Abstract
Early-life exposure to stressors can shape the phenotype of the offspring resulting in changes that may affect their prehatch and posthatch development. This can be modeled indirectly through maternal exposure to stressors (natural model) or by offspring exposure to stress hormones (pharmacological model). In this study, both models were used to investigate the effects of genetic line on hatchability, late embryonic mortality, sex ratio, and body weight until 17 wk of age. To form the parent stock, fertilized eggs of 4 commercial genetic lines — two brown (brown 1 and 2), two white (white 1 and 2), and a pure line White Leghorn — were incubated, hatched, and housed identically in 4 flocks of 27 birds (24 females and 3 males) per strain. Each strain was equally separated into 2 groups: “maternal stress,” where hens were subjected to a series of acute psychological stressors (e.g., physical restraint, transportation) for 8 D before egg collection, and “control,” where hens received routine husbandry. At 3 maternal ages, fertile eggs from both treatments were collected, and additional eggs from the control group were injected with corticosterone (10 ng/mL egg content) (“CORT”). A “vehicle” treatment was included to account for effects of egg manipulation. Each maternal age comprised a replicate over time. Eggs were incubated and hatched, and the offspring (N = 1,919) were brooded until 17 wk under identical conditions. The results show that prenatal stress interacted with strain to decrease embryonic survival and growth. Among all strains, brown 2 was consistently the most affected line in both prehatch and posthatch development. Our study shows that embryonic survival and offspring growth are mostly affected by the pharmacological model and that strain differences may increase susceptibility to prenatal stress. Moreover, it suggests that the natural stressor model may be useful for quantifying the response of the mother to stressors, whereas the pharmacological model may be useful for quantifying the response of the embryo to increased levels of corticosterone.
Key words: breeder flock, chicken, corticosterone, genetics, prenatal stress
Introduction
The prenatal bond between the mother and offspring involves mechanisms that can shape the phenotype of the progeny in both genetic and nongenetic ways. In birds, stressful events experienced by the female can modify the deposition of nutrients and resources into the egg. It has been suggested that these changes may signalize the future environment to the embryo (Gluckman et al., 2005, Williams and Groothuis, 2015), allowing for adaptive phenotypic modifications in the offspring (Mousseau and Fox, 1998, Gluckman et al., 2005, Podmokła et al., 2018).
The effects of maternal stress on egg composition have been extensively studied in birds (e.g., hormones (Carter et al., 2018), antioxidants (Possenti et al., 2018), and immunoglobulins (Roth et al., 2018)). Gonadal steroid hormones, and particularly androgens, are known to be important mediators of maternal effects. In addition, the steroid hormone corticosterone has attracted considerable attention as a maternal cue because of its characteristics as the final product of the stress response and its physiological capacity to mediate different traits on the developing embryo (reviewed in the study by Groothuis et al., 2019). Embryonic production of corticosterone naturally stimulates the synthesis and maturation of vital organs such as the lung, small intestine, liver, adrenals, and kidney, among other functions (Jenkins and Porter, 2004, Wada, 2008). However, increased concentrations of this hormone can impair offspring traits such as embryonic development, hatchability, and body weight (Eriksen et al., 2003, Henriksen et al., 2013, Tissier et al., 2014). Moreover, differences in the sex ratio due to prenatal stress have been reported in quails (Bonier et al., 2007) and passerines (Love and Williams, 2008) and might also be related to increased levels of glucocorticoids in the egg.
In laboratory conditions, 2 approaches can be used to increase the levels of corticosterone exposure experienced by the embryo: a natural stress model that consists of maternal exposure to stressors (either naturally or through corticosterone injections or implants) and a pharmacological model that involves egg manipulation. Maternal stress presents little risk of damaging the egg or harming the embryo (Podmokła et al., 2018) and does not preclude other mediators that might also have an effect on embryonic development. However, it is difficult to accurately measure the concentration of the hormone reaching the embryo owing to the hen's ability to adjust circulating steroids in the egg (Groothuis and Schwabl, 2008, Williams and Groothuis, 2015) and the embryonic capability to modulate corticosterone levels through rapid metabolization (Vassallo et al., 2014). Contrarily, manipulation of the egg allows a precise hormone increase but through the use of an invasive mechanical procedure involving injection, normally harmful to the embryo (Heiblum et al., 2001, Janczak et al., 2007a). Moreover, as pointed out by Groothuis et al. (2019), responses to the hormone are often dose dependent, and the actual physiological concentrations delivered to the egg from the hen remain largely unknown (Rettenbacher et al., 2013a).
A vast body of literature already exists with respect to the long-term effects of prenatal corticosterone on avian morphology (reviewed in the study by Henriksen et al., 2011), but to our knowledge, the influence of genetics on this matter has not yet been regarded. Domestication of the wild jungle fowl and artificial genetic selection for traits desired by humans have not only created a large number of breeds with several phenotypical differences but have also increased prenatal stress susceptibility in chickens (Nätt et al., 2012); however, this has only been tested in one strain of birds, and it is possible that phylogenetically distant groups, such as brown and white strains, respond differently to the same effect.
Herein, we aimed to investigate how genetics interacts with prenatal stress to affect embryonic survival and growth in the offspring of layer breeders. For this, we tested 2 stress models, maternal stress and corticosterone injections “CORT” in 5 genetic lines of breeder hens. Based on the phenotypic differences observed across strains and the differences of each stress model, we hypothesized that offspring response to prenatal stress will depend on the genetics of the breeder flock and will be more easily observed in the pharmacological model.
Materials and methods
The effects of 2 prenatal stress models (maternal stress vs. CORT) and a vehicle control treatment were assessed on the hatchability, late embryonic mortality, sex ratio, and body weight from hatch to the age of 17 wk of the offspring of 5 strains of layer breeders. Stressors were applied at 3 maternal ages (32, 52, and 72 wk), resulting in different cohorts of the progeny that were treated as replicates across time. Treatments aimed for 2 repetitions of 20 birds per strain, but the vehicle and CORT treatments frequently failed to achieve these numbers owing to high embryonic mortality. All birds were treated in accordance with the Canadian Council on Animal Care, and all procedures were approved by the University of Guelph Animal Care Committee (Animal Utilization Protocol #1946).
Parent Stock: Management
A total of 2,600 fertilized eggs of parent stock were provided by genetic companies 1 and 2 (brown 1 and white 1, brown 2 and white 2) and the University of Guelph's Arkell Poultry Research Station (pure line White Leghorn). To guarantee similar experiences, eggs from all strains were collected from grandparent hens between 40 and 50 wk of age and subjected to identical incubation and husbandry conditions. The eggs were stored at 4°C for a maximum of 9 D and hatched at the University of Guelph's Arkell Poultry Research Station using commercial-grade incubators and hatchers (NatureForm, Jacksonville, FL). On day 19, eggs were candled, and only the ones containing live embryos were transferred to the hatcher. The birds were sexed and vaccinated for bronchitis (spray), Marek's disease (injection), and Immucox (gel droplet) on day 1 at the hatchery. The vaccination program also included the following: Newcastle-bronchitis vaccine (spray) at 3 wk, ILT Vectormune FP-LT-AE (wing web) at 6 wk, and Newcastle-bronchitis at 10 (spray) and 16 (intramuscular) wk.
