Abstract
Vitamin D is essential for the metabolism of calcium (Ca) and phosphorus (P) in birds. The objective of the study was to evaluate the effect of different isoforms of dietary vitamin D on Ca and P utilization, egg quality, and bone mineralization of laying hens. A total of 42 Lohmann white laying hens at 57 wk of age were randomly assigned to 7 dietary treatments for 6 wk. Dietary treatments were: 3,000 IU/kg Vit D3 as control, and control with additional 3,000 IU/kg 25-hydroxyvitamin D3 (T1), 9,000 IU/kg 25-hydroxyvitamin D3 (T2), 3,000 IU/kg vitamin D3 (T3), 9,000 IU/kg vitamin D3 (T4), 3,000 IU/kg of vitamin D2 (T5), or 9,000 IU/kg of vitamin D2 (T6). Egg production and egg quality were measured weekly. Fecal samples were collected at weeks 2 and 6 to measure Ca and P utilization. After 6 wk, the left tibia and femurs were collected to measure bone mineral density (BMD) and bone mineral content (BMC). A 1-way ANOVA with Tukey HSD means separation test was used for statistical analysis. There were no significant differences in egg production, egg quality, BMD, or BMC of tibia and femurs among the treatments (P > 0.05). T6 significantly reduced feed intake (P < 0.05). The apparent total tract digestibility (ATTD) of Ca was higher (P < 0.012) in treatments supplemented with additional vitamin D, irrespective of forms. The ATTD of P was higher (P < 0.0001) in T5 compared to the other treatments at both time points. The utilization of Ca and P by laying hens can be improved through the addition of different isoforms of vitamin D in diets. However, additional vitamin D supplementation to laying hens, regardless of forms, had no effect on either bone mineralization or measures of egg quality.
Key words: digestibility, egg quality, layers, minerals, tibia, vitamin D
INTRODUCTION
Optimum concentrations of vitamins in poultry diets allow the birds to perform to their genetic potential. Vitamin requirements established decades ago do not consider today's genetically superior birds with increased growth, egg production, and improved feed efficiency. Vitamin intake per unit of output is continually declining. The yearly decline for layers is around 1% per egg produced, while for broilers it has been 0.6 to 0.8% for body gain (Leeson, 2007). Signs of vitamin D deficiency begin to occur in laying hens in confinement within 4 wk (Tsang et al., 1990; Mattila et al., 2011). When laying hens are fed a diet deficient in vitamin D, the first sign of deficiency is a thinning of the eggshells. Commercial layers will continue to lay eggs with reduced shell quantity for weeks. If the diet is completely devoid of vitamin D3, egg production decreases rapidly and eggs with very thin shells or no shell will be produced.
Vitamin D3 has traditionally been used as a major source of vitamin D by the poultry industry. Since 2006, 25-hydroxyvitamin D3 (25-OHD) has been permitted and is widely used as another source of vitamin D in the poultry industry (Mattila et al., 2011). However, the advantage of 25-OHD over vitamin D3 on Ca utilization, egg production, and performance are a subject of debate. Body weight and bone ash at days 21 and 42 were higher, and the incidence of Tibia dyschondroplasia (TD) was lower in broilers fed 25-OHD as compared to vitamin D3 in their diets (Fritts and Waldroup, 2003). Keshavaraz (2003) conducted 2 studies, the one with different levels of Ca (3.34, 4.3, 4.73, and 4.94) and 2 sources of vitamin D (vitamin D3 and 25-OHD), and the other with 3 non-phytate P levels, 2 levels of phytase, and 2 sources of vitamin D. The results suggest that use of 25-OHD did not have any advantage over vitamin D3 in increasing the performance and eggshell quality of laying hens.
Vitamin D is a fat-soluble vitamin essential for the proper metabolism of calcium (Ca) and phosphorus (P), and the maintenance of normal skeletal integrity in animals (Bikle, 1994). Vitamin D also plays a role in Ca and P absorption, regulation of parathyroid hormone, bone mineralization and mobilization, and it controls the incidence of bone disorders (Garcia et al., 2013). For the laying hen, calcium is one of the key nutrients required for optimal eggshell quality in laying hens (Kebreab et al., 2009) for shell formation and calcification which requires about 2 to 3 g of Ca per day (Bar, 2009). Inadequate utilization of Ca and P and insufficient mineralization of the bones results in skeletal problems in laying hen. Due to extensive mobilization of Ca from bones to eggs, laying hens may suffer from variety of skeletal problems including osteoporosis, cage layer fatigue, and keel bone deformities (Whitehead et al., 2003). Low egg production, cracked egg shells, mortality of birds, and welfare issues are associated with low Ca and P utilization and vitamin D deficiency in laying hens (Lavelin et al., 2000; Whitehead, 2004). Vitamin D plays a major role in Ca and P metabolism in the intestine and bones of birds in order to maintain Ca homeostasis. Vitamin D is responsible for normal growth, egg and shell quality, and reproduction in laying hen, and it is of particular importance when hens are raised indoors (Mattila et al., 2003).
Vitamin D2 is considered as an ineffective form of vitamin D in poultry feed with no metabolic effect on birds (Hoy et al., 1988). Vitamin D3 and vitamin D2 included at 6,000 and 15,000 IU/kg feed to laying hen diets increased vitamin D3 and D2 concentration in their egg yolk (Mattila et al., 2004). This gives us an evidence that vitamin D2 is absorbed through the intestine and metabolized in laying hens, which gets deposited in the eggs. However, there is no study to quantify the effect of vitamin D2 on Ca and P utilization in laying hens. There is a lack of understanding about the effect of extra dosage of vitamin D3, 25-OHD, and vitamin D2 on Ca and P digestibility and skeletal health when supplemented to laying hen diets at varying concentrations.
The objectives of this study were: 1) to evaluate the effects of extra dosage of dietary vitamin D2, vitamin D3, and 25-OHD on Ca and P utilization in laying hens, and 2) to evaluate the effect of feeding different forms of vitamin D to laying hens on skeletal health, egg production, and egg quality parameters.
