Abstract
Zinc is an essential nutritional trace element for all forms of life as it plays an important role in numerous biological processes. In poultry, zinc is provided by in-feed supplementation, mainly as zinc oxide or zinc sulfate. Alternatively zinc can be supplemented as organic sources, which are characterized by using an organic ligand that may be an amino acid, peptide, or protein to bind zinc and have a higher bioavailability than inorganic zinc sources. There are limited number of studies directly comparing the effects of inorganic vs. organic zinc sources on performance and intestinal health in broilers. Therefore, a digestibility and a performance study were conducted to evaluate and compare the effect of an amino acid-complexed zinc source vs. an inorganic zinc source on intestinal health. The experiment consisted of 2 treatments: either a zinc amino acid complex or zinc sulfate was added to a wheat–rye based diet at 60 ppm Zn, with 10 replicates (34 broilers per pen) per treatment. Effects on performance, intestinal morphology, microbiota composition, and oxidative stress were measured. Supplementing zinc amino acid complexes improved the zinc digestibility coefficient as compared to supplementation with zinc sulfate. Broilers supplemented with zinc amino acid complexes had a significantly lower feed conversion ratio in the starter phase compared to birds supplemented with zinc sulfate. A significantly higher villus length was observed in broilers supplemented with zinc amino acid complexes at days 10 and 28. Supplementation with zinc amino acid complexes resulted in a decreased abundance of several genera belonging to the phylum of Proteobacteria. Plasma malondialdehyde levels and glutathione peroxidase activity showed an improved oxidative status in broilers supplemented with zinc amino acid complexes. In conclusion, zinc supplied in feed as amino acid complex is more readily absorbed, potentially conferring a protective effect on villus epithelial cells in the starter phase.
Key words: zinc amino acid complex, broiler, intestinal morphology, microbiota, oxidative stress
INTRODUCTION
Zinc is an essential nutritional trace element for all forms of life as it plays an important role in numerous biological processes (Faa et al., 2008; Ranaldi et al., 2013; Bonaventura et al., 2015). Zinc not only contributes to the synthesis, stability, and catalytic activity of many proteins (Stefanidou et al., 2006), but also influences nucleic acid metabolism and immunological responses. Moreover, it plays an important role in wound healing and in restoring the integrity of damaged tissues (Batal et al., 2001; Jahanian and Rasouli, 2015). Zinc also has antioxidant effects (Gammoh and Rink, 2017) as it is a cofactor of the Cu/Zn superoxide dismutase, which plays a crucial role in the protection of cells against oxygen radicals (Oteiza, 2012). Finally, zinc ensures normal growth, health and fertility, development of bones and feathers, and regulates appetite in broilers (Shao et al., 2014; Kwiecien et al., 2017).
Cellular zinc homeostasis is strictly regulated by uptake and elimination of zinc through specialized transporters and by sequestration of zinc by carrier proteins such as metallothioneins (Bonaventura et al., 2015). Even minor changes in zinc homeostasis can lead to clinical consequences which are most distinct in tissues with a high cell turnover, such as the skin, the gastro-intestinal mucosa, and the immune system (Bonaventura et al., 2015). Due to the absence of a specialized zinc storage system, a daily intake of zinc through the diet is necessary to ensure the homeostasis that allows zinc to maintain and support its numerous functions (Bonaventura et al., 2015). In plants, zinc is mostly bound to phytate, forming an insoluble complex that hampers absorption. This can be solved by adding phytases to poultry diets (Lönnerdal, 2000; Tamim and Angel, 2003). Nevertheless, additional supplementation is recommended to meet the dietary requirements as described by NRC (1994), Mohanna and Nys (1999) and Ma et al. (2011). A diet without supplemental zinc provides insufficient zinc; therefore, supplementing broiler diets with zinc is a common industry practice (Sunder et al., 2008).
The source of zinc in the feed impacts the absorption rate of zinc. Mainly inorganic zinc, such as ZnO and ZnSO4, is supplemented in poultry feed (Summers, 1997). In organic zinc sources, zinc is coupled to an organic ligand, typically an amino acid, peptide, or protein, and these have a higher bioavailability than inorganic zinc sources (Star et al., 2012; Swiatkiewicz et al., 2014). Research has already been conducted to evaluate the effects of different zinc sources on bioavailability in broilers, but there is little information available in the literature on effects on performance directly comparing supplementation with ZnSO4 (ZnS) vs. zinc amino acid complexes (ZnAA) included at the same level in the feed. Moreover, to the best of our knowledge, no information is available in the literature regarding the effects of zinc source in broilers on intestinal health and microbiota composition. Therefore, the aim of the present study was to compare ZnAA as opposed to inorganic ZnSO4 in the feed of broilers, focusing on performance and on intestinal and general health parameters.
MATERIALS AND METHODS
All experimental procedures in this study were in compliance with the European guidelines for the care and use of animals in research (Directive 2010: 63: EU) and were approved by the Ethical committee of the Research Institute for Agriculture, Fisheries and Food (ILVO), Merelbeke, Belgium, under authorization number 2016: 301.
Trial 1: Performance Study
Experimental Design and Dietary Treatments
A total of 680 one-day-old male Ross 308 broilers were randomly allocated to 20 floor pens (10 pens per treatment and 34 broilers per pen) in an alternating block design, 1 replicate per treatment in each block. Broilers were housed on a solid floor covered with wood shavings (2.38 kg/m²). Up to day 7, the broilers were subjected to a light schedule of 23 h light and 1 h dark. From day 7 onwards the animals were subjected to a light schedule of 18 h light and 6 h dark. Broilers were orally vaccinated with Paracox-5 (Intervet UK Ltd., Milton Keynes, UK) on day 3. Dietary treatments included a wheat–rye based diet (Table 1) supplemented with 60 ppm Zn either as ZnSO4 (ZnS, ZnSO4.7H2 O, Sigma-Aldrich, St. Louis, MO) or 60 ppm Zn as ZnAA (AvailaZn, Zinpro Corporation, Eden Prairie, MN) (Table 2). AvailaZn is a zinc chelate based on single amino acids from hydrolyzed soy protein and zinc bound in a one to one molar ratio. The wheat–rye based diet contained a high level of crude protein (20%) and non-starch polysaccharides (15%) (NSP) without the addition of NSP enzymes in order to create a nutritional challenge at the intestinal level. All feed contain zinc levels that comply with the dietary needs as described by NRC (1994). The starter diet was fed from day 0 up to day 10 and was provided in a crumbled form. The grower and finisher diets were fed as pellets from day 10 up to day 28 and from day 28 up to day 36, respectively. Feed and drinking water were provided ad libitum. The levels of zinc present in the total feed were measured by Inductive Coupled Plasma-Mass Spectrometry after microwave destruction in closed recipients (Agilent 7500ce ICP-MS, Agilent Technologies, Santa-Clara, CA), according to the method described by Ashoka, et al. (2009) (Table 2). The total amount of zinc is delivered by zinc naturally present in the feed ingredients and by supplemented ZnS or ZnAA.
Table 1.
Dietary composition of the diets.