Groups of 130 females and 20 males per strain were formed and placed into floor pens bedded with litter, with a space allowance of 98 cm2/bird. Birds were wing banded and beak treated by professionals on days 7 and 11, respectively, and the analgesic (acetylsalicylic acid) diluted in water was provided for 3 D after the latter procedure (Machin, 2005). At 6 wk of age, each strain was equally distributed to 4 parent flocks of 27 birds (24 females and 3 males) and kept until 76 wk of age. Each pen (3.7 m2) contained pine shavings and one perch. To cover account for unexpected losses (e.g., mortality, sickness, low body weight), extra birds (1 male and 2 females) were kept in each group until 21 wk of age. The flocks were reared under identical feeding, temperature, and lighting programs. At 18 wk, 5 nest boxes were added into each one of the pens. Egg collection was performed and recorded daily from 21 to 75 wk between 7:30 and 9:00 h. Despite routine husbandry, all human interactions with the birds were avoided to prevent possible habituation.
Parent Stock: Control and Stress Treatments
Each strain of parent stock was equally assigned to control and maternal stress treatments with 2 replicate flocks per strain and treatment. Control groups were strictly subjected to regular husbandry, whereas maternal stress groups were subjected to daily sessions of acute psychological procedures that have been shown to elevate plasma corticosterone (Table 1). The average time window for egg production, from the beginning of vitellogenesis until laying, is 8 D. Thus, each flock in the maternal stress group received a minimum of 8 consecutive days of stressors before the beginning of egg collection. For stressors 3, 4, and 5 (Table 1), females were crated and moved to a testing arena measuring 100 cm × 100 cm × 200 cm (length × width × height), constructed of solid panels with 2 doors located on opposite walls and 2 light emitting diode (LED) lights on the ceiling. The birds were immediately returned to home pens after application of stressors.
Table 1.
List of acute psychological stressors applied to breeder stock females.
| Stressor | Reference | |
|---|---|---|
| 1 | Crating followed by 15 min of transportation | Zulkifli et al., 2009 |
| 2 | Physical restraint in a cloth bag for 10 min | Ericsson et al., 2016 |
| 3 | Crating followed by 30 min in a testing arena and 3 simulated predator attacks of 30 s each | Pitk et al., 2012 |
| 4 | Crating followed by 15 min in a testing arena and 3 acute auditory stressors (air horn) | Guibert et al., 2011 |
| 5 | Crating followed by 30 min of social interaction with another strain in a testing arena | Siegel, 1980 |
Overall, stress sessions respected the following criteria: (1) Flocks were subjected to one stressor a day; (2) The total number of sessions depended on the egg production of each flock; (3) The same stressors were never applied consecutively to avoid habituation; (4) Sessions ran randomly from 9:00 to 16:00 h. Procedures were performed at 3 maternal ages (32, 52, and 72 wk), resulting in different offspring groups. Each group was treated as a replicate comprised over time. This design allowed us to work with a large sample size, but it also resulted in replicates confounded with incubatory settings, chick placement, and egg composition because the nutritional value of the egg changes as a hen ages (Nielsen, 1998). The eggs were collected and stored for a maximum of 9 D at 4°C until incubation.
Parent Stock: Vehicle and CORT Treatments
The CORT treatment aimed to simulate eggs that received an increased concentration of corticosterone from a stressed hen. The concentration of corticosterone in egg yolks has been previously reported to range from 0.77 to 2.8 ng/g in Hy-Line Brown (Navara and Pinson, 2010, Ahmed et al., 2014, Engel et al., 2019) to average 1.6 ng/g in Hy-Line White (Navara and Pinson, 2010) and 2.13 ng/g in Bovan White (Haussmann et al., 2012) under control conditions. However, analytical validation of enzyme immunoassay and radioimmunoassay techniques showed the presence of cross-reactive substances that hamper quantification of corticosterone in the yolk and albumen of eggs (Rettenbacher et al., 2013a). Because the exact concentration of corticosterone in eggs remains unknown, we followed the methodology and dosage developed by Janczak et al. (2007a) and subsequently used by Haussmann et al. (2012). The CORT treatment group received a final concentration of 10 ng of corticosterone/mL of egg content (corticosterone: Sigma-Aldrich Chemical Co., St. Louis, MO) diluted in sesame oil (Fisher Scientific Co., Fair Lawn, NJ), while the vehicle treatment group received the same concentration of sesame oil only. The average weight of egg content (90% of egg weight) (Beuving and Vonder, 1981) per offspring group was 50, 50, and 59 g. Thus, 50 μL of solution was injected into eggs from breeders of 32 and 52 wk of age, whereas 60 μL was injected into eggs from 72-wk-old hens.
When breeders were at 25 wk of age, we collected eggs from all strains and conducted a pilot study to estimate hatchability levels. Eggs used to form the different offspring groups were selected according to weight (between 52 and 70 g) and day of laying (recent over old). One day before incubation, a 5 x 5 mm layer of silicone sealant (General Electric, Boston, MA) was applied onto the basal tip of the eggs designed to form the injected treatments to prevent gas exchange and contamination. On the morning of each incubation day, a stock solution of 2.5 mg corticosterone was diluted in 2.5 mL sesame oil, warmed to 100°C, and sonicated for 15 min. Both CORT and vehicle solutions were sterilized at 180°C for 30 min and let to cool down to room temperature. Moments before incubation, 1-ml syringes were filled, and treatments were injected 5 mm into the albumen through the silicone seal using 23-gauge needles.
Offspring Stock: Incubation and Embryonic Data Collection
Egg collection, incubation, and hatch occurred under similar conditions at different maternal ages, resulting in 3 offspring groups replicated over time. On day 19 of incubation, the eggs were candled and transferred to the hatcher. After observing an unusual number of dead embryos at different ontogenetic stages in the first replicate group, we began to record day of embryonic mortality for replicates 2 and 3 (N = 1,602), which was determined by comparison with pictures of embryos at different ontogenetic phases (Hamburger and Hamilton, 1951). For statistical reasons, data were organized in 2 categories: early mortality, if before 10 D of development, and late mortality, from 11 to 16 D. Immediately after hatch, we recorded the number and sex of the healthy progeny to determine hatchability and sex ratio (N = 1,919).
Offspring Stock: Housing Condition and Posthatch Data Collection
The offspring were wing banded at hatch and equally distributed to 3.72 m2 pens with a perch and litter floor. Our experimental design aimed for 2 replicates with 20 birds each (10 females and 10 males) per treatment and strain. However, final numbers varied owing to low hatchability of injected treatments (Table 2). All birds (N = 1,919) were weighed at hatch and at 2, 4, 8, 11, 13, 15, and 17 wk of age.
Table 2.
