Abstract
The effectiveness of rice protein coatings enriched with essential oils on maintaining interior quality of fresh eggs was evaluated during storage at 20°C for 6 wk. Egg quality was assessed by weight loss, Haugh unit (HU), albumen pH, and yolk index (YI) in uncoated eggs (control treatment) and eggs coated with rice protein concentrate at 8% enriched or not with different essential oils (1%): tea tree (Melaleuca alternifolia), copaíba (Copaifera langsdorffii), or thymo (Thymus vulgaris). The HU and YI were higher in coated eggs (P < 0.001). Data were submitted to variance analysis, and the statistical models included the effects of treatments (coating types), storage periods (weeks), and interaction (treatments by storage periods). Weight loss increased (P < 0.001) during long-term storage. Uncoated eggs showed the highest weight loss (5.43%), whereas coatings of rice protein alone (4.23%) or enriched with tea tree (4.10%), copaíba (3.90%), and thymo (4.08%) solutions were effective in preventing weight lost (P < 0.001). The coating use preserved the internal quality of the eggs for up to 3 wk longer than uncoated eggs in terms of HU, YI, and pH. Uncoated eggs had the worst (P < 0.001) HU (58.46), albumen pH (9.48), and YI (0.33) after 6 wk of storage. In conclusion, the use of coatings based on rice protein concentrate enriched with different essential oils influences the internal quality of eggs during storage and may be an effective alternative for increasing the shelf life of commercial eggs.
Key words: copaíba, phytochemicals, storage, tea tree, thymo
INTRODUCTION
Eggs are perishable products and lose quality if they are not handled and stored properly. From the oviposition, egg is subject to physical and chemical changes in the albumen and yolk that could result in changes in the flavor, freshness, and palatability. The longer is the storage time, the greater is the deterioration of internal quality and the higher is the carbon dioxide movement through the shell, especially at room temperature (Oliveira and Oliveira, 2013). In Brazil, washing the eggs before breaking is a recommended process that must be done by mechanical devices with procedures that prevent the microbial penetration into the egg (Brazil, 1990). The United States of America, Japan, and Australia also adopt egg-washing procedures, while many countries—including the United Kingdom and EU—have resisted to the practice (Jones et al., 2018). Previous studies have shown that the use of protein coatings after egg washing can help maintain internal egg quality during storage for long periods (Biladeau and Keener, 2009; Caner and Yuceer, 2015).
Despite the diversity of feedstock already available, the development of coatings from by-products is an economically interesting alternative for the industry. In this context, the rice by-products probably deserve highlight due to its availability in many regions, such as Asia and Brazil. Studies had described the use of rice as a feedstock for the preparation of edible coating (Dias et al., 2010; Das et al., 2013). Rice protein coating was also studied, and previous research suggests its effect in preserving the internal quality of raw eggs (Pires et al., 2019).
Essential oils are the secondary metabolites of aromatic plants, which have a wide range of biological activity (Abd-Elsalam and Khokhlov, 2015). Tea tree is an essential oil of Melaleuca alternifolia, and it is a complex mixture of terpen hydrocarbons and tertiary alcohols. Its main components are terpinen‐4‐ol and 1.8‐cineole (Jamróz et al., 2018). The copaíba presents different amounts of substances in the oil composition. About 80% are sesquiterpenes, a class of terpenes, and 20% are diterpenes. Among the sesquiterpenes, about 50% of the composition is β-caryophyllene, followed by α-humulene, α-copaene, α-bergamotene, and δ-cadinene (Tobouti et al., 2017). Thyme contains high concentrations of phenolic compounds including carvacrol, thymol, p-cymene, and γ-terpinene (Marino et al., 1999). Due to the presence of these substances, essential oils can be used in different applications, such as antimicrobials and antioxidants.
Essential oil-edible coatings are considered as an effective and innovative method in maintaining food quality by increasing their distribution in the food areas where microorganisms grow and proliferate, as well as by enhancing their antimicrobial activity. Coatings with essential oils are a layer of the mixture of essential oils and biological polymers, which are able to carry oil (protein, natural gum, modified starch, lipids, etc.). It can not only prevent the exchange of oxygen, water, and carbon dioxide, but also can delay the deterioration of food, so as to play a role in preservation (Ju et al., 2018). Upadhyaya et al. (2016) reported that the phytochemicals, especially carvacrol and eugenol, when applied in pectin and gum arabic-based coating were effective in reducing Salmonella Enteritidis on shell eggs. However, there are no previous reports on the use of coating enriched with phytochemicals to maintain egg quality.
Thus, the aim of the study was, therefore, to evaluate the speed of deterioration of internal quality of the eggs after application of rice protein coating enriched with different essential oils in eggs during 6 wk of storage at room temperature (20°C).