| Starter diet | Grower diet | Finisher diet | |
|---|---|---|---|
| Ingredient (%) | |||
| Wheat | 49.29 | 55.62 | 59.00 |
| Rye | 5.00 | 5.00 | 5.00 |
| Soybean meal (48) | 29.37 | 23.16 | 20.11 |
| Soybeans | 7.50 | 7.50 | 7.50 |
| Rapeseed meal | 2.00 | 2.00 | 2.00 |
| Animal fat | 2.50 | 2.60 | 2.70 |
| Soy oil | 1.00 | 1.00 | 1.00 |
| Vitamin + trace (vitamix)1 | 1.000 | 1.000 | 1.000 |
| CaCO3 | 0.820 | 0.908 | 0.826 |
| Di-Ca-phosphate | 0.650 | 0.361 | 0.107 |
| NaCl | 0.264 | 0.226 | 0.268 |
| Na-bicarbonate | 0.104 | 0.157 | 0.101 |
| L-Lys-HCl | 0.160 | 0.175 | 0.154 |
| DL-Methonine | 0.256 | 0.208 | 0.167 |
| L-Threonine | 0.071 | 0.064 | 0.049 |
| Phytase | 0.020 | 0.020 | 0.020 |
| Calculated nutrient composition | |||
| Crude protein (%) | 23.00 | 21.00 | 20.00 |
| Crude fat (%) | 6.43 | 6.41 | 6.46 |
| Non-soluble polysaccharides (%) | 15.37 | 15.00 | 14.83 |
| Metabolizable energy (MCal/kg) | 2.63 | 2.70 | 2.75 |
| Dig. Lysine (%) | 1.12 | 1.03 | 0.95 |
| Dig. Methionine + Cysteine (%) | 0.86 | 0.77 | 0.71 |
| Dig. Threonine (%) | 0.75 | 0.67 | 0.62 |
| Dig. Valine (%) | 0.89 | 0.81 | 0.76 |
| Ca (%) | 0.85 | 0.80 | 0.70 |
| Available P (%) | 0.40 | 0.35 | 0.31 |
| NaCl + KCl (mEq/kg) | 254 | 226 | 213 |
| Linoleic acid (18:2) (%) | 2.10 | 2.07 | 2.06 |
Provided per kg of diet: vitamin A (retinylacetate 3a672a, 10,000 IU), vitamin D3 (E671, 3,000 IU), vitamin E (all-rac-α-tocopherol acetate, 50 IU), vitamin K (2.5 mg), vitamin B1 (thiamine mononitrate, 2 mg), riboflavin (5 mg), calcium D-pantothenate (15 mg), vitamin B6 (4 mg), vitamin B12 (0.025 mg), niacinamide (30 mg), folic acid (1 mg), biotin (0.2 mg), choline (choline chloride, 689.7 mg), Cu (CuSO4.5H2 O, 12 mg), Mn (MnSO4.H2 O, 95.9 mg), Fe (FeSO4.H2 O), 49.2 mg; I (KI, 1.2 mg), Se (Na2 SeO3, 0.4 mg), sepioliet (7.0 mg), propylgallate (2.0 mg), BHT (3.0 mg).
Table 2.
Analyzed zinc concentrations in the diets for broilers (mg/kg, as-fed basis).
| Zinc source |
Analyzed Zn (mg/kg) |
||
|---|---|---|---|
| Starter | Grower | Finisher | |
| ZnS | 115 ± 1.4 | 104 ± 5.9 | 97 ± 1.3 |
| ZnAA | 129 ± 4.2 | 112 ± 4.9 | 109 ± 12.0 |
ZnS: ZnSO4; ZnAA: zinc amino acid complexes.
Data represent mean ± standard deviation expressed in mg/kg.
Mortality was recorded daily, and this information was used to correct the performance parameters. At days 10, 28, and 36, all broilers and feed left overs were weighed per pen to determine body weight gain (BWG), feed intake (FI), and feed conversion ratio (FCR). At the same time point, 3 broilers per pen were euthanized by an intravenous overdose of sodium pentobarbital 20% (Kela NV, Hoogstraten, Belgium) and venous blood samples were collected in serum and heparin tubes (Vacutest Plast, Kima, Arzergrande, Italy). Tissue samples of the duodenal loop were collected for fixation in 4% of formaldehyde at days 10 and 28. Content of ileum and cecum was collected aseptically (day 10) and stored at −20°C. At the end of the trial, litter quality was scored (per pen, scores ranging from 0 to 3). Footpad and hock lesions were scored (of 8 birds per pen, scores ranging from 0 to 4) based on the Welfare quality assessment protocols (Welfare Quality Consortium, 2009) (Table 3).
Table 3.
Score system for litter quality and lesion scoring.
| Score system litter quality | ||||
|---|---|---|---|---|
| Score 0 | Score 1 | Score 2 | Score 3 | |
| Dry or nearly sticky litter | Litter starts to stick | Litter starts to clot | The litter is very greasy and forms a “cake” like structure | |
| Score footpad lesions and hock burns | ||||
| Score 0 | Score 1 | Score 2 | Score 3 | Score 4 |
| No damage or inflammation | Minor damaging | Intermediate damaging | Severe damage and clear inflammation | Severe damage and clear inflammation of larger areas |
Intestinal Morphology
Formalin-fixed intestinal segments of the duodenum were dehydrated in xylene, embedded in paraffin, and sectioned in 4 µm slides for hematoxylin-eosin staining and CD3 immunohistochemistry. The sections were automatically (Shandon Varistain-Gemini, Thermofisher Scientific, Cheshire, UK) deparaffinized in xylene and rehydrated in isopropylene, 95% ethanol, and 50% ethanol, and stained with hematoxylin and eosin. The sections were examined using a light microscope (Leica DM LB2 Digital, Leica Microsystems, Wetzlar, Germany). Villus length was measured from the tip of the villus to the crypt–villus junction. Crypt depth was measured from the crypt base up to the crypt–villus junction. Measurements were performed on 12 random selected duodenal villi and crypts per section (one section per animal) using Leica DM LB2 Digital and a computer-based image analysis program, Leica Application Suite V4.1. The average villus length and crypt depth were calculated per animal. The thickness of the tunica muscularis was determined at 20 locations per section (1 section per animal) of which the average was calculated per animal. The above-mentioned measurements were performed for 3 birds per pen (10 pens per treatment).
CD3 Immunohistochemistry
CD3 immunohistological staining of duodenal sections was performed as described by Aguirre et al. (2019). Slides were analyzed with Leica DM LB2 Digital and a computer-based image analysis program LAS V4.1 (Leica Application Suite V4, Germany). The CD3+ area percentage in the duodenal tissue was quantified using 3 representative fields of view per section (1 section per animal) in 3 birds per pen (10 pens per treatment).
Microbiota Composition
DNA was extracted from the cecal and ileal content of broilers aged 10 D using the CTAB method as previously described (De Maesschalck et al., 2015). Amplification and sequencing of the V3-V4 regions of the 16S rRNA gene was done by Macrogen (Seoul, South Korea), using the primers S-D-Bact-0341-b-S-17 and S-D-Bact-0785-a-A-21, as described by Klindworth et al. (2013), extended with Illumina-specific adaptors. Amplicons were sequenced in a single run using Illumina MiSeq v3 technology (2 × 300 bp, paired-end) and using 30% PhiX DNA as spike-in. Demultiplexing of the amplicon dataset and deletion of the barcodes were done by the sequencing provider (Macrogen). The sequences were processed using a pipeline combining PANDAseq (Masella et al., 2012) and QIIME (v1.9.1). The paired-end sequences were assembled using PANDAseq, with a quality threshold of 0.9 and length cut-off values for the merged sequences between 400 and 500 bp. Open-reference operational taxonomic unit (OTU) picking was performed at 97% sequence similarity using USEARCH (v6.1) and converted to an OTU table (Edgar, 2010). OTU taxonomy was assigned against the Silva database (v123, clustered at 97% identity) using the PyNast algorithm with QIIME default parameters (Caporaso et al., 2010; Quast et al., 2013). OTUs with a total abundance below 0.01% of the total sequences were discarded (Bokulich et al., 2013). Alpha rarefaction curves were generated using QIIME, and a subsampling depth of 10,000 reads was selected. No samples were eliminated following subsampling.
Bioinformatics and Statistical Analysis of 16S rRNA Gene Amplicon Data
Further analysis of alpha diversity (observed OTUs, Chao1 richness estimator, and Shannon diversity estimator) and beta diversity (Bray–Curtis dissimilarities) was performed using the phyloseq (McMurdie and Holmes, 2013) pipeline in R (v3.4.3). Normality of the alpha diversity data was tested using the Shapiro–Wilk test, and subsequently a t-test was used for normal distributed data. Differences in beta diversity were examined using the ANOSIM function from the Vegan package. Differences in relative abundance at the phylum level were assessed using the 2-sided Welch t-test from the mt wrapper in phyloseq. To detect differentially abundant taxa between the different diet groups, DESeq2 was applied on the non-rarified community composition data for either cecal or ileal communities (Love et al., 2014). Significant differences were calculated using a Wald test followed by a Benjamini–Hochberg multiple hypothesis correction. For all tests, a P-value < 0.05 was considered significant and considered as tendency at 0.05 < P < 0.1.
