Abstract
There is a necessity for the implementation of in-feed probiotics in the poultry production industry, following strict regulations around the use of antibiotic growth promoters (AGP). Bacillus spp. are becoming an attractive alternative because of their functionality and stability. This study aims to evaluate the effect of a novel multi-strain Bacillus based probiotic on growth performance and gut health in male Ross 308 broiler chickens challenged with Clostridium perfringens Type A. Broilers on a 4 phase feeding program were fed diets containing either a standard metabolizable energy (ME) (100%) or a reduced ME (98%) level. The test probiotic was compared to an un-supplemented negative control and a commercial benchmark product as positive control over a 35 D feeding trial, using a 2 × 3 factorial experimental design. Chicks were inoculated with a once-off dose of C. perfringens on day 14. Growth performance was measured weekly to calculate body weight (BW), feed intake (FI) and feed conversion ratio (FCR). Villi histomorphology, gut lesions, and liver weight were assessed at day 35. Broilers fed the reduced ME diet with the test probiotic achieved higher final BWs (P = 0.037) and FCR (P = 0.014) than the negative control. Broilers fed the standard ME diet with the test probiotic showed improved (P = 0.001) FCR than the negative control from day 21 onwards. Increased duodenal villi height (P = 0.012) and villi height to crypt depth ratio in the duodenum (P < 0.0001) and jejunum (P = 0.0004) were observed in broilers fed the reduced ME diet containing the test probiotic. Additionally, the test probiotic resulted in significantly reduced relative liver weights in both ME groups. Consequently, the results suggest that the novel multi-strain Bacillus based probiotic enhanced broiler performance and improved gut health and is thus attractive as an alternative to AGP's in broiler production.
Key words: Bacillus subtilis, Bacillus velezensis, probiotic, broiler
INTRODUCTION
Probiotics are becoming increasingly relevant in the poultry industry, due to the phasing out of antibiotic growth promoters (AGP) worldwide. Probiotics are “live microbes that, when administered in adequate amounts, confer a health benefit to the host” (FAO/WHO, 2002). Probiotics support a healthier digestive tract, due to varying modes of action, such as improving digestion, regulating intestinal microflora, improving gut barrier function, preventing gastrointestinal diseases, and modulating the immune system (Edens, 2003; Kabir, 2009; Gaggìa et al., 2010). The genus Bacillus confers several advantages such as survival in the harsh feed manufacturing processes, longer shelf life and robustness to the fluctuating conditions within the chicken gastrointestinal tract (GIT) (Cutting, 2011; Vasquez, 2016; Grant et al., 2018; Mingmongkolchai and Panbangred, 2018).
It has been shown that Bacillus spp. resulted in improved broiler growth rate and feed utilization efficiency (Gil De Los Santos et al., 2005; Sen et al., 2012; Harrington et al., 2016), They also enhance immune response (Yurong et al., 2005; Huang et al., 2008), confer protection against pathogens (La Ragione et al., 2001; La Ragione and Woodward, 2003; Teo and Tan, 2005; Jayaraman et al., 2013), colonize the GIT of chickens (La Ragione and Woodward, 2003), and improve the histomorphology of intestinal villi (Samanya and Yamauchi, 2002; Al-Baadani et al., 2016). These effects endorse research into their use as natural alternatives to AGP products.
The ban on the use of AGPs in poultry production has challenged the industry need to prevent poultry diseases. Although zoonotic diseases, such as salmonellosis and listeriosis, have negative effects on the industry, losses in broiler production is most severe due to Clostridium perfringens infections, which causes acute necrotic enteritis (NE), resulting in production losses (Immerseel et al., 2004). Casewell et al. (2003) correlated an increased incidence of NE with the banning of AGPs. This pathogen also causes sub-clinical necrotic enteritis (SNE), presenting as necrotic dermatitis, cholangiohepatitis, gizzard erosion, and lower production efficiency (Lovland and Kaldhusdal, 2001; Hafez, 2011; M'Sadeq et al., 2015).
Feed contributes up to 70% of broiler production costs; therefore, nutritionists continuously strive to decrease feed costs by incorporating cheaper raw materials with lower metabolizable energy (ME) values (Świątkiewicz and Koreleski, 2008; Mendes et al., 2013). To address this challenge, exogenous enzymes are added to feed to improve digestibility (Choct, 2006) with varying degrees of success (Leeson et al., 1996). Probiotics may provide a solution to this problem by enhancing the digestibility of lower ME feeds (Torres-Rodriguez et al., 2005).
When developing probiotics, the use of microorganisms indigenous to poultry is preferred, as it not only gives the best chance of probiotic survival and colonization of the GIT but also alleviates many of the challenges associated with the inclusion of foreign bacteria (Dunne et al., 2001; Schrezenmeir and de Vrese, 2001). This study evaluates the in-vivo efficacy of a novel multi-strain probiotic comprising of Bacillus spp. on growth performance and intestinal histomorphological parameters of broilers fed diets containing 2 ME levels. The broilers were exposed to C. perfringens at sub-clinical levels to challenge the birds such that the probiotic effect could be thoroughly evaluated.
MATERIALS AND METHODS
Ethics Approval
This study was approved by the Animal Ethics Committee of the University of Pretoria (South Africa) (EC005-18). Further approval from the Council of Scientific and Industrial Research (CSIR) (Pretoria, South Africa) Research Ethics Committee (248/2018) was granted prior to the commencement of the trial.
Bird Husbandry
A total of 1,656 Ross 308 broiler chicks (males) were obtained from a commercial hatchery where they were vaccinated against New Castle Disease. Chicks were delivered to the 96-pen broiler test facility (Hillcrest, University of Pretoria, South Africa) the morning after hatched (day 1). The temperature and ventilation of the broiler test facility were constantly regulated by an automatic climate controller (Model DOL 539, Skov, Denmark) for the duration of the trial. Environmental temperatures were gradually decreased weekly from day 0 to day 21 and then maintained according to breeder guidelines (Aviagen, 2007) and litter temperature was checked twice daily. Each pen (2.25 m2) was covered with clean pine shavings and fitted with 1 tube feeder and 5 nipple drinkers. Chicks had easy access to feed and potable water ad libitum throughout the entire trial period. They were exposed to 23 h of light up to 7 D of age, then from there onwards they received 8 h of darkness continuously in a 24-h period. All birds orally received booster vaccines against New Castle Disease and Gumboro respectively, at day 14 and day 21 of age.
Experimental Design
A 35-D trial was conducted under challenged conditions by infection with C. perfringens. Upon arrival at the test facility, broiler chicks were randomly allocated to 6 treatment groups, with 12 pen replicates for each treatment and 23 chicks per pen. Pen replications were arranged according to a randomized complete block design, with each of the 12 blocks containing 1 replicate per treatment. The average body weight (BW) of chicks at day 0 was 40 g and the weight did not differ significantly between any of the experimental groups.
Subclinical Necrotic Enteritis (SNE) Challenge
All broilers were subjected to conditions previously described (Pedersen et al., 2008; Lensing et al., 2010; M'Sadeq et al., 2015) for creating SNE. At an age of 10 D, the broilers received a coccidial vaccine containing live, attenuated oocysts of Eimeria acervulina, E. brunetti, E. maxima, E. mitis, E. necatrix, E. praecox, and E. tenella at 10 times the dosage prescribed by the manufacturer (Paracox-8, Schering-Plough, Kenilworth, NJ). On day 14, all birds were orally administered 1 mL liver broth containing 1 × 108 CFU mL−1C. perfringens Type A. The C. perfringens culture was obtained from Thermo Fischer Scientific (USA) and grown at a biosafety level II facility at Deltamune (Centurion, South Africa).
