Abstract
Many requirements are necessary to meet the European Union rules to export poultry, including the amount of physiological water and water-protein ratio (WPR) in carcasses. Therefore, the aim of this study was to identify if strain, nutrition, and age affect the amount of collagen and fat and the WPR in cuts and verify whether the latter meets the international export standards. A total of 3,240 male chicks were housed in a completely randomized design in a 3 × 3 × 5 factorial arrangement, which included 3 nutritional densities (regular, medium, and high), 3 strains (021 Embrapa and 2 commercial strains identified as A and B), and 5 ages. Twelve broilers from each treatment (totaling 540 birds) were slaughtered at 28, 35, 42, 49, and 56 D of age to determine collagen and fat levels and WPR (through the calculation of moisture and protein percentage) in broiler breasts and legs using the near-infrared spectroscopy method. The use of feeds with different nutritional densities presented in this study has no effect on the WPR in the breast and legs of broilers slaughtered between 28 and 56 D of age. However, nutritional density influences liveweight and percentage of fat in the breast and legs. Collagen percentage in the legs decreases with increasing nutritional density. The 021 Embrapa strain cuts present a lower WPR than those of other commercial strains. However, the values found for all strains studied are within the limits of the Europe Union and Brazilian legislations. The liveweight, breast weight, leg weight, and leg fat increases linearly with age. Quite the opposite, water protein ratio, breast fat level, and breast collagen level decrease linearly with age. Leg WPR and leg collagen level are not affected by age. Despite the differences found for strains, nutritional densities and age readers should be aware that these factors may interact with each other depending on the response variable studied.
Key words: broiler, genetics, nutrition, water-protein ratio, WPR
Introduction
Considering the importance of Brazilian poultry farming in the global market, meat export, and the dependence on foreign genetic material, research on the production of genetically improved and commercially competitive national bird strains has become urgent (Ledur et al., 2011). Thus, the Brazilian Agricultural Research Corporation/Swine and Poultry (Embrapa) developed 5 breeding programs of commercial broiler strains for egg and poultry production.
These breeding programs were developed to reduce the dependence on importing basic genetic material. One of these programs resulted in the 021 Embrapa broiler strain, a hybrid broiler for industrial poultry production that originated from the crossing of 4 pure lines. Although this animal presents less intense growth rates, at 42 D of age, this product has high viability (96%), liveweight of 2,125 g, feed conversion of 1.84, carcass yield of 73.6%, and breast yield of 20% (Ledur et al., 2011).
Bird strain is important for economic return in poultry farming because broiler growth and development directly influence the age at slaughter and yield of the carcass and noble parts, such as breast and legs (Mendes et al., 1993, Cotta, 1994, Moreira et al., 2003). According to Bilgili et al. (1992), age at slaughter, sex, and strain are the main factors that affect broiler performance. However, in addition to productive characteristics, those related to poultry meat quality have become important owing to the increasing demand in the consumer market (Beraquet, 2000).
The world consumption of poultry meat has increased annually, and Brazil has stood out as the main exporting country. The European Union is one of the main markets for Brazilian poultry; it imported more than 62,000 tons of broiler cuts in 2017 (ABPA, 2018). However, to keep this export market open and meet this great demand, it is necessary to fulfill many requirements, including the quantity of physiological water and water-protein ratio (WPR) present in these cuts. Physiological (or metabolic) water is the water naturally present in all animal species, that is, found in carcasses that have not undergone any processing stages involving external water (Dias et al., 2017).
The current regulations to quantify physiological water and WPR in cuts, such as whole legs and broiler breasts, are the EU Regulation No. 543/2008 of 06/16/2008 and Brazilian Normative Instruction—MAPA, No. 32 (Brazil/MAPA, 2010). In the EU legislation, acceptable WPR for broiler breast meat and whole legs are within 3.19 ± 0.12 (3.07–3.31) and 3.78 ± 0.19 (3.59–3.97), respectively. Similarly, the Brazilian Normative Instruction 32 also establishes WPR limits, but its values for total water content differ from the abovementioned values, with acceptable WPR for broiler breast meat and whole legs between 3.03 to 3.55 and 3.59 to 4.67, respectively.
The chemical composition related to moisture and protein contents in the cuts can be analyzed by different methods. Some methods are time-consuming and expensive, involving the generation of chemical residues and pollutants in the environment (Pesti and Bakalli, 1997, Silva et al., 2003), such as the traditional Kjeldahl and Dumas (combustion) methods. They involve long processes and can take several hours to produce results, and in many food-production industries, they hinder real-time production and product quality decisions (Anderson, 2007). However, there are some studies on the use of more practical and nonpolluting methods to obtain the chemical composition of foods.
Near-infrared (NIR) spectroscopy provides fast results for multiple constituents and is suitable for high-capacity production environments. NIR has been routinely used in the meat industry for product results. According to the manufacturer's manual (Foss, 2009), FoodScan equipment has been approved by the AOAC (1995) and AQIS (Australian Quarantine and Inspection Service) for meat and meat product analysis. At Embrapa Swine and Poultry, in Santa Catarina (SC) State, this technique was used in several studies, demonstrating technical efficiency and robustness and a reduction in the analysis residue.
