Abstract
This study was conducted to investigate the effects of supplementation with nicotinamide (NAM) and sodium butyrate (BA) on meat quality and expression of muscle development genes in broilers reared at a high stocking density. A total of 567, 21-day-old AA broilers were randomly assigned to 5 treatment groups and 2 control groups, with 7 replicates of each group. The control groups included a low stocking density (LD; 12.9 birds/m2) and were fed a basal diet. The treatment groups were kept at a high stocking density (HD; 18.6 birds/m2) and received either a low dose of NAM (50 mg/kg; treatment LN), a high dose of NAM (100 mg/kg; treatment HN), a low dose of BA (500 mg/kg; treatment LB), a high dose of BA (1,000 mg/kg; treatment HB), or a compound supplement (50 mg/kg NAM+500 mg/kg BA; treatment COMB); broilers were reared till 42 D of age. The control groups were kept at HD or at LD (12.9 birds/m2) and were fed a basal diet. The heterophil-to-lymphocyte ratio was significantly higher in the HD control group than that in the LD group; this ratio was significantly lower in treatments LN, HN, HB, and COMB than that in the HD control group. The lightness of breast muscles at 45 min and 24 h after slaughter was significantly higher in the HD group than that in the LD group, and the HD group showed the highest drip loss at 24 h and 48 h. Lightness and drip loss were lower in the HN, LB, and COMB treatments than those in the HD group. HD rearing significantly reduced gene expression of myogenic regulatory factor 5 (MYF5) while significantly increased expression of the protein ubiquitin degradation genes FBXO9, FBXO22, and FBXO32. All treatments significantly reduced FBXO9 and FBXO32 expression. Our results suggest dietary supplementation with NAM and BA can improve meat quality of broilers under high stocking density by upregulating the expression of myogenic genes, and inhibiting protein ubiquitination.
Key words: stocking density, broiler, nicotinamide, sodium butyrate, meat quality
Introduction
During the past decades, stocking density in poultry breeding has dramatically increased to improve profitability (Kiani and von Borstel, 2019). However, high stocking density has adverse effects on production performance and animal welfare in broilers (Averos and Estevez, 2018, Goo et al., 2019). In a previous study, we found that high stocking density reduced the pectoral muscle's total antioxidant capacity (T-AOC) and reduced expression of antioxidant proteins such as regucalcin and catalase in the liver (Wu et al., 2018). Furthermore, high stocking density has been shown to cause oxidative stress in broilers (Najafi et al., 2015), and oxidative stress is associated with high drip loss and lowered pH of broiler breast muscle (Chen et al., 2010, Wang et al., 2017). Moreover, high stocking density also affects meat quality in terms of reduced tenderness (Patria et al., 2016, Zhang et al., 2018).
Oxidative stress can overactivate the ubiquitin-proteasome system, which causes protein degradation and skeletal muscle atrophy (Abrigo et al., 2018). The F-box proteins (FBXO) are crucial for the process of ubiquitin-dependent proteolysis in eukaryotes (Yoshida, 2007). FBXO32, a member of the F-box protein family, is a commonly used marker of accelerated proteolysis and atrophy processes (Cohen et al., 2015). Thus, FBXO32 expression is considered as a suitable marker for assessing muscle mass in broiler chickens for breeding selection (Nakashima et al., 2009).
Nicotinamide (NAM) can upregulate the expression of antioxidant genes (John et al., 2012) and improve stress resistance ability (Tran et al., 2016). In addition, NAM reduces the formation of carbonylated proteins in the liver of high-fat-fed mice (Mitchell et al., 2018). Dietary supplementation with high doses of NAM (150 mg/kg) can increase the content of unsaturated fatty acids in breast muscle of broilers reared at high stocking densities (Piva et al., 1993). Furthermore, supplementation with NAM can improve muscle quality, and dietary supplementation with 60 mg/kg niacin (the precursor of NAM) reduced drip loss in broiler breast muscle (Jiang et al., 2011).
Sodium butyrate (BA) was shown to improve meat quality in broilers subjected to experimental corticosterone injections (Zhang et al., 2011) and to affect muscle structure by increasing the ratio of slow-type muscle fibers in the quadriceps of mice (Henagan et al., 2015). In a previous study, we identified a synergistic effect of NAM and BA on growth performance and mitochondrial function in broilers (Wu et al., 2019). However, the individual contribution of each of these 2 compounds to meat quality in broilers has not yet been investigated.
Therefore, this study was conducted to assess the effect of supplementation with NAM and BA on meat quality and to investigate the expression of putative genes associated with meat quality.
Material and Methods
Ethics Statement
All procedures used in this experiment were approved by the Institutional Animal Care and Use Committee of the China Agricultural University, Beijing, China.
Experimental Animals and Grouping
A total of 567, 21-day-old AA broilers were randomly assigned to 7 groups, which included 2 control groups and 5 treatment groups, using 7 replicates of each group.
The control groups were kept at a low stocking density (LD; 12.9 birds/m2; 9 birds per cage) or high stocking density (HD; 18.6 birds/m2; 12 birds per cage) and were fed a basal diet. Stocking densities used in this study are based on the study of Vargas-Galicia et al. (2017). The treatment groups were kept at HD, and their diet was supplemented with a low dose of NAM (50 mg/kg; treatment LN), a high dose of NAM (100 mg/kg; treatment HN), a low dose of BA (500 mg/kg; treatment LB), high dose of BA (1,000 mg/kg; treatment HB), or compound addition (50 mg/kg NAM plus 500 mg/kg BA; treatment COMB); broilers were reared to an age of 42 D.
The feed additives NAM (99%; Jiangxi Brothers Medicine Co. Ltd., Jiujiang, China) and BA (30%, encapsulated; Hangzhou King Technology Feed Co. Ltd., Hangzhou, China) were purchased from a commercial distributor.
Experimental Diets
The composition and nutrient levels of basal diet are shown in Table 1. The diets were formulated to meet or exceed minimum nutrient requirements as recommended by the National Research Council (1994).
Table 1.