Probiotic and Dietary Treatments
A 6-strain probiotic containing 4 Bacillus subtilis (CPB 011, CPB 029, HP 1.6, and D 014) and 2 Bacillus velezensis (CBP 020 and CPB 035) strains were previously developed and provided by the CSIR (Biosciences, Pretoria, South Africa). The Bacillus spp. were selected for inclusion in the test probiotic product on the basis of their overall performance with regards to chicken probiotic attributes including growth and survival in the pH of the proventriculus and of the intestine, survival in bile salts, production of extracellular enzymes (amylase, protease, cellulose, and xylanase), the adherence to epithelial cells and possession of antagonistic activity against selected common poultry pathogens (Escherichia coli, Salmonella enteritis, Listeria monocytogenes, and C. perfringens). The selection criteria for the microorganisms contained in the test probiotic was based on a comprehensive review of previous studies (Ehrmann et al., 2002; Barbosa et al., 2005; Taheri et al., 2009; Wolfenden et al., 2010). The test probiotic efficacy was compared to a commercially available product containing a B. subtilis strain supplied by the manufacturer. The probiotics were added in powdered form to the mash feed at 50 g and 100 g per ton of feed for the commercial probiotic (specified as 2 × 109 CFU g−1) and test probiotic (specified as 1 × 109 CFU g−1), respectively. The viability of the Bacillus spp. in mixed feed was assessed using the standard plate count method. Prior to analysis, the feed samples were first subjected to heat treatment (55°C for 20 min) followed by homogenization using a bench top homogenizer (T 25 digital ULTRA-TURRAX, IKA, Germany) to ensure that only Bacillus spores were counted.
This study was conducted on broilers reared on rations containing 2 different levels of ME, and either no probiotics or one of the 2 probiotic products, in a 2 × 3 factorial arrangement. ME content of the standard diet was formulated to meet or exceed recommendations for Ross 308 broilers. The reduced ME diet contained 98% ME of that of the standard diet. Probiotics were included in the diets from commencement to termination of the trial.
A 4 phase feeding program was followed: pre-starter (fed from day 1 to 7), starter (fed from day 7 to 14), grower (fed from day 17 to 28), and finisher (fed from day 28 to 35). The feed ingredients and formulated nutrient composition of the diets are shown in Table 1. The treatments are as follows: 1) Reduced ME diet with no probiotic (negative control), 2) Reduced ME diet + commercial probiotic (positive control), 3) Reduced ME diet + test probiotic, 4) Standard ME diet with no probiotic (negative control), 5) Standard ME diet + commercial probiotic (positive control), and 6) Standard ME diet + test probiotic.
Table 1.
Feed ingredient (%) and calculated nutrient composition (%) of the basal diets.
| Pre-starter |
Starter |
Grower |
Finisher |
|||||
|---|---|---|---|---|---|---|---|---|
| Feed ingredient | Standard ME | Reduced ME | Standard ME | Reduced ME | Standard ME | Reduced ME | Standard ME | Reduced ME |
| Yellow maize | 60.7 | 60.8 | 61.7 | 63.4 | 67.8 | 68.3 | 71.1 | 71.7 |
| Soya oil cake (46.5%) | 30.3 | 29.2 | 30.6 | 29.7 | 24 | 21.3 | 19.8 | 17.4 |
| Sunflower oil cake (36%) | 3 | 4 | 2.5 | 3.2 | 4 | 7.2 | 5 | 7.9 |
| Lysine (78%) | 0.276 | 0.293 | 0.189 | 0.204 | 0.201 | 0.246 | 0.314 | 0.353 |
| Methionine (98%) | 0.261 | 0.257 | 0.213 | 0.209 | 0.173 | 0.164 | 0.199 | 0.19 |
| Threonine (98%) | 0.06 | 0.062 | 0.018 | 0.02 | 0.006 | 0.011 | 0.051 | 0.055 |
| Soya oil (Degummed) | 1.061 | 0 | 1.492 | 0 | 1.046 | 0 | 1.091 | 0 |
| Wheat bran | 1 | 2.2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Limestone | 1.64 | 1.64 | 1.64 | 1.64 | 1.44 | 1.43 | 1.29 | 1.28 |
| Mono-di-calcium phosphate | 0.81 | 0.79 | 0.82 | 0.81 | 0.51 | 0.5 | 0.31 | 0.3 |
| Salt (fine) | 0.335 | 0.326 | 0.366 | 0.359 | 0.183 | 0.163 | 0.143 | 0.126 |
| Sodium bicarbonate | 0.18 | 0.193 | 0.137 | 0.147 | 0.322 | 0.35 | 0.379 | 0.404 |
| Axtra Phy10000 P (100 g/t) | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| Salinomycin (12%) | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
| Vitamin and mineral premix | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| Calculated nutrient levels | ||||||||
| Dry matter | 89.22 | 89.13 | 89.22 | 89.06 | 89.03 | 89.00 | 88.97 | 88.92 |
| ME poultry (ckal/kg) | 2,849 | 2,763 | 2,894 | 2,808 | 2,930 | 2,844 | 2,971 | 2,885 |
| Crude protein | 20.28 | 20.32 | 20.01 | 20.00 | 18.01 | 18.01 | 16.89 | 16.91 |
| Crude fat | 4.20 | 3.16 | 4.63 | 3.19 | 4.25 | 3.19 | 4.32 | 3.22 |
| Crude fiber | 4.24 | 4.50 | 4.08 | 4.22 | 4.20 | 4.70 | 4.26 | 4.72 |
| Ash | 5.60 | 5.61 | 5.59 | 5.57 | 4.70 | 4.68 | 4.16 | 4.15 |
| Calcium | 0.92 | 0.92 | 0.92 | 0.92 | 0.79 | 0.79 | 0.70 | 0.70 |
| Phosphorous (total) | 0.52 | 0.53 | 0.52 | 0.52 | 0.44 | 0.45 | 0.39 | 0.40 |
| Sodium | 0.18 | 0.18 | 0.18 | 0.18 | 0.16 | 0.16 | 0.16 | 0.16 |
| Chloride | 0.30 | 0.30 | 0.30 | 0.30 | 0.20 | 0.20 | 0.20 | 0.20 |
| Potassium | 0.85 | 0.85 | 0.84 | 0.84 | 0.74 | 0.73 | 0.68 | 0.67 |
| Lysine | 1.25 | 1.25 | 1.18 | 1.18 | 1.04 | 1.03 | 1.03 | |
Feed Manufacture and Analysis
Feed was manufactured by SimpleGrow Agricultural Services (Pretoria, South Africa). The Official Methods of Analysis (AOAC, 2000) were followed for analyses of dry matter (no. 934.01), ash (no. 942.05), crude protein (Leco-Dumas Method no. 986.06), crude fiber (no. 962.09), ether extract (number 920.39), calcium (no. 935.13), and phosphorus (no. 965.17) of the 2 basal diets from each of the 3 feeding phases. The nutrient composition of the feed is shown in Table 2. The apparent ME was calculated using the following equation, which was described by Alvarenga et al. (2015):
Table 2.
