Abstract
The present study was undertaken to investigate the effect of aqueous Withania somnifera root (WSR) extract in broiler chicks experimentally infected with Escherichia coli O78 @ 107 CFU/0.5 ml intraperitoneally. Clinical signs and mortality due to colibacillosis observed in infected chicks were mild and lasted for short duration in WSR extract supplemented group as compared with the nonsupplemented group. A significant increase in serum alanine transaminase, aspartate transaminase, lactate dehydrogenase, and creatine phosphokinase activities and a decrease in total protein and albumin concentrations were observed in the infected groups, though these changes were of lower magnitude in WSR extract supplemented group. A significantly higher activity of oxidative blood parameters such as superoxide dismutase, catalase, glutathione reductase, and glutathione-S-transferase enzymes were noticed in WSR extract supplemented group. The WSR extract supplemented group revealed significantly higher E. coli–specific antibody titer and enhanced lymphocyte proliferation response as compared with the nonsupplemented group. The gross and histopathological lesions of colibacillosis were mild in the WSR extract–supplemented infected group as compared with the nonsupplemented infected group. Withania somnifera root extract supplementation produced 31.48 and 34.38% protection in the gross and histopathological lesions in E. coli infected chicks, respectively. It is concluded that supplementation of 20% WSR extract @ 20 ml/L of water caused a reduction in the severity, mortality, and recovery period of E. coli infection and enhanced the humoral and cellular immune responses suggesting its protective effect on limiting the pathology of E. coli infection in broiler chickens.
Key words: Withania somnifera, Escherichia coli, chicken, immune response, lesions
Introduction
Avian colibacillosis caused by Escherichia coli (E. coli) is of great concern as it burdens the poultry farmers with heavy economic losses. The acute form of the disease is characterized by septicemia and the subacute form by airsacculitis and fibrinous polyserositis (e.g., pericarditis, perihepatitis, and peritonitis). Consequently, it decreases egg production and increases condemnations, mortality, and with increased costs of vaccination and chemotherapy results in economic losses (Kabir, 2010, Lau et al., 2010).
The E. coli strains are found to be resistant to various antimicrobials such as cephradine, tetracyclines, chloramphenicol, sulfonamides, β-lactam antibiotics, aminoglycosides, and fluoroquinolones (Blanco et al., 1997, Bass et al., 1999, Li et al., 2007, Roth et al., 2019). Attributed to the extensive use of antibiotics and lack of new drugs and vaccines, multidrug resistance is now a worldwide problem. A remedy can be found in the use of ethnoveterinary medicines. One of the approaches in developing successful drugs from medicinal plants is a phytotherapeutic approach wherein standardized crude drug preparations (extracts or active fractions) can be used as drugs with modern standards of safety and efficacy.
The prime objective of Ayurveda, the ancient Indian system of medicine, is the prevention of the disease process. Withania somnifera is an important medicinal plant, a small, woody shrub 60 to 200 cm high, in the Solanaceae family, which is described under many common names such as ginseng and ashwagandha. It is widely distributed in eastern Asia, Africa, and Australia. The roots are the main portions of the plant used therapeutically (Girdhari and Rana, 2007). The major biochemical constituents of ashwagandha roots are steroidal alkaloids and steroidal lactones in a class of constituents called withanolides (Oberholzer et al., 2008, Varma et al., 2011). Withaferin A and Withanolide D are the 2 main withanolides that contribute to most of the biological activity of W. somnifera (Harikrishnan et al., 2008, Mirjalilli et al., 2009). Recently, the plant was investigated to be effective in the treatment of some bacterial infections and was tested for antibacterial properties (Arora et al., 2004, Owais et al., 2005, Teixeira et al., 2006). Besides its use as an antibacterial product, several reports have demonstrated immunomodulator and tumor inhibiting activity of its root extract (Ziauddin et al., 1996, Dhuley, 1997, Davis and Kuttan, 2000). W. somnifera has been used as an antioxidant, adaptogen, aphrodisiac, liver tonic, antiinflammatory agent, and astringent agent (Rastogi and Mehrotra, 1998). Ashwagandha root powder (@ 0.5%) supplementation in feed has shown a reduction in the severity of S. gallinarum infection in chicks (Kumari et al., 2015a).
However, the literature on in vivo studies regarding the effect of this medicinal plant on pathological lesions and immunological response against E. coli infection in poultry is sparse. Keeping in view the above facts, the present study was undertaken to study clinical, biochemical, immunological indices, and pathological changes in E. coli-infected broiler chicks supplemented with W. somnifera root (WSR) extract.