Number of chicks placed in pens by strain, treatment, and offspring group.
| Strain | Treatment | Offspring group |
|||||
|---|---|---|---|---|---|---|---|
| 1 |
2 |
3 |
|||||
| Pen 1 | Pen 2 | Pen 1 | Pen 2 | Pen 1 | Pen 2 | ||
| Brown 1 | Control | 20 | 20 | 20 | 19 | 20 | 20 |
| Maternal stress | 20 | 20 | 20 | 20 | 20 | 20 | |
| Vehicle | 11 | 11 | 20 | 20 | 19 | 18 | |
| CORT | 12 | 12 | 21 | 0 | 20 | 0 | |
| Brown 2 | Control | 20 | 20 | 19 | 20 | 20 | 20 |
| Maternal stress | 20 | 20 | 20 | 20 | 20 | 20 | |
| Vehicle | 10 | 0 | 20 | 20 | 16 | 15 | |
| CORT | 10 | 0 | 15 | 0 | 17 | 0 | |
| White 1 | Control | 20 | 20 | 20 | 20 | 20 | 20 |
| Maternal stress | 20 | 20 | 20 | 20 | 18 | 18 | |
| Vehicle | 13 | 12 | 17 | 16 | 20 | 20 | |
| CORT | 15 | 0 | 14 | 13 | 18 | 0 | |
| White 2 | Control | 20 | 20 | 20 | 20 | 20 | 20 |
| Maternal stress | 17 | 18 | 20 | 20 | 20 | 20 | |
| Vehicle | 18 | 0 | 20 | 20 | 20 | 20 | |
| CORT | 11 | 12 | 23 | 0 | 17 | 16 | |
| White Leghorn | Control | 14 | 14 | 20 | 20 | 17 | 17 |
| Maternal stress | 16 | 17 | 20 | 20 | 17 | 16 | |
| Vehicle | 11 | 0 | 14 | 12 | 23 | 0 | |
| CORT | 10 | 0 | 15 | 0 | 10 | 0 | |
Abbreviation: CORT, corticosterone.
Statistical Analyses
The Glimmix procedure of SAS 9.4 (SAS Institute, Cary, NC) was used to perform all statistical analyses. The basic statistical model included fixed effects of treatment, strain, and treatment-by-strain interaction. Further preplanned comparisons included treatment (control vs. all other treatments [referred to as stressors in tables] and control vs. each stress model) and strain (white vs. brown and genetic company 1 vs. genetic company 2). Tests for normality included Shapiro–Wilk and Anderson–Darling measurements in conjunction with visual plots. The level for assessment of statistical significance of differences between means was set at P < 0.05. The analyses controlled for the multiple testing error using the percentage of false positives, which estimates the false discovery rate (FDR) (García, 2016).
Hatchability, Late Embryonic Mortality, and Sex Ratio
Observations are presented as the percentage of healthy chicks at hatch, embryos that died at the late stage of development, and females at hatch. Data were subjected to a Poisson distribution, and differences between least squares means were tested using a chi-square test. Significance after FDR correction was set at P < 0.021 for hatchability and P < 0.026 for late embryonic mortality.
Body Weight
Body weight data were subjected to ANOVA. The model was partitioned by offspring age, accounted for unbalanced repeated measures, and random effects included the offspring replicate and pen nested within the room, with the bird as the experimental unit. A log-normal transformation was performed to meet the assumption of a normal distribution of residuals. Significance after FDR correction was set at P < 0.012, followed by a power analysis (alpha = 0.01). The least squares means and SE were back transformed and are presented in the results (g).
Results
Hatchability
Descriptive statistics of the number of viable embryos and percent hatchability for the pilot study and offspring replicates at 3 maternal ages are given in Table 3. Although not statistically analyzed, embryonic viability in injected treatments consistently decreased as breeder hens aged.
Table 3.
Number of eggs set at embryonic day 0, number of viable embryos at embryonic day 10 or 19, and percentage of viable embryo.
| Offspring replicate |
Pilot |
Offspring 1 |
Offspring 2 |
Offspring 3 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Breeder flock age |
25 wk |
32 wk |
52 wk |
72 wk |
|||||||||
| Embryonic day | 0 | 10 | % | 0 | 19 | % | 0 | 19 | % | 0 | 19 | % | |
| Brown 1 | Control | 34 | 32 | 94 | 46 | 42 | 91 | 60 | 52 | 87 | 69 | 51 | 74 |
| Maternal stress | - | - | - | 45 | 43 | 96 | 48 | 42 | 88 | 66 | 44 | 67 | |
| Vehicle | 14 | 9 | 64 | 33 | 22 | 67 | 99 | 43 | 43 | 115 | 39 | 34 | |
| CORT | 15 | 9 | 60 | 44 | 24 | 55 | 89 | 21 | 24 | 221 | 20 | 9 | |
| Brown 2 | Control | 32 | 28 | 88 | 49 | 42 | 86 | 53 | 46 | 87 | 74 | 63 | 85 |
| Maternal stress | - | - | - | 48 | 42 | 88 | 47 | 41 | 87 | 67 | 58 | 87 | |
| Vehicle | 14 | 10 | 71 | 33 | 10 | 30 | 101 | 42 | 42 | 181 | 34 | 19 | |
| CORT | 11 | 7 | 64 | 47 | 10 | 21 | 129 | 16 | 12 | 275 | 19 | 7 | |
| White 1 | Control | 30 | 26 | 87 | 49 | 45 | 92 | 58 | 52 | 90 | 64 | 61 | 95 |
| Maternal stress | - | - | - | 44 | 35 | 80 | 56 | 52 | 93 | 60 | 54 | 90 | |
| Vehicle | 15 | 12 | 80 | 45 | 18 | 40 | 112 | 55 | 49 | 152 | 57 | 38 | |
| CORT | 19 | 16 | 84 | 41 | 23 | 56 | 100 | 24 | 24 | 208 | 41 | 20 | |
| White 2 | Control | 40 | 37 | 93 | 50 | 49 | 98 | 51 | 48 | 94 | 71 | 56 | 79 |
| Maternal stress | - | - | - | 49 | 47 | 96 | 50 | 48 | 96 | 69 | 54 | 78 | |
| Vehicle | 15 | 14 | 93 | 45 | 25 | 56 | 87 | 33 | 38 | 201 | 63 | 31 | |
| CORT | 16 | 11 | 69 | 69 | 15 | 22 | 96 | 28 | 29 | 246 | 23 | 9 | |
| White Leghorn | Control | 40 | 35 | 88 | 54 | 28 | 52 | 52 | 44 | 85 | 57 | 34 | 60 |
| Maternal stress | - | - | - | 48 | 33 | 69 | 59 | 54 | 92 | 55 | 42 | 76 | |
| Vehicle | 11 | 7 | 64 | 44 | 11 | 25 | 78 | 26 | 33 | 70 | 24 | 34 | |
| CORT | 11 | 4 | 36 | 46 | 10 | 22 | 106 | 16 | 15 | 115 | 10 | 9 | |
Data are displayed by breeder flock's age strain and treatment.
Abbreviation: CORT, corticosterone.