Metabolic Function Prediction of the Microbial Communities
To gain more insight into the possible functional pathways of the microbial communities in the ileum, the functional composition was predicted using PICRUSt (Phylogenetic Investigation of Communities by Reconstruction of Unobserved States; Langille et al., 2013). PICRUSt uses precomputed ancestral state reconstructions based on the Greengenes database. Therefore, OTU picking was reperformed as described above with following modifications: closed-reference OTU picking was used, and OTU taxonomy was assigned against the Greengenes database (v 13.5) (DeSantis et al., 2006) after which the OTU counts were normalized by their expected 16s copy number using QIIME (Kembel et al., 2012; Angly et al., 2014). Metagenome predictions were performed against the KEGG database (Kyoto Encyclopedia of Genes and Genomes; Kanehisa and Goto, 2000). The resulting function prediction (KEGG orthologs [KO]) was analyzed using the HUMAnN2 algorithm to get KEGG modules and pathways (http://huttenhower.sph.harvard.edu/humann2). Alpha diversity (observed OTUs, Chao1 richness estimator, and Shannon diversity estimator) was determined using the phyloseq package. Bray–Curtis dissimilarity was used to quantify the difference in predicted KO populations using the phyloseq package. Differences in Bray–Curtis dissimilarity were examined using the anosim function from the vegan package as described above. Differentially abundant KOs were identified using DESeq2 analysis and plotted in a heatmap using the pheatmap package in R. Differentially abundant modules are identified using the phyloseq mt wrapper (Wilcoxon test with multiple hypothesis correction and calculation of false discovery rate).
Biochemical Analyses
Serum zinc concentrations were determined as described by van Riet et al. (2015). Shortly, serum samples were mixed with an equal volume of trichloroacetic acid to deproteinate samples before centrifugation at 10,000 × g for 10 min. The remaining supernatant was used within 2 h to determine serum zinc concentrations. Therefore, the deproteinated serum was diluted 5 times with a color reagent (Randox kit ZN2341, Randox Laboratories Limited, Crumlin, UK), and incubated 5 min at 25°C. Absorbance was measured at 560 nm. Serum zinc concentration was calculated from a zinc standard calibration curve. Malondialdehyde (MDA) concentration in plasma was measured by the reaction of MDA with thiobarbituric acid as described by Vossen et al. (2011). The absorbance of the colored complex was measured spectrophotometrically at 532 nm. A standard curve with 1,1,3,3-tetramethoxypropane was used, and the thiobarbituric acid reactive substance (TBARS) concentration was expressed in nmol MDA per mL of plasma. Glutathione peroxidase (GPx) activity was determined in plasma samples by measuring the oxidation of nicotinamide adenine dinucleotide phosphate in the presence of reduced glutathione and hydrogen peroxide. A decrease in absorbance was kinetically monitored at 340 nm for 5 min as described by Vossen et al. (2011).
Trial 2: Digestibility Study
Birds used for the digestibility study were housed under the same conditions as the broilers of the performance study up to day 9. From day 9 on, the broilers were housed in digestibility cages. The digestibility study consisted of 6 replicates per treatment with 4 or 5 male broilers (Ross 308) per replicate. After a 5-D adaptation period, the balance period was executed during 5 consecutive days between 14 and 18 D of age according to the European reference method of Bourdillon et al. (1990). Total FI was monitored during this balance period, and excreta was collected daily and stored at −20°C. Excreta was pooled per cage and was mixed to prepare a representative sample and subsequently freeze-dried and grounded. Dry matter (EC 1971), crude protein (ISO 5983-2, 2009), and crude fat (ISO 6492, 1999) were determined from the pooled samples according to ISO standards to calculate digestibility coefficients. Zinc content in feed and excreta (2 samples per treatment were analyzed in duplicate) was determined with Inductively Coupled Plasma Mass Spectrometry after microwave destruction in closed recipients (Agilent 7500ce ICP-MS, Agilent Technologies), according to the method described by Ashoka et al. (2009).
Statistical Analysis
Statistical analysis was performed in R for Windows (version 3.5.1). The pen was considered as the experimental unit for all analyzed variables except for the nutrient digestibility, where the digestibility cage was considered as the experimental unit. All data were checked for outliers and normality of the residuals. Normality of the sample distribution was performed with the Kolmogorov–Smirnov test. Data were compared using an independent samples t-test. Mortality and lesion scores (footpad lesions and hock burn) and litter quality were analyzed using an ordered logistic regression model with treatment as a fixed factor and animal or pen as the experimental unit. Statistical analysis on the gut microbiota was performed using R, as described above. The differences were considered statistically significant at P ≤ 0.05 and considered as tendency at 0.05 < P < 0.1.
RESULTS
Digestibility
A higher zinc digestibility coefficient (P = 0.02) was observed for broilers fed a diet supplemented with ZnAA (N = 5) compared to broilers fed a diet supplemented with ZnS (N = 6) (36.4 vs. 32.5%) (Table 4). Zinc contents were determined in all experimental diets in order to exclude that there was a difference in zinc concentration in the diets of the different treatments (Table 2). There were no distinct differences in zinc concentration between treatments, so the difference in digestibility coefficient is not due to a difference in zinc intake. There were no differences found in FI or amount of excreta (P > 0.01) nor were there significant differences in proximate nutrient digestibility.
Table 4.
Effect of supplementation with ZnS or ZnAA on digestibility parameters and calculated digestibility coefficients in broilers.
| ZnS | ZnAA | P-value | |
|---|---|---|---|
| Parameters | |||
| Feed intake (FI) (g) | 1,256 ± 49 | 1,168 ± 90 | 0.059 |
| Wet excreta (WE) (g) | 1,797 ± 72 | 1,720 ± 146 | 0.271 |
| WE/FI* | 0.300 ± 0.02 | 0.304 ± 0.01 | 0.731 |
| Digestibility coefficients (%) | |||
| Gross energy | 72.0 ± 2.3 | 71.8 ± 1.7 | 0.878 |
| Crude protein | 58.2 ± 2.9 | 57.1 ± 2.2 | 0.512 |
| Crude fat | 78.8 ± 2.5 | 77.0 ± 4.3 | 0.423 |
| Zinc | 32.7 ± 1.3 | 36.4 ± 2.7 | 0.020 |
ZnS: ZnSO4; ZnAA: zinc amino acid complexes.
Data represent mean ± standard deviation (ZnS, N = 6; ZnAA, N = 5).
Performance Parameters
A decreased FCR was observed for the starter (day 0 to 10) period for broilers fed the diet supplemented with ZnAA (P = 0.03) (Table 5). Moreover, there was a trend for a higher body weight from day 0 to 10 and improved FCR from day 0 to 28 for broilers supplemented with ZnAA as compared to ZnS. There was no effect of the source of supplemented zinc on the performance parameters during the overall period. Dietary treatment did not affect mortality rates, litter quality, or lesion scores (data not shown).
Table 5.
Effect of supplementation with ZnSO4 or ZnAA on performance in broilers.
| Period | BW (g/animal) | BWG (g/animal/day) | FI (g/animal/day) | FCR | |
|---|---|---|---|---|---|
| 0–10 D | ZnS | 284.7 ± 5.6 | 24.4 ± 0.5 | 28.6 ± 0.9 | 1.172 ± 0.018 |
| ZnAA | 290.5 ± 5.8 | 25.0 ± 0.5 | 28.7 ± 0.9 | 1.149 ± 0.012 | |
| P-value | 0.063 | 0.061 | 0.860 | 0.029 | |
| 10–28 D | ZnS | 1,572 ± 38 | 71.5 ± 2.0 | 104.2 ± 3.2 | 1.456 ± 0.022 |
| ZnAA | 1,597 ± 40 | 72.6 ± 2.0 | 104.7 ± 2.8 | 1.441 ± 0.017 | |
| P-value | 0.207 | 0.283 | 0.720 | 0.167 | |
| 28–36 D | ZnS | 2,423 ± 43 | 106.4 ± 3.1 | 171.6 ± 3.9 | 1.613 ± 0.051 |
| ZnAA | 2,456 ± 46 | 107.4 ± 3.7 | 174.7 ± 3.9 | 1.627 ± 0.046 | |
| P-value | 0.188 | 0.487 | 0.170 | 0.405 | |
| 0–28 D | ZnS | 54.7 ± 1.4 | 77.2 ± 2.2 | 1.411 ± 0.019 | |
| ZnAA | 55.6 ± 1.4 | 77.5 ± 2.0 | 1.394 ± 0.014 | ||
| P-value | 0.207 | 0.733 | 0.073 | ||
| 0–36 D | ZnS | 67.3 ± 1.2 | 98.2 ± 2.4 | 1.459 ± 0.019 | |
| ZnAA | 68.2 ± 1.3 | 99.1 ± 2.1 | 1.453 ± 0.016 | ||
| P-value | 0.187 | 0.405 | 0.375 |
ZnS: ZnSO4; ZnAA: zinc amino acid complexes.