This study aimed at meeting the demand of the broiler production chain, to better understand the variability of WPR results in cuts and possible factors that may impact these results, based on more modern analytical techniques, for increased quality control in slaughterhouses.
Therefore, the objective of this study was to identify the effect of strain, nutrition, and age on collagen, fat, and WPR results and determine whether the latter meets the international standards for broiler meat production using the NIR method.
Materials and methods
The experiment was approved by the Ethics Committee on the Use of Animals of the Agricultural Sciences Department of the Federal University of Paraná—UFPR, number 116/2016.
The study took place at the Canguiri Experimental Farm, Poultry Farming Department of the Federal University of Paraná—UFPR, in Pinhais/PR. The poultry shed was 70 m long and 8 m wide, with a total of 116 pens, each measuring 2.10 m2.
Animals, Experimental Design, and Treatments
The study included a total of 3,240 male chicks from 021 Embrapa and 2 commercial strains identified as A and B, totaling 1,080 animals from each strain. These animals were separated in groups of 30 and housed in 108 pens with pine wood shavings bedding. Each pen was considered an experimental unit.
The treatments were defined through the 3 × 3 × 5 factorial design system, testing 3 strains, 3 nutritional densities of the diet, and 5 ages. The animals were distributed in a completely randomized design, totaling 9 treatments and 12 replications per treatment that were evaluated at 5 different ages. The nutritional densities used followed the Brazilian Tables for Poultry and Swine (Rostagno et al., 2011), aiming at regular, medium, and superior performance broilers, as shown in Table 1, for 4 feeding phases: prestarter (0–7 D), starter (8–21 D), grower (22–35 D), and finisher (36–56 D).
Table 1.
Nutritional composition of experimental feeds.
| Ingredients (%) | Prestarter (1–7 D) |
Starter (8–21 D) |
Grower (22–35 D) |
Finisher (36–56 D) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nutritional density/AME | ||||||||||||
| Regular |
Medium |
High |
Regular |
Medium |
High |
Regular |
Medium |
High |
Regular |
Medium |
High |
|
| 2.803 (kcal/kg) | 2.950 (kcal/kg) | 3.098 (kcal/kg) | 2.850 (kcal/kg) | 3.000 (kcal/kg) | 3.150 (kcal/kg) | 2.945 (kcal/kg) | 3.100 (kcal/kg) | 3.255 (kcal/kg) | 2.992 (kcal/kg) | 3.150 (kcal/kg) | 3.307 (kcal/kg) | |
| Corn | 57.8 | 46.58 | 36.04 | 62.96 | 54.65 | 44.57 | 66.95 | 57.69 | 48.42 | 70.19 | 61.30 | 52.02 |
| Soybean meal, 46% | 38.08 | 44.86 | 51.64 | 32.01 | 37.94 | 44.30 | 28.82 | 34.38 | 39.94 | 26.07 | 31.24 | 36.80 |
| Soybean oil | 0.130 | 4.050 | 7.990 | 0.000 | 3.335 | 7.239 | 0.400 | 4.250 | 8.100 | 0.420 | 4.260 | 8.150 |
| Limestone | 0.971 | 0.971 | 0.971 | 0.982 | 0.983 | 0.982 | 0.917 | 0.916 | 0.915 | 0.782 | 0.780 | 0.779 |
| Dicalcium phosphate | 1.841 | 1.783 | 1.726 | 1.471 | 1.418 | 1.365 | 1.231 | 1.186 | 1.141 | 0.934 | 0.893 | 0.848 |
| Salt | 0.530 | 0.531 | 0.532 | 0.505 | 0.506 | 0.507 | 0.480 | 0.481 | 0.482 | 0.454 | 0.455 | 0.456 |
| DL-Methionine | 0.244 | 0.293 | 0.343 | 0.205 | 0.243 | 0.291 | 0.180 | 0.224 | 0.269 | 0.151 | 0.199 | 0.233 |
| L-Lysine | 0.191 | 0.165 | 0.138 | 0.205 | 0.188 | 0.161 | 0.195 | 0.179 | 0.163 | 0.199 | 0.181 | 0.152 |
| L-Threonine | 0.077 | 0.077 | 0.089 | 0.064 | 0.074 | 0.081 | 0.052 | 0.060 | 0.067 | 0.046 | 0.059 | 0.055 |
| Choline 60 | 0.050 | 0.050 | 0.050 | 0.050 | 0.050 | 0.050 | 0.050 | 0.050 | 0.050 | 0.050 | 0.050 | 0.050 |
| Kaolin | 0.100 | 0.100 | 0.100 | 0.888 | 0.100 | 0.100 | 0.100 | 0.100 | 0.100 | 0.100 | 0.100 | 0.100 |
| Toxin adsorbent | 0.200 | 0.200 | 0.200 | 0.200 | 0.200 | 0.200 | 0.200 | 0.200 | 0.200 | 0.200 | 0.200 | 0.200 |
| BHT | 0.010 | 0.010 | 0.010 | 0.010 | 0.010 | 0.010 | 0.010 | 0.010 | 0.010 | 0.010 | 0.010 | 0.010 |
| Vitamin premix1 | 0.120 | 0.120 | 0.120 | 0.100 | 0.100 | 0.100 | 0.100 | 0.100 | 0.100 | 0.100 | 0.100 | 0.100 |
