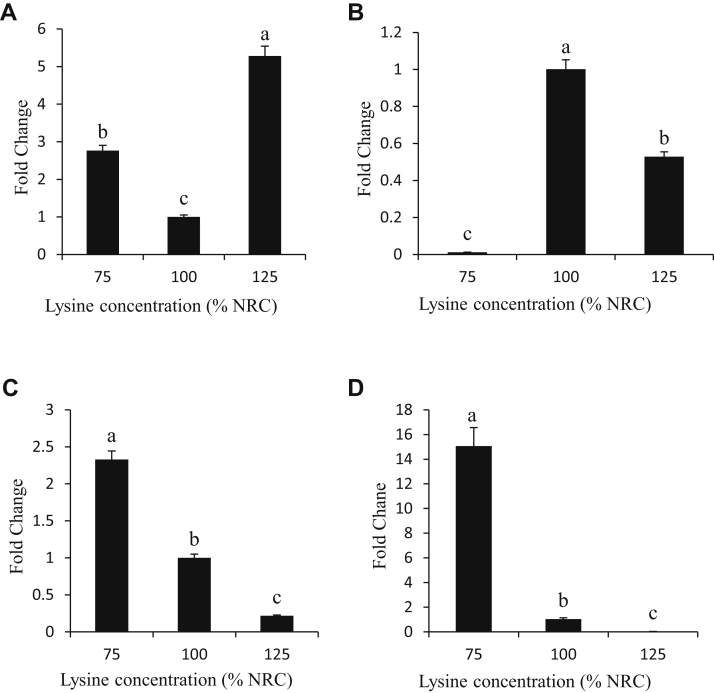
Expression of Ghrelin Receptor
The expression of the ghrelin receptor gene in the liver, intestines, hypothalamus, and pancreas (A, B, C, and D, respectively) of chicken broilers fed diets containing 75, 100, and 125% of supplemental lysine is shown in Figure 1. Varying dietary lysine concentrations alters the expression of the ghrelin receptor gene in the liver, intestines, hypothalamus, and pancreas in broiler chickens. Birds that received the diet with the highest amount of lysine (125%) showed a significant increase (5.3-fold difference) in expression of ghrelin receptor in the liver when compared with birds fed the control and low-lysine diets (1.0- and 2.8-fold difference, respectively) (Figure 1A). The expression of ghrelin gene receptor in the intestines showed lower expression in the low- and high-lysine diets (0.01- and 0.5-fold difference) compared with the control diet (Figure 1B). There was an increased expression (2.3-fold difference) of ghrelin receptor in the hypothalamus (Figure 1C) of low-lysine diet compared with the expression in the control and high-lysine diets (1.0- and 0.2-fold difference, respectively). Birds that received the low-lysine diet showed a significant increase (15.1-fold difference) in expression of ghrelin receptor in the pancreas, whereas birds receiving the high-lysine diet showed a significant decrease (0.03-fold difference) in the expression of ghrelin in the pancreas when compared with birds fed the control diet (Figure 1D). There was a general significant decrease (P < 0.05) in the expression of ghrelin in the hypothalamus and pancreas with an increase in lysine concentration in the broiler diet.
Figure 1.
Tissue expression (fold change) of ghrelin in the liver, intestines, hypothalamus, and pancreas (A, B, C, and D, respectively) of chicken broilers fed diets containing 75, 100, and 125% lysine of National Research Council (NRC) requirement. The chicken glyceraldehyde-3-phosphate dehydrogenase (GAPDH) “house-keeping” gene was used as endogenous control. a,b,cWithin individual charts, bars representing mean gene expression with no common superscripts differ significantly (P < 0.05).
