Abstract
This study evaluated the growth performance, immunity, and jejunum morphology of chicks hatched from laying breeder hens given dietary additive supplementation, as well as chicks receiving direct antibiotic supplementation in early life. Hy-line breeder hens were allotted to 2 groups with 3 replicates. A control group (CON) was fed a basal diet, and the treatment group (CCAB) received β-carotene, curcumin, allicin, and sodium butyrate in addition to basal diet for 5 wk. Breeder–hen eggs were collected and hatched. The chicks hatched from the CON group were assigned to 2 treatments: a chick control group (cCON) and a chick treatment group (Cipro) given ciprofloxacin lactate into drinking water; the cCON group, Cipro group, and the chicks hatched from the CCAB group (cCCAB) were fed the same diet for 4 wk. The results demonstrated that there were significant differences between the CON and CCAB groups in the serum levels of IgA, IgG, IgM (triple P < 0.01), lysozyme (P < 0.05), and β-defensin (P < 0.05). The body weights of the cCCAB group's chicks increased at 1, 7, and 28 D of age (P < 0.05, P < 0.05, P < 0.01, respectively), and those of the Cipro group's chicks increased at 7 and 21 D of age (P < 0.01, P < 0.05). The tibial lengths of the cCCAB group's chicks increased at 1, 7, 14, 21, and 28 D of age (P < 0.01, P < 0.05, triple P < 0.01), and the lengths in the Cipro group increased at 7 and 14 D of age (P < 0.01, P < 0.01). Intestinal development, including intestinal length, jejunum morphology, and IgA positive cells, helps to explain these results. The breeder eggs from the CCAB group had higher IgG (P < 0.05) and IgM (P < 0.05) levels in the egg whites and higher IgA, IgG, and IgM levels (triple P < 0.01) in the egg yolks. In conclusion, β-carotene, curcumin, allicin, and sodium butyrate supplementation of laying breeder hen diets produced more advantages in growth performance and intestinal development in offspring than in chicks directly supplemented with antibiotics.
Key words: laying breeder hen, β-carotene, maternal antibody, antibiotic, intestinal health
INTRODUCTION
Eggs play an important role in human diets and provide a variety of nutrients. Therefore, development of the egg-laying industry is important (Wilson, 2017). However, egg production in modern farming is a complex process that includes breeder–hen farming, hatching, rearing periods, and laying periods. Each stage in this process presents potential hazards for production. Importantly, the health of the chicks directly determines production performance and egg quality during the laying period (Lang et al., 2019). According to previous studies, newborn chicks experience relatively high mortality rates, posing challenges for the laying industry. To guarantee the growth and health of chicks, antibiotics have been widely used to increase feed conversion, prevent disease, and promote growth during early life (Emami et al., 2012; Chattopadhyay, 2014). However, a substantial amount of evidence suggests that the increasing problem of antibiotic resistance is associated with the large-scale use of antibiotics in the poultry industry (Forgetta et al., 2012), and the high prevalence of multiresistant Salmonella, E. coil, and Campylobacter spp. influences meat and egg quality (Schwaiger et al., 2012; Yulistiani et al., 2017). A number of strategies have been proposed to reduce the use of antibiotics in the poultry-farming industry, including phytogenic feed additives, phytoncides, and organic acids (Mehdi et al., 2018).
β-carotene is a type of pro-vitamin A carotenoid with beneficial effects on antioxidation and immunity and has been widely used in different areas (Vrolijk et al., 2015). Curcumin has long been used as a dietary spice, and recent research has shown that curcumin possesses immunomodulatory, antioxidant, and anti-inflammatory properties and is used as an herbal medication for the treatment of inflammation (Sharma et al., 2005). Allicin, a sulfur-containing and volatile compound, is found in white garlic and possesses a variety of beneficial biological effects, including antimicrobial, antioxidant, and immunomodulatory activities (Salehi et al., 2019). Butyrate is a short-chain fatty acid produced by microbiota in the large intestine of animals and has multiple functions that benefit the cells of the gut, including immune modulation and oxidative stress reduction (Bedford and Gong, 2018). Therefore, because of their natural and beneficial effects, this study used these 4 substances as dietary additives to promote animal health.
β-carotene, curcumin, allicin, and sodium butyrate were used to supplement breeder laying hen diets in an attempt to improve their immunity and to observe the growth performance, immunity, and intestinal morphology of their offspring in the early life period. These characteristics were compared with those of chicks directly given antibiotic supplementation in their diets.
MATERIALS AND METHODS
This experiment was approved by the Animal Ethical Committee of Jilin Agricultural University.
Bird Management
A total of 162 Hy-line Brown laying breeder hens (Rhode Island White) at the age of 45 wk of age and with a similar physiological status were randomly allotted to 2 treatment groups (control group [CON] and a β-carotene, curcumin, allicin, and sodium butyrate supplementation group [CCAB]), with 3 replications of 27 hens in each group. Hens were kept in cages (60 × 40 × 40 cm3) equipped with 2 nipple drinkers and 1 feeder, with 3 hens per cage. Semen was collected from heathy male chickens (Rhode Island Red) and injected into the ovaries of the hens. Artificial insemination was conducted once every 5 D in the afternoon. A total of 150 eggs from each group was collected on the last 3 D of the experimental period and hatched under standard conditions of 70 to 80% humidity at 37.8°C with intermittent rotation. The experiment was carried out at the Changchun Academy of Agricultural Science, Changchun, China. The number of dead embryos and fertile eggs were determined by candling eggs on 19 D of incubation. After incubation, 60 healthy male chicks from the CON group with similar body weights were divided into 2 groups (a chick control group [cCON] and a ciprofloxacin lactate treatment group [Cipro]) with each group comprising of 30 chicks each. Another 30 male chicks were selected from the CCAB-group eggs and formed a group (cCCAB). These chicks were housed in 3 cages (70 × 65 × 40 cm3) at the same altitude under a standard brooding management system with gradually deceasing temperatures ranging from 36 to 26°C. The details of the chick brooding management are shown in Table 1, and the management was in accordance with the management guide provided by Hy-line International Co., Ltd, USA. The body weights and tibial lengths of all the chicks were recorded at 7, 14, 21, and 28 D of age.
Table 1.
Chick-brooding management.
| Age | 0–3 D | 4–7 D | 8–14 D | 15–21 D | 22–28 D |
|---|---|---|---|---|---|
| Air temperature (°C) | 35–36 | 33–35 | 31–33 | 29–31 | 26–27 |
| Light intensity (Lux) | 30–50 | 30–50 | 25 | 25 | 25 |
| Light hours (h) | 22 | 21 | 20 | 19 | 18 |
| Relative humidity (%) | 60 | 60 | 60–40 | 60–40 | 60–40 |
Experimental Design and Diets
After 1-wk acclimation period, the breeder hens of the CON group were fed a basal diet, as shown in Table 2, while the hens of the CCAB group were fed the same basal diet supplemented with 60 mg/kg β-carotene, 250 mg/kg curcumin, 250 mg/kg allicin, and 500 mg/kg sodium butyrate (Shaanxi Kingreg Biotech Co., Ltd, China). All hens were allowed to eat and drink freely. The laying breeder hens experiment lasted 5 wk and included a 1-wk acclimation period. All chicks were fed a single basal diet (Table 2) and allowed to eat and drink at will. The Cipro group had 100 mg/L ciprofloxacin lactate (Shaanxi Kingreg Biotech Co., Ltd, China) added to their drinking water, and they were allowed to eat and drink at will. The direct ciprofloxacin lactate supplementation experiment lasted 4 wk.
