Abstract
The objective of this study was to determine the impact of pre-rigor salting using KCl on the technological properties of ground chicken breast. Chicken breast muscle (M. pectoralis major and minor) was hot-debonded and salted with 2% NaCl (w/w), 1% NaCl+1% KCl mixture, or 2% KCl, respectively, within 30 min after slaughter. Post-rigor salting treatment was prepared with 2% NaCl at 24 h postmortem. All pre-rigor salting treatments showed higher ultimate pH, protein solubility, and final yield than post-rigor salting treatment (P < 0.05). However, the positive effects of pre-rigor salting on chicken breast differed by salt type. Pre-rigor salting with KCl resulted in higher ultimate pH and R-values of chicken breast than pre-rigor salting with NaCl (P < 0.05). Despite the high ultimate pH, pre-rigor salting with KCl resulted in lower protein solubility, final yield, and hardness of chicken breast than pre-rigor salting with NaCl (P < 0.05). These results indicate that pre-rigor salting with KCl could contribute to the maintenance of relatively excellent technological properties of pre-rigor chicken breasts compared to post-rigor salted chicken breast. However, this current study also suggests that the impact of KCl on technological properties in pre-rigor chicken breast, such as water-holding capacity, protein solubility, and texture, could be less effective than pre-rigor salting with NaCl at an identical percentage concentration.
Key words: chicken breast, hot-boning, low sodium, pre-rigor salting, potassium chloride
INTRODUCTION
During the conversion of skeletal muscle to meat, in general, the occurrence of rigor-mortis negatively influences water-holding capacity and protein functionality (Sadler and Swan, 1997; Kim et al., 2015). Immediately after slaughter, in this regard, the use of pre-rigor muscle is well-known to have several advantages on meat product manufacturing, improving water-holding capacity, protein solubility/extractability, and emulsifying capacity (Pisula and Tyburcy, 1996).
Unfortunately, the excellent quality attributes of pre-rigor muscle decline due to postmortem metabolisms rigor-mortis and pH decline (Hamm, 1977; Kim et al., 2015). A pre-rigor salting technique is one possible approach for sustaining the technological properties of pre-rigor muscle (Coon et al., 1983). This processing technique is based on the inactivation of endogenous enzymes involved in glycolysis at high ionic strength, which can in turn serve to minimize the decline in pH (Hamm, 1977; Kim et al., 2015). Thus, the effects of pre-rigor salting are greatly dependent upon the amount of salt added (Coon et al., 1983; Kim et al., 2011), and it has been suggested that the addition of at least 2% NaCl is required to guarantee the beneficial effects of pre-rigor salting on chicken breast (Kim et al., 2015).
Sodium chloride (NaCl) is one of the essential ingredients in meat processing, because it plays an important role in producing the desirable water-holding capacity, texture, and microbial properties of final products, as well as acceptable sensory attributes (Ruusunen and Puolanne, 2005; Desmond, 2006). Although various chloride salts such as potassium chloride (KCl), calcium chloride, and magnesium chloride (MgCl2) can be used as a food additive, NaCl is preferentially used in processed meat products owing to its sensory acceptability (Inguglia et al., 2017). Nevertheless, the use of alternative chloride salts has been attempted, especially now that excessive consumption of sodium is known to negatively impact human health (Inguglia et al., 2017). To reduce sodium content in meat products, the partial replacement of NaCl with KCl has been commercially applied; in general, it has been recognized that a replacement of 50% of NaCl with KCl (below 1% based on a total weight) may be acceptable, without adverse impacts on sensory properties (Desmond, 2006).
While pre-rigor salting effects have been mainly evaluated through the addition of NaCl, some studies have indicated that the effects could vary with the types of salt added (Rees et al., 2002; Kim et al., 2015). Therefore, it could be hypothesized that the salt type would be one of the critical factors affecting technological properties of pre-rigor salted meat, since the impact of salt type on postmortem metabolism in pre-rigor muscle may be as important as ionic strength. However, despite the practical use of KCl in the meat industry, little information is available about the impact of pre-rigor salting with KCl on technological properties.
Therefore, the objective of this study was to determine the processing characteristics of pre-rigor chicken breasts salted with NaCl, NaCl+KCl mixture (1:1 ratio), and KCl.
