Abstract
The liver is the main site of de novo lipogenesis in poultry, and hepatic lipid metabolism disorder will lead to excessive abdominal fat deposition or fatty liver disease, finally causing huge economic loss. The present study was conducted to investigate developmental changes in hepatic lipid metabolism of chicks from embryonic periods to the first week after hatching. Liver samples were collected from embryonic day 11 (E11) to the age of day 7 posthatch (D7) for lipid metabolism analysis. Hematoxylin–eosin and Oil Red O staining analysis showed that hepatic lipids increased gradually during embryonic period and declined posthatch; The sum of hepatic triglycerides and cholesterol reached the peak at E19 and D1 by ELISA analysis (P < 0.05). Acetyl-CoA carboxylase, fatty acid synthase, and acyl-CoA desaturase 1 mRNA expression in the liver were higher from E17 to D1 with the peak at E19 when compared with those at E13 and E15 (P < 0.05). Hepatic elongase of very long-chain fatty acids 6 and microsomal triglyceride transfer protein mRNA abundance were lower during embryonic periods but reached relative higher level after hatching (P < 0.05). On the contrary, hepatic carbohydrate response element binding protein (ChREBP), carnitine palmitoyltransferase 1, and peroxisome proliferators–activated receptor α expression were higher during embryonic periods but decreased posthatch (P < 0.05). The mRNA abundance of sterol-regulatory element binding protein 1c was the lowest at E13 and E15, then increased gradually from E17 to D1, while decreased from D3 to D7 little by little (P < 0.05). In summary, hepatic lipogenesis genes have different expression patterns during the embryonic periods and the first week of posthatch, which might be activated by ChREBP during embryonic periods; fatty acid oxidation was enhanced around the hatched day but declined posthatch. These findings will broaden the understanding of physiological characteristics and dynamic pattern about hepatic lipid metabolism in chicks.
Key words: chick, hepatic lipid metabolism, gene expression, embryonic period, posthatch
Introduction
For a long period of time, the genetic breeding goal of meat-type chickens was to improve the growth rate, but excessive abdominal fat deposition has also been accompanied because of its positive correlation with body weight (Moreira et al., 2018). Physiologically excessive fat has negative effects on feed efficiency, meat yield, and economic benefit, which is a pendent problem to be settled urgently. There exist differences in the sites of de novo lipogenesis for different animals; the predominant site is adipose tissue in ruminants (Ingle et al., 1972); both of liver and adipose tissue all have equal importance for rodents and rabbit (Chiluard, 1994); while in birds, the liver is considered as the main site for de novo fatty acid synthesis, accounting for 95% in chicks (Leveille et al., 1975). In other words, hepatic lipid metabolism is closely related to abdominal fat accumulation to some extent in poultry. It was reported that adipose tissue development during embryonic and early postnatal periods determines its growth process for the whole life in chicks (Ailhaud et al., 1992, Guo, 2011). On the other hand, during the last 7 D of embryonic period and the first few days of posthatch, 90% energy requirement for growth development was derived from lipid fatty acid oxidation (Noble and Cocchi, 1990). Therefore, it is essential for researcher to understand dynamic changes of hepatic lipid metabolism during the embryonic and starter periods of posthatch, which may broaden the understanding of physiological characteristics about hepatic lipid metabolism in chickens.
Moreover, fatty liver is 1 of metabolic diseases caused by lipid metabolism disorder in laying hens, and it is widespread especially in the period of high-egg-laying-rate (Trott et al., 2014). However, fatty liver is often neglected because its symptoms are unapparent and difficult to diagnose before death, which leads to huge financial losses in poultry industry (Shini, 2014). Many studies have established experimental models by dietary intervention in vivo (Rozenboim et al., 2016, Zhang et al., 2018, Gao et al., 2019) or by drug-induction in vitro (Matteo et al., 2010, Chen et al., 2018, Zhou et al., 2018) to carry out studies for mechanism exploration about lipid metabolism disorder. Our previous study has compared hepatic lipid contents among new-born, adult, and fatty liver chickens by hematoxylin–eosin (HE) and Oil Red O staining and found that hepatic characteristics were similar between new-born and fatty liver chickens, suggesting that the liver from new-born chicks might model fatty liver in early stages and be used in vitro to some extent (Liu et al., 2018). On the other hand, chicks have been widely used for human fatty liver disease study because of ∼70% homologous to human in their genetic makeup (Shi et al., 2014). The same as chicks, the main site of lipogenesis in human is also the liver, thus previous reports pointed that chicks would be a better model for human nonalcoholic fatty liver disease (NAFLD) (Ayala et al., 2009, Makovicky et al., 2011). Laying hens were ever used to identify potential plasma biomarkers for human NAFLD (Tsai et al., 2017). But detailed mechanism and sufficient evidences about fatty liver model using chicks for human NAFLD are rare.
Therefore, the objective of the current study was to determine developmental changes of hepatic genes expression pattern and lipid contents from embryonic periods to the first week after hatching, aiming to provide theoretical basis on physiological characteristics about hepatic lipid metabolism in chickens.