Table 2.
Basal diet composition of experimental laying breeder hens and chicks.
| Items | Laying breeder hen diet | Chick diet |
|---|---|---|
| Ingredient (%) | ||
| Corn | 60.00 | 56.19 |
| Soybean meal | 22.30 | 19.61 |
| Limestone | 9.00 | 1.53 |
| Wheat middling and red dog | 3.00 | 8.00 |
| Soybean oil | 1.50 | − |
| Puffed soybean | − | 8.00 |
| Corn gluten meal | − | 2.00 |
| Fish meal | − | 2.00 |
| CaHPO4 | 1.50 | 1.10 |
| Premix1 | 1.40 | 1.00 |
| Choline chloride | 0.45 | 0.06 |
| NaCl | − | 0.25 |
| Lys | 0.35 | 0.15 |
| Thr | 0.30 | 0.10 |
| Met | 0.20 | 0.01 |
| Total | 100 | 100 |
| Calculated composition2 (%) | ||
| ME(KJ/kg) | 11.22 | 12.90 |
| CP | 15.85 | 20.20 |
| Ca | 3.76 | 1.05 |
| Available phosphorus | 0.460 | 0.380 |
| Lys | 1.100 | 1.128 |
| Methionine | 0.430 | 0.487 |
| Met+Cys | 0.726 | 0.847 |
The premix provided the following per kg of diet: Vitamin A 8,000 IU, Vitamin D 3,750 IU, Vitamin E 100 mg, Vitamin K3 3 mg, Vitamin B2 12.5 mg, Vitamin B6 9 mg, Vitamin B12 0.03 mg, pantothenic acid 18 mg, niacin 60 mg, folic acid 1.5 mg, biotin 0.225 mg, Fe 80 mg, Cu 9 mg, I 0.9 mg, Se 0.3 mg, Mn 12.55 mg, and Zn 25.2 mg.
Values were calculated from data provide by the China Feed Database (2013).
Sample Collection
At the termination of the experimental period, blood samples were collected from the wing vein of each chick replicate and placed into vacuum blood-collection tubes. The serum was centrifuged at 3,000 r for 15 min and stored at −80°C. To observe IgA-positive cells and intestinal morphology, the intermediate part of duodenum, jejunum, and ileum of each chick was fixed in 4% paraformaldehyde. Intestinal lengths were measured following euthanasia.
Detection of IgA-positive Cells
To prepare formalin-fixed and paraffin-embedded (FFPE) tissue sections for immunofluorescence, samples from the duodenum, jejunum, and ileum were fixed in 4% paraformaldehyde and embedded in paraffin according to conventional methods. Briefly, 5-μm thick sections were cut onto gelatinized slides. The slides were deparaffinized and rehydrated and placed in an antigen retrieval solution (SolarBio, China) in a boiling water bath for 30 min. The slides were blocked using goat serum and incubated at room temperature for 20 min. Sections were then stained with mouse anti-chicken IgA-FITC (SouthernBiotech, USA) in humidified chambers overnight at 4°C. After washing with PBS, the nuclei were stained using Hoechst 33,258 (SolarBio, China). The autofluorescence of the FFPE tissue sections were diminished using sodium borohydride according to the method of Davis et al. (2014). A total of 162 sections (3 groups × 6 birds per group × 3 intestinal segments with 3 replicates) were observed using an inverted microscope (Axio Examiner ZEISS, Germany), and ZEN lite for Windows was used to photograph images under 100× magnification. It should be ensured that more villus and crypt appeared under the microscope; the number of IgA-positive cells of per villus was calculated by Image J software (NIH, USA).
Determination of Serum and Egg Immune Parameters
The IgA, IgG, IgM, lysozyme (LZM), and β-defensin (β-DF) in the serum of laying breeder hens and their offspring were determined using commercial ELISA kits (MeiMianBio, China) according to the manufacturer's instructions. Immunoglobulins were extracted from egg yolks and egg whites according to the method of Hamal et al. (2006) and detected by ELISA.
Observations of Intestinal Morphology
The FFPE duodenum, jejunum, and ileum sections (5-μm thickness) were stained using hematoxylin and eosin (HE) according to standard methods and photographed under 100× magnification. Villous length and crypt depths were measured using ZEN lite under 40× magnification.
Statistical Analyses
Statistical analyses were conducted by Student's t-test and one-way ANOVA using SPSS 23.0 for MacOS, and the results are expressed as the mean ± SEM. A value of P < 0.05 and P < 0.01 was considered statistically significant.
RESULTS
Serum Immune Parameters of Laying Breeder Hens
The effects of dietary β-carotene, curcumin, allicin, and sodium butyrate supplementation on serum immunoglobulins and immunologically active substances of laying breeder hens are shown in Table 3. The serum levels of IgA, IgG, IgM, LZM, and β-DF in the CCAB group were significantly higher (P < 0.01, P < 0.01, P < 0.01, P < 0.05, P < 0.05) than those of the CON group at the end of the experimental period (28 D).
Table 3.
Effects of β-carotene, curcumin, allicin, and sodium butyrate supplementation on serum immune parameters of breeder hens.
| IgA | IgG | IgM | LZM | β-DF | |
|---|---|---|---|---|---|
| CON | 4772.53 ± 94.45 | 45.70 ± 3.07 | 1735.34 ± 187.58 | 21.53 ± 1.67 | 20.03 ± 0.92 |
| CCAB | 8585.07 ± 123.28** | 62.06 ± 4.76** | 2756.53 ± 200.37** | 28.02 ± 0.68* | 22.51 ± 1.26* |
CON, the control group; CCAB, the β-carotene, curcumin, allicin and sodium butyrate supplementation group; IgA, immunoglobulin A (ng/L); IgG, immunoglobulin G (μg/mL); IgM, immunoglobulin M (ng/mL); LZM, lysozyme (U/L); β-DF, β-defensin (ng/L). Significant differences in comparison with the CON group are expressed as *P < 0.05 and **P < 0.01 by Student's t-test. n = 10 breeder hens per group.
Growth Performance of Offspring
The body weights and tibial lengths of the chicks are shown in Table 4. Body weight in the Cipro group was significantly higher than that in the cCON group at the age of 7 D (P < 0.05) and 21 D (P < 0.05), and tibial length was longer at the age of 7 D (P < 0.01), 14 D (P < 0.01), and 28 D (P < 0.05). Body weight in the cCCAB group was higher than that in the cCON group at the age of 1 D (P < 0.05), 7 D (P < 0.05), 21 D (P < 0.01), and 28 D (P < 0.01), and the tibial length was longer at the age of 1 D (P < 0.01), 7 D (P < 0.05), 14 D (P < 0.01), 21 D (P < 0.01) and 28 D (P < 0.01).
Table 4.
