Abstract
There is no information regarding the influence of heat stress (HS) on host metabolic profile. In this study, we investigated the effects of different environmental temperatures on oxidative status, hormone levels, HS indicators, and plasma metabolites in broilers. A total of 1,680 yellow-feather broilers (28 D old) were randomly allotted to 4 groups with 6 replicates. The broilers (29–57 D old) were maintained in thermostatic rooms (20°C, 25°C, 28°C, and 30°C) for 28 consecutive days. The results showed that the plasma cortisol and adrenocorticotropic hormone levels and creatine kinase and lactate dehydrogenase activities gradually increased when the temperature increased from 20°C to 30°C. However, the insulin-like growth factor-І level decreased gradually. Furthermore, heat shock protein 70 expression significantly increased in the liver and breast muscle (P < 0.01). As the temperature increased, the total anti-oxidant capacity in the plasma and liver gradually decreased, whereas the malondialdehyde level increased. The activity of plasma glutathione peroxidase and total superoxide dismutase in the liver showed a similar increasing trend (P < 0.01). In addition, 15 metabolites were identified at higher (P < 0.05) levels, whereas 2 metabolites were identified at lower (P < 0.05) levels in the 30°C treatment group than those in the 25°C treatment group. Most of these potentially diagnostic biomarkers are involved in carbohydrate, amino acid, lipid, or gut microbiome-derived metabolism, indicating that HS affected the metabolic pathways in broilers. Six candidate metabolites (tartronic acid, l-bethreine, tartaric acid, allose, glutaric acid, and neohesperidin) were selected as biomarkers, as they showed high sensitivity, specificity, and accuracy in diagnosing broilers under HS (P < 0.01). In conclusion, in the final stage of growth, we identified 6 plasma differential metabolites as potential biomarkers of HS-induced metabolic disorders in yellow-feathered broilers. This work offers new insights into the metabolic alterations of broilers exposed to HS and provides a new perspective for further study.
Key words: animal comfort, heat stress, metabolomics, oxidative damage, yellow-feathered broiler
Introduction
Heat is one of the most important stressors challenging poultry production worldwide (Lara and Rostagno, 2013). The resultant heat stress (HS) is from the interactions of air temperature, humidity, radiant heat, and air speed, among which the air temperature plays a major role (Lin et al., 2006). The effects of high ambient temperature on survival, performance, and product quality are well described in poultry and continue to be economically detrimental to several farms (Sahin et al., 2017). In broiler chickens, body temperature above the thermoneutral zone disturbs physiological homeostasis (Pawar et al., 2016) and suppresses the functions of both immune and digestive systems (Quinteiro-Filho et al., 2017, Sugiharto et al., 2017), leading to several adverse effects such as lower production performance (Wan et al., 2017), oxidative stress (Liu et al., 2013), gut inflammation and dysfunction (Strong et al., 2015), mitochondrial damage (Gu et al., 2015), energy metabolic disturbance (He et al., 2019), health problem, and significant economic losses (Luo et al., 2017). Moreover, modern poultry genotypes have resulted in the production of more body heat due to high metabolic activity (Deeb and Cahaner, 2002) and the lack of sweat glands also enhances their susceptibility to HS (Narinç et al., 2016). Therefore, understanding and controlling environmental conditions is crucial to achieve successful poultry production and animal welfare.
Stress response, associated with the activation of the hypothalamic–pituitary–adrenal (HPA) axis and orthosympathetic nervous system, can aggravate the detrimental effect of high body temperature (Lin et al., 2006). Exposure of chickens to extreme HS alters the activity of the neuroendocrine system, resulting in the activation of the HPA axis, producing higher level of cortisol (Cort), which is an effective HS indicator in chickens (Quinteiro-Filho et al., 2017). The hypothalamic–pituitary–adrenal and sympathetic–adrenal medullar axes constitute the main pathways through which the immune response can be altered (Butts and Sternberg, 2008, Marketon and Glaser, 2008). Therefore, it is crucial to provide more insights on the interactions among the nervous, endocrine, and immune systems.
Previous research on the performance of broilers has focused on HS-induced changes in behaviors, meat quality, energy intake, growth, and inflammatory and oxidative responses (Altan et al., 2003, Sahin et al., 2017, Wang et al., 2018a, Wang et al., 2018b). To the authors' understanding, there is no previous report on metabolic changes of broilers in the final stage of growth exposed to HS, and the mechanisms underlying these changes remain unknown. Metabolomics is an emerging research area, which can quantitatively measure a series of small molecular metabolites in biological samples using high-throughput approaches (Wang et al., 2018a, Wang et al., 2018b). Metabolite identification and integrative analysis at the molecular and cellular levels under a given set of physiological conditions can enable a comprehensive characterization of metabolism (Wei et al., 2018). In broilers, blood can be collected very conveniently, as it can be collected from live animals at different growth phases. It also provides information on the changes in the metabolic and physiological responses of broilers. Therefore, blood is regarded as the ideal biological sample for monitoring physiological alterations. In a previous study, ultrafast liquid chromatography–tandem mass spectrometry was used to study the difference in plasma metabolic profiles between HS-free and HS lactating dairy cows and to verify the reliability of metabolites as a biomarker candidate (Tian et al., 2015). However, no metabolomic studies on the plasma have been conducted to identify biomarkers of HS in broilers. Therefore, metabolomic analysis can provide novel insights into the changes in the metabolic status in biological systems and help identify predictive biomarkers of HS using the plasma metabolome. More notably, HS also induces metabolomic modifications, which do not affect animals' health status (Quinteiro-Filho et al., 2012, Lara and Rostagno, 2013). However, no information is available regarding the influence of HS on host metabolic profile. Therefore, the purposes of this study were to investigate distinctive metabolic changes and physiological responses in the plasma samples of HS chickens and identify metabolic candidate biomarkers for reliable diagnosis of HS using a metabolomic platform.
Materials and methods
All procedures of this study were in accordance with the Chinese guidelines for animal welfare and were approved by the Zhejiang University Institutional Animal Care and Use Committee.