Materials and methods
Liver Sampling
Hatching embryos and Arbor Acre chicks used in the current study were bought from the Yangling Julong Poultry Industry Co. Ltd. (Yangling, China). At embryonic day 13 (E13), E15, E17, and E19, 7 eggs with similar size and weight were selected and taken out from the incubator every day. Chick embryos were obtained and weighted after removing surface liquid with a filter paper. Liver tissues were rinsed thoroughly with ice-cold phosphate-buffered saline to remove blood contamination on the surface and also weighted for the liver index calculation. About 1 cm3 liver samples were fixed in 4% formaldehyde for subsequent histological analysis using HE and Oil Red O staining. Livers were collected in centrifuge tubes and stored at −80°C. Likewise, at the age of 1, 3, 5, and 7 D after hatching (D1, D3, D5, D7), chicks and liver tissues were weighted and collected as described in embryonic sampling above. All animal procedures in the study were approved by the Committee for the Care and Use of Experimental Animal at Northwest A&F University (Shaanxi, China).
HE and Oil Red O Staining
After fixing, the liver samples were processed through a series of procedures including dehydration, paraffin embedding, sectioning, and staining. All these procedures were performed by Wuhan Goodbio Technology Co., Ltd. (Wuhan, China). Stained sections were observed for lipid analysis using the microscope.
Triglyceride and Cholesterol Detection
Liver tissues were firstly defrosted, washed, and homogenized together with phosphate-buffered saline. Then, the supernatant was collected after centrifuging at 3,000 rpm for 15 min at 4°C to examine triglyceride (TG) and total cholesterol (TC) contents using commercial kits (Enzyme-linked Biotechnology Co., Ltd, Shanghai, China) based on kits instructions. Meanwhile, protein level was also determined using BCA Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA). The TG and TC contents were expressed by mmol/g of protein.
Gene Expression
Total RNA extraction and cDNA synthesis in liver were performed according to reagent protocols using TRIzol and Primer Script RT Reagent Kits (TaKaRa, Dalian, China). Genes relative expression was quantified by real-time quantitative PCR. The assay was carried out via SYBR Premix Ex Taq kit (TaKaRa) on the IQ5 (Bio-Rad, Hercules, CA). Detailed reaction system was referred to our previous publishment (Liu et al., 2017). Primer sequences used in the current study were all obtained from GenBank and shown in Table 1. All samples were run in triplicate, and the average cycle threshold (Ct) values were calculated for quantification using the 2−ΔΔCt method (Livak and Schmittgen, 2001).
Table 1.
Forward and reverse primer sequences for PCR analysis.
| Gene | Accession number | Primer sequences, 5′ to 3′ | Product size, bp |
|---|---|---|---|
| β-actin | L08165 | F: ATTGTCCACCGCAAATGCTTC | 113 |
| R: AAATAAAGCCATGCCAATCTCGTC | |||
| ACC | J03541 | F: GCTTCCCATTTGCCGTCCTA | 185 |
| R: GCCATTCTCACCACCTGATTACTG | |||
| FAS | J03860 | F: TTTGGTGGTTCGAGGTGGTA | 215 |
| R: CAAAGGTTGTATTTCGGGAGC | |||
| CPT1 | AY675193 | F: TAGAGGGCGTGGACCAATAA | 229 |
| R: TGGGATGCGGGAGGTATT | |||
| PPARα | AF163809 | F: TTTAACGGAGTTCCAATCGC | 224 |
| R: AACCCTTACAACCTTCACAAGC | |||
| SCD1 | NM204890.1 | F: GTTTCCACAACTACCACCATACATT | 175 |
| R: CCATCTCCAGTCCGCATTTT | |||
| SREBP-1c | XM015294109 | F: GCCCTCTGTGCCTTTGTCTTC | 130 |
| R: ACTCAGCCATGATGCTTCTTC | |||
| ELOVL6 | NM001031539 | F: GGTGGTCGGCACCTAATGAA | 169 |
| R: TCTGGTCACACACTGACTGC | |||
| ChREBP | EU152408 | F: ATTGACCCGACCCTGACG | 160 |
| R: CATACTGGATGTACCACGCTCT | |||
| MTTP | NM001109784 | F: GCAGATGGACAGAGTTGGCT | 224 |
| R: ACACCAAAAGTGCAAGGTGC |
Abbreviations: ACC, acetyl CoA carboxylase; ChREBP, carbohydrate responsive element binding protein; CPT1, carnitine palmitoyltransferase 1; ELOVL6, elongase of very long chain fatty acids 6; FAS, fatty acid synthase; MTTP, microsomal triglyceride transfer protein; PPAR, peroxisome proliferator-activated receptor; SCD1, stearoyl-CoA desaturase 1; SRRBP, sterol regulatory element binding proteins.
Statistical Analysis
All data were shown as means and analyzed by one-way ANOVA using the GLM procedure of SPSS 20.0 (SPSS Inc., Chicago, IL). P-values less than 0.05 were considered to be statistically significant, and the notable differences between groups were identified by Duncan's multiple comparisons test.