Materials and methods
This study was performed after the approval from the Institutional Animal Ethics Committee of the university.
Experimental Birds, Housing, and Feeding
One hundred and twenty, 1-day-old, healthy and unvaccinated Cobb broiler chicks were procured from a local commercial hatchery and reared under cage system under strict hygienic conditions in a temperature controlled and ventilated animal house of the department. The housing, lighting, ambient temperature, relative humidity, and other physical conditions of the in-door facility were maintained in accordance to standard guidelines (Aviagen, 2014). These birds were fed ad libitum on commercial broiler feed (Godrej Agrovet, Pvt. Ltd., Mumbai, India) in square-type bottom feeders attached outside the cages, and fresh water was provided by good quality plastic fountain-type drinkers.
Preparation of Aqueous Extract of Withania somnifera Roots
W. somnifera roots were procured from the Medicinal, Aromatic, and Potential Crops Section, Department of Genetics and Plant Breeding, CCS Haryana Agriculture University, Hisar, India and were chopped, washed, shade-dried, and powdered. Twenty percent aqueous root extract of W. somnifera was prepared and filtered through Whatmann (No. 1) filter paper (Pujari and Gandhi, 2012), which was given to chickens through oral route in drinking water @ 20 ml per liter. The dose used in the present experimental study was calculated on the basis of in vitro antibacterial effect of WSR extract on E. coli (Kumari and Gupta, 2015). Results of other workers revealed that total alkaloids content of WSR collected from the same source as that of present study varied between 0.26 and 0.31%, and tannins content varied between 0.66 and 0.84 mg/g (Gulati et al., 2017).
Experimental Design and Sampling
The 1-day-old chicks were divided into 2 groups viz. Group A and B containing 60 birds each. The birds of group B were supplemented with 20% aqueous WSR root extract through oral route in the drinking water @ 20 ml per liter of water. The amount of water provided and the amount left after drinking within 24 h was quantified daily to calculate the average amount of the extract consumed per day per bird (Table1). The birds of group A were provided drinking water without any supplementation.
Table 1.
The average extract consumed per bird per day (in ml) in different experimental groups at different intervals.
| Groups | Age in D |
|||||
|---|---|---|---|---|---|---|
| 1 | 7 | 14 | 21 | 28 | 35 | |
| B1 | 0.48 | 1.37 | 4.0 | 4.2 | 4.7 | 7.83 |
| B2 | 0.47 | 1.41 | 4.0 | 3.56 | 4.8 | 8.67 |
After 7 D of age, the birds of both the groups were further divided into 2 subgroups (Group A into A1 and A2, Group B into B1 and B2) of 25 and 35 birds, respectively. All the chicks of groups A2 and B2 were injected with E. coli O78 resuspended in the sterile normal saline solution @ 1 × 107 CFU/0.5 ml intraperitoneally, whereas chicks of group A1 and B1 were injected with 0.5 ml sterile normal saline solution intraperitoneally. The birds of the group A1 and B1 (noninfected) were kept in one room and those of groups A2 and B2 (infected) at a distance in another room with the same housing and environmental conditions. Blood was collected directly from the heart from 5 chicks from each group at 0, 7, 14, 21, and 28 D postinfection in sterilized test tubes without anticoagulant for serum separation and in sterile heparinized vials. Blood was also collected in sterile vials containing 2 mg/ml of EDTA for antioxidant enzymes studies. Thereafter, these birds from each group were sacrificed, and a thorough postmortem examination was conducted. During necropsy, tissue samples from lungs, heart, liver, spleen, proventriculus, intestines, and bursa of Fabricius were collected from all the birds in 10% buffered formalin for histopathological examination. A part of the liver from all the groups was also collected in sterilized Petri dish under aseptic conditions for viable bacterial (E. coli) count.
Clinical Signs and Mortality
Each bird was closely observed daily for clinical signs and mortality, if any. The clinical signs score was calculated on a scale of 0 to 4 (No signs—0; Mild signs—1; Moderate signs—2; Moderately severe signs—3; Severe signs—4).
Body Weight
All the birds from each group were weighed at 0, 7, 14, 21, and 28 D postinfection to assess the growth response.
Biochemical Studies
The serum samples obtained at different intervals were analyzed for total protein concentration, albumin concentration, and the enzyme activities of aspartate transaminase, alanine transaminase, lactate dehydrogenase, and creatine phosphokinase using single-step reagent kits (Transasia Bio-Medicals Ltd., Mumbai, India) employing a Semiautomatic Biochemistry Analyzer.