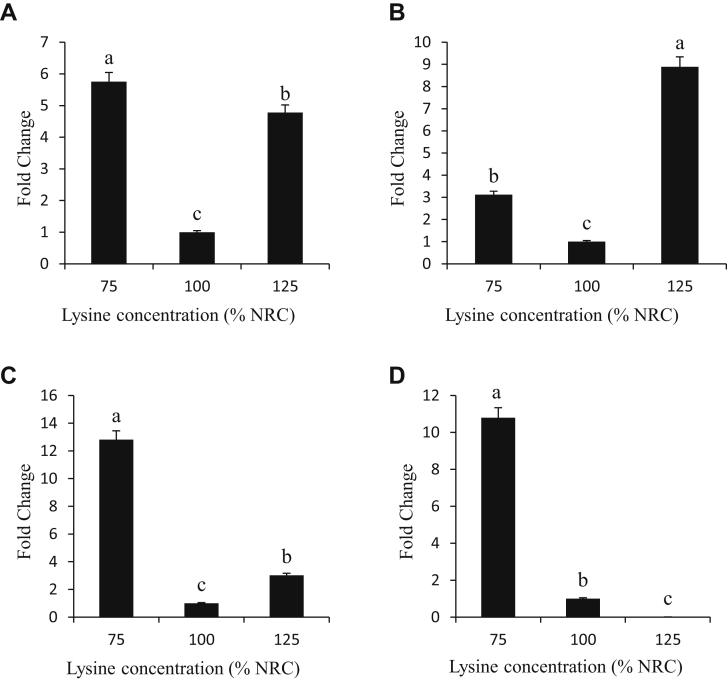
Expression of Leptin Receptor
The expression of leptin in the liver, intestines, hypothalamus, and pancreas (A, B, C, and D, respectively) of chicken broilers fed diets containing 75, 100, and 125% of supplemental lysine is shown in Figure 2. Varying concentrations of lysine significantly (P < 0.05) affected the expression of leptin receptor in various tissues. The expression of leptin in the liver (Figure 2A) in the low-lysine diet was the highest (5.8-fold change) followed by the expression in the high-lysine diet (4.8-fold change) compared with the control diet. The expression of leptin receptor gene in the intestines of birds fed high- and low-lysine diets increased (8.9- and 3.1-fold difference) compared with the birds fed the control diet. In the hypothalamus (Figure 2C), there was an increase (P < 0.05) in the expression of leptin receptor in the birds fed the low- and high-lysine diets (12.8 and 3-fold difference) compared with birds fed the control diets. The expression of leptin receptor in the pancreas increased (10.8-fold difference) in low-lysine diets and decreased (0.01-fold change) compared with the control group (Figure 2D).
Figure 2.
Tissue expression (fold change) of leptin in the liver, intestines, hypothalamus, and pancreas (A, B, C, and D, respectively) of chicken broilers fed diets containing 75, 100, and 125% lysine of National Research Council (NRC) requirement. The chicken glyceraldehyde-3-phosphate dehydrogenase (GAPDH) “house-keeping” gene was used as an endogenous control. a,b,cWithin individual charts, bars representing mean gene expression with no common superscripts differ significantly (P < 0.05).
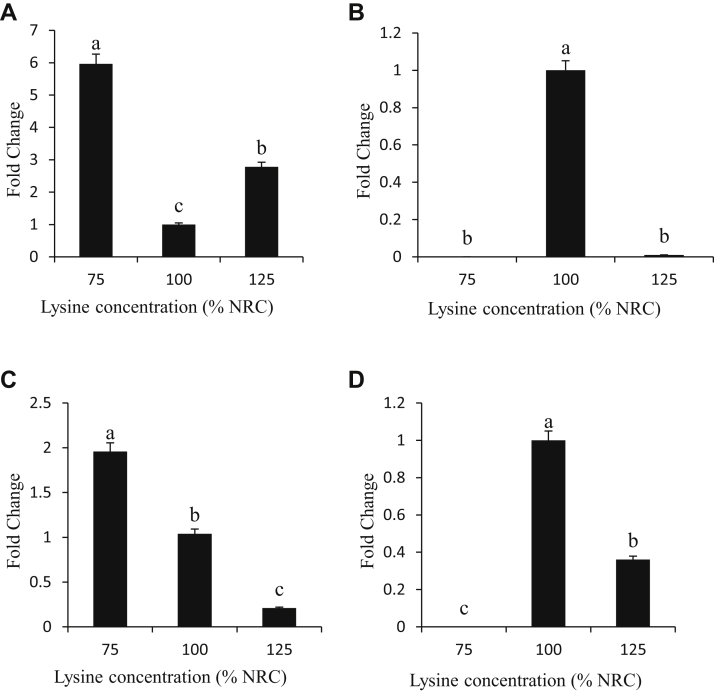
Expression of Adiponectin Receptor
The expression of the adiponectin receptor gene in the liver, intestines, hypothalamus, and pancreas (A, B, C, and D, respectively) of chicken broilers fed diets containing 75, 100, and 125% of supplemental lysine is shown in Figure 3. Birds that received the diet with the lowest and highest amount of lysine showed a significantly higher (P < 0.05) expression of adiponectin receptor gene in the liver (6- and 2.8-fold difference, respectively) when compared with birds fed the control diets (Figure 3A). The expression of adiponectin in the high-lysine diets was significantly higher (P < 0.05) than the control diets. The expressions of adiponectin in the intestines of birds fed diets containing low and high lysine concentrations (0.0003- and 0.01-fold change, respectively) were not different from each other (Figure 3B). However, the expression in the low- and high-lysine diet groups differed significantly (P < 0.05) when compared with the control group. The expression of adiponectin receptor gene in the hypothalamus of birds fed diets containing low lysine concentration showed a significant increase (P < 0.05) in expression (2.0-fold change), whereas the high-lysine diets significantly decreased (P < 0.05) the expression of adiponectin receptor gene (0.1-fold change) when compared with the control group (Figure 3C). As shown in Figure 3D, the expression of adiponectin receptor gene in the liver was highly altered by changes in dietary lysine concentrations with great variability. There was almost no or significantly decreased (P < 0.05) expression of adiponectin receptor gene in low and high lysine concentrations, respectively (0.002- and 0.4-fold change, respectively) compared with the birds fed a control diet.
