Abstract
The regulatory roles of mitogen-activated protein kinase (MAPK) signaling pathways on the hypothalamic−pituitary−gonadal axis (HPG axis) of Wenchang chicks under heat stress (HS) were investigated. Additionally, the crosstalk between these signaling pathways was explored. Immunohistochemical experiments and Western blotting were employed to quantify extracellular regulated protein kinases (ERK), c-Jun N-terminal kinases (JNK), and p38MAPK (P38). In female chicks, hypothalamic ERKs were upregulated in Weeks 1 and 2 in the HS group compared with the control group (CK), while JNK and p38 were downregulated (P < 0.05). Pituitary MAPKs were all downregulated in the HS group compared with the CK group in Week 3, but p38 was upregulated in Week 4 (P < 0.01). In the HS group, ovarian MAPKs were all upregulated compared with the CK group during Week 5, whereas ERK was downregulated in Week 6 (P < 0.01). In contrast to the patterns of MAPK expression in female chicks in the HS and CK groups, ERK in male chicks showed a completely opposite pattern in Weeks 1, 2, and 5, while p38 and JNK were downregulated in both female and male chicks under HS during Weeks 2 and 3. In the HS group, pituitary and testis MAPKs showed a pattern opposite to that observed in female chicks under HS in Week 5; MAPKs were all downregulated (P < 0.05). Thus, there are gender differences in the MAPK signaling pathways in the HPG axis in chicks, and these pathways showed plasticity. Early HS can enhance chick growth and development as well as promote developing in the MAPK signaling pathways in the HPG axis. However, after heated brooding was discontinued in chicks, long-term HS obstructed chick development and caused tissue and function injury to the HPG axis.
Key words: heat stress, HPG axis, MAPKs, Wenchang chick
INTRODUCTION
Chickens have been domesticated for thousands of years (Liu et al., 2006; Kanginakudru et al., 2008; Peters et al., 2016), and many local high production performance varieties have been bred to date (Mosca et al., 2016; Bungsrisawat et al., 2018). However, the irrational exploitation of natural resources by mankind and continuous environmental deterioration has resulted in global warming (Lefevre et al., 2017; Dosio et al., 2018). This is particularly pronounced in tropical and subtropical regions in which high temperatures and humidity often result in heat stress (HS) in poultry. Heat stress is a state of negative balance between the net amount of energy flowing from an animal to its surrounding environment and the amount of heat energy produced by the animal (Ajakaiye et al., 2011). Heat stress is the sum of non-specific responses produced by the animal in response to a high-temperature environment. Therefore, HS poses a significant challenge to the poultry industry (Saeed et al., 2017). In poultry, HS not only disrupts the structure and function of major organs such as the hypothalamus, pituitary gland, small intestine, thymus, Bursa of Fabricius, and gonads through cell apoptosis but also drastically decreases reproduction rate, egg yield, meat quality, and other production performance measures. Heat stress can also increase the mortality rate in poultry (Chen et al., 2016; Liang et al., 2016; Lu et al., 2018).
In order to resist HS, poultry can synthesize and release hormones through the hypothalamic− pituitary−gonadal axis (HPG axis) to regulate immunity and reproduction. Relevant studies have pointed out that the HPG axis in poultry exhibits plasticity (Ottinger et al. 1995; Sockman et al., 2004). At the same time, although heat resistance in poultry is heritable (Wan et al., 2017; Wang et al., 2017; Wol et al., 2019), Heat stress tends to occur when the upper temperature limit is exceeded. Mitogen-activated protein kinase (MAPK) pathways are intracellular serine/threonine kinase signal transduction pathways that are widely distributed in various tissues. These signal transduction pathways consist of a tertiary conserved signal transduction network of MAPK, MAPK kinase, and MAPKK kinase, and these are divided into 3 subfamilies: extracellular regulated protein kinases (ERK), c-Jun N-terminal kinases (JNK), and p38 (Chang and Karin 2001). These pathways can transmit cell surface signals inside the cells and nuclei, and crosstalk exists between the pathways. These pathways play important regulatory roles in cell proliferation, differentiation, and apoptosis (Lewis et al., 1998; Junttila et al., 2008). In addition, KEGG signaling pathway analysis showed that HS can activate Ras-MAPK pathways in the livers of poultry (Zeng et al., 2015). In view of this, we examined the changes and interactions of ERK, JNK, and p38 in the hypothalamus, pituitary glands, and testis or ovaries across different periods of the brooding stage at the tissue and protein levels in order to elucidate the survival strategies of chicks towards HS.
MATERIALS AND METHODS
Housing and Management of Experimental Animals
A total of 288 1-day-old healthy chicks (Hainan Yongji Livestock Co., Ltd., Hainan, China) were used in this study. The chicks were divided into 2 groups by gender, and then randomized into gender-specific control (CK) groups and HS groups, with 72 chicks per group. The chicks were weighed and numbered. There were no significant differences in the weight or feed intake between the 2 groups. All chicks were given an ad libitum diet of basic feedstuff (Yilong Feed Factory, Zhanjiang, China), and distilled water was used for drinking. The chicks in the different groups were all housed under routine management conditions for 6 weeks, and rectal temperature and food and water statuses were recorded. The basic feed conformed to NPC (1994) standards.
HS Treatment
Each day, chicks from the HS group were placed in LRH-800-GS climatic cabinets (Tomorrow Environmental Protection Instrument, Shaoguan, China) with a temperature of 40 ± 0.5°C and a relative humidity of 70% ± 5% for 2 h of HS treatment (12:00 to 14:00). Simultaneously, chicks from the CK group were placed in non-heated climatic cabinets. After the HS treatment, all chicks were returned to their cages for routine housing (Ramnath et al., 2008; Chen et al., 2014). The experiments were conducted with the approval from the Hainan Normal University Animal Experimentation Ethics Committee.
Sample Collection and Processing
At the end of each week during the first 6 wk of age, rectal temperatures of the chicks were measured after the HS treatment. The chicks were then weighed, along with chicks from the CK group. In total, 12 chicks were randomly selected from each group, and their hypothalamus, pituitary glands, and ovaries or testes were collected. After these organs were weighed, they were rapidly placed into Bouin's fixative solution for fixation before paraffin embedding (n = 6 for each group). These organs from another set of chicks (n = 6 for each group) were placed into cryovials and then snap frozen in liquid nitrogen. This was followed by storage in a −80°C freezer.