The composition and nutrient level of basal diet.
| Ingredient | % | Nutrient parameters | Nutrient level (%) |
|---|---|---|---|
| Corn | 60.73 | AMEn | 3,110 kcal/kg |
| Soybean meal | 22.70 | Crude protein | 19.05 |
| Corn gluten meal | 6.00 | Lysine | 1.12 |
| Wheat bran | 2.00 | Methionine | 0.53 |
| Soybean oil | 4.10 | Threonine | 0.71 |
| DL- Methionine | 0.22 | Tryptophan | 0.19 |
| L-Lysine sulphate | 0.60 | Calcium | 0.93 |
| Threonine | 0.03 | Available phosphorus | 0.41 |
| Sodium chloride | 0.30 | Met + Cys3 | 0.80 |
| Choline chloride (50%) | 0.20 | ||
| Trace mineral premix1 | 0.20 | ||
| Vitamin premix2 | 0.02 | ||
| Dicalcium phosphate | 1.70 | ||
| Limestone | 1.20 |
The trace mineral premix provided the following per kg of diets: Cu, 16 mg (as CuSO4·5H2O); Zn, 110 mg (as ZnSO4); Fe, 80 mg (as FeSO4·H2O); Mn, 120 mg (as MnO);Se, 0.3 mg (as Na2SeO3); I, 1.5 mg (as KI); Co, 0.5 mg.
The vitamin premix provided the following per kg of diets: vitamin A, 10,000 IU; vitamin D3, 2,400 IU; vitamin E, 20 mg; vitamin K3, 2 mg; vitamin B1, 2 mg; vitamin B2, 6.4 mg; VB6, 3 mg; VB12, 0.02 mg; biotin, 0.1 mg; folic acid, 1 mg; pantothenic acid, 10 mg; nicotinamide, 30 mg.
Met + Cys: methionine + cysteine.
Raising Management
The experiment was conducted on the experimental chicken farm of the College of Animal Science and Technology, China Agricultural University, Beijing, China. Broilers were raised from 21 to 42 D of age on a plastic wire floor. The respective diet and clean drinking water were continuously provided ad libitum. Ambient temperature was kept at 20–21°C, and the illumination period was 18 h per day. The animals' health status was monitored daily.
Data Collection and Sampling
On day 42 and after 5 h of starvation, the broilers' body weight (BW) and the remaining feed per cage were weighed. Feed intake (FI) and body weight gain (BWG) were determined to calculate the feed conversion rate (FCR).
One bird per cage was randomly selected to collect serum and breast muscle samples. A 4-mL blood sample was collected from the wing vein of one broiler per replicate and was placed in a coagulation tube. After centrifugation at 3,000 × g and 4°C for 10 minutes, serum was separated and stored at −20°C. Blood samples of 2 mL were collected using anticoagulation tubes to conduct routine blood tests. The broilers were killed by cervical dislocation after blood collection.
Immediately after slaughter, the breast muscle on the animal's right side was removed for meat quality evaluation.
Meat Quality Evaluation
To determine drip loss, approximately 10 g of muscle was weighed (W1) and placed in a sealed polyethylene bag at for storage 4°C. The muscle was reweighed (W2) after 24 h, and drip loss was calculated as (W1–W2)/W2 × 100%.
Cooking loss was determined according to the method suggested in the study by Cai et al. (2018) and was calculated according to the the following formula: (initial weight − final weight)/initial weight × 100%.
At 45 min and 24 h postmortem, meat color was measured using an automatic Chroma meter (3nh NR-10QC; Shenzhen Three nh Technology Co., Ltd., Shenzhen, China) set to the color system of lightness (L*), redness (a*), and yellowness (b*). Each sample was measured 3 times at 3 different locations (cranial, medial, and caudal portion) on the medial surface of each fillet.
The pH of breast muscle was measured at 45 min, 24 h, and 48 h after slaughter using a pH meter (Testo 205; Testo, Berlin, Germany), and pH of leg muscle was measured for 45 min and 24 h after slaughter.
Enzyme Activity Determination in Serum
Blood routine tests were performed by a conductivity method using a blood biochemical analyzer (XS-500i; Sysmex Co., Kobe, Japan).
T-AOC (cat. # A015); enzymatic activities of superoxide dismutase (cat. # A001-3), lactate dehydrogenase (cat. # A020-2), creatine kinase (cat. # A032), and xanthine oxidase (XOD; cat. # A002-1); and content of malondialdehyde (cat. # A003-1) in serum were determined according to the instructions of the Jian Cheng Bioengineering Institute (Nanjing, China).
Expression of Muscle Development Genes
Total RNA of broiler muscle samples was extracted using Trizol reagent (TAKARA BIO Inc., Kusatsu, Japan), and RNA purity and concentration were measured using a nucleic acid analyzer (NanoDrop 2000; Thermo Fisher Scientific, Waltham, MA). The cDNA was produced using a reverse transcription kit (PrimeScript RT Reagent Kit with gDNA Eraser [Perfect Real Time]; cat. # RR047 A; TAKARA BIO Inc., Kusatsu, Japan) and was stored at −80°C for further analysis. Fluorescence quantitative PCR was performed using a TB GreenTM Premix Ex TaqTM kit (Tli RNaseH Plus; cat. # RR420 A; TAKARA BIO Inc., Kusatsu, Japan) according to the manufacturer's instructions. The detection device was a 7500-fluorescence detection system (Applied Biosystems, Foster City, CA). The PCR reaction conditions were as follows: initial denaturation at 95°C for 30 s was followed by 40 cycles of 95°C for 5 s and 60°C for 34 s. After PCR amplification, a dissolution curve was produced, and agarose gel electrophoresis was performed to confirm the respective fragment size. PCR primer sequences for the target gene fragments and the internal reference gene (beta-actin) are shown in table 2. Gene expression values were calculated using the 2−ΔΔCT method.
Table 2.
Primer sequences of real time PCR.