MATERIAL AND METHODS
A total of 372 non-fertile eggs, freshly laid (1-day-old) from ISA Brown hens, were supplied by a commercial farm (Rio Grande do Sul, Brazil). All eggs were obtained from birds of the same age, maintained under similar environment, handling, and feeding conditions. The eggs were randomly divided into 5 treatments. Uncoated eggs were used as a control treatment. The other treatments consisted of coatings based on rice protein concentrate (RPC). The coatings were prepared at 8% (w/w protein) using RPC (MidWay Labs, FL) enriched or not with different essential oils at 1%: tea tree (Melaleuca alternifolia), copaíba (Copaifera langsdorffii), or thymo (Thymus vulgaris). All essential oils were commercial available products (Phytoterápica, São Paulo, Brazil). The tea tree and thyme oils were extracted by steam distillation method. Copaiba oil is produced by extraction of the trunk of the trees belonging to the genera Copaifera. The essential oil can also be extracted through the fractional distillation method.
Preparation of Coating Solutions and Coating of Shell Eggs
Glycerol (Neon, São Paulo, Brazil) was added to give a protein: plasticizer ratio of 1:2 w/w. The solutions were kept on a magnetic stirrer for 5 min and then heated in a water bath (90°C) for 30 min (Antunes, 2003). Then, the temperature was reduced to 25°C and the pH was adjusted to 10 with 1 N NaOH solution, for the dissolution of the proteins in the film-forming solution.
All eggs were washed with water at 42°C, and chlorine (50 ppm) was used as a sanitizer (Brazil, 1990). The eggs were immersed for 1 min each followed by a drying time of 5 min. The clean eggs were individually submerged in the coating solutions at 24°C for 1 min, so that the coating visibly covered the entire shell surface. The eggs were then dried (Caner and Cansız, 2008) and stored at a controlled ambient temperature (20°C) for up to 6 wk in plastic trays specific for eggs. The uncoated washed eggs served as a control treatment.
A total of 12 eggs were immediately submitted to the quality analysis to represent the characteristics of fresh eggs (0 D of storage). Weekly during the study, 12 eggs from each group were randomly separated for quality evaluation (weight loss, Haugh unit [HU], yolk index, and albumen pH) at each storage interval (1 to 6 wk).
Weight Loss
The eggs were weighed individually using a digital precision (±0.001 g) scale (Bel, Mark M 214A, Milano, Italy). Weight loss (%) during storage was calculated weekly in relation to the respective egg weight at the beginning of the trial, as described by Caner and Cansız (2008), using the following equation:
Haugh Unit
The albumen height was measured with a digital caliper (TMX PD–150, China) at a distance of 10 mm from the yolk. Afterwards, the HU was obtained through the equation proposed by Haugh (1937):
where h is the thickness of albumen (mm) and W is the mass of the entire egg (g).
Based on the HU results, the eggs were graded as follows: class AA, when HU was higher than 72; class A, eggs with HU from 71 to 60; class B, eggs with HU from 59 to 31; or class C, when HU was lower than 30 (Yuceer and Caner, 2014).
Yolk Index
The width and height of the yolk were measured with a digital caliper (TMX PD–150, China). Afterwards, the yolk index was calculated through the equation (Sharp and Powell, 1930):
pH Measurements
After the separation of yolk and albumen, the dense and the fluid albumen were homogenized for 20 s, and then the pH was determined using a digital pH meter (Kasvi model k39-2014B, Paraná, Brazil) previously calibrated with buffer solutions of pH 7 and 10 (Brazil, 1999).
Ultrastructural Assessment
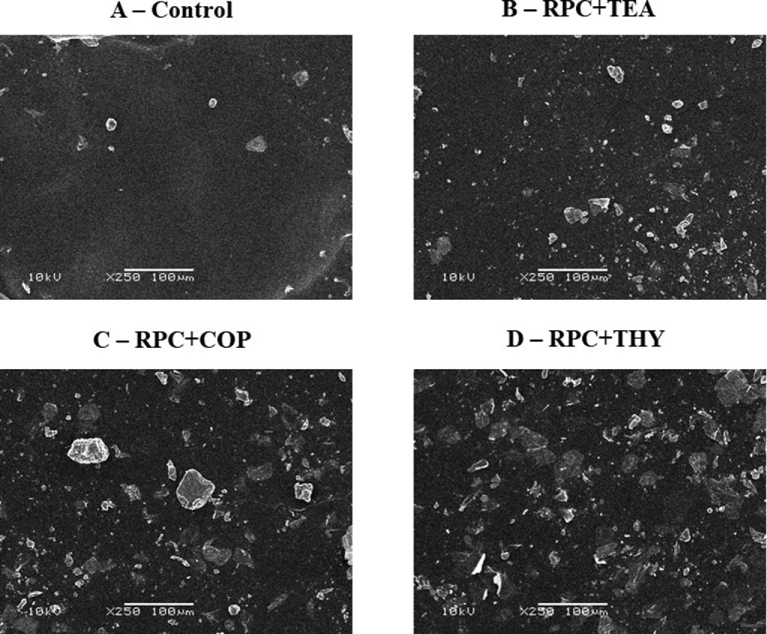
At the end of the project, 3 eggs from each treatment were randomly selected and lightly broken. Afterwards, their eggshells were segmented with scissors in 3 parts corresponding to the apical, equatorial, and basal regions. Residual albumen was removed. Then, fragments of approximately 0.5 cm² were removed from each egg region. The samples were mounted on a stub, coated with gold–palladium of 35 nm for 3 min (Sputter Coater—SCD 050 Balzers, Germany) and analyzed through a scanning electron microscope (JEOL 6060, Japan) at a standard magnification of ×250.