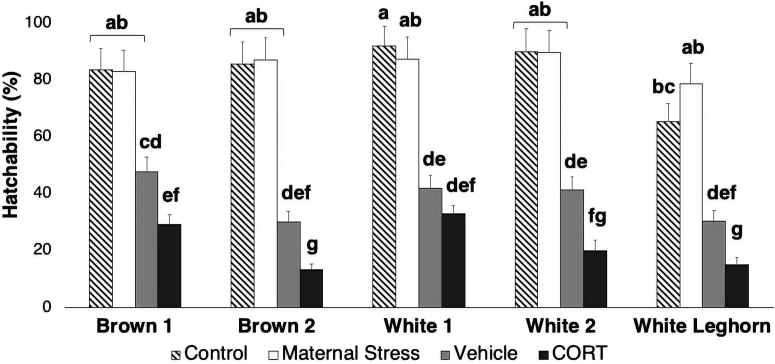
Hatchability was affected by in ovo injections (P < 0.001) (Figure 1). Overall differences between control (83.1 ± 2.4%) and vehicle (38.0 ± 1.6%; P < 0.001) treatments displayed the effects of mechanical damage on the egg, which was intensified by the addition of corticosterone (CORT: 20.9 ± 1.2%; CORT vs. control: P < 0.001). Maternal stress (85.4 ± 2.4%) treatment did not affect hatchability compared with control treatment (P = 0.493).
Figure 1.
Hatchability. Results are displayed by strain and treatment (±SE). Means with different superscripts differ (P < 0.021). CORT, corticosterone.
A chi-square test showed interactive effects between strain and treatment (P < 0.001) (Table 4). Among all strains, White Leghorn showed the lowest hatchability values in the control treatment and the second lowest values in vehicle and CORT treatments, only higher than brown 2 (Figure 1). Maternal stress treatment displayed no differences across strains. Contrast analyses determined a difference between strains from genetics company 1 and 2 (P < 0.001). Overall, brown 1 and white 1 were the most resilient strains to the effects of vehicle and CORT treatments, whereas brown 2 was the most susceptible strain.
Table 4.
Effects of strain and treatment and contrast analyses on hatchability.
| Strain × treatment |
Degrees of freedom |
χ2 |
P-value |
|---|---|---|---|
| 12 |
3.51 |
<0.001 |
|
| Contrasts | |||
| F value | Pr > F | ||
| Treatment | Control vs. stressors | 344.50 | <0.001 |
| Control vs. maternal stress | 0.47 | 0.493 | |
| Control vs. in ovo injections | 556.01 | <0.001 | |
| Control vs. CORT | 453.12 | <0.001 | |
| Control vs. vehicle | 68.63 | <0.001 | |
| Vehicle vs. CORT | 236.39 | <0.001 | |
| Maternal stress vs. CORT | 474.33 | <0.001 | |
| Strain | White vs. brown | 0.08 | 0.780 |
| Genetic company 1 vs. 2 | 22.68 | <0.001 | |
Abbreviation: CORT, corticosterone.
Sex Ratio
The percentage of females at hatch was independent of treatment and strain (P = 0.730). Preplanned comparisons also failed to show strain differences (brown vs. white: P = 0.251; genetic company 1 vs. genetic company 2: P = 0.599) (Table 5).
Table 5.
Sex ratio (%).
| Strain | Treatment | Sex ratio (%) |
|
|---|---|---|---|
| Female | Male | ||
| Brown 1 | Control | 54 | 46 |
| Maternal stress | 50 | 50 | |
| Vehicle | 53 | 47 | |
| CORT | 39 | 61 | |
| Brown 2 | Control | 53 | 47 |
| Maternal stress | 57 | 43 | |
| Vehicle | 51 | 49 | |
| CORT | 49 | 51 | |
| White 1 | Control | 44 | 56 |
| Maternal stress | 57 | 43 | |
| Vehicle | 46 | 54 | |
| CORT | 42 | 58 | |
| White 2 | Control | 39 | 61 |
| Maternal stress | 51 | 49 | |
| Vehicle | 46 | 54 | |
| CORT | 53 | 47 | |
| White Leghorn | Control | 50 | 50 |
| Maternal stress | 51 | 49 | |
| Vehicle | 47 | 53 | |
| CORT | 49 | 51 | |
Data are displayed by strain and treatment.
Abbreviation: CORT, corticosterone.
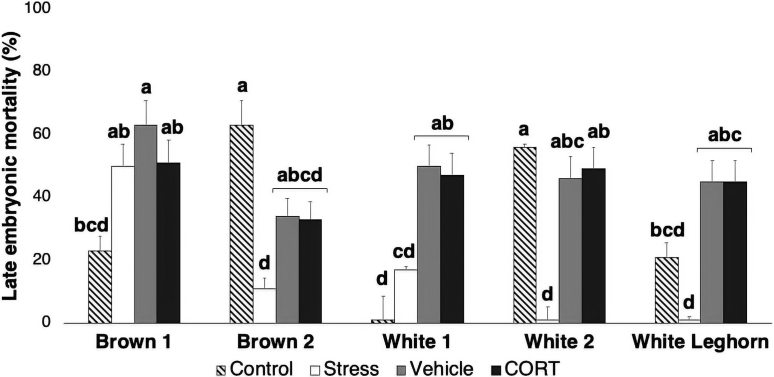
Late Embryonic Mortality
Interactive effects of treatment and strain (P < 0.001) affected embryonic mortality between 11 and 16 D of development (Table 6). Compared with the control treatment, both the maternal stress (P = 0.004) and in ovo injection (P < 0.001) treatments showed effects on commercial strains, but with inconsistent patterns. Brown 1 and white 1 strains showed an increased percentage of embryonic mortality in response to maternal stressors compared with control treatment, whereas a decreased response was observed in brown 2 and white 2 strains (Figure 2). Curiously, White Leghorn responded in opposite directions for each stress model: although maternal stress treatment decreased the occurrence of late-stage embryonic mortality, in ovo injection treatment increased it. No differences between genetic companies (P = 0.793) were observed. Further contrast analyses determined a statistical difference between control (17.6 ± 3.7%) and all other treatments (maternal stress: 6.2 ± 1.9%, P = 0.004; vehicle: 46.7 ± 3.1%, P < 0.001; CORT: 44.5 ± 3.0%; P < 0.001) and between maternal stress and CORT (P < 0.001) treatments, but no differences between vehicle and CORT (P = 0.612) treatments.
Table 6.
Effects of strain and treatment and contrast analyses on late embryonic mortality.
| Strain × treatment |
Degrees of freedom |
χ2 |
P-value |
|---|---|---|---|
| 12 |
98.84 |
<0.001 |
|
| Contrasts | |||
| F value | Pr > F | ||
| Treatment | Control vs. stressors | 1.47 | 0.225 |
| Control vs. maternal stress | 8.22 | 0.004 | |
| Control vs. in ovo injections | 19.10 | <0.001 | |
| Control vs. CORT | 17.30 | <0.001 | |
| Control vs. vehicle | 19.21 | <0.001 | |
| Vehicle vs. CORT | 0.26 | 0.612 | |
| Maternal stress vs. CORT | 42.35 | <0.001 | |
| Strain | White vs. brown | 26.14 | <0.001 |
| Genetic company 1 vs. 2 | 0.07 | 0.793 | |
Abbreviation: CORT, corticosterone.
Figure 2.
Late embryonic mortality. Results are displayed by strain and treatment (±SE). Means with different superscripts differ (P < 0.026). CORT, corticosterone.