Effects of laying breeder hen dietary β-carotene, curcumin, allicin, and sodium butyrate supplementation on growth performance of offspring and the effects of ciprofloxacin lactate treatment on chick growth performance.
| Body weight (g) | |||||
|---|---|---|---|---|---|
| Age | 1 D | 7 D | 14 D | 21 D | 28 D |
| cCON | 41.36 ± 0.97 | 60.19 ± 2.61 | 131.30 ± 2.60 | 208.07 ± 2.60 | 318.02 ± 5.70 |
| Cipro | 43.65 ± 0.69 | 65.56 ± 3.73** | 133.30 ± 3.11 | 223.80 ± 3.11* | 321.77 ± 8.67 |
| cCCAB | 44.15 ± 0.81* | 64.53 ± 3.91* | 137.02 ± 2.68 | 235.6 ± 2.68** | 377.93 ± 6.75** |
| Tibial length (mm) | |||||
| cCON | 28.28 ± 0.41 | 33.25 ± 1.25 | 42.86 ± 0.46 | 52.43 ± 0.46 | 59.16 ± 0.78 |
| Cipro | 29.39 ± 0.27 | 34.41 ± 0.64** | 45.46 ± 0.31** | 52.48 ± 0.31 | 60.70 ± 0.96 |
| cCCAB | 30.71 ± 0.26** | 34.09 ± 0.84* | 46.39 ± 0.47** | 57.71 ± 0.47** | 66.52 ± 0.68** |
cCON, chick control group; Cipro, ciprofloxacin lactate treatment group; cCCAB, chicks from the CCAB group. Significant differences in comparison with the cCON group are expressed as *P < 0.05 and **P < 0.01 by ANOVA and post-hoc least square difference test. n = 30 chicks per group at 1 and 7 D of age; n = 24 chicks per group at the 14 D of age; n = 18 chicks per group at the 21 D of age; n = 12 chicks per group at the 28 D of age.
Serum Immune Parameters of Offspring
The effects of breeder hen dietary β-carotene, curcumin, allicin, and sodium butyrate supplementation on the serum immune parameters of the offspring, and the effects of ciprofloxacin lactate treatment on serum immune parameters at the ages of 7, 14, 21, and 28 D are shown in Figure 1. Compared with the cCON group, the serum IgA levels (Figure 1A) of the Cipro and cCCAB groups were significantly decreased at the age of 14 D (P < 0.01, P < 0.01) and 21 D (P < 0.01, P < 0.01), but then increased at the age of 28 D (P < 0.01, P > 0.05). The serum IgG (Figure 1B) levels in the Cipro and cCCAB groups were decreased at 7 D (P < 0.01, P < 0.01), but increased at the age of 14 D (P < 0.01, P < 0.01) and 28 D (P < 0.01, P < 0.01). The serum IgM (Figure 1C) levels in the Cipro and cCCAB groups were increased at the age 7 D (P < 0.01, P < 0.05), but then decreased at the age of 14 D (P < 0.01, P < 0.01) and 21 D (P < 0.05, P < 0.01). The levels of serum LZM (Figure 1D) in the Cipro and cCCAB groups were increased at the age of 14, 21, and 28 D (all P < 0.01). The levels of β-DF (Figure 1E) in the Cipro and cCCAB groups were increased at the age of 21 D (P < 0.01, P < 0.05) and 28 D (P < 0.01, P < 0.01), and in the cCCAB group the levels were increased at the age of 14 D (P < 0.01). In addition, there was a significant difference between the Cipro group and the cCCAB group on at the age of 14 D. Compared with the Cipro group, the serum IgG and LZM levels of the cCCAB group were decreased (P < 0.01, P < 0.01), but the serum IgA and β-DF levels were increased (P < 0.05, P < 0.01).
Figure 1.
Effects of laying breeder hen dietary β-carotene, curcumin, allicin and sodium butyrate supplementation on the serum immune parameters of offspring chicks and the effects of ciprofloxacin lactate treatment on serum immune parameters. cCON, chick control group; Cipro, ciprofloxacin lactate treatment group; cCCAB, chicks from the CCAB group. IgA, immunoglobulin A (ng/L); IgG, immunoglobulin G (μg/mL); IgM, immunoglobulin M (ng/mL); LZM, lysozyme (U/L); β-DF, β-defensin (ng/L). Data are expressed as the mean ± SEM at each time point, n = 6 chicks per group. Significant differences compared with the cCON group are expressed as *P < 0.05 and **P < 0.01, and significant differences between the Cipro group and the cCCAB group are expressed as #P < 0.05 and ##P < 0.01 by least square difference test.
Jejunum Morphology of Offspring
The intestinal lengths of the chicks are shown in Table 5. The Cipro group exhibited increased lengths at the age of 7 D (P < 0.01) and 14 D (P < 0.05), while the cCCAB group showed increases at the age of 7 D (P < 0.01) and 28 D (P < 0.05).
Table 5.
Effects of laying breeder hen dietary β-carotene, curcumin, allicin, and sodium butyrate supplementation on the intestinal length (cm) of chicks and the effects of ciprofloxacin lactate treatment on chick intestinal length.
| Age | 7 D | 14 D | 21 D | 28 D |
|---|---|---|---|---|
| cCON | 46.67 ± 2.14 | 56.33 ± 1.33 | 74.00 ± 1.44 | 77.17 ± 1.62 |
| Cipro | 58.50 ± 0.62** | 61.67 ± 1.76* | 75.67 ± 1.41 | 74.33 ± 0.67 |
| cCCAB | 56.00 ± 1.06** | 59.00 ± 1.69 | 74.17 ± 2.70 | 83.50 ± 2.08* |
cCON, chick control group; Cipro, ciprofloxacin lactate treatment group; cCCAB, chicks from the CCAB group. Significant differences in comparison with the cCON group are expressed as *P < 0.05 and **P < 0.01 by ANOVA and post-hoc least square difference test. n = 6 chicks per group at each time point.
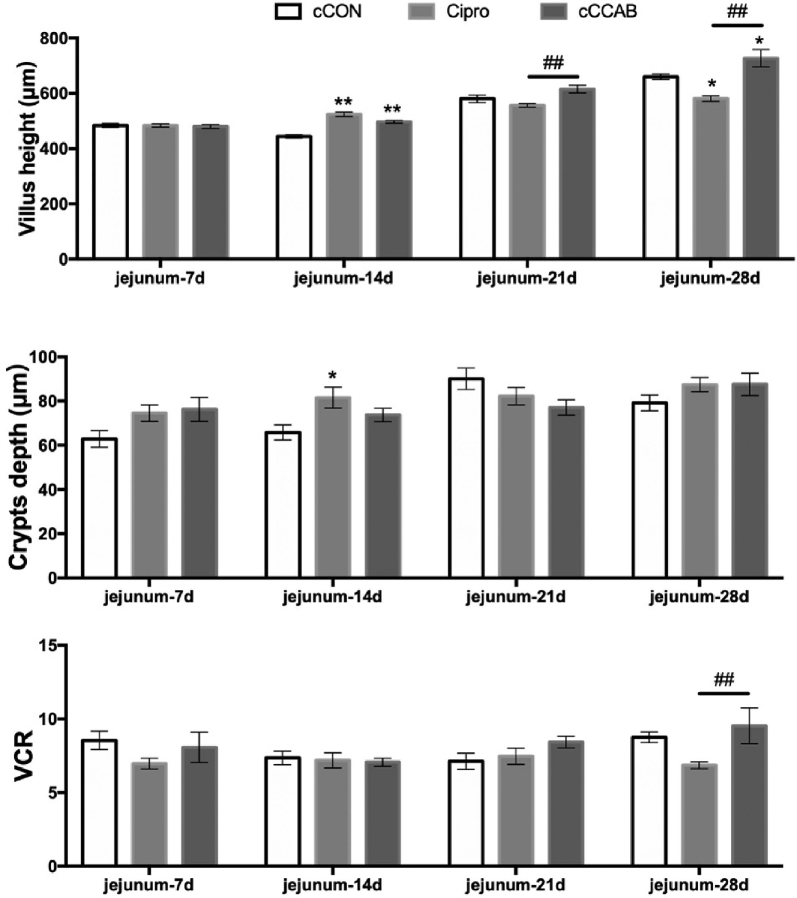
The effects of breeder hen dietary β-carotene, curcumin, allicin, and sodium butyrate supplementation on the jejunum morphology of their progeny and the direct effects of ciprofloxacin lactate treatment on jejunum morphology at the age of 7, 14, 21, and 28 D are shown in Figure 2. At the age of 7 D, the villus height, crypt depth, and villus height to crypt depth ratio (VCR) of the jejunum among the cCON, Cipro, and cCCAB groups remained even and without apparent differences (P > 0.05). However, at the age of 14 D, the height of the villi in the jejunums from the Cipro and cCCAB groups became higher (P < 0.01, P < 0.01) than in the cCON group, and jejunum crypt depths of the Cipro group was increased (P < 0.05) compared with that of the cCON group. The villous height of the jejunums from the cCCAB group was significantly increased (P < 0.01) in comparison to the Cipro group at the age of 21 D. When it comes to the age of 28 D, in a comparison with the cCON group, the villous height of the jejunums in the cCCAB group was increased (P < 0.05), but in the Cipro group was decreased (P < 0.05), there was a significant difference between the cCCAB and Cipro groups: the VCR in the jejunums from the cCCAB group was higher (P < 0.01) than in the Cipro group.