Immunohistochemical Experiments
Paraffin-embedded hypothalamus, pituitary glands, and ovaries or testes were made into 5-μm serial sections, and the immunohistochemistry (IHC) streptavidin−biotin complex method was employed (Yin et al., 2011). Sections underwent conventional IHC steps. The sections were observed under a microscope; a brown coloration indicated a positive reaction. A total of 3 sections were randomly selected, and 10 fields were selected for each section. After incubating with primary antibodies of rabbit phospho-ERK (Cat# bs-1646R), phospho-JNK (Cat# bs-17591R), or phospho-p38 (Cat# bs-2210R) (Bioss Biotech, Beijing, China) at 1:400 dilution, the sections were further incubated with the secondary antibody (Yeasen Biotech Co. Ltd., Shanghai, China). The experiment was carried out according to the manufacturer's instructions. An Olympus CX31 microscope (Olympus, Tokyo, Japan) was used to observe positive immune reactions in tissues and cells in the prepared tissue sections, and a microscope-mounted digital camera MSHOT MC50 (Micro-shot Technology Co., Ltd., Guangzhou, China) was used for photography. Afterwards, Image-pro Plus 6.0 software (Media Cybernetics, Rockville, MD) was used for image analysis, and the mean integral optical densities (MIODs) of phosphoERK-, phosphoJNK-, and phosphoP38-positive cells within the field were measured (Song et al., 2005). The MIODs were used to perform a comprehensive percentage analysis of phospho-MAPKs in each week.
Western Blotting
A volume of 100 μL of RIPA lysis buffer, 20 μL of 1 M mol/L PMSF, and 10 μL of phosphatase inhibitors were added per 20 mg of tissue. The tissues were lysed on ice for 30 min before mincing. This was followed by liquid nitrogen freezing for 15 min before thawing on ice. An electric tissue high-speed homogenizer (model OSE-Y10, Tiangen Biotech, Beijing, China) was used for homogenization until a particulate-free viscous state was achieved. Next, the lysates were centrifuged at 12,000 rpm for 20 min at 4°C. The supernatants were collected, and the BCA assay for the microvolume UV−Vis spectrophotometer NanoDrop One (Thermo Fisher Scientific, Waltham, MA) was used for protein quantitation of the sample. The protein concentrations and loading volumes were calculated. Subsequently, 2 × SDS-PAGE protein loading buffer was added to the total protein samples. The samples were heated in a 100°C water bath for 5 min and stored at −20°C. A miniVE vertical electrophoresis and electrotransfer unit (GE Healthcare, New York, NY) was used for SDS-PAGE electrophoresis (constant voltage of 90 V, 40 min) before proteins were transferred to membranes (constant current of 200 mA for 2 h). Next, 1 × TBST was used for 3 washes with shaking for 10 min per wash. After this step, the membranes were blocked with 5% BSA blocking solution on a shaking incubator for 1 h before primary antibodies [rabbit Anti-ERK (Cat# bs-2637R), phospho-ERK, JNK (Cat# bs-0501R), phospho-JNK, p38 (Cat# bs-28027R), and phospho-p38 (Bioss Biotech, Beijing, China) at a 1:500 dilution and β-actin (Cat # 30102ES40) at a 1:1,000 dilution] were incubated with the membranes at room temperature for 10 min with gentle shaking before incubation at 4°C overnight. On the second day, the membranes were washed 3 times with 1 × TBST for 10 min per wash. Subsequently, the membranes were incubated with the secondary antibody and peroxidase-conjugated goat anti-rabbit IgG (H+L) (Cat# 33101ES60, 1:5,000) at room temperature for 1 h before 1 × TBST was used to wash the membranes 3 times for 10 min per wash. Finally, the SuperSignal West Pico PLUS chemiluminescent substrate (Cat# No. 34580) (Thermo Fisher Scientific) was used for detection. The ChemiDoc XRS+ System (Bio-Rad, Hercules, CA) was used for exposure and photography of the PVDF membranes, and Image Lab software (Bio-Rad) was used for the analysis. Other reagents and consumables were purchased from Yeasen Biotech (Shanghai, China).
Statistical Analysis
The Image Lab image analysis system was used for the semi-quantitation of bands in each Western blot image. The experimental results were expressed as Mean ± SEM. Excel 2003 and SPSS 22.0 (IBM, Armonk, NY) were used for data analysis. One-way ANOVA was used to analyze the differences between groups. An alpha level of P < 0.05 was considered significant, and an alpha level of P < 0.01 was considered extremely significant. The percentage method (Juárez-Rojas et al., 2015) was used to compare the activation status of crucial proteins in the 3 MAPK signaling pathways.