MATERIALS AND METHODS
Animal and Housing
The experiment was conducted following the guidelines of an animal use protocol for research, as approved by the Protocol Management and Review Committee, University of Manitoba. The experimental birds were selected from the main laying flock of the University of Manitoba that had been fed a wheat- and soybean-based diet. A total of 48 Lohmann white laying hens, 57 wk of age, were selected for the study. The hens were individually housed in metabolic cages located in a climate-controlled layer facility. The lighting schedule was maintained on a cycle of 16 h light and 8 h dark. Feed and water were provided to permit ad libitum consumption. After initial placement, hens were adapted to the metabolic cages for 7 d, during which they consumed a wheat and soybean meal-based control diet. Egg production was recorded throughout the adaptation period, and a total of 42 birds were selected based on egg production, body weight, and feed intake (FI) for inclusion into the study. Individual birds were randomly assigned to dietary treatments (n = 6/treatment).
Experimental Diets
Diets were formulated to meet or above the recommendations established for Lohmann hens (Lohmann brown Management Guild). The control (basal) diet was formulated based on a wheat and soybean meal and contained 3,000 IU of vitamin D3/kg of diet (Table 1). Other treatments consisted of the control diet plus: T1) 3,000 IU of 25-OHD/kg of diet; T2) 9,000 IU 25-OHD3/kg of diet; T3) 3,000 IU vitamin D3/kg of diet; T4) 9,000 IU vitamin D3/kg of diet; T5) 3,000 IU vitamin D2/kg of diet; and T6) 9,000 IU vitamin D2/kg of diet (Table 2). Chromium dioxide (0.3%) was used as an indigestible marker for Ca and P digestibility. Samples of mixed feed were retained for analysis of Ca, P, and chromium. The experimental diets were fed for a total of 6 wk. The experimental diets were mixed every 3 wk to minimize degradation of supplemented vitamin D from diets. FI for each hen was measured weekly.
Table 1.
Ingredients and chemical composition of the control diet fed to laying hens at late stage of egg production for 6 wk.
| Ingredient | Amount (%) |
|---|---|
| Wheat | 64.49 |
| Soybean | 18.75 |
| Veg. oil | 3.70 |
| Limestone | 7.30 |
| Shell/bone builder | 2.10 |
| Biophos | 1.50 |
| Lysine | 0.09 |
| DL-Met | 0.17 |
| Threonine | 0.10 |
| Vitamin premix1 | 1.00 |
| Mineral premix2 | 0.50 |
| Marker | 0.30 |
| Calculated nutrient content | |
| ME (Kcal/kg) | 2,864.00 |
| Crude protein (%) | 16.8 |
| Ca (%) | 3.9 |
| Available P (%) | 0.42 |
Vitamin premix supplied per kilogram of complete feed: vitamin A, 8,250 IU; vitamin E, 30 IU; vitamin B12, 0.013 mg; vitamin K3, 2.0 mg; niacin, 23.6 mg; choline chloride, 1081 mg; folic acid, 4.0 mg; biotin, 0.25 mg; pyridoxine, 4.0 mg; thiamine, 4.0 mg; vitamin D3, 3,000 IU.
Mineral premix supplied per kg of complete feed: manganese oxide, 70 mg; zinc oxide 80 mg, ferrous sulfate, 80 mg, copper sulfate, 10 mg; sodium selenium, 0.3 mg; calcium iodate premix, 0.5 mg.
Table 2.
Amount (IU/kg of diet) of vitamin D3, vitamin D2, and 25-OHDroxyvitamin D3 (25-OHDOHD) supplemented to dietary treatments fed to laying hens at late stage of egg production.
| Treatment | Vitamin D2 | 25-OHD | Vitamin D3 |
|---|---|---|---|
| Control1 | 0 | 0 | 0 |
| Control + 3,000 IU 25-OHD | 0 | 3,000 | 0 |
| Control + 9,000 IU 25-OHD | 0 | 6,000 | 0 |
| Control + 3,000 IU D3 | 0 | 0 | 3,000 |
| Control + 9,000 IU D3 | 0 | 0 | 6,000 |
| Control + 3,000 IU D2 | 3,000 | 0 | 0 |
| Control + 9,000 IU D2 | 6,000 | 0 | 0 |
Control diet contains 3,000 IU of vitamin D3/kg of feed.
Excreta Sampling and Analysis
A total of 24-h excreta samples were collected from each bird at weeks 2 and 6. Clean plastic trays were placed below each hen to permit excreta collection, and the samples were collected over 2 consecutive 12-h periods, pooled together and stored at −20°C until further analyses. Excreta samples were freeze-dried, ground through a 1 mm mesh screen, and mixed thoroughly before analyses. Diet and excreta Ca, P, and chromium (Cr) were measured using an inductively coupled plasma optical emission spectrometer (Varian ICP, VISTA MPX, CCD Simultaneous) according to AOAC method 985.01. Briefly, for Ca and P analysis, 1 g of sample was ashed at 600°C overnight in a muffle furnace. The resulting ash was digested and diluted in a 100 mL volumetric flask with deionized water. For Cr analysis, 1 g of feed or 0.20 to 0.25 g of excreta were ashed at 600°C overnight in a muffle furnace and digested on a hot plate. As the color of the sample changed to violet, the digested samples were diluted with deionized water in a 200 mL volumetric flask. The resulting filtrate was used to detect Ca, P, and Cr in diet and fecal samples.
Apparent Total Tract Digestibility of Calcium and Phosphorus
The ATTD of Ca was calculated according to the following equation (Al-Masri, 1995):
where Crd is the chromium content in the diet, Crf is the chromium content in feces, Caf is the Ca content in feces, and Cad is the Ca content in the diet. ATTD of P was also calculated using the above equation.
Hen Performance and Egg Quality Measurement
Egg production and mortality were recorded daily. Eggs produced over 3 consecutive days were collected at the end of the first week and weeks 2, 4, and 6 for egg quality assessment, including egg weight, specific gravity, albumen height, and egg shell thickness. Individual weights of eggs collected over 3 consecutive days were measured in grams and recorded. The specific gravity of eggs was determined using salt solutions of different gradients, as previously described (Bennett, 1993). Albumen height was measured with a micrometer (mm) placing the tripod meter above albumin while avoiding chalaza and yolk. For eggshell thickness, shells were washed to remove albumin and dried at room temperature overnight. Three pieces from different parts of the eggshell were measured (mm), and the average of 3 measurements was taken to compute the eggshell thickness value.