Figure 2.
Effects of laying breeder hen dietary β-carotene, curcumin, allicin and sodium butyrate supplementation on jejunum morphology of offspring chicks and effects of direct ciprofloxacin lactate treatment on chick jejunum morphology at the age of 7, 14, 21, and 28 D. cCON, chick control group; Cipro, ciprofloxacin lactate treatment group; cCCAB, chicks from the CCAB group; VCR, villus height to crypt depth ratio. Bar = 100 μm (100×). Data are expressed as the mean ± SEM, n = 6 chicks per group. Significant differences compared with the cCON group are expressed as *P < 0.05 and **P < 0.01, and significant differences between the Cipro group and the cCCAB group are expressed as #P < 0.05 and ##P < 0.01 by least square difference test.
IgA-Positive Cells in the Small Intestine of Offspring
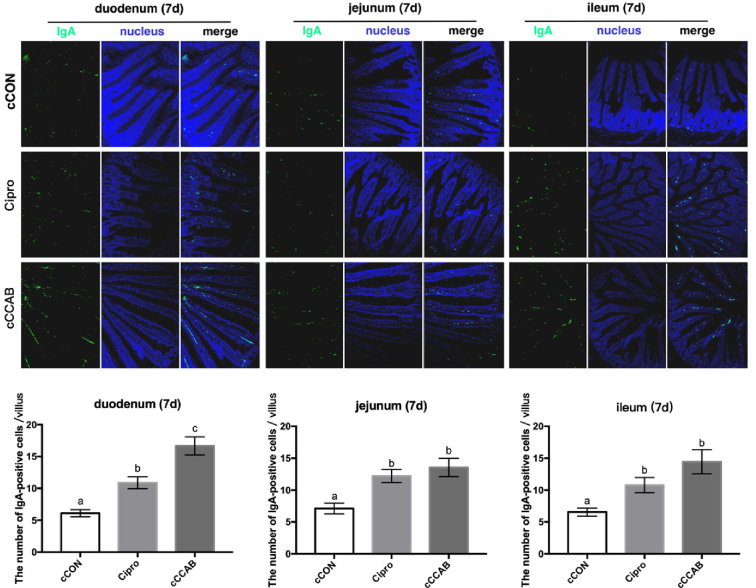
In chicks at the age of 7 D (Figure 3), the IgA-positive cells in the duodenums, jejunums, and ilea of the Cipro and cCCAB groups were significantly increased (P < 0.05) compared with the cCON group. The number of IgA-positive cells in the duodenums of the cCCAB group were also significantly increased (P < 0.05) compared with those in the Cipro group.
Figure 3.
Effects of laying breeder hen dietary β-carotene, curcumin, allicin and sodium butyrate supplementation on IgA-positive cells of the small intestine in offspring chicks and the effects of ciprofloxacin lactate treatment on IgA-positive cells of the small intestine at the age of 7 D. cCON, chick control group; Cipro, ciprofloxacin lactate treatment group; cCCAB, chicks from the CCAB group. 100×.
Data are represented as mean ± SEM, a,b,c Means with no common superscripts within a row differ significantly (P < 0.05) by Duncan's multiple range test, n = 60 villi (6 chicks of 10 villi each) per group.
In chicks at the age of 14 D (Figure 4), the IgA-positive cells were increased (P < 0.05) in the duodenums, jejunums, and ilea of the Cipro and cCCAB group compared with the cCON group, and the number of IgA-positive cells in the duodenums and ilea of the Cipro group were higher (P < 0.05) than the cCCAB group.
Figure 4.
Effects of laying breeder hen dietary β-carotene, curcumin, allicin and sodium butyrate supplementation on IgA-positive cells of the small intestine in offspring chicks and the effects of ciprofloxacin lactate treatment on IgA-positive cells of the small intestine at the age of 14 D. cCON, chick control group; Cipro, ciprofloxacin lactate treatment group; cCCAB, chicks from the CCAB group. 100×.
Data are represented as mean ± SEM, a,b,c Means with no common superscripts within a row differ significantly (P < 0.05) by Duncan's multiple range test, n = 60 villi (6 chicks of 10 villi each) chicks per group.
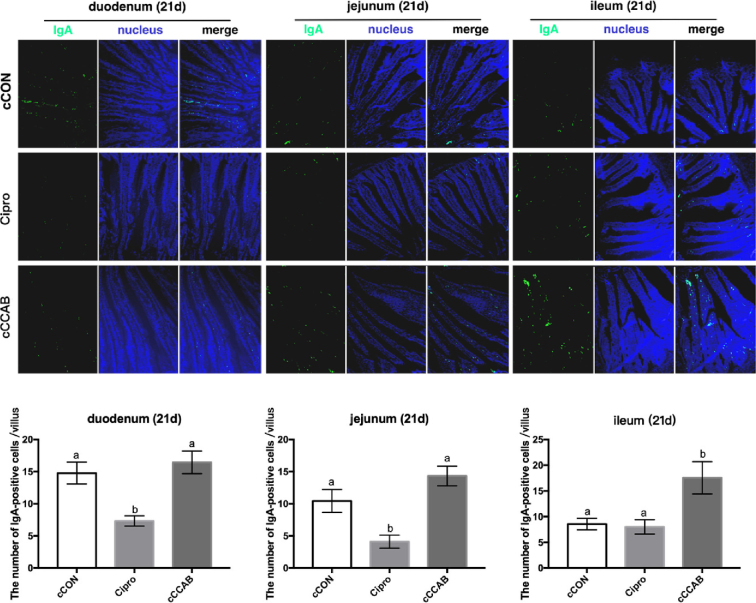
In chicks at the age of 21 D (Figure 5), the IgA-positive cells in the ilea from the cCCAB group were increased (P < 0.05) compared to the cCON and Cipro groups, while the duodenum and jejunum IgA-positive cells from the cCCAB and cCON groups were increased compared (P < 0.05, P < 0.05) with those of the Cipro group.