Chickens, Diet, and Management
One-day-old broiler chicks of Lingnan strain were reared in floor pens covered with fresh wood shavings. The chicks were raised to 28 D of age under a standard broiler management program. Feed and water were provided ad libitum. At 29 D of age, the chicks with extreme heavy or light weight were excluded, leaving 1,680 chicks. The chicks were wing banded and placed in floor pens (area of 2 m × 4 m). The 1,680 29-day-old broilers were randomly allotted to 4 environment-controlled rooms in the same horizontal position. Each room contained 6 pens with 70 chicks (35 males and 35 females) per pen, and each pen served as a replicate. The chicks were maintained in thermostatic rooms (20°C, 25°C, 28°C, and 30°C). The temperature in the rooms was controlled using an air conditioner of the same model and year of production. Each air conditioner was placed in the same location of each room and humidity was maintained within the optimal range. The chicks had free access to feed and water throughout the experimental period with light–dark cycles per day. Experimental diet was designed according to the NRC (1994) requirements. The composition and nutrient levels of the basal diets are shown in Table 1.
Table 1.
Composition and nutrient level of the basal diet used in different phases of trial (% air-dry basis).
| Items | Starter (1–21 D) | Grower (22–42 D) | Finisher (43–56 D) |
|---|---|---|---|
| Ingredients, % | |||
| Corn | 62.50 | 67.50 | 75.00 |
| Soybean meal | 31.00 | 23.50 | 14.50 |
| Corn gluten meal | 2.00 | 4.00 | 5.00 |
| Soy oil | 0.50 | 1.00 | 1.50 |
| Salt | 0.30 | 0.30 | 0.30 |
| Calcium hydrogen phosphate | 1.20 | 1.00 | 0.80 |
| Limestone | 1.50 | 1.30 | 1.20 |
| Zeolite | - | 0.40 | 0.70 |
| Premix1,2 | 1.00 | 1.00 | 1.00 |
| Total | 100.00 | 100.00 | 100.00 |
| Nutrient levels3 (%) | |||
| ME (MJ/kg) | 12.22 | 12.59 | 12.97 |
| CP | 21.09 | 19.16 | 16.07 |
| Lys | 1.09 | 0.99 | 0.87 |
| Met | 0.49 | 0.38 | 0.35 |
| Met + Cys | 0.87 | 0.73 | 0.65 |
| Calcium | 0.9 | 0.85 | 0.69 |
| Total phosphorus | 0.58 | 0.52 | 0.45 |
Supplied per kilogram of diet: from 1 D to 21 D, Fe 80 mg, Cu 8 mg, Zn 60 mg, Mn 80 mg, I 0.35 mg, Se 0.15 mg, VA 6000 IU, VD3 1500 IU, VE 20 mg, Vk3 1 mg, VB1 2.2 mg, VB2 4.2 mg, VB6 4.2 mg, VB12 0.012 mg, nicotinamide 42 mg, D-calcium pantothenate 12 mg, folic acid 1.0 mg, D-biotin 0.18 mg, choline 800 mg.
From 22 D to 56 D, Fe 80 mg, Cu 8 mg, Zn 60 mg, Mn 80 mg, I 0.35 mg, Se 0.15 mg, VA 6000 IU, VD3 1500 IU, VE 20 mg, Vk3 1 mg, VB1 2.2 mg, VB2 4.2 mg, VB6 4.2 mg, VB12 0.012 mg, nicotinamide 42 mg, D-calcium pantothenate 12 mg, folic acid 1.0 mg, D-biotin 0.18 mg, choline 800 mg.
ME is calculated value; other nutrient levels are measured values.
Sample Collection
At 57 D of age, the chicks were deprived of feed overnight. Two chicks, excluding those with extreme body weight, were chosen randomly from each pen. They were weighed and blood samples were collected from the wing vein into heparinized tubes; the plasma was obtained after centrifugation at 3,000 g for 10 min. After blood collection, the chicks were electrically stunned, exsanguinated, and dissected by a trained team to collect tissue samples. The right pectoralis muscle and liver tissues were carefully collected, dabbed dry, and placed in liquid nitrogen. Frozen tissue and plasma samples were stored at −80°C until further analysis.
Biochemical and Enzyme Analysis
One gram of frozen liver and pectoralis muscle was homogenized separately on ice with 9 mL of 0.9% saline water (pH 7.0). The homogenate was centrifuged at 8,000 × g for 10 min at 4°C and the supernatant was stored at −80°C. Total anti-oxidative capacity (T-AOC); glutathione peroxidase (GSH-PX), total superoxide dismutase (T-SOD), and catalase (CAT) activities; and malondialdehyde (MDA) level were measured using the respective assay kits (Nanjing Jiancheng Institute of Bioengineering, Nanjing, China) and colorimetric methods with a spectrophotometer (Biomate 5, Thermo Electron Corporation, Rochester, NY, USA).
Enzyme-Linked Immunosorbent Assay
The level of secretory Cort, adrenocorticotropic hormone (ACTH), and insulin-like growth factor-І (IGF-І) in the plasma was measured using the enzyme-linked immunosorbent assay (ELISA). Moreover, the heat shock protein (HSP) level in the liver and breast muscle was determined colorimetrically using the ELISA kits (Nanjing Jiancheng Institute of Bioengineering, Nanjing, China) according to manufacturer's instructions. Briefly, 50 μL of samples, reagents, and standards was pipetted into wells of microplates coated with the capture antibody specific for a protein of interest, Bio Ab, and the plates were incubated at 37°C for 30 min. After washing 5 times with washing solution, HRP conjugate reagent was added, and the sample was incubated at 37°C for 30 min. After incubation and washing to remove the excess HRP Conjugate, 50 μL of chromogen solutions A and B was added, and the sample was incubated for another 30 min at 37°C. Thereafter, 50 μL of stop solution was added and converted to a detectable form using the enzyme to a detectable form (color signal), and then the OD was measured within 10 min.
Sample Preparation for the Gas Chromatography Time-of-Flight/Mass Spectrometry (GC-TOF/MS) Analysis
The samples were prepared for the metabolomic analysis as described previously (Dunn et al., 2011). Briefly, 100 μL of plasma was added to 350 μL of extraction liquid (methanol:acetonitrile:water = 2:2:1, volume ratio) and 20 μL of L-2-chlorophenylalanine. The sample mixture was maintained at 4°C for 30 min and subsequently centrifuged at 13,000 rpm for 15 min. The supernatant (400 μL) was collected into a 1.5-mL tube, and then transferred to a glass vial and dried under vacuum. Methoxyamine salt reagent (methoxyamine hydrochloride dissolved in pyridine 20 mg/mL) was added and the mixture was incubated in an oven at 80°C for 30 min, and then derivatized using N,O-Bis(trimethylsilyl)trifluoroacetamide and incubated at 70°C for 1 h. The final mixture was strongly vortexed for 1 min and subjected to further analyses (Li et al., 2018).