Macroscopic Pathological Tests
At the end of study, all the birds were euthanized. Necropsy was performed in all birds. Visceral organs were separated, and nutritional status (breast muscles, and fat pad), skeletal condition (bones), and calcification of soft tissue (kidney, liver, heart, and lungs) were assessed macroscopically for any injurious effect due to excess supplementation of vitamin D.
Bone Quality Measurement
At the end of week 6, all hens were euthanized by cervical dislocation. Femur and tibia bones from the left legs were collected to measure bone mineral density (BMD), bone mineral content (BMC), and bone area. Tibia and femur samples were stored at −20°C until further processing and analyses. Bone samples were cleaned of non-bone tissues (muscle, fat, tendon, etc.) before the measurement. BMD, BMC, and bone area of the femur and tibia bones were measured using a dual energy x-ray absorptiometry (DEXA; pDEXA, Bone Densitometer, Norland Medical System, Inc. WI). Scanning was performed across each tibia and femur placing each bone at the same position and orientation during the measurement. All scans were obtained at a scan speed of 2.5 mm/s, with a voxel resolution of 0.07 × 0.07 × 0.50 mm.
Statistical Analysis
Data on Ca and P digestibility, egg quality parameters, and bone measurement were subjected to analysis using the general linear model procedure of SAS (SAS Institute Inc., Cary, NC). One-way ANOVA was used with model Yij = µ + αi + εij. Significant differences between treatments were determined using Tukey's honestly significant difference test. Data were considered significantly different if P ≤ 0.05.
RESULTS AND DISCUSSION
Laying Hen Performance and Egg Quality
Laying hen performance and egg quality were compared among the dietary treatments. The hens fed the diet supplemented with 3,000 IU of vitamin D2 had significantly higher (P < 0.05) FI compared to hens fed the diet supplemented with 9,000 IU of vitamin D2 at week 1 (105.05 vs. 87.97), week 2 (104.7 vs. 90.85), week 3 (107.8 vs. 90.34), and week 6 (101.96 and 90.11) (Table 3). Hens fed the diet with 9,000 IU of vitamin D2 had significant lower FI than the ones fed the diet with 3,000 IU of vitamin D3 at week 3 and the ones fed the diet with 3,000 IU of vitamin D3 and the control at week 6. However, there was no difference in FI between hens fed diets supplemented with different levels of vitamin D3 and 25-OHD (Table 3). There was no significant difference in egg production (Table 4) and egg weight (Table 5) by vitamin D2, vitamin D3, or 25-OHD at all weeks of the study. Recently, Akbari Moghaddam Kakhki et al. (2018) also reported that additional 25-OHD supplementation (69 or 138 µ g/kg) on the basal diet containing 3,300 IU of vitamin D3/kg did not show any egg production improvement in aged Lohmann LSL-lite layers (72 to 81 wk old), whereas 25-OHD supplementation significantly increased egg weight and mass. This may be attributed to age; 25-OHD may have better effects on egg weight and mass in old laying hens.
Table 3.
Effect of various levels of vitamin D3, vitamin D2, and 25-OHDroxyvitamin D3 (25-OHDOHD) on feed intake/bird/day of laying hen at late stage of egg production measured each week during 6 wk of study.
| (Feed intake (g)/hen/d) |
|||||||
|---|---|---|---|---|---|---|---|
| Week |
|||||||
| Treatment | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
| Control1 | 97.07 | 98.98a,b | 98.67a,b | 102.28a,b | 93.51 | 102.72a | 98.82a,b |
| Control + 3,000 IU OHD | 85.95 | 91.41a,b | 92.61a,b | 95.62a,b | 89.5 | 110.00a | 93.10a–c |
| Control + 9,000 IU OHD | 84.85 | 96.51a,b | 93.05a,b | 95.03b | 88.84 | 101.66a | 90.11b,c |
| Control + 3,000 IU D3 | 86.25 | 96.78a,b | 98.29a,b | 103.21 a | 94.09 | 105.82a | 99.48a,b |
| Control + 9,000 IU D3 | 92.26 | 94.20a,b | 97.84a,b | 99.58a,b | 93.33 | 103.10a | 94.69a–c |
| Control + 3,000 IU D2 | 102.94 | 105.05a | 104.70a | 107.80a | 97.86 | 109.65a | 101.96a |
| Control + 9,000 IU D2 | 80.85 | 87.97b | 90.85b | 90.34b | 85.1 | 99.10a | 90.11c |
| SEM2 | 5.82 | 3.491 | 2.745 | 2.89 | 2.97 | 6.41 | 2.361 |
| P value3 | 0.13 | 0.043 | 0.018 | 0.003 | 0.091 | 0.769 | 0.0005 |
Means with different superscripts within a column differ significantly (P < 0.05).
Control diet contains 3,000 IU/kg of vitamin D3 in the diet.
SEM, standard error of the mean.
P values from 1-way ANOVA and Tukey's test.
Table 4.
Effect of various levels of vitamin D3, vitamin D2, and 25-OHDroxyvitamin D3 (25-OHDOHD) on hen day egg production of laying hens at late stage of egg production for 6 wk.
| Treatment | Hen-day egg production, % |
|---|---|
| Control1 | 98.33 |
| Control + 3,000 IU 25-OHD | 97.30 |
| Control + 9,000 IU 25-OHD | 96.93 |
| Control + 3,000 IU D3 | 95.27 |
| Control + 9,000 IU D3 | 96.62 |
| Control + 3,000 IU D2 | 95.60 |
| Control + 9,000 IU D2 | 95.60 |
| SEM2 | 1.82 |
| P value3 | 0.893 |
Control diet contains 3,000 IU/kg of vitamin D3 in the diet.
SEM, standard error of the mean.
P values from Tukey's test.
Table 5.
Effect of various levels of vitamin D3, vitamin D2, and 25-OHDroxyvitamin D3 (25-OHOHD) on egg weights of laying hens at late stage of egg production measured at different weeks of the study.
| Egg mass, g/hen/d |
||||
|---|---|---|---|---|
| Week |
||||
| Treatment | 0 | 2 | 4 | 6 |
| Control1 | 64.36 | 61.86 | 59.61 | 63.00 |
| Control + 3,000 IU 25-OHD | 60.48 | 60.58 | 61.37 | 60.98 |
| Control + 9,000 IU 25-OHD | 59.68 | 62.11 | 59.84 | 62.87 |
| Control + 3,000 IU D3 | 64.46 | 63.18 | 63.57 | 65.22 |
| Control + 9,000 IU D3 | 60.85 | 60.23 | 59.88 | 60.72 |
| Control + 3,000 IU D2 | 63.27 | 65.18 | 62.77 | 64.83 |
| Control + 9,000 IU D2 | 61.03 | 58.11 | 56.15 | 59.69 |
| SEM2 | 1.45 | 1.41 | 1.67 | 1.46 |
| P value3 | 0.137 | 0.085 | 0.075 | 0.080 |
Control diet contains 3,000 IU/kg of vitamin D3 in the diet.