RESULTS
Effects of HS on Hypothalamic MAPK Expression in Chicks
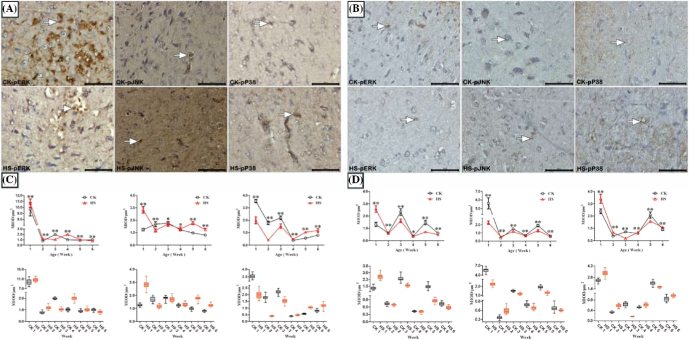
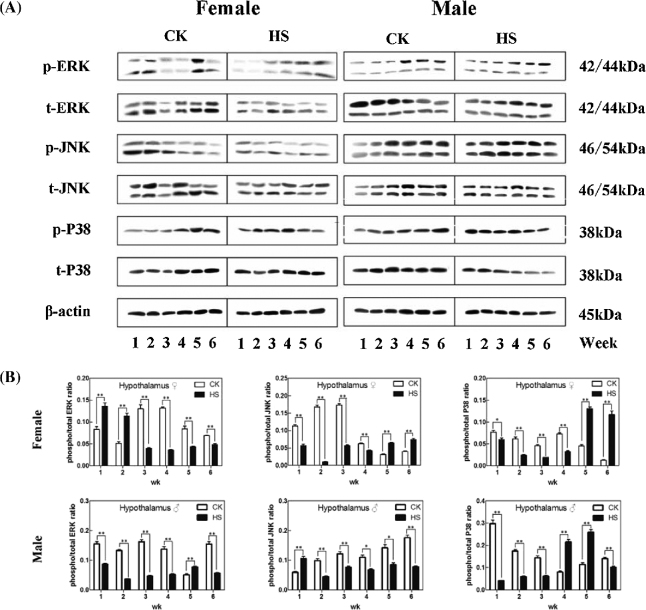
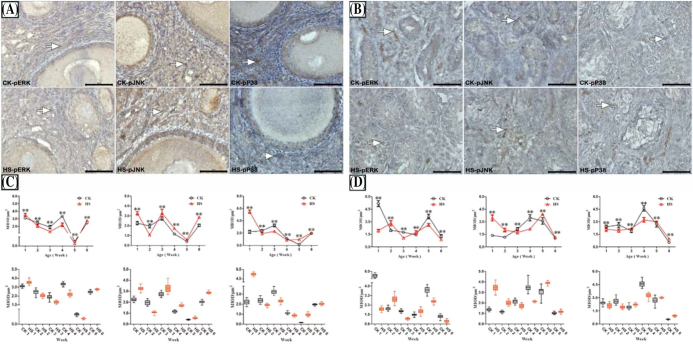
The IHC staining results of phosphorylated ERK, JNK, and p38 in the hypothalamus of chicks showed that positive cells presented brown cytoplasms and were sporadically distributed in hypothalamic tissues (Figures 1A and 1B). Significant MIOD changes in hypothalamic ERK, JNK, and p38 phosphorylation were observed (Figures 1C and 1D). For females, MIODs of hypothalamic MAPKs in the HS group were all lower than those of the CK group in Week 3, but this trend was reversed in Week 5 (P < 0.01). For males, MIODs in HS were lower than those of the CK group in both Weeks 3 and 5 (P < 0.01). The Western blot results of phosphorylated ERK, JNK, and P38 proteins in hypothalamic tissues (Figures 2A and 2B) showed that HS treatment downregulated the level of phosphorylated MAPK proteins in Weeks 3 and 4 in females, but in Weeks 2, 3, and 6 in males (P < 0.01).
Figure 1.
Effect of heat stress on phospho-MAPKs in the hypothalamus of chicks (× 400, Bar = 50 μm, MIOD/μm2). Group A and group B are female and male chicks, respectively, followed by group C and group D. Control check (CK); heat stress (HS); and the numbers after CK/HS detail weeks from 1 to 6; phospho-ERK (pERK); phospho-JNK (pJNK); phospho-P38 (pP38); mean integral optical density (MIOD). Positive reactions are denoted with an arrow. * indicates P < 0.05 and ** indicates P < 0.01 between the CK group and the HS group in the same week, and the same below.
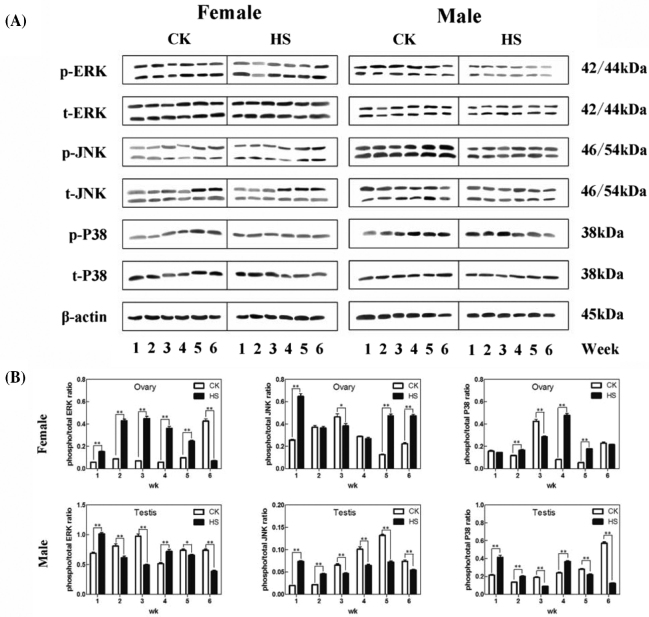
Figure 2.
Effect of heat stress on phospho-/total MAPKs in the hypothalamus of chicks. Group A is the histogram result of the Western Blot followed by group B. Control check (CK); heat stress (HS); phospho-ERK (pERK); phospho-JNK (pJNK); phospho-P38 (pP38); total ERK (tERK); total JNK (tJNK); total P38 (tP38). * indicates P < 0.05 and ** indicates P < 0.01 between the CK group and the HS group in the same week, and the same below.