| Mineral premix2 | 0.050 | 0.050 | 0.050 | 0.050 | 0.050 | 0.050 | 0.050 | 0.050 | 0.050 | 0.050 | 0.050 | 0.050 |
| K carbonate | 0.332 | 0.166 | 0.001 | 0.304 | 0.154 | 0.000 | 0.263 | 0.130 | 0.000 | 0.247 | 0.125 | 0.000 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Nutritional profile | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total calcium | 0.920 | 0.920 | 0.920 | 0.820 | 0.820 | 0.820 | 0.730 | 0.730 | 0.730 | 0.600 | 0.600 | 0.600 |
| Crude fiber | 3.005 | 3.183 | 3.360 | 2.786 | 2.956 | 3.119 | 2.685 | 2.820 | 2.954 | 2.595 | 2.716 | 2.850 |
| Prestarter (1–7 D) |
Starter (8–21 D) |
Grower (22–35 D) |
Finisher (36–56 D) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nutritional density/AME | ||||||||||||
| Regular |
Medium |
High |
Regular |
Medium |
High |
Regular |
Medium |
High |
Regular |
Medium |
High |
|
| 2.803 (kcal/kg) | 2.950 (kcal/kg) | 3.098 (kcal/kg) | 2.850 (kcal/kg) | 3.000 (kcal/kg) | 3.150 (kcal/kg) | 2.945 (kcal/kg) | 3.100 (kcal/kg) | 3.255 (kcal/kg) | 2.992 (kcal/kg) | 3.150 (kcal/kg) | 3.307 (kcal/kg) | |
| Available phosphorus | 0.470 | 0.470 | 0.470 | 0.390 | 0.390 | 0.390 | 0.340 | 0.340 | 0.340 | 0.280 | 0.280 | 0.280 |
| Fat | 2.859 | 6.474 | 10.10 | 2.841 | 5.942 | 9.546 | 3.325 | 6.894 | 10.46 | 3.420 | 6.983 | 10.59 |
| Digestible methionine | 0.529 | 0.601 | 0.675 | 0.465 | 0.524 | 0.594 | 0.427 | 0.490 | 0.553 | 0.388 | 0.452 | 0.505 |
| Digestible lysine | 1.180 | 1.310 | 1.440 | 1.050 | 1.170 | 1.290 | 0.970 | 1.080 | 1.190 | 0.910 | 1.010 | 1.110 |
| Crude protein | 21.51 | 23.86 | 26.21 | 19.16 | 21.28 | 23.47 | 17.96 | 19.85 | 21.74 | 16.92 | 18.66 | 20.53 |
| Digestible threonine | 0.770 | 0.850 | 0.940 | 0.680 | 0.760 | 0.840 | 0.630 | 0.700 | 0.770 | 0.590 | 0.660 | 0.720 |
| Digestible tryptophan | 0.236 | 0.269 | 0.303 | 0.204 | 0.2343 | 0.265 | 0.188 | 0.215 | 0.243 | 0.174 | 0.199 | 0.226 |
Abbreviation: AME, apparent metabolizable energy; BHT, butylated hydroxytoluene.
Product composition (guaranteed levels per kg of product): vit A = 11.000.000 I.U.; vit D3 = 4.000.000 I.U.; vit E = 55.000 I.U.; vit K3 = 3.000 mg; vit B1 = 2.300 mg; vit B2 = 7.000 mg; pantothenic acid = 12 g; vit B6 = 4.000 mg; vit B12 = 25.000 mcg; nicotinic acid = 60 g; folic acid = 2.000 mg; biotin = 250 mg; selenium = 300 mg. 2Product composition (guaranteed levels per kg of product): iron = 100 g; copper = 20 g; manganese = 130 g; zinc = 130 g; iodine = 2.000 mg.
The treatments carried out are T1: 021 Embrapa strain and regular nutritional density; T2: 021 Embrapa strain and medium nutritional density; T3: 021 Embrapa strain and high nutritional density; T4: Commercial strain A and regular nutritional density; T5: Commercial strain A and medium nutritional density; T6: Commercial strain A and high nutritional density; T7: Commercial strain B and regular nutritional density; T8: Commercial strain B and medium nutritional density; T9: Commercial strain B and high nutritional density. All of these treatments were evaluated at 28, 35, 42, 49, and 56 D of age.
The feeds were pelleted (80°C, 20 s) and ground at different degrees for the prestarter, starter, and grower phases. The finisher feed was only pelleted.
Samples
Twelve broilers from each treatment were weighed and slaughtered at 28, 35, 42, 49, and 56 D of age, totaling 540 birds, to determine the liveweight, WPR, and fat and collagen percentages of broiler breast and leg. The animals were euthanized at the poultry slaughterhouse of Canguiri Experimental Farm—UFPR. Electronarcosis was used before 3-min bleeding. All processes were conducted by a previously trained team.
During the process of sample manipulation to extract skinless breasts and whole right legs (thigh and drumstick) with skin, scalding was omitted to avoid contact of the carcasses with water, and plucking was manual. Then, the samples were placed in plastic bags and frozen for subsequent processing.