Figure 3.
Tissue expression (fold change) of adiponectin in the liver, intestines, hypothalamus, and pancreas (A, B, C, and D, respectively) of chicken broilers fed diets containing 75, 100 and 125% lysine of National Research Council (NRC) requirement. The chicken glyceraldehyde-3-phosphate dehydrogenase (GAPDH) “house-keeping” gene was used as an endogenous control. a,b,cWithin individual charts, bars representing mean gene expression with no common superscripts differ significantly (P < 0.05).
Correlations
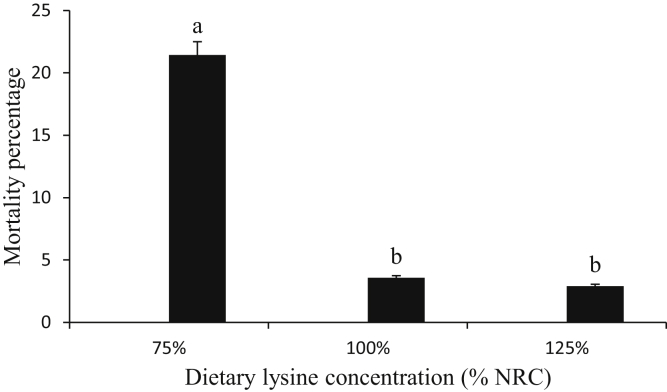
Correlation coefficients of lysine and performance parameters of broilers fed diets varying in lysine concentrations from hatch to 8 WOA are presented in Table 7. Lysine concentrations were strongly positively correlated to BWG (0.91474) and FC (0.85867) at P < 0.05. The FCR is negatively correlated to lysine concentrations. High mortality of birds (21.43%) was recorded at low lysine concentration compared with the control and high-lysine diets (3.57 and 2.91%, respectively). Low lysine was characterized by poor development of some birds' body parts, low BWG, and high mortality rates due to poor growth (Figure 4). This poor performance may be attributed to induced deficiency of lysine, the most limiting amino acid for growth or weight gain, which resulted in a decrease in protein synthesis for essential functions to sustain life in these birds. Ghrelin was negatively correlated to adiponectin and leptin (P < 0.05) in the liver, hypothalamus, and pancreas at both low and high lysine concentrations, which is suggested to cause a change in food regulation and hence a change in homeostatic profiles of lysine. Leptin is positively correlated to adiponectin as shown in the hypothalamus and liver at P < 0.05 (Table 7), which shows similar functions.
Table 7.
Pearson correlation coefficients among lysine concentrations, BWG, FC, FCR, and fold change of genes regulated by lysine in various tissues.