| Gene | Primer sequence (5′–3′) | Size | Accession no. |
|---|---|---|---|
| Beta-actin | Forward: CAACACAGTGCTGTCTGGTGGTAC Reverse: CTCCTGCTTGCTGATCCACATCTG | 199 | L08165.1 |
| MSTN | Forward: AGTGGCTCTGGATGGCAGTAGTC Reverse: TCTGTCTCCACGTACAAGCATTGC |
80 | NM_001001461.1 |
| FBXO32 | Forward: GCCAGTACCACTTCACAGACAGAC Reverse: GCGTGTCACCATACTGCTCCTTC |
132 | NM_001030956.1 |
| MYF5 | Forward: GCGGAAGGCAGCCACTATGAG Reverse: CGATGTACCTGATGGCGTTCCTC |
143 | NM_001030363.1 |
| MYOG | Forward: AGCAGGAGCGTGAGCAGAGG Reverse: CGATGGAGGAGAGCGAGTGGAG |
198 | NM_204184.1 |
| MYF6 | Forward: CTGCTGCACAGGCTGGATCAG Reverse: AGGCCGACGACTCCACCATG |
199 | NM_001030746.1 |
| TNNI1 | Forward: AGAAGGAGGACACGGAGAAGGAG Reverse: TTGGCGGCGTCGAACATCTTC |
110 | XM_004934839.3 |
| TNNI2 | Forward: TGGAGGACCTGAGCCAGAAGC Reverse: ACGCAGCATGGCATCAGCAG |
95 | NM_205417.1 |
| FBXO7 | Forward: AGTGCCACTGATGCCTTGATTGTC Reverse: GCATAGACACTGCCTTGGCTTCTG |
94 | NM_001012537.2 |
| FBXO8 | Forward: CAGCAACTGCAACACCAAGGATTC Reverse: TTCCGGTGGCTTGTATTGGAGAC |
101 | NM_001006446.1 |
| FBXO9 | Forward: CTATAGAGCGTGGCACCAAGTGG Reverse: TGTGGATCTTCAGGCGTTGTAAGC |
93 | NM_001006414.1 |
| FBXO22 | Forward: CCAGAGTGCCACGGTTTTGT Reverse: CTCGGGGATGTTTGCTGCTT |
94 | NM_001030545.1 |
| FBXO34 | Forward: TGGCTACGGAGCGAGGTGTG Reverse: CAGCGTGAGCAAGTCCTGAACC |
122 | NM_001031213.1 |
| FBXO38 | Forward: AGTATGGCTTGGCTGATGTGGTTG Reverse: CATCGCACCAATGTGAGGTCTACC |
190 | JX290204.1 |
| VEGF | Forward: CGGAGTTGTCGAAGGCTGCTC Reverse: CGCACATCTCATCAGAGGCACAC |
191 | AB011078.1 |
| ANGPT1 | Forward: GGATGGTGGTTCGATGCCTGTG Reverse: GTGGTGGAACGCAAGGAGTAGC |
134 | NM_001199447.3 |
| ANGPT2 | Forward: TCTGGACTCACAACAAGTGGAACC Reverse: GCTGCCACCTTCACGTCTCTG |
129 | NM_204817.1 |
| ANGPTL2 | Forward: GCTGCTGTAGGAACTGCTGCTAG Reverse: CCTGTGGATCACTGGAACGCTTG |
112 | NM_001277699.1 |
| ANGPTL3 | Forward: ACGTTACGTCGAGTATGCGTTCAC Reverse: CCATGTCACGGTCCGCAGTTG |
140 | NM_001135122.1 |
Statistical Analysis
Results were shown as means and standard errors of the mean. A one-way ANOVA was applied for single-factor analysis using SPSS 20.0 (SPSS Inc. Chicago, IL). Statistical significance is reported at P < 0.05. Trends are reported at P < 0.1.
Results
Performance and Meat Quality
Stocking density had no significant effect on FI, BW, BWG, and FCR, and supplementation with NAM or BA produced no significant effect on broiler production performance (Table 3).
Table 3.
Effect of NAM and BA supplementation and stocking density on production performance of broilers from 21 to 42 D old.
| Items | LD | HD | LN | HN | LB | HB | COMB | SEM | P value |
|---|---|---|---|---|---|---|---|---|---|
| BW/g | 2,448 | 2,285 | 2,256 | 2,274 | 2,282 | 2,251 | 2,282 | 21.6 | 0.204 |
| BWG/g | 1,517 | 1,358 | 1,326 | 1,356 | 1,340 | 1,333 | 1,361 | 21.2 | 0.210 |
| FI/g | 2,691 | 2,536 | 2,496 | 2,534 | 2,483 | 2,495 | 2,556 | 23.8 | 0.267 |
| FCR | 1.78 | 1.87 | 1.89 | 1.88 | 1.85 | 1.88 | 1.89 | 0.01 | 0.299 |
Data are presented as mean ± SEM.
BA, sodium butyrate; COMB, compound addition 50 mg/kg NAM plus 500 mg/kg BA + HD; LB, low dose of BA (500 mg/kg);LD, low stocking density (12.9 birds/m2) group; LN, low dose of NAM (50 mg/kg) + HD; HB, high dose of BA (1,000 mg/kg); HD, high stocking density group (18.6 birds/m2) group; HN, high dose of NAM (100 mg/kg) + HD; NAM, nicotinamide; 1, SEM, standard error of mean.
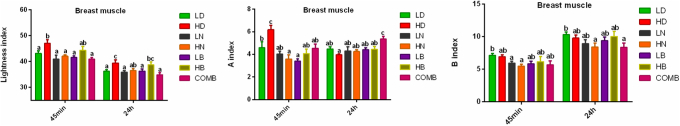
However, stocking density significantly affected breast muscle lightness at 45 min and 24 h, with higher values in the HD group than in the LD group (P < 0.05). Supplementation with BA or NAM significantly decreased breast muscle lightness at 45 min and 24 h after slaughter, compared with the control, apart from the HB treatment (P < 0.05) (Figure 1).
Figure 1.
Meat color of breast muscle at 45 min and 24 h Values with different superscript letters in the same column differ significantly (P < 0.05). Data are presented as mean ± SEM. SEM, standard error of mean.
Breast muscle redness at 45 min after slaughter was significantly higher in the HD group than in the LD group (P < 0.05) (Figure 1) but was lower in the HD group than in the LD group after 24 h. At 45 min, redness values of the LN, HN, LB, and COMB groups were significantly lower than that of the HD group (P < 0.05), and at 24 h, redness of COMB was significantly higher than that of the HD group (P < 0.05). No significant difference was observed between groups HD and LD.
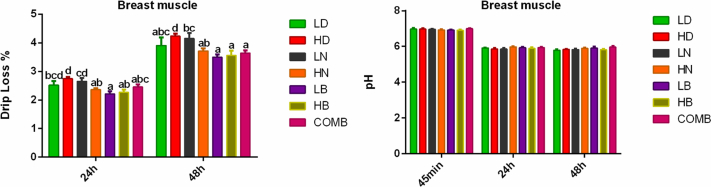
There was a significant difference in the drip loss of breast muscle between groups (P < 0.05), and the highest drip loss was observed in the HD group at 24 h and 48 h. The drip loss in HN, LB, HB, and COMB group was lower than that in the HD group at 24 h and 48 h (P < 0.05) (Figure 2).
Figure 2.
Drip loss and pH of breast muscle. Values with different superscript letters in the same column differ significantly (P < 0.05). Data are presented as mean ± SEM. SEM, standard error of mean.
Stocking density had no significant effect on the pH of the breast muscle at 45 min, 24 h, and 48 h (Figure 2).