GC-TOF/MS Analysis
The derivatized samples were analyzed using the Agilent 7890 GC system equipped with Pegasus 4D TOFMS (LECO, St. Joseph, MI) with the DB-5MS capillary column (30 m × 250 μm inner diameter, 0.25 μm film thickness coated with 95% dimethylpolysiloxane cross-linked with 5% diphenyl). The chromatography conditions were as follows: initial temperature was maintained at 80°C for 12 s, increased to 180°C at a rate of 10°C/min, to 240°C at a rate of 5°C/min, and further to 290°C at a rate of 20°C/min, and maintained for 11 min. One microliter of sample solution was injected with helium as the carrier gas at a flow rate of 1 mL/min. The temperature of the transfer line and ion source was 245°C and 220°C, respectively. The MS data were acquired at a mass-to-charge ratio (m/z) range of 20 to 600 in a full-scan mode (Li et al., 2018).
GC-TOF/MS Data Acquisition and Processing
The raw peak extraction, data baseline filtering and calibration, peak alignment, deconvolution analysis, peak identification, and peak area integration were carried out using Chroma TOF4.3X software (LECO) and the LECO-Fiehn Rtx5 database. The peaks were identified using the retention time index method. The missing values in the original data were filled by half of the minimum value using the numerical simulation method. Noise removal was performed based on an interquartile range to filter data and then normalized using the area normalization method. SIMCA14.1 software package (Umetrics, Umea, Sweden) was used for the following multivariate variable pattern recognition analyses: principal component analysis (PCA), partial least squares discriminant analysis (PLS-DA), and orthogonal partial least-squares discriminant analysis (OPLS-DA). The PCA was used to show the internal structure of the data and display the similarities and differences. OPLS-DA was applied to obtain a higher level of group separation and better explain the variables. To evaluate the predictive ability and fitting level of the model, the parameters R2Y and Q2 were applied. The metabolites that differentiated the 2 groups were filtered using the following requirements: variable importance in the projection (VIP) > 1 and P values of 0.05 (threshold) with 95% Hotelling's T-squared ellipse.
Statistical Analysis
The data were examined using the one-way analysis of variance (ANOVA) with IBM SPSS 19.0 and expressed as mean with SEM derived from the ANOVA error mean square. Differences between the means were examined using Duncan's multiple range test with P < 0.05. Moreover, Student's t-test was used to profile metabolite differences between the 2 groups.
Results
Stress Indicators in the Tissues and Plasma
Effects of environmental temperature on stress indicators in the tissues and plasma are shown in Table 2. When the temperature was increased from 20°C to 30°C, the plasma Cort and ACTH levels and CK and LDH activities gradually increased. However, the IGF-І level gradually decreased as the temperature increased. Moreover, higher environmental temperatures increased HSP70 protein expression in both the liver and breast muscle. The plasma Cort (P < 0.01) and ACTH (P < 0.05) levels and CK (P < 0.01) and LDH (P < 0.01) activities at 30°C were significantly increased compared with those at 20°C. Furthermore, HSP70 protein expression in the liver and breast muscle was the highest at 30°C. Except for the Cort level, there was no significant difference in parameters mentioned above between the 25°C and 28°C treatment groups (P > 0.05).
Table 2.
Effects of environmental temperature on physiological and biochemical indexes of tissues and plasma in broilers (mean ± SEM, n = 12 per treatment).
| Items | Environmental temperature |
SEM | |||
|---|---|---|---|---|---|
| 20°C | 25°C | 28°C | 30°C | ||
| Plasma | |||||
| Cort (μg/L) | 101.00c | 107.00c | 131.00b | 154.00a | 3.994 |
| ACTH (ng/L) | 39.00b | 44.50a,b | 46.70a,b | 49.50a | 1.813 |
| CK (U/mL) | 1.57c | 1.62b,c | 1.80a,b | 1.85a | 0.030 |
| LDH (U/L) | 463.00b | 474.00a,b | 482.00a,b | 531.00a | 11.440 |
| IGF-І (μg/L) | 16.80a | 15.40a,b | 14.20b | 13.60b | 0.395 |
| Liver | |||||
| HSP70 (ng/gprot) | 248.00c | 267.00b,c | 304.00a,b | 334.00a | 8.482 |
| Breast muscle | |||||
| HSP70 (ng/gprot) | 211.00b | 226.00a,b | 244.00a,b | 266.00a | 6.478 |
a–cMean values within a row with no common superscript differ significantly (P < 0.05).
Abbreviations: ACTH, adrenocorticotropic hormone; CK, creatine jubase; Cort, Cortisol; HSP, heat shock protein; IGF-І, insulin-like growth factor-І; LDH, lactate dehydrogenase; SEM, standard error of mean.
Antioxidant-Related Parameters in the Plasma and Liver
Antioxidant parameters in the plasma and liver are shown in Table 3. At 30°C, the T-AOC activity in both the plasma and liver decreased compared with that at 20°C and 25°C (P < 0.05), whereas the level of MDA increased (P < 0.05). Moreover, at 20°C and 25°C, the GSH-Px activity increased by 37.32 and 36.05% and the T-SOD activity increased by 30.11 and 29.03%, respectively, compared with those at 30°C. As except for plasma MDA level, no significant difference was observed for parameters between the 28°C and 30°C treatment groups (P > 0.05).
Table 3.