| BWG | FC | FCR | LGhr | LLept | LAdip | HGhr | HLept | HAdip | IGhr | ILept | IAdip | PGhr | PLept | PAdip | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lysine1 | 0.615** | 0.859** | −0.791** | 0.513* | -0.018 | −0.463* | −0.790** | −0.182 | −0.900** | 0.181 | −0.673** | 0.001 | −0.540* | −0.699** | −0.243 |
| BWG | 0.755** | −0.603** | 0.115 | 0.236 | −0.445* | −0.592* | −0.806** | −0.549* | 0.737** | −0.185 | −0.315 | −0.331 | −0.670** | −0.698** | |
| FC | −0.048 | 0.754** | 0.108 | 0.116 | −0.330 | −0.124 | −0.461* | 0.230 | 0.139 | −0.675** | 0.368 | −0.321 | −0.788** | ||
| FCR | −0.488* | −0.281 | −0.068 | −0.369 | −0.220 | −0.541* | 0.112 | 0.585* | 0.388 | 0.325 | −0.020 | 0.414* | |||
| LGhr | 0.488* | −0.526* | 0.528* | −0.976** | −0.828** | 0.319 | −0.570* | 0.690** | 0.404* | −0.066 | −0.895** | ||||
| LLept | 0.626** | −0.596* | 0.001 | 0.133 | −0.306 | 0.154 | −0.099 | −0.817** | −0.407* | 0.679** | |||||
| LAdip | −0.956** | 0.145 | 0.613** | −0.682** | 0.698** | 0.103 | −0.116 | −0.491* | 0.359 | ||||||
| HGhr | 0.469* | −0.311 | 0.107 | −0.647** | 0.578* | 0.340 | −0.201 | −0.068 | |||||||
| HLept | 0.635** | 0.089 | 0.302 | 0.421* | −0.564* | 0.283 | 0.713** | ||||||||
| HAdip | −0.157 | 0.397 | 0.395 | −0.073 | 0.246 | 0.672** | |||||||||
| IGhr | 0.855** | 0.112 | 0.678** | 0.341 | −0.196 | ||||||||||
| ILept | 0.105 | −0.148 | −0.051 | −0.136 | |||||||||||
| IAdip | −0.184 | 0.336 | 0.847** | ||||||||||||
| PGhr | −0.720** | −0.423* | |||||||||||||
| PLept | 0.855** |
* denotes significance: P < 0.05; ** denotes significance: P < 0.01.
Abbreviations: BWG, BW gain; FC, feed consumption; FCR, feed conversion ratio; HAdip, hypothalamus adiponectin; HGhr, hypothalamus ghrelin; HLept, hypothalamus leptin; IAdip, intestine adiponectin; IGhr, intestine ghrelin; ILept, intestine leptin; LAdip, liver adiponectin; LGhr, liver ghrelin; LLept, liver leptin; PAdip, pancreas adiponectin; PGhr, pancreas ghrelin; PLept, pancreas leptin.
Lysine concentration.
Figure 4.
Percentage mortality of broilers fed varying concentrations of dietary lysine from hatch to 8 weeks of age. a,bWithin individual charts, bars representing mean percentage mortality with no common superscript differ significantly (P < 0.05). NRC, National Research Council.
Discussion
Lysine is an essential amino acid required for animal growth and maintenance. It is used as a reference amino acid to formulate animal diets because it represents one of the most limiting amino acids in practical corn–soybean meal in poultry production. There were interactions among lysine concentrations, sex of the birds, and time (week) that were significant (P < 0.05), and therefore, means of BWG, FC, LC, and FCR were separated by lysine concentrations, sex, and time. Reports from previous studies have shown that BWG, FC, LC, and FCR are affected by the sex of broiler birds (Acar et al., 1991, Han and Baker, 1991, Gorman and Balnave, 1995, Bhogoju et al., 2017). The highest performance of broiler chicken of both sexes was recorded in the 125% lysine diets, followed by 100 and 75% lysine diets, respectively. Kerr et al. (1999) and Nasr and Kheiri (2011) also confirmed that increasing dietary lysine concentration increases BWG because there is an increased breast meat yield. Our results confirmed findings of previous studies that demonstrated that lysine requirements for growing chickens are higher than the NRC's (1994) recommendation for maximum BWG (Kidd et al., 1997; Kerr et al., 1999). Supplemental lysine in broiler chicken diets has been shown to increase breast yield. Previous reports show that lysine is used in broiler production to improve body protein accretion, specifically targeting development of breast muscle fibers (Leclercq, 1998). Higher feed intake results in higher nutrient intake such as other essential amino acids and energy. Baker et al. (1975) reported that increasing lysine levels in monogastric animals such as chickens and pigs increased FC. Consumption of more feed by birds means that more nutrients such as energy, microminerals, macrominerals, essential amino acids, and vitamins would most likely be absorbed. Therefore, there is expectation that an increase in protein synthesis and a decrease in protein degradation result in protein accretion (Tesseraud et al., 1996). Low lysine concentration is characterized by early body developmental problems due to a hindered growth rate and reduced muscle development and, hence, high mortality. This is a confirmation obtained by Benevenga and Blemings (2007) that lysine plays a role in the early development of avian and mammalian organisms.