Results
Hepatic Morphology
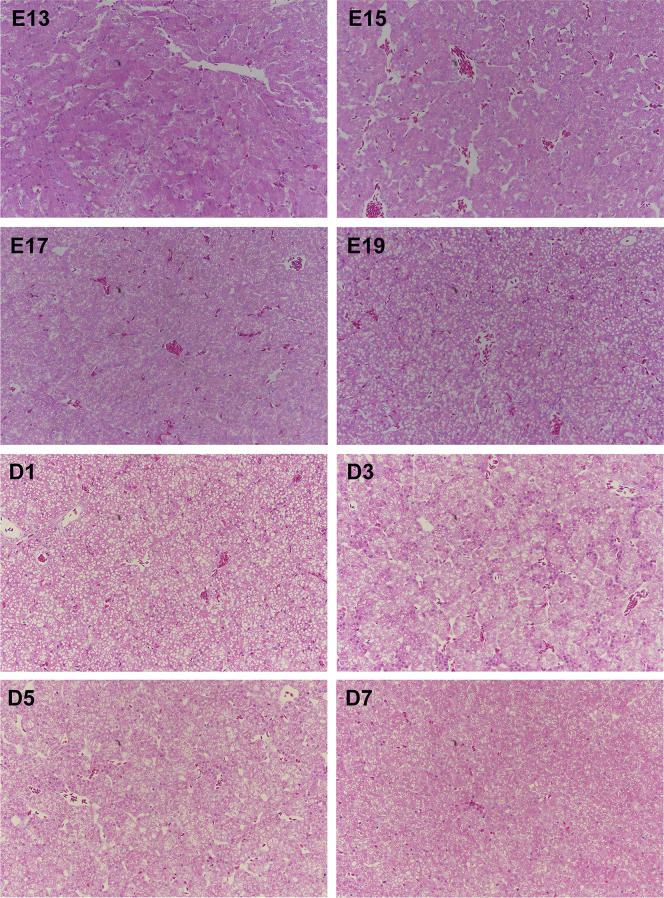
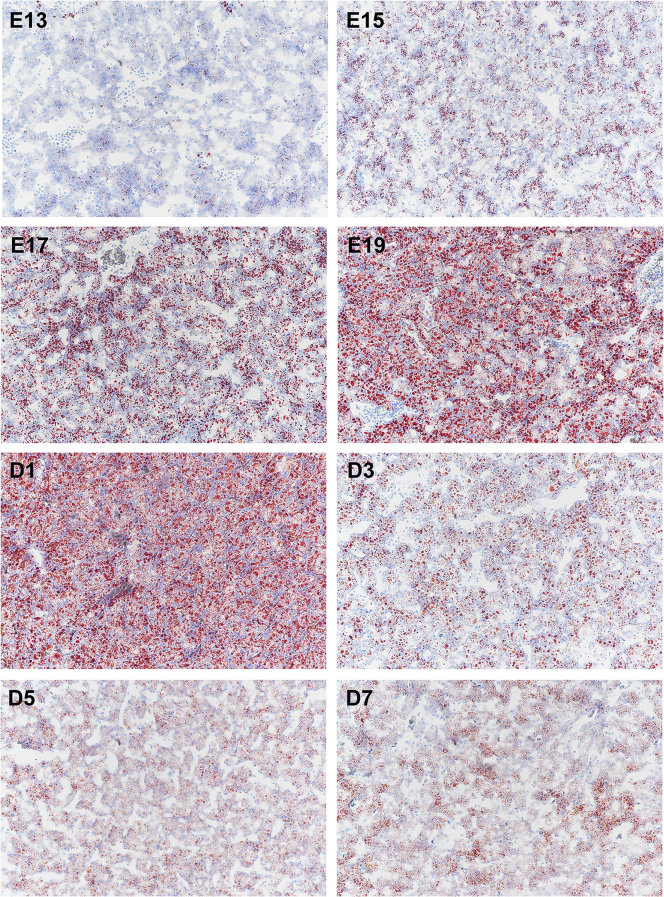
As exhibited in Figure 1, Figure 2 for HE and Oil Red O staining, respectively, hepatic texture and reticular formations was not intact, and there existed some interstice at E13, suggesting that the liver organ was not fully developed before E15. In addition, HE staining results showed that small white cavities appeared from E15 and became more and more until the hatched day, then decreased with age during the first week after hatching. Meanwhile, Oil Red O staining also revealed the same phenomenon that red circle drops became more and more from E15 to D1, then declined. These white cavities or red circle drops all mean fat droplets.
Figure 1.
Hepatic hematoxylin-eosin staining analysis of chicks from embryonic day 13 to posthatch day 7. White cavities with different sizes all mean fat droplets (magnification:10 × 20), which are located in the cytoplasm. The liver with high lipid contents lost the normal reticular formation, otherwise, hepatic texture is flexible and has clear reticular formations with less fat droplets.
Figure 2.
Hepatic Oil Red O staining analysis of chicks from embryonic day 13 to posthatch day 7. Red circle drops are on behalf of fat droplets (magnification:10 × 20).
Hepatic Growth
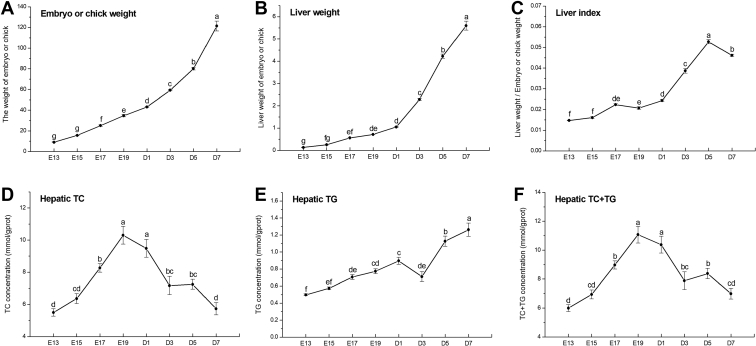
As shown in Figures 3A-3C, the weight of embryo or chick increased gradually with age as well as the liver weight (P < 0.05), and growth rate was higher during the first week posthatch than that during embryonic period (P < 0.05). Liver index first increased from E15 to E17, then keep relative steady until D1, next ascended rapidly from D1 to D5 (P < 0.05).