Analysed nutrient composition of the basal diets (% on as is basis).
| Pre-starter |
Starter |
Grower |
Finisher |
|||||
|---|---|---|---|---|---|---|---|---|
| (Standard ME) | (Reduced ME) | (Standard ME) | (Reduced ME) | (Standard ME) | (Reduced ME) | (Standard ME) | (Reduced ME) | |
| Dry matter | 89.23 | 89.33 | 89.55 | 88.65 | 88.73 | 88.58 | 88.60 | 88.25 |
| AMEn (kcal/kg) | 2,600 | 2,482 | 2,649 | 2,555 | 2,754 | 2,658 | 2,831 | 2,750 |
| Crude protein | 20.94 | 21.61 | 20.83 | 20.77 | 18.59 | 18.71 | 18.09 | 18.72 |
| Crude fat | 3.23 | 2.45 | 4.10 | 2.47 | 3.35 | 2.20 | 3.52 | 2.55 |
| Crude fiber | 4.71 | 4.78 | 4.76 | 4.29 | 4.34 | 4.86 | 4.05 | 4.55 |
| Ash | 5.30 | 5.65 | 5.30 | 5.28 | 4.43 | 4.45 | 4.08 | 4.03 |
| Calcium | 0.733 | 0.763 | 0.717 | 0.727 | 0.683 | 0.655 | 0.535 | 0.485 |
| Phosphorous (total) | 0.527 | 0.528 | 0.550 | 0.527 | 0.460 | 0.478 | 0.396 | 0.412 |
Max CV < 20% between analyzed and calculated nutrient composition.
AMEn = Nitrogen-corrected apparent metabolizable energy (as is basis).
Broiler Performance
BW and feed intake (FI) were recorded weekly (day 0, 7, 14, 21, 28, and 35) as an average per pen. Weights of all the birds that died throughout the trial period were recorded. Feed conversion ratio (FCR) adjusted for mortalities was calculated for each pen as FI (g)/BW gain (g) over a specified period of time. Mortality rate per treatment was calculated for the entire trial period.
Liver Weights, Lesion Scoring, and Histomorphology of the Gastrointestinal Tract
All birds were weighed individually on day 35 and then 2 birds with a BW closest to the average of their respective pen were selected for sampling. Birds were sacrificed humanely by cervical dislocation and the GIT and liver were carefully removed from each bird for further investigation.
Relative Liver Weight
The livers of the birds were weighed on a precision balance (Model 1502e, Adam Equipment PGW, United Kingdom). Liver weight was expressed as the percentage of the birds' BW, and a pen average was calculated.
Lesion Scoring
The entire GIT was carefully removed and opened by longitudinal incision. It was then examined for lesions possibly caused by NE and subsequently scored based on the severity of the lesions presented, using the guidelines described by Shojadoost et al. (2012).
Histomorphology of the Gastrointestinal Tract
Samples (2 cm in length) were taken from the duodenum, jejunum, and ileum. Each section was carefully rinsed with 10% (v·v−1) phosphate buffered formalin (Merck, Germany) to remove digesta and stored in sample bottles containing formalin. Samples were embedded in paraffin (Merck, Germany), transverse sections of approximately 4 to 5 µm in thickness were cut and every 10th section was collected. These sections were transferred to microscope slides and stained with haematoxylin and eosin (Merck, Germany). Slides were viewed at a 5× magnification with an AXIO Imager M2 light microscope (Zeisis, Germany). Images of 10 randomly selected intact villi per section were taken and villi height and crypt depth measured using Image J software. Villus height was measured from the tip of the villus to the villus-crypt junction, and crypt depth was defined as the depth of the invagination between adjacent villi. Villi height (µM) to crypt depth (µM) ratio (VCR) was calculated.
Statistical Analysis
Statistical analysis was performed using the Statistical Analysis System statistical software program (Statistical Analysis System, 2017). The significance between treatments was determined by an analysis of variance with the general linear model. Means and standard error of means for the different treatments were calculated and differences (P < 0.05) between means were determined by Fischer's test (Ryman, 2006) at the 95% confidence level. In all cases the level of statistical significance was P < 0.05. Differences between treatments for mortalities were calculated with a chi square.
RESULTS
Analysis of Feed
The nutrient analyses of the feeds produced for this study are presented in Table 2. The resultant nutritional values for all parameters were similar to the theoretically calculated values as per Table 1 (max CV < 20%).
Broiler Performance
Within the treatment groups that received the standard energy diets there were no significant differences observed with BW and FI (Table 3). However, the broiler groups supplemented with either the commercial or the test probiotics resulted in significantly improved FCRs compared to the un-supplemented group (without probiotics) after day 35. Furthermore, the group supplemented with the test probiotic resulted in significantly improved FCR over the un-supplemented control group, consistently from 21 D onwards.
Table 3.
Growth performance of broilers fed diets containing either a standard (100%) or reduced (98%) metabolizable energy level with or without Bacillus spp. based probiotics.
| Standard ME |
Reduced ME |
P value |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Without probiotics | Commercial probiotic | Test probiotic | Without probiotics | Commercial probiotic | Test probiotic | SEM | Probiotic treatment | ME | Energy × probiotic treatment | |
| Body weight (g) | ||||||||||
| Day 1 | 40.87 | 40.69 | 40.5 | 41.05 | 41.05 | 40.65 | 0.23 | 0.242 | 0.233 | 0.883 |
| Day 7 | 177.8 | 178.9 | 179.5 | 177.6 | 177.1 | 177.4 | 1.75 | 0.904 | 0.340 | 0.844 |
| Day 14 | 448.5 | 451.3 | 450.7 | 443.1 | 442. 6 | 446.8 | 5.04 | 0.845 | 0.152 | 0.885 |
| Day 21 | 827.4 | 820.5 | 821.5 | 818.1 | 803.1 | 817.4 | 9.54 | 0.635 | 0.122 | 0.756 |
| Day 28 | 1,366a,b | 1,363a,b | 1,388a | 1,349a,b | 1,324b | 1,367a,b | 18.01 | 0.170 | 0.087 | 0.808 |
| Day 35 | 1,906a,b | 1,940a,b | 1,957a,b | 1,881b | 1,892a,b | 1,974a | 30.66 | 0.065 | 0.470 | 0.564 |
| Feed intake (g/bird) | ||||||||||
| Day 0-7 | 174.4 | 176.5 | 172.7 | 177.7 | 180.6 | 174.9 | 2.78 | 0.239 | 0.167 | 0.941 |
| Day 0 to 14 | 522.1 | 520.2 | 514.3 | 527.5 | 530.3 | 526.1 | 6.46 | 0.963 | 0.488 | 0.771 |
| Day 0 to 21 | 1,222a | 1,164b | 1,160b | 1,116b | 1,126b | 1,121b | 19.7 | 0.166 | 0.001 | 0.312 |
| Day 0 to 28 | 2,218a | 2,163a | 2,181a | 2,021b | 2,026b | 2,050b | 30.5 | 0.682 | <0.0001 | 0.505 |
| Day 0 to 35 | 3,203a | 3,144a,c | 3,176a,d | 2,992b | 2,977b | 3,065b–d | 43.9 | 0.393 | <0.0001 | 0.526 |
| Feed conversion ratio (g: g) | ||||||||||
| Day 0 to 7 | 1.27a,b | 1.28a,b,c | 1.22b | 1.30a,c | 1.33c | 1.28a,c | 0.02 | 0.023 | 0.005 | 0.732 |
| Day 0 to 14 | 1.58a,b,c | 1.53a,b | 1.50a | 1.59b,c | 1.62c | 1.58a,b,c | 0.03 | 0.757 | 0.043 | 0.599 |
| Day 0 to 21 | 1.72b | 1.62a | 1.63a | 1.60a | 1.61a | 1.58a | 0.03 | 0.074 | 0.137 | 0.120 |
| Day 0 to 28 | 1.78d | 1.72c,d | 1.69b,c | 1.64a,b | 1.65a,b | 1.63a | 0.02 | 0.142 | <0.0001 | 0.099 |
| Day 0 to 35 | 1.78c | 1.69b | 1.68b | 1.69b | 1.68a,b | 1.61a | 0.02 | 0.002 | 0.0003 | 0.263 |
Values without a common superscript in the same row differ significantly (P < 0.05, n = 12).