This study did show that the higher efficiency of the diet with high lysine allowed a better transformation of amino acid intake into tissue synthesis and accretion. This is possibly related to a higher amino acid availability to synthesize muscle. Diets having high lysine promote the conversion of amino acids into protein and, hence, the high carcass yield (Eits et al., 2003; Dozier III et al., 2008). Previous reports indicate that higher BWG and better FCR were attributed to increased levels of lysine supplementation in the diets of broilers (Viola et al., 2009). These observations are also in agreement with reports of Corzo et al. (2002) that increasing dietary lysine concentrations in diets of broiler chickens improved FCR.
Gene expression assays demonstrated that varying lysine concentrations in diets of broilers alter the expression of leptin receptor, ghrelin receptor, and adiponectin receptor genes in the hypothalamus, pancreas, intestines, and the liver. The expression of ghrelin receptor gene in the liver and intestines was highly variable, whereas the expression in the hypothalamus and pancreas showed a consistent increase with decreased lysine concentration (Figure 1). Our findings from the liver and hypothalamus show that the liver serves as an amino acid–sensing organ through its own innervated system that communicates with the hypothalamus. These findings are supported by the study from Jensen et al. (2013) who deduced that the liver senses amino acids via the brain. Our results from expression of ghrelin and leptin in the pancreas show that the pancreas also has some amino acid–sensing capabilities. Ghrelin, also known as the hunger hormone, is a peptide hormone produced by ghrelinergic cells in the gastrointestinal tract when the stomach is empty (Inui et al., 2004, Sakata and Sakai, 2010). Ghrelin acts on hypothalamic brain cells both to increase hunger and to increase gastric acid secretion and gastrointestinal motility to prepare the body for food intake (Schwartz et al., 2000). As a result, appetite and energy expenditure is regulated. The role of anorexigenic and orexigenic hormones such as leptin and ghrelin in the control of food absorption has been thoroughly studied (Kojima and Kangawa, 2006; Gao and Horvath, 2007, Nirmala et al., 2009). Payne et al. (2016) found out that an amino acid has an effect on the activities of these hormones and can cause a change in food intake and, hence, a change in the overall performance of birds. Ohkubo et al. (2008) and Xu et al. (2013) argue that the leptin/insulin signaling cascade regulates food intake. Our study results are supported by Seroussi et al. (2016) who found that expression of leptin can establish functional pathways that respond to and are regulated by factors such as nutrients. A decrease in leptin levels acts as a starvation signal, whereas an increase in leptin levels educes feed intake (Denbow et al., 2000, Pandit et al., 2017). We propose that when the concentration of lysine is low, there is decreased ghrelin in the intestines, whereas an increase in lysine concentration increases ghrelin to the brain through the blood or the vagus nerve. The correlation results obtained from the expressions of leptin showed an inverse of ghrelin expression. This confirms that the effects of leptin are opposite to those of ghrelin; leptin decreases neuropeptide-Y release to suppress appetite and BW (Kojima and Kangawa, 2006). Therefore, with low lysine concentrations, the leptin receptor is highly expressed. The expression of adiponectin in the liver and hypothalamus was similar to that of leptin. Many studies have found that the effect of adiponectin is inversely correlated with body mass index in animals (Ukkola and Santaniemi, 2002). Concentrations of circulating adiponectin increase during caloric restriction in animals. Increased adiponectin shows increased energy expenditure, which is highly associated with protein uncoupling. Similar studies carried out by Nedvidkova et al. (2005) found that adiponectin exerts some of its weight reduction effects through the brain. This is similar to the action of leptin, but the 2 hormones perform complementary actions and can have synergistic effects, which were confirmed by the correlation results of this study. This study proposes that lysine mediates leptin and ghrelin functions through the endocrine system by the hypothalamus and liver system and that the effects of adiponectin are similar to leptin. The pancreas has some amino acid–sensing capabilities that may need further investigation. There is the likelihood that low dietary lysine suppressed FC through hypothalamic signaling, creating a state of hunger and, therefore, an increase in intestinal expression of ghrelin.