MATERIALS AND METHODS
Raw Materials and Pre-/Post-Rigor Salting
Animal production were approved by Gyeongnam National University of Science and Technology Animal Care and Use Committee (GNTECH—ACUC, # 2018-7). A total of 12 male birds (Dark Cornish, 42 wk, averaged live weight of 4.8 ± 0.5 kg) were housed in covered floor pens and fed with a commercial diet based on the recommendations for nutrients by NRC (1994). Feed and water were allowed until 12 h and 2 h before slaughter, respectively. The birds were conventionally slaughtered in accordance with the poultry slaughter procedure described by Alvarado and Sams (2000). In brief, the birds were electrically stunned, bled for 3 min, scaled at 62°C for 1 min, and defeathered using a rotary drum plucker. After evisceration, both sides of chicken breast muscle (M. pectoralis major and minor) were manually de-boned within 15 min after slaughter. Left and right sides of chicken breasts were assigned into pre-rigor salting treatments and post-rigor salting treatment, respectively. The left side chicken breasts assigned into pre-rigor salting treatment were immediately ground using a meat grinder (M-12S, Hankook fujee, Hwaseong, Korea) equipped with an 8-mm plate. The ground chicken breast was divided into 3 portions and manually mixed with 2% NaCl (w/w), 2% NaCl+KCl mixture (1:1 ratio, w/w), or 2% KCl (w/w) for 3 min, respectively, in which calculated ionic strengths were as follows; 0.342 (2% NaCl), 0.305 (2% NaCl+KCl mixture), and 0.268 (2% KCl). In terms of post-rigor salting treatment, the intact chicken breasts (right side) were vacuum-packaged in nylon/polyethylene bags and stored in a 4°C refrigerator. At 24 h postmortem, the chicken breasts were ground and mixed with 2% NaCl (w/w) in the same way mentioned above. The pre-/post-rigor salted chicken breasts were vacuum-packaged, stored in the 4°C refrigerator for 24 h, and used for further analysis. A total of 3 independent batches were repeated.
pH Measurement
A total of 3 g of sample were homogenized with 27 mL of distilled water (DW) at 8,000 rpm for 1 min. The pH value of pre-/post-rigor salted chicken breasts was measured in triplicate using an electric pH meter (Orion star A211, Thermo scientific, Beverly, MA).
R-value
R-value was determined according to the method of Koh et al. (1993) with minor modifications. A total of 4 g of sample were homogenized with 20 mL of 6% perchloric acid (v/v) at 5,000 rpm for 90 s and centrifuged at 3,000 × g for 10 min (4°C). A total of 10 mL of the supernatant was taken, and the pH was adjusted with 2 M KOH to 6.0−6.5 and stored at 4°C for 60 min. The supernatant was filtrated through Whatman No. 1, and 0.1 mL of the filtrate was mixed with 2.9 mL of 0.1 M sodium phosphate buffer (pH 6.5). The absorbance of mixture was read at 250 and 260 nm using an UV/VIS spectrophotometer (Uvikon 943, Kontron, Milan, Italy). R-value was expressed as the ratio of absorbance at 250 nm (IMP) to absorbance at 260 nm (adenine nucleotides; ATP, ADP, and AMP).
Color Measurement
The surface color of sample was determined using a colorimeter (Minolta Chroma meter CR-400, Minolta Ltd., Osaka, Japan; illuminate C, calibrated with a white plate, L* = +97.83, a* = −0.43, b* = +1.98). CIE L* (lightness), a* (redness), and b* (yellowness) values were recorded from 5 random locations on the surface of each sample. Chroma (color intensity) was calculated using the following expression; chroma = [(a*2+b*2)1/2] (AMSA, 2012).
Water-Holding Capacity
Water-holding capacity was determined by the serial procedure of Bowker and Zhuang (2016) with minor modifications; water uptake, cooking loss, and final yield. A total of 5 g of sample was placed in a 50 mL centrifuge tube and mixed with 20 mL of DW for 1 min. The mixture was kept at 4°C for 15 min and centrifuged at 3,000 × g for 15 min (4°C). Unlike the original procedure, the addition of salt was not considered in this study, since the sample was already salted. The supernatant (unabsorbed water layer) was carefully discarded, and water uptake was calculated as follows; water uptake (%) = [(swollen sample weight (g)−initial sample weight (g))/initial sample weight (g) × 100]. The swollen samples were cooked at 80°C for 20 min, and cooking loss was calculated as follows; cooking loss (%) = [(swollen sample weight (g)−cooked sample weight (g))/swollen sample weight (g) × 100]. Final yield was calculated as follows; final yield (%) = [(cooked sample weight (g)/initial sample weight (g)) × 100].
Protein Solubility
The solubility of salt-soluble protein was determined using the procedure described by Saffle and Galbreath (1964). A total of 5 g of sample was homogenized in 30 mL of 2% NaCl solution (w/v). The homogenates were centrifuged at 3,000 × g for 30 min at 4°C. The supernatant containing salt-soluble protein fraction was filtered through filter paper (Whatman No. 1). The protein concentrations of the extractable protein fraction were measured using a biuret assay with BSA standard curve (Gornall et al., 1949).
Emulsion Activity Index (EAI)
EAI was determined by the method described by Bowker and Zhuang (2016). The protein concentration of extractable fraction was adjusted to 1.5 mg/mL and mixed with corn oil in a 3:1 of volume ratio and homogenized at 14,000 rpm for 60 s. Aliquots of 35 μL from the emulsified layer were diluted to 3.5 mL of 0.1% SDS buffer (w/v). Immediately, the turbidity was read at 500 nm using a UV/VIS spectrophotometer (Uvikon 943, Kontron, Milan, Italy). EAI was calculated as follows: EAI = 2.33 × Absorbance at 500 nm (Selmane et al., 2008).