Blood Indices
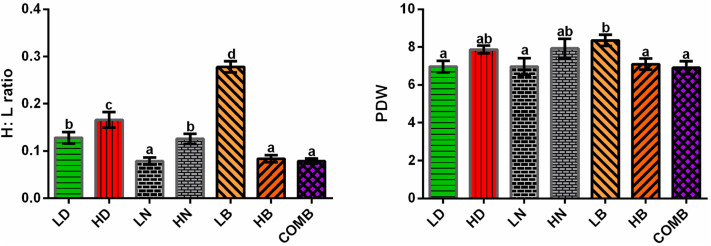
The heterophil-to-lymphocyte ratio was significantly higher in the HD group than that in the LD group (P < 0.05). In the LN, HN, HB, and COMB treatments, this ratio was significantly lower than that in the HD group (P < 0.05). Platelet distribution width showed a similar pattern as the heterophil-to-lymphocyte ratio (Figure 3).
Figure 3.
The blood heterophil-to-lymphocyte (H:L) ratio and platelet distribution width (PDW) of broilers. Values with different superscript letters in the same column differ significantly (P < 0.05). Data are presented as mean ± SEM. SEM, standard error of mean.
Stocking density had no significant effect on serum superoxide dismutase activity or T-AOC. Similarly, stocking density had no significant effect on oxidative stress markers, that is, creatine kinase and lactate dehydrogenase, and neither on malondialdehyde or XOD. However, serum XOD activity was significantly lower in the LN treatment than that in the HD group (P < 0.05) (Table 4).
Table 4.
Effect of NAM and BA supplementation and stocking density on serum antioxidant and oxidative stress indicators.
| Items | LD | HD | LN | HN | LB | HB | COMB | SEM | P value |
|---|---|---|---|---|---|---|---|---|---|
| SOD/U/mL | 68.74 | 70.15 | 79.43 | 69.28 | 78.95 | 78.78 | 70.05 | 1.69 | 0.148 |
| T-AOC/U/mL | 1.68 | 1.53 | 1.81 | 1.73 | 1.60 | 1.74 | 1.82 | 0.05 | 0.823 |
| CK/U/mL | 3.23a,b | 3.36a,b,c | 3.62b,c,d | 3.93c,d | 4.04d | 3.94c,d | 2.94a | 0.09 | 0.001 |
| LDH/U/L | 7,542.37a,b | 7,244.55a,b | 8,916.01b,c | 8,324.88b,c | 9,495.72c | 9,128.37b,c | 7,449.33a,b | 239.72 | 0.006 |
| MDA/nmol/mL | 0.25 | 0.23 | 0.19 | 0.18 | 0.24 | 0.42 | 0.35 | 0.03 | 0.136 |
| XOD/U/L | 0.85b,c,d | 0.84b,c,d | 0.30a | 0.93c,d | 0.59a,b,c | 0.29a,b | 1.52d,e | 0.07 | <0.001 |
Values with different superscript letters in the same column differ significantly (P < 0.05).
Data are presented as mean ± SEM.
BA, sodium butyrate; CK, creatine kinase; COMB, compound addition 50 mg/kg NAM plus 500 mg/kg BA + HD; LB, low dose of BA (500 mg/kg);LD, low stocking density (12.9 birds/m2) group; LDH, lactate dehydrogenase; LN, low dose of NAM (50 mg/kg) + HD; HB, high dose of BA (1,000 mg/kg); HD, high stocking density group (18.6 birds/m2) group; HN, high dose of NAM (100 mg/kg) + HD; MDA, the content of malondialdehyde; NAM, nicotinamide; SEM, standard error of mean1,; SOD, superoxide dismutase; T-AOC, total antioxidant capacity; XOD, xanthine oxidase.
Expression of Muscle Development Genes
Gene expression of myogenic regulatory factor 5 (MYF5) was significantly reduced in the HD group, compared with the LD group. The expression of MYF5 was significantly higher in the LB treatment than that in the HD group (P < 0.05). Stocking density had no significant effect on the expression of myogenic regulatory factor 6 (MYF6), myogenin (MYOG), and myostatin (MSTN). Relative gene expression of MYOG was higher in treatments LB and COMB than in HD group (P < 0.05). In contrast, gene expression of MSTN was significantly lower in treatments LN, HN, and HB groups than that in the HD group (P < 0.05) (Table 5).
Table 5.
Effect of NAM and BA supplementation and stocking density on mRNA expression of muscle development–related genes of broilers from day 21 to 42.
| Gene Name | LD | HD | LN | HN | LB | HB | COMB | SEM | P value |
|---|---|---|---|---|---|---|---|---|---|
| MYF5 | 1.00d | 0.53b,c | 0.13a | 0.31a,b | 0.97d | 0.74c,d | 0.70c,d | 0.12 | <0.001 |
| MYF6 | 1.00a,b,c | 1.48c | 0.88a,b,c | 0.37a | 0.68a,b | 0.94a,b,c | 1.25b,c | 0.08 | 0.016 |
| MYOG | 1.00a,b | 0.86a,b | 0.92a,b | 0.77a | 1.58c,d | 1.24b,c | 1.77d | 0.08 | <0.001 |
| MSTN | 1.00b | 0.98b | 0.56a | 0.42a | 1.21b | 0.50a | 1.21b | 0.07 | <0.001 |
Values with different superscript letters in the same column differ significantly (P < 0.05).
Data are presented as mean ± SEM.
BA, sodium butyrate; COMB, compound addition 50 mg/kg NAM plus 500 mg/kg BA + HD; LB, low dose of BA (500 mg/kg); LD, low stocking density (12.9 birds/m2) group; LN, low dose of NAM (50 mg/kg) + HD; HB, high dose of BA (1,000 mg/kg); HD, high stocking density group (18.6 birds/m2) group; HN, high dose of NAM (100 mg/kg) + HD; 1,NAM, nicotinamide; SEM, standard error of mean.
Compared with the LD treatment, the HD group showed a significant increase in gene expression of FBXO9, FBXO22, and FBXO32. Expression levels of FBXO9 and FBXO32 were significantly lower in all treatment groups than those in the HD group (P < 0.05), and expression levels of FBXO22 and FBXO38 were significantly lower in LN, HN, LB, and HB treatments than in the HD group (P < 0.05) (Table 6).
Table 6.