Salmonella spp.
At the laboratory, 3 eggs per treatment were analyzed on days 0, 28, and 42 for Salmonella spp. presence. After sterilization of smaller diameter end of the eggs with 70% alcohol, each egg was broken. Samples were individually homogenized, and 25 mL of yolk and albumen was placed in an Erlenmeyer flask containing 225 mL of 1% peptone solution. Samples in 1% peptone water were incubated at 37°C for 18 to 20 h. After this period, samples were homogenized, and 1 mL was transferred to 9 mL of Selenite Cystine Broth (SC) and 1 to 10 mL of Rappaport Vassiliadis broth (RV), followed by incubation at 37°C for 24 h. Using a nichrome inoculation loop, aliquots were the streaked on the surface of XLT4, Hektoen, and brilliant green agar plates, and again incubated at 37°C for 24 h. Colony-forming units (CFU) with characteristic Salmonella morphology were selected, and 3 to 5 CFU per plate were transferred to tubes containing triple sugar iron agar (TSI) and incubated at 37°C for 24 h. TSI cultures suggestive of Salmonella were subjected to urease, indole production, methyl red, motility, lysine decarboxylase, Simmons citrate, and malonate tests.
Statistical Analysis
A completely randomized design was used in the study. Statistical procedures were performed using SAS statistical software (9.4, SAS Inst. Inc., Cary, NC). The normality of the data was verified using the Shapiro–Wilks test through the UNIVARIATE procedure. Afterwards, the data were submitted to analysis of variance using PROC GLM, considering each egg an experimental unit. Statistical models included the effects of treatments (coating types), storage periods (weeks), and interaction (treatments by storage periods). Eventual differences (P < 0.05) were assessed with a Tukey multiple comparison test.
RESULTS AND DISCUSSION
The eggs evaluated at day zero presented mean HU values of 81.99, assuring their excellent quality (AA grade) standard according to the USDA (2000) recommendation. The other quality parameters evaluated at the beginning of the trial were also in accordance with the Brazilian legislation (Brazil, 1997), which determine minimum internal quality conditions for yolk (translucent, firm, consistent, and without germ) and albumen (transparent, consistent, limpid, no stain, and intact chalaza). Salmonella spp. was not identified in any evaluated sample.
Weight Loss
Egg size and weight are measures that will influence other variables such as HU and shell thickness; consequently, the resistance of the shell is affected by the size of the eggs (Oliveira and Oliveira, 2013). However, in this study, the egg weight did not differ (P > 0.05) between uncoated eggs (67.93 g) and eggs coated with RPC (69.25 g), neither those coated with RPC combined with tea tree (69 g), copaíba (68.72 g), and thymo (69.08 g) at the beginning of the study.
Weight loss of eggs is one of the most important measurements in monitoring the change in quality of fresh eggs during storage (Suppakul et al., 2010). The accumulated weight loss of the eggs during the 6 wk of storage is shown in Table 1. Weight loss increased (P < 0.001) with storage time, which was already reported in previous studies (Biladeau and Keener, 2009; Yuceer and Caner, 2014) and is caused primarily by evaporation of water and loss of carbon dioxide through the pores of shells (Scott and Silversides, 2000). Water loss depends on temperature, airflow, and relative humidity during storage. The longer the storage period, the more critical these factors become, especially under room temperature (Feddern, et al., 2017).
Table 1.
Effect of rice protein concentrate (RPC) coatings enriched with essential oils tea tree (TEA), copaiba (COP), or thymo (THY) on cumulative weight loss (% in relation to week 0) of eggs during 6 wk of storage at 20°C.¹
| Week | Control | RPC | RPC+TEA | RPC+COP | RPC+THY |
|---|---|---|---|---|---|
| 1 | 1.05 ± 0.02F,a | 0.79 ± 0.03F,b | 0.74 ± 0.03F,c | 0.74 ± 0.04F,c | 0.76 ± 0.03F,b,c |
| 2 | 1.32 ± 0.09E,a | 1.04 ± 0.07E,c | 1.04 ± 0.05E,c | 1.06 ± 0.08E,c | 1.16 ± 0.11E,b,c |
| 3 | 2.62 ± 0.11D,a | 1.77 ± 0.10D,b | 1.49 ± 0.05D,c | 1.35 ± 0.11D,c | 1.51 ± 0.12D,d |
| 4 | 3.46 ± 0.12C,a | 2.42 ± 0.16C,b | 1.99 ± 0.10C,c | 2.32 ± 0.11C,d | 2.57 ± 0.21C,c |
| 5 | 4.56 ± 0.17B,a | 3.59 ± 0.13B,b | 3.21 ± 0.17B,c | 3.42 ± 0.19B,b,c | 3.43 ± 0.23B,b,c |
| 6 | 5.43 ± 0.17A,a | 4.23 ± 0.19A,b | 4.10 ± 0.18A,b,c | 3.90 ± 0.18A,c | 4.08 ± 0.22A,b,c |
Data are expressed as means ± standard deviations. Information was collected in 12 eggs per treatment. Statistical models included the effects of treatments (P < 0.001), storage periods (P < 0.001), and interaction (treatments by storage periods, P < 0.001).