Texture Profile Analysis (TPA)
TPA was performed using a texture analyzer (TA-XT plus, Stable Micro Systems Ltd., Surrey, England). For temperature equilibrium, the cooked samples used for final yield determination were stored at room temperature for 3 h. Cylindrical samples (20 mm in height) were taken from the central portion of the cooked samples. TPA test condition was as follows; pre-test speed 2.0 mm/s, post-test speed 5.0 mm/s, head speed 2.0 mm/s, distance 14.0 mm (70% compression), force 5 g, and maximum load 50 kg (Choi et al., 2010). Hardness (kg), springiness (ratio), cohesiveness (unitless), gumminess (kg), and chewiness (kg) were determined according to the method of Bourne (1978).
Statistical Analysis
The experimental design of this study was a completely randomized block design with 3 independent batches (n = 3). For pre-rigor salting treatments, one-way ANOVA was performed using SPSS 18.0 program (SPSS Inc., Chicago, IL), in which the salt type was considered as a main effect. Duncan's multiple range test was used for determining the differences between means (P < 0.05). Between each pre-rigor salted chicken breast and post-rigor salted chicken breast, the significance of difference was determined using Student t-test.
RESULTS AND DISCUSSION
pH and R-value
The pH value and R-value of pre-rigor salted chicken breasts are shown in Table 1. All pre-rigor salted chicken breasts showed higher pH values than post-rigor salted chicken breasts, regardless of salt type (P < 0.01). Pre-rigor chicken breasts salted with KCl had a significantly higher pH value (6.00) than those salted with NaCl (5.95) or NaCl+KCl mixture (5.96). Many previous studies have reported that pre-rigor salting could contribute to the maintenance of high pH values in muscle after postmortem rigor-mortis (Bernthal et al., 1989; Farouk and Swan, 1997; Lee et al., 2012; Kim et al., 2015). Van Hoof and Hamm (1973) suggested that the addition of 2% NaCl to ground pre-rigor muscle inhibits the formation of glucose-6-phosphate, which is an intermediate derivative of the glycolytic pathway. Hamm (1977) found that the presence of NaCl deactivates glycolytic enzymes such as phosphorylase and phosphofructokinase. Coon et al. (1983) noted that the addition of salt to pre-rigor muscle resulted in a higher ultimate pH than post-rigor muscle, and they suggested that the high pH value of pre-rigor salted muscle could be attributed to a slowed rate of postmortem glycolysis, eventually resulting in low production of lactate.
Table 1.
pH and R-value of pre-rigor salted ground chicken breasts with NaCl and/or KCl.
| Post-rigor salting1 |
Pre-rigor salting |
||||
|---|---|---|---|---|---|
| Traits | NaCl2 | NaCl | NaCl+KCl (1:1 ratio) | KCl | Significance of P value3 |
| pH | 5.48 ± 0.03 | 5.95 ± 0.02B** | 5.96 ± 0.02B** | 6.00 ± 0.01A** | 0.000 |
| R-value (250 nm/260 nm) | 1.37 ± 0.01 | 1.35 ± 0.03B | 1.35 ± 0.01B** | 1.41 ± 0.01A** | 0.003 |
All values are mean ± standard deviation. (n = 3).
Ground chicken breasts were salted within postmortem 30 min (pre-rigor salting) or at 24 h (post-rigor salting).
The salt concentration of all samples was equally fixed as 2% (w/w, based on total sample weight).
The significance of the result for one-way ANOVA within 3 pre-rigor salting treatments.
Asterisks mean the significance of t-test between post-rigor salting treatment with NaCl and each pre-rigor salting treatment. **P < 0.01.
Means sharing different letters in the same row are significantly different among pre-rigor salting treatments (P < 0.05).
Moreover, the result of this study shows that the salt type (NaCl vs. KCl) could affect the ultimate pH of pre-rigor salted chicken breasts, although the pH difference due to the salt effect was numerically small. Some previous studies have noted that the addition of KCl could slightly increase the pH of processed meat products compared to NaCl (Keeton, 1984; Barbut et al., 1988; Horita et al., 2011). While a similar impact of KCl on the pH value has been previously observed in post-rigor muscle (Terrell et al., 1981; Hand et al., 1982; Soglia et al., 2014), a clear explanation has proven elusive. According to Aliño et al. (2009), K+ ion has a greater ability to penetrate into muscle tissue than Na+ ion. As a possible explanation, thus, pre-rigor salting with KCl might be more effective in promoting high ionic strength within muscle cell/tissue compared to pre-rigor salting with NaCl owing to rapid penetration. This phenomenon might in turn contribute to the slightly higher ultimate pH value in pre-rigor chicken breasts salted with KCl, thereby effectively inhibiting postmortem glycolysis.