Evaluations
The samples were processed at the Laboratory of Physical and Chemical Analyses (LAFQ) of the Brazilian Agricultural Research Corporation (Embrapa), Swine and Poultry Unit, in the district of Tamanduá, Concórdia, SC.
Before analyses, breast and leg samples were removed from the cold room (−7°C) and maintained for 48 h in refrigeration (0°C–7°C). For evaluation, the breasts were boned and legs were kept whole (with skin and bone) and were ground using a HM 297 homogenizer grinder (Foss). All samples were ground semi-thawed so that the temperature at the end of the process was >10°C.
After grinding, each sample was placed in the FoodScan scanning platform. The equipment was calibrated to read 16 points in the same sample to obtain the fat, collagen, moisture, and protein parameters.
According to the method and numerical formula proposed by Regulation No. 543/2008 (EU), the WPR was calculated as follows:
Statistical Analysis
Data were analyzed using the R package emmeans: Estimated marginal means (R Core Team, 2019). When a significant F was observed, the mean values were compared using the Tukey method with a significance level of 0.05. The interaction between strain, nutritional density, and age, when significant, was unfolded according to the involved factors.
First, the outlier data were identified and removed using the following calculation criteria: lower limit = first quartile – 1.5 (third quartile – first quartile), and upper limit = third quartile + 1.5 (third quartile – first quartile).
Moreover, to study the relationship between response variables, Pearson correlation procedure was used. In addition, linear and quadratic effects of age on liveweight and breast and leg characteristics were used. Models were selected by the coefficient of determination (R2); by the significance of regression and the lack of adjustment (tested by F test); by the significance of regression coefficients (tested by F test).
Results and discussion
Table 2 shows the results for breast characteristics of the different broiler strains fed with different nutritional densities and slaughtered at 28, 35, 42, 49, and 56 D of age. The effect of strain was detected on liveweight and for all breast variables. The 021 Embrapa presented the lowest mean of liveweight, breast weight, and WPR and the highest amount of fat and collagen in the breast (P < 0.05), differing from the mean values for strains A and B. Although the 021 Embrapa presents less intense growth rates, at 42 D of age, this product has high viability (96%), liveweight of 2,125 g, feed conversion of 1.84, carcass yield of 73.6%, and breast yield of 20% (Ledur et al., 2011).
Table 2.
Liveweight and characteristics of broiler breasts of 3 different strains fed with 3 nutritional densities and slaughtered at different ages.
| Item | Liveweight | Breast weight | Breast WPR | Breast fat | Breast collagen |
|---|---|---|---|---|---|
| Effect of strain | |||||
| 021 Embrapa | 2,372a | 458a | 3.207a | 2.42b | 0.730b |
| A | 2,961b | 769b | 3.248b | 2.18a | 0.667a |
| B | 3,140c | 797c | 3.283c | 2.07a | 0.667a |
| SEM | 18.3 | 7.95 | 0.007 | 0.04 | 0.132 |
| Effect of nutritional density | |||||
| Regular | 2,810a,b | 669 | 3.244 | 2.36b | 0.705 |
| Medium | 2,869b | 687 | 3.250 | 2.27b | 0.680 |
| High | 2,794a | 668 | 3.244 | 2.04a | 0.678 |
| SEM | 18.3 | 7.95 | 0.007 | 0.04 | 0.132 |
| Effect of age | |||||
| 28 | 1,621a | 367a | 3.34d | 2.4b,c | 0.707 |
| 35 | 2,218b | 530b | 3.30c | 2.56c | 0.690 |
| 42 | 2,697c | 631c | 3.24b | 2.19a,b | 0.694 |
| 49 | 3,715d | 905d | 3.18a | 1.99a | 0.673 |
| 56 | 3,871e | 941d | 3.17a | 1.97a | 0.675 |
| SEM | 23.7 | 10.3 | 0.01 | 0.06 | 0.017 |
| P value | |||||
| Strain | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Density | 0.01 | 0.1528 | 0.82 | <0.0001 | 0.271 |
| Age | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.602 |
| Strain:Density | 0.45 | 0.6234 | 0.024 | 0.225 | 0.1014 |
| Strain:Age | <0.0001 | <0.0001 | 0.279 | 0.007 | 0.511 |
| Density:Age | 0.89 | 0.9451 | 0.210 | 0.07 | 0.191 |
| Strain:Density:Age | 0.53 | 0.7275 | 0.666 | 0.316 | 0.131 |
a-eMean values followed by different lowercase letters in the column indicate significant difference (P < 0.05) between effects.
Abbreviations: SEM, standard error of the mean; WPR, water protein ratio.