SEM, standard error of the mean.
P values from Tukey's test.
In the current study, the addition of different isoforms of vitamin D (vitamin D2, vitamin D3, or 25-OHD) did not improve egg quality parameters measured. This is in agreement with other studies. Mattila et al. (2004, 2011) reported that FI, egg mass, Haugh unit, specific gravity and calcium content in egg shells were not significantly affected by vitamin D3 or D2 in the treatment diets during the 48-wk experimental period. Keshavarz (2003) and Kappeli et al. (2011) reported no effect on body weight gain, egg production, and feed conversion in laying hens when 2,760 IU of D3 or 25-OHD was supplemented to the treatment diets. Increasing the concentration of vitamin D3 up to 102,200 IU/kg of diet did not show any negative effect on hen performance and egg quality measurements (Persia et al., 2013). On the basis of previous studies (Persia et al., 2013), improvement in egg quality parameters was not expected in hen fed vitamin D3 and 25-OHD dietary treatments. The reason behind no effect of different forms and levels of vitamin D on egg quality parameters during our study may be due to inclusion of adequate levels of Ca, available P, and vitamin D3 in the control diet. Thus, additional supplementation of different isoforms of vitamin D in access amount may not increase the egg quality of laying hens in a late stage production cycle.
Macroscopic observation of soft tissues did not show any signs of calcification, indicating that there was no negative effect of high dosages of vitamin D even if 12,000 IU/kg of vitamin D3 was included in laying hen diets in the current study. The finding is similar to Mattila et al. (2003) that used up to 12,000 IU/kg of vitamin D3 in laying hen diet without any lesions of high Ca deposition in histological sections of soft tissues. Baker et al. (1998) demonstrated no toxic effect in broilers fed up to 50,000 IU vitamin D3/kg feed, and Persia et al. (2013) stated that inclusion of up to 102,200 IU of D3/kg of diet did not consistently affect laying hen performance or egg quality over the 19 to 58 wk of age feeding period.
Calcium and Phosphorus Utilization
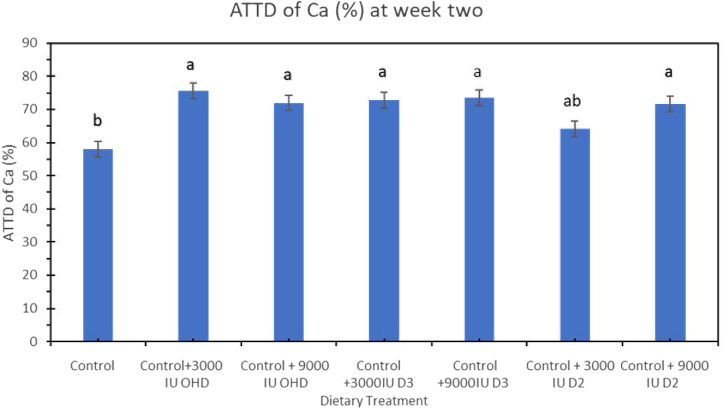
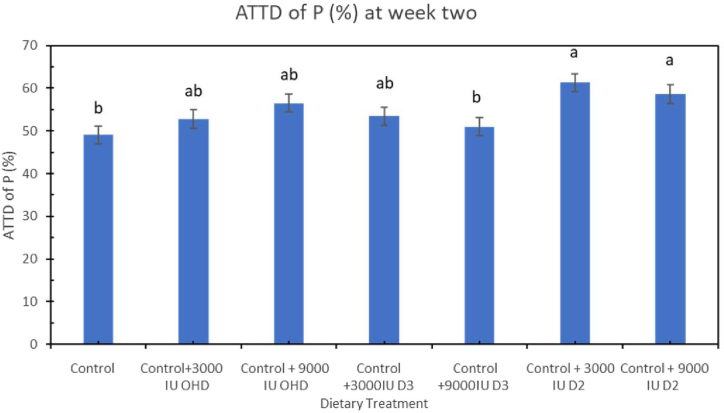
Ca and P utilization were increased by different forms of vitamin D in the diets. At week 2, ATTD of Ca was significantly higher (P < 0.001) in hens fed dietary treatments supplemented with additional vitamin D compared to hens fed control diet (Figure 1). Hens fed dietary treatment supplemented with 3,000 IU of vitamin D3/kg had the highest ATTD of Ca compared to other 6 treatments. ATTD of P at week 2 was significantly higher (P = 0.0031) in hens fed diet supplemented with 3,000 or 9,000 IU vitamin D2/kg (61.23 or 58.03%) compared to hens fed positive control diet (49.05%) (Figure 2).
Figure 1.
Effect of vitamin D3, vitamin D2, and 25-OHDroxyvitamin D3 on apparent total tract digestibility (ATTD) of calcium (Ca) in laying hens fed adequate Ca and P at week 2 of study. Bars with different letters (a, b) differ significantly (P < 0.05, n = 6, error bars = SEM).
Figure 2.
Effect of vitamin D3, vitamin D2, and 25-OHDroxyvitamin D3 on apparent total tract digestibility (ATTD) of phosphorus (P) in laying hens fed adequate Ca and P at week 2 of study. Bars with different letters (a, b) differ significantly (P < 0.05, n = 6, error bars = SEM).