Figure 5.
Effects of laying breeder hen dietary β-carotene, curcumin, allicin and sodium butyrate supplementation on IgA-positive cells of the small intestine in offspring chicks and the effects of ciprofloxacin lactate treatment on IgA-positive cells of the small intestine at the age of 21 D. cCON, chick control group; Cipro, ciprofloxacin lactate treatment group; cCCAB, chicks from the CCAB group. 100×.
Data are represented as mean ± SEM, a,b Means with no common superscripts within a row differ significantly (P < 0.05) by Duncan's multiple range test, n = 60 villi (6 chicks of 10 villi each) chicks per group.
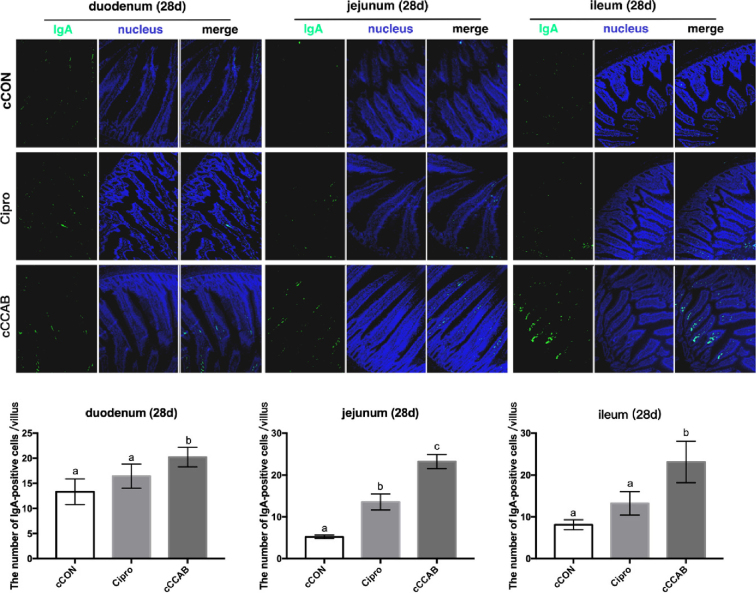
In chicks at the age of 28 D (Figure 6), the IgA-positive cells in the duodenums, jejunums, and ilea of the Cipro and cCON groups were significantly lower (P < 0.05) than that of the cCCAB group.
Figure 6.
Effects of laying breeder hen dietary β-carotene, curcumin, allicin and sodium butyrate supplementation on IgA-positive cells of the small intestine in offspring chicks and the effects of ciprofloxacin lactate treatment on IgA-positive cells of the small intestine at the age of 21 D. cCON, chick control group; Cipro, ciprofloxacin lactate treatment group; cCCAB, chicks from the CCAB group. 100×.
Data are represented as mean ± SEM, a,b,c Means with no common superscripts within a row differ significantly (P < 0.05) by Duncan's multiple range test, n = 60 villi (6 chicks of 10 villi each) chicks per group.
Immunoglobulins in Breeder Eggs
The effects of laying breeder hen dietary β-carotene, curcumin, allicin, and sodium butyrate supplementation on immunoglobulin concentrations in breeder eggs are shown in Table 6. Our results show that the levels of IgG and IgM in the egg whites of the CCAB group were significantly increased (P < 0.05, P < 0.05) and that the levels of IgA, IgG, and IgM in egg yolks were higher (triple P < 0.01) than in the CON group.
Table 6.
Effects of laying breeder hen dietary β-carotene, curcumin, allicin, and sodium butyrate supplementation on immunoglobulin content of breeder eggs.
| Egg white |
Egg yolk |
|||||
|---|---|---|---|---|---|---|
| IgA | IgG | IgM | IgA | IgG | IgM | |
| CON | 7.34 ± 0.64 | 114.18 ± 12.74 | 14.42 ± 1.41 | 20.78 ± 0.75 | 380.85 ± 20.11 | 29.83 ± 0.86 |
| CCAB | 12.67 ± 2.68 | 175.18 ± 12.14* | 19.63 ± 1.02* | 29.01 ± 0.89** | 564.54 ± 49.34** | 44.78 ± 2.59** |
CON, the control group; CCAB, the β-carotene, curcumin, allicin and sodium butyrate supplementation group; IgA, immunoglobulin A (μg/mL); IgG, immunoglobulin G (μg/mL); IgM, immunoglobulin M (μg/mL). Significant differences in comparison with the CON group are expressed as *P < 0.05 and **P < 0.01 by Student's t-test. n = 6 breeder eggs per group.
Fertilizing Capacity and hatchability of Breeder Eggs
The effects of laying breeder hen dietary β-carotene, curcumin, allicin, and sodium butyrate supplementation on fertilizing capacity and hatchability of breeder eggs are shown in Table 7. The embryo mortality of the CCAB group was significantly decreased (P < 0.05) than that of the CON group.
Table 7.
Effects of laying breeder hen dietary β-carotene, curcumin, allicin, and sodium butyrate supplementation on fertilizing capacity and hatchability of breeder eggs.
| Items | CON | CCAB |
|---|---|---|
| Embryo mortality | 3.33 ± 0.67% | 0.67 ± 0.07%* |
| Hatchability of fertile eggs | 64.67 ± 6.33% | 76.67 ± 1.86% |
| Hatchability of set eggs | 56.67 ± 4.81% | 67.33 ± 1.76% |
| Fertility of set eggs | 88.00 ± 1.16% | 88.00 ± 1.16% |
CON, the control group; CCAB, the β-carotene, curcumin, allicin, and sodium butyrate supplementation group. Significant differences in comparison with the CON group are expressed as *P < 0.05 by Student's t-test. n = 5 plates of 30 eggs each.
Embryo morality = (dead embryos number/set eggs number) × 100%.
Hatchability of fertile eggs = (hatched eggs number/fertile eggs number) × 100%.
Hatchability of set eggs = (hatched eggs number/set eggs number) × 100%.
Fertility of set eggs = (fertile eggs number/set eggs number) × 100%.
DISCUSSION
Immunoglobulins play an essential role in immune regulation. IgA is crucial for protection at mucosal surfaces by preventing the entry (Bienenstock et al., 1973), IgM performs three main functions: the regulation of subsequent immune response, facilitating the production of IgG and the first immune response against foreign antigens (Ehrenstein et al., 2000), β-DF and LZM belong to innate immunity with antimicrobial activity. In this study, the increasing levels of IgA, IgG, IgM, β-DF, and LZM in serum (Table 3) show that β-carotene, curcumin, allicin, and sodium butyrate supplementation in the diets of breeder laying hens had a notable effect on their humoral immunity. Previous reports have shown that higher β-carotene supplementation in Gray Partridge diets improved the immune conditions of female birds (Cucco et al., 2006), while fermented garlic powder administration may also enhance immunity in broilers (Ao et al., 2011); curcumin supplementation has also been shown to improve the total serum Ig and IgG titers after SRBC injection in Hy-line hens (Arshami et al., 2013), and butyrate supplementation in poultry enhances gut health and egg production (Bedford and Gong, 2018), which suggests that β-carotene, curcumin, allicin, and sodium butyrate improved breeders immunocompetence via physiological functioning.