Figure 3.
Developmental changes of embryo or chick growth parameters and hepatic lipid contents. Embryo or chick body weight (A); liver weight of embryo or chick (B); liver index relative to body weight of embryo or chick (C); hepatic TC concentration (D); hepatic TG concentration (E); total TC+TG concentration in the liver (F). Data are expressed as mean ± SEM (n =7), and bars with different lowercase letters indicated statistically significant differences (one-way ANOVA, P < 0.05). Abbreviations: TC, total cholesterol; TG, triglycerides.
Hepatic TG and TC Contents
Developmental changes of hepatic TG and TC in embryos or chicks were demonstrated in Figures 3D-3F. TC contents in the liver raised gradually from chick embryonic period to D1, then declined during the first week posthatch (P < 0.05). Hepatic TG levels also increased during embryonic periods but decreased at D3, then increased from D3 to D7 (P < 0.05). Total lipid contents (the sum of TG and TC) in the liver of chicks reached higher levels around the hatched day (P < 0.05).
Developmental Expression Pattern of Genes
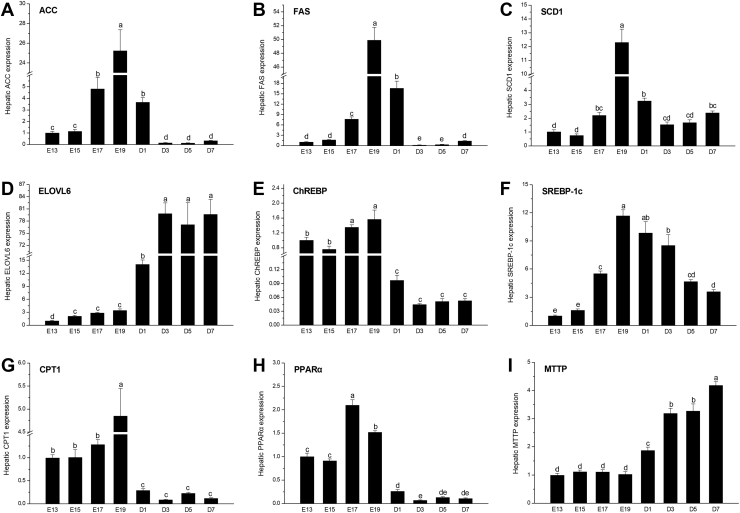
As shown in Figure 4, developmental changes of hepatic genes expression about lipid metabolism were indicated. The acetyl CoA carboxylase (ACC) mRNA level were higher with the peak at E19 from E17 to D1 than that at other observed age, and the same phenomenon was found about hepatic fatty acid synthase (FAS) expression pattern (P < 0.05). The highest abundance of stearoyl-CoA desaturase 1 (SCD1) was also observed at E19, then declined from E19 to D3 and hold steady from D3 to D7 (P < 0.05). Different from ACC and FAS, elongase of very long-chain fatty acids 6 (ELOVL6) mRNA expression is lower during embryonic periods but at relative higher level after hatching (P < 0.05). Developmental microsomal triglyceride transfer protein (MTTP) expression was similar with ELOVL6 (P < 0.05). On the contrary, hepatic carbohydrate responsive element binding protein (ChREBP), carnitine palmitoyltransferase 1 (CPT1), and peroxisome proliferator-activated receptor α (PPARα) expressions were higher at the age of embryonic periods but decreased posthatch (P < 0.05). The mRNA abundance of sterol regulatory element binding proteins 1c was the lowest at E13 and E15, then increased gradually from E17 to E19, but decreased little by little from D3 to D7 (P < 0.05).
Figure 4.
Developmental changes of genes expression related to lipogenesis and lipolysis in the liver. Results of hepatic ACC, FAS, SCD1, ELOVL6, ChREBP, SREBP-1c, CPT1, PPARα, and MTTP mRNA expression, respectively (A-I). Data are expressed as mean ± SEM (n = 7), and bars with different lowercase letters indicated statistically significant differences (one-way ANOVA, P < 0.05). Abbreviations: ACC, acetyl CoA carboxylase; ChREBP, carbohydrate responsive element binding protein; CPT1, carnitine palmitoyltransferase 1; ELOVL6, elongase of very long chain fatty acids 6; FAS, fatty acid synthase; MTTP, microsomal triglyceride transfer protein; PPAR, peroxisome proliferator-activated receptor; SCD1, stearoyl-CoA desaturase 1; SRRBP, sterol regulatory element binding proteins.