Within the treatment groups that received the reduced ME diets, the test probiotic treatment resulted in a significantly higher final BW (day 35) compared to the negative control (P = 0.03) and was 4.1% higher than the commercial probiotic, but this difference was not significant (Table 3). FCR within the reduced ME diets were positively influenced by supplementation of the test probiotic from day 28 onwards, resulting in significantly better FCR than the negative control (P < 0.05). The FCR of the test probiotic was also 4.3% better than the commercial probiotic but the difference was not significant.
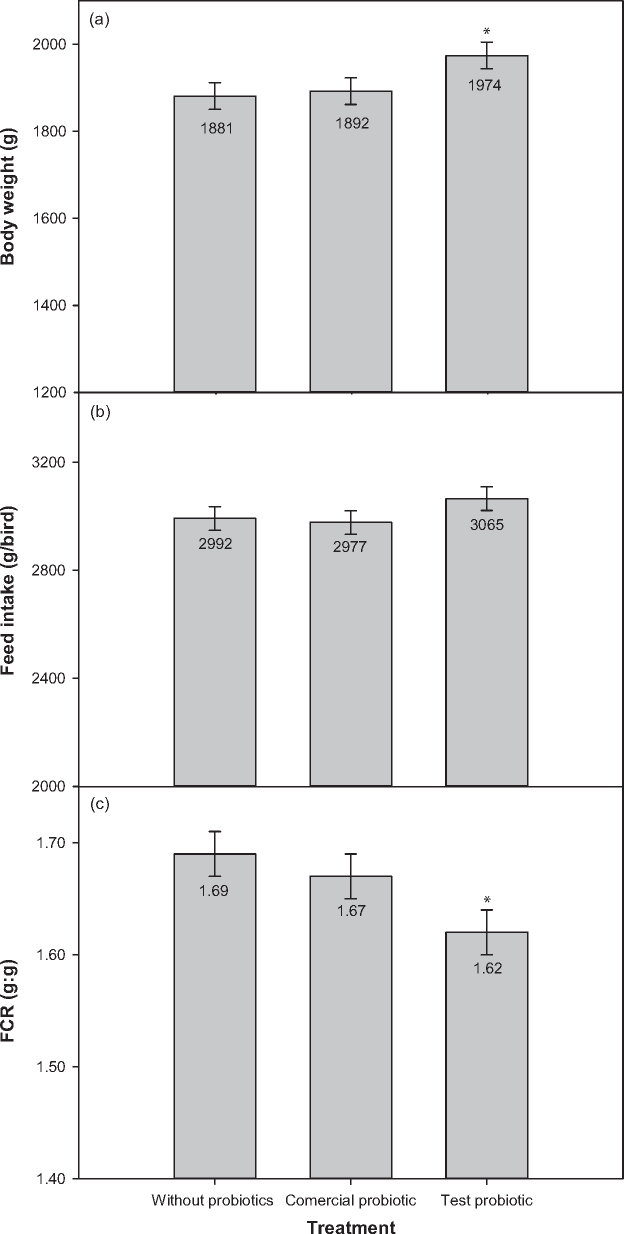
The benefit of probiotic supplementation on broilers fed with the reduced ME diet is illustrated in Figure 1. In all of the responses (BW, FI, and FCR), the test probiotic supplementation resulted in improved performance than the un-supplemented and commercial probiotic treatment groups, but was only significantly better than the un-supplemented group for final BW and FCR.
Figure 1.
The broiler performance of birds fed the reduced ME diet supplemented with or without probiotics. a) The body weights of birds at day 35. b) The cumulative feed intake from day 0 to 35. c) The cumulative mortality adjusted FCR from day 0 to 35.* are represented as significant difference (P < 0.05) over the control.
The total mortality rate for all 6 experimental groups was only 3.7% for the duration of the trial and no effect of treatment could be correlated to mortality.
A significant effect of probiotic treatment was noted for FCR at day 35 (Table 3). Irrespective of probiotic supplementation, a suppressing effect on FI from day 21 onwards was observed, when broilers were fed a reduced ME diet in comparison to the standard ME diet (P < 0.0001). No significant interaction effects between ME level and probiotic treatment were observed.
Lesion Scoring and Liver Weight
In broilers fed the standard ME diets, the cumulative lesion scores across the 3 regions of the small intestine (duodenum, jejunum, and ileum) were 22, 17, and 19 for the un-supplemented control, the commercial probiotic and the test probiotic treatments, respectively. Similarly, in the reduced ME diets the cumulative lesion counts were 32, 12, and 26, respectively. No significant differences were found in the numbers and severity of lesions between any of the treatment groups.
For both levels of dietary ME, the livers of broilers that received the test probiotic weighed significantly less than those of both the commercial probiotic and un-supplemented groups (Table 4). Irrespective of probiotic treatment, the ME content of the diets had a highly significant (P < 0.0001) effect on the liver weight of broilers when expressed as a percentage of BW. The livers of broilers that received the reduced ME diets were heavier than those fed the standard ME diet.
Table 4.
Intestinal villi histomorphology and liver weight of broilers fed diets containing either a standard (100%) or reduced (98%) metabolizable energy level with or without Bacillus spp. based probiotics.
| Standard ME |
Reduced ME |
P value |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Without probiotics | Commercial probiotic | Test probiotic | Without probiotics | Commercial probiotic | Test probiotic | SEM | Probiotic treatment | ME | Energy × probiotic treatment | |
| Duodenum | ||||||||||
| Villi height (μM) | 1,634b | 1,834a,b | 1,807b,c | 1,664b | 1,711b | 1,936a,c | 73.9 | 0.015 | 0.844 | 0.238 |
| Crypt depth (μM) | 195.3b | 184.0b,c | 200.2b | 252.6a | 165.4c | 201.8b | 9.43 | <0.0001 | 0.088 | 0.0006 |
| Villi: crypt (μM: μM) | 8.430c | 10.24a,d | 9.138c,d | 6.713b | 10.52a | 9.763a,c | 0.49 | <0.0001 | 0.499 | 0.042 |
| Jejunum | ||||||||||
| Villi height (μM) | 1,184a,b | 1,166a,b | 1,275a | 1,093b | 1,179a,b | 1,243a,b | 59.7 | 0.126 | 0.459 | 0.684 |
| Crypt depth | 174.1b | 182.9b | 195.5a,b | 219.0a | 133.5c | 171.1b | 11.9 | 0.007 | 0.325 | 0.0006 |
| Villi: crypt (μM: μM) | 6.853a | 6.883a | 6.524a | 5.108b | 8.925c | 7.477a | 0.45 | 0.0003 | 0.258 | 0.0003 |
| Ileum | ||||||||||
| Villi height (μM) | 775.3a,b | 801.1a,b | 872.2a | 758.5b | 818.3a,b | 857.0a,b | 39.4 | 0.054 | 0.880 | 0.889 |
| Crypt depth (μM) | 176.8 | 151.9 | 179.1 | 195.9 | 158.2 | 190.1 | 17.6 | 0.144 | 0.401 | 0.936 |
| Villi: crypt (μM: μM) | 4.437b,c | 5.493a,d | 4.953c,d | 3.915b,c | 6.133a | 4.895b,d | 0.36 | 0.0002 | 0.946 | 0.281 |
| Liver weight (%) | 2.32b | 2.44b,d | 2.06c | 2.61a,d | 2.68a | 2.35b | 0.06 | <0.0001 | <0.0001 | 0.930 |
Values without a common superscript in the same row differ significantly (P < 0.05).