To study the effects of β-carotene, curcumin, allicin, and sodium butyrate supplementation on the offspring of breeders, the body weights and tibial lengths of chicks were measured in their early life. The results showed that the chicks of the cCCAB group consistently exhibited better growth performance. However, the growth performance of chicks given ciprofloxacin lactate supplementation was enhanced before 14 D, but soon afterwards, the effects of growth promotion was no longer significant. A study by Yazdi et al. (2014) also found that newly hatched broiler chicks treated with antibiotics improved their body weights at the age of 14 D, but there was a significant decline by 42 D of age. A widely accepted mechanism underlying how antibiotics promote growth involves their control of pathogen proliferation in the gut, which establishes a healthy intestinal environment and ensures digestion and absorption (Goodarzi et al., 2014; Rasouli and Jahanian 2019; Smith, 2019).
The gut is an important organ system affecting digestion, absorption and host defense, and the health status and growth performance of poultry is influenced by their gut health (Sugiharto, 2016). Therefore, jejunum morphology and small intestinal IgA-positive cells were examined to evaluate intestinal development. In this study, the β-carotene, curcumin, allicin, and sodium butyrate supplements in the diet of the hens improved their offspring's intestinal length from 7 to 28 D of age, and the intestinal length of the chicks directly supplemented with ciprofloxacin lactate was significantly improved at 7 and 14 D of age, but worsened at 28 D of age. Intestinal length is a key parameter of intestinal development and corresponds to growth performance (Faure et al., 2013).
Jejunum, the longest segment in chicken intestine, is the main region for nutrient absorption in the chicken intestine (Leeson and Summer, 2001). We know that longer villi indicate more intestinal surface area and more effective nutrient absorption to ensuing growth. A deeper crypt may indicate faster tissue turnover to permit renewal of the villus (Baurhoo et al., 2007), and the VCR is a useful criterion for estimating the digestive capacity in the small intestine (Montagne et al., 2003). The data on jejunum morphology in our study showed that the directly supplemented with ciprofloxacin lactate in chicks improved the jejunum villous height and crypts depth at 14 D of age, but decreased in villous height at 28 D of age; Rasouli and Jahanian (2019) reported that antibiotics improved jejunum villous height in broiler chicks. However, the chicks from the β-carotene, curcumin, allicin, and sodium butyrate supplemented hens had higher villi at 21 and 28 D of age and higher VCR at 28 D than that chicks directly supplemented with ciprofloxacin lactate, so these results of intestinal length and jejunum morphology may help explain the change of growth performance in the cCCAB and Cipro groups. Moreover, antibiotics are an effective tool that modify gut microbiota and decrease bacterial proliferation to improve animal growth performance and has been widely used. Nevertheless, the long-term use of antibiotics leads to antibiotic-resistant “superbugs” (M'Sadeq et al., 2015). Hence, the abundance distribution of jejunum contents at the age of 28 D was analyzed, and the results (Figure S1) showed that the relative abundance of microbiota in chicks directly supplemented with ciprofloxacin lactate emerged apparent changes: the decreasing of Firmicutes and the increasing of Proteobacteria at the phylum level compared with the cCON and cCCAB groups. The dominant phyla in the intestines of poultry are Firmicutes, Bacteroidetes, and Proteobacteria (Wei et al., 2013), although Proteobacteria is a minor constituent, this group included many pathogenic bacteria, such as Escherichia coli, Salmonella, Vibrio cholerae, Helicobacter, etc. (Shin et al., 2015). Villi play an important role in the absorption of nutrients in the small intestine; therefore, the health status of the intestinal villi can affect animal growth (Suzuki et al., 2009). Sections of the jejunums stained with HE (Figure S2) demonstrate that the villi in the jejunum from the chicks directly ciprofloxacin lactate supplemented appeared split and damaged at the 28 D of age, which may be caused by long-term supplementation of ciprofloxacin lactate to permit increase of Proteobacteria, and the proliferation of pathogenic bacteria in the intestine causes gut inflammation and damage to the villi. The villus height, crypts depth, VCR and sections images of duodenums and ilea are shown in Supplementary Figure 3–5.
IgA-positive cells are a type of B cell within the lamina propria that can secrete IgA, which forms an important part of the intestinal immune barrier and defends against pathogens in the gut, thus maintaining intestinal health (Kim and Lillehoj, 2019). In regard to the small intestine IgA-positive cells, we found that the cCCAB group had elevated or normal levels of IgA-positive cells compared to the cCON group during the 7 to 28 D age range, these results suggest that the chicks from the β-carotene, curcumin, allicin, and sodium butyrate supplemented hens had well-developed intestinal immunity that safeguarded their growth. While the chicks supplemented with ciprofloxacin lactate had more IgA-positive cells at 7 and 14 D in small intestinal, these decreased to the level of the control group after 14 D; IgA-positive cells deficiency results in bacterial overgrowth, adherence, and translocation (Langkamp-Henken et al., 1992). The change in intestinal IgA-positive cells in the chicks supplemented with ciprofloxacin lactate during the 7 to 28 D period also suggest improvements in growth performance.
We detected serum immunoglobulins and levels of immunologically active substances at different times in the chicks supplemented with ciprofloxacin lactate and those without supplementation, and found that the serum levels of IgA and IgM in chicks' early life displayed the same decreasing tendencies from 7 to 21 D of age but then increased after 21 D of age. Serum IgG levels were stable before 21 D of age and then increased after 21 D and that of LZM and β-DF increased from 7 to 28 D. The changing trends of immunoglobulin levels are in line with previous research that found maternal antibodies decrease with the increasing age of the chick, up to 21 to 28 D of age and that newly synthesized IgG is present in the circulation at least 3 D after hatching (Rose and Orlans, 1981). The decrease of IgA and IgM in the Cipro and cCCAB groups at 14 and 21 D of age is likely to be contributed by advance immune independence, because immune protection of the domestic fowl depends on transfer of maternal antibodies, and maternal IgA and IgM will be depleted prior to immune independence (Bar-Shira et al., 2014). Avian β-defensin, one kind of host defense peptides belonging to the innate immune system with broad antimicrobial activity, is highly expressed in many different tissues (Zhang and Wong, 2019). Previous studies show that expression of avian β-defensin mRNA was upregulated to self-protect under pathogens challenge (Yoshimura et al., 2006; Akbari et al., 2008; Garcia et al., 2018). However, the regulatory mechanisms are uncertain now. Therefore, the increase of β-defense in the Cipro and cCCAB groups at the 14, 21, and 28 D of age implies better innate immune abilities, as well as LZM. Moreover, the improvement of serum IgA and IgG in the cCCAB and Cipro groups at 28 D also suggests that chicks from the CCAB hen group and chicks given ciprofloxacin lactate supplementation had better immune abilities after depletion of maternal antibiotics about at 21 D of age (Rose and Orlans, 1981).