Effect of NAM and BA supplementation and stocking density on mRNA expression of F-Box Protein Family Genes from day 21 to 42.
| Gene Name | LD | HD | LN | HN | LB | HB | COMB | SEM | P value |
|---|---|---|---|---|---|---|---|---|---|
| FBXO7 | 1.00a,b | 1.32a,b | 0.77a | 0.68a | 1.25a | 0.95a | 1.22b | 0.04 | <0.001 |
| FBXO8 | 1.00 | 1.49 | 1.23 | 1.11 | 1.01 | 1.15 | 1.51 | 0.04 | 0.078 |
| FBXO9 | 1.00b | 1.71c | 1.02b | 0.11a | 0.71b | 0.89b | 1.15b | 0.05 | <0.001 |
| FBXO22 | 1.00a | 1.55b | 0.95a | 0.81a | 0.66a | 0.70a | 1.08a,b | 0.05 | 0.011 |
| FBXO32 | 1.00b | 2.07c | 0.25a | 0.30a | 0.14a | 0.27a | 0.36a | 0.05 | <0.001 |
| FBXO34 | 1.00 | 1.40 | 1.56 | 1.36 | 1.08 | 1.14 | 1.29 | 0.05 | 0.201 |
| FBXO38 | 1.00a,b | 1.21b | 0.78a | 0.69a | 0.72a | 0.60a | 1.02a,b | 0.05 | 0.019 |
Values with different superscript litters in the same column differ significantly (P < 0.05).
Data are presented as mean ± SEM.
BA, sodium butyrate; COMB, compound addition 50 mg/kg NAM plus 500 mg/kg BA + HD; LB, low dose of BA (500 mg/kg); LD, low stocking density (12.9 birds/m2) group; LN, low dose of NAM (50 mg/kg) + HD; HB, high dose of BA (1,000 mg/kg); HD, high stocking density group (18.6 birds/m2) group; HN, high dose of NAM (100 mg/kg) + HD; 1,NAM, nicotinamide; SEM, standard error of mean.
Stocking density had no significant effect on gene expression of troponin I type I (TNNI1) and troponin I type 2 (TNNI2) genes. Noticeably, the TNNI1:TNNI2 ratio was significantly higher in the LN treatment than in the HD group (P < 0.05). Gene expression of the vascular endothelial growth factor (VEGF) was significantly higher in the HD group than in the LD group (P < 0.05). Expression levels of VEGF were significantly lower in the HN, LB, and HB treatments than in the HD group (P < 0.05). Stocking density had no significant effect on expression of ANGPT1, ANGPT2, ANGPTL2, and ANGPTL3 (P > 0.05); however, ANGPTL2 expression was significantly higher (P < 0.05) in LB and HB than in the HD group (Table 7).
Table 7.
Effect of NAM and BA supplementation and stocking density on mRNA expression of fiber type and vascular development genes from day 21 to 42.
| Gene Name | LD | HD | LN | HD | LB | HB | COMB | SEM | P value |
|---|---|---|---|---|---|---|---|---|---|
| TNNI1 | 1.00 | 1.04 | 0.88 | 0.78 | 0.79 | 0.71 | 1.05 | 0.05 | 0.468 |
| TNNI2 | 1.00 | 0.85 | 0.58 | 0.66 | 0.63 | 0.59 | 0.97 | 0.06 | 0.052 |
| TNNI1-2 | 1.00a | 1.17a | 1.47b | 1.09a | 1.19a | 1.14a | 1.07a | 0.03 | <0.001 |
| ANGPT1 | 1.00 | 0.85 | 0.91 | 0.79 | 0.86 | 0.68 | 0.74 | 0.04 | 0.314 |
| ANGPT2 | 1.00 | 0.90 | 0.85 | 0.87 | 0.89 | 1.12 | 0.99 | 0.03 | 0.276 |
| ANGPTL2 | 1.00a,b | 0.90a,b | 0.70a | 0.88a,b | 1.39c | 1.45c | 1.21b,c | 0.06 | <0.001 |
| ANGPTL3 | 1.00 | 1.23 | 0.95 | 0.95 | 1.00 | 1.01 | 0.95 | 0.03 | 0.221 |
| VEGF | 1.00a,b | 1.84c | 1.92c | 0.69a | 0.87a,b | 0.92a,b | 1.45b,c | 0.06 | 0.001 |
Values with different superscript letters in the same column differ significantly (P < 0.05).
Data are presented as mean ± SEM.
BA, sodium butyrate; COMB, compound addition 50 mg/kg NAM plus 500 mg/kg BA + HD; LB, low dose of BA (500 mg/kg); LD, low stocking density (12.9 birds/m2) group; LN, low dose of NAM (50 mg/kg) + HD; HB, high dose of BA (1,000 mg/kg); HD, high stocking density group (18.6 birds/m2) group; HN, high dose of NAM (100 mg/kg) + HD; 1,NAM, nicotinamide; SEM, standard error of mean; TNNI1-2, the TNNI1-to-TNNI2 ratio.
Discussion
In the present study, stocking density had no significant effect on FI, BW, and FCR of broilers. In line with these results, a previous study found that increasing stocking density from 10 birds/m2 to 16 birds/m2 had no adverse effects on BW, BWG, and FCR of caged broilers (Najafi et al., 2015). However, effects of stocking density on growth performance of broilers may depend on the rearing system.
The heterophil-to-lymphocyte ratio is an established tool to measure stress in poultry (Qaid et al., 2016). In the present study, the heterophil-to-lymphocyte ratio was higher in the HD group than that in the LD group, suggesting that high stocking density increases oxidative stress. Supplementation with NAM can upregulate expression of antioxidant genes (John et al., 2012) and can also enhance stress tolerance (Tran et al., 2016). In the present study, the activity of serum XOD was lower in the LN treatment than in the HD group, and inhibition of XOD is known to reduce production of reactive oxygen species (Fernandez et al., 2002). Therefore, dietary supplementation with NAM likely improves antioxidant capacity in broilers. Similarly, BA appears to enhance broiler antioxidant capacity (Wu et al., 2018). In the present study, the heterophil-to-lymphocyte ratio in the LN, HN, HB and COMB treatments was lower than that in the HD group, suggesting that both BA and NAM may help improve resistance against oxidative stress tolerance.
Meat color is one of the most important factors affecting consumer purchase decisions (Mancini and Hunt, 2005). Our results showed that high stocking density increased the lightness of breast muscle. High meat lightness is typically associated with muscle diseases such as woody meat and pale soft exudative meat (Petracci et al., 2004, Cai et al., 2018). Stress conditions such as heat stress and high CO2 concentrations have been shown to increase the lightness of breast muscle (Lu et al., 2017, Xu et al., 2018), and high lightness values in the HD group of present study may be due to oxidative stress induced by high stocking density. In the present study, we observed that dietary supplementation with either NAM or BA alone or in combination reversed the increase in lightness of breast muscle, suggesting antioxidant effects NAM and BA.