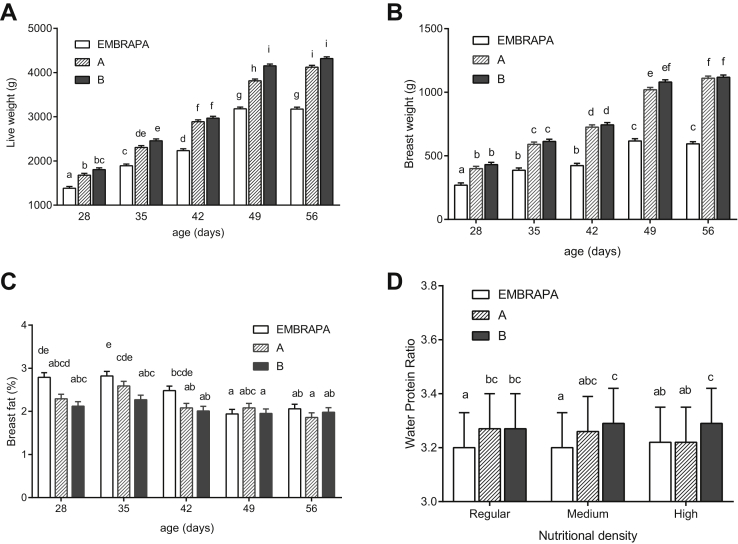
Nutritional density affected the liveweight and the percentage of fat in breast (P < 0.05). The effect of age was significant for all variables, except for breast collagen. However, the unfolding interaction shows that the magnitude of the effect of the strain on liveweight, breast weight, and breast fat depends on the age of the birds (Figure 1). Significant interaction (P < 0.0001) between strain and age was observed for liveweight, breast weight, and breast fat (P = 0.007). Significant interaction was also found between strain and nutritional density (P = 0.02) for breast WPR. No significant interactions between factors were observed for breast collagen. In this case, the 021 Embrapa strain showed higher amount of collagen in the breast than strains A and B (P < 0.0001).
Figure 1.
Unfolding of strain × age interactions for liveweight (A), breast weight (B), and breast fat percentage (C) and strain × nutritional density for water protein ratio (WPR) (D) of broilers fed at different nutritional densities. Means followed by different lowercase letters differ significantly (P < 0.05).
In the interaction graphs, we can see that all strains show a linear increase in liveweight (Figure 1A) and breast weight (Figure 1B), but in all ages, the 021 Embrapa had a significantly lower mean than strains A and B. This difference becomes much more evident at older ages, making the interaction between strain and age significant (P < 0.0001).
According to Havenstein et al. (2003), the genetic selection carried out increased the yields of edible meat and doubled the breast muscle proportion but was also associated with increased fatness. This statement was not corroborated in the present study regarding breast fat. It can be observed in Figure 1C that 021 Embrapa, which is a strain with lower selection pressure, at 28 D presented significantly higher percentage of fat than strains A and B. However, these differences were not significant at older ages. There is still much to know about the effects of genetic selection and physiological mechanisms that affect the quality of meat quality and carcass of broilers (Sandercock et al. 2009).
The 021 Embrapa strain showed lower mean WPR (3.207) than strains A (3.248) and B (3.283) (P < 0.0001). The present study showed no interaction effect of strain × age on the evaluation of WPR. But despite this, WPR results for strains at each age deserve special attention.
The WPR of the 021 Embrapa at 28 D of age showed the lowest mean WPR (3.30 ± 0.01) but did not differ from that of strain A (3.34 ± 0.01) and B (3.38 ± 0.01). The mean WPR in broilers slaughtered at 28 D of age showed that only the 021 Embrapa strain was within the European Commission Legislation values of 3.07 to 3.31 European Union (2008). The other mean values were within the Brazilian legislation limits of 3.03 to 3.55 for WPR in broiler breasts (Brazil/MAPA, 2010).
The most common slaughter age for broilers is 42 D. However, the values found (Embrapa 3.21 ± 0.01; strain A 3.24 ± 0.01; strain B 3.26 ± 0.01) were within the limits of the EU (3.07–3.31) and Brazilian (3.03–3.55) legislations.
A study carried out by the European Commission in 2012 (Commission of the European Communities, 2012) to evaluate the content of physiological water in poultry farmed and slaughtered in the EU showed that the WPR limits in the European legislation for breast and legs are very low for the poultry currently being raised. One of the recommendations was to amend the legislation to adapt the water and protein values based on the 2012 study, as commercial strains are in constant technological development to achieve maximum performance.
Strain had an effect on the WPR in breasts at 49 D of age. The 021 Embrapa strain had a significantly lower mean (3.11 ± 0.01) than the value obtained by strain B (3.25 ± 0.01), but did not differ statistically from strain A (3.19 ± 0.01). All WPR values found in breasts of broilers slaughtered at 49 D met both the EU (3.07–3.31) European Union (2008) and Brazilian (3.03–3.55) (Brazil/MAPA, 2010) demands.
According to Leeson and Summers (2009), there has been much discussion about the effects of diet on the percentage of carcass components, but in some situations, the change occurred because there was a corresponding change in the level of another component. As the percentage of protein in carcasses is not influenced considerably by nutrition, and assuming there is no amino acid deficiency, the real protein yield is regulated by genetics.
No differences between strains were observed in the WPR in breasts of broilers slaughtered at 56 D of age. All WPR values found in breasts of broilers slaughtered at 56 D met the demands of the European (3.07–3.31) and Brazilian (3.03–3.55) legislations.
However, Table 2 and Figure 1D show a significant interaction between strain and nutritional density for WPR. Strain A numerically reduced WPR as the nutritional density of the diet went from low to high while strain B remained at the highest average and 021 Embrapa at the lowest average uniformly at all nutritional densities. Nevertheless, all observed averages were in accordance with European Commission and Brazilian Legislation.