Means in the same row with different lowercase letters are significantly different (P < 0.001).
Means in the same column with different capital letters are significantly different (P < 0.001).
Treatment by time interaction (P < 0.001) was found for weight loss, with differences (P < 0.001) among treatments observed in all studied periods. Eggs from the control group (uncoated) had the highest weight loss during the entire when compared to eggs coated with RPC and RPC plus tea tree, copaíba, or thymo. According to FAO (2003), a 2 to 3% loss of egg weight during storage is acceptable. In this study, the eggs coating kept the weight loss within the acceptable range up to 4 wk of storage, which was not observed in uncoated eggs (3.46% at this same time). Various studies have shown the enhancement effects of using coatings on the moisture loss of the eggs during storage. These effects were associated mainly with the use of protein-based coatings (Biladeau and Keener, 2009; Caner and Yuceer, 2015; Xu et al., 2017; Pires et al., 2019).
Eggshells coated with RPC and RPC plus tea tree, copaíba, or thymo showed a lower surface porosity in the ultrastructural assessment (Figure 1), which may have contributed to the lower weight loss during storage. The use of essential oils in the elaboration of eggs films can minimize the loss of mass due to a hydrophobic characteristic. In addict, an emulsifying capacity of the oils is able to form a more homogeneous film, which can facilitate its adhesion to the egg, according to ultrastructural assessment (Figure 1).
Figure 1.
Scanning electron microscopy (×250) of uncoated eggshell (A) and coated eggs (B to D) after 6 wk of storage. RPC: rice protein concentrate coating; TEA: tea tree; COP: copaíba; THY: thymol.
Haugh Unit
The liquefaction of the dense albumen is evidenced by the reduction of HU values. Haugh unit results of uncoated and coated eggs are shown in Table 2. The initial HU value (81.99) decreased throughout the storage time (P < 0.001). The reduction of HU value can be attributed to ovomucine proteolysis, cleavage of disulfide bridges, or by the interaction between α and β ovomucines. During the storage, the enzymes present in the albumen hydrolyze the amino acid chains and, by destroying the protein structure, release the water that was bound to the large protein molecules occurs, which leads to the fluidization of the albumen and the loss of the viscosity of the dense albumen (Oliveira and Oliveira, 2013). The liquefaction of the dense albumen is evidenced by the reduction of HU values. These results were in agreement with Biladeau and Keener (2009) and Pires et al. (2019), who demonstrated that HU decreased during storage.
Table 2.
Effect of rice protein concentrate (RPC) coatings enriched with essential oils tea tree (TEA), copaiba (COP), or thymo (THY) on Haugh unit (HU) and egg grade1 (designated after each mean. in the parenthesis) during 6 wk of storage at 20°C.²
| Week | Control | RPC | RPC+TEA | RPC+COP | RPC+THY |
|---|---|---|---|---|---|
| 0 | 81.99(AA) ± 0.39A,a | 81.99(AA) ± 0.39A,a | 81.99(AA) ± 0.39A,a | 81.99(AA) ± 0.39A,a | 81.99(AA) ± 0.39A,a |
| 1 | 79.62(AA) ± 0.45B,a | 81.10(AA) ± 0.52B,b | 81.36(AA) ± 0.36A,b | 81.19(AA) ± 0.61A,B,b | 81.23(AA) ± 0.63A,B,b |
| 2 | 75.28(AA) ± 0.34C,c | 78.43(AA) ± 0.32C,b | 80.23(AA) ± 1.13B,a | 80.52(AA) ± 1.44B,a | 80.23(AA) ± 0.84B,a |
| 3 | 70.92(A) ± 0.55D,b | 76.92(AA) ± 0.36D,a | 77.21(AA) ± 1.14C,a | 77.60(AA) ± 0.67C,a | 77.50(AA) ± 0.92C,a |
| 4 | 66.47(A) ± 0.53E,c | 71.90(A) ± 0.40E,b | 77.17(AA) ± 0.58C,a | 77.07(AA) ± 0.40C,a | 77.23(AA) ± 0.76C,a |
| 5 | 63.29(A) ± 0.49F,c | 68.58(A) ± 0.48F,b | 74.06(AA) ± 0.58D,a | 72.12(AA) ± 2.40D,a | 73.90(AA) ± 1.16D,a |
| 6 | 58.46(B) ± 0.52 G,c | 61.47(A) ± 0.62 G,b | 71.53(A) ± 0.75E,a | 71.67(A) ± 2.90D,a | 72.76(AA) ± 1.30D,a |
Egg grades: AA. HU > 72; A. HU = 71–60; B. HU = 59–31; C. HU < 30.
Data are expressed as means (egg grades) ± standard deviations. Information was collected in 12 eggs per treatment. Statistical models included the effects of treatments (P < 0.001), storage periods (P < 0.001), and interaction (treatments by storage periods, P < 0.001).
Means in the same row with different lowercase letters are significantly different (P < 0.001).
Means in the same column with different capital letters are significantly different (P < 0.001).