Data represents means based on 12 replicates per treatment.
Villi: crypt = villi height to crypt depth ratio.
Liver weight expressed as percentage of body weight.
Histomorphology of the Gastrointestinal Tract
In broilers fed the standard ME diet, there was no significant difference in villi height or crypt depth of the small intestine between any of the treatments (Table 4). However, broilers fed the standard ME diet supplemented with the commercial probiotic resulted in significantly higher VCR (µM: µM) within the duodenum and ileum when compared to the negative control. Similarly, the test probiotic treatment also resulted in higher ratios, but the differences were not significant.
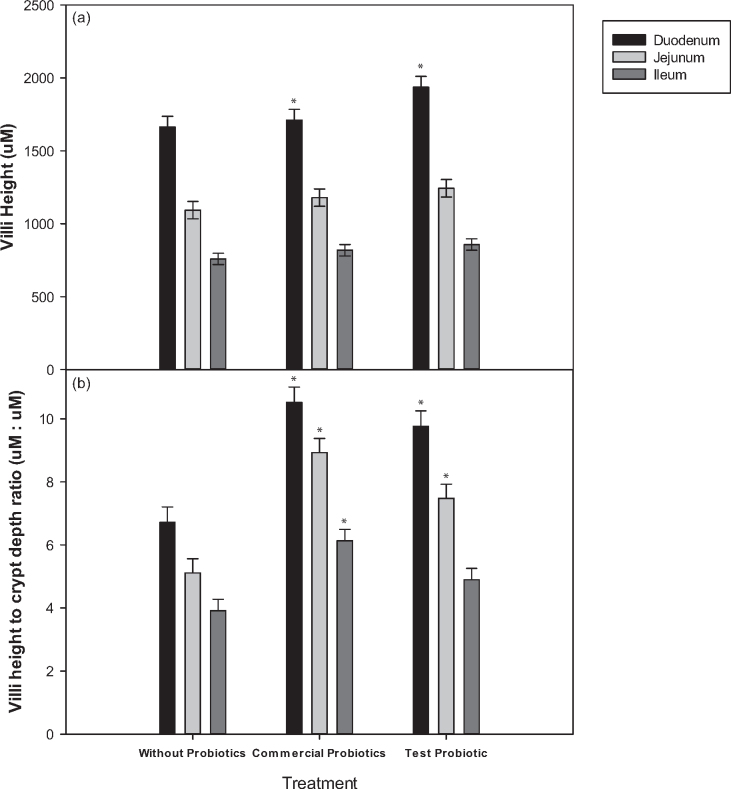
Broilers fed the reduced ME diets containing the test probiotic, displayed significantly longer duodenal villi when compared to both the un-supplemented (P = 0.01) and the commercial probiotic (P = 0.03) groups (Figure 2). Both duodenal and jejunal crypt depths of broilers fed either of the probiotics supplemented in the reduced ME diets were significantly shallower than in the un-supplemented treatment group. Additionally, the VCR in both the duodenum and the jejunum were significantly higher for the broilers that received either one of the probiotic treatments under reduced ME conditions (Table 4 and Figure 2).
Figure 2.
The histomorphology of the small intestine of broilers fed the reduced ME diet supplemented with or without probiotics, showing effects on the duodenum, jejunum, and ileum. a) The villi height. b) The villi height to crypt depth ratio. * represents significant difference (P < 0.05) over the control.
DISCUSSION
The main aim of the present trial was to evaluate a novel multi-strain Bacillus based probiotic in an AGP-free environment and to compare the efficacy to an un-supplemented control. Furthermore, a commercially available Bacillus based benchmark product was used as a positive control.
When the test probiotic product was evaluated within standard ME diets against the un-supplemented control, the test probiotic resulted in a significantly improved FCR (P < 0.001). Although not significantly different from the commercial probiotic, the absolute FCR value for the test probiotic was also better (Table 3). The improved FCR in both the test and commercial probiotic supplemented groups was ascribable predominantly to higher body mass gain when compared to the un-supplemented control, indicating improved nutrient and energy utilization due to probiotic supplementation. Other researchers have also shown the positive effect on FCR, when studying Bacillus based probiotics supplemented into standard ME basal diets (Mountzouris et al., 2010; Liu et al., 2012; Zhang et al., 2013; Zaghari et al., 2015; Li et al., 2016).
Interesting, this study showed a significant improvement in FCR, but the differences in FI and BW were insignificant, possibly due to the nutrient sufficiency of the standard ME diet coupled to the optimal environmental conditions of the field trial. These test conditions potentially attenuate the full probiotic effects, which are more likely to be realized under challenging commercial broiler production conditions. Infante-Rodríguez (2016) published similar findings, showing that broilers receiving a standard energy diet, under ideal growth conditions, showed a significant improvement in FCR compared to diets with a moderate decrease in ME, but not in BW, for similar reasons.
In the groups fed the reduced ME diets, the test probiotic performed better (BW and FCR, Table 3) than within standard ME diets, when compared to the un-supplemented control. There was no significant difference in the FI between any of the treatments (CV < 1.5% at 35 D), indicating that the main contributor to enhanced FCR was actually BW gain. This can thus infer that the test probiotic enhanced the efficiency of nutrient adsorption and utilization, predominantly ascribable to the production of exogenous enzymes by the test probiotic. The test probiotic comprised of strains that displayed a high diversity and production level of digestion enzymes (amylase, protease, cellulose, and xylanase) (U. Ramlucken, CSIR, Pretoria, Gauteng, personal communication). Bacilli are known to produce numerous digestive enzymes that allow for the efficient breakdown of feed into smaller molecules thus improving the efficiency of absorption and assimilation (Latorre et al., 2016). Another probiotic characteristic that may have attributed to improved performance could have been superior survival and persistence in the GIT, which enhances the probiotic effect.
When comparing different ME diets, the probiotic effects on broiler performance was enhanced in the reduced ME diets. The reasons for this remain unclear; however, one of the probable causes is the greater impact of exogenous enzyme activity in lower energy diets, compared to diets that provide sufficient nutrients. Goodarzi Boroojeni et al. (2018) also concluded that the probiotic effects were more pronounced in nutrient-deficient diets, when testing a B. subtilis probiotic on broilers. Furthermore, the observations were consistent with previous findings by Harrington et al. (2016), who also reported that broilers supplemented with B. subtilis, resulted in improved final BW and FCR in reduced ME diets (2%). In their study, an economic saving of $0.018/kg BW gain was calculated when broilers were fed a B. subtilis probiotic incorporated into a reduced ME diet (2% ME reduction). Knap et al. (2011) showed that B. subtilis supplementation significantly improved FCR, but not BW, in reduced ME diets (4% ME reduction). This study further contributes to the existing body of evidence indicating commercial attractiveness of probiotic supplementation particularly in reduced ME diets.