R-value is an indicator of ATP degradation (a ratio of ATP, ADP, and AMP to IMP), and a high number implies a relatively low ATP content (Honikel and Hamm, 1978). The R-value of pre-rigor salted chicken breasts ranged from 1.35 to 1.41, and was affected by salt type (P < 0.05). Papa and Fletcher (1988) reported that R-values of hot-bonded chicken breast muscle reached above 1.20 to 1.30 at 2 to 4 h postmortem. In this study, the R-value of pre-rigor chicken breasts salted with KCl (1.41) was significantly higher than those salted with NaCl (1.35). This shows the possibility that the rapid depletion of adenine nucleotides, mainly ATP, might occur in pre-rigor chicken breasts salted with NaCl. Previously, Coon et al. (1983) found that an increase in pre-rigor salting level could accelerate ATP depletion during the first 72 h postmortem, resulting in an increased R-value, while higher ultimate pH value (above 5.80) was observed. In particular, a rapid increase in R-value has been observed in pre-rigor salted muscle during the initial 24 h postmortem, despite high ionic strength and low storage temperature (Coon et al., 1983). In this regard, our result, showing that pre-rigor salting with KCl led to an increased R-value coupled with high ultimate pH, was consistent with the previous observations. Further studies elucidating the mechanisms of early ATP depletion in pre-rigor salted muscle and its impact on processing characteristics are warranted.
Color Characteristics
The color characteristics of pre-rigor salted chicken breasts are shown in Table 2. No differences in CIE L* (lightness), CIE b* (yellowness), and chroma were found between pre- and post-rigor salted chicken breasts (P > 0.05), but a significant difference in CIE a* (redness) was found only between pre- and post-rigor salted chicken breast with NaCl (P < 0.05). A previous study, conducted by Farouk and Swan (1997), has reported that pre-rigor salted beef muscle showed lower lightness and yellowness than unsalted pre-rigor beef muscle. They suggested the color characteristics of pre-rigor salted beef muscle could be affected by low pH at salting, since a decrease in pH increases the rate of lipid oxidation (Farouk and Swan, 1997). However, the result of this study shows little to no differences in color characteristics between pre- and post-rigor salted chicken breasts at the same salt concentration. Among pre-rigor salting treatments, pre-rigor chicken breast salted with KCl or NaCl+KCl mixture resulted in a slightly higher lightness compared to NaCl (P < 0.05); it is likely that the differences in lightness between pre-rigor salted treatments were too numerically small to produce a functional difference.
Table 2.
Color characteristic of pre-rigor salted ground chicken breasts with NaCl and/or KCl.
| Post-rigor salting1 |
Pre-rigor salting |
||||
|---|---|---|---|---|---|
| Traits | NaCl2 | NaCl | NaCl+KCl (1:1 ratio) | KCl | Significance of P value3 |
| CIE L* (lightness) | 68.00 ± 1.43 | 67.15 ± 0.93B | 69.37 ± 0.57A | 69.00 ± 0.39A | 0.014 |
| CIE a* (redness) | 2.08 ± 0.27 | 3.05 ± 0.47* | 2.26 ± 0.34 | 2.48 ± 0.37 | 0.114 |
| CIE b* (yellowness) | 13.82 ± 0.69 | 12.80 ± 0.64 | 13.46 ± 0.38 | 13.73 ± 0.78 | 0.250 |
| Chroma | 13.98 ± 0.65 | 13.17 ± 0.51 | 13.66 ± 0.32 | 13.96 ± 0.70 | 0.264 |
All values are mean ± standard deviation. (n = 3).
Ground chicken breasts were salted within postmortem 15 min (pre-rigor salting) or at 24 h (post-rigor salting).
The salt concentration of all samples was equally fixed as 2% (w/w, based on total sample weight).
The significance of the result for one-way ANOVA within 3 pre-rigor salting treatments.
Asterisks mean the significance of t-test between post-rigor salting treatment with NaCl and each pre-rigor salting treatment. *P < 0.05.
Means sharing different letters in the same row are significantly different among pre-rigor salting treatments (P < 0.05).
Water-Holding Capacity
The water-holding capacity of pre-rigor salted chicken breasts is shown in Figure 1. Pre-rigor chicken breast salted with NaCl showed a higher water uptake than post-rigor chicken breast salted with NaCl (P < 0.05), but no difference in water uptake between pre-rigor salting with KCl and post-rigor salting with NaCl was found (P > 0.05). All pre-rigor salted treatments showed significantly lower cooking loss than post-rigor salted chicken breast. As a result, higher final yields were found in pre-rigor salted chicken breasts (P < 0.05), compared to post-rigor, and the order of final yield in pre-rigor salted treatments was as follows: pre-rigor salting with NaCl (79.5%) > pre-rigor salting with NaCl+KCl mixture (73.5%) ≥ pre-rigor salting with KCl (69.6%). Thus, pre-rigor salting with KCl decreased the water uptake of pre-rigor chicken breasts, which in turn caused the decreased final yield (P < 0.05).
Figure 1.