At the end of the tested storage time (week 6), the HU means ranged from 58.46 (uncoated eggs) to 61.47 (RPC). The HU of the uncoated eggs decreased more rapidly than coated eggs, with the differences between treatments observed early as the first week and maintained up to the end of the project. These results support previous observations that different protein coatings (Biladeau and Keener, 2009; Canner and Yuccer, 2015), may be effective in preserving the albumen quality of eggs.
The HU values indicated that uncoated eggs changed in quality from grade “AA” to “A” after 3 wk, and to grade “B” after 6 wk. Meanwhile, eggs coated with RPC changed from “AA” to “A” after 5 wk of storage and eggs coated with RPC combined with essential oil changed from “AA” to “A” only after 6 wk of storage at 20°C. This demonstrated that the use of coatings allowed preserving the internal egg quality (grade A maintenance) for 2 to 3 wk longer compared to uncoated eggs. Advantages of coatings (grade A maintenance) were already reported by Wardy et al. (2011) and Nongtaodum et al. (2013) for stored eggs.
Yolk Index
The yolk index of uncoated and coated eggs also decreased (P < 0.001) throughout the storage (Table 3), as already reported in previous studies (Canner and Yuccer, 2015; Xu et al., 2017; Drabik et al., 2018). In the current study, interaction was observed between storage time and different treatments (P < 0.001). The effect of the coating was observed from the second week of storage, when all coatings tested had a higher yolk index (P < 0.001) compared to control treatment. The higher the yolk index, the better is the quality of the yolk (Yuceer and Caner, 2014). At the end of the project, the best yolk index was observed in the treatment that combined RPC and essential oils, followed by the eggs coated with RPC without other substances. This study demonstrated that the use of coating was able to preserve the yolk quality for a longer time than uncoated eggs, which are in agreement with previous studies (Caner and Yuccer, 2015; Xu et al., 2017). These results also indicated a positive effect of essential oils in preserving the yolk quality.
Table 3.
Effect of rice protein concentrate (RPC) coatings enriched with essential oils tea tree (TEA), copaiba (COP), or thymo (THY) on yolk index during 6 wk of storage at 20°C.¹
| Week | Control | RPC | RPC+TEA | RPC+COP | RPC+THY |
|---|---|---|---|---|---|
| 0 | 0.49 ± 0.01A,a | 0.49 ± 0.01A,a | 0.49 ± 0.01A,a | 0.49 ± 0.01A,a | 0.49 ± 0.01A,a |
| 1 | 0.44 ± 0.04B,a,b | 0.46 ± 0.03B,a | 0.45 ± 0.01B,a,b | 0.45 ± 0.01B,a,b | 0.45 ± 0.01A,a,b |
| 2 | 0.40 ± 0.04C,d | 0.42 ± 0.06C,c,d | 0.45 ± 0.02B,a | 0.43 ± 0.01C,D,b,c | 0.44 ± 0.01B,a,b |
| 3 | 0.38 ± 0.04D,d | 0.40 ± 0.0D,c | 0.42 ± 0.01C,b | 0.44 ± 0.01B,C,a | 0.44 ± 0.01B,a |
| 4 | 0.36 ± 0.05E,d | 0.38 ± 0.07E,c | 0.41 ± 0.01C,D,b | 0.42 ± 0.01D,a | 0.42 ± 0.01C,a |
| 5 | 0.36 ± 0.04F,d | 0.38 ± 0.06E,F,c,d | 0.40 ± 0.01D,b | 0.42 ± 0.01D,a | 0.42 ± 0.01C,a |
| 6 | 0.33 ± 0.03 G,d | 0.37 ± 0.04F,c | 0.38 ± 0.01E,b,c | 0.39 ± 0.02E,a,b | 0.40 ± 0.01D,a |
Data are expressed as means ± standard deviations. Information was collected in 12 eggs per treatment. Statistical models included the effects of treatments (P < 0.001), storage periods (P < 0.001), and interaction (treatments by storage periods, P < 0.001).
Means in the same row with different lowercase letters are significantly different (P < 0.001).
Means in the same column with different capital letters are significantly different (P < 0.001).
Coatings seem to be efficient to reduce the mass transfer rate (water and CO2 loss) from the albumen through the eggshell during long-term storage. The increasing the width of the yolk is a process that caused by the diffusion of water through the vitelline membrane (from albumen to yolk). This process inhibits albumen liquefaction and water absorption by the yolk and minimizes a reduction in yolk quality (Caner and Yuceer, 2015), which could explain the advantages for coated eggs in the present research.
pH Measurement in Albumen and Yolk
The increase in albumin pH occurs due to the dissociation of carbonic acid (H2 CO3), forming water and carbon dioxide (Figueiredo et al., 2013). The pH determination of the albumen is a suitable measure to evaluate the freshness of the eggs, since there is less influence of the strain and age of the bird on the pH compared with other quality measurements (Silversides and Scott, 2001). The albumen pH of the freshly laid egg usually ranges from 7.6 to 7.9. However, the albumen pH increases with the storage period of the egg and can reach 9.5 (Alleoni and Antunes, 2001). On the other hand, the increase in yolk pH (6.0) has little variation (6.4 to 6.9) even after long storage periods (Oliveira and Oliveira, 2013). During storage, CO2 escapes through the eggshell pores. So, the increase in albumen pH over time may be due to the loss of CO2 and/or a change in the bicarbonate buffer system (Biladeau and Keener, 2009).