In reduced ME diets, the duodenal villi height was significantly greater (P < 0.05) in broilers fed diets containing the test probiotic compared to the un-supplemented control. The resultant greater villi height, ascribable to probiotic treatment, increased the intestinal surface area and enabled more efficient absorption of available nutrients (Figure 2). This is potentially one of the contributing factors to the improved broiler performance (BW and FCR) (Figure 1). The improvements in gut morphology and exogenous enzyme activity are likely to be the major contributing factors to the improved FCR due to the test probiotic supplementation.
VCR was also significantly improved in the duodenal and jejunal compartments by supplementation of both the test and commercial probiotic when compared to the un-supplemented control. Longer villi and a greater VCR are indicators of a healthy GIT (Xu et al., 2003). Besides increasing the surface area for optimal nutrient absorption, these indicators of gut morphology are also directly associated with improved epithelial turnover and activation of cell mitosis (Samanya and Yamauchi, 2002). The crypt is responsible for the synthesis of villi and a deeper crypt indicates a higher demand for tissue turnover, which is an energy consuming process, resulting in poorer energy efficiency. Shorter villi and deeper crypts lead to poor nutrient absorption, presence of toxins, an increase of mucus secretion in the GIT, reduced disease resistance, and overall decreased broiler performance (Sen et al., 2012). The results from our study indicate improved gut health and contributing to enhanced broiler growth performance. These results correlate with other studies (Samanya and Yamauchi, 2002; Sen et al., 2012) who also reported similar gut morphology observations due to supplementation with Bacillus based probiotics.
Depression of broiler performance due to C. perfringens infection was not demonstrated in this study because inclusion of an uncontaminated control group in the same facility was not possible. However, judged by poor overall performance of all broilers challenged with this pathogen compared to breeder standards (Aviagen, 2007) and previously recorded performance for this genetic strain at the same facility, C. perfringens infection and its consequent SNE posed a mild challenge. Besides a reduction in broiler performance, subclinical C. perfringens infection may also cause enlargement and inflammation of the liver, typically associated with hepatitis or cholangiohepatitis (Hafez, 2011). Increased liver weight may be indicative of the presence of subclinical infection (Lovland and Kaldhusdal, 2001). The smaller livers in broilers fed diets supplemented with the test probiotic may indicate a higher resistance of these birds to the C. perfringens challenge. However, due to a lack of liver histopathology data in this study, we cannot, based solely on the liver weight of the birds, infer that the test probiotic reduced C. perfringens infection, but that there are potential health benefits indicated by significantly reduced liver weight in birds treated with the test probiotic. A coccidiostat was added to all treatments to safe guard against severe coccidiosis. In South Africa, the broiler industry currently supplements most broiler feed with both an AGP and a coccidiostat. For the purpose of this study we only replaced the AGP with the novel probiotic to keep the test feed as close to the commercial feed as possible. Future trials will evaluate the probiotic effect on exclusion of both AGP and coccidiostat.
The purpose of the study was not to specifically investigate the response of broilers to dietary ME concentration, nevertheless, some interesting effects of ME level on broiler performance were observed. Analyzed crude fat content followed the same pattern as formulated values for crude fat (Table 2), which confirmed the feeding of the respective groups with the intended ME levels. The standard ME diet was supplemented with soya oil to create the differences in dietary energy levels. Since, diets were formulated on an iso-protein basis, a higher CP: ME (g: ckal) ratio was expected for the reduced ME diets. Lower ME level resulted in a significantly higher FI from day 21 to slaughter. According to the theory of FI and growth proposed by Gous et al. (1999), broilers would attempt to grow at their genetic potential, and therefore would consume a given feed at a level that would allow them to grow to that genetic potential. It appears as if the standard ME diet with the lower CP: ME ratio was somewhat limiting in CP, which restricted growth rate to below genetic potential during the last 2 to 3 wk of the feeding trial, thus resulting in the difference observed. Burnham et al. (1992) showed that broilers increase FI in response to a limiting nutrient (CP) in the feed, in an attempt to obtain more of the limiting nutrient. The broilers in the standard ME (100%) group thus consumed higher levels of feed and thus energy, possibly causing higher levels of lipid deposition (not measured).
Reduced dietary ME, resulted in higher relative liver weight % (of bird BW) in comparison to the standard ME diets. The lower oil content in the reduced ME diet could have been a further contributory factor for liver enlargement and increased incidence of SNE. In contrast, the soya oil added to the standard ME diet could have somewhat protected the broilers against C. perfringens proliferation and restricted liver enlargement. Also in the standard ME diet, higher lipid intake may have contributed to the increased production of bile acids (Smits et al., 1998), which can elicit an antibacterial effect in the small intestine, thus attenuating the challenge from C. perfringens (Inagaki et al., 2006).
CONCLUSIONS
The multi-strain Bacillus probiotic product improved growth performance and generally had a positive effect on C. perfringens challenged-broiler well-being, indicated by gut and liver health observations. Furthermore, its probiotic effect appeared to be better than the commercial probiotic product. Some of the key driving factors for industry adoption include improved performance in cheaper reduced ME diets and better resistance to C. perfringens challenge. Our results further substantiate the attractiveness of multi-strain Bacillus probiotics as a replacement to other undesirable in-feed growth promoting and antibacterial additives. Future considerations include testing the novel probiotic under commercial conditions, as well as elucidating the modes of action of its functionality.
ACKNOWLEDGEMENTS
The authors would like to thank the Department of Science and Technology (Pretoria, South Africa) for funding. Further funding support was provided by the CSIR (Pretoria, South Africa), who also produced the microorganisms, which were productized by OptimusBio (PTY) Ltd (Pretoria, South Africa). The authors are also grateful to Ceva Animal Health (Pty) Ltd (Johannesburg, South Africa) for the sponsorship of the coccidial vaccine, Roelf Coertze (University of Pretoria, Pretoria, South Africa) for his contribution in the statistical analysis and the Department of Animal Science, (University of Pretoria, Pretoria, South Africa) for technical assistance during the trial.
REFERENCES
- Al-Baadani H., Abudabos A., Al-Mufarrej S., Alzawqari M. Effects of dietary inclusion of probiotics, prebiotics and synbiotics on intestinal histological changes in challenged broiler chickens. S. Afr. J. Anim. Sci. 2016;46:157–165. [Google Scholar]
- Alvarenga R., Rodrigues P., Zangeronimo M., Oliveira E., Mariano F., Lima E., Garcia A., Jr, Naves L., Nardelli N. Validation of prediction equations of energy values of a single ingredient or their combinations in male broilers. Asian-Australasian J. Anim. Sci. 2015;28:1335–1344. doi: 10.5713/ajas.14.0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AOAC . 9th rev. ed. AOAC; Maryland, USA: 2000. Official Method Of Analysisw. [Google Scholar]
- Aviagen R. P. B., 2007. Ross 308 Broiler Management Guide. Nutrition Supplement.