Effects of HS on Pituitary MAPK Expression in Chicks
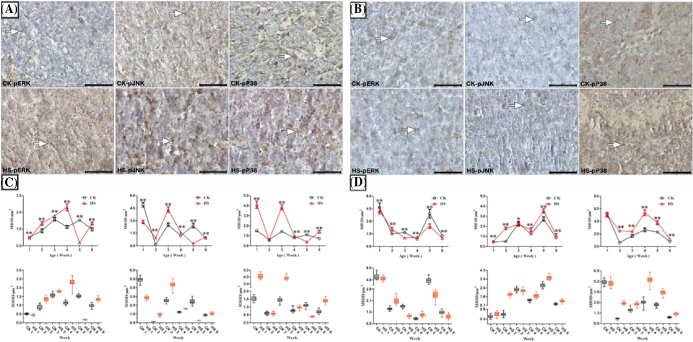
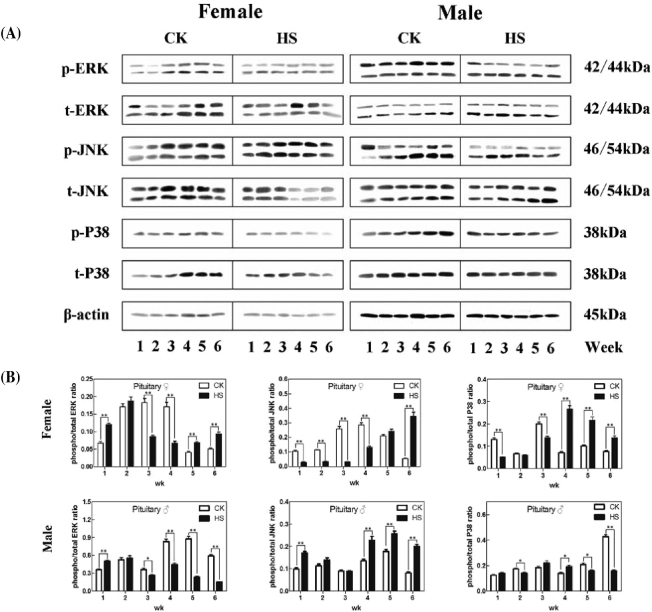
Observation of the IHC staining results of the pituitary in chicks showed that positive ERK, JNK, and p38 phosphorylation, which appeared as a brown coloration, were sporadically distributed in tissues of the pituitary, mainly appearing in the cytoplasm (Figures 3A and 3B). MIODs of ERK, JNK, and p38 phosphorylation were obtained after statistical analysis (Figures 3C and 3D). For females, MIODs of MAPKs of the pituitary in the HS group were all lower than those of the CK group in Week 5, but an opposite trend was observed in Weeks 3 and 6 (P < 0.01). For males, MIODs in HS were lower than those of the CK group in both Weeks 2 and 4 (P < 0.01). In addition, ERK, JNK, and P38 proteins in the pituitary were assessed in chicks (Figures 4A and 4B). The results indicated that the expression of MAPK proteins in the HS group of females was higher than in the CK group in Weeks 5 and 6, but this was a reversal of the trend in Week 3 (P < 0.01). However, male chicks showed a different pattern; in Week 1 the MAPKs of the HS group were expressed at a higher level than those of the CK group.
Figure 3.
Effect of heat stress on phospho-MAPKs in the pituitary of chicks (× 400, Bar = 50 μm, MIOD/μm2).
Figure 4.
Effect of heat stress on phospho-/total MAPKs in the pituitary of chicks.
Effects of HS on Ovarian MAPK Expression in Chicks
In IHC, positive ERK, JNK, and p38 phosphorylation appeared as a brown coloration that showed a scattered distribution in tissues of the ovary (Figure 5A). Those MIODs were obtained after statistical analysis (Figure 5C). MIODs of MAPKs in the HS group were higher than those of the CK group in Weeks 1 and 6, but an opposite trend was observed in Week 2 (P < 0.01). Furthermore, the detection of ERK, JNK, and P38 by Western blot showed a similar trend (Figure 6). The analysis showed that the expression level of MAPK proteins in the HS group was higher than that of the CK group in Week 5 (P < 0.01).
Figure 5.
Effect of heat stress on phospho-MAPKs in the gonads of chicks (× 400, Bar = 50 μm, MIOD/μm2).
Figure 6.
Effect of heat stress on phospho-/total MAPKs in the gonads of chicks.
Effects of HS on Testicular MAPK Expression in Chicks
Positive MAPK phosphorylation appeared as a brown coloration that showed scattered distribution in testicular tissues (Figure 5B). MIODs of ERK, JNK, and p38 phosphorylation were obtained after statistical analysis (Figure 5D). There was no collective trend in the same week among the MAPKs (ERK, JNK, and P38) when comparing groups CK and HS. The MAPK phosphorylation status in the testes was observed (Figure 6). The HS group experienced a relative upregulation in MAPK proteins compared to the CK group during the first week; this trend was reversed in Weeks 3, 5, and 6 (P < 0.01).
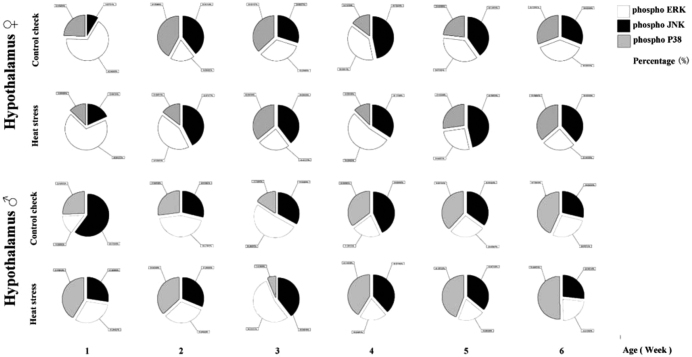
Effects of HS on MAPK Signaling Crosstalk in the Hypothalamus in Chicks
The percentages of phosphorylation in the 3 MAPK signaling pathways in the hypothalamus of chicks were recorded (Figure 7). For female chicks, the results showed that ERK phosphorylation played a major role in the HS group in Weeks 1, 2, and 4, as ERK phosphorylation was relatively higher in the HS group than in the CK group during these weeks. In Week 5, JNK signaling was dominant in the HS group. In Weeks 2 and 3, p38 signaling was dominant in the CK group. In contrast, for male chicks, the CK group mainly showed JNK phosphorylation during Week 1, whereas p38 had a dominant role in the HS group. During Week 3, the CK and HS groups showed an increase in the percentage of ERK phosphorylation. This switched to dominant JNK- and p38 phosphorylation in Weeks 4 to 5, and then p38 phosphorylation played a dominant role in Week 6.
Figure 7.
Effect of crosstalk among MAPKs signaling pathways in the hypothalamus of chicks. ♀ and ♂ reflect female and male chicks, respectively. phospho-ERK (pERK); phospho-JNK (pJNK); phospho-P38 (pP38), and the same below.