Conclusion
In conclusion, this study showed that increasing the lysine requirement for broiler chickens beyond NRC (1994) recommendation increases performance. A change in lysine concentration creates an amino acid imbalance, which alters the overall performance of broilers. Low levels of lysine cause a significant change in the growth of broilers at early and later stages of growth. Low lysine hinders growth and causes poor development of major bird organs such as the liver and pancreas. Decreased lysine concentration in broiler chicken diets was characterized by high mortality rates in birds during early development. BW gains decreased tremendously with depleted levels of lysine. The FCR generally decreased with increasing lysine concentrations, which is a good factor to a broiler producer. This is as a result of decreased feed intake by birds at low lysine concentrations. Various lysine concentrations caused changes in the expressions of neuroendocrine molecules such as ghrelin, leptin, and adiponectin which caused significant changes in the BW of birds. The expression of these genes in the hypothalamus revealed possible signaling pathways involved in the lysine-brain axis. Low lysine levels triggered an increase in leptin levels that created a state of satiety. There is a possible role of adiponectin creating a sense of satiety when the concentrations of lysine are low. Leptin and adiponectin suppress the effect of ghrelin in inducing appetite due to combined interactions. The action of ghrelin is highly influenced by adiponectin and leptin receptor alterations in the liver, hypothalamus, and pancreas when lysine concentrations are varied. Therefore, lysine homeostasis is mediated through the hypothalamus and liver by ghrelin, leptin and adiponectin that regulates energy homeostasis hence affecting performance of the birds. This cause changes in the growth patterns of organisms. More investigation should be done to elucidate the effect of lysine on metabolic pathways and effect on other neuroendocrine peptides.
Acknowledgments
The birds used in this study were provided by Aviagen in Huntsville, AL, USA. This research was supported by United States Department of Agriculture -NIFA, United States of America.
References
- Acar N., Moran E.T., Jr., Bilgili S.F. Live performance and carcass yield of male broilers from two commercial strain crosses receiving rations containing lysine below and above the established requirement between six and eight weeks of age. Poult. Sci. 1991;70:2315–2321. [Google Scholar]
- Adachi H., Takemoto Y., Bungo T., Ohkubo T. Chicken leptin receptor is functional in activating JAK-STAT pathway in vitro. J. Endocrinol. 2008;197:335–342. doi: 10.1677/JOE-08-0098. [DOI] [PubMed] [Google Scholar]
- Baker D.H., Katz R.S., Easter R.A. Lysine requirement of growing pigs at two levels of dietary protein. J. Anim. Sci. 1975;40:851–856. doi: 10.2527/jas1975.405851x. [DOI] [PubMed] [Google Scholar]
- Benevenga N.J., Blemings K.P. Unique aspects of lysine nutrition and metabolism. J. Nutr. 2007;197:1610–1615. doi: 10.1093/jn/137.6.1610S. [DOI] [PubMed] [Google Scholar]
- Bhogoju S., Nahashon S.N., Donkor J., Kimathi B., Johnson D., Khwatenge C., Bowden-Taylor T. Effect of varying dietary concentrations of lysine on growth performance of the Pearl Grey Guinea fowl. Poult. Sci. 2017;96:1306–1315. doi: 10.3382/ps/pew395. [DOI] [PubMed] [Google Scholar]
- Boling S.D., Firman J.D. Digestible lysine requirement of female turkeys during the starter period. Poult. Sci. 1998;77:547–551. doi: 10.1093/ps/77.4.547. [DOI] [PubMed] [Google Scholar]
- Bungo T., Shimojo M., Masuda Y., Tachibanab T., Tanaka S., Sugahara K., Furuse M. Intracerebroventricular administration of mouse leptin does not reduce food intake in the chicken. Brain Res. 1999;817:196–198. doi: 10.1016/s0006-8993(98)01223-2. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Mackey K. Short technical report. Modification of the TRI reagent procedure for isolation of RNA from polysaccharide-and proteoglycan-rich sources. Biotechniques. 1995;19:942–945. [PubMed] [Google Scholar]