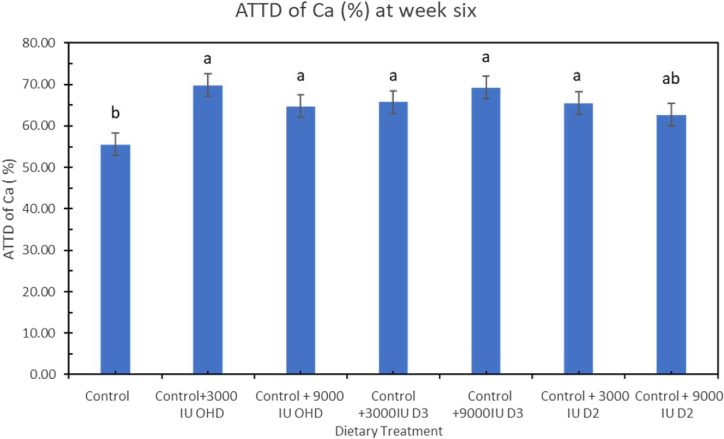
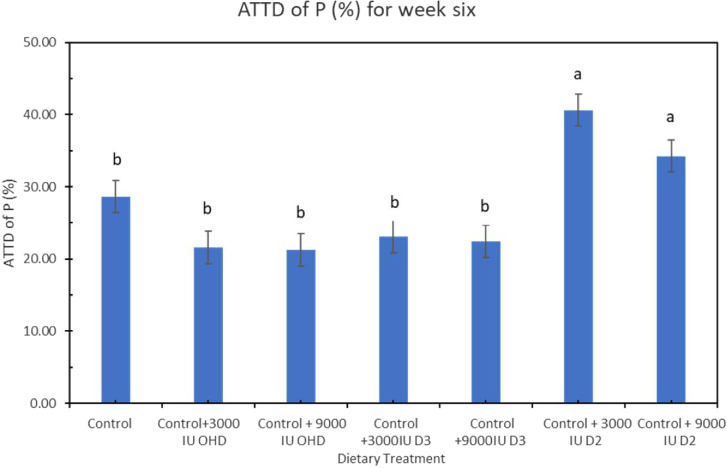
At week 6, ATTD of Ca was significantly higher in hens fed all other dietary treatments except for 3,000 IU vitamin D2/kg compared to control diet (P = 0.0127) (Figure 3). Hens fed diet supplemented with 9,000 IU of D3 (69.2%) and 3,000 IU of 25-OHD (79.76%) had the highest ATTD of Ca. At week 6, ATTD of P was significantly higher (P < 0.0001) in hens fed dietary treatment supplemented with both levels of vitamin D2 compared to hens fed other treatment diet (Figure 4). This is the first study that investigates the effect of extra dosages of vitamin D2 in Ca and P utilization when supplied to laying hen diets containing 3,000 IU vitamin D3/kg. Addition of vitamin D2 in laying hens diet together with basal level of vitamin D3 increased P utilization in laying hens.
Figure 3.
Effect of vitamin D3, vitamin D2, and 25-OHDroxyvitamin D3 on apparent total tract digestibility (ATTD) of calcium (Ca) in laying hens fed adequate Ca and P at week 6 of study. Bars with different letters (a, b) differ significantly (P < 0.05, n = 6, error bars = SEM).
Figure 4.
Effect of vitamin D3, vitamin D2, and 25-OHDroxyvitamin D3 on apparent total tract digestibility (ATTD) of phosphorus (P) in laying hens fed adequate Ca and P at week 6 of study. Bars with different letters (a, b) differ significantly (P < 0.05, n = 6, error bars = SEM).
Supplementation of higher concentration of vitamin D improved Ca digestibility in laying hen. Positive effect on Ca utilization was seen in all 3 forms of vitamin D used in this study. There was no difference (P = 0.201) in ATTD of Ca and P in laying hens when similar amounts of 25-OHD and vitamin D3 were added in the diet for up to 6 wk. Supplementation of higher concentration of 25-OHD in normal level of Ca and P diet synergistically improved digestibility of Ca and P in broiler (Qian et al., 1997; Keshavarz, 2003), which is similar to the finding of our study. A study was conducted to study the effect of different levels of vitamin D3, phytase, Ca, and P on mineral retention in broilers. The results concluded that supplementation of vitamin D3 in the diet increased retention of Ca and P in broilers (Delezie et al., 2012).
No study has been conducted to determine the effect of vitamin D2 in Ca and P utilization in poultry. It is believed that vitamin D2 is not as potent as vitamin D3 to make an effect on Ca and P utilization in poultry. Most of the study conducted to determine the effect of different metabolites of vitamin D has been done in rachitic chickens (Chen and Bosmann, 1964; Hibberd and Norman, 1969; Fraser et al., 2013). Degradation of vitamin D and its turnover can be different in between healthy chicks and chicks with vitamin D deficiency. Favus and Langman (1984) found out that 25-OHD turnover was greatly reduced when vitamin D-deficient animals were used compared to normal animals. The author also presented that the physiological amount of 25-OHD turnover increased when deficient animals were supplemented with 25-OHD at dose of 10 ng/d for 4 d as compared to the vitamin D non-deficient animals which showed no turnover of 25-OHD. It has been demonstrated that vitamin D2 and vitamin D3 have similar rate of metabolism in blood compared to the other metabolites of respective vitamins (Hoy et al., 1988; Lehmann and Meurer, 2010). The difference between previous studies and studies from Hoy et al. (1988) is use of rachitic chicken vs. healthy chicken. Thus, vitamin D2 may show metabolic effect in laying hen when used with adequate vitamin D3 in the diet. Here we report that utilization of dietary Ca and phosphorus is increased in laying hens when combination of vitamin D2 and D3 is supplemented to the diet.
Mattila et al. (2004, 2011) have discussed D3 to be more effective than D2 in deposition of vitamin in egg yolk. Nevertheless, the time required to reach a top concentration of vitamin D3 in yolk was like vitamin D2. This indicates that there is absorption and metabolism of vitamin D2 in birds, which could have produced a beneficial effect on Ca and P metabolism.
Despite their structural differences in molecular forms, vitamin D2 and vitamin D3 exhibit identical sets of biological response around the body through same VDR-mediated regulations of gene expression (Jones, 2013). It has been stated that D2 is not potentially active in avian species with speculations and question raising over the similar effect of vitamin D2 over D3 in other animals and humans. One of the constant markers of this measurement in human studies has been measurement of rise in plasma levels of 25-OH-D3. There are studies that indicate that vitamin D2 is less active than vitamin D3 in its potential function and maintaining 25-OH-D level in blood (Trang et al., 1998; Logan et al., 2013). However, there are studies that have argued both analogs of vitamin D have similar effect in maintaining blood 25-OH-D (Holick et al., 2008). The difference in the studies can be due to variation with study design, dose, size, and frequency of the experimental vitamin D used in the study (Jones, 2013). A systematic review by Tripkovic et al. (2012) indicated that vitamin D3 was highly effective in raising 25-OHD compared to D2; however, when the frequency of dose administration was compared, administration of bolus dose resulted in a significant increase of serum 25-OHD from vitamin D3 in compared to D2, but there was no difference when daily supplementation was done.