In the current study, the albumen pH varied (P < 0.001) throughout the storage period (Table 4). The average initial albumen pH of the eggs was 8.05 and this value increased to 9.48 after 6 wk in the uncoated eggs. Coated and uncoated treatments differed (P < 0.001) in terms of albumen pH early from the first week up to the end of the project. The results agree with previous studies (Caner and Yuceer, 2015; Pires et al. 2019) which reported that different coatings were able to extend the shelf life of eggs in relation to albumen pH. The treatments with essential oils showed similar albumen pH at the end of the project when compared to the eggs coated with RPC alone.
Table 4.
Effect of rice protein concentrate (RPC) coatings enriched with essential oils tea tree (TEA), copaiba (COP), or thymo (THY) on pH during 6 wk of storage at 20°C.¹
| Week | Control | RPC | RPC+TEA | RPC+COP | RPC+THY |
|---|---|---|---|---|---|
| 0 | 8.05 ± 0.02E,a | 8.05 ± 0.02F,a | 8.05 ± 0.02C,a | 8.05 ± 0.02D,a | 8.05 ± 0.02C,a |
| 1 | 8.33 ± 0.19D,a | 8.09 ± 0.05E,b | 8.14 ± 0.16B,C,a,b | 8.07 ± 0.17C,D,a,b | 8.07 ± 0.07C,b |
| 2 | 8.71 ± 0.05C,a | 8.37 ± 0.06D,b | 8.24 ± 0.09A,b | 8.22 ± 0.10C,b | 8.26 ± 0.07A,b |
| 3 | 9.08 ± 0.04B,a | 8.48 ± 0.10C,b | 8.18 ± 0.10B,b | 8.13 ± 0.08A,B,b | 8.13 ± 0.11B,b |
| 4 | 9.21 ± 0.06B,a | 9.09 ± 0.07B,c | 9.23 ± 0.12A,a | 9.16 ± 0.04A,a,b | 9.16 ± 0.07A,a,b |
| 5 | 9.44 ± 0.16A,a | 9.16 ± 0.06A,B,b,c | 9.24 ± 0.09A,b | 9.06 ± 0.08B,c | 9.16 ± 0.08A,b,c |
| 6 | 9.48 ± 0.11A,a | 9.20 ± 0.04A,b | 9.26 ± 0.10A,b | 9.16 ± 0.08A,b | 9.19 ± 0.04A,b |
Data are expressed as means ± standard deviations. Information was collected in 12 eggs per treatment. Statistical models included the effects of treatments (P < 0.001), storage periods (P < 0.001), and interaction (treatments by storage periods, P < 0.001).
Means in the same row with different lowercase letters are significantly different (P < 0.001).
Means in the same column with different capital letters are significantly different (P < 0.001).
The yolk pH varied (P < 0.001) over the storage period (Table 5). After 6 wk of storage, the pH of the uncoated eggs decreased from 6.22 to 7.05. Previous research has documented a maximum increase in yolk pH of 6.0 to 6.27 (Biladeau and Keener, 2009). From week 3 to 5, the pH of the yolk in coated eggs was lower than of the uncoated eggs. However, at week 6, there was no difference among the pH of the yolk in control and any coated eggs. Few variation in pH of egg yolk was expected because the pH of the albumen increases during storage due to CO2 loss and migration of water from the albumen into the yolk during storage (Biladeau and Keener, 2009).
Table 5.
Effect of rice protein concentrate (RPC) coatings enriched with essential oils tea tree (TEA), copaiba (COP), or thymo (THY) on yolk pH during 6 wk of storage at 20°C.¹
| Week | Control | RPC | RPC+TEA | RPC+COP | RPC+THY |
|---|---|---|---|---|---|
| 0 | 6.22 ± 0.15C,a | 6.24 ± 0.15C,a | 6.24 ± 0.15B,a | 6.24 ± 0.15C,a | 6.24 ± 0.15C,a |
| 1 | 6.47 ± 0.14B,a | 6.30 ± 0.12C,a,b | 6.14 ± 0.32B,a,b | 6.12 ± 0.21C,a,b | 6.29 ± 0.26B,b |
| 2 | 6.59 ± 0.17B,a | 6.45 ± 0.19C,a | 6.42 ± 0.23B,a | 6.46 ± 0.50B,a | 6.46 ± 0.51A,a |
| 3 | 6.92 ± 0.05A,a | 6.45 ± 0.23C,b | 6.42 ± 0.49B,b | 6.44 ± 0.37B,B | 6.53 ± 0.31B,b |
| 4 | 6.96 ± 0.04A,a | 6.50 ± 0.10B,C,b | 6.50 ± 0.47B,b | 6.52 ± 0.29A,b | 6.50 ± 0.43A,b |
| 5 | 6.97 ± 0.03A,a | 6.75 ± 0.20A,B,b | 6.59 ± 0.80A,b | 6.66 ± 0.30A,b | 6.56 ± 0.22A,b |
| 6 | 7.05 ± 0.04A,a,b | 6.81 ± 0.06A,a | 6.58 ± 0.36A,b | 6.60 ± 0.41A,b | 6.64 ± 0.22A,b |
Data are expressed as means ± standard deviations. Information was collected in 12 eggs per treatment. Statistical models included the effects of treatments (P < 0.001), storage periods (P < 0.001), and interaction (treatments by storage periods, P < 0.001).