Stressors such as heat stress can reduce the redness of breast muscle (Wen et al., 2019). In the present study, the HD group showed the lowest redness values at 24 h after slaughter. In addition, our study found the value of 24 h redness in COMB group to be higher than that in the HD group. Generally, low lightness and increased redness values are desired as this indicates high myoglobin concentrations and an improvement in meat color (Kim et al., 2010). Higher redness values also indicate higher oxidative capacity of muscles (Gentry et al., 2004). Dietary supplementation with BA can increase the proportion of type I fibers in skeletal muscle in mice (Henagan et al., 2015), and similarly, supplementation with niacin, a precursor of NAM, increased the number of oxidative type I fibers in skeletal muscle of growing pigs (Khan et al., 2013) and induced muscle fiber transition from type II to type I in sheep (Khan et al., 2013). The TNNI1 and TNNI2 genes were only found in slow and fast fibers, respectively. In this study, stocking density had no significant effect on expression of TNNI1 or TNNI2; however, low doses of NAM significantly increased the TNNI1:TNNI2 ratio. Therefore, higher redness values may be associated with an increase in the ratio of oxidized slow fiber to fast fiber.
Stress conditions such as heat stress can cause increased drip loss in breast muscle (Lu et al., 2017), and high stocking densities increased drip loss in breast muscle of Peking ducks (Zhang et al., 2018). In the present study, the HD group showed the highest drip loss at 24 h and 48 h after slaughter, suggesting that high stocking densities may decrease water-holding capacity in broilers, perhaps because of increased oxidative stress. Previous studies indicated that dietary supplementation with niacin at 60 mg/kg helps reduce drip loss in chicken breast muscle (Jiang et al., 2011) and that supplementation with BA may partially reduce meat quality deterioration (Zhang et al., 2011). In the present study, dietary supplementation with NAM or BA reduced drip loss in breast muscle at 24 h after slaughter. In pork meat, high drip loss seems to be associated with reduced activity of antioxidant enzymes (Chen et al., 2010), and it has been shown that dietary supplementation with organic essential oils or antioxidants such as quercetin can help reduce drip loss in meat by enhancing the activity of antioxidant enzymes (Zou et al., 2016). NAM and BA are known to exert antioxidant effects, thus improved water-holding capacity may be associated with higher antioxidant capacity.
Environmental stressors can lead to enhanced muscle proteolysis (Milan et al., 2015), and muscle disorders such as woody meat and striped muscle are often accompanied by severe proteolytic processes (Baldi et al., 2018). The F-box protein family plays a crucial role in protein ubiquitination degradation (Milan et al., 2015), and the protein FBXO32 is a common marker for accelerated proteolysis and atrophy processes (Cohen et al., 2015). Compared with the LD group, HD rearing significantly increased expression of ubiquitin degradation genes such as FBXO9 and FBXO32, which are key genes regarding muscle atrophy. The ubiquitin-proteasome system affects drip loss by regulating the degradation of myofibrils (Milan et al., 2015), and upregulation of the ubiquitination gene UBE3B can increase damage to sarcoplasmic and myofibrillar proteins (Huynh et al., 2013), whereas increased expression of the deubiquitination gene ZRANB1 would enhance the water-holding capacity by promoting protein stability (Ponsuksili et al., 2010, Huynh et al., 2013). The expression levels of FBXO9 and FBXO32 were significantly lower in all treatment groups than those in the HD group. Therefore, supplementation with NAM and BA may improve muscle water-holding capacity by inhibiting the ubiquitination degradation process.
MYF5 is an important transcriptional activator that induces fibroblast differentiation into myoblasts, promotes transcription of muscle-specific target genes, and plays a role in muscle differentiation (Yamamoto et al., 2018). MYOG is essential for muscle homeostasis and for regulating myocyte fusion by determining the number and size of muscle fibers (Ganassi et al., 2018). In the present study, expression of the myogenic regulatory factor gene MYF5 was significantly reduced in the HD rearing group, compared with that in the LD group. Furthermore, MYOG expression was higher in the treatments LB and COMB than in the HD group. The protein MSTN is a key negative regulator of muscle growth and development, and inhibiting MSTN signaling can increase muscle mass (Shin et al., 2015). In the present study, expression of MSTN was lower in the LN, HN, and HB treatments than in the HD group. These results suggest that high stocking density may impede muscle development by inhibiting the expression of myogenic genes. NAM and BA can accelerate muscle development by elevating the expression of myogenic genes and inhibiting expression of MSTN. VEGF expression was higher in the HD group than in the LD group, as well as with the treatments HN, LB, and HB. Deep chest muscle disease is typically associated with increased VEGFA expression (Yalcin et al., 2018). Thus, HD rearing may increase the risk of muscle disorders; however, dietary supplementation with NAM and BA may help prevent the occurrence of muscle disorder by inhibiting VEGFA expression.
In summary, high stocking density rearing caused abnormal color and reduced water-holding capacity in broiler breast muscle. NAM and BA supplementation may improve meat quality by increasing antioxidants, upregulating the expression of myogenic genes, and inhibiting protein ubiquitination.
Acknowledgements
The authors acknowledge the National Key Research and Development Program of China (2016YFD0500509-06) and the System for Poultry Production Technology, Beijing Agriculture Innovation Consortium (project number: BAIC04-2019) for supporting this research.
Footnotes
Conflict of interest: All authors declare no conflicts of interest.