Effects of environmental temperature on anti-oxidation function in broilers (mean ± SEM, n = 12 per treatment).
| Items | Environmental temperature |
SEM | |||
|---|---|---|---|---|---|
| 20°C | 25°C | 28°C | 30°C | ||
| Plasma | |||||
| T-AOC (U/mL) | 10.51a | 10.33a | 9.92a,b | 8.41b | 0.317 |
| GSH-Px (U/mL) | 1,078.00a | 1,068.00a | 802.00b | 785.00b | 3.817 |
| T-SOD (U/mL) | 142.00 | 144.00 | 142.00 | 140.00 | 1.509 |
| CAT (U/mL) | 2.36 | 2.43 | 2.21 | 2.17 | 0.047 |
| MDA (nmol/mL) | 3.22b | 3.29b | 3.33b | 4.62a | 0.128 |
| Liver | |||||
| T-AOC (U/mgprot) | 1.88a | 1.85a | 1.49b | 1.43b | 0.033 |
| GSH-Px (U/mgprot) | 57.60 | 57.30 | 56.00 | 57.00 | 0.794 |
| T-SOD (U/mgprot) | 121.00a | 120.00a | 107.00a,b | 93.00b | 0.950 |
| CAT (U/mgprot) | 3.19 | 3.21 | 3.14 | 2.90 | 0.074 |
| MDA (nmol/mgprot) | 0.43b | 0.44b | 0.53a | 0.57a | 0.018 |
a–cMean values within a row with no common superscript differ significantly (P < 0.05).
Abbreviations: CAT, catalase; GSH-Px, glutathione peroxidase; MDA, malondialdehyde; SEM, standard error of mean; SOD, superoxide dismutase; T-AOC, total anti-oxidant capability.
Metabolic Differences in the Plasma
The total GC–TOF–MS ion chromatogram of the thermoneutral (25°C) and HS groups (30°C) acquired using the UPLC–MS platform is presented in Figure 1. After pretreatment and standardization using MZ mine 2.0 software, 402 integral peaks following extraction ion chromatography were detected in the quality control samples and 459 peaks in the test samples.
Figure 1.
Total GC-TOF-MS ion flow about metabolites in the broilers' plasma (n = 10 per treatment).
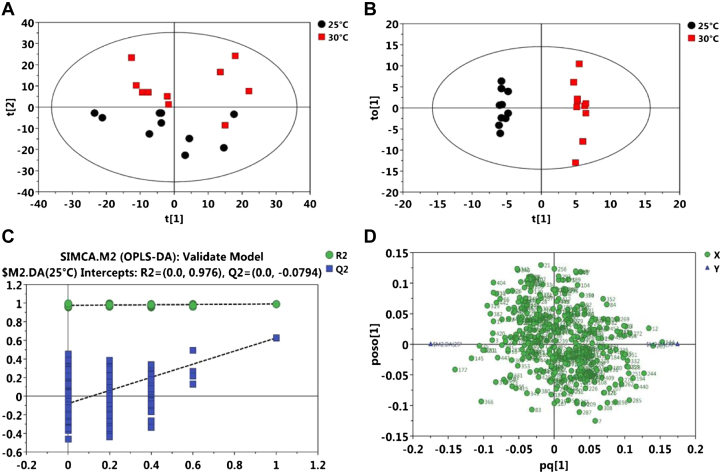
To explore the functional changes in the gut metabolome, the plasma of the 25°C and 30°C treatment groups was analyzed using multiple MS platforms. The PCA was first performed to show a trend of intergroup separation in the scores plot (Figure 2A), in which the HS-treated broilers were clearly separated from the thermoneutral controls. This method also enabled detection and exclusion of any outliers, defined as observations located outside the 95% confidence region of the model. The OPLS-DA was performed to better understand the different metabolic patterns. Figure 2B shows significantly separated clusters between the 25°C (black circle) and 30°C (red square) treatment groups. The values of R2X and Q2 and the results of permutation tests indicated that the samples were of reasonable quality (Figure. 2C). Furthermore, 17 biomarker metabolites were filtered. These metabolites in the plasma, including lipids, amino acids, carbohydrates, organic acids, and amines, are involved in multiple biochemical processes (Table 4). In addition, 15 metabolites were identified at higher (P < 0.05) levels, whereas 2 metabolites were identified at lower (P < 0.05) levels in the HS treatment group than those in the thermoneutral group. Therein, 6 candidate metabolites (tartronic acid, l-bethreine, tartaric acid, allose, glutaric acid, and neohesperidin) were selected as biomarkers, as they showed high sensitivity, specificity, and accuracy in diagnosing broilers under HS (P < 0.01).
Figure 2.
(A) Principal component analysis (PCA) score plots, (B) orthogonal projections to latent structure-discriminant analysis (OPLS-DA) score plots (R2X = 0.0, R2Y = 0.976, Q2 = -0.0794), (C) permutation test of OPLS-DA, and (D) loading plots derived from the LC-TOF/MS metabolite profiles of plasma for broilers under 25°C and 30°C treatment groups. The squares closer to the left triangle indicate higher levels of metabolites in the left group in relation to the right group; correspondingly, those closer to the right triangle suggested higher levels of metabolites in the right group in relation to the left group. n = 10 per treatment. Black circle: 25°C; red square: 30°C; green circle: R2; blue square: Q2. The dashed line represents the regression line for R2 and Q2.
Table 4.
Plasma differential metabolites between 25°C and 30°C treatment groups (n = 10 per treatment).
| Items | 25°C |
30°C |
VIP1 | P-value |
|---|---|---|---|---|
| Mean | Mean | |||
| Tartronic acid | 0.0003 | 2.7900 × 10−5 | 2.8095 | 1.240 × 10−5 |
| l-bethreine | 0.0011 | 0.0002 | 2.3342 | 0.002 |
| Lactose | 2.0400 × 10−8 | 0.0012 | 2.3133 | 0.044 |
| Galactose | 0.0084 | 0.0419 | 2.0957 | 0.024 |
| D-talose | 2.0400 × 10−8 | 0.0017 | 2.0683 | 0.018 |
| Tartaric acid | 0.0001 | 0.0005 | 2.0634 | 0.005 |
| Allose | 0.0011 | 0.0036 | 1.8943 | 0.009 |
| Ribose | 0.0042 | 0.0104 | 1.8769 | 0.010 |
| Glutaric acid | 0.0005 | 0.0016 | 1.8717 | 0.006 |
| Xylose | 0.0007 | 0.0013 | 1.7776 | 0.027 |
| DL-3-aminoisobutyric acid | 0.0071 | 0.0082 | 1.7256 | 0.020 |
| Neohesperidin | 0.0005 | 0.0025 | 1.6003 | 0.004 |
| Galactinol | 0.0008 | 0.0035 | 1.4865 | 0.010 |
| Citrulline | 0.0116 | 0.0158 | 1.3011 | 0.041 |
| Maleamic acid | 0.0031 | 0.0057 | 1.2087 | 0.039 |
| Galactonic acid | 0.0005 | 0.0008 | 1.0695 | 0.046 |
| Propanedioic acid | 0.0079 | 0.0113 | 1.0032 | 0.040 |
VIP: variable importance in the projection.