Means in the same row with different lowercase letters are significantly different (P < 0.001).
Means in the same column with different capital letters are significantly different (P < 0.001).
Coating Effects
The rice protein coating exhibited sufficient hydrophobicity and sealing properties required to effectively retard water loss during the storage at room temperature for up to 6 wk. The addition of material lipids to the coating may improve the barrier properties of moisture. Essential oils can provide a barrier to mass and oxygen loss due to lipophilic characteristics. The loss of albumen and yolk quality can be influenced by the capacity of the coating to block the pores on the surface of the shell. In general, the effects of coatings on albumen and yolks are favorable, indicating that the use of RPC-based coating plus essential oil may be a viable alternative to maintain functional properties (HU, yolk index, pH) of the eggs, which are adversely affected by storage period.
CONCLUSIONS
The present results indicate that egg weight loss, albumen pH, HU, and YG are parameters that are greatly influenced by RPC-based coating and essential oils and storage time. Rice protein coating can be used for extending the shelf life of eggs. This study demonstrated that coating with RPC could preserve albumen quality for 2 to 3 wk longer compared to uncoated eggs.
ACKNOWLEDGEMENTS
We thank Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for funding this study. We also thank Granja Filippsen for the donation of the eggs and Phytoterápica for the donation of the essential oils. Finally, we acknowledge the Center for Microscopy and Microanalysis of Universidade Federal do Rio Grande do Sul.
REFERENCES
- Abd-Elsalam K.A., Khokhlov A.R. Eugenol oil nanoemulsion: antifungal activity against Fusarium oxysporum f. sp. vasinfectum and phytotoxicity on cottonseeds. Appl. Nanosci. 2015;5:255–265. [Google Scholar]
- Alleoni A.C.C., Antunes A.J. Haugh unit as a quality measure of laying hens eggs stored under temperature. Sci. Agri. 2001;58:681–685. [Google Scholar]
- Antunes A.J. Manole; São Paulo: 2003. Functionality of Bovine Whey Proteins; p. 135. [Google Scholar]
- Biladeau A., Keener K. The effects of edible coatings on chicken egg quality under refrigerated storage. Poult. Sci. 2009;88:1266–1274. doi: 10.3382/ps.2008-00295. [DOI] [PubMed] [Google Scholar]
- BRASIL. 1990. Ministry of Agriculture, Livestock and Food Supply. Ordinance n° 1, 21 February of 1990. Meat and Derivatives Inspection Division. General Rules for the Inspection of Eggs and Derivatives. Brasília, Distrito Federal, Brazil.
- BRASIL. 1997. Regulation of Industrial and Sanitary Inspection of Productis of Animal Origin. Ordinance n° 30.691, 29 march of 1952, and changes. DOU. Updated in 1997. Ministry of Agriculture, Livestock and Food Supply. Brasília, Distrito Federal, Brazil.
- BRASIL. 1999. Normative Instruction n° 20, 21 July of 1999. Physical-chemical analytical methods for the control of meat products and their ingredients – salt and brine: DIPOA, Ministry of Agriculture, Livestock and Food Supply. Brasília, Distrito Federal, Brazil.