- Corzo A., Moran E.T., Jr., Hoehler D. Lysine need of heavy broiler males applying the ideal protein concept. Poult. Sci. 2002;81:1863–1868. doi: 10.1093/ps/81.12.1863. [DOI] [PubMed] [Google Scholar]
- Denbow D.M., Meade S., Robertson A., McMurtry J.P., Richards M., Ashwell C. Leptin-induced decrease in feed intake in chickens. Physiol. Behav. 2000;69:359–362. doi: 10.1016/s0031-9384(99)00258-9. [DOI] [PubMed] [Google Scholar]
- Dozier W.A., III, Kidd M.T., Corzo A. Dietary amino acid responses of broiler chickens. J. Appl. Poult. Res. 2008;17:157–167. [Google Scholar]
- Eits R.M., Kwakkel R.P., Verstegen M.W.A., Emmans G.C. Responses of broiler chickens to dietary protein. Effects of early life protein nutrition on later responses. Br. Poult. Sci. 2003;44:398–409. doi: 10.1080/0007166031000035544. [DOI] [PubMed] [Google Scholar]
- Gao Q., Horvath T.L. Neuronal control of energy homeostasis. FEBS Lett. 2007;582:132–141. doi: 10.1016/j.febslet.2007.11.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman I., Balnave D. The effect of dietary lysine and methionine on the growth characteristics and breast meat yield of Australian broiler chickens. Aust. J. Agr. Res. 1995;46:1569–1577. [Google Scholar]
- Han Y., Baker D.H. Lysine requirements of fast- and slow-growing broiler chicks. Poult. Sci. 1991;70:2108–2114. doi: 10.3382/ps.0702108. [DOI] [PubMed] [Google Scholar]
- Horev G., Einat P., Aharoni T., Eshdat Y., Friedman-Einat M. Molecular cloning and properties of the chicken leptin-receptor (CLEPR) gene. Mol. Cell Endocrinol. 2000;162:95–106. doi: 10.1016/s0303-7207(00)00205-7. [DOI] [PubMed] [Google Scholar]
- Inui A., Asakawa A., Bowers C.Y., Mantovani G., Laviano A., Meguid M.M., Fujimiya M. Ghrelin, appetite, and gastric motility: the emerging role of the stomach as an endocrine organ. FASEB J. 2004;18:439–456. doi: 10.1096/fj.03-0641rev. [DOI] [PubMed] [Google Scholar]
- Jensen K.J., Alpini G., Glaser S. Hepatic nervous system and neurobiology of the liver. Compr. Physiol. 2013;3:655–665. doi: 10.1002/cphy.c120018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr B.J., Kidd M.T., Halpin K.M., McWard G.W., Quarles C.L. Lysine level increases live performance and breast yield in male broilers and breast yield in male broilers. J. Appl. Poult. Res. 1999;8:381–390. [Google Scholar]
- Kidd M.T., Kerr B.J., Anthony N.B. Dietary interactions between lysine and threonine in broilers. Poult. Sci. 1997;76:608–614. doi: 10.1093/ps/76.4.608. [DOI] [PubMed] [Google Scholar]
- Kojima M., Kangawa K. Drug Insight: the functions of ghrelin and its potential as a multitherapeutic hormone. Nat. Clin. Pract. Endocrino. Metabol. 2006;2:80–88. doi: 10.1038/ncpendmet0080. [DOI] [PubMed] [Google Scholar]
- Leclercq B. Specific effects of lysine on broiler production: comparison with threonine and valine. Poult. Sci. 1998;77:118–123. doi: 10.1093/ps/77.1.118. [DOI] [PubMed] [Google Scholar]
- Liu X., Dunn I.C., Sharp P.J., Boswell T. Molecular cloning and tissue distribution of a short form chicken leptin receptor mRNA. Domest. Anim. Endocrinol. 2007;32:155–166. doi: 10.1016/j.domaniend.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Nasr J., Kheiri F. Effect of different lysine levels on Arian broiler performances. Ital. J. Anim. Sci. 2011;10:32. [Google Scholar]
- National Research Council . 9th rev. Natl. Acad. Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- Nedvidkova J., Smitka K., Kopsky V., Hainer V. Adiponectin, an adipocyte-derived protein. Physiol. Res. 2005;54:133–140. [PubMed] [Google Scholar]
- Nirmala G., Suchitra B., Pavankumar K. Appetite regulating hormones. Vet. World. 2009;2:242–246. [Google Scholar]
- Ohkubo T., Adachi H. Leptin signaling and action in birds. J. Poult. Sci. 2008;45:233–240. [Google Scholar]