Oxidative Stress Parameters
Preparation of Erythrocyte Lysate
Blood samples collected in EDTA were centrifuged at 1,500 rpm for 10 min. The pellet was suspended in and washed thrice with normal saline solution. Finally, 10% erythrocyte lysate was prepared by adding chilled distilled water. Hemoglobin concentration of erythrocyte lysate was measured as per the method of Benjamin (1985).
Assay of Superoxide Dismutase
The activity of superoxide dismutase (SOD) in erythrocyte lysate was determined by the method of Marklund and Marklund (1974). Absorbance (OD) values were read at 420 nm, and the unit of enzyme activity was defined as the amount of enzyme causing 50% inhibition of autooxidation of pyrogallol observed in control.
Assay of Catalase
The activity of catalase in erythrocyte lysate was determined according to the method described by Aebi (1983). In blank test tube, only phosphate buffer (50 mM, pH 7) was taken, and the base was set zero with it. In test sample tubes, 10% erythrocyte lysate was added to phosphate buffer (50 mM, pH 7). After adding H2O2 (30 mM), absorbance was read at 240 nm for 1 min. The results were expressed as μmol H2O2 decomposed per min per mg Hb.
Assay of Glutathione Reductase
The assay of glutathione reductase (GR) was performed according to the method described by Carlberg and Mannervik (1985). Absorbance (OD) was read at 340 nm for 4 min. A unit of GR activity was defined as the amount of enzyme that catalyzes the oxidation of 1 μmol of NADPH per minute using 6.22 × 103 as the molar extinction coefficient of NADPH.
Assay of Glutathione-S-Transferase
The activity of glutathione-S-transferase (GST) in erythrocyte lysate was determined by the method of Habig et al. (1974). Absorbance (OD) was read at 340 nm for 3 min. The unit of enzyme activity was defined as the amount of enzyme that catalyzes the formation of 1 μmol conjugate of reduced glutathione and 1-Chloro-2,4-dinitrobenzene per minute per mg of Hb.
Viable Bacterial Cell Count of E. coli in the Liver
Viable bacterial cell count of all the groups that is infected (A2 and B2) as well as noninfected (A1 and B1) was determined in the liver at 7, 14, 21, and 28 D postinfection under aseptic conditions by the plate-counting method described by Cruickshank et al. (1975). The tissue samples were mixed with brain–heart infusion broth in the ratio of 1:2. Ten-fold dilutions were prepared, and 0.1 ml of each dilution was spread over the surface of Mackonkey's lactose agar plates in duplicate and incubated at 37ᵒC for 24 h. The number of colonies so obtained was multiplied with respective dilution factor to get the original number of viable organisms per gram of the tissue.
Immunological Studies
Indirect ELISA
Antigen Preparation
Stock culture of E. coli O78 was grown in brain–heart infusion broth for 24 h at 37°C and then inoculated on Mackonkey's lactose agar plates. The growth was harvested in sterile normal saline solution and after washing the pellet 3 times, and the cell suspension was sonicated (Labsonic 1510 sonifier). The sonicated bacterial cell suspension was termed as E. coli O78 sonicated antigen for coating the ELISA plates. The optimum concentration of sonicated bacterial cell antigen, serum dilution (1:400), and conjugate dilution (1:3,000) used in the assay was determined using checkerboard titration method.
Procedure
Flat-bottomed ELISA plates (Nunc, Roskilde, Denmark) were coated with E. coli (50 μl) antigen in 0.05 M carbonate-bicarbonate buffer (pH 9.6) and incubated at 4°C overnight. The plates were washed 5 times in phosphate buffer saline containing 0.05 percent between Tween 20 (PBST, pH 7.2). Unreactive sites in well were blocked with blocking buffer (lactalbumin hydrolysate 3% v/v, new born calf serum 5%v/v, and normal rabbit serum mixed in PBST). After washing, the plates with PBST, 50 μl of each serum sample (1:400 in blocking buffer) in 2-fold serial dilution was dispensed into respective wells and incubated at 37°C for 1 h followed by washings with PBST. Then 50 μl of antichicken IgY (IgG) peroxidase conjugate diluted to 1:3,000 in blocking buffer was added to all wells of the plate. The plates were incubated at 37°C for 1 h and washed, and finally, 50 μl of substrate solution was added to the wells. The reaction was stopped by the addition of 50 μl of 1 M H2SO4 after the development of color in positive control well. Absorbance values were read at 492 nm using an automatic micro-ELISA reader (Tecan, Sunrise model Belgium, version 1.21). The results were expressed as endpoint titers that is the reciprocal of the serum dilution immediately above the baseline for positivity. The baseline (cut-off) selected was 5 times the standard deviation higher than the mean optical density obtained by using serum from negative control chickens. The endpoint titers were converted to log10x, where x was the serum dilution.