- Caner C., Cansiz Ö. Chitosan coating minimises eggshell breakage and improves egg quality. J. Sci. Food Agric. 2008;88:56–61. [Google Scholar]
- Caner C., Yüceer M. Efficacy of various protein-based coating on enhancing the shelf life of fresh eggs during storage. Poult. Sci. 2015;94:1665–1677. doi: 10.3382/ps/pev102. [DOI] [PubMed] [Google Scholar]
- Das D.K., Dutta H., Mahanta C.L. Development of a rice starch-based coating with antioxidant and microbe-barrier properties and study of its effect on tomatoes stored at room temperature. LWT - Food Sci. Tech. 2013;50:272–278. [Google Scholar]
- Dias A.B., Müller C.M., Larotonda F.D., Laurindo J.B. Biodegradable films based on rice starch and rice flour. J. Cereal Sci. 2010;51:213–219. [Google Scholar]
- Drabik K., Chabroszewska P., Vasiukov K., Agnieszka Adamczuk A., Batkowska J. Glycerin as a factor for moderating quality changes in table eggs during storage. Arch. Anim. Breed. 2018;61:285–292. [Google Scholar]
- FAO (Food and Agriculture Organization of the United Nations) Egg marketing—a guide for the production and sale of eggs. 2003. Agricultural Services Bulletin 150. Rome, IT. Available from http://www.fao.org/3/y4628e/y4628e00.htm
- Feddern V., De Prá M.C., Mores R., Nicoloso R.S., Coldebella A., Abreu P.A. Egg quality assessment at different storage conditions, seasons and laying hen strains. Ciênc. Agrotec. 2017;41:322–333. [Google Scholar]
- Figueiredo T., Viegas R., Lara L., Baiao N., Souza M., Heneine L., Cancado S. Bioactive amines and internal quality of commercial eggs. Poult. Sci. 2013;92:1376–1384. doi: 10.3382/ps.2012-02735. [DOI] [PubMed] [Google Scholar]
- Haugh R.R. The Haugh unit for measuring egg quality. US Egg Poult. Magazine. 1937;43:552–555. [Google Scholar]
- Jamróz E., Juszczak L., Kucharek M. Development of starch-furcellaran-gelatin films containing tea tree essential oil. J. Appl. Polym. Sci. 2018;135 doi: 10.1016/j.ijbiomac.2018.04.014. [DOI] [PubMed] [Google Scholar]
- Jones D.R., Ward G.E., Regmi P., Karche D.M. Impact of egg handling and conditions during extended storage on egg quality. Poult. Sci. 2018;97:716–723. doi: 10.3382/ps/pex351. [DOI] [PubMed] [Google Scholar]
- Ju J., Guo Y., Cheng Y., Qian H., Yao W. Application of edible coating with essential oil in food preservation. Crit. Rev. Food Sci. Nutr. 2018;8:1–62. doi: 10.1080/10408398.2018.1456402. [DOI] [PubMed] [Google Scholar]
- Marino M., Bersani C., Comi G. Antimicrobial activity of the essential oils of thymus vulgaris L. measured using a bioimpedometric method. J. Food. Prot. 1999;62:1017–1023. doi: 10.4315/0362-028x-62.9.1017. [DOI] [PubMed] [Google Scholar]
- Nongtaodum S., Jangchud A., Jangchud K., Dhamvithee P., No H.K., Prinyawiwatkul W. Oil coating affects internal quality and sensory acceptance of selected attributes of raw eggs during storage. J. Food Sci. 2013;78:329–335. doi: 10.1111/1750-3841.12035. [DOI] [PubMed] [Google Scholar]
- Oliveira B.L., Oliveira D.D. UFLA; Lavras: 2013. Egg Quality and Technology; p. 223. [Google Scholar]
- Pires P.G.S., Machado G.S., Franceschi C.H., Kindlein L., Andretta I. Rice protein coating in extending the shelf-life of conventional eggs. Poult. Sci. 2019;98:1918–1924. doi: 10.3382/ps/pey501. [DOI] [PubMed] [Google Scholar]
- Scott T., Silversides F.G. The effect of storage and strain of hen on egg quality. Poult. Sci. 2000;79:1725–1729. doi: 10.1093/ps/79.12.1725. [DOI] [PubMed] [Google Scholar]
- Sharp P.F., Powell C.K. Decrease in interior quality of hens' eggs during storage as indicated by the yolk. Ind. Eng. Chem. 1930;22:908–910. [Google Scholar]
- Silversides F., Scott T. Effect of storage and layer age on quality of eggs from two lines of hens. Poult. Sci. 2001;80:1240–1245. doi: 10.1093/ps/80.8.1240. [DOI] [PubMed] [Google Scholar]
- Suppakul P., Jutakorn K., Bangchokedee Y. Efficacy of cellulose-based coating on enhancing the shelf life of fresh eggs. J. Food Eng. 2010;98:207–213. [Google Scholar]
- Tobouti P.L., Martins T.C.M., Pereira T.J., Mussi M.C.M. Antimicrobial activity of copaiba oil: a review and a call for further research. Biomed. Pharmacother. 2017;94:93–99. doi: 10.1016/j.biopha.2017.07.092. [DOI] [PubMed] [Google Scholar]
- Upadhyaya I., Yin H.B., Surendran Nair M., Chen C.H., Lang R., Darre M.J., Venkitanarayanan K. Inactivation of Salmonella Enteritidis on shell eggs by coating with phytochemicals. Poult. Sci. 2016;95:1898–1904. doi: 10.3382/ps/pew152. [DOI] [PubMed] [Google Scholar]
- USDA U.S. Department of Agriculture . AMS, USDA; Washington, D.C.: 2000. United States Standards, Grades, and Weight Classes for Shelleggs. AMS 56.210. [Google Scholar]
- Wardy W., Torrico D.D., Jirangrat W., No H.K., Saalia F.K., Prinyawiwatkul W. Chitosan-soybean oil emulsion coating affects physico-functional and sensory quality of eggs during storage. LWT - Food Sci. Tech. 2011;44:2349–2355. [Google Scholar]
- Xu L., Zhang H., Lv X., Chi Y., Wu Y., Shao H. Internal quality of coated eggs with soy protein isolate and montmorillonite: Effects of storage conditions. Int. J. Food Prop. 2017;20:1921–1934. [Google Scholar]
- Yuceer M., Caner C. Antimicrobial lysozyme–chitosan coatings affect functional properties and shelf life of chicken eggs during storage. J. Sci. Food Agric. 2014;94:153–162. doi: 10.1002/jsfa.6322. [DOI] [PubMed] [Google Scholar]