Discussion
To the best of our knowledge, this study is the first to identify predictive plasma biomarkers of HS that could be associated with oxidative status, hormone levels, and HS indicators of the host through high-performance liquid chromatography with quadrupole time-of flight/mass spectrometry (HPLC-QTOF/MS). The results would help understand heat acclimatization mechanisms in poultry in regions where sudden thermal increases may be expected. The results of the present study demonstrated that exposure of broilers to high temperature caused a significant increase in the level of Cort and ACTH (Table 2). High environmental temperatures alter the activity of the neuroendocrine system in poultry, resulting in the activation of the HPA axis, and elevated plasma Cort level can affect cellular transportation, proliferation, cytokine secretion, antibody production, and cytolytic activity (Garriga et al., 2006, Butts and Sternberg, 2008, Marketon and Glaser, 2008, Star et al., 2008, Quinteiro-Filho et al., 2012). Similarly, previous studies found that HS tended to elevate plasma Cort and ACTH levels (Sahin et al.,2002). In addition, both plasma CK and LDH activities increased as the ambient temperature increased. This was probably because the birds spent more time drinking and panting, as well as more time with their wings elevated, less time moving or walking, and more time resting (Mack et al.,2013). In addition, the IGF-І level in the plasma decreased. IGF-І is synthesized in the liver under the regulation of growth hormones and secreted into the bloodstream (endocrine action), which leads to the promotion of growth and inhibition of apoptosis. IGFs also act in an autocrine/paracrine manner in the peripheral tissues (Blum et al., 2018). These results could explain poor poultry production due to HS.
Birds exposed to high ambient temperature presented a significant increase in HSP70 expression in the breast muscle and liver compared with that in the 20°C treatment group. In addition, HS is known to delay the synthesis of most proteins except for heat shock proteins (Wang et al., 2018a, Wang et al., 2018b). It has been reported that HS induced an increase in the levels of HSP70 protein and mRNA in the heart, liver, and kidney of broiler chickens (Guerreiro et al., 2004). The HSP70 level increased significantly in broilers under HS, with a relatively higher expression in the heart (Mahmoud et al., 2004), liver, brain (Gabriel et al., 1996, Guerreiro et al., 2004), and immune organs (the bursa of Fabricius, thymus, and spleen) (Liu et al., 2013). Koelkebeck et al. (1995) showed that the overexpression of heat shock protein might be the reason for the increase in ATP after HS. Therefore, the higher HSP70 expression suggests that the proteins are involved in stress caused by heat shock exposure in chickens.
Antioxidant defense mechanisms could minimize the oxidative damage, which can protect the cells against cellular oxidants and repair systems that prevent the accumulation of oxidation-damaged molecules (Altan et al., 2003). Studies in broiler chickens, laying hens, and Japanese quails reported that HS could disturb the balance between the production of reactive oxygen species and the antioxidant systems (Sahin et al., 2002, Lin et al., 2006). As we know, oxidative stress has been associated with not only the elevated production of free radicals but also changes in the scavenging capacity of the antioxidant systems (Azad et al., 2010). Antioxidant enzymes (T-AOC, GSH-Px, T-SOD, and CAT) play a vital role in protecting against cellular damage from harmful effects of reactive oxygen species. Therefore, T-AOC, GSH-Px, T-SOD, and CAT activities were used to estimate the response of enzymatic scavenging systems. Compared with those of birds in the 20°C treatment group, birds in the higher temperature treatment groups showed a decrease in the plasma T-AOC and GSH-Px activities. Meanwhile, the T-AOC and T-SOD activities in the liver showed a similar trend, and the minimum levels of those parameters were observed in the 30°C treatment group (Table 3). These results are consistent with those of Ezzat et al. (2017), who reported that HS caused a significant decrease in SOD and GPX activities compared with those in thermoneutral control broiler chicks (Ezzat et al., 2017). On the contrary, Azad et al. (2010) observed that the anti-oxidation ability was disturbed under chronic stress condition. However, it has been reported that exposing birds to HS resulted in a significant increase in the SOD, CAT, and GPx activities (Altan et al., 2003). The reason for the discrepancy between our results and theirs with respect to the response of anti-oxidative system to HS may be the differences in breed and heat protocol. Moreover, HS increased lipid peroxidation due to increased free radical generation, as indicated by MDA level. MDA is a major breakdown product of lipid peroxides and has been used to assess lipid peroxidation (Biswas et al., 2018). Changes of MDA level have been considered as a HS response of broilers, and the results of the present study demonstrated higher levels of MDA in both the plasma and liver of birds (from 29–57 D) exposed to HS (Table 3). These results are consistent with those of studies, which reported that in chickens, the MDA level increased in response to HS treatment (Altan et al., 2003, Azad et al., 2010).