- Ohkubo T., Nishio M., Tsurudome M., Ito M., Ito Y. Existence of leptin receptor protein in chicken tissues: isolation of a monoclonal antibody against chicken leptin receptor. Gen. Comp. Endocrinol. 2007;151:269–273. doi: 10.1016/j.ygcen.2007.01.021. [DOI] [PubMed] [Google Scholar]
- Ohkubo T., Tanaka M., Nakashima K. Structure and tissue distribution of chicken leptin receptor (cOb-R) mRNA. Biochim. Biophys. Acta. 2000;1491:303–308. doi: 10.1016/s0167-4781(00)00046-4. [DOI] [PubMed] [Google Scholar]
- Pandit R., Beerens S., Adan R.A. Role of leptin in energy expenditure: the hypothalamic perspective. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017;312:938–947. doi: 10.1152/ajpregu.00045.2016. [DOI] [PubMed] [Google Scholar]
- Payne A., Wang X., Ivy M.T., Stewart A., Nelson K., Darris C., Nahashon S.N. Lysine mediation of neuroendocrine food regulation in Guinea fowl. Poult. Sci. 2016;95:276–286. doi: 10.3382/ps/pev326. [DOI] [PubMed] [Google Scholar]
- Prokop J.W., Schmidt C., Gasper D., Duff R.J., Milsted A., Ohkubo T., Ball H.C., Shawkey M.D., Mays H.L., Jr., Cogburn L.A. Discovery of the elusive leptin in birds: identification of several ‘missing links’ in the evolution of leptin and its receptors. PLoS ONE. 2014;9:e92751. doi: 10.1371/journal.pone.0092751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata I., Sakai T. Ghrelin cells in the gastrointestinal tract. Int. J. Pept. 2010;2010:945056. doi: 10.1155/2010/945056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS Institute . SAS Inst. Inc.; Cary, NC: 2011. SAS/STAT User’s Guide. Version 9.3. [Google Scholar]
- Schmittgen T.D., Livak K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008;3:1101–11007. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Schwartz M.W., Woods S.C., Porte D., Seeley R.J., Baskin D.G. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- Seroussi E., Cinnamon Y., Yosefi S., Genin O., Smith J.G., Rafati N., Bornelov S., Andersson L., Friedman-Einat M. Identification of the long-sought leptin in chicken and duck: expression pattern of the highly GC-rich avian leptin fits an autocrine/paracrine rather than endocrine function. Endocrinology. 2016;157:737–751. doi: 10.1210/en.2015-1634. [DOI] [PubMed] [Google Scholar]
- Struys E.A., Jakobs C. Metabolism of lysine in alpha-aminoadipic semialdehyde dehydrogenase-deficient fibroblasts: evidence for an alternative pathway of pipecolic acid formation. FEBS Lett. 2010;584:181–186. doi: 10.1016/j.febslet.2009.11.055. [DOI] [PubMed] [Google Scholar]
- Tesseraud S., Peresson R., Chagneau A.M. Dietary lysine deficiency greatly affects muscle and liver protein turnover in growing chickens. Br. J. Nutr. 1996;75:853–865. doi: 10.1079/bjn19960191. [DOI] [PubMed] [Google Scholar]
- Tome D., Bos C. Lysine requirement through the human life cycle. J. Nutr. 2007;137:1642–1645. doi: 10.1093/jn/137.6.1642S. [DOI] [PubMed] [Google Scholar]
- Toride Y. FAO; 2000. Lysine and Other Amino Acids for Feed: Production and Contribution to Protein Utilization in Animal Feeding.http://www.fao.org/3/y5019e/y5019e0a.htm Accessed January 2019. [Google Scholar]
- Ukkola O., Santaniemi M. Adiponectin: a link between excess adiposity and associated comorbidities? J. Mol. Med. 2002;80:696–702. doi: 10.1007/s00109-002-0378-7. [DOI] [PubMed] [Google Scholar]
- Viola T.H., Kessler A.M., Ribeiro A.M.L., Viola E.S., Trevizan L., Goncalves T.A. Performance and weight of body fractions, in the growing supplementation of lysine, at 40 days of age in broilers. Rural Sci. 2009;39:515–521. [Google Scholar]
- Wijtten P.J.A., Prak R., Lemme A., Langhout D.J. Effect of different dietary ideal protein concentrations on broiler performance. Br. Poult. Sci. 2004;45:504–511. doi: 10.1080/100071660412331286217. [DOI] [PubMed] [Google Scholar]
- Xu Y., Kim E.R., Zhao R., Myers M.G., Jr., Munzberg H., Tong Q. Glutamate release mediates leptin action on energy expenditure. Mol. Metab. 2013;2:109–111. doi: 10.1016/j.molmet.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]