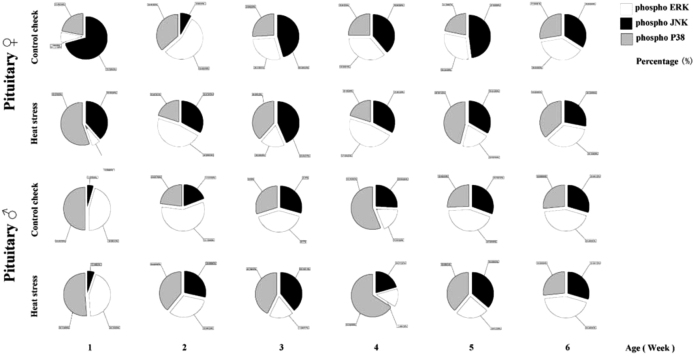
Effects of HS on MAPK Signaling Crosstalk in the Pituitary Glands in Chicks
The percentages of phosphorylation in the 3 MAPK signaling pathways in the pituitary glands of chicks were recorded (Figure 8). For females, the CK group mainly showed JNK phosphorylation in Week 1, while p38 played a dominant role in the HS group. During Week 2, the CK and HS groups mainly exhibited ERK phosphorylation; this was converted to an increase in the percentage of JNK phosphorylation during Week 3. The CK group showed JNK phosphorylation throughout Week 5, while p38 played a dominant role in the HS group at that time. All percentages became stable in Week 6.
Figure 8.
Effect of crosstalk among MAPKs signaling pathways in the pituitary of chicks.
Results for males showed that the CK and HS groups had dominant ERK and p38 phosphorylation in Week 1. During Week 2, in the CK group, the percentage of p38 phosphorylation decreased, and the percentage of JNK phosphorylation increased; thus, ERK phosphorylation was dominant. Conversely, the percentages of phosphorylation of the 3 MAPKs were balanced in the HS group. In Week 4, the percentage of p38 phosphorylation increased and was more noticeable in the HS group. Subsequently, during Weeks 5 and 6, the percentages of phosphorylation of the 3 MAPKs were comparable.
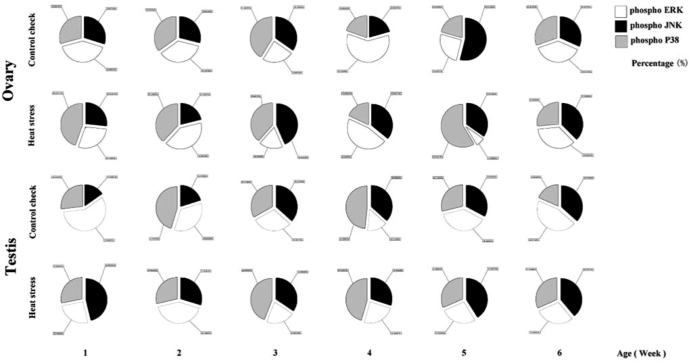
Effects of HS on MAPK Signaling Crosstalk in the Gonads in Chicks
The percentages of phosphorylation in the 3 MAPK signaling pathways in the gonads of chicks were recorded (Figure 9). The CK group exhibited dominant JNK and p38 phosphorylation in Week 3, and this changed to dominant ERK phosphorylation in Week 4 and then dominant JNK phosphorylation for the remaining time. In the HS group, p38 phosphorylation was dominant in Weeks 1 and 5, both ERK and p38 phosphorylation dominated in Week 2, p38 and JNK phosphorylation dominated in Week 3, ERK and JNK phosphorylation dominated in Week 4, and the phosphorylation of these 3 MAPKs were comparable in Week 6. However, the CK group exhibited dominant JNK and p38 phosphorylation during Week 1. This changed to p38 and ERK phosphorylation in Week 2, followed by dominant JNK (and p38) phosphorylation during Week 4. The CK group displayed dominant ERK and JNK phosphorylation in Week 6. In the HS group, JNK phosphorylation dominated in Week 1, and this changed to dominant p38 phosphorylation in Weeks 3 and 4. The phosphorylation levels of the 3 MAPKs were comparable in Weeks 5 and 6.
Figure 9.
Effect of crosstalk among MAPKs signaling pathways in the gonads of chicks. Ovary and testis are gonads belonging to female and male chicks, respectively.
DISCUSSION
The hypothalamus is a nexus that regulates body temperature and thirst in chickens. Collectively, Weeks 1 and 2 are an extremely crucial brooding stage for chicks. This is because the thermoregulation system of chicks has not yet matured, and they are particularly sensitive to ambient temperature (Tzschentke and Basta, 2000, 2002). Under a low ambient temperature, chicks will maintain their body temperature by flocking or by eating feed. The MAPK signaling pathways are in a relatively disordered state at this early stage of life. Therefore, HS can promote upregulation of the hypothalamic ERK signaling pathway in female chicks during Weeks 1 and 2, and this is also accompanied by a higher expression of the stress response JNK signaling pathway, particularly in Week 1. During Weeks 3 and 4, the hypothalamic MAPKs showed relatively identical expression levels in the HS and CK groups. Following this stage, chicks enter a rapid growth phase during Weeks 5 and 6. Long-term HS damages the hypothalamus of chicks, reducing the neuronal cell body area and distribution area (Lu et al., 2018). In our study, HS caused JNK and p38 expression levels to be higher for female chicks in the HS group than in the CK group, resulting in functional compensation. However, male chicks showed some different expression levels of hypothalamic MAPKs as compared to that of female chicks. This occurs because male chicks enter the stage of fighting for a place in the pecking order. Male chicks from the non-heat stress group had more energy than those from the HS group. Therefore, the hypothalamic JNK signals (stress signals) of male chicks in the non-heat stress group were higher than in the HS group.
The pituitary gland is the most complex endocrine organ in chickens. Female chicks are at the brooding stage in the first 2 wk of life, and protein expression levels of ERK in the HS group were found to be relatively higher than those of the CK group, but JNK and P38 were lower. At this time, the environment did not significantly affect the chicks' thermoregulation system. Then, the HS group displayed downregulated MAPK proteins from Weeks 3 to 4, except for P38 in Week 4. Interestingly, the MIOD for JNK in the HS group was higher than that of the CK group; however, the protein phosphorylation levels were lower. This suggests that HS increases tolerance to high temperatures in female chicks to some extent. Finally, MAPKs had been upregulated in HS compared with CK in Weeks 5 and 6. However, the weights of the pituitary glands in the HS group were lower than those of the CK group (Liang et al., 2018), suggesting that metabolic compensation (alkalosis) occurred in the pituitary glands of chicks that experienced 5 to 6 wk of HS. Compared with female chicks, there were several differences in the male chicks: the protein expression of JNK was upregulated under HS in the first 2 wk, and P38 of the HS group experienced an upregulation relative to that of the CK group in first week. Ultimately, except JNK to keep level in both sexes of chicks; ERK and P38 in the last 2 wk were downregulated under HS. In a nutshell, compared to female chicks, the ERK and p38 signaling pathways developed much earlier in male chicks during the brooding stage. Furthermore, the percentage of JNK signaling pathway activation was higher in Weeks 3 and 5. This suggests that there are gender-specific differences in the anti-heat stress strategies in chicks.