As both the analogs of vitamin D share similar pathway, it could be possible that one form of vitamin D helps another form of vitamin D in absorption of Ca and P form intestine of birds. We hypothesize that there may be an interaction pathway between vitamin D3 and vitamin D2 which can enhance the absorption P in the intestine of laying hens. Further research needs to be considered to study the effect of vitamin D2 when it is used in combination with other potentially effective vitamins.
Bone Mineralization
Femurs and tibia separated from laying hens of each treatment group were measured for BMD, BMC, and bone area using DEXA. Inclusion of different levels and forms of vitamin D did not affect BMD, BMC, and bone area of femurs and tibia in mature laying hens (Table 6). Supplementation of different isoform and increasing concentration of vitamin D did not make any difference in bone strength of laying hens at late stage production. DEXA has been used as a tool to evaluate BMD, BMC, and bone area in laying hens (Shahnazari et al., 2006). BMD and BMC measurements from DEXA have been positively correlated with breakage strength, bone ash, and bone concentration in laying hens and broilers (Kim et al., 2006, 2011).
Table 6.
Effect of various levels of vitamin D3, vitamin D2, and 25-OHDOHD on bone mineral density (BMD), bone mineral content (BMC), and bone area of laying hens fed adequate levels of calcium and phosphorus at late stage of egg production.
| Femur |
Tibia |
|||||
|---|---|---|---|---|---|---|
| Treatment | BMD (g/cm2) | BMC (g) | Bone area (cm2) | BMD (g/cm2) | BMC (g) | Bone area (cm2) |
| Control1 | 0.205 | 2.08 | 10.149 | 0.189 | 2.55 | 13.471 |
| Control + 3,000 IU 25-OHD | 0.217 | 2.17 | 9.983 | 0.199 | 2.68 | 13.461 |
| Control + 9,000 IU 25-OHD | 0.198 | 1.97 | 9.971 | 0.186 | 2.486 | 13.38 |
| Control + 3,000 IU D3 | 0.203 | 2.01 | 9.836 | 0.184 | 2.433 | 13.193 |
| Control + 9,000 IU D3 | 0.196 | 1.93 | 9.839 | 0.182 | 2.393 | 13.14 |
| Control + 3,000 IU D2 | 0.198 | 1.97 | 9.976 | 0.183 | 2.457 | 13.391 |
| Control + 9,000 IU D2 | 0.219 | 2.18 | 9.845 | 0.203 | 2.662 | 13.147 |
| SEM2 | 0.006 | 0.07 | 0.133 | 0.006 | 0.083 | 0.17 |
| P value3 | 0.109 | 0.20 | 0.698 | 0.103 | 0.12 | 0.614 |
Control diet contains 3,000 IU/kg of vitamin D3 in the diet.
SEM, standard error of the mean.
P values from Tukey's test.
The result obtained in this study is in agreement with the finding by Keshavarz (1996) where supplementation of different level of vitamin D in laying hens diet with sufficient levels of Ca and P did not significantly increased tibia ash. Similar to our finding, laying hens fed up to 102,200 IU vitamin D/kg of diet for 40 wk did not show significant difference in tibia ash determination (Persia et al., 2013). A study conducted to compare the effect of vitamin D3 and 25-OHD (12,000 IU/kg of diet) in broiler breeders concluded that 25-OHD was more potent compared to vitamin D3 on bone ash of progeny in broiler breeders (Atencio et al., 2005).
Laying hen need constant supply of Ca for daily egg shell formation and bone maintenance. When inclusion of Ca or vitamin D is low in diets fed to laying hens, Ca is released from bones and deposited into the egg shell. Laying hens control their calcium balance either through absorption of dietary Ca in the intestine or through bone resorption (Świątkiewicz et al. 2010). Inclusion of adequate Ca and P in laying hen diets allows constant supply of Ca and P to maintain Ca homeostasis in birds during egg production. Birds more than 55 wk old are called late-laying stage laying hens. As birds get older, change in bone mineral content and density in these birds are gradually decreased due to higher bone resorption and lower bone formation of the birds (Browning and Cowieson, 2015). It is important to maximizing bone mineralization because it will increase bone strength and decrease problems related to leg weakness, morbidity, and mortalities (Kakhki et al., 2019). During the laying period, formation of normal structural lamellar bone ceases and only medullary bone is formed, which does not attribute to functional strength (Fleming, 2008). Medullary bones offer minimal functional strength to the bones, and turnover of compact bone is very slow as birds gets older. The current study may not be long enough to detect any difference in composition of bone at this age of laying hens, especially when adequate amounts of Ca, P, and vitamin D3 have been used in the basal diet. Further study can be considered to evaluate the skeletal health of young laying hens fed low Ca and P diets supplemented with different combination of vitamin D isoforms in the future. This would give more in depth understanding if BMD and BMC reduction while feeding low Ca and P would be minimized and restored by supplementation of different forms of vitamin D in the diet.
In conclusion, feeding diets supplemented with vitamin D2 in combination with vitamin D3 could result in an increase in Ca and P utilization in laying hens in late laying cycle. However, there is no difference between vitamin D3 and 25-OHD in Ca and P utilization in laying hens at late laying stage. Ca and P utilization can be improved by increasing the combined isoforms of vitamin D in diets; however, they do not have effects on egg production, bone health, and egg quality parameters of laying hens when adequate amounts of Ca, P, and vitamin D are used in the diet.
Adequate supplementation of Ca, P, and vitamin D in the diet is sufficient to maintain bone health of laying hen at a late laying cycle. There is no positive effect of increasing dietary vitamin D or replacing different isoform of vitamin D in laying hen for first 6 wk when they are fed adequate Ca, P, and vitamin D in the diet.
ACKNOWLEDGMENT
We are grateful to Jerry Levandoski and Harry Muc for care of experimental animals and helping with lab works.