Body weight (BW) was determined at the last day of each period (day 10, 28, and 36). Body weight gain (BWG), feed intake (FI), and feed conversion ratio (FCR) were determined at the end of the 3 periods.
ZnSO4 (ZnS) and zinc amino acid complexes (ZnAA). Data represent mean ± standard deviation (N = 10).
Intestinal Morphology and T-cell Abundance
Duodenal villus length and crypt depth were measured and villus length/crypt depth ratio were calculated at the end of the starter (day 10) and grower periods (day 28). At the end of the starter period and the grower period, an increase in villus length (P < 0.05) and villus length to crypt depth ratio (P < 0.05) was observed in broilers fed a diet supplemented with ZnAA as compared to birds fed a diet supplemented with ZnS (Table 6). The supplementation of different zinc sources did not affect the thickness of the tunica muscularis. The amount of CD3+ T cells was determined in duodenal sections as a marker for intestinal inflammation. No changes were observed in the duodenal T-cell abundance at the end of the starter and grower periods.
Table 6.
Effect of supplementation of ZnS or ZnAA on intestinal morphology and T-cell abundance in broilers on days 10 and 28 in duodenal sections.
| ZnS | ZnAA | P-value | |
|---|---|---|---|
| Day 10 | |||
| Villus length (VL) | 1,206 ± 221 | 1308 ± 155 | 0.046 |
| Crypt depth (CD) | 314.5 ± 76.6 | 301.6 ± 62.1 | 0.494 |
| Ratio (VL:CD) | 3.99 ± 1.05 | 4.52 ± 1.04 | 0.049 |
| Thickness tunica muscularis | 94.8 ± 30.4 | 91.3 ± 21.8 | 0.626 |
| CD3+ | 7.68 ± 2.51 | 6.32 ± 1.61 | 0.167 |
| Day 28 | |||
| Villus length (VL) | 1,489 ± 230 | 1,667 ± 262 | 0.012 |
| Crypt depth (CD) | 313.6 ± 62.3 | 311.4 ± 63.2 | 0.896 |
| Ratio (VL:CD) | 5.06 ± 1.13 | 5.80 ± 1.47 | 0.046 |
| Thickness tunica muscularis | 132.8 ± 36.3 | 129.7 ± 35.1 | 0.758 |
| CD3+ | 12.29 ± 2.82 | 11.54 ± 1.70 | 0.480 |
ZnS: ZnSO4; ZnAA: zinc amino acid complexes.
Analysis based on 10 measurements per section per bird for villus length (μm) and crypt depth (μm) or 3 microscopic fields per section for CD3+ measurements (area %). Data represent mean ± standard deviation (N = 10).
Blood Parameters
Zinc levels were determined in serum samples collected from broilers included in the performance trial. No differences in serum zinc levels were observed between dietary treatments at different ages (Table 7).
Table 7.
Effect of supplementation with ZnS or ZnAA on serum zinc levels (ug/dL) in broilers measured on days 10, 28, and 36.
| ZnS | ZnAA | P-value | |
|---|---|---|---|
| Serum zinc level | |||
| Day 10 | 212.7 ± 7.4 | 216.2 ± 5.2 | 0.703 |
| Day 28 | 206.6 ± 5.7 | 195.6 ± 5.0 | 0.160 |
| Day 36 | 210.4 ± 8.2 | 210.2 ± 13.1 | 0.987 |
ZnS: ZnSO4; ZnAA: zinc amino acid complexes.
Data represent mean ± standard deviation (N = 10) and are expressed in μg/dL.
MDA concentration and GPx activity were measured in plasma to evaluate oxidative status. MDA is commonly known as a marker for oxidative stress and is an end product of lipid peroxidation. Glutathione peroxidase plays an important role in the cascade which protects cells from oxidative damage. A lower MDA level (P < 0.01) was observed at the end of the starter period level in broilers fed a diet supplemented with ZnAA (Table 8). There was no difference in plasma MDA level at slaughter age. However, a lower plasma GPx (P = 0.02) was observed at slaughter age in broilers supplemented with ZnAA as compared to broilers supplemented with ZnS (Table 8).
Table 8.
Effect of supplementation with ZnS or ZnAA supplementation on plasma malondialdehyde concentration (MDA, mmol/L) measured on days 10, 28, and 36 and effect on plasma glutathione peroxidase activity (GPx, μmol/min. mL) at day 36.
| ZnS | ZnAA | P-value | |
|---|---|---|---|
| MDA day 10 | 16.72 ± 0.90 | 15.08 ± 1.16 | 0.007 |
| MDA day 28 | 13.88 ± 2.72 | 14.32 ± 1.85 | 0.678 |
| MDA day 36 | 12.64 ± 0.60 | 12.00 ± 0.76 | 0.140 |
| GPx day 36 | 0.72 ± 0.04 | 0.60 ± 0.15 | 0.021 |
ZnS: ZnSO4; ZnAA: zinc amino acid complexes.
Data represent mean ± standard deviation (N = 10, expressed as mmol/L for MDA and expressed as μmol/min.mL plasma for GPx).
Microbiota Composition
Influence on the Cecal and Ileal Microbial Diversity
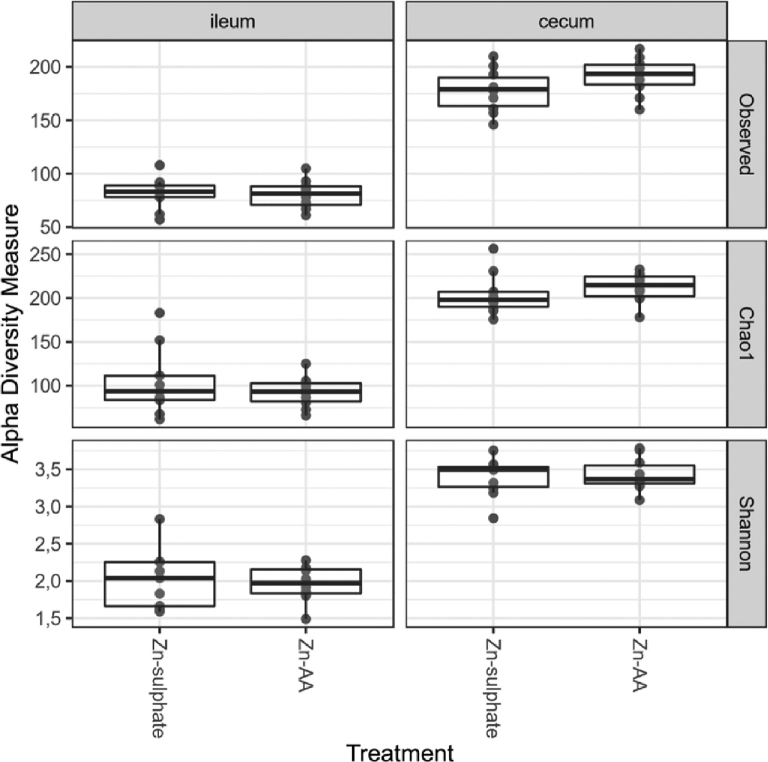
No differences were observed in either the cecal or ileal bacterial richness or diversity (Figure 1), which was determined by calculating the number of observed OTUs, the estimated OTU richness (Chao1), and the estimated community diversity (Shannon index).
Figure 1.
Alpha diversity metrics of the ileal and cecal microbial community from birds fed a diet supplemented with ZnS or ZnAA complexes. Observed: observed OTUs, Chao1: estimated OTU richness, and Shannon: estimated community diversity. ZnS: ZnSO4; ZnAA: zinc amino acid complexes.