To date, however, there have been no metabolomic studies of broilers affected by HS. A previous targeted quantitative metabolomic analysis reported 8 predictive biomarkers of disease status in transition dairy cows (Hailemariam et al., 2014). In this study, plasma samples from the 25°C (thermoneutral group) and 30°C treatment group birds (HS group) were analyzed by GC–MS-based metabolomic method to reveal significant differences in metabolites between the thermoneutral and HS groups and to evaluate the negative effect and relative mechanism of HS. In addition, plasma metabolomics is used to validate the metabolic changes associated with specific diseases. Some previous metabolomic studies have attempted to discover biomarkers that might aid in the early detection of colorectal cancer (Ritchie et al., 2010), breast cancer (Huang et al., 2015, Hadi et al., 2017), toxoplasmosis infection (Zhou et al., 2016), and Behcet's disease (Ahn et al., 2018). In the present study, the differentially expressed metabolites were primarily involved in the metabolic processes of amino acids, carbohydrates, and nucleotides. Of the identified metabolites, 15 metabolites were found at higher levels, whereas 2 metabolites were found at lower levels in the 30°C treatment group (Table 4). Moreover, Tian et al. (2015) demonstrated that LC–MS techniques can be used to identify potential biomarkers for HS-induced metabolic disorders in lactating dairy cows. In this study, the plasma metabolite profiles obtained by GC–MS analysis successfully distinguished the HS group from the thermoneutral group. Furthermore, the selected 6 candidate metabolic biomarkers according to high statistical significance between the 25°C and 30°C treatment groups, namely tartronic acid, l-bethreine, tartaric acid, allose, glutaric acid, and neohesperidin, showed high sensitivity, specificity, and accuracy in diagnosing broilers under HS (Table 4). Most of the potential biomarkers discovered in metabolic pathways indicated the metabolic pathway shifts induced by HS. Moreover, Wang et al. (2011) suggested that the active compounds such as tartaric acid and crataegolic acid in CHM could promote the secretion of gastric juice and digestive enzyme activities. A previous study found that HS birds had higher levels of citrulline and tartaric acid. As plasma citrulline and tartaric acid levels change in response to HS, they may be valuable indicators in birds resistant to HS. Cain et al. (1974) reported that when 2- and 3-hydroxypyridine were oxidized to 2,5-dihydroxypyridine, the production of maleamic acid occurred through ring cleavage. Subsequently, Hegarty et al. (1979) reported that 2,5-dihy-droxypyridine accumulated during the transformation of 3-hydroxypyridine by a gram-negative bacterium isolated from soil. In our study, higher levels of plasma maleamic acid were found in HS birds. Galactinol is a galactosyl donor for the synthesis of raffinose (raffinose family oligosaccharides-trisaccharide) and stachyose (raffinose family oligosaccharide-tetrasaccharide), and its synthesis by galactinol synthase (GolS) is the first committed step of the raffinose family oligosaccharides biosynthetic pathway (Sprenger and Keller, 2000). However, galactose is an energy-providing nutrient and a necessary basic substrate for the biosynthesis of several macromolecules in the body. Altered galactose metabolism can cause a variety of clinical manifestations in animals and humans, and the current therapy for these problems mainly consists of hormonal and antioxidant therapy (Liu et al., 2000). The conversion of galactose to glucose has been observed to be enhanced via an increase in the GALT activity in the liver and insulin action (Rogers and Segal, 1981). Therefore, understanding the effect of different environmental temperatures on oxidative status, hormone levels, HS indicators, and metabolomics differences in the plasma can help develop hormonal and antioxidant therapy for treating and alleviating HS in broilers. Moreover, to the best of our knowledge, this is the first report on the detection of plasma characteristic metabolic profile in broilers under HS using GC–MS.
In conclusion, anti-stress function and anti-oxidative capacity of broilers gradually decreased as the ambient temperature increased and were found to be negatively affected by HS. Six metabolites, including tartronic acid, l-bethreine, tartaric acid, allose, glutaric acid and neohesperidin, were identified as potential biomarkers of HS in broilers. The results presented here provide new insights into HS-induced shifts in metabolic changes and physiological responses of broilers. Further research is needed to evaluate these biomarkers in practical applications and to elucidate the physiological mechanisms of HS-induced metabolic disorders.
Acknowledgments
The financial support provided by National Key R&D Program of China (project: 2016YFD05005, Beijing, China), Zhejiang Province Key R&D Program of China (project: 2018C02035, Hangzhou, China), China Agriculture Research System (project: CARS-42-G19, Beijing, China) is gratefully acknowledged.
References
- Ahn J.K., Kim J., Hwang J., Song J., Kim K.H., Cha H.S. Potential metabolomic biomarkers for reliable diagnosis of Behcet's disease using gas chromatography/time-of-flight-mass spectrometry. Joint Bone Spine. 2018;85:337–343. doi: 10.1016/j.jbspin.2017.05.019. [DOI] [PubMed] [Google Scholar]
- Altan Ö, Pabuçcuoğlu A., Altan A., Konyalioğlu S., Bayraktar H. Effect of heat stress on oxidative stress, lipid peroxidation and some stress parameters in broilers. Br. Poult. Sci. 2003;44:545–550. doi: 10.1080/00071660310001618334. [DOI] [PubMed] [Google Scholar]