REFERENCES
- Akbari Moghaddam Kakhki R., Heuthorst T., Mills A., Neijat M., Kiarie E. Interactive effects of calcium and top-dressed 25-hydroxy vitamin D3 on egg production, egg shell quality, and bones attributes in aged Lohmann LSL-lite layers. Poult. Sci. 2018;98:1254–1262. doi: 10.3382/ps/pey446. [DOI] [PubMed] [Google Scholar]
- Al-Masri M.R. Absorption and endogenous excretion of phosphorus in growing broiler chicks, as influenced by calcium and phosphorus ratios in feed. Br. J. Nutr. 1995;74:407–415. doi: 10.1079/bjn19950144. [DOI] [PubMed] [Google Scholar]
- Atencio A., Pesti G.M., Edwards H.M., Jr. Twenty-five OHDroxycholecalciferol as a cholecalciferol substitute in broiler breeder hen diets and its effect on the performance and general health of the progeny. Poult. Sci. 2005;84:1277–1285. doi: 10.1093/ps/84.8.1277. [DOI] [PubMed] [Google Scholar]
- Baker D.H., Biehl R.R., Emmert J.L. Vitamin D3 requirement of young chicks receiving diets varying in calcium and available phosphorus. Br. Poult. Sci. 1998;39:413–417. doi: 10.1080/00071669888980. [DOI] [PubMed] [Google Scholar]
- Bar A. Calcium transport in strongly calcifying laying birds: mechanisms and regulation. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2009;152:447–469. doi: 10.1016/j.cbpa.2008.11.020. [DOI] [PubMed] [Google Scholar]
- Bennett C.D. Measuring table egg shell quality with one specific gravity salt solution. J. App. Poult. Res. 1993;2:130–134. [Google Scholar]
- Bikle D.D. Role of vitamin D, its metabolites, and analogs in the management of osteoporosis. Rheum. Dis. Clin. North. Am. 1994;20:759–775. [PubMed] [Google Scholar]
- Browning L.C., Cowieson A.J. Interactive effects of vitamin D3 and strontium on performance, nutrient retention and bone mineral composition in laying hens. J. Sci. Food Agric. 2015;95:1080–1087.. doi: 10.1002/jsfa.6801. [DOI] [PubMed] [Google Scholar]
- Chen P.S., Jr., Bosmann H.B. Effect of vitamins D2 and D3 on serum calcium and phosphorus in rachitic chicks. J. Nutr. 1964;83:133–139. doi: 10.1093/jn/83.2.133. [DOI] [PubMed] [Google Scholar]
- Delezie E., Maertens L., Huyghebaert G. Consequences of phosphorus interactions with calcium, phytase, and cholecalciferol on zootechnical performance and mineral retention in broiler chickens. Poult. Sci. 2012;91:2523–2531. doi: 10.3382/ps.2011-01937. [DOI] [PubMed] [Google Scholar]
- Favus M.J., Langman C.B. Effects of 1,25-diOHDroxyvitamin D3 on colonic calcium transport in vitamin D-deficient and normal rats. Am. J. Physiol. 1984;246:G268–G273. doi: 10.1152/ajpgi.1984.246.3.G268. [DOI] [PubMed] [Google Scholar]
- Fleming R.H. Nutritional factors affecting poultry bone health. Proc. Nutr. Soc. 2008;67:177–183. doi: 10.1017/S0029665108007015. [DOI] [PubMed] [Google Scholar]
- Fraser D., Duncan I.J., Edwards S.A., Grandin T., Gregory N.G., Guyonnet V., Hemsworth P.H., Huertas S.M., Huzzey J.M., Mellor D.J., Mench J.A. General principles for the welfare of animals in production systems: the underlying science and its application. Vet. J. 2013;198:19–27. doi: 10.1016/j.tvjl.2013.06.028. [DOI] [PubMed] [Google Scholar]
- Fritts C.A., Waldroup P.W. Effect of source and level of vitamin d on live performance and bone development in growing broilers. J. Appl. Poult. Res. 2003;12:45–52. [Google Scholar]
- Garcia A.F.Q.M., Murakami A.E., do Amaral Duarte C.R., Rojas I.C.O., Picoli K.P., Puzotti M.M. Use of vitamin D3 and its metabolites in broiler chicken feed on performance, bone parameters and meat quality. Asian-Australas. J. Anim. Sci. 2013;26:408–415. doi: 10.5713/ajas.2012.12455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibberd K.A., Norman A.W. Comparative biological effects of vitamins D2 and D3 and diOHDrotachysterol2 and diOHDrotachysterol3 in the chick. Biochem. Pharmacol. 1969;18:2347–2355. doi: 10.1016/0006-2952(69)90349-9. [DOI] [PubMed] [Google Scholar]
- Holick M.F., Biancuzzo R.M., Chen T.C., Klein E.K., Young A., Bibuld D., Reitz R., Salameh W., Ameri A., Tannenbaum A.D. Vitamin D2 is as effective as vitamin D3 in maintaining circulating concentrations of 25-OHDroxyvitamin D. J. Clin. Endocrinol. Metab. 2008;93:677–681. doi: 10.1210/jc.2007-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoy D.A., Ramberg C.F., Jr., Horst R.L. Evidence that discrimination against ergocalciferol by the chick is the result of enhanced metabolic clearance rates for its mono- and diOHDroxylated metabolites. J. Nutr. 1988;118:633–638. doi: 10.1093/jn/118.5.633. [DOI] [PubMed] [Google Scholar]
- Jones G. Extrarenal vitamin D activation and interactions between vitamin D-2, vitamin D-3, and vitamin D analogs. In: Cousins R.J., editor. Annual Review of Nutrition, Vol 33. Annual Review of Nutrition No. 33. Annual Reviews, Palo Alto. 2013. pp. 23–44. [DOI] [PubMed] [Google Scholar]
- Kakhki R., Heuthorst T., Mills A., Neijat M., Kiarie E. Interactive effects of calcium and top-dressed 25-hydroxy vitamin D3 on egg production, egg shell quality, and bones attributes in aged Lohmann LSL-lite layers. Poult. Sci. 2019;98:1254–1262. doi: 10.3382/ps/pey446. [DOI] [PubMed] [Google Scholar]