- Barbosa T.M., Serra C.R., La Ragione R.M., Woodward M.J., Henriques A.O. Screening for bacillus isolates in the broiler gastrointestinal tract. Appl. Environ. Microbiol. 2005;71:968–978. doi: 10.1128/AEM.71.2.968-978.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham D., Emmans G., Gous R. Isoleucine responses in broiler chickens. Interactions with leucine and valine. Br. Poult. Sci. 1992;33:71–87. doi: 10.1080/00071669208417445. [DOI] [PubMed] [Google Scholar]
- Casewell M., Friis C., Marco E., McMullin P., Phillips I. The European ban on growth-promoting antibiotics and emerging consequences for human and animal health. J. Antimicrob. Chemother. 2003;52:159–161. doi: 10.1093/jac/dkg313. [DOI] [PubMed] [Google Scholar]
- Choct M. Enzymes for the feed industry: past, present and future. World's Poult. Sci. J. 2006;62:5–16. [Google Scholar]
- Cutting S.M. Bacillus probiotics. Food Microbiol. 2011;28:214–220. doi: 10.1016/j.fm.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Dunne C., O'Mahony L., Murphy L., Thornton G., Morrissey D., O'Halloran S., Feeney M., Flynn S., Fitzgerald G., Daly C. In vitro selection criteria for probiotic bacteria of human origin: correlation with in vivo findings. Am. J. Clin. Nutr. 2001;73:386s–392s. doi: 10.1093/ajcn/73.2.386s. [DOI] [PubMed] [Google Scholar]
- Edens F. An alternative for antibiotic se in poultry: probiotics. Rev. Bras. Ciênc. Avíc. 2003;5:75–97. [Google Scholar]
- Ehrmann M., Kurzak P., Bauer J., Vogel R. Characterization of lactobacilli towards their use as probiotic adjuncts in poultry. J. Appl. Microbiol. 2002;92:966–975. doi: 10.1046/j.1365-2672.2002.01608.x. [DOI] [PubMed] [Google Scholar]
- FAO/WHO Guidelines For The Evaluation of Probiotics in Food. 2002. https://www.who.int/foodsafety/fs_management/en/probiotic_guidelines.pdf
- Gaggìa F., Mattarelli P., Biavati B. Probiotics and prebiotics in animal feeding for safe food production. Int. J. Food Microbiol. 2010;141:S15–S28. doi: 10.1016/j.ijfoodmicro.2010.02.031. [DOI] [PubMed] [Google Scholar]
- Gil De Los Santos J., Storch O., Gil-Turnes C. Bacillus cereus var. Toyoii and S accharomyces boulardii increased feed efficiency in broilers infected with S almonella enteritidis. Br. Poult. Sci. 2005;46:494–497. doi: 10.1080/00071660500181461. [DOI] [PubMed] [Google Scholar]
- Goodarzi Boroojeni F., Vahjen W., Männer K., Blanch A., Sandvang D., Zentek J. Bacillus subtilis in broiler diets with different levels of energy and protein. Poult. Sci. 2018;97:3967–3976. doi: 10.3382/ps/pey265. [DOI] [PubMed] [Google Scholar]
- Gous R., Moran E., Jr, Stilborn H., Bradford G., Emmans G. Evaluation of the parameters needed to describe the overall growth, the chemical growth, and the growth of feathers and breast muscles of broilers. Poult. Sci. 1999;78:812–821. doi: 10.1093/ps/78.6.812. [DOI] [PubMed] [Google Scholar]
- Grant A.Q., Gay C.G., Lillehoj H.S. Bacillus spp. as direct-fed microbial antibiotic alternatives to enhance growth, immunity, and gut health in poultry. Avian Pathol. 2018;47:339–351. doi: 10.1080/03079457.2018.1464117. [DOI] [PubMed] [Google Scholar]
- Hafez H. M., 2011. Enteric diseases of poultry with special attention to C, lostridium perfringens.
- Harrington D., Sims M., Kehlet A.B. Effect of B acillus subtilis supplementation in low energy diets on broiler performance. J. Appl. Poult. Res. 2016;25:29–39. [Google Scholar]
- Huang J.-M., La Ragione R.M., Nunez A., Cutting S.M. Immunostimulatory activity of bacillus spores. FEMS Immunol. Med. Microbiol. 2008;53:195–203. doi: 10.1111/j.1574-695X.2008.00415.x. [DOI] [PubMed] [Google Scholar]
- Immerseel F.V., Buck J.D., Pasmans F., Huyghebaert G., Haesebrouck F., Ducatelle R. Clostridium perfringens in poultry: an emerging threat for animal and public health. Avian Pathol. 2004;33:537–549. doi: 10.1080/03079450400013162. [DOI] [PubMed] [Google Scholar]
- Inagaki T., Moschetta A., Lee Y.-K., Peng L., Zhao G., Downes M., Ruth T.Y., Shelton J.M., Richardson J.A., Repa J.J. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc. Natl. Acad. Sci. 2006;103:3920–3925. doi: 10.1073/pnas.0509592103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infante-Rodríguez F., Salinas-Chavira J., Montaño-Gómez M., Manríquez-Nuñez O., González-Vizcarra V., Guevara-Florentino O., De León J.R. Effect of diets with different energy concentrations on growth performance, carcass characteristics and meat chemical composition of broiler chickens in dry tropics. SpringerPlus. 2016;5 doi: 10.1186/s40064-016-3608-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman S., Thangavel G., Kurian H., Mani R., Mukkalil R., Chirakkal H. Bacillus subtilis pb6 improves intestinal health of broiler chickens challenged with C lostridium perfringens-induced necrotic enteritis. Poult. Sci. 2013;92:370–374. doi: 10.3382/ps.2012-02528. [DOI] [PubMed] [Google Scholar]
- Kabir S. The role of probiotics in the poultry industry. Int. J. Mol. Sci. 2009;10:3531–3546. doi: 10.3390/ijms10083531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knap I., Kehlet A., Bente T. 2011. B. Subtilis-improved protein digestibility and equal performance in energy-reduced diets for broilers [conference poster]. Actes des 9èmes Journées de la Recherche Avicole, Tours, France, 29 et 30 mars 2011. 349–352.