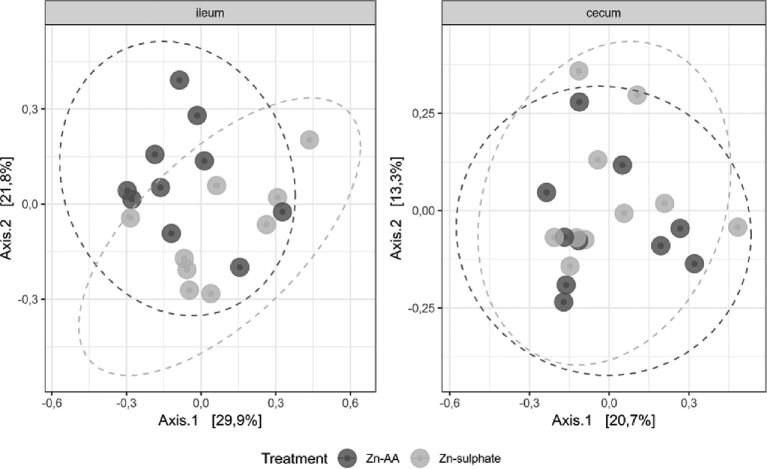
Bray–Curtis dissimilarity was used to investigate beta diversity between either the cecal or ileal microbiota in broilers fed a diet supplemented with ZnS or ZnAA complexes (Figure 2). A trend for a changing microbial composition was observed in the ileum (ANOSIM statistic R = 0.1177, P = 0.069), but no differences could be observed in the cecum (ANOSIM statistic R = 0.002333, P = 0.447).
Figure 2.
Principle coordinate analysis (PCoA) plot with of Bray–Curtis dissimilarities of the ileal or cecal microbiota of broilers fed either the ZnS (ZnSO4) or ZnAA (zinc amino acid complexes) supplemented diet. Each dot represents a single chicken.
Influence on the Taxonomic Composition of the Microbiota
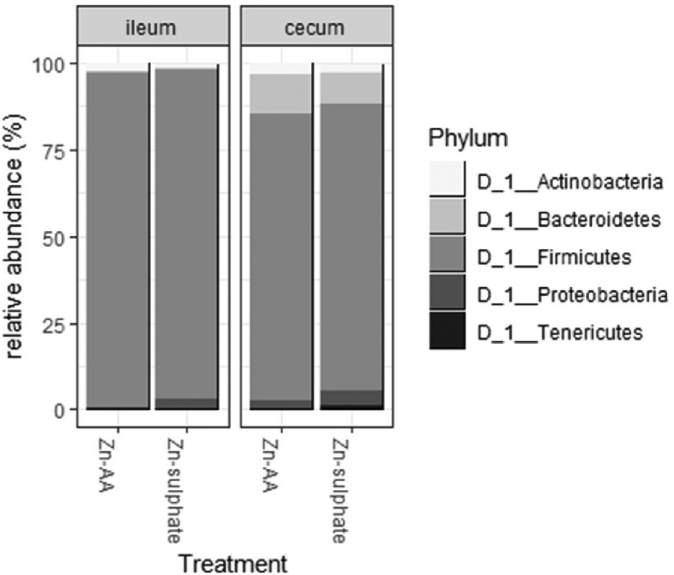
The ileal microbiota was characterized by a high abundance of Firmicutes (94.4% in the ZnS supplemented group, 96.6% in the ZnAA supplemented group), followed by Actinobacteria as the second most abundant phylum (1.0% and 2.6%, respectively). The phylum Proteobacteria accounted for 3.2% in the ileal content of the broilers supplemented with ZnS, whereas a relative abundance of 0.7% was observed in broilers supplemented with ZnAA (Figure 3). In the cecum, Firmicutes (82.6 or 82.8% for birds supplemented with ZnS or ZnAA, respectively) and Bacteroidetes (9.1 or 11.6% for birds supplemented with ZnS or ZnAA, respectively) were the most abundant phyla. The phylum Proteobacteria accounted for 4.3% of the total sequences found in the ceca of birds fed a diet supplemented with ZnS, whereas a relative abundance of 2.4% was observed in the ceca from birds receiving a diet supplemented with ZnAA. Dietary treatment did not significantly affect the above-mentioned differences in both ileum and cecum.
Figure 3.
Relative abundance (%) of the 4 most abundant phyla in the ileum or cecum from broilers fed a diet supplemented with a ZnS or ZnAA. ZnS: ZnSO4; ZnAA: zinc amino acid complexes.
Differentially abundant genera (Table 9) in the cecal and ileal microbial composition were identified using DESeq2. In the ileal content, the relative abundance of 13 genera was decreased in broilers fed a diet supplemented with ZnAA compared to broilers fed a diet compared to ZnS. The other genera belonged to the families Lachnospiraceae, Ruminococcaceae, and Streptococcaceae, which belong to the phylum Firmicutes and the families Helicobacteraceae, Sphinghomonadaceae, Comamonadaceae, Burkholderiaceae, and Pseudomonaceae which belong to the phylum Proteobacteria. In the cecal content of broilers fed a diet supplemented with ZnAA, the abundance of the genus Defluviitaleaceae UCG-011 was decreased compared to broilers fed a diet supplemented with ZnS.
Table 9.
Significant differences in genus level abundance in the ileal and cecal microbiota from broilers fed ZnS (ZnSO4) or ZnAA (zinc amino acid complexes) supplemented.
| Phylum |
Class |
Order |
Family |
Genus |
Mean relative abundance (%) |
Log2 fold change |
Adjusted P-value |
|
|---|---|---|---|---|---|---|---|---|
| ZnAA | ZnS | |||||||
| Ileum | ||||||||
| Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | uncultured | 0.039 | 0.499 | −3.35 | 0.00081 |
| Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | Blautia | 0.020 | 0.158 | −3.20 | 0.00119 |
| Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | Lachnospiraceae NK4A136 group | 0.018 | 0.176 | −5.20 | 0.00115 |
| Firmicutes | Clostridia | Clostridiales | Ruminococcaceae | Butyriciccocus | 0.007 | 0.086 | −3.70 | 3.54E-05 |
| Firmicutes | Clostridia | Clostridiales | Ruminococcaceae | Anaerotruncus | 0.001 | 0.025 | −4.96 | 0.00132 |
| Firmicutes | Clostridia | Clostridiales | Ruminococcaceae | uncultured | 0.004 | 0.026 | −3.49 | 0.00209 |
| Firmicutes | Clostridia | Clostridiales | Ruminococcaceae | Subdoligranulum | 0.047 | 0.560 | −2.97 | 0.02537 |
| Firmicutes | Bacilli | Lactobacillales | Streptococcaceae | Streptococcus | 0.021 | 0.872 | −3.932 | 0.00196 |
| Proteobacteria | Epsilonproteobacteria | Campylobacterales | Helicobacteraceae | Helicobacter | 0.019 | 0.510 | −4.78 | 4.63E-05 |
| Proteobacteria | Alphaproteobacteria | Sphingomonadales | Sphingomonadaceae | Sphingomonas | 0.013 | 0.313 | −4.87 | 4.63E-05 |
| Proteobacteria | Betaproteobacteria | Burkholderiales | Comamonadaceae | Delftia | 0.045 | 0.455 | −3.41 | 0.00030 |
| Proteobacteria | Betaproteobacteria | Burkholderiales | Burkholderiaceae | Ralstonia | 0.030 | 0.384 | −4.35 | 4.63E-05 |
| Proteobacteria | Gammaproteobacteria | Pseudomonadales | Pseudomonadaceae | Pseudomonas | 0.023 | 0.475 | −2.90 | 0.02537 |
| Cecum | ||||||||
| Firmicutes | Clostridia | Clostridiales | Defluviitaleaceae | Defluviitaleaceae UCG-011 | 0.003 | 0.002 | −5.93 | 0.00094 |
The taxonomic classification, the mean relative abundance and the log2 fold change (of the DESeq2 normalized abundance of each species are shown).