Discussion
In the present study, we analyzed the developmental pattern of hepatic lipid metabolism in chicks during embryonic periods and the first week after hatching. We found that hepatic lipid contents in chicks increased with age from E15 to the hatched day then decreased gradually from D1 to D7 based on HE and Oil Red O staining; total contents of TG and TC reached the peak around the hatched day. The previous study has reported that the liver accumulated very high lipid levels with cholesterol eaters accounting for 70% of the total before hatching (Noble and Ogunyemi, 1989), which was consistent with our data in this study that hepatic TC levels were well above the TG concentration. In addition, hepatic TC contents declined quickly, while the TG level increased in the study during the first week posthatch, indicating that hepatic TC provided main energy maintenance for rapid growth development of chicks in the immediate posthatch period, and newly TG were also accumulated in the liver from the diets or de novo lipogenesis (Noy and Sklan, 1999). As we all know, adipose cell number and size contribute to abdominal fat weight. It was reported that adipocyte hyperplasia occurred from 3 D posthatch to 42 D, and the hypertrophic growth occurred before 35 D in chickens (Guo, 2011). The endogenous body lipids are mainly from hepatic origin (O'Hea and Leveille, 1968); hence, it is important to do dietary nutrition management well in the energy utilization switch period from hepatic lipid storage to feeds intake to avoid excessive fat deposition in the starter chicks.
To deeply understand the molecular mechanism of lipid metabolism changes, we also detected hepatic genes expression about lipogenesis and lipolysis. ACC, FAS, SCD1, and ELOVL6 are involved in hepatic de novo lipogenesis directly and could be activated by ChREBP and sterol regulatory element binding proteins 1c transcription factors (Ntambi, 2016). As the rate-limiting enzyme in fatty acid synthesis, ACC is responsible for catalyzing the carboxylation of acetyl-CoA to malonyl-CoA; FAS utilizes acetyl-CoA and malonyl-CoA to produce palmitate (C16:0) through a complex seven-step reaction (Kim et al., 2017). In the current study, hepatic ACC and FAS expression began to increase from E17 and reached the peak at E19 but declined at D1, which agreed with previous findings that FAS mRNA level peaked just before hatching, followed by a decline at hatching (Zhao et al., 2010b). These results suggested that embryonic liver possess the ability of de novo fatty acid synthesis during late embryonic growth. In addition, ChREBP showed higher abundance during embryonic periods than those after hatching, implying that fatty acid synthesis might be activated by ChREBP before hatching. Near the hatched day, SREBP mRNA levels were also higher at E19 and D1, indicating that ChREBP and SREBP might all contribute to hepatic de novo fatty acid synthesis around the hatched day.
SCD1 preferred palmitoyl-CoA (C16:0) substrates for the biosynthesis of monounsaturated fatty acids (C16:1), which were key sources for the formation of TC or TG esters (Mauvoisin and Mounier, 2011). ELOVL6 was involved in the elongation of saturated and monounsaturated fatty acids (Matsuzaka et al., 2012). Developmental changes of hepatic SCD1 expression was the same with ACC and FAS in the study, but ELOVL6 expression pattern showed the difference from other genes about lipogenesis. Higher ELOVL6 mRNA abundance in the liver was detected after hatching. These results could account for the phenomenon of increasing hepatic TG contents gradually from embryonic periods to the first week posthatch. In addition, different genes expression patterns related to lipogenesis in the current study also support the report that there were changes in the compositions and proportions of hepatic fatty acid during embryos and newly hatched chicks (Noble and Ogunyemi, 1989).
Meanwhile, genes expression about lipolysis in the liver was evaluated. CPT1 is a rate-limiting enzyme which transfers the long-chain fatty acyl group from CoA to carnitine for subsequent fatty acid oxidation (Yao et al., 2018). PPARα was reported to regulate genes transcription about lipolysis and be in negative correlation with fatty acid oxidation (Larter et al., 2010). The previous study estimated that more than 90% energy requirement was derived from fatty acid oxidation during chick embryonic development (Noble and Cocchi, 1990). In this study, hepatic CPT1 and PPARα expression reached a peak before hatching, which agreed with other report (Zhao et al., 2010a). This finding implied that fatty acid oxidation was improved based on the aerobic respiratory way to provide more energy for pipping eggshell. However, these 2 gene expression declined after hatching, and hepatic MTTP mRNA level was found to significantly improve gradually from D1 to D7. The MTTP subunit has lipid transfer activity and owns the ability to transfer lipids to apoB for subsequent processing, leading to chylomicrons and VLDL secretion in the liver (Gordon et al., 1995, Rubin et al., 2010). To our knowledge, on the basis of these findings, we raise a presumption that energy requirement for new-born chick growth is not from hepatic fatty acid oxidation because of lower CPT1 and PPARα expression; it is possible that hepatic lipids are transferred to developmental organs for energy usage, after all total lipids in the liver declined rapidly, and MTTP showed an obvious increase in the first week of posthatch.