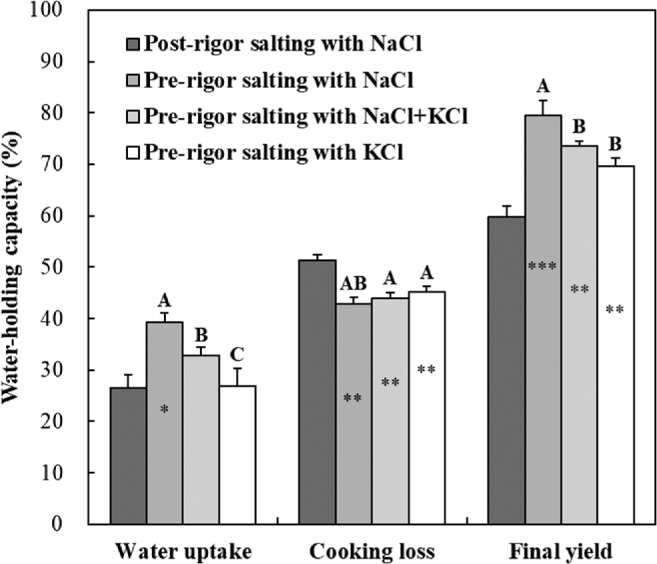
Water-holding capacity of pre-rigor salted chicken breasts with NaCl and/or KCl. Treatments: post-rigor salting with NaCl, post-rigor ground chicken breasts with 2% NaCl; pre-rigor salting with NaCl, pre-rigor ground chicken breasts with 2% NaCl; pre-rigor salting with NaCl+KCl, pre-rigor ground chicken breasts with 1% NaCl and 1% KCl; pre-rigor salting with KCl, pre-rigor ground chicken breasts with 2% KCl. A-C Means with different letters within pre-rigor salting treatments are significantly different (P < 0.05). Asterisk in bars means the significance difference in t-test between post-rigor salted with NaCl and each pre-rigor salting treatments. *P < 0.05; **P < 0.01; and ***P < 0.001.
In general, ion binding to myofibrillar proteins including myosin and actin causes muscle swelling, which in turn contribute to the improvement of water uptake in salted meat (Offer and Trinick, 1983). Hamm (1957) reported that Na+ ion has an increased ability to hydrate muscle compared to K+ ions at the same ionic strength and within the isoelectric point (pH 5.5 and 6.4). Further, Hamm (1961) explained that the low hydration ability of K+ ion was likely due to the fact that K+ ions can bind to myosin and actomyosin, but not to actin. Thus, the decreased water uptake in pre-rigor chicken breast salted with KCl might be related to a relatively limited hydration ability of K+ ion as compared with Na+ ion. As another possible explanation, the actual ionic strength in pre-rigor salted chicken breasts was slightly different, even at the same percentage salt concentration: 2% NaCl (0.342 of ionic strength), 1% NaCl+1% KCl mixture (0.305 of ionic strength), and 2% KCl (0.268 of ionic strength). Although this study was conducted with percentage replacement, as with the most previous studies, the decrease in ionic strength that correlates with increasing the replacement ratio of KCl would be one explanation for the decreased water uptake in pre-rigor salted chicken breast.
Protein Functionality (Protein Solubility and EAI)
The protein solubility and EAI of pre-rigor salted chicken breast are shown in Table 3. All pre-rigor salted chicken breasts showed a higher protein solubility than post-rigor salted chicken breasts (P < 0.05), in which the highest protein solubility was found in pre-rigor chicken breast salted with NaCl. However, the addition of KCl decreased the protein solubility of pre-rigor salted chicken breasts (P < 0.05). Protein solubility, particularly of salt-soluble myofibrils, is one of the most important technological properties affecting water-holding capacity, emulsifying capacity, and texture of meat products (Offer and Trinick, 1983; Joo et al., 1999). Wu et al. (2016) reported that myofibrillar protein solubility was dependent on the type of cations (NaCl and KCl), and Na+ ion showed a higher solubility than K+. Whiting and Jenkins (1981) reported that protein extractability was higher in 0.6 M NaCl solution than in 0.6 M KCl solution, when the pH was within 6.0 to 6.5. As mentioned in the results for water-holding capacity, the decreased protein solubility in pre-rigor chicken breast salted with KCl could be explained by the different hydration ability of Na+ and K+ ions and/or the difference in actual ionic strength.
Table 3.
Protein solubility and emulsion activity index (EAI) of pre-rigor salted ground chicken breasts with NaCl and/or KCl.
| Post-rigor salting1 |
Pre-rigor salting |
||||
|---|---|---|---|---|---|
| Traits | NaCl2 | NaCl | NaCl+KCl (1:1 ratio) | KCl | Significance of P value3 |
| Protein solubility (mg/g) | 104.4 ± 5.6 | 212.6 ± 5.5A*** | 202.0 ± 4.0A,B*** | 194.0 ± 10.2B** | 0.048 |
| EAI | 0.77 ± 0.05 | 0.87 ± 0.12 | 0.80 ± 0.13 | 0.76 ± 0.09 | 0.165 |
All values are mean ± standard deviation. (n = 3).
Ground chicken breasts were salted within postmortem 15 min (pre-rigor salting) or at 24 h (post-rigor salting).
The salt concentration of all samples was equally fixed as 2% (w/w, based on total sample weight).
The significance of the result for one-way ANOVA within 3 pre-rigor salting treatments.
Asterisks mean the significance of t-test between post-rigor salting treatment with NaCl and each pre-rigor salting treatment. **P < 0.01; ***P < 0.001.
Means sharing different letters in the same row are significantly different among pre-rigor salting treatments (P < 0.05).