Influence on Metabolic Function Prediction of the Microbial Communities
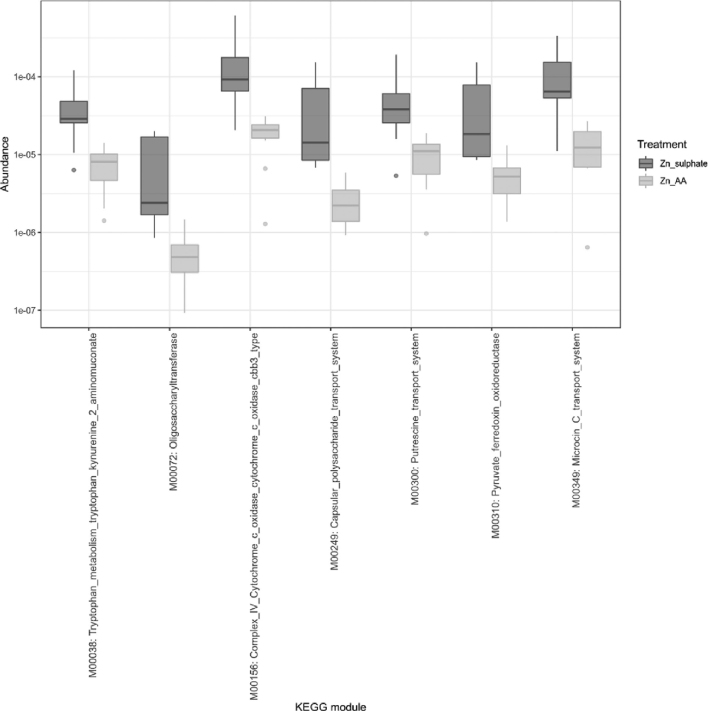
Alpha diversity did not show any significant differences (Additional file 1). Bray—Curtis dissimilarity (Additional file 2) was used to quantify the difference in predicted KO between treatments populations (ANOSIM statistic R = 0.138, P = 0.043). In total, 1,215 differentially abundant KO were identified using DESeq2 and plotted in a heatmap. Samples were clustered based on the similarity of these KOs and resulted in clustered samples according to the treatment (Additional file 3). In order to get more insight into the physiological processes these KOs contribute to, all predicted KOs are grouped into KEGG modules. Two modules were significantly affected (P < 0.05 and FDR < 0.05) and for another 5 modules there was a tendency (P < 0.01 and FDR < 0.05) (Table 10). Some systems which are characterized by oxidative processes like complex IV cytochrome c oxidase and pyruvate ferredoxin oxidoreductase are enriched in ZnS group (Furdui and Ragsdale, 2000). Putrescine transport system is also enriched in the ZnS group. Putrescine is a cellular polyamine produced by both eukaryotic and prokaryotic cells and which is produced by some bacteria (mainly Escherichia coli) in response to oxidative stress (Shah and Swlatlo, 2008). Capsular polysaccharide transport system and microcin C transport system are enriched in the ZnS group and are mainly produced by enterobacteria as part of their defense mechanism triggered in stress conditions (Severinov and Nair, 2012; He et al., 2016) (Figure 4).
Table 10.
Significantly enriched modules in broilers fed a diet supplemented with ZnS compared to broilers fed a diet supplemented with ZnAA.
| ZnS enriched | Functional description | Adjusted P-value | FDR |
|---|---|---|---|
| M00249 | Capsular_polysaccharide_transport_system | 0.016 | 0.03 |
| M00156 | Complex_IV_Cytochrome_c_oxidase_ | 0.042 | 0.03 |
| cytochrome_c_oxidase_cbb3_type | |||
| M00310 | Pyruvate_ferredoxin_oxidoreductase | 0.053 | 0.03 |
| M00038 | Tryptophan_metabolism_tryptophan_ | 0.063 | 0.03 |
| kynurenine_2_aminomuconate | |||
| M00349 | Microcin_C_transport_system | 0.072 | 0.03 |
| M00072 | Oligosaccharyltransferase | 0.072 | 0.03 |
| M00300 | Putrescine_transport_system | 0.089 | 0.04 |
ZnS: ZnSO4; ZnAA: zinc amino acid complexes; FDR: False Discovery Rate.
Figure 4.
Significantly enriched modules in broilers fed a diet supplemented with ZnS compared to broilers fed a diet supplemented with ZnAA. ZnS: Zn sulfate, ZnSO4; ZnAA: zinc amino acid complexes.
DISCUSSION
In the present study, we observed a higher digestibility coefficient for ZnAA complexes compared to ZnS, confirming the higher bioavailability reported by Star et al. (2012). Supplementation with ZnAA complexes did not influence the digestibility of other nutrients which is in accordance with studies where the supplementation of zinc-glycine chelates was compared to ZnS (Ma et al., 2011; Kwiecien et al., 2017). The bioavailability of zinc supplements is affected by competition with other minerals or inhibition by antagonists present in the diet (Lönnerdal, 2000; Sauer et al. 2017). Therefore, it is important to supply a zinc source which is taken up by a route which is not inhibited or saturated by Zn and other trace metals. It has been shown that ZnAA complexes are taken up by amino acid transporters as opposed to zinc salts which are taken up by zinc transporters (Gao et al., 2014; Sauer et al., 2017). The latter can be inhibited by zinc uptake antagonists (Lönnerdal, 2000). This alternative supply route of zinc might explain the higher bioavailability of ZnAA. Although ZnAA are characterized by an increased bioavailability, no differences in zinc serum levels were observed, which is in accordance with previous observations (Abd Mohanna and Nys, 1999; Zakaria et al., 2017; El-Hack et al., 2018).
A lower level of zinc was observed in excreta from broilers fed a diet supplemented with ZnAA as opposed to ZnS due to the improved bioavailability of ZnAA. To our knowledge, no literature is available about the effect of supplementation of different zinc sources on the microbiota composition in broilers. A study conducted by Ishaq et al. (2019) in yearling rams revealed that supplementation of ZnAA supplementation alters bacterial communities compared to the supplementation of ZnS. In the present study, the microbial composition in the ileum did not differ in bacterial diversity or richness but does show a trend towards a changing microbial composition. Single genera belonging to the phylum Firmicutes were reduced when supplementing ZnAA without affecting the overall relative abundance of the phylum. Several genera belonging to the phylum Proteobacteria were less abundant in the ileum content of the group supplemented with ZnAA compared to the ZnS group and overall relative abundance was also reduced. Expansion of Proteobacteria has been proposed as a microbial signature of gut dysbiosis and epithelial dysfunction (Litvak et al., 2017; Weiss and Hennet, 2017), and this may partly explain the lower villus length and villus length to crypth depth ratio observed in broilers supplemented with ZnS as compared to ZnAA.
This study showed that the use of ZnAA instead of ZnSO4 significantly reduced FCR. This decrease in FCR is not due to an increased FI, but seems to be an effect of increased weight gain when zinc is provided as ZnAA complexes as opposed to ZnS. A performance study conducted by Saenmahayak et al. (2010) showed that partially replacement of ZnS supplementation by ZnAA supplementation was able to improve body weight and FCR. Jahanian et al., (2008) showed that supplementation with Zn-methionine improved performance as compared to ZnS in broilers.
Supplementation with ZnAA increased villus length and villus length to crypt depth ratio up to day 28. The crypts constantly renew the epithelial cells lining the intestinal lumen by migration of new cells from the crypts to the villus tip. During this migration cells mature and become more efficient in nutrient absorption. The villous epithelial cells come directly in contact with the lumen content and are therefore prone to damage, which often results in an increased loss of villous epithelial cells in cases of intestinal health problems (Zhang et al., 2015). The improved villus length without accompanying increase in crypt depth may indicate that there is less villous epithelial cell loss at the villus tip compared to supplementation with ZnS. An increased villus length is associated with an increased digestion and absorption of nutrients, and an increase of brush border enzymes and nutrient transport systems (Awad et al., 2017). According to Collet (2012), the intestinal surface is directly proportional to digestive and absorptive efficiency and thus also to feed conversion efficiency. Taking this in consideration, the improved villus morphology may partly explain the lowered FCR during the starter and grower phases when supplementing with ZnAA complexes.
This study showed that supplementation with ZnAA complexes seems to alleviate oxidative stress by decreased MDA plasma levels and reduction in GPx activities. MDA has been determined as a marker for oxidative stress, as it is the most important end product in the chain reaction of lipid peroxidation caused by radicals (Del Rio et al., 2005). This study showed a decrease of plasma MDA levels in broilers fed a diet supplemented with ZnAA complexes at day 10 (end of starter phase). This might indicate a beneficial impact of ZnAA complexes on the oxidative status in broilers in the starter phase. At slaughter age (day 36), no differences in plasma MDA levels were found, but supplementation with ZnAA complexes showed a significantly lower activity for GPx activity in the plasma indicating a lower need for antioxidant activity for birds supplemented with ZnAA to maintain the same oxidative status. Analysis of the metabolic function prediction of the microbial communities shows enrichment of pathways involved in oxidative reactions in the group of ZnS supplemented broilers. A hypothesis could be that in the group supplemented with ZnS more pro-oxidative molecules were present, which led to a higher activity of the GPx in the plasma of broilers fed a diet supplemented with ZnS compared to broilers supplemented a diet with ZnAA. The observation of metabolic pathways responding to oxidative stress in the intestinal microbiota is in line with this hypothesis. A study conducted by Ma et al. (2011) showed that zinc supplementation can decrease MDA levels in liver extracts compared to a non-supplemented control group, which confirms the importance of zinc supplementation, although Ma et al. (2011) did not find differences between ZnS and ZnAA supplementation in liver extracts.