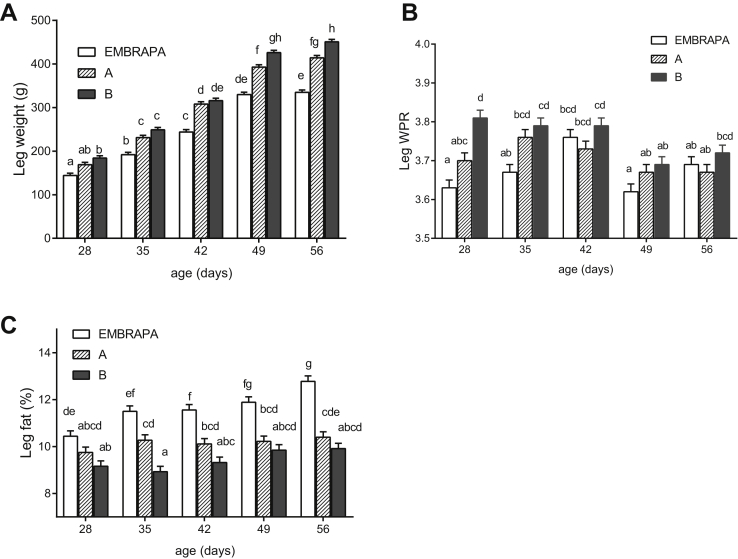
Table 3 shows the results for leg characteristics of 3 different strains fed with 3 nutritional densities and slaughtered at 28, 35, 42, 49, and 56 D of age. There were significant interactions between strain and age for all leg characteristics (P < 0.001).
Table 3.
Characteristics of broiler legs of 3 different strains fed with 3 nutritional densities and slaughtered at different ages.
| Item | Leg weight | Leg WPR | Leg fat | Leg collagen |
|---|---|---|---|---|
| Effect of strain | ||||
| 021 Embrapa | 249a | 3.675a | 11.63c | 1.29a |
| A | 303b | 3.707b | 10.15b | 1.41b |
| B | 325c | 3.761c | 9.43a | 1.50b |
| SEM | 2.41 | 0.0086 | 0.103 | 0.0312 |
| Effect of nutritional density | ||||
| Regular | 292 | 3.724 | 11.3c | 1.82c |
| Medium | 296 | 3.703 | 10.6b | 1.31b |
| High | 289 | 3.716 | 9.3a | 1.06a |
| SEM | 2.41 | 0.0086 | 0.103 | 0.0312 |
| Effect of age | ||||
| 28 | 166a | 3.71b,c | 9.78a | 1.21a |
| 35 | 224b | 3.74c,d | 10.23a,b | 1.13a |
| 42 | 289c | 3.76d | 10.33b | 1.43b |
| 49 | 283d | 3.66a | 10.66b,c | 1.72c |
| 56 | 400e | 3.70a,b | 11.03c | 1.50b |
| SEM | 3.11 | 0.0111 | 0.132 | 0.0403 |
| P value | ||||
| Strain | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Density | 0.1578 | 0.2047 | <0.0001 | <0.0001 |
| Age | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Strain:Density | 0.3055 | 0.7877 | 0.3752 | <0.0001 |
| Strain:Age | <0.0001 | <0.0001 | 0.0046 | <0.0001 |
| Density:Age | 0.8070 | 0.0740 | 0.2834 | 0.1506 |
| Strain:Density:Age | 0.8389 | 0.0812 | 0.1173 | <0.0001 |
a-eMean values followed by different lowercase letters in the column indicate significant difference (P < 0.05) between effects.
Abbreviations: SEM, standard error of the mean; WPR, water protein ratio.
Figure 2A shows that all strains show a linear increase in leg weight, but in all ages, the 021 Embrapa had a significantly lower mean than strains A and B. This difference gets bigger as the bird gets older.
Figure 2.
Unfolding of strain × age interactions for leg weight (A), leg water protein ratio (WPR) (B), and leg fat percentage (C) of broilers fed with different nutritional densities. Means followed by different lowercase letters differ significantly (P < 0.05).
A significant interaction between strain and age was reported for the WPR in legs (Figure 2B). The WPR for legs at 28 D of age was similar to that of breasts at this age, which was only affected by strain. The 021 Embrapa strain presented the lowest mean, followed by strain A, which presented an intermediate WPR value, and strain B, which presented the highest mean. A similar pattern was found at 35 and 49 D but not at 42 and 56 D of age. This is the reason that explains the significant interaction between age and strain for WPR in legs. Despite these differences, the mean WPR values in legs at 28, 35, 42, 49, and 56 D of age met both the European (3.59–3.97) European Union (2008) and Brazilian (3.59–4.67) (Brasil/MAPA, 2010) legislation demands.
Figueiredo et al. (2016) reported a difference in the physiological WPR of specific cuts (breast and whole legs) in different genotypes of broilers slaughtered at 42 D of age. However, the values found in those samples were also within the EU R. 543/2008 limits. Dias et al. (2015) studied 2 broiler strains slaughtered from 42 to 50 D of age and reported that the WPR values in different cuts (breast, boneless breast, and whole leg) were in accordance with Brazilian law.
In general, lower WPR values are better because they indicate a higher protein concentration and less water in the cuts, as we have the amount of moisture divided by the amount of protein in the WPR formula.