No significant difference in EAI value between all treatments was found (Table 3). According to Li-Chan et al. (1985), emulsifying properties of muscle proteins are related to the exposure level of internal hydrophobic groups. Xiong and Brekke (1990) reported no difference in protein unfolding or conformational change between salt-soluble proteins extracted from pre- and post-rigor chicken breasts. In addition, Zhang et al. (2015) reported that the replacement of NaCl with KCl had no impact on surface hydrophobicity of myofibrils extracted from chicken. Thus, our findings for unchanged EAI in this study, regardless of different salting time and salt type, are consistent with the previous observations.
Textural Properties
The textural properties of pre-rigor salted chicken breasts are shown in Table 4. No differences in hardness, springiness, cohesiveness, and chewiness were found between pre- and post-rigor salted chicken breasts (P > 0.05). Among pre-rigor salting treatments, the lowest hardness, gumminess, and chewiness were found in pre-rigor chicken breast salted with KCl (P < 0.05). In addition, pre-rigor chicken breasts salted with KCl or NaCl+KCl mixture showed slightly lower gumminess than post-rigor chicken breast salted with NaCl (P < 0.05), while there were no significant differences in primary textural parameters such as hardness, springiness, and cohesiveness. Previously, Barbut et al. (1988) have found that firmness in turkey frankfurters formulated with different salts at the same ionic strength was ordered as follows; NaCl> KCl> MgCl2. Gordon and Barbut (1989) reported that the hardness of the meat batter (frankfurters) was affected by salt type, and the results showed that hardness was higher with 2.5% NaCl than with 3.19% KCl at identical ionic strengths. They suggested that the hardness of meat batter was affected by the type and amount of extracted proteins as well as cation−protein interaction during the cooking process (Gordon and Barbut, 1989). Likewise, in this study, the decreased hardness of pre-rigor chicken breast salted with KCl was likely due to decreased protein solubility upon KCl addition, which in turn negatively affects secondary parameters and textural properties such as gumminess and chewiness.
Table 4.
Textural properties of pre-rigor salted ground chicken breasts with NaCl and/or KCl.
| Post-rigor salting1 |
Pre-rigor salting |
||||
|---|---|---|---|---|---|
| Traits | NaCl2 | NaCl | NaCl+KCl (1:1 ratio) | KCl | Significance of P value3 |
| Hardness (kg) | 23.29 ± 0.66 | 23.78 ± 0.53A | 22.34 ± 0.43B | 21.98 ± 0.49B | 0.009 |
| Springiness (ratio) | 0.75 ± 0.01 | 0.77 ± 0.01 | 0.76 ± 0.01 | 0.77 ± 0.01 | 0.154 |
| Cohesiveness | 0.34 ± 0.01 | 0.35 ± 0.01 | 0.34 ± 0.01 | 0.34 ± 0.01 | 0.140 |
| Gumminess (kg) | 8.01 ± 0.24 | 8.36 ± 0.24A | 7.57 ± 0.07B* | 7.38 ± 0.21B* | 0.002 |
| Chewiness (kg) | 6.43 ± 0.33 | 6.44 ± 0.22A | 5.71 ± 0.06B | 5.65 ± 0.20B | 0.003 |
All values are mean ± standard deviation. (n = 3).
Ground chicken breasts were salted at postmortem 15 min (pre-rigor salting) or 24 h (post-rigor salting).
The salt concentration of all samples was equally fixed as 2% (w/w, based on total sample weight).
The significance of the result for one-way ANOVA within 3 pre-rigor salting treatments.
Asterisks mean the significance of t-test between post-rigor salting treatment with NaCl and each pre-rigor salting treatment. *P < 0.05.
Means sharing different letters in the same row are significantly different among pre-rigor salting treatments (P < 0.05).
In conclusion, the results of this study show that 2% KCl could have pre-rigor salting effects that maintain superior technological properties of pre-rigor chicken breasts compared to post-rigor salted chicken breasts with 2% NaCl. However, the impact of 2% KCl on technological properties, such as water-holding capacity, protein solubility, and texture, could be less effective than pre-rigor salting with 2% NaCl, while pre-rigor salting with KCl slightly increased the ultimate pH value of chicken breast. The decreased efficiency of pre-rigor salting with KCl may be associated with the lower hydration ability of K+ ion than that of Na+ ion, along with its relatively low ionic strength at the same percentage concentration. In pre-rigor salting with NaCl and/or KCl, therefore, it may be that ion interaction with muscle proteins, but not their effect on postmortem metabolism, may have a greater impact on the technological properties of pre-rigor salted meat. To clearly confirm the impacts of pre-rigor salting with KCl, further studies comparing pre-rigor salting effect of NaCl and KCl at equal ionic strength are warranted.
ACKNOWLEDGEMENTS
This research was supported by Technology Development Program (PJ013809012018) for rural development administration, Republic of Korea.