Body Weight From Hatch to 17 Wk of Age
A treatment-by-strain interaction (P < 0.003) was observed at all tested ages. As expected, comparisons of sex (P < 0.001) and white vs. brown strains (P < 0.001) were consistently significant throughout the development of the offspring. Contrast analyses determined that maternal stress treatment had no effect on body weight (P > 0.125). However, egg injections consistently decreased weight gain (P < 0.001), with both CORT (P < 0.001) and vehicle (P < 0.042) treatments affecting the progeny in comparison with the control treatment. Although the white 1 and brown 2 strains were consistently susceptible to CORT treatment from hatch until 17 wk, White Leghorn displayed effects of injections only at hatch (Table 7).
Table 7.
Body weight (g).
| Age | Strain | Control | Maternal stress | Vehicle | CORT |
|---|---|---|---|---|---|
| Hatch | Brown 1 | 41.57 ± 0.6a | 40.38 ± 0.5a | 40.82 ± 0.6a | 40.08 ± 0.6a |
| Brown 2 | 39.98 ± 0.5a,b | 40.27 ± 0.5a | 38.77 ± 0.6b | 38.10 ± 0.7b | |
| White 1 | 39.40 ± 0.5a | 38.97 ± 0.5a | 38.59 ± 0.6a,b | 36.96 ± 0.5b | |
| White 2 | 41.10 ± 0.5a | 40.80 ± 0.5a | 39.66 ± 0.5a | 39.40 ± 0.6a | |
| White Leghorn | 38.76 ± 0.5a,b | 39.66 ± 0.6a | 37.20 ± 0.6b,c | 36.28 ± 0.7c | |
| 2 wk | Brown 1 | 166.90 ± 1.5a | 165.86 ± 1.5a | 165.24 ± 1.6a | 157.82 ± 1.8b |
| Brown 2 | 159.53 ± 1.5a,b | 162.39 ± 1.5a | 153.85 ± 1.6b | 142.51 ± 2.1c | |
| White 1 | 157.20 ± 1.4a | 155.62 ± 1.5a | 152.91 ± 1.6a | 142.93 ± 1.5b | |
| White 2 | 165.67 ± 1.5a | 160.97 ± 1.5a | 164.39 ± 1.6a | 150.85 ± 1.9b | |
| White Leghorn | 142.61 ± 1.4a | 142.42 ± 1.4a | 140.47 ± 1.7a | 139.51 ± 2.2a | |
| 4 wk | Brown 1 | 362.96 ± 4.3a | 363.74 ± 4.2a | 361.85 ± 4.4a | 357.37 ± 4.8a |
| Brown 2 | 351.42 ± 4.1a | 356.22 ± 4.2a | 351.51 ± 4.5a | 328.39 ± 5.1b | |
| White 1 | 345.00 ± 4.0a | 350.31 ± 4.2a | 344.82 ± 4.4a | 328.69 ± 4.2b | |
| White 2 | 357.01 ± 4.2a | 355.46 ± 4.2a | 350.97 ± 4.2a | 333.99 ± 4.6b | |
| White Leghorn | 321.96 ± 3.9a | 324.26 ± 3.9a | 311.50 ± 4.2a | 310.36 ± 5.0a | |
| 8 wk | Brown 1 | 895.89 ± 38.2a | 874.70 ± 37.2a | 882.62 ± 37.7a | 872.48 ± 37.5a |
| Brown 2 | 872.40 ± 37.2a,b | 883.47 ± 37.6a | 850.41 ± 36.5c,b | 819.84 ± 35.8c | |
| White 1 | 813.36 ± 34.6a | 800.51 ± 34.1a | 809.19 ± 34.8a | 767.77 ± 32.9b | |
| White 2 | 815.07 ± 34.7a | 805.79 ± 34.3a | 793.14 ± 33.8a,b | 763.61 ± 32.9b | |
| White Leghorn | 745.88 ± 31.9a | 751.02 ± 32.1a | 737.44 ± 31.7a | 747.28 ± 32.8a | |
| 11 wk | Brown 1 | 1,415.97 ± 48.7a | 1,379.66 ± 47.4a | 1,398.02 ± 48.2a | 1,361.49 ± 47.3a |
| Brown 2 | 1,408.03 ± 48.5a,b | 1,437.03 ± 49.3a | 1,374.03 ± 47.6b | 1,307.22 ± 46.5c | |
| White 1 | 1,201.60 ± 41.3a | 1,191.71 ± 41.0a | 1,201.41 ± 41.9a | 1,136.68 ± 39.3b | |
| White 2 | 1,196.23 ± 41.1a | 1,197.34 ± 41.2a | 1,183.77 ± 40.7a | 1,163.80 ± 40.7a | |
| White Leghorn | 1,130.63 ± 38.9a | 1,145.44 ± 39.5a | 1,112.71 ± 38.7a | 1,111.58 ± 39.8a | |
| 13 wk | Brown 1 | 1,534.42 ± 17.1a | 1,481.30 ± 16.2a,b | 1,479.20 ± 17.1a,b | 1,466.59 ± 18.0b |
| Brown 2 | 1,538.72 ± 17.3a,b | 1,566.13 ± 17.2a | 1,489.08 ± 17.8b | 1,418.28 ± 20.7b | |
| White 1 | 1,275.91 ± 14.1a | 1,254.40 ± 14.1a,b | 1,274.47 ± 16.1a | 1,207.9 ± 14.1b | |
| White 2 | 1,274.26 ± 14.0a | 1,280.42 ± 14.2a | 1,264.32 ± 14.2a,b | 1,217.79 ± 15.9b | |
| White Leghorn | 1,204.29 ± 13.6a | 1,222.43 ± 13.9a | 1,197.56 ± 14.8a | 1,186.17 ± 18.2a | |
| 15 wk | Brown 1 | 1,740.36 ± 18.0a | 1,700.43 ± 17.3a,b | 1,715.57 ± 18.2a,b | 1,654.2 ± 18.9b |
| Brown 2 | 1,782.20 ± 18.5a | 1,790.39 ± 18.3a | 1,700.66 ± 19.0b | 1,619.16 ± 22.1c | |
| White 1 | 1,433.72 ± 14.7a | 1,428.38 ± 14.8a | 1,412.63 ± 16.5a | 1,339.57 ± 14.5b | |
| White 2 | 1,419.49 ± 14.5a | 1,432.10 ± 14.7a | 1,403.45 ± 14.6a,b | 1,355.07 ± 16.6b | |
| White Leghorn | 1,371.20 ± 14.2a | 1,389.43 ± 14.5a | 1,341.88 ± 15.5a | 1,348.31 ± 19.5a | |
| 17 wk | Brown 1 | 1,844.09 ± 37.9a | 1,784.35 ± 36.5a,b | 1,798.07 ± 37.3a,b | 1,747.67 ± 37.4b |
| Brown 2 | 1,887.71 ± 38.9a | 1,907.23 ± 39.0a | 1,781.31 ± 37.9b | 1,701.68 ± 39.5b | |
| White 1 | 1,513.25 ± 31.0a | 1,505.38 ± 31.0a | 1,492.29 ± 32.2a | 1,372.59 ± 28.7b | |
| White 2 | 1,481.70 ± 30.5a | 1,459.50 ± 29.9a | 1,470.59 ± 30.4a | 1,419.39 ± 31.2a | |
| White Leghorn | 1,462.08 ± 30.1a | 1,448.01 ± 29.9a | 1,430.44 ± 30.8a | 1,412.93 ± 33.9a |
Averages (±SE) are displayed by offspring age, strain, and stress model. Means with different superscripts within the age-group and strain differ (P < 0.012).