It acknowledged NAFLD is considered as a pathological ranging from simple steatosis through to steatohepatitis and cirrhosis of varying severity (Mann et al., 2016). Hepatic simple steatosis is the initial step and the early stage with increased lipogenesis, decreased fatty acid oxidation, and more TG or cholesterol deposition; then the liver progresses from steatosis to steatohepatitis with increased inflammation and oxidative stress; finally, the steatohepatitis can ultimately lead to cirrhosis, and the pathogenesis among these different stages are involved in a multistep process (Imajo et al., 2013). It was reported that about 10-20% with each phase of disease could develop into the following stage, whereas, there is also a possibility of reversibility especially in the first 2 stages (Fan and Farrell, 2009, Ekstedt et al., 2015). Our results showed much lipids accumulation in the liver around the chick birth day such as E19 and D1. In addition, higher gene expression about lipogenesis and lower gene levels about fatty acid oxidation were found at D1. These characteristics are similar to those in NAFLD with simple steatosis (Bertola, 2018) and the adult fatty hen reported by previous studies (Hu et al., 2017, Zhou et al., 2018). The previous study has employed adult laying hens as the model to identify potential biomarkers for human NAFLD (Tsai et al., 2017). Owing to the difficulty of collecting enough patient samples and individual differences, rodent models were used to imitate particular characteristics of NAFLD by dietary induction or genetic manipulations (Maher, 2011, Imajo et al., 2013). But no rodent model could encompass the full spectrum of disease progression. Primary human hepatocytes are regarded as “gold standard” for lipid metabolism studies, but there exist many shortcomings such as interdonor differences and inability to proliferate (Pramfalk et al., 2016). Primary hepatocytes from new-born chicks are full of lipid droplets based on Oil Red O staining and electron microscopy analysis (Liu et al., 2018). On the other hand, chicks also showed the same site for de novo lipogenesis as human 90% mainly in the liver (Leveille et al., 1975), but for mouse or pigs, their adipose tissues and livers contributed equally to de novo lipogenesis (Laliotis et al., 2010). Therefore, we consider that the new-born chick is likely to be a better model for primary hepatocytes isolation to carry out research in vitro about NAFLD during earlier stage.
In conclusion, we demonstrated dynamic patterns of hepatic lipid metabolism including genes expression about lipogenesis and lipolysis in chicks from embryonic periods to the first week after hatching. Our results found lipid accumulation reached a peak around the hatched day; hepatic lipogenesis genes have different expression patterns; fatty acid oxidation was enhanced before hatching but declined posthatch. These findings provide a theoretical basis for physiological characteristics and dynamic pattern about hepatic lipid metabolism in chickens. In addition, it might be worthy of consideration that newly hatched chicks could be used for primary hepatocytes isolation to carry out studies about human NAFLD diseases or fatty liver in poultry in vitro, which will need to be verified in the future.
Acknowledgments
This work was funded by the National Key Research and Development Program of China (2017YFD0500500, 2017YFD0502200), the Program for Shaanxi Science & Technology (2018ZDCXL-NY-0201, 2018ZDXM-NY-051), and the Program for Yangling Agricultural High-tech Industries Demonstration Zone (2018CXY-10).
The authors declare that they have no conflicts of interest.
References
- Ailhaud G., Grimaldi P., Negrel R. Cellular and molecular aspects of adipose tissue development. Annu. Rev. Nutr. 1992;12:207–233. doi: 10.1146/annurev.nu.12.070192.001231. [DOI] [PubMed] [Google Scholar]
- Ayala I., Martín C.A., Adánez G., Fernándezrufete A., García Pérez B., Castells M.T. Hyperlipidemic chicken as a model of non-alcoholic steatohepatitis. Exp. Biol. Med. 2009;234:10–16. doi: 10.3181/0807-RM-219. [DOI] [PubMed] [Google Scholar]
- Bertola A. Rodent models of fatty liver diseases. Liver Res. 2018;2:3–13. [Google Scholar]
- Chen X., Li L., Liu X., Luo R., Liao G., Li L., Liu J., Cheng J., Lu Y., Chen Y. Oleic acid protects saturated fatty acid mediated lipotoxicity in hepatocytes and rat of non-alcoholic steatohepatitis. Life Sci. 2018;203:291–304. doi: 10.1016/j.lfs.2018.04.022. [DOI] [PubMed] [Google Scholar]
- Chiluard Y. Dietary fat and adipose tissue metabolism in ruminants, pigs and rodents: a review. J. Dairy Sci. 1994;76:3897–3931. doi: 10.3168/jds.S0022-0302(93)77730-9. [DOI] [PubMed] [Google Scholar]
- Ekstedt M., Hagstrom H., Nasr P., Fredrikson M., Stal P., Kechagias S., Hultcrantz R. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61:1547–1554. doi: 10.1002/hep.27368. [DOI] [PubMed] [Google Scholar]