REFERENCES
- Aliño M., Grau R., Toldrá F., Blesa E., Pagán M.J., Barat J.M. Influence of sodium replacement on physicochemical properties of dry-cured loin. Meat Sci. 2009;83:423–430. doi: 10.1016/j.meatsci.2009.06.022. [DOI] [PubMed] [Google Scholar]
- Alvarado C.Z., Sams A.R. Rigor mortis development in turkey breast muscle and the effect of electrical stunning. Poult. Sci. 2000;79:1694–1698. doi: 10.1093/ps/79.11.1694. [DOI] [PubMed] [Google Scholar]
- AMSA . American Meat Science Association; IL: 2012. Meat Color Measurement Guidelines; pp. 1–17. [Google Scholar]
- Barbut S., Maurer A.J., Lindsay R.C. Effects of partial sodium chloride replacement with other chloride salts on the physical and sensory properties of turkey frankfurters. Food Res. Int. 1988;21:90–96. [Google Scholar]
- Bernthal P.H., Booren A.M., Gray J.I. Effect of sodium chloride concentration on pH, water-holding capacity and extractable protein of prerigor and postrigor ground beef. Meat Sci. 1989;25:143–154. doi: 10.1016/0309-1740(89)90029-6. [DOI] [PubMed] [Google Scholar]
- Bourne M.C. Texture profile analysis. Food Technol. 1978;32:62–66. [Google Scholar]
- Bowker B., Zhuang H. Impact of white striping on functionality attributes of broiler breast meat. Poult. Sci. 2016;95:1957–1965. doi: 10.3382/ps/pew115. [DOI] [PubMed] [Google Scholar]
- Choi Y.S., Choi J.H., Han D.J., Kim H.Y., Lee M.A., Kim H.W., Lee J.W., Chung H.J., Kim C.J. Optimization of replacing pork back fat with grape seed oil and rice bran fiber for reduced-fat meat emulsion systems. Meat Sci. 2010;84:212–218. doi: 10.1016/j.meatsci.2009.08.048. [DOI] [PubMed] [Google Scholar]
- Coon F.P., Calkins C.R., Mandigo R.W. Pre-and post-rigor sectioned and formed beef steaks manufactured with different salt levels, mixing times and tempering times. J. Food Sci. 1983;48:1731–1734. [Google Scholar]
- Desmond E. Reducing salt: a challenge for the meat industry. Meat Sci. 2006;74:188–196. doi: 10.1016/j.meatsci.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Farouk M.M., Swan J.E. Effect of pH at time of salting on the functional properties of pre-rigor beef. Meat Sci. 1997;45:463–472. doi: 10.1016/s0309-1740(96)00125-8. [DOI] [PubMed] [Google Scholar]
- Gordon A., Barbut S. The effect of chloride salts on the texture, microstructure and stability of meat batters. Food Structure. 1989;8:271–283. [Google Scholar]
- Gornall A.G., Bardawill C.J., David M.M. Determination of serum protein by means of the biuret reaction. J. Biol. Chem. 1949;177:751–766. [PubMed] [Google Scholar]
- Hamm R. Über das Wasserbindungsvermögen des Saugetiermuskels. III Mitt. Die Wirkung von Neutralsalzen. Zeitschrift für Lebensmittel-Untersuchung und -Forschung. 1957;106:281. [Google Scholar]
- Hamm R. Biochemistry of meat hydration. Adv. Food Nutr. Res. 1961;10:355–463. doi: 10.1016/s0065-2628(08)60141-x. [DOI] [PubMed] [Google Scholar]
- Hamm R. Postmortem breakdown of ATP and glycogen in ground muscle: a review. Meat Sci. 1977;1:15–39. doi: 10.1016/0309-1740(77)90029-8. [DOI] [PubMed] [Google Scholar]
- Hand L.W., Terrell R.N., Smith G.C. Effects of complete or partial replacement of sodium chloride on processing and sensory properties of hams. J. Food Sci. 1982;47:1776–1778. [Google Scholar]
- Honikel K.O., Hamm R. Influence of cooling and freezing of minced pre-rigor muscle on the breakdown of ATP and glycogen. Meat Sci. 1978;2:181–188. doi: 10.1016/0309-1740(78)90003-7. [DOI] [PubMed] [Google Scholar]
- Horita C.N., Morgano M.A., Celeghini R.M.S., Pollonio M.A.R. Physico-chemical and sensory properties of reduced-fat mortadella prepared with blends of calcium, magnesium and potassium chloride as partial substitutes for sodium chloride. Meat Sci. 2011;89:426–433. doi: 10.1016/j.meatsci.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Inguglia E.S., Zhang Z., Tiwari B.K., Kerry J.P., Burgess C.M. Salt reduction strategies in processed meat products – a review. Trends Food Sci. Technol. 2017;59:70–78. [Google Scholar]
- Joo S.T., Kauffman R.G., Kim B.C., Park G.B. The relationship of sarcoplasmic and myofibrillar protein solubility to colour and water-holding capacity in porcine longissimus muscle. Meat Sci. 1999;52:291–297. doi: 10.1016/s0309-1740(99)00005-4. [DOI] [PubMed] [Google Scholar]
- Keeton J.T. Effects of potassium chloride on properties of country-style hams. J. Food Sci. 1984;49:146–148. [Google Scholar]