Abbreviation: CORT, corticosterone.
Discussion
Effects of Stress Treatments
The primary goal of this study was to understand how prenatal stress affects different genetic lines of layers. As displayed on hatchability, late embryonic mortality, and body weight, stressors seem to interact with the genotype to affect the progeny of laying hens. However, the results of this study must be interpreted with caution because they are accompanied by several factors.
First, virtually no effects of maternal stress treatment were found on the progeny in contrast to the CORT treatment. This could be because the stress sessions did not induce a similar magnitude of corticosterone deposition as occurred in CORT treatment or because both the hen and embryo possess the capacity to regulate maternally derived corticosterone. As previously reported (Vassallo et al., 2014, Vassallo et al., 2019, Carter et al., 2018), embryos can modulate their developmental environment through rapid metabolization of free corticosterone in the egg. In addition, the catalytic activity of enzymes such as 11β-hydroxysteroid dehydrogenase located in the ovary follicles of hens can reduce the concentration of corticosterone in the yolk (Rettenbacher et al., 2013b). Together, these mechanisms act as metabolic buffering agents, regulating the progeny's exposure to corticosterone. Previous studies have successfully demonstrated the effects of maternal exposure to stressors (Pappas et al., 2006, Goerlich et al., 2012). However, these studies used stressors that activated different physiological and metabolic pathways, such as maternal undernutrition, temporary feed restriction, or temperature stress. Here, we report that psychological stressors did not impair either embryonic survival or offspring growth.
A second note of caution is that the actual concentration of corticosterone transferred from the mother to egg remains largely unknown (Rettenbacher et al., 2009, Rettenbacher et al., 2013a, Almasi et al., 2012) and may differ across strains (Navarra and Pinson, 2010). This caveat may have significant impact on our study because the effects observed in the CORT treatment might be due to hormone manipulation outside of the physiological range of eggs for different strains of breeder hens. The injected concentration of corticosterone may also have overwhelmed the capacity of the embryo to inactivate corticosterone, consequently impacting phenotypical traits important to fitness such as hatchability and body weight. Mechanical damage can also result from breaching the egg because puncturing and disrupting eggshell membranes can increase embryonic mortality (Heiblum et al., 2001). Furthermore, we observed that hatchability and body weight were affected by the vehicle treatment. Sesame oil contains 43% of polyunsaturated fatty acids (Fukuda et al., 1986), which have been reported to affect growth, feeding behavior, and fearfulness in poultry (Aigueperse et al., 2013, Baéza et al., 2017).
The relatively high mortality observed in this study compared with some others in the literature (Janczak et al., 2007a, Haussmann et al., 2012) may be related to differences in breeder flock age because hatchability decreases as hens age (Lapão et al., 1999). We found that all strains showed a progressive increase in embryonic mortality as the breeder flocks aged, but the mortality rates from younger breeders (at 25 or 32 wk of age) were much more similar to values previously reported (Janczak et al., 2007a, Janczak et al., 2007b). Unfortunately, the majority of these studies did not report the age of the breeder flocks, and therefore, any association between breeder flock's age and susceptibility to prenatal stress remains strictly speculative. Finally, similar to the studies by Aslam et al. (2014) and Pinson et al. (2015), the present study failed to show a treatment effect on sex ratio.
Effects of Genetics
Although a vast body of literature has already investigated the long-term effects of prenatal corticosterone on embryonic survival and offspring growth, not many studies have considered the effects of genetics as a predisposing factor on this matter. The present study observed significant differences across genetic lines in hatchability, late embryonic mortality, and body weight of the progeny, regardless of stress treatment. White Leghorn displayed the lowest hatchability level in both control and injected treatments and was also the least affected strain in body weight, suggesting that the highest vulnerability to injections resulted in a more robust progeny. Interestingly, White Leghorn in the control group showed lower hatchability than that in the maternal stress treatment for the same strain. An inconsistent genetic selection program may help explain this finding as the White Leghorn flock used in this study belongs to the University of Guelph and consequently does not follow a strict genetic selection program. Moreover, because eggs used in CORT and vehicle treatment were a subsample of the control group, an additive correlation between hatchability of injection and control treatment is naturally expected.
The brown 2 line was the most affected strain in both prehatch and posthatch development, displaying the lowest hatchability and the most persistent effects of injections on body weight. In addition, maternal stress and CORT treatments decreased late embryonic mortality of the brown 2 line, suggesting that embryos died more frequently within the first 10 D of development, which is when the embryonic metabolism of free corticosterone starts (Vassallo et al., 2014, Vassallo et al., 2019). Oppositely, the brown 1 and white 1 lines from genetic company 1 showed the best hatchability and had late embryonic mortality, increased by both CORT and maternal stress treatments, suggesting that genetic selection strategies by genetic companies in conjunction with specific management and nutritional recommendations may potentially play a critical role in embryonic response to prenatal stress.
As expected, white strains showed a marked difference in body weight compared with brown strains. This may be explained by phylogenetic differences between brown and white birds. Brown commercial lines originate from the Rhode Island Red, an originally dual-purpose breed with medium genetic diversity (Lyimo et al., 2014), whereas white commercial lines are derived from the White Leghorn breed, which has low genetic diversity (Lyimo et al., 2014). Moreover, the process of domestication resulted in phenotypical and genetic differences between the White Leghorn and red jungle fowl (Kerje et al., 2003), and genetic selection from pure into commercial lines may have acted similarly, resulting in phenotypical differences produced by genetic changes. Although the actual concentration of corticosterone in the egg of chickens remains unknown (Rettenbacher et al., 2013a), studies have shown that they might differ between white and brown strains (Navara and Pinson, 2010). If so, the dosage provided in the CORT treatment might have exceeded the physiological range of corticosterone in the eggs of some strains while remaining in the physiological range of others, therefore explaining the strain differences observed in this study.
Conclusion
The present study successfully demonstrated that prenatal stress affects embryonic survival and growth of the offspring and that these effects are highly dependent on the stress model and genotype of layer breeders. As hypothesized, CORT treatment consistently impaired all analyzed traits, whereas maternal stress treatment only displayed effects on early embryonic mortality. This difference in treatments may be because maternal stress treatment was less effective in increasing corticosterone concentration in the egg than CORT treatment. In addition, mechanical disruption of the shell followed by injection may have contributed to impairment of the progeny. Among all strains, the brown 2 line was consistently the most affected in both prehatch and posthatch development. Oppositely, the pure line White Leghorn displayed the highest posthatch resiliency. Phylogenetic differences and genetic selection for productive traits might help explain these differences; however, some of these findings may be an artifact of the pharmacological model because the physiological range of egg corticosterone may differ across strains. Overall, the results show that embryonic survival and offspring growth are mostly affected by in ovo injections and that some genotypes might be more susceptible to prenatal stress than others.