- Azad M.A.K., Kikusato M., Maekawa T., Shirakawa H., Toyomizu M. Metabolic characteristics and oxidative damage to skeletal muscle in broiler chickens exposed to chronic heat stress. Comp. Biochem. Physiol. A: Mol. Integr. Physiol. 2010;155:401–406. doi: 10.1016/j.cbpa.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Butts C.L., Sternberg E.M. Neuroendocrine factors alter host defense by modulating immune function. Cell Immunol. 2008;252:7–15. doi: 10.1016/j.cellimm.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum W.F., Alherbish A., Alsagheir A., Awwa E.A., Kaplan W., Koledova M., Savage M.O. The growth hormone–insulin-like growth factor-I axis in the diagnosis and treatment of growth disorders. Endocr. Connect. 2018;7:R212–R222. doi: 10.1530/EC-18-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas P., Bhattacharya P., De M. Lipid peroxidation: a biomarker of oxidative stress in type-2 diabetes melltus. Malaysian J. Med. Res. 2018;2:61–66. [Google Scholar]
- Cain R.B., Houghton C., Wright K.A. Microbial metabolism of the pyridine ring. Metabolism of 2-and 3-hydroxypyridines by the maleamate pathway in Achromobacter sp. Biochemical Journal. 1974;140:293–300. doi: 10.1042/bj1400293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeb N., Cahaner A. Genotype-by-environment interaction with broiler genotypes differing in growth rate. 3. Growth rate and water consumption of broiler progeny from weight-selected versus nonselected parents under normal and high ambient temperatures. Poult. Sci. 2002;81:293–301. doi: 10.1093/ps/81.3.293. [DOI] [PubMed] [Google Scholar]
- Dunn W.B., Broadhurst D., Begley P., Zelena E., Francis-McIntyre S., Anderson N., Brown M., Knowles J.D., Halsall A., Haselden J.N. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat. Protoc. 2011;6:1060. doi: 10.1038/nprot.2011.335. [DOI] [PubMed] [Google Scholar]
- Ezzat W., Abdallah E., Rizk A., Ouda M., Abd El-krim R.E. Impact of chromiun picolinate supplementation on productive performance, immune response and heat shock proteins of broiler chickens under heate-stress condition. Egyptian Poultry Science Journal. 2017;2:37. [Google Scholar]
- Gabriel J.E., Ferro J.A., Stefani R.M.P., Ferro M.I.T., Gomes S.L., Marcos M. Effect of acute heat stress on heat shock protein 70 messenger RNA and on heat shock protein expression in the liver of broilers. Br. Poul. Sci. 1996;37:443–449. doi: 10.1080/00071669608417875. [DOI] [PubMed] [Google Scholar]
- Garriga C., Hunter R.R., Amat C., Planas J.M., Mitchell M.A., Moretó M. Heat stress increases apical glucose transport in the chicken jejunum. Am. J. Physio.Regul. Integr. Comp. Physiol. 2006;290:195–201. doi: 10.1152/ajpregu.00393.2005. [DOI] [PubMed] [Google Scholar]
- Gu Z., Li L., Wu F., Zhao P., Yang H., Liu Y., Geng Y., Zhao M., Su L. Heat stress induced apoptosis is triggered by transcription-independent p53, Ca 2+ dyshomeostasis and the subsequent Bax mitochondrial translocation. Sci. Rep. 2015;5:11497. doi: 10.1038/srep11497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerreiro E.N., Giachetto P.F., Givisiez P.E.N., Ferro J.A., Ferro M.I.T., Jane E.G., Renato L.F., Marcos M. Brain and hepatic Hsp70 protein levels in heat-acclimated broiler chickens during heat stress. Braz. J. Poult. Sci. 2004;6:201–206. [Google Scholar]
- Hadi N.I., Jamal Q., Iqbal A., Shaikh F., Somroo S., Musharraf S.G. Serum metabolomic profiles for breast cancer diagnosis, grading and staging by gas chromatography-mass spectrometry. Sci. Rep. 2017;7:1715. doi: 10.1038/s41598-017-01924-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hailemariam D., Mandal R., Saleem F., Dunn S., Wishart D., Ametaj B. Identification of predictive biomarkers of disease state in transition dairy cows. J. Dairy Sci. 2014;97:2680–2693. doi: 10.3168/jds.2013-6803. [DOI] [PubMed] [Google Scholar]
- He S., Li S., Arowolo M.A., Yu Q., Chen F., Hu R., He J. Effect of resveratrol on growth performance, rectal temperature and serum parameters of yellow-feather broilers under heat stress. Anim. Sci. J. 2019;90:401–411. doi: 10.1111/asj.13161. [DOI] [PubMed] [Google Scholar]
- Hegarty M., Lee C.P., Christie G., Haydock K. The goitrogen 3-Hydroxy-4 (1H)-Pyridone, a RuminaI metabolite from Leucaena Leucocephala: effects in mice and rats. Australian Journal of Biological Sciences. 1979;32:27–40. [PubMed] [Google Scholar]
- Huang J.H., Fu L., Li B., Xie H.L., Zhang X., Chen Y., Qin Y., Wang Y., Zhang S., Huang H. Distinguishing the serum metabolite profiles differences in breast cancer by gas chromatography mass spectrometry and random forest method. RSC Adv. 2015;5:58952–58958. [Google Scholar]
- Koelkebeck K.W., Odom T.W. Laying hen responses to acute heat stress and carbon dioxide supplementation: II. Changes in plasma enzymes, metabolites and electrolytes. Comparative Biochemistry and Physiology Part A: Physiology. 1995;112:119–122. doi: 10.1016/0300-9629(95)00081-h. [DOI] [PubMed] [Google Scholar]
- Lara L., Rostagno M. Impact of heat stress on poultry production. Animals. 2013;3:356–369. doi: 10.3390/ani3020356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Yuan., Yong G., Zheng S.W., Jiang X.M., Ma X., Y Han X. Weaning stress Perturbs gut microbiome and its metabolic profile in Piglets. Sci. Rep. 2018;8:18068. doi: 10.1038/s41598-018-33649-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H., Jiao H., Buyse J., Decuypere E. Strategies for preventing heat stress in poultry. World's Poult. Sci. J. 2006;62:71–86. [Google Scholar]
- Liu G., Hale G.E., Hughes C.L. Galactose metabolism and ovarian toxicity. Reprod. Toxicol. 2000;14:377–384. doi: 10.1016/s0890-6238(00)00096-4. [DOI] [PubMed] [Google Scholar]
- Liu L., He J., Xie H., Yang Y., Li J., Zou Y. Resveratrol induces antioxidant and heat shock protein mRNA expression in response to heat stress in black-boned chickens. Poult. Sci. 2013;93:54–62. doi: 10.3382/ps.2013-03423. [DOI] [PubMed] [Google Scholar]
- Luo J., Song J., Liu L., Xue B., Tian G., Yang Y. Effect of epigallocatechin gallate on growth performance and serum biochemical metabolites in heat-stressed broilers. Poult. Sci. 2017;97:599–606. doi: 10.3382/ps/pex353. [DOI] [PubMed] [Google Scholar]
- Mack L.A., Felver-Gant J.N., Dennis R.L., Cheng H.W. Genetic variations alter production and behavioral responses following heat stress in 2 strains of laying hens. Poult. Sci. 2013;92:285–294. doi: 10.3382/ps.2012-02589. [DOI] [PubMed] [Google Scholar]