References
- Abrigo J., Elorza A.A., Riedel C.A., Vilos C., Simon F., Cabrera D., Estrada L., Cabello-Verrugio C. Role of oxidative stress as key regulator of muscle wasting during Cachexia. Oxid Med. Cell Longev. 2018;2018:2063179. doi: 10.1155/2018/2063179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averos X., Estevez I. Meta-analysis of the effects of intensive rearing environments on the performance and welfare of broiler chickens. Poult. Sci. 2018;97:3767–3785. doi: 10.3382/ps/pey243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldi G., Soglia F., Mazzoni M., Sirri F., Canonico L., Babini E., Laghi L., Cavani C., Petracci M. Implications of white striping and spaghetti meat abnormalities on meat quality and histological features in broilers. Animal. 2018;12:164–173. doi: 10.1017/S1751731117001069. [DOI] [PubMed] [Google Scholar]
- Cai K., Shao W., Chen X., Campbell Y.L., Nair M.N., Suman S.P., Beach C.M., Guyton M.C., Schilling M.W. Meat quality traits and proteome profile of woody broiler breast (pectoralis major) meat. Poult. Sci. 2018;97:337–346. doi: 10.3382/ps/pex284. [DOI] [PubMed] [Google Scholar]
- Chen T., Zhou G.H., Xu X.L., Zhao G.M., Li C.B. Phospholipase A2 and antioxidant enzyme activities in normal and PSE pork. Meat Sci. 2010;84:143–146. doi: 10.1016/j.meatsci.2009.08.039. [DOI] [PubMed] [Google Scholar]
- Cohen S., Nathan J.A., Goldberg A.L. Muscle wasting in disease: molecular mechanisms and promising therapies. Nat. Rev. Drug Discov. 2015;14:58–74. doi: 10.1038/nrd4467. [DOI] [PubMed] [Google Scholar]
- Fernandez L., Heredia N., Grande L., Gomez G., Rimola A., Marco A., Gelpi E., Rosello-Catafau J., Peralta C. Preconditioning protects liver and lung damage in rat liver transplantation: role of xanthine/xanthine oxidase. Hepatology. 2002;36:562–572. doi: 10.1053/jhep.2002.34616. [DOI] [PubMed] [Google Scholar]
- Ganassi M., Badodi S., Ortuste Q.H., Zammit P.S., Hinits Y., Hughes S.M. Myogenin promotes myocyte fusion to balance fibre number and size. Nat. Commun. 2018;9:4232. doi: 10.1038/s41467-018-06583-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry J.G., McGlone J.J., Miller M.F., Blanton J.J. Environmental effects on pig performance, meat quality, and muscle characteristics. J. Anim. Sci. 2004;82:209–217. doi: 10.2527/2004.821209x. [DOI] [PubMed] [Google Scholar]
- Goo D., Kim J.H., Choi H.S., Park G.H., Han G.P., Kil D.Y. Effect of stocking density and sex on growth performance, meat quality, and intestinal barrier function in broiler chickens. Poult. Sci. 2019;98:1153–1160. doi: 10.3382/ps/pey491. [DOI] [PubMed] [Google Scholar]
- Henagan T.M., Stefanska B., Fang Z., Navard A.M., Ye J., Lenard N.R., Devarshi P.P. Sodium butyrate epigenetically modulates high-fat diet-induced skeletal muscle mitochondrial adaptation, obesity and insulin resistance through nucleosome positioning. Br. J. Pharmacol. 2015;172:2782–2798. doi: 10.1111/bph.13058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh T.P., Murani E., Maak S., Ponsuksili S., Wimmers K. UBE3B and ZRANB1 polymorphisms and transcript abundance are associated with water holding capacity of porcine M. longissimus dorsi. Meat Sci. 2013;95:166–172. doi: 10.1016/j.meatsci.2013.04.033. [DOI] [PubMed] [Google Scholar]
- Jiang R.R., Zhao G.P., Chen J.L., Zheng M.Q., Zhao J.P., Li P., Hu J., Wen J. Effect of dietary supplemental nicotinic acid on growth performance, carcass characteristics and meat quality in three genotypes of chicken. J. Anim. Physiol. N. 2011;95:137–145. doi: 10.1111/j.1439-0396.2010.01031.x. [DOI] [PubMed] [Google Scholar]
- John C.M., Ramasamy R., Al N.G., Al-Nuaimi A.H., Adam A. Nicotinamide supplementation protects gestational diabetic rats by reducing oxidative stress and enhancing immune responses. Curr. Med. Chem. 2012;19:5181–5186. doi: 10.2174/092986712803530449. [DOI] [PubMed] [Google Scholar]
- Khan M., Couturier A., Kubens J.F., Most E., Mooren F.C., Kruger K., Ringseis R., Eder K. Niacin supplementation induces type II to type I muscle fiber transition in skeletal muscle of sheep. Acta Vet. Scand. 2013;55:85. doi: 10.1186/1751-0147-55-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M., Ringseis R., Mooren F.C., Kruger K., Most E., Eder K. Niacin supplementation increases the number of oxidative type I fibers in skeletal muscle of growing pigs. BMC Vet. Res. 2013;9:177. doi: 10.1186/1746-6148-9-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiani A., von Borstel U.K. Impact of different group sizes on plumage cleanliness and leg disorders in broilers. Livest Sci. 2019;221:52–56. [Google Scholar]
- Kim G., Jeong J., Hur S., Yang H., Jeon J., Joo S. The relationship between meat color (CIE L* and a*), myoglobin content, and their influence on muscle fiber characteristics and pork quality. Korean J. Food Sci. 2010;30:626–633. [Google Scholar]
- Lu Z., He X., Ma B., Zhang L., Li J., Jiang Y., Zhou G., Gao F. Chronic heat stress impairs the quality of breast-muscle meat in broilers by affecting redox status and Energy-Substance metabolism. J. Agr Food Chem. 2017;65:11251–11258. doi: 10.1021/acs.jafc.7b04428. [DOI] [PubMed] [Google Scholar]
- Mancini R.A., Hunt M.C. Current research in meat color. Meat Sci. 2005;71:100–121. doi: 10.1016/j.meatsci.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Milan G., Romanello V., Pescatore F., Armani A., Paik J.H., Frasson L., Seydel A., Zhao J., Abraham R., Goldberg A.L., Blaauw B., DePinho R.A., Sandri M. Regulation of autophagy and the ubiquitin-proteasome system by the FoxO transcriptional network during muscle atrophy. Nat. Commun. 2015;6:6670. doi: 10.1038/ncomms7670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell S.J., Bernier M., Aon M.A., Cortassa S., Kim E.Y., Fang E.F., Palacios H.H., Ali A., Navas-Enamorado I., Di Francesco A., Kaiser T.A., Waltz T.B., Zhang N., Ellis J.L., Elliott P.J., Frederick D.W., Bohr V.A., Schmidt M.S., Brenner C., Sinclair D.A., Sauve A.A., Baur J.A., de Cabo R. Nicotinamide improves Aspects of Healthspan, but not Lifespan, in mice. Cell Metab. 2018;27:667–676. doi: 10.1016/j.cmet.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najafi P., Zulkifli I., Jajuli N.A., Farjam A.S., Ramiah S.K., Amir A.A., O'Reily E., Eckersall D. Environmental temperature and stocking density effects on acute phase proteins, heat shock protein 70, circulating corticosterone and performance in broiler chickens. Int. J. Biometeorol. 2015;59:1577–1583. doi: 10.1007/s00484-015-0964-3. [DOI] [PubMed] [Google Scholar]