- Fan J.G., Farrell G.C. Epidemiology of non-alcoholic fatty liver disease in China. J. Hepatol. 2009;50:204–210. doi: 10.1016/j.jhep.2008.10.010. [DOI] [PubMed] [Google Scholar]
- Gao X., Liu P., Wu C., Wang T., Liu G., Cao H., Zhang C., Hu G., Guo X. Effects of fatty liver hemorrhagic syndrome on the AMP-activated protein kinase signaling pathway in laying hens. Poult. Sci. 2019;98:2201–2210. doi: 10.3382/ps/pey586. [DOI] [PubMed] [Google Scholar]
- Gordon D.A., Wetterau J.R., Gregg R.E. Microsomal triglyceride transfer protein: a protein complex required for the assembly of lipoprotein particles. Trends Cell Biol. 1995;5:317–321. doi: 10.1016/s0962-8924(00)89054-6. [DOI] [PubMed] [Google Scholar]
- Guo L. Comparison of adipose tissue cellularity in chicken lines divergently selected for fatness. Poult. Sci. 2011;90:2024–2034. doi: 10.3382/ps.2010-00863. [DOI] [PubMed] [Google Scholar]
- Hu Y., Sun Q., Liu J., Jia Y., Cai D., Idriss A.A., Omer N.A., Zhao R. In ovo injection of betaine alleviates corticosterone-induced fatty liver in chickens through epigenetic modifications. Sci. Rep-UK. 2017;7:40152–40165. doi: 10.1038/srep40251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imajo K., Yoneda M., Kessoku T., Ogawa Y., Maeda S., Sumida Y., Hyogo H., Eguchi Y., Wada K., Nakajima A. Rodent models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Int. J. Mol. Sci. 2013;14:21833–21857. doi: 10.3390/ijms141121833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingle D.L., Bauman D.E., Garrigus U.S. Lipogenesis in the ruminant: in vivo site of fatty acid synthesis in sheep. J. Nutr. 1972;102:617–623. doi: 10.1093/jn/102.5.617. [DOI] [PubMed] [Google Scholar]
- Kim C.W., Addy C., Kusunoki J., Anderson N.N., Deja S., Fu X., Burgess S.C., Li C., Chakravarthy M., Previs S. Acetyl CoA carboxylase inhibition reduces hepatic steatosis but elevates plasma triglycerides in mice and humans: a bedside to bench investigation. Cell Metab. 2017;26:394–406. doi: 10.1016/j.cmet.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larter C.Z., Yeh M.M., Jenny C., Jacqueline W., Sandie B., Aileen D.P., Bell-Anderson K.S., Farrell G.C. Activation of peroxisome proliferator-activated receptor alpha by dietary fish oil attenuates steatosis, but does not prevent experimental steatohepatitis because of hepatic lipoperoxide accumulation. J. Gastroenterol. Hepatol. 2010;23:267–275. doi: 10.1111/j.1440-1746.2007.05157.x. [DOI] [PubMed] [Google Scholar]
- Laliotis G.P., Bizelis I., Rogdakis E. Comparative approach of the de novo fatty acid synthesis (Lipogenesis) between ruminant and non ruminant mammalian species: from biochemical level to the main regulatory lipogenic genes. Curr. Genomics. 2010;11:168–183. doi: 10.2174/138920210791110960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leveille G.A., Romsos D.R., Yeh Y.Y., O'Hea E.K. Lipid biosynthesis in the chick. A consideration of site of synthesis, influence of diet and possible regulatory mechanisms. Poult. Sci. 1975;54:1075–1093. doi: 10.3382/ps.0541075. [DOI] [PubMed] [Google Scholar]
- Liu Y.L., Shen J., Yang X., Sun Q.Z., Yang X.J. Folic acid reduced triglycerides deposition in primary chicken hepatocytes. J. Agric. Food Chem. 2018;66:13162–13172. doi: 10.1021/acs.jafc.8b05193. [DOI] [PubMed] [Google Scholar]
- Liu Y.L., Yin R.Q., Liang S.S., Duan Y.L., Yao J.H., Yang X.J. Effect of dietary Lycium barbarum polysaccharide on growth performance and immune function of broilers. J. Appl. Poult. Res. 2017;26:200–208. [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Maher J.J. New insights from rodent models of fatty liver disease. Antioxid. Redox Signal. 2011;15:535–550. doi: 10.1089/ars.2010.3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makovicky P., Dudova M., Tumova E., Rajmon R., Vodkova Z. Experimental study of non-alcoholic fatty liver disease (NAFLD) on a model of starving chickens: is generalization of steatosis accompanied by fibrosis of the liver tissue? Pathol. Res. Pract. 2011;207:151–155. doi: 10.1016/j.prp.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Mann J.P., Semple R.K., Armstrong M.J. How useful are monogenic rodent models for the study of human non-alcoholic fatty liver disease? Front. Endocrinol. 2016;16:145–156. doi: 10.3389/fendo.2016.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaka T., Atsumi A., Matsumori R., Nie T., Shinozaki H., Suzukikemuriyama N., Kuba M., Nakagawa Y., Ishii K., Shimada M. Elovl6 promotes nonalcoholic steatohepatitis. Hepatology. 2012;56:2199–2208. doi: 10.1002/hep.25932. [DOI] [PubMed] [Google Scholar]
- Matteo R., Maria R.O., Lucia C., Claudia A., Stefano B., Adriano P., Luca I.F., Fabio M., Marco B., Sebastiano B. Differential effect of oleic and palmitic acid on lipid accumulation and apoptosis in cultured hepatocytes. J. Gastroenterol. Hepatol. 2010;24:830–840. doi: 10.1111/j.1440-1746.2008.05733.x. [DOI] [PubMed] [Google Scholar]