- La Ragione R.M., Casula G., Cutting S.M., Woodward M.J. Bacillus subtilis spores competitively exclude Escherichia coli O78:K80 in poultry. Vet. Microbiol. 2001;79:133–142. doi: 10.1016/s0378-1135(00)00350-3. [DOI] [PubMed] [Google Scholar]
- La Ragione R.M., Woodward M.J. Competitive exclusion by B acillus subtilis spores of S almonella enterica serotype enteritidis and C lostridium perfringens in young chickens. Vet. Microbiol. 2003;94:245–256. doi: 10.1016/s0378-1135(03)00077-4. [DOI] [PubMed] [Google Scholar]
- Latorre J.D., Hernandez-Velasco X., Wolfenden R.E., Vicente J.L., Wolfenden A.D., Menconi A., Bielke L.R., Hargis B.M., Tellez G. Evaluation and selection of bacillus species based on enzyme production, antimicrobial activity, and biofilm synthesis as direct-fed microbial candidates for poultry. Front. Vet. Sci. 2016;3:95. doi: 10.3389/fvets.2016.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeson S., Caston L., Summers J. Broiler response to diet energy. Poult. Sci. 1996;75:529–535. doi: 10.3382/ps.0750529. [DOI] [PubMed] [Google Scholar]
- Lensing M., van der Klis J.D., Fabri T., Cazemier A., Else A.J. Efficacy of a lactylate on production performance and intestinal health of broilers during a subclinical C lostridium perfringens infection. Poult. Sci. 2010;89:2401–2409. doi: 10.3382/ps.2010-00942. [DOI] [PubMed] [Google Scholar]
- Li Y., Xu Q., Huang Z., Lv L., Liu X., Yin C., Yan H., Yuan J. Effect of B acillus subtilis cgmcc 1.1086 on the growth performance and intestinal microbiota of broilers. J. Appl. Microbiol. 2016;120:195–204. doi: 10.1111/jam.12972. [DOI] [PubMed] [Google Scholar]
- Liu X., Yan H., Lv L., Xu Q., Yin C., Zhang K., Wang P., Hu J. Growth performance and meat quality of broiler chickens supplemented with bacillus licheniformis in drinking water. Asian-Australasian J. Anim. Sci. 2012;25:682. doi: 10.5713/ajas.2011.11334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovland A., Kaldhusdal M. Severely impaired production performance in broiler flocks with high incidence of C lostridium perfringens-associated hepatitis. Avian Pathol. 2001;30:73–81. doi: 10.1080/03079450020023230. [DOI] [PubMed] [Google Scholar]
- M'Sadeq S.A., Wu S., Swick R.A., Choct M. Towards the control of necrotic enteritis in broiler chickens with in-feed antibiotics phasing-out worldwide. Anim. Nutr. 2015;1:1–11. doi: 10.1016/j.aninu.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes A., Ribeiro T., Correia B., Bule P., Macas B., Falcao L., Freire J., Ferreira L., Fontes C., Lordelo M. Low doses of exogenous xylanase improve the nutritive value of triticale-based diets for broilers. J. Appl. Poult. Res. 2013;22:92–99. [Google Scholar]
- Mingmongkolchai S., Panbangred W. Bacillus probiotics: an alternative to antibiotics for livestock production. J. Appl. Microbiol. 2018;124:1334–1346. doi: 10.1111/jam.13690. [DOI] [PubMed] [Google Scholar]
- Mountzouris K., Tsitrsikos P., Palamidi I., Arvaniti A., Mohnl M., Schatzmayr G., Fegeros K. Effects of probiotic inclusion levels in broiler nutrition on growth performance, nutrient digestibility, plasma immunoglobulins, and cecal microflora composition. Poult. Sci. 2010;89:58–67. doi: 10.3382/ps.2009-00308. [DOI] [PubMed] [Google Scholar]
- Pedersen K., Bjerrum L., Heuer O.E., Wong D.M.A.L.F., Nauerby B. Reproducible infection model for C lostridium perfringens in broiler chickens. Avian Dis. 2008;52:34–39. doi: 10.1637/7955-022307-Reg. [DOI] [PubMed] [Google Scholar]
- Ryman N. Chifish: a computer program testing for genetic heterogeneity at multiple loci using chi-square and Fisher's exact test. Mol. Ecol. Notes. 2006;6:285–287. [Google Scholar]
- Samanya M., Yamauchi K.-E. Histological alterations of intestinal villi in chickens fed dried B acillus subtilis var. Natto. Comp. Biochem. Physiol. A: Mol. Integr. Physiol. 2002;133:95–104. doi: 10.1016/s1095-6433(02)00121-6. [DOI] [PubMed] [Google Scholar]
- Schrezenmeir J., de Vrese M. Probiotics, prebiotics, and synbiotics—approaching a definition. Am. J. Clin. Nutr. 2001;73:361s–364s. doi: 10.1093/ajcn/73.2.361s. [DOI] [PubMed] [Google Scholar]
- Sen S., Ingale S., Kim Y., Kim J., Kim K., Lohakare J., Kim E., Kim H., Ryu M., Kwon I. Effect of supplementation of B acillus subtilis LS 1–2 to broiler diets on growth performance, nutrient retention, caecal microbiology and small intestinal morphology. Res. Vet. Sci. 2012;93:264–268. doi: 10.1016/j.rvsc.2011.05.021. [DOI] [PubMed] [Google Scholar]
- Shojadoost B., Vince A.R., Prescott J.F. The successful experimental induction of necrotic enteritis in chickens by C lostridium perfringens: a critical review. Vet. Res. 2012;43:74. doi: 10.1186/1297-9716-43-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits C., Veldman A., Verkade H., Beynen A. The inhibitory effect of carboxymethylcellulose with high viscosity on lipid absorption in broiler chickens coincides with reduced bile salt concentration and raised microbial numbers in the small intestine. Poult. Sci. 1998;77:1534–1539. doi: 10.1093/ps/77.10.1534. [DOI] [PubMed] [Google Scholar]
- Statistical Analysis Systems . SAS Institute Inc.; Cary, NC, USA: 2017. SAS User's Guide: Statistics Version 9.3. [Google Scholar]
- Świątkiewicz S., Koreleski J. The use of distillers dried grains with solubles (ddgs) in poultry nutrition. World's Poult. Sci. J. 2008;64:257–266. [Google Scholar]
- Taheri H., Moravej H., Tabandeh F., Zaghari M., Shivazad M. Screening of lactic acid bacteria toward their selection as a source of chicken probiotic. Poult. Sci. 2009;88:1586–1593. doi: 10.3382/ps.2009-00041. [DOI] [PubMed] [Google Scholar]
- Teo A. Y.-L., Tan H.-M. Inhibition of C lostridium perfringens by a novel strain of B acillus subtilis isolated from the gastrointestinal tracts of healthy chickens. Appl. Environ. Microbiol. 2005;71:4185–4190. doi: 10.1128/AEM.71.8.4185-4190.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Rodriguez A., Sartor C., Higgins S., Wolfenden A., Bielke L., Pixley C., Sutton L., Tellez G., Hargis B. Effect of aspergillus meal prebiotic (fermacto) on performance of broiler chickens in the starter phase and fed low protein diets. J. Appl. Poult. Res. 2005;14:665–669. [Google Scholar]
- Vasquez A. Bacillus species are superior probiotic feed-additives for poultry. J. Bacteriol. Mycol. Open Access. 2016;2 [Google Scholar]
- Wolfenden R., Pumford N., Morgan M., Shivaramaiah S., Wolfenden A., Tellez G., Hargis B. Evaluation of a screening and selection method for bacillus isolates for use as effective direct-fed microbials in commercial poultry. Int. J. Poult. Sci. 2010;9:317–323. [Google Scholar]
- Xu Z., Hu C., Xia M., Zhan X., Wang M. Effects of dietary fructooligosaccharide on digestive enzyme activities, intestinal microflora and morphology of male broilers. Poult. Sci. 2003;82:1030–1036. doi: 10.1093/ps/82.6.1030. [DOI] [PubMed] [Google Scholar]
- Yurong Y., Ruiping S., ShiMin Z., Yibao J. Effect of probiotics on intestinal mucosal immunity and ultrastructure of cecal tonsils of chickens. Arch. Anim. Nutr. 2005;59:237–246. doi: 10.1080/17450390500216928. [DOI] [PubMed] [Google Scholar]
- Zaghari M., Zahroojian N., Riahi M., Parhizkar S. Effect of B acillus subtilis spore (gallipro®) nutrients equivalency value on broiler chicken performance. Ital. J. Anim. Sci. 2015;14 [Google Scholar]
- Zhang Z., Cho J., Kim I. Effects of B acillus subtilis UBT-MO2 on growth performance, relative immune organ weight, gas concentration in excreta, and intestinal microbial shedding in broiler chickens. Livest. Sci. 2013;155:343–347. [Google Scholar]