Acknowledgments
This research is funded by Egg Farmers of Canada, the Ontario Ministry of Agriculture and Rural Affairs (OMAFRA), the Canadian Poultry Research Council and two anonymous genetics companies. We thank the personnel at the University of Guelph's “Arkell Poultry Research Station” for the technical assistance and maintenance of the birds.
References
- Ahmed A.A., Ma W., Ni Y., Wang S., Zhao R. Corticosterone in ovo modifies aggressive behaviors and reproductive performances through alterations of the hypothalamic-pituitary-gonadal axis in the chicken. Anim. Reprod. Sci. 2014;146:193–201. doi: 10.1016/j.anireprosci.2014.02.013. [DOI] [PubMed] [Google Scholar]
- Aigueperse N., Calandreau L., Bertin A. Maternal diet influences offspring feeding behavior and fearfulness in the precocial chicken. PLoS One. 2013;8 doi: 10.1371/journal.pone.0077583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almasi B., Rettenbacher S., Müller C., Brill S., Wagner H., Jenni L. Maternal corticosterone is transferred into the egg yolk. Gen. Comp. Endocrinol. 2012;178:139–144. doi: 10.1016/j.ygcen.2012.04.032. [DOI] [PubMed] [Google Scholar]
- Aslam M.A., Groothuis T.G.G., Smits M.A., Woelders H. Effect of corticosterone and hen body Mass on primary sex ratio in laying hen (Gallus gallus), using Unincubated Eggs1. Biol. Reprod. 2014;90:1–9. doi: 10.1095/biolreprod.113.115352. [DOI] [PubMed] [Google Scholar]
- Baéza E., Chartrin P., Bordeau T., Lessire M., Thoby J.M., Gigaud V., Blanchet M., Alinier A., Leterrier C. Omega-3 polyunsaturated fatty acids provided during embryonic development improve the growth performance and welfare of Muscovy ducks (Cairina moschata) Poult. Sci. 2017;96:3176–3187. doi: 10.3382/ps/pex147. [DOI] [PubMed] [Google Scholar]
- Beuving G., Vonder G.M.A. The influence of ovulation and oviposition on corticosterone levels in the plasma of laying hens. Gen. Comp. Endocrinol. 1981;44:382–388. doi: 10.1016/0016-6480(81)90016-2. [DOI] [PubMed] [Google Scholar]
- Bonier F., Martin P.R., Wingfield J.C. Maternal corticosteroids influence primary offspring sex ratio in a free-ranging passerine bird. Behav. Ecol. 2007;18:1045–1050. [Google Scholar]
- Carter A.W., Bowden R.M., Paitz R.T. Evidence of embryonic regulation of maternally derived yolk corticosterone. J. Exp. Biol. 2018;221 doi: 10.1242/jeb.182600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J.M., Widowski T.M., Tilbrook A.J., Butler K.L., Hemsworth P.H. The effects of floor space and nest box access on the physiology and behavior of caged laying hens. Poult. Sci. 2019;98:533–547. doi: 10.3382/ps/pey378. [DOI] [PubMed] [Google Scholar]
- Ericsson M., Henriksen R., Bélteky J., Sundman A.S., Shionoya K., Jensen P. Long-term and transgenerational effects of stress experienced during different life phases in chickens (Gallus gallus) PLoS One. 2016;11 doi: 10.1371/journal.pone.0153879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen M.S., Haug A., Torjesen P.A., Bakken M. Prenatal exposure to corticosterone impairs embryonic development and increases fluctuating asymmetry in chickens (Gallus gallus domesticus) Br. Poult. Sci. 2003;44:690–697. doi: 10.1080/00071660310001643660. [DOI] [PubMed] [Google Scholar]
- Fukuda Y., Nagata M., Osawa T., Namiki M. Chemical aspects of the antioxidative activity of roasted sesame seed oil, and the effect of using the oil for frying. Agric. Biol. Chem. 1986;50:857–862. [Google Scholar]
- García L.V. Controlling the false discovery rate in ecological research. Trends Ecol. Evol. 2016;18:553–554. [Google Scholar]
- Gluckman P.D., Hanson M.A., Spencer H.G. Predictive adaptive responses and human evolution. Trends Ecol. Evol. 2005;20:527–533. doi: 10.1016/j.tree.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Goerlich V.C., Nätt D., Elfwing M., Macdonald B., Jensen P. Transgenerational effects of early experience on behavioral, hormonal and gene expression responses to acute stress in the precocial chicken. Horm. Behav. 2012;61:711–718. doi: 10.1016/j.yhbeh.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Groothuis T.G.G., Hsu B.-Y., Kumar N., Tschirren B. Revisiting mechanisms and functions of prenatal hormone-mediated maternal effects using avian species as a model. Philos. Trans. R. Soc. B Biol. Sci. 2019;374:20180115. doi: 10.1098/rstb.2018.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groothuis T.G.G., Schwabl H. Hormone-mediated maternal effects in birds: mechanisms matter but what do we know of them? Philos. Trans. R. Soc. B Biol. Sci. 2008;363:1647–1661. doi: 10.1098/rstb.2007.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guibert F., Richard-Yris M.A., Lumineau S., Kotrschal K., Bertin A., Petton C., Möstl E., Houdelier C. Unpredictable mild stressors on laying females influence the composition of Japanese quail eggs and offspring’s phenotype. Appl. Anim. Behav. Sci. 2011;132:51–60. [Google Scholar]
- Hamburger V., Hamilton H.L. A series of normal stages in the development of the chick embryo. J. Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- Haussmann M.F., Longenecker A.S., Marchetto N.M., Juliano S.A., Bowden R.M. Embryonic exposure to corticosterone modifies the juvenile stress response, oxidative stress and telomere length. Proc. R. Soc. B Biol. Sci. 2012;279:1447–1456. doi: 10.1098/rspb.2011.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiblum R., Arnon E., Chazan G., Robinzon B., Gvaryahu G., Snapir N. Glucocorticoid administration during incubation: embryo mortality and Posthatch growth in chickens. Poult. Sci. 2001;80:1357–1363. doi: 10.1093/ps/80.9.1357. [DOI] [PubMed] [Google Scholar]
- Henriksen R., Rettenbacher S., Groothuis T.G.G. Maternal corticosterone elevation during egg formation in chickens (Gallus gallus domesticus) influences offspring traits, partly via prenatal undernutrition. Gen. Comp. Endocrinol. 2013;191:83–91. doi: 10.1016/j.ygcen.2013.05.028. [DOI] [PubMed] [Google Scholar]
- Henriksen R., Rettenbacher S., Groothuis T.G.G. Prenatal stress in birds: pathways, effects, function and perspectives. Neurosci. Biobehav. Rev. 2011;35:1484–1501. doi: 10.1016/j.neubiorev.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Janczak A.M., Haung A., Bakken M. Evaluation of experimental methods for manipulating chicken egg Hormone.pdf. J. Anim. Vet. Adv. 2007;6:500–504. [Google Scholar]