- Nakashima K., Ishida A., Katsumata M. Comparison of proteolytic-related gene expression in the skeletal muscles of layer and broiler chickens. Biosci. Biotechnol. Biochem. 2009;73:1869–1871. doi: 10.1271/bbb.90134. [DOI] [PubMed] [Google Scholar]
- National Research Council . National Academy Press; Washington DC: 1994. Nutrient requirement of poultry, 9th ed. [Google Scholar]
- Patria C.A., Afnan R., Arief I.I. Physical and microbiological qualities of kampong-broiler crossbred chickens meat raised in different stocking densities. Media Peternakan. 2016;39:141–147. [Google Scholar]
- Petracci M., Betti M., Bianchi M., Cavani C. Color variation and characterization of broiler breast meat during processing in Italy. Poult. Sci. 2004;83:2086–2092. doi: 10.1093/ps/83.12.2086. [DOI] [PubMed] [Google Scholar]
- Piva G., Morlacchini M., Prandini A. Proceedings of the 10th National Congress. Scientific Association of Animal Production; Bologna, Italy: 1993. The effect of nicotinamide (NIC) supplementation on meat quality in broilers reared in overcrowded conditions; pp. 513–518. [Google Scholar]
- Ponsuksili S., Murani E., Schwerin M., Schellander K., Wimmers K. Identification of expression QTL (eQTL) of genes expressed in porcine M. longissimus dorsi and associated with meat quality traits. BMC Genomics. 2010;11:572. doi: 10.1186/1471-2164-11-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qaid M., Albatshan H., Shafey T., Hussein E., Abudabos A.M. Effect of stocking density on the performance and immunity of 1-to 14-d- old broiler Chicks. Braz. J. Poult. Sci. 2016;18:683–691. [Google Scholar]
- Shin S., Song Y., Ahn J., Kim E., Chen P., Yang S., Suh Y., Lee K. A novel mechanism of myostatin regulation by its alternative splicing variant during myogenesis in avian species. Am. J. Physiol. Cell Physiol. 2015;309:C650–C659. doi: 10.1152/ajpcell.00187.2015. [DOI] [PubMed] [Google Scholar]
- Tran M.T., Zsengeller Z.K., Berg A.H., Khankin E.V., Bhasin M.K., Kim W., Clish C.B., Stillman I.E., Karumanchi S.A., Rhee E.P., Parikh S.M. PGC1alpha drives NAD biosynthesis linking oxidative metabolism to renal protection. Nature. 2016;531:528–532. doi: 10.1038/nature17184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas-Galicia A.J., Sosa-Montes E., Rodriguez-Ortega L.T., Pro-Martinez A., Ruiz-Feria C.A., Gonzalez-Ceron F., Gallegos-Sanchez J., Arreola-Enriquez J., Bautista-Ortega J. Effect of litter material and stocking density on bone and tendon strength, and productive performance in broilers. Can J. Anim. Sci. 2017;97:673–682. [Google Scholar]
- Wang R.H., Liang R.R., Lin H., Zhu L.X., Zhang Y.M., Mao Y.W., Dong P.C., Niu L.B., Zhang M.H., Luo X. Effect of acute heat stress and slaughter processing on poultry meat quality and postmortem carbohydrate metabolism. Poult. Sci. 2017;96:738–746. doi: 10.3382/ps/pew329. [DOI] [PubMed] [Google Scholar]
- Wen C., Chen Y., Leng Z., Ding L., Wang T., Zhou Y. Dietary betaine improves meat quality and oxidative status of broilers under heat stress. J. Sci. Food Agr. 2019;99:620–623. doi: 10.1002/jsfa.9223. [DOI] [PubMed] [Google Scholar]
- Wu W., Xiao Z., An W., Dong Y., Zhang B. Dietary sodium butyrate improves intestinal development and function by modulating the microbial community in broilers. Plos One. 2018;13:e0197762. doi: 10.1371/journal.pone.0197762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Li J., Qin X., Sun S., Xiao Z., Dong X., Shahid M.S., Yin D., Yuan J. Proteome and microbiota analysis reveals alterations of liver-gut axis under different stocking density of Peking ducks. Plos One. 2018;13:e198985. doi: 10.1371/journal.pone.0198985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Wang Y., Yin D., Wu W., Sun X., Zhang Y., Guo X., Chen J., Yuan J. Effect of supplementation of nicotinamide and sodium butyrate on the growth performance, liver mitochondrial function and gut microbiota of broilers at high stocking density. Food & Funct. 2019;10:7081–7090. doi: 10.1039/c9fo00904c. [DOI] [PubMed] [Google Scholar]
- Xu L., Zhang H., Yue H., Wu S., Yang H., Wang Z., Qi G. Gas stunning with CO2 affected meat color, lipid peroxidation, oxidative stress, and gene expression of mitogen-activated protein kinases, glutathione S-transferases, and Cu/Zn-superoxide dismutase in the skeletal muscles of broilers. J. Anim. Sci. Biotechnol. 2018;9:37. doi: 10.1186/s40104-018-0252-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalcin S., Sahin K., Tuzcu M., Bilgen G., Ozkan S., Izzetoglu G.T., Isik R. Muscle structure and gene expression in pectoralis major muscle in response to deep pectoral myopathy induction in fast- and slow-growing commercial broilers. Br. Poult. Sci. 2018;19:1–7. doi: 10.1080/00071668.2018.1430351. [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Legendre N.P., Biswas A.A., Lawton A., Yamamoto S., Tajbakhsh S., Kardon G., Goldhamer D.J. Loss of MyoD and Myf5 in skeletal muscle Stem Cells results in Altered myogenic Programming and Failed regeneration. Stem Cell Rep. 2018;10:956–969. doi: 10.1016/j.stemcr.2018.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida Y. F-box proteins that contain sugar-binding domains. Biosci. Biotechnol. Biochem. 2007;71:2623–2631. doi: 10.1271/bbb.70074. [DOI] [PubMed] [Google Scholar]
- Zhang W.H., Gao F., Zhu Q.F., Li C., Jiang Y., Dai S.F., Zhou G.H. Dietary sodium butyrate alleviates the oxidative stress induced by corticosterone exposure and improves meat quality in broiler chickens. Poult. Sci. 2011;90:2592–2599. doi: 10.3382/ps.2011-01446. [DOI] [PubMed] [Google Scholar]
- Zhang Y.R., Zhang L.S., Wang Z., Liu Y., Li F.H., Yuan J.M., Xia Z.F. Effects of stocking density on growth performance, meat quality and tibia development of Pekin ducks. Anim. Sci. J. 2018;89:925–930. doi: 10.1111/asj.12997. [DOI] [PubMed] [Google Scholar]
- Zou Y., Xiang Q., Wang J., Wei H., Peng J. Effects of oregano essential oil or quercetin supplementation on body weight loss, carcass characteristics, meat quality and antioxidant status in finishing pigs under transport stress. Livest Sci. 2016;192:33–38. [Google Scholar]