In conclusion, zinc supplied in feed as amino acid complex is more bioavailable and significantly enhances broiler performance during the early life. ZnAA complex supplementation results in an increased villus length and villus length to crypt depth ratio, indicating an improved intestinal morphology. Moreover, a decreased abundance of several genera belonging to the phylum Proteobacteria was observed when supplementing ZnAA complexes, indicating a positive impact on intestinal health. A reduction in plasma MDA levels and GPx activity indicates that supplementation with ZnAA complexes might have a positive impact on oxidative status compared to supplementation with ZnS. These results open opportunities for further studies, where the effect of supplementation of ZnAA complexes on intestinal health and performance under more challenging conditions should be investigated.
ACKNOWLEDGEMENTS
The authors acknowledge Jolien Vander Linden, the animal care takers at the ILVO and the colleagues of the department at Ghent University for their help with feeding, taking care of the birds and the offered help with taking samples. Special thanks to Evy Goossens for the help with processing microbiota data. We are grateful to Zinpro Corporation for financial support of this work.
Footnotes
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.3382/ps/pez525.
Supplementary Material
REFERENCES
- Abd El-Hack E.M., Alagawany M., Salah A.S., Abdel-Latif M.A., Farghly M.F.A. Effects of dietary supplementation of zinc oxide and zinc methionine on layer performance, egg quality, and blood serum indices. Biol. Trace Elem. Res. 2018;184:456–462. doi: 10.1007/s12011-017-1190-0. [DOI] [PubMed] [Google Scholar]
- Aguirre M., Vuorenmaa J., Valkonen E., Kettunen H., Callens C., Haesebrouck F., Ducatelle R., Van Immerseel F., Goossens E. In-feed resin acids reduce matrix metalloproteinase activity in the ileal mucosa of healthy broilers without inducing major effects on the gut microbiota. Vet. Res. 2019;50 doi: 10.1186/s13567-019-0633-3. doi ARTN 1510.1186/s13567-019-0633-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angly F.E., Dennis P.G., Skarshewski A., Vanwonterghem I., Hugenholtz P., Tyson G.W. CopyRighter: a rapid tool for improving the accuracy of microbial community profiles through lineage-specific gene copy number correction. Microbiome. 2014;2:11. doi: 10.1186/2049-2618-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashoka S., Peake B.M., Bremner G., Hageman K.J., Reid M.R. Comparison of digestion methods for ICP-MS determination of trace elements in fish tissues. Anal. Chim. Acta. 2009;653:191–199. doi: 10.1016/j.aca.2009.09.025. [DOI] [PubMed] [Google Scholar]
- Awad W.A., Hess C., Hess M. Enteric pathogens and their toxin-induced disruption of the intestinal barrier through alteration of tight junctions in chickens. Toxins. 2017;9 doi: 10.3390/toxins9020060. doi ARTN 6010.3390/toxins9020060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batal A.B., Parr T.M., Baker D.H. Zinc bioavailability in tetrabasic zinc chloride and the dietary zinc requirement of young chicks fed a soy concentrate diet. Poult. Sci. 2001;80:87–90. doi: 10.1093/ps/80.1.87. [DOI] [PubMed] [Google Scholar]
- Bokulich N.A., Subramanian S., Faith J.J., Gevers D., Gordon J.I., Knight R., Mills D.A., Caporaso J.G. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods. 2013;10:57–59. doi: 10.1038/nmeth.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaventura P., Benedetti G., Albarede F., Miossec P. Zinc and its role in immunity and inflammation. Autoimmun. Rev. 2015;14:277–285. doi: 10.1016/j.autrev.2014.11.008. [DOI] [PubMed] [Google Scholar]
- Bourdillon A., Carre B., Conan L., Francesch M., Fuentes M., Huyghebaert G., Janssen W.M.M.A., Leclercq B., Lessire M., Mcnab J., Rigoni M., Wiseman J. European reference method of invivo determination of metabolizable energy in poultry - reproducibility, effect of age, comparison with predicted values. Br. Poult. Sci. 1990;31:567–576. doi: 10.1080/00071669008417288. [DOI] [PubMed] [Google Scholar]
- Caporaso J.G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F.D., Costello E.K., Fierer N., Pena A.G., Goodrich J.K., Gordon J.I., Huttley G.A., Kelley S.T., Knights D., Koenig J.E., Ley R.E., Lozupone C.A., McDonald D., Muegge B.D., Pirrung M., Reeder J., Sevinsky J.R., Tumbaugh P.J., Walters W.A., Widmann J., Yatsunenko T., Zaneveld J., Knight R. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett S.R. Nutrition and wet litter problems in poultry. Anim. Feed. Sci. Tech. 2012;173:65–75. [Google Scholar]
- De Maesschalck C., Eeckhaut V., Maertens L., De Lange L., Marchal L., Nezer C., De Baere S., Croubels S., Daube G., Dewulf J., Haesebrouck F., Ducatelle R., Taminau B., Van Immerseel F. Effects of Xylo-oligosaccharides on broiler chicken performance and microbiota. Appl. Environ. Microb. 2015;81:5880–5888. doi: 10.1128/AEM.01616-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Rio D., Stewart A.J., Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2005;15:316–328. doi: 10.1016/j.numecd.2005.05.003. [DOI] [PubMed] [Google Scholar]
- DeSantis T.Z., Hugenholtz P., Larsen N., Rojas M., Brodie E.L., Keller K., Huber T., Dalevi D., Hu P., Andersen G.L. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microb. 2006;72:5069–5072. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EC Determination of moisture. Comission directive 71/393/EEC establishing Community methods of analysis for the official control of feedingstuffs. Official Journal of the European Communities L279, 20 December 1971. 1971:7. [Google Scholar]
- Edgar R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- Faa G., Nurchi V.M., Ravarino A., Fanni D., Nemolato S., Gerosa C., Eyken P.V., Geboes K. Zinc in gastrointestinal and liver disease. Coord. Chem. Rev. 2008;252:1257–1269. [Google Scholar]
- Furdui C., Ragsdale S.W. The role of pyruvate ferredoxin oxidoreductase in pyruvate synthesis during autotrophic growth by the Wood-Ljungdahl pathway. J. Biol. Chem. 2000;275:28494–28499. doi: 10.1074/jbc.M003291200. [DOI] [PubMed] [Google Scholar]
- Gammoh N.Z., Rink L. Zinc in infection and inflammation. Nutrients. 2017;9 doi: 10.3390/nu9060624. >doi ARTN 62410.3390/nu9060624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S., Yin T.J., Xu B.B., Ma Y., Hu M. Amino acid facilitates absorption of copper in the Caco-2 cell culture model. Life Sci. 2014;109:50–56. doi: 10.1016/j.lfs.2014.05.021. [DOI] [PubMed] [Google Scholar]
- He Q., Li X.P., Liu C., Su L.L., Xia Z.K., Li X., Li Y., Li L.L., Yan T., Feng Q., Xiao L. Dysbiosis of the fecal microbiota in the TNBS-induced Crohn's disease mouse model. Appl. Microbiol. Biot. 2016;100:4485–4494. doi: 10.1007/s00253-015-7205-x. [DOI] [PubMed] [Google Scholar]
- International Organization for Standardization (ISO). 1999. ISO 6492, Animal Feeding Stuffs - Determination of fat content (last reviewed in 2016).
- International Organization for Standardization (ISO). 2009. ISO 5983-2, Animal feeding stuffs - Determination of nitrogen content and calculation of crude protein content - Part 2: Block digestion and steam distillation method (last reviewed in 2014).