The degree of gonadal development determines the production performance of the chick and its progeny. For female chicks, HS chicks have smaller individual size, lower egg yield (calcium loss), and early maturing of ovaries (Leeson and Summers, 1981). In the first 3 wk, HS upregulated ERK phosphorylation levels; a turning point occurred in Week 4, and compensation occurred during Weeks 5 and 6. The weights of ovaries from the HS group were higher than in the CK group during Weeks 2 and 3 (Liang et al., 2018). Although HS activated JNK stress proteins during Week 1, it also promotes ovary development to some extent, which may lead to signs of early maturation. In the HS group, this was shown as higher GnRH levels in the first 2 wk compared with the CK group (Lu, 2018), which also promotes the development of the HPG axis. Although daily 2 h acute HS treatments caused the MIOD of JNK in the HS group to be upregulated in Weeks 3 and 4, protein phosphorylation levels were lower compared with those of the CK group. This shows that HS activated JNK stress signals in Week 1 and strengthened the heat adaptability of the HS group until metabolic compensation by JNK signaling took place during Weeks 5 and 6. In contrast, JNK expression was low throughout Weeks 3 and 4 in the CK group. This ultimately caused the ovary weight of female chicks in the CK group to be higher than that of the HS group (Liang et al., 2018). Unlike JNK, the p38 phosphorylation level of the HS group in the first 3 wk was downregulated compared with that of the CK group. However, the p38 phosphorylation levels of the HS group became upregulated from Weeks 4 to 6. This indicates that long-term HS causes female chicks to generate a survival strategy; this strategy consists of mainly differentiating small cells and adjusting body temperature by accelerating the metabolism. For male chicks, HS was found to upregulate ERK phosphorylation levels in the first 2 wk of the brooding stage. In Week 1, the weight of the testis in the HS group was higher than that of the CK group before becoming comparable in Week 2 (Chen et al., 2015). This finding suggests that HS accelerates testicular development. During the last 4 wk, in the HS group, ERK phosphorylation levels and total ERK protein in the testis were downregulated compared with those of the CK group. This suggests that persistent HS not only disrupts testicular structure but also affects the normal regulation of the ERK signaling pathway. Further studies are needed to determine how regulation occurs; this would involve measuring the levels of related hormones. Throughout the first 3 wk, changes in testicular JNK phosphorylation were consistent with pituitary JNK phosphorylation levels in both the CK and HS groups; this was due to positive regulation by the pituitary gland. However, the MIOD and protein phosphorylation levels for p38 showed opposite trends, and this result is key for examining how p38 participates in the regulation of HS.
The 3 MAPK signaling pathways in the HPG axis in chicks were relatively disordered in the first 3 wk before becoming stable from Weeks 4 to 6. In addition, as the chicks aged, the BW, cell diameter, and density of various tissues in the HPG axis in the HS group became significantly lower than in the CK group (Liang et al., 2018; Lu et al., 2018). The p38 expression level in the HS group was higher than that of the CK group, opposite to the principle of positive regulation. This is because high p38 expression in the CK group was due to cell differentiation, which promotes cell proliferation. However, high p38 expression in the HS group was mainly due to damaged tissues and physiological functions resulting in the promotion of cell differentiation, ultimately inhibiting excessive apoptosis in cells.
When animals experience nutrient deficiency or physical injury, they maximize, not optimize, their growth rate for compensation in order to recover from slow development (Metcalfe and Monaghan, 2001). The HPG axis in birds primarily develops in the embryo and during the first few weeks after birth. The HPG axis exhibits plasticity in the few months following incubation as well as during reproduction and exhibits sexual dimorphism due to differences in the levels of serum hormones including testosterone (T) and the ratios of T to estradiol (E2) and estrone to E2 during the incubation period (Ottinger et al., 2001; MacManes et al., 2017; Wang et al., 2019). Under stress conditions, the housing environment changes the resource allocation in birds and affects the HPG axis, causing changes in reproduction (Farrell et al., 2015). Additionally, during the early stages of life after chicks are first hatched, they are sensitive to the external environment, and temperature regulation and immune systems are not yet mature (Liu, 2012). Heat stress can trigger MAPK crosstalk to initiate the hyperosmotic response pathway (Dunayevich et al., 2018). Therefore, during the early brooding stage, high temperature and humidity challenge training may aid chicks in surviving warm environments. In particular, this training may facilitate the development of temperature-sensitive neurons in the MAPK signaling pathway in the HPG axis and can improve the regulation and immune tolerance of chicks to external environmental changes, particularly in tropical and subtropical regions (i.e., high temperatures mid-day and drastic decrease in temperatures at night). Heat training in young broiler chicks at an early stage may result in better heat resistance at the physiological level (Borges et al., 2003; Hassan and Reddy, 2012), which is a heritable trait (Lara and Rostagno, 2013; Wan et al., 2017). Therefore, stress also has a positive aspect (Dhabhar, 2014). However, the duration and intensity of HS/heat attack training should be combined with local conditions. As chicks grow and gradually mature, various systems of the chick body become stable. If chicks remain in a high-temperature and high-humidity environment, premature compensation in the growth and development of various tissues and organs, as well as their signaling pathways, will occur. This manifests as calcium loss, shorter adult stature, and chickens with poorer meat quality. Egg quality can also be affected (Sahin et al., 2002; Zhang et al., 2012).
CONCLUSION
In summary, the effects of HS on the growth and development of chicks are significant during the early brooding stage as the MAPK signaling pathways in the HPG axis are developed. However, after heated brooding is discontinued, if chicks have undergone HS training and possess some heat resistance, the environment must be controlled. Specifically, the temperature must be made comfortable for chicks in order to avoid HS-induced injuries.