- Kappeli S., Fröhlich E., Gebhardt-Henrich Sabine G., Pfulg A., Schäublin Heidi, Zweifel R., Wiedmer H., Stoffel M.H. Effects of dietary supplementation with synthetic vitamin D3 and 25-hydroxycholecalciferol on blood calcium and phosphate levels and performance in laying hens. Arch. Geflügelk. 2011;75:179–184. [Google Scholar]
- Kebreab E., France J., Kwakkel R.P., Leeson S., Kuhi H.D., Dijkstra J. Development and evaluation of a dynamic modelof calcium and phosphorus flows in layers. Poult. Sci. 2009;88:680–689. doi: 10.3382/ps.2008-00157. [DOI] [PubMed] [Google Scholar]
- Keshavarz K. The effect of different levels of vitamin C and cholecalciferol with adequate or marginal levels of dietary calcium on performance and eggshell quality of laying hens. Poult. Sci. 1996;75:1227–1235. doi: 10.3382/ps.0751227. [DOI] [PubMed] [Google Scholar]
- Keshavarz K. A comparison between cholecalciferol and 25-OH-cholecalciferol on performance and eggshell quality of hens fed different levels of calcium and phosphorus. Poult. Sci. 2003;82:1415–1422. doi: 10.1093/ps/82.9.1415. [DOI] [PubMed] [Google Scholar]
- Kim W.K., Bloomfield S.A., Ricke S.C. Effects of age, vitamin D3, and fructooligosaccharides on bone growth and skeletal integrity of broiler chicks. Poult. Sci. 2011;90:2425–2432. doi: 10.3382/ps.2011-01475. [DOI] [PubMed] [Google Scholar]
- Kim W., Donalson L.M., Mitchell A.D., Kubena L.F., Nisbet D.J., Ricke S.C. Effects of alfalfa and fructooligosaccharide on molting parameters and bone qualities using dual energy X-ray absorptiometry and conventional bone assays. Poult. Sci. 2006;85:15–20. doi: 10.1093/ps/85.1.15. [DOI] [PubMed] [Google Scholar]
- Lavelin I., Meiri N., Pines M. New insight in eggshell formation. Poult. Sci. 2000;79:1014–1017. doi: 10.1093/ps/79.7.1014. [DOI] [PubMed] [Google Scholar]
- Leeson S. Vitamin requirements: is there basis for re-evaluating dietary specifications? World's Poult. Sci. J. 2007;63:255–266. [Google Scholar]
- Lehmann B., Meurer M. Vitamin D metabolism. Derma. Therapy. 2010;23:2–12. doi: 10.1111/j.1529-8019.2009.01286.x. [DOI] [PubMed] [Google Scholar]
- Logan V.F., Gray A.R., Peddie M.C., Harper M.J., Houghton L.A. Long-term vitamin D3 supplementation is more effective than vitamin D2 in maintaining serum 25-OHDroxyvitamin D status over the winter months. Br. J. Nutr. 2013;109:1082–1088. doi: 10.1017/S0007114512002851. [DOI] [PubMed] [Google Scholar]
- Mattila P., Rokka T., Könkö K., Valaja J., Rossow L., Ryhänen E.L. Effect of cholecalciferol-enriched hen feed on egg quality. J. Agric. Food. Chem. 2003;51:283–287. doi: 10.1021/jf020743z. [DOI] [PubMed] [Google Scholar]
- Mattila P., Valaja J., Rossow L., Venalainen E., Tupasela T. Effect of vitamin D2- and D3-enriched diets on egg vitamin D content, production, and bird condition during an entire production period. Poult. Sci. 2004;83:433–440. doi: 10.1093/ps/83.3.433. [DOI] [PubMed] [Google Scholar]
- Mattila P.H., Valkonen E., Valaja J. Effect of different vitamin D supplementations in poultry feed on vitamin D content of eggs and chicken meat. J. Agric. Food. Chem. 2011;59:8298–8303. doi: 10.1021/jf2012634. [DOI] [PubMed] [Google Scholar]
- Persia M.E., Higgins M., Wang T., Trample D., Bobeck E.A. Effects of long-term supplementation of laying hens with high concentrations of cholecalciferol on performance and egg quality. Poult. Sci. 2013;92:2930–2937. doi: 10.3382/ps.2013-03243. [DOI] [PubMed] [Google Scholar]
- Qian H., Kornegay E.T., Denbow D.M. Utilization of phytate phosphorus and calcium as influenced by microbial phytase, cholecalciferol, and the calcium:total phosphorus ratio in broiler diets. Poult. Sci. 1997;76:37–46. doi: 10.1093/ps/76.1.37. [DOI] [PubMed] [Google Scholar]
- Shahnazari M., Sharkey N.A., Fosmire G.J., Leach R.M. Effects of strontium on bone strength, density, volume, and microarchitecture in laying hens. J. Bone Miner. Res. 2006;21:1696–1703. doi: 10.1359/jbmr.060724. [DOI] [PubMed] [Google Scholar]
- Świątkiewicz S., Koreleski J., Arczewska A. Effect of organic acids and prebiotics on bone quality in laying hens fed diets with two levels of calcium and phosphorus. Acta Vet. BRNO. 2010;79:185–193. [Google Scholar]
- Tsang W.T., Wu M.C., Yang L., Chen Y.K., Sergent A.M. Strained-layer 1.5 μm wavelength InGaAs/InP multiple quantum well lasers grown by chemical beam epitaxy. Electr Lett. 1990;26:2035–2036. [Google Scholar]
- Trang H.M., Cole D.E., Rubin L.A., Pierratos A., Siu S., Vieth R. Evidence that vitamin D3 increases serum 25-OHDroxyvitamin D more efficiently than does vitamin D2. Am. J. Clin. Nutr. 1998;68:854–858. doi: 10.1093/ajcn/68.4.854. [DOI] [PubMed] [Google Scholar]
- Tripkovic L., Lambert H., Hart K., Smith C.P., Bucca G., Penson S., Chope G., Hyppönen E., Berry J., Vieth R., Lanham-New S. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-OHDroxyvitamin D status: a systematic review and meta-analysis. Am. J. Clin. Nutr. 2012;95:1357–1364. doi: 10.3945/ajcn.111.031070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead C.C. Overview of bone biology in the egg-laying hen. Poult. Sci. 2004;83:193–199. doi: 10.1093/ps/83.2.193. [DOI] [PubMed] [Google Scholar]
- Whitehead C.C., Fleming R.H., Julian R.J., Sørensen P. Skeletal problems associated with selection for increased production. Poult. Genet. Breed. Biotechnol. 2003;3:29–52. [Google Scholar]