The WPR in breast and legs was correlated with other response variables to discover how strong is their association, regardless of the treatment being tested. For breast WPR, significantly negative correlations were found with age (Pearson −0.98, P = 0.002), breast weight (Pearson −0.98, P = 0.0001), and leg weight (Pearson −0.93, P = 0.01) and positive correlations with breast collagen (Pearson 0.91, P = 0.02) and breast fat (Pearson 0.91, P = 0.02). Surprisingly, leg WPR was not correlated to any other response variable. These nonsignificant correlations possible may be explained by the tissue composition of the legs. According to Bochno et al. (2003), in the second week of life, approximately 36 and 35% of lean meat is located in the breast and legs, respectively. As chickens grow, the percentage of lean tissue increases to 44% in the breast and reduces to 32% in the legs, relative to the total lean tissue in the carcass. This situation makes the measuring of linear correlation of whole leg WPR with other measures not too much precise. Once we know that WPR in breast is closely related to age, we can estimate this value and others using the regression equations that are presented in table 4.
Table 4.
Regression equations to estimate the characteristics of broiler carcass of 3 different strains fed with 3 nutritional densities and slaughtered at 28, 35, 42, 49, and 56 D of age.
| Item | Linear equation | R2 | P | Quadratic equation |
|---|---|---|---|---|
| Liveweight | -773.8 + 85.671x | 0.97 | 0.002 | NS |
| Breast weight | -239.0 + 21.757x | 0.96 | 0.003 | NS |
| Breast WPR | 3.522-0.006x | 0.97 | 0.002 | NS |
| Breast fat | 3.08-0.0204x | 0.77 | 0.049 | NS |
| Breast collagen | 0.736-0.0012x | 0.82 | 0.032 | NS |
| Leg weight | −83.8 + 8.957x | 0.97 | 0.002 | NS |
| Leg WPR | NS | NS | ||
| Leg fat | 8.648 + 0.041x | 0.97 | 0.001 | NS |
| Leg collagen | NS | NS |
Abbreviations: NS, nonsignificant; WPR, water protein ratio.
There was an increasing linear effect (P < 0.05) for liveweight, breast weight, leg weight, and leg fat with increasing age. Quite the opposite, there was a decreasing linear effect for breast WPR, breast fat, and breast collagen. Only the models for estimating leg WPR and leg collagen were nonsignificant (P > 0.05). When birds get older, there is an increase in fat content and a reduction in body protein and water deposition (Rutz et al., 1999, Gonzales and Sartori, 2002).
Figure 2C shows that all strains show a linear increase in leg fat (%), but in all ages, the 021 Embrapa had a significantly higher mean than strains A and B. This difference becomes more important in older birds.
The leg collagen (%) was affected by a triple significant interaction between strain × nutritional density × age (Table 3). It is generally observed that the 021 lineage Embrapa and high nutritional density diet produce lower collagen content in legs. However, this response pattern will depend on the age of the birds.
Conclusions
The use of feeds with different nutritional densities presented in this study has no effect on the WPR in breast and legs of broilers slaughtered between 28 and 56 D of age. However, nutritional density influences liveweight and percentage of fat in the breast and leg. Collagen percentage in leg decreases with increasing nutritional density.
The 021 Embrapa strain cuts present a lower WPR than those of other commercial strains. However, the values found for all strains studied are within the limits of the Europe Union and Brazilian legislations.
The liveweight, breast weight, leg weight, and leg fat increases linearly with age. Quite the opposite, WPR, breast fat, and breast collagen decrease linearly with age. Leg WPR and leg collagen are not affected by age.
Despite the differences found for strains, nutritional densities and age readers should be aware that these factors may interact with each other depending on the response variable studied.
References
- AOAC. (1995) ASSOCIATION OF OFFICIAL ANALYTICAL CHEMISTS. Methods of Analysis of the Association of Official Analytical Chemists. 16 ed., Washington, D.C., 1995. 1094p.