- Ishaq S.L., Page C.M., Yeoman C.J., Murphy T.W., Van Emon M.L., Stewart W.C. Zinc AA supplementation alters yearling ram rumen bacterial communities but zinc sulfate supplementation does not. J. Anim. Sci. 2019;97:687–697. doi: 10.1093/jas/sky456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahanian R., Moghaddam H.N., Rezaei A. Improved broiler chick performance by dietary supplementation of organic zinc sources. Asian Austral. J. Anim. 2008;21:1348–1354. [Google Scholar]
- Jahanian R., Rasouli E. Effects of dietary substitution of zinc-methionine for inorganic zinc sources on growth performance, tissue zinc accumulation and some blood parameters in broiler chicks. J. Anim. Physiol. Anim. Nutr. 2015;99:50–58. doi: 10.1111/jpn.12213. [DOI] [PubMed] [Google Scholar]
- Kanehisa M., Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kembel S.W., Wu M., Eisen J.A., Green J.L. Incorporating 16S gene copy number information improves estimates of microbial diversity and abundance. PLoS Comput. Biol. 2012;8 doi: 10.1371/journal.pcbi.1002743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klindworth A., Pruesse E., Schweer T., Peplies J., Quast C., Horn M., Glockner F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic. Acids Res. 2013;41 doi: 10.1093/nar/gks808. doi ARTN e110.1093/nar/gks808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiecien M., Winiarska-Mieczan A., Milczarek A., Klebaniuk R. Biological response of broiler chickens to decreasing dietary inclusion levels of zinc glycine chelate. Biol. Trace Elem. Res. 2017;175:204–213. doi: 10.1007/s12011-016-0743-y. [DOI] [PubMed] [Google Scholar]
- Langille M.G., Zaneveld J., Caporaso J.G., McDonald D., Knights D., Reyes J.A., Clemente J.C., Burkepile D.E., Vega Thurber R.L., Knight R., Beiko R.G., Huttenhower C. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013;31:814–821. doi: 10.1038/nbt.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvak Y., Byndloss M.X., Tsolis R.M., Baumler A.J. Dysbiotic Proteobacteria expansion: a microbial signature of epithelial dysfunction. Curr. Opin. Microbiol. 2017;39:1–6. doi: 10.1016/j.mib.2017.07.003. [DOI] [PubMed] [Google Scholar]
- Lönnerdal B. Dietary factors influencing zinc absorption. J. Nutr. 2000;130:1378S–1383S. doi: 10.1093/jn/130.5.1378S. [DOI] [PubMed] [Google Scholar]
- Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15 doi: 10.1186/s13059-014-0550-8. doi ARTN 55010.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W., Niu H., Feng J., Wang Y. Effects of zinc chelate on oxidative stress, contents of trace elements, and intestinal morphology in broilers. Biol. Trace Elem. Res. 2011;142:546–556. doi: 10.1007/s12011-010-8824-9. [DOI] [PubMed] [Google Scholar]
- Masella A.P., Bartram A.K., Truszkowski J.M., Brown D.G., Neufeld J.D. PANDAseq: PAired-eND Assembler for Illumina sequences. BMC Bioinform. 2012;13 doi: 10.1186/1471-2105-13-31. doi Artn 3110.1186/1471-2105-13-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurdie P.J., Holmes S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One. 2013;8 doi: 10.1371/journal.pone.0061217. doi ARTN e6121710.1371/journal.pone.0061217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanna C., Nys Y. Effect of dietary zinc content and sources on the growth, body zinc deposition and retention, zinc excretion and immune response in chickens. Br. Poult. Sci. 1999;40:108–114. doi: 10.1080/00071669987926. [DOI] [PubMed] [Google Scholar]
- NRC . 9th rev. ed. Natl. Acad. Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- Oteiza P.I. Zinc and the modulation of redox homeostasis. Free Radic. Biol. Med. 2012;53:1748–1759. doi: 10.1016/j.freeradbiomed.2012.08.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quast C., Pruesse E., Yilmaz P., Gerken J., Schweer T., Yarza P., Peplies J., Glockner F.O. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic. Acids Res. 2013;41:D590–D596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranaldi G., Ferruzza S., Canali R., Leoni G., Zalewski P.D., Sambuy Y., Perozzi G., Murgia C. Intracellular zinc is required for intestinal cell survival signals triggered by the inflammatory cytokine TNF alpha. J. Nutr. Biochem. 2013;24:967–976. doi: 10.1016/j.jnutbio.2012.06.020. [DOI] [PubMed] [Google Scholar]
- Saenmahayak B., Bilgili S.F., Hess J.B., Singh M. Live and processing performance of broiler chickens fed diets supplemented with complexed zinc. J. Appl. Poult. Res. 2010;19:334–340. [Google Scholar]
- Sauer A.K., Pfaender S., Hagmeyer S., Tarana L., Mattes A.K., Briel F., Kury S., Boeckers T.M., Grabrucker A.M. Characterization of zinc amino acid complexes for zinc delivery in vitro using Caco-2 cells and enterocytes from hiPSC. Biometals. 2017;30:643–661. doi: 10.1007/s10534-017-0033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severinov K., Nair S.K. Microcin C: biosynthesis and mechanisms of bacterial resistance. Future Microbiol. 2012;7:281–289. doi: 10.2217/fmb.11.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah P., Swlatlo E. A multifaceted role for polyamines in bacterial pathogens. Mol. Microbiol. 2008;68:4–16. doi: 10.1111/j.1365-2958.2008.06126.x. [DOI] [PubMed] [Google Scholar]
- Shao Y.X., Lei Z., Yuan J.M., Yang Y., Guo Y.M., Zhang B.K. Effect of zinc on growth performance, gut morphometry, and cecal microbial community in broilers challenged with S almonella enterica serovar Typhimurium. J. Microbiol. 2014;52:1002–1011. doi: 10.1007/s12275-014-4347-y. [DOI] [PubMed] [Google Scholar]
- Star L., van der Klis J.D., Rapp C., Ward T.L. Bioavailability of organic and inorganic zinc sources in male broilers. Poult. Sci. 2012;91:3115–3120. doi: 10.3382/ps.2012-02314. [DOI] [PubMed] [Google Scholar]
- Stefanidou M., Maravelias C., Dona A., Spiliopoulou C. Zinc: a multipurpose trace element. Arch. Toxicol. 2006;80:1–9. doi: 10.1007/s00204-005-0009-5. [DOI] [PubMed] [Google Scholar]
- Summers S.L.J.D. 2nd ed. Univerity Books; Nottingham, England: 1997. Commercial Poultry Nutrition. [Google Scholar]
- Sunder G.S., Panda A.K., Gopinath N.C.S., Rao S.V.R., Raju M.V.L.N., Reddy M.R., Kumar C.V. Effects of higher levels of zinc supplementation on performance, mineral availability, and immune competence in broiler chickens. J. Appl. Poult. Res. 2008;17:79–86. [Google Scholar]
- Swiatkiewicz S., Arczewska-Wlosek A., Jozefiak D. The efficacy of organic minerals in poultry nutrition: review and implications of recent studies. World Poult. Sci. J. 2014;70:475–485. [Google Scholar]
- Tamim N.M., Angel R. Phytate phosphorus hydrolysis as influenced by dietary calcium and micro-mineral source in broiler diets. J. Agric. Food Chem. 2003;51:4687–4693. doi: 10.1021/jf034122x. [DOI] [PubMed] [Google Scholar]
- van Riet M.M.J., Millet S., Nalon E., Langendries K.C.M., Cools A., Ampe B., Du Laing G., Tuyttens F.A.M., Maes D., Janssens G.P.J. Fluctuation of potential zinc status biomarkers throughout a reproductive cycle of primiparous and multiparous sows. Br. J. Nutr. 2015;114:544–552. doi: 10.1017/S0007114515002238. [DOI] [PubMed] [Google Scholar]
- Vossen E., Ntawubizi M., Raes K., Smet K., Huyghebaert G., Arnouts S., De Smet S. Effect of dietary antioxidant supplementation on the oxidative status of plasma in broilers. J. Anim. Physiol. Anim. Nutr. 2011;95:198–205. doi: 10.1111/j.1439-0396.2010.01041.x. [DOI] [PubMed] [Google Scholar]
- Weiss G.A., Hennet T. Mechanisms and consequences of intestinal dysbiosis. Cell. Mol. Life Sci. 2017;74:2959–2977. doi: 10.1007/s00018-017-2509-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welfare Quality. 2009. Welfare Quality Assessment Protocol for Poultry. Lelystad, The Netherlands.
- Zakaria H.A., Jalal M., Al-Titi H.H., Souad A. Effect of sources and levels of dietary zinc on the performance, carcass traits and blood parameters of broilers. Braz. J. Poult. Sci. 2017;19:519–526. [Google Scholar]
- Zhang K.Y., Hornef M.W., Dupont A. The intestinal epithelium as guardian of gut barrier integrity. Cell. Microbiol. 2015;17:1561–1569. doi: 10.1111/cmi.12501. [DOI] [PubMed] [Google Scholar]
Uncited Reference
- Council N.R. 9th rev. ed. Natl. Acad. Press.; Washington, DC: 1994. Nutrient requirements of poultry. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.