ACKNOWLEDGEMENTS
This work was supported by research grants from National Natural Science Foundation of China (NFSC 31560680, Beijing). We express our high appreciation to Jia-ying Tian, Hainan Normal University, for the assistance with sample collection.
REFERENCES
- Ajakaiye J.J., Perez-Bello A., Mollineda-Trujillo A. Impact of heat stress on egg quality in layer hens supplemented with I-ascorbic acid and dl-tocopherol acetate. Vet. Arhiv. 2011;81:119–132. [Google Scholar]
- Borges S.A., Silva A.V.F.D., Ariki J., Hooge D.M., Cummings K.R. Dietary electrolyte balance for broiler chickens exposed to thermoneutral or heat-stress environments. Environ. Poult. Sci. 2003;82:428–435. doi: 10.1093/ps/82.3.428. [DOI] [PubMed] [Google Scholar]
- Bungsrisawat P., Tumwasorn S., Loongyai W., Nakthong S., Sopannarath P. Genetic parameters of some carcass and meat quality traits in Betong chicken (KU line) Agr. Nat. Resour. 2018;52:1–20. [Google Scholar]
- Chang L., Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- Chen Z., Xie J., Wang B., Tang J. Effect of γ-aminobutyric acid on digestive enzymes, absorption function, and immune function of intestinal mucosa in heat-stressed chicken. Poult. Sci. 2014;93:2490–2500. doi: 10.3382/ps.2013-03398. [DOI] [PubMed] [Google Scholar]
- Chen Z., Zhang J.R., Zhou Y.W., Liang C., Jiang Y.Y. Effect of heat stress on the pituitary and testicular development of Wenchang chicks. Archiv. Anim. Breed. 2015;58:373–378. [Google Scholar]
- Chen Z., Zhou Y.W., Liang C., Jiang Y.Y., Xie L.J. Effects of γ-aminobutyric acid on the tissue structure, antioxidant activity, cell apoptosis, and cytokine contents of bursa of Fabricius in chicks under heat stress. Archiv. Anim. Breed. 2016;59:97–105. [Google Scholar]
- Dhabhar F.S. Effects of stress on immune function: the good, the bad, and the beautiful. Immunol. Res. 2014;58:193–210. doi: 10.1007/s12026-014-8517-0. [DOI] [PubMed] [Google Scholar]
- Dosio A., Mentaschi L., Fischer E.M., Wyser K. Extreme heat waves under 1.5 °C and 2 °C global warming. Environ. Res. Lett. 2018;13 [Google Scholar]
- Dunayevich P., Baltanás R., Clemente J.A., Couto A., Sapochnik D., Vasen G., Colman-Lerner A. Heat-stress triggers MAPK crosstalk to turn on the hyperosmotic response pathway. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-33203-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell T.M., Morgan A., Sarquis-Adamson Y., MacDougall-Shackleton S.A. Effects of early-developmental stress on growth rates, body composition and developmental plasticity of the HPG-axis. Gen. Comp. Endocr. 2015;222:134–143. doi: 10.1016/j.ygcen.2015.08.001. [DOI] [PubMed] [Google Scholar]
- Hassan A.M., Reddy P.G. Early age thermal conditioning improves broiler chick's response to acute heat stress at marketing age. Am. J. Anim. Vet. Sci. 2012;7:1–6. [Google Scholar]
- Juárez-Rojas A.L., García-Lorenzana M., Aragón-Martínez A., Gómez-Quiroz L.E., del Socorro Retana-Márquez M. Intrinsic and extrinsic apoptotic pathways are involved in rat testis by cold water immersion-induced acute and chronic stress. Syst. Biol. Reprod. Med. 2015;61:211–221. doi: 10.3109/19396368.2015.1030473. [DOI] [PubMed] [Google Scholar]
- Junttila M.R., Li S.P., Westermarck J. Phosphatase-mediated crosstalk between MAPK signaling pathways in the regulation of cell survival. FASEB J. 2008;22:954–965. doi: 10.1096/fj.06-7859rev. [DOI] [PubMed] [Google Scholar]
- Kanginakudru S., Metta M., Jakat R., Nagaraju J. Genetic evidence from Indian red jungle fowl corroborates multiple domestication of modern day chicken. BMC Evol. Biol. 2008;8:174. doi: 10.1186/1471-2148-8-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara L.J., Rostagno M.H. Impact of heat stress on poultry production. Ainmals. 2013;3:356–369. doi: 10.3390/ani3020356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeson S., Summers J.D. Effect of rearing diet on performance of early maturing pullets. Can. J. Anim. Sci. 1981;61:743–749. [Google Scholar]
- Lefevre S., McKenzie D.J., Nilsson G.E. In modelling effects of global warming, invalid assumptions lead to unrealistic projections. Global Change Biol. 2017;24:553–556. doi: 10.1111/gcb.13978. [DOI] [PubMed] [Google Scholar]
- Lewis T.S., Shapiro P.S., Ahn N.G. Signal transduction through MAP kinase cascades. Adv. Cancer. Res. 1998;74:49–139. doi: 10.1016/s0065-230x(08)60765-4. [DOI] [PubMed] [Google Scholar]
- Liang C., Xie X.Z., Zhou Y.W., Jiang Y.Y., Xie L.J., Chen Z. Effects of γ-aminobutyric acid on the thymus tissue structure, antioxidant activity, cell apoptosis, and cytokine levels in chicks under heat stress. Czech. J. Anim. Sci. 2016;61:539–550. [Google Scholar]
- Liang W., Lu B.B., Li Q.H., Chen Z. Effect of heat stress on the growth and development of pituitary and ovary in Wenchang chicks. Heilongjiang Anim. Sci. Vet. Med. 2018;36:20–23. [Google Scholar]
- Liu C. Feeding and management measures in during stage of laying hen. China Poult. 2012;34:57–58. [Google Scholar]
- Liu Y.P., Wu G.S., Yao Y.G., Miao Y.W., Luikart G., Baig M., Ding Z.L., Palanichamy M.C., Zhang Y.P. Multiple maternal origins of chickens: out of the Asian jungles. Mol. Phylogenet. Evol. 2006;38:12–19. doi: 10.1016/j.ympev.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Lu B.B. Hainan Normal Univ.; Haikou: 2018. Effect of heat stress on development of GABAergic neurons and reproductive hormone cells in HPT axis of Wenchang chicks. MSc Diss. [Google Scholar]