- Janczak A.M., Heikkilä M., Valros A., Torjesen P., Andersen I.L., Bakken M. Effects of embryonic corticosterone exposure and post-hatch handling on tonic immobility and willingness to compete in chicks. Appl. Anim. Behav. Sci. 2007;107:275–286. [Google Scholar]
- Jenkins S.A., Porter T.E. Ontogeny of the hypothalamo-pituitary-adrenocortical axis in the chicken embryo: a review. Domest. Anim. Endocrinol. 2004;26:267–275. doi: 10.1016/j.domaniend.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Kerje S., Lind J., Schütz K., Jensen P., Andersson L. Melanocortin 1-receptor (MC1R) mutations are associated with plumage colour in chicken. Anim. Genet. 2003;34:241–248. doi: 10.1046/j.1365-2052.2003.00991.x. [DOI] [PubMed] [Google Scholar]
- Lapão C., Gama L.T., Soares M.C. Effects of broiler breeder age and length of egg storage on albumen characteristics and hatchability. Poult. Sci. 1999;78:640–645. doi: 10.1093/ps/78.5.640. [DOI] [PubMed] [Google Scholar]
- Love O.P., Williams T.D. The adaptive value of stress-Induced phenotypes: effects of maternally derived corticosterone on sex-Biased Investment, Cost of Reproduction, and maternal fitness. Am. Nat. 2008;172:E135–E149. doi: 10.1086/590959. [DOI] [PubMed] [Google Scholar]
- Lyimo C.M., Weigend A., Msoffe P.L., Eding H., Simianer H., Weigend S. Global diversity and genetic contributions of chicken populations from African, Asian and European regions. Anim. Genet. 2014;45:836–848. doi: 10.1111/age.12230. [DOI] [PubMed] [Google Scholar]
- Machin K.L. Controlling avian Pain. Compend. Contin. Educ. Pract. Vet. 2005;5:299–309. [Google Scholar]
- Mousseau T.A., Fox C.W. Maternal effects as adaptation. Trends Ecol. Evol. 1998;13:403–407. doi: 10.1016/s0169-5347(98)01472-4. [DOI] [PubMed] [Google Scholar]
- Nätt D., Nätt D., Rubin C.-J., Wright D., Johnsson M., Beltéky J., Andersson L., Jensen P. Heritable genome-wide variation of gene expression and promoter methylation between wild and domesticated chickens. BMC Genomics. 2012;13:59. doi: 10.1186/1471-2164-13-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navara K.J., Pinson S.E. Yolk and albumen corticosterone concentrations in eggs laid by white versus brown caged laying hens. Poult. Sci. 2010;89:1509–1513. doi: 10.3382/ps.2009-00416. [DOI] [PubMed] [Google Scholar]
- Nielsen H. Hen age and fatty acid composition of egg yolk lipid. Br. Poult. Sci. 1998;39:53–56. doi: 10.1080/00071669889394. [DOI] [PubMed] [Google Scholar]
- Pappas A.C., Acamovic T., Surai P.F., McDevitt R.M. Maternal organo-selenium compounds and polyunsaturated fatty acids affect progeny performance and levels of selenium and docosahexaenoic acid in the chick tissues. Poult. Sci. 2006;85:1610–1620. doi: 10.1093/ps/85.9.1610. [DOI] [PubMed] [Google Scholar]
- Pinson S.E., Wilson J.L., Navara K.J. Timing matters: corticosterone injections 4 h before ovulation bias sex ratios towards females in chickens. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2015;185:539–546. doi: 10.1007/s00360-015-0897-5. [DOI] [PubMed] [Google Scholar]
- Pitk M., Tilgar V., Kilgas P., Mänd R. Acute stress affects the corticosterone level in bird eggs: a case study with great tits (Parus major) Horm. Behav. 2012;62:475–479. doi: 10.1016/j.yhbeh.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Podmokła E., Drobniak S.M., Rutkowska J. Chicken or egg? Outcomes of experimental manipulations of maternally transmitted hormones depend on administration method – a meta-analysis. Biol. Rev. 2018;93:1499–1517. doi: 10.1111/brv.12406. [DOI] [PubMed] [Google Scholar]
- Possenti C.D., Secomandi S., Schiavon A., Caprioli M., Rubolini D., Romano A., Saino N., Parolini M. Independent and combined effects of egg pro- and anti-oxidants on gull chick phenotype. J. Exp. Biol. 2018;221:jeb174300. doi: 10.1242/jeb.174300. [DOI] [PubMed] [Google Scholar]
- Rettenbacher S., Groothuis T.G., Henriksen R., Möstl E. Corticosterone in bird eggs: the importance of analytical validation. Wien. Tierarztl. Monatsschr. 2013;100:283–290. [Google Scholar]
- Rettenbacher S., Henriksen R., Groothuids T.G., Lepschy M. Corticosterone metabolism by chicken follicle cells does not affect ovarian reproductive hormone synthesis in vitro. Gen. Comp. Endocrinol. 2013;184:67–74. doi: 10.1016/j.ygcen.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettenbacher S., Möstl E., Groothuis T.G.G. Gestagens and glucocorticoids in chicken eggs. Gen. Comp. Endocrinol. 2009;164:125–129. doi: 10.1016/j.ygcen.2009.05.019. [DOI] [PubMed] [Google Scholar]
- Roth O., Beemelmanns A., Barribeau S.M., Sadd B.M. Recent advances in vertebrate and invertebrate transgenerational immunity in the light of ecology and evolution. Heredity (Edinb) 2018;121:225–238. doi: 10.1038/s41437-018-0101-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel H.S. Physiological stress in birds. Bioscience. 1980;30:529–534. [Google Scholar]
- Tissier M.L., Williams T.D., Criscuolo F. Maternal effects underlie ageing costs of growth in the zebra finch (Taeniopygia guttata) PLoS One. 2014;9 doi: 10.1371/journal.pone.0097705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassallo B.G., Litwa H.P., Haussmann M.F., Paitz R.T. In ovo metabolism and yolk glucocorticoid concentration interact to influence embryonic glucocorticoid exposure patterns. Gen. Comp. Endocrinol. 2019;272:57–62. doi: 10.1016/j.ygcen.2018.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassallo B.G., Paitz R.T., Fasanello V.J., Haussmann M.F. Glucocorticoid metabolism in the in ovo environment modulates exposure to maternal corticosterone in Japanese quail embryos (Coturnix japonica) Biol. Lett. 2014;10 doi: 10.1098/rsbl.2014.0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada H. Glucocorticoids: mediators of vertebrate ontogenetic transitions. Gen. Comp. Endocrinol. 2008;156:441–453. doi: 10.1016/j.ygcen.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Williams T.D., Groothuis T.G.G. Egg quality, embryonic development, and post-hatching phenotype: an integrated perspective. Nests, Eggs, and Incubation. 2015:113–126. [Google Scholar]
- Zulkifli I., Al-Aqil A., Omar A.R., Sazili A.Q., Rajion M.A. Crating and heat stress influence blood parameters and heat shock protein 70 expression in broiler chickens showing short or long tonic immobility reactions. Poult. Sci. 2009;88:471–476. doi: 10.3382/ps.2008-00287. [DOI] [PubMed] [Google Scholar]