- Kim H.W., Choi J.H., Choi Y.S., Kim H.Y., Han D.J., Kim T.H., Lee S.K., Kim C.J. Effects of salt concentration in soybean sauce on the physicochemical properties of pre-rigor ground Hanwoo muscle. Korean J. Food Sci. An. 2011;31:389–397. [Google Scholar]
- Kim H.W., Hwang K.E., Song D.H., Kim Y.J., Ham Y.K., Yeo E.J., Jeong T.J., Choi Y.S., Kim C.J. Effect of pre-rigor salting levels on physicochemical and textural properties of chicken breast muscles. Korean J. Food Sci. An. 2015;35:577–584. doi: 10.5851/kosfa.2015.35.5.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh K.C., Binder T.D., McMillan K.W., Kim M.B. The relationship between ATP and R-values in postmortem bovine longissimus dorsi muscle. Meat Sci. 1993;33:253–263. doi: 10.1016/0309-1740(93)90063-N. [DOI] [PubMed] [Google Scholar]
- Lee S.H., Choi J.H., Choi Y.S., Kim H.Y., Kim H.W., Park J.H., Song D.H., Kim Y.J., Kim C.J. Effects of pre-rigor salting on the physicochemical and textural properties of ground duck breast muscle. Korean J. Food An. 2012;32:756–762. [Google Scholar]
- Li-Chan E., Nakai S., Wood D.F. Relationship between functional (fat binding, emulsifying) and physicochemical properties of muscle proteins. Effects of heating, freezing, pH and species. J. Food Sci. 1985;50:1034–1040. [Google Scholar]
- NRC . 9th rev. ed. Natl. Acad. Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- Offer G., Trinick J. On the mechanism of water holding in meat: the swelling and shrinking of myofibrils. Meat Sci. 1983;8:245–281. doi: 10.1016/0309-1740(83)90013-X. [DOI] [PubMed] [Google Scholar]
- Papa C.M., Fletcher D.L. Pectoralis muscle shortening and rigor development at different locations within the broiler breast. Poult. Sci. 1988;67:635–640. doi: 10.3382/ps.0670635. [DOI] [PubMed] [Google Scholar]
- Pisula A., Tyburcy A. Hot processing of meat. Meat Sci. 1996;43:S125–S134. doi: 10.1016/0309-1740(96)00060-5. [DOI] [PubMed] [Google Scholar]
- Rees M.P., Trout G.R., Warner R.D. Effect of calcium infusion on tenderness and ageing rate of pork m. longissimus thoracis et lumborum after accelerated boning. Meat Sci. 2002;61:169–179. doi: 10.1016/s0309-1740(01)00181-4. [DOI] [PubMed] [Google Scholar]
- Ruusunen M., Puolanne E. Reducing sodium intake from meat products. Meat Sci. 2005;70:531–541. doi: 10.1016/j.meatsci.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Sadler D.N., Swan J.E. Chilled storage life of hot-boned, pre-rigor, salted minced beef. Meat Sci. 1997;45:427–437. doi: 10.1016/s0309-1740(96)00132-5. [DOI] [PubMed] [Google Scholar]
- Saffle R.L., Galbreath J.W. Quantitative determination of salt-soluble protein in various types of meat. Food Technol. 1964;18:1934–1944. [Google Scholar]
- Selmane D., Christophe V., Gholamreza D. Extraction of proteins from slaughterhouse by-products: Influence of operating conditions on functional properties. Meat Sci. 2008;79:640–647. doi: 10.1016/j.meatsci.2007.10.029. [DOI] [PubMed] [Google Scholar]
- Soglia F., Petracci M., Mudalal S., Vannini L., Gozzi G., Camprini L., Cavani C. Partial replacement of sodium chloride with potassium chloride in marinated rabbit meat. Int. J. Food Sci. Technol. 2014;49:2184–2191. [Google Scholar]
- Terrell R.N., Ming C.G., Jacobs J.A., Smith G.C., Carpenter Z.L. Effect of chloride salts, acid phosphate and electrical stimulation on pH and moisture loss from beef clod muscles. J. Ani. Sci. 1981;53:658–662. [Google Scholar]
- Van Hoof J., Hamm R. Influence of diphosphate (pyrophosphate) on the breakdown of adenosine triphosphate and glycogen in ground beef muscle postmortem. Eur. Food Res. Technol. 1973;153:271–282. [Google Scholar]
- Whiting R.C., Jenkins R.K. Partial substitution of sodium chloride by potassium chloride in frankfurter formulation. J. Food Qual. 1981;4:259–269. [Google Scholar]
- Wu L., Wu T., Wu J., Chang R., Lan X., Wei K., Jia X. Effects of cations on the “salt in” of myofibrillar proteins. Food Hydrocoll. 2016;58:179–183. [Google Scholar]
- Xiong Y.L., Brekke C.J. Physicochemical and gelation properties of pre-and postrigor chicken salt-soluble proteins. J. Food Sci. 1990;55:1544–1548. [Google Scholar]
- Zhang Z., Yang Y., Tang X., Chen Y., You Y. Chemical forces study of heat-induced myofibrillar protein gel as affected by partial substitution of NaCl with KCl, MgCl2 and CaCl2. CyTA J. Food. 2015;14:239–247. [Google Scholar]
Uncited Reference
- Dalrymple R.H., Hamm R. Postmortem glycolysis in prerigor ground bovine and rabbit muscle. J. Food Sci. 1975;40:850–853. [Google Scholar]