- Mahmoud K.Z., Edens F.W., Eisen E.J., Havenstein G.B. Ascorbic acid decreases heat shock protein 70 and plasma corticosterone response in broilers (Gallus gallus domesticus) subjected to cyclic heat stress. Comp. Biochem. Physiol. B: Biochem. Mol. Biol. 2004;137:35–42. doi: 10.1016/j.cbpc.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Marketon J.I.W., Glaser R. Stress hormones and immune function. Cell Immunol. 2008;252:16–26. doi: 10.1016/j.cellimm.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Narinç D., Erdoğan S., Tahtabiçen E., Aksoy T. Effects of thermal manipulations during embryogenesis of broiler chickens on developmental stability, hatchability and chick quality. Animal. 2016;10:1328–1335. doi: 10.1017/S1751731116000276. [DOI] [PubMed] [Google Scholar]
- NRC . Nutrient requirements of poultry. 9th rev. ed. National Academy Press; Washington, DC: 1994. [Google Scholar]
- Pawar S., Sajjanar B., Lonkar V., Kurade N., Kadam A., Nirmal A., Brahmane M., Bal S. Assessing and mitigating the impact of heat stress on poultry. Adv. Anim. Vet. Sci. 2016;4:332–341. [Google Scholar]
- Quinteiro-Filho W.M., Gomes A., Pinheiro M.L., Ribeiro A., Ferraz-de-Paula V., Astolfi-Ferreira C.S., Ferreira A.J.P., Palermo-Neto J. Heat stress impairs performance and induces intestinal inflammation in broiler chickens infected with Salmonella Enteritidis. Avian Pathol. 2012;41:421–427. doi: 10.1080/03079457.2012.709315. [DOI] [PubMed] [Google Scholar]
- Quinteiro-Filho W., Calefi A., Cruz D., Aloia T., Zager A., Astolfi-Ferreira C., Ferreira J.P., Sharif S., Palermo-Neto J. Heat stress decreases expression of the cytokines, avian β-defensins 4 and 6 and Toll-like receptor 2 in broiler chickens infected with Salmonella Enteritidis. Vet. Immunol. Immunol. 2017;186:19–28. doi: 10.1016/j.vetimm.2017.02.006. [DOI] [PubMed] [Google Scholar]
- Ritchie S.A., Ahiahonu P.W., Jayasinghe D., Heath D., Liu J., Lu Y., Jin W., Kavianpour A., Yamazaki Y., Khan A.M. Reduced levels of hydroxylated, polyunsaturated ultra long-chain fatty acids in the serum of colorectal cancer patients: implications for early screening and detection. BMC Med. 2010;8:13. doi: 10.1186/1741-7015-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers S., Segal S. Changing activities of galactose-metabolizing enzymes during perfusion of suckling-rat liver. Am. J. Physiol. 1981;240:333–339. doi: 10.1152/ajpendo.1981.240.3.E333. [DOI] [PubMed] [Google Scholar]
- Sahin K., Osman K., Nurhan S., Mustafa S. Effects of vitamin C and vitamin E on lipid peroxidation status, serum hormone, metabolite, and mineral concentrations of Japanese quails reared under heat stress (34º C) Int. J. Vitam. Nutr. Res. 2002;72:91–100. doi: 10.1024/0300-9831.72.2.91. [DOI] [PubMed] [Google Scholar]
- Sahin N., Hayirli A., Orhan C., Tuzcu M., Akdemir F., Komorowski J., Sahin K. Effects of the supplemental chromium form on performance and oxidative stress in broilers exposed to heat stress. Poult. Sci. 2017;96:4317–4324. doi: 10.3382/ps/pex249. [DOI] [PubMed] [Google Scholar]
- Sprenger N., Keller F. Allocation of raffinose family oligosaccharides to transport and storage pools in Ajuga reptans: the roles of two distinct galactinol synthases. Plant J. 2000;21:249–258. doi: 10.1046/j.1365-313x.2000.00671.x. [DOI] [PubMed] [Google Scholar]
- Star L., Decuypere E., Parmentier H.K., Kemp B. Effect of single or combined climatic and hygienic stress in four layer lines: 2. Endocrine and oxidative stress responses. Poult. Sci. 2008;87:1031–1038. doi: 10.3382/ps.2007-00143. [DOI] [PubMed] [Google Scholar]
- Strong R.A., Hester P.Y., Eicher S.D., Hu J., Cheng H.W. The effect of cooled perches on immunological parameters of caged white leghorn hens during the hot summer months. PloS One. 2015;10:e0141215. doi: 10.1371/journal.pone.0141215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiharto S., Yudiarti T., Isroli I., Widiastuti E., Kusumanti E. Dietary supplementation of probiotics in poultry exposed to heat stress–a review. Ann. Anim. Sci. 2017;17:591–604. [Google Scholar]
- Tian H., Wang W., Zheng N., Cheng J., Li S., Zhang Y., Wang J. Identification of diagnostic biomarkers and metabolic pathway shifts of heat-stressed lactating dairy cows. J. Proteomics. 2015;125:17–28. doi: 10.1016/j.jprot.2015.04.014. [DOI] [PubMed] [Google Scholar]
- Wang H.F., Yang W.R., Wang Y.X., Yang Z.B., Cui Y.H. The study on the effects of Chinese herbal mixtures on growth, activity of post-ruminal digestive enzymes and serum antioxidant status of beef cattle. Agricultural Sciences in China. 2011;10:448–455. [Google Scholar]
- Wan X., Jiang L., Zhong H., Lu Y., Zhang L., Wang T. Effects of enzymatically treated Artemisia annua L. on growth performance and some blood parameters of broilers exposed to heat stress. Anim. Sci. J. 2017;88:1239–1246. doi: 10.1111/asj.12766. [DOI] [PubMed] [Google Scholar]
- Wang W., Li Z., Gan L., Fan H., Guo Y. Dietary supplemental Kluyveromyces marxianus alters the serum metabolite profile in broiler chickens. Food Funct. 2018;9:3776–3787. doi: 10.1039/c8fo00268a. [DOI] [PubMed] [Google Scholar]
- Wang Y., Zhao H., Liu J., Shao Y., Li J., Luo L., Xing M. Copper and arsenic-induced oxidative stress and immune imbalance are associated with activation of heat shock proteins in chicken intestines. Int. Immunopharmacol. 2018;60:64–75. doi: 10.1016/j.intimp.2018.04.038. [DOI] [PubMed] [Google Scholar]
- Wei Z., Zhou H., Zhang Y., Zhang Q., Zhang W., Mai K. Integrative analysis of transcriptomics and metabolomics profiling on flesh quality of large yellow croaker Larimichthys crocea fed a diet with hydroxyproline supplementation. Br. J. Nutr. 2018;119:359–367. doi: 10.1017/S0007114517003968. [DOI] [PubMed] [Google Scholar]
- Zhou C.X., Zhou D.H., Elsheikha H.M., Zhao Y., Suo X., Zhu X.Q. Metabolomic profiling of mice serum during toxoplasmosis progression using liquid chromatography-mass spectrometry. Sci. Rep. 2016;6:19557. doi: 10.1038/srep19557. [DOI] [PMC free article] [PubMed] [Google Scholar]