- Mauvoisin D., Mounier C. Hormonal and nutritional regulation of SCD1 gene expression. Biochimie. 2011;93:78–86. doi: 10.1016/j.biochi.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Moreira G.C.M., Boschiero C., Cesar A.S.M., Reecy J.M., Godoy T.F., Pertille F., Ledur M.C., Moura A., Garrick D.J., Coutinho L.L. Integration of genome wide association studies and whole genome sequencing provides novel insights into fat deposition in chicken. Sci. Rep. 2018;8:16222–16236. doi: 10.1038/s41598-018-34364-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble R., Cocchi M. Lipid metabolism and the neonatal chicken. Prog. Lipid Res. 1990;29:107–140. doi: 10.1016/0163-7827(90)90014-c. [DOI] [PubMed] [Google Scholar]
- Noble R.C., Ogunyemi D. Lipid changes in the residual yolk and liver of the chick immediately after hatching. Biol. Neonate. 1989;56:228–236. doi: 10.1159/000243127. [DOI] [PubMed] [Google Scholar]
- Noy Y., Sklan D. Energy utilization in newly hatched chicks. Poult. Sci. 1999;78:1750–1756. doi: 10.1093/ps/78.12.1750. [DOI] [PubMed] [Google Scholar]
- Ntambi J.M. Department of Biochemistry, University of Wisconsin-Madison; Madison, WI: 2016. Hepatic de novo lipogenesis and regulation of metabolism. [Google Scholar]
- O'Hea E.K., Leveille G.A. Lipogenesis in isolated adipose tissue of the domestic chick (Gallus domesticus) Comp. Biochem. Physiol. 1968;26:111–120. doi: 10.1016/0010-406x(68)90317-4. [DOI] [PubMed] [Google Scholar]
- Pramfalk C., Larsson L., Härdfeldt J., Eriksson M., Parini P. Culturing of HepG2 cells with human serum improve their functionality and suitability in studies of lipid metabolism. Biochim. Biophys. Acta. 2016;1861:51–59. doi: 10.1016/j.bbalip.2015.10.008. [DOI] [PubMed] [Google Scholar]
- Rozenboim I., Mahato J., Cohen N.A., Tirosh O. Low protein and high-energy diet: a possible natural cause of fatty liver hemorrhagic syndrome in caged White Leghorn laying hens. Poult. Sci. 2016;95:612–621. doi: 10.3382/ps/pev367. [DOI] [PubMed] [Google Scholar]
- Rubin D., Schneidermuntau A., Klapper M., Nitz I., Helwig U., Fölsch U.R., Schrezenmeir J., Döring F. Functional analysis of promoter variants in the microsomal triglyceride transfer protein (MTTP) gene. Hum. Mutat. 2010;29:123–129. doi: 10.1002/humu.20615. [DOI] [PubMed] [Google Scholar]
- Shi L., Ko M.L., Huang C.Y., Park S.Y., Hong M.P., Wu C., Ko G.Y.P. Chicken embryos as a potential new model for early onset type I diabetes. J. Diabetes Res. 2014;2014:354094. doi: 10.1155/2014/354094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shini A. The University of Queensland; Brisbane, Australia: 2014. Fatty Liver Haemorrhagic Syndrome in Laying Hens: Field and Experimental Investigations: Fatty Liver Haemorrhagic Syndrome. [Google Scholar]
- Trott K.A., Giannitti F., Rimoldi G., Hill A., Woods L., Barr B., Anderson M., Mete A. Fatty liver hemorrhagic syndrome in the backyard chicken: a retrospective histopathologic case series. Vet. Pathol. 2014;51:787–795. doi: 10.1177/0300985813503569. [DOI] [PubMed] [Google Scholar]
- Tsai M.T., Chen Y.J., Chen C.Y., Tsai M.H., Han C.L., Chen Y.J., Mersmann H.J., Ding S.T. Identification of potential plasma biomarkers for nonalcoholic fatty liver disease by integrating transcriptomics and proteomics in laying hens. J. Nutr. 2017;147:293–303. doi: 10.3945/jn.116.240358. [DOI] [PubMed] [Google Scholar]
- Yao C.H., Liu G.Y., Wang R., Moon S.H., Gross R.W., Patti G.J. Identifying off-target effects of etomoxir reveals that carnitine palmitoyltransferase I is essential for cancer cell proliferation independent of β-oxidation. Plos Biol. 2018;16:e2003782. doi: 10.1371/journal.pbio.2003782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Liu Z., Liu R., Wang J., Zheng M., Li Q., Cui H., Zhao G., Wen J. Alteration of hepatic gene expression along with the inherited phenotype of acquired fatty liver in chicken. Genes. 2018;9:e199. doi: 10.3390/genes9040199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S., Ma H., Huang G., Zou S. Hepatic lipolysis in broiler chickens with different fat deposition during embryonic development. Res. Vet. Sci. 2010;88:321–325. doi: 10.1016/j.rvsc.2009.07.007. [DOI] [PubMed] [Google Scholar]
- Zhao S., Ma H., Zou S., Chen W., Zhao R. Hepatic lipogenesis in broiler chickens with different fat deposition during embryonic development. J. Vet. Med. A Physiol. Pathol. Clin. Med. 2010;54:1–6. doi: 10.1111/j.1439-0442.2007.00898.x. [DOI] [PubMed] [Google Scholar]
- Zhou Z., Kim J.W., Zhao J., Qi J., Choi S.J., Lim C.W., Lee M.Y., Lee K., Kim B. Treatment of cigarette smoke extract and condensate differentially potentiates palmitic acid-induced lipotoxicity and steatohepatitis in vitro. Toxicol. Vitro. 2018;52:33–40. doi: 10.1016/j.tiv.2018.05.017. [DOI] [PubMed] [Google Scholar]