Embryonic development of breeder eggs is completely dependent on nutrients deposited by hens (Oviedo-Rondón, 2019). Hens transfer naturally binding IgG antibodies to protect neonatal chicks, as they cannot produce these antibodies themselves (Ismiraj et al., 2019). This maternal effect led us to investigate immunoglobulins in breeder eggs, and the results demonstrated that dietary β-carotene, curcumin, allicin, and sodium butyrate supplementation in breeder hen diets improved immunoglobulin concentrations in egg yolks and egg whites. Al-Natour et al. (2004) reported that the amount of IgG transferred into egg yolk was proportionally related to maternal serum IgG concentration, and the amount of IgA and IgM was transferred into egg yolk and egg white from the dam. Although mechanisms are uncertain, our study implies the improvement of immunoglobulin levels in breeder eggs may be caused by improvements in the hen's serum immunoglobulins after β-carotene, curcumin, allicin, and sodium butyrate supplementation. Furthermore, differences in immunoglobulin concentrations in eggs may be associated with embryo mortality and a difference in immune system development and offspring growth.
In conclusion, β-carotene, curcumin, allicin, and sodium butyrate supplementation of breeder hen diets had a positive effect on hen immunity, and their offspring maintained better growth performance and immunity in early life. It may be that chicks with stronger intestinal villi and intestinal immune system development gained these positive effects from the laying breeder hens through maternal antibodies transferred into the eggs, thus ensuring their development. Moreover, in comparison with chicks supplemented with ciprofloxacin lactate, we found that chicks hatched from immune-enhanced hens had more natural advantages in terms of intestinal health and growth. Our study indicates that nutritional interventions in breeder hens is a potential method to change how antibiotics are used in the early life of chicks by using maternal protection to improve chick health instead of administering antibiotics directly.
ACKNOWLEDGEMENTS
This study was supported by the Science and Technology Development Plan Program of Jilin Province (NO.20170204043NY) and the Agricultural Science and Technology Innovation Program for Outstanding Youth of Jilin Province (NO. CXGC2017JQ009).
Footnotes
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.3382/ps/pez584.
Figure S1. Abundance distribution at the phylum level in chicks' jejunum contents at the age of 28 D. cCON, chick control group; Cipro, ciprofloxacin lactate treatment group; cCCAB, chicks from the CCAB group. n = 6 chicks per group.
Figure S2. Sections of the jejunums stained with HE at the age of 7, 14, 21, and 28 D. cCON, chick control group; Cipro, ciprofloxacin lactate treatment group; cCCAB, chicks from the CCAB group. Bar = 100 μm, 100×. n = 6 chicks per group.
Figure S3. Duodenum and ileum morphology of offspring chicks and chicks direct ciprofloxacin lactate treatment at the age of 7, 14, 21, and 28 D. cCON, chick control group; Cipro, ciprofloxacin lactate treatment group; cCCAB, chicks from the CCAB group. Significant differences compared with the cCON group are expressed as *P < 0.05 and **P < 0.01, and significant differences between the Cipro group and the cCCAB group are expressed as #P < 0.05 and ##P < 0.01 by least square difference test. n = 6 chicks per group.
Figure S4. Sections of the duodenums stained with HE at the age of 7, 14, 21, and 28 D. cCON, chick control group; Cipro, ciprofloxacin lactate treatment group; cCCAB, chicks from the CCAB group. Bar=100 μm, 100×. n = 6 chicks per group.
Figure S5. Sections of the ilea stained with HE at the age of 7, 14, 21, and 28 D. cCON, chick control group; Cipro, ciprofloxacin lactate treatment group; cCCAB, chicks from the CCAB group. Bar = 100 μm, 100×. n = 6 chicks per group.
Supplementary Material
REFERENCES
- Akbari M.R., Haghighi H.R., Chambers J.R., Brisbin J., Read L.R., Sharif S. Expression of antimicrobial peptides in cecal tonsils of chickens treated with probiotics and infected with Salmonella enterica serovar typhimurium. Clin. Vaccine Immunol. 2008;15:1689–1693. doi: 10.1128/CVI.00242-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Natour M.Q., Ward L.A., Saif Y.M., Stewart-Brown B., Keck L.D. Effect of different levels of maternally derived antibodies on protection against infectious bursal disease virus. Avian Dis. 2004;48:177–182. doi: 10.1637/5319. [DOI] [PubMed] [Google Scholar]
- Ao X., Yoo J.S., Zhou T.X., Wang J.P., Meng Q.W., Yan L., Cho J.H., Kim I.H. Effects of fermented garlic powder supplementation on growth performance, blood profiles and breast meat quality in broilers. Livest. Sci. 2011;141:85–89. [Google Scholar]
- Arshami J., Pilevar M., Aami Azghadi M., Raji A.R. Hypolipidemic and antioxidative effects of curcumin on blood parameters, humoral immunity, and jejunum histology in Hy-line hens. Avicenna J. Phytomed. 2013;3:178–185. [PMC free article] [PubMed] [Google Scholar]
- Bar-Shira E., Cohen I., Elad O., Friedman A. Role of goblet cells and mucin layer in protecting maternal IgA in precocious birds. Dev. Comp. Immunol. 2014;44:186–194. doi: 10.1016/j.dci.2013.12.010. [DOI] [PubMed] [Google Scholar]
- Baurhoo B., Phillip L., Ruiz-Feria C.A. Effects of purified lignin and mannan oligosaccharides on intestinal integrity and microbial populations in the ceca and litter of broiler chickens. Poult. Sci. 2007;86:1070–1078. doi: 10.1093/ps/86.6.1070. [DOI] [PubMed] [Google Scholar]
- Bedford A., Gong J. Implications of butyrate and its derivatives for gut health and animal production. Anim. Nutr. 2018;4:151–159. doi: 10.1016/j.aninu.2017.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienenstock J., Gauldie J., Perey D.Y. Synthesis of IgG, IgA, IgM by chicken tissues: immunofluorescent and 14C amino acid incorporation studies. J. Immunol. 1973;111:1112–1118. [PubMed] [Google Scholar]
- Chattopadhyay M.K. Use of antibiotics as feed additives: a burning question. Front. Microbiol. 2014;5:334. doi: 10.3389/fmicb.2014.00334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cucco M., Guasco B., Malacarne G., Ottonelli R. Effects of β-carotene supplementation on chick growth, immune status and behaviour in the grey partridge, Perdix perdix. Behav. Processes. 2006;73:325–332. doi: 10.1016/j.beproc.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Davis A.S., Richter A., Becker S., Moyer J.E., Sandouk A., Skinner J., Taubenberger J.K. Characterizing and diminishing autofluorescence in Formalin-fixed Paraffin-embedded human respiratory tissue. J. Histochem. Cytochem. 2014;62:405–423. [Google Scholar]
- Ehrenstein M.R., Cook H.T., Neuberger M.S. Deficiency in serum immunoglobulin (Ig)M predisposes to development of IgG autoantibodies. J. Exp. Med. 2000;191:1253–1258. doi: 10.1084/jem.191.7.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emami N.K., Samie A., Rahmani H.R., Ruiz-Feria C.A. The effect of peppermint essential oil and fructooligosaccharides, as alternatives to virginiamycin, on growth performance, digestibility, gut morphology and immune response of male broilers. Anim. Feed Sci. Technol. 2012;175:57–64. [Google Scholar]