- Lu B.B., Liang W., Xie X.Z., Chen Z. Effects of heat stress on growth and development of HPT axis tissues in Wenchang chicks. China Poult. 2018;40:34–39. [Google Scholar]
- MacManes M.D., Austin S.H., Lang A.S., Booth A., Farrar V., Calisi R.M. Widespread patterns of sexually dimorphic gene expression in an avian hypothalamic–pituitary–gonadal (HPG) axis. Sci. Rep. 2017;7 doi: 10.1038/srep45125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe N.B., Monaghan P. Compensation for a bad start: grow now, pay later? Trends Ecol. Evol. 2001;16:254–260. doi: 10.1016/s0169-5347(01)02124-3. [DOI] [PubMed] [Google Scholar]
- Mosca F., Kuster C.A., Stella S., Farina G., Madeddu M., Zaniboni L., Cerolini S. Growth performance, carcass characteristics and meat composition of Milanino chickens fed on diets with different protein concentrations. Br. Poult. Sci. 2016;57:531–537. doi: 10.1080/00071668.2016.1174768. [DOI] [PubMed] [Google Scholar]
- Ottinger M.A., Nisbet I.C.T., Finch C.E. Aging and reproduction: comparative endocrinology of the common tern and Japanese quail. Am. Zool. 1995;35:299–306. [Google Scholar]
- Ottinger M.A., Pitts S., Abdelnabi M.A. Steroid hormones during embryonic development in Japanese quail: plasma, gonadal, and adrenal levels. Poult. Sci. 2001;80:795–799. doi: 10.1093/ps/80.6.795. [DOI] [PubMed] [Google Scholar]
- Peters J., Lebrasseur O., Deng H., Larson G. Holocene cultural history of Red jungle fowl (Gallus gallus) and its domestic descendant in East Asia. Quat. Sci. Rev. 2016;142:102–119. [Google Scholar]
- Ramnath V., Rekha P.S., Sujatha K.S. Amelioration of heat stress induced disturbances of antioxidant defense system in chicken by brahma rasayana. Evid. Based Complement. Alternat. Med. 2008;5:77–84. doi: 10.1093/ecam/nel116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed M., Babazadeh D., Naveed M., Arain M.A., Hassan F.U., Chao S. Reconsidering betaine as a natural anti-heat stress agent in poultry industry: a review. Trop. Anim. Health. Pro. 2017;49:1329–1338. doi: 10.1007/s11250-017-1355-z. [DOI] [PubMed] [Google Scholar]
- Sahin K., Sahin N., Onderci M. Vitamin E supplementation can alleviate negative effects of heat stress on egg production, egg quality, digestibility of nutrients and egg yolk mineral concentrations of Japanese quails. Res. Vet. Sci. 2002;73:307–312. doi: 10.1016/s0034-5288(02)00126-1. [DOI] [PubMed] [Google Scholar]
- Sockman K.W., Williams T.D., Dawson A., Ball G.F. Prior experience with photostimulation enhances photo-induced reproductive development in female European Starlings: a possible basis for the age-related increase in avian reproductive performance. Biol. Reprod. 2004;71:979–986. doi: 10.1095/biolreprod.104.029751. [DOI] [PubMed] [Google Scholar]
- Song Y., Cen Y., Xu X.W., Liu Y. Nerve regeneration of hypertrophic scars and keloids after deep burns. J. Sichuan. Univ. (Med. Sci. Ed.). 2005;36:797–799. [PubMed] [Google Scholar]
- Tzschentke B., Basta D. Development of hypothalamic neuronal thermosensitivity in birds during the perinatal period. J. Therm. Biol. 2000;25:119–123. [Google Scholar]
- Tzschentke B., Basta D. Early development of neuronal hypothalamic thermosensitivity in birds: influence of epigenetic temperature adaptation. Comp. Biochem. Phys. A. 2002;131:825–832. doi: 10.1016/s1095-6433(02)00020-x. [DOI] [PubMed] [Google Scholar]
- Wan Y., Ma C.D., Wei P.P., Fang Q., Guo X., Zhou B.Y., Jiang R.S. Dynamic expression of HSP90B1 mRNA in the hypothalamus of two Chinese chicken breeds under heat stress and association analysis with a SNP in Huainan chickens. Czech. J. Anim. Sci. 2017;62:82–87. [Google Scholar]
- Wang Y.L., Jin G.F., Ma M.H., Xiang X.L. Sex differences in serum steroid hormone levels during embryonic development in hen eggs. Poult. Sci. 2019 doi: 10.3382/ps/pez270. [DOI] [PubMed] [Google Scholar]
- Wang Y., Saelao P., Chanthavixay K., Gallardo R., Bunn D., Lamont S.J., Dekkers J.M., Kelly T., Zhou H. Physiological responses to heat stress in two genetically distinct chicken inbred lines. Poult. Sci. 2017;97:770–780. doi: 10.3382/ps/pex363. [DOI] [PubMed] [Google Scholar]
- Wol A., Arango J., Settar P., Fulton J.E., O'Sullivan N.P., Dekkers J.C.M. Genome wide association study for heat stress induced mortality in a white egg layer line. Poult. Sci. 2019;98:92–96. doi: 10.3382/ps/pey403. [DOI] [PubMed] [Google Scholar]
- Yin F., Chen Z., Li Z.W., Tang J. Influence of acute heat stress on the development of GABAergic neurons in HPA-axies of mouse embryos. J. Therm. Biol. 2011;36:486–491. [Google Scholar]
- Zeng T., Zhang L.P., Li J.J., Wang D.Q., Tian Y., Lu L.Z. De novo assembly and characterization of Muscovy duck liver transcriptome and analysis of differentially regulated genes in response to heat stress. Cell Stress Chaperones. 2015;20:483–493. doi: 10.1007/s12192-015-0573-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z.Y., Jia G.Q., Zuo J.J., Zhang Y., Lei J., Ren L., Feng D.Y. Effects of constant and cyclic heat stress on muscle metabolism and meat quality of broiler breast fillet and thigh meat. Poult. Sci. 2012;91:2931–2937. doi: 10.3382/ps.2012-02255. [DOI] [PubMed] [Google Scholar]