- Anderson S. Determination of fat, moisture, and protein in meat and meat products by using the FOSS FoodScan near-infrared spectrophotometer with FOSS artificial neural network calibration model and associated database: collaborative study. J. AOAC Int. 2007;90:1073–1083. [PubMed] [Google Scholar]
- Beraquet N.J. Influence of ante and post mortem factors on poultry meat quality. Braz. Rev. Poult. Sci. 2000;1:155–166. [Google Scholar]
- Bilgili S.F., Moran J.R., Acar N. Strain-cross response of male broilers to dietary lysine in the finisher feed: live performance further-processing yields. Poult. Sci. 1992;71:850–858. doi: 10.3382/ps.0710850. [DOI] [PubMed] [Google Scholar]
- Bochno R., Brzozowski W., Murawska D. Age-related changes in the distribution of meat, fat with skin and bones in broiler chicken carcasses. Polish J. Nat. Sci. 2003;14:335–345. [Google Scholar]
- Brazil/MAPA . Establishes the Parameters for Evaluation of the Total Content of Water Contained in Chilled, Chilled and Frozen Cuts. Official Gazette of the Federative Republic of Brazil; Brasilia, DF: 2010. Ministry of Agriculture, Livestock and Supply. Normative Instruction Nº 32 of December 3. [Google Scholar]
- Brazilian Association of Animal Protein - ABPA Annual Report Brazilian association of animal protein - ABPA. Annual poultry Report. 2018. http://abpa-br.com.br/storage/files/relatorio-anual-2018.pdf
- Commission of the European Communities . Luxembourg: Office for Official Publications of the European Communities; Luxembourg: 2012. Study of physiological water content of poultry.http://ec.europa.eu/agriculture/externalstudies/index_en.htm [Google Scholar]
- Cotta J.T.B. Poultry Science and Technology Foundation. Slaughtering and Processing of Chickens, 1994. Annals Campinas: Facta 1994; Campinas: 1994. Zootechnical, microbiological and sensory aspects of broiler carcass quality; pp. 77–95. [Google Scholar]
- Dias R., Krabbe E., de Figueiredo E.A.P., de AVILA V.S. International meat market: the challenge of meeting the parameters of the water: protein ratio. Ind. Poult. 2017;9:20–24. [Google Scholar]
- Dias V.H.C., Kovacs T.A.S., Herrera R.A., Dos Santos T.G., Dos Santos J.M.G., Andreazzi M.A. VIII Internal Show of Scientific Initiation Work, I Internal Show of Technological Initiation Work and Innovation. Unicesumar - Maringá University Center, Annals; Maringá: 2015. Moisture ratio: protein in broiler chicken carcasses; pp. 4–8. [Google Scholar]
- European Union, Commission Regulation (EC) Nº 543/2008 Laying Down Detailed Rules for the Application of Council Regulation (EC) Nº 1234/2007 as Regards the Marketing Standards for Poultry Meat. Official Journal of the European Communities, Brussels.
- Figueiredo E.A.P., Krabbe E.L., de Avila V.S., Klein C.H., Lopes L.D.S., Kawski V. Apinco Conference on Poultry Science and Technology, 2016. Annals. Campinas: Facta; Campinas, SP: 2016. Moisture: protein ratio in broiler genotypes. [Google Scholar]
- Foss . Edition 1. BR; July 2009. Reliable Meat Analysis with the FoodScan™, P/N 1026284. [Google Scholar]
- Gonzales E., Sartori J.S. FUNEP/UNESP; Jaboticabal: 2002. Muscle Growth and Metabolism. Avian Physiology Applied to Broilers; pp. 279–298. [Google Scholar]
- Havenstein G.B., Ferket P.R., Qureshi M.A. Carcass composition and yield of 1957 versus 2001 broilers when fed representative 1957 and 2001 broiler diets. Poult. Sci. 2003;82:1509–1518. doi: 10.1093/ps/82.10.1509. [DOI] [PubMed] [Google Scholar]
- Ledur M.C., Figueiredo E.A.P., Schmidt G.S., Avila V.S., Peixoto J.O.O. Concórdia, SC; 2011. Breeding of Poultry in Brazil and the Contributions of Embrapa Swine and Poultry. [Google Scholar]
- Leeson S., Summers J.D. Feeding Programs for Broiler Chickens. Nottingham University Press; 2009. Commercial poultry nutrition; pp. 281–282. [Google Scholar]
- Mendes A.A., Garcia E.A., Gonzales E. Effect of strain and slaughter age on carcass yield of broiler chickens. Braz. J. Anim. Sci. 1993;22:466–472. [Google Scholar]
- Moreira J., Mendes A.A., Garcia E.A., de Oliveira R.P., Garcia R.G., de Almeida I.C.L. Performance evaluation, carcass yield and breast meat quality in conformation versus conventional broiler chickens. Braz. J. Anim. Sci. 2003;32(Suppl. 1):1663–1673. [Google Scholar]
- Pesti G., Bakalli R. Estimation of the composition of broiler carcasses from their specific gravity. Poult. Sci. 1997;76:948–951. doi: 10.1093/ps/76.7.948. [DOI] [PubMed] [Google Scholar]
- R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2019. R: A Language and Environment for Statistical Computing.https://www.R-project.org/ [Google Scholar]
- Rostagno H.S., Albino L.F.T., Donzele J.L., Gomes P.C., Oliveira R.F., Lopes D.C., Ferreira A.S., Barreto S.L.T., Euclides R.F. In: Brazilian tables for poultry and swine: food composition and nutritional requirements. 3. ed. Viçosa M.G., editor. UFV, DZO; 2011. p. 252. [Google Scholar]
- Rutz F., Xavier E.G., Dadlt G.M. International Symposium on Cutting Chicken Production in the Final Stage. Annals; Campinas: 1999. Nutrition requirements for the final phase (Energy, amino acids, vitamins, minerals and additives) pp. 29–54. [Google Scholar]
- Sandercock D.A., Nute G.R., Hocking P.M. Quantifying the effects of genetic selection and genetic variation for body size, carcass composition, and meat quality in the domestic fowl (Gallus domesticus) Poult. Sci. 2009;88:923–931. doi: 10.3382/ps.2008-00376. [DOI] [PubMed] [Google Scholar]
- Silva J.H.V., Albino L.F.T., Nascimento A.H. Estimates of carcass anatomical composition of broilers based on feed protein level and carcass weight. Braz. J. Anim. Sci. 2003;32:344–352. [Google Scholar]