- Faure S., Georges M., McKey J., Sagnol S., de Santa Barbara P. Expression pattern of the homeotic gene Bapx1 during early chick gastrointestinal tract development. Gene Expr. Patterns. 2013;13:287–292. doi: 10.1016/j.gep.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Forgetta V., Rempel H., Malouin F., Vaillancourt R., Jr., Topp E., Dewar K., Diarra M.S. Pathogenic and multidrug-resistant Escherichia fergusonii from broiler chicken. Poult. Sci. 2012;91:512–525. doi: 10.3382/ps.2011-01738. [DOI] [PubMed] [Google Scholar]
- Garcia J.S., Byrd J.A., Wong E.A. Expression of nutrient transporters and host defense peptides in Campylobacter challenged broilers. Poult. Sci. 2018;97:3671–3680. doi: 10.3382/ps/pey228. [DOI] [PubMed] [Google Scholar]
- Goodarzi M., Nanekarani S., Landy N. Effect of dietary supplementation with onion (allium cepa l.) on performance, carcass traits and intestinal microflora composition in broiler chickens. Asian Pac. J. Trop. Dis. 2014;4:S297–S301. [Google Scholar]
- Hamal K.R., Burgess S.C., Pevzner I.Y., Erf G.F. Maternal antibody transfer from dams to their egg yolks, egg whites, and chicks in meat lines of chickens. Poult. Sci. 2006;85:1364–1372. doi: 10.1093/ps/85.8.1364. [DOI] [PubMed] [Google Scholar]
- Ismiraj M.R., Arts J.A.J., Parmentier H.K. Maternal transfer of natural (auto-) antibodies in chickens. Poult. Sci. 2019;98:2380–2391. doi: 10.3382/ps/pez017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W.H., Lillehoj H.S. Immunity, immunomodulation, and antibiotic alternatives to maximize the genetic potential of poultry for growth and disease response. Anim. Feed Sci. Technol. 2019;250:41–50. [Google Scholar]
- Langkamp-Henken B., Glezer J., Kudsk K. Immunologic structure and function of the gastrointestinal tract. Nutr. Clin. Prac. 1992;7:100–108. doi: 10.1177/0115426592007003100. [DOI] [PubMed] [Google Scholar]
- Lang W., Hong P., Li R., Zhang H., Huang Y., Zheng X. Growth performance and intestinal morphology of Hyline chickens fed diets with different diet particle sizes. J. Anim. Physio. Anim. Nutr. 2019;103:518–524. doi: 10.1111/jpn.13046. [DOI] [PubMed] [Google Scholar]
- Leeson S., Summers J.D. 4th revised edition. University Books; Guelph, ON, Canada: 2001. Scott's Nutrition of the Chicken. [Google Scholar]
- Mehdi Y., Letourneau-Montminy M.P., Gaucher M.L., Chorfi Y., Suresh G., Rouissi T., Brar S.K., Cote C., Ramirez A.A., Godbout S. Use of antibiotics in broiler production: global impacts and alternatives. Anim. Nutr. 2018;4:170–178. doi: 10.1016/j.aninu.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagne L., Pluske J.R., Hampson D.J. A review of interactions between dietary fibre and the intestinal mucosa, and their consequences on digestive health in young non-ruminant animals. Anim. Feed Sci. Technol. 2003;108:95–117. [Google Scholar]
- M'Sadeq S.A., Wu S., Swick R.A., Choct M. Towards the control of necrotic enteritis in broiler chickens with in-feed antibiotics phasing-out worldwide. Anim. Nutr. 2015;1:1–11. doi: 10.1016/j.aninu.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oviedo-Rondón E.O. Holistic view of intestinal health in poultry. Anim. Feed Sci. Technol. 2019;250:1–8. [Google Scholar]
- Rasouli E., Jahanian R. Comparative effects of genistein and antibiotics on performance, meat oxidative stability, jejunal morphology, and ileal microbial community in broiler chicks. Anim. Feed Sci. Technol. 2019;256 [Google Scholar]
- Rose M.E., Orlans E. Immunoglobulins in the egg, embryo and young chick. Dev. Comp. Immunol. 1981;5:15–20. doi: 10.1016/s0145-305x(81)80003-1. [DOI] [PubMed] [Google Scholar]
- Salehi B., Zucca P., Orhan I.E., Azzini E., Adetunji C.O., Mohammed S.A., Banerjee S.K., Sharopov F., Rigano D., Sharifi-Rad J., Armstrong L., Martorell M., Sureda A., Martins N., Selamoğlu Z., Ahmad Z. Allicin and health: a comprehensive review. Trends Food Sci. Technol. 2019;86:502–516. [Google Scholar]
- Schwaiger K., Huther S., Holzel C., Kampf P., Bauer J. Prevalence of antibiotic-resistant enterobacteriaceae isolated from chicken and pork meat purchased at the slaughterhouse and at retail in Bavaria, Germany. Int. J. Food Microbiol. 2012;154:206–211. doi: 10.1016/j.ijfoodmicro.2011.12.014. [DOI] [PubMed] [Google Scholar]
- Sharma R.A., Gescher A.J., Steward W.P. Curcumin: the story so far. Eur. J. Cancer. 2005;41:1955–1968. doi: 10.1016/j.ejca.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Shin N.R., Whon T.W., Bae J.W. Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015;33:496–503. doi: 10.1016/j.tibtech.2015.06.011. [DOI] [PubMed] [Google Scholar]
- Smith J.A. Broiler production without antibiotics: United States field perspectives. Anim. Feed Sci. Technol. 2019;250:93–98. [Google Scholar]
- Sugiharto S. Role of nutraceuticals in gut health and growth performance of poultry. J. Saudi Soc. Agric. Sci. 2016;15:99–111. [Google Scholar]
- Suzuki T., Mochizuki K., Goda T. Localized expression of genes related to carbohydrate and lipid absorption along the crypt-villus axis of rat jejunum. Biochim. Biophys. Acta. 2009;1790:1624–1635. doi: 10.1016/j.bbagen.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Vrolijk M.F., Opperhuizen A., Jansen E.H., Godschalk R.W., Van Schooten F.J., Bast A., Haenen G.R. The shifting perception on antioxidants: the case of vitamin E and beta-carotene. Redox Biol. 2015;4:272–278. doi: 10.1016/j.redox.2014.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S., Morrison M., Yu Z. Bacterial census of poultry intestinal microbiome. Poult. Sci. 2013;92:671–683. doi: 10.3382/ps.2012-02822. [DOI] [PubMed] [Google Scholar]
- Wilson P.B. Recent advances in avian egg science: A review. Poult. Sci. 2017;96:3747–3754. doi: 10.3382/ps/pex187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdi F.F., Ghalamkari G., Toghyani M., Modaresi M., Landy N. Efficiency of Tribulus terrestris L. as an antibiotic growth promoter substitute on performance and immune responses in broiler chicks. Asian Pac. J. Trop. Dis. 2014;4:S1014–S1018. [Google Scholar]
- Yoshimura Y., Ohashi H., Subedi K., Nishibori M., Isobe N. Effects of age, egg-laying activity, and Salmonella-inoculation on the expressions of gallinacin mRNA in the vagina of the hen oviduct. J. Reprod. Dev. 2006;52:211–218. doi: 10.1262/jrd.17070. [DOI] [PubMed] [Google Scholar]
- Yulistiani R., Praseptiangga D., Raharjo D., Shirakawa T. Prevalence of Antibiotic-resistance enterobacteriaceae strains isolated from chicken meat at traditional markets in Surabaya, Indonesia. IOP Conf. Ser.: Mater. Sci. Eng. 2017;193 [Google Scholar]
- Zhang H., Wong E.A. Expression of avian beta-defensin mRNA in the chicken yolk sac. Dev. Comp. Immunol. 2019;95:89–95. doi: 10.1016/j.dci.2019.02.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.