Lymphocyte Proliferation Assay
The blood samples were evaluated in the lymphocyte proliferation assay to assess cellular immune responses. Peripheral lymphocytes were harvested from blood and incubated with mitogen (phytohemagglutinin [PHA]) or E. coli antigen. The degree of lymphocyte proliferation was assayed by tetrazolium-based lymphocyte proliferation assay (Mosmann, 1983). The conversion of 3-(4, 5-dimethylthiazo-2-lyl)-2, 5-diphenyl tetrazolium bromide to formazan is directly proportional to the number of proliferating cells and measured by a shift in the absorbance with an ELISA reader. The results were expressed as the response by stimulated cells relative to unstimulated cells.
The optimum concentration of sonicated bacterial cell antigen (1:20 dilution) and PHA (20 μg/ml) used in this assay was determined using checkerboard titration method.
Procedure
Blood was collected in heparinized vials and diluted with equal volume of RPMI-1640 medium. Diluted blood was then carefully layered onto histopaque-1077 (Sigma, St Louis, MO) column and centrifuged at 3,000 rpm for 20 min at 4ᵒC in a cooling centrifuge (Velocity R Centrifuge). The white interface layer so obtained containing lymphocytes was harvested carefully, and the cells were resuspended and washed twice with RPMI-1640 and then once with RPMI complete medium at 2,500 rpm for 10 min in the cooling centrifuge. The viability of mononuclear cells was estimated by trypan blue exclusion.
The cells were finally resuspended in RPMI (Roswell Park Memorial Institute Medium) complete medium at a concentration of 1 × 106 live cells/ml. To perform the lymphocyte proliferation assay, 100 μL of cell suspension (1 × 106 cells) was dispensed in each of the 3 wells of the microtiter plate. These cells were then stimulated by adding 50 μl of optimally diluted sonicated bacterial cell antigen (1:20) or mitogen (PHA [Sigma] @ 20 μg/ml). Control wells received RPMI-1640 medium only. The plates were then incubated at 37°C in CO2 incubator for 72 h. After that, 20 μl of working solution (5 mg/ml) of 3-(4, 5-dimethylthiazo-2-lyl)-2, 5-diphenyl tetrazolium bromide dye (Sigma) was added in each well, and plates were further incubated at 37°C in CO2 incubator for 4 h. Following incubation, the supernatant of each well was discarded carefully, and 100 μl of dimethyl sulfoxide was added into each well to solubilize the formazan crystals. Finally, optical density was recorded using microplate reader (Tecan, Sunrise model, Belgium version 1.21) at 540 nm wavelength, and the stimulation index (SI) was calculated by the following formulae (Denizot and Lang, 1986):
Gross Pathology
The chicks sacrificed or died naturally during the experiment were thoroughly examined for gross lesions, if any.
Histopathology
The formalin-fixed tissues were processed by paraffin-embedding technique. After trimming and properly washing the tissues overnight in running tap water, they were dehydrated in graded ethanol, cleared in benzene, and embedded in paraffin wax (melting point 60°C–62°C). Sections of 3 to 4 μm thickness were cut using semiautomatic microtome and stained with hematoxylin and eosin (Luna, 1968).
Lesion Score
The colibacillosis-specific gross lesion score (GLS) and histopathological lesion score (HLS) in different experimental groups were calculated for different organs/tissues at scale of 0 to 4 [No lesion—0; Mild lesions (congestion)—1; Moderate lesions (thin fibrin layer over organs)—2; Moderately severe lesions (thick fibrin layer over organs)—3; Severe lesions (very thick layer/mass of fibrin over organs, adhesions, necrosis—4])
Percent mean gross and histopathological lesions were calculated as per the method described by Witter (1982).
Protective Effect
Percent protective effect due to WSR extract supplementation in E. coli-infected chicks was calculated on the basis of GLS and HLS as per the method of Witter (1982) using the following formula:
Statistical Analysis
The data of body weight and biochemical parameters including oxidative stress and lymphocyte proliferation assay were statistically analyzed by applying Analysis of Variance using Statistical Package for Social Sciences (SPSS), 17th version. Antibody titer and viable bacterial cell counts were analyzed with a t-test. Clinical signs and lesion scores were analyzed with a chi-square test. For all the parameters, the results were compared at P < 0.05.
Results
Clinical Signs and Mortality
Mean score of clinical signs of chickens in the infected groups at different intervals is given in Table 2. The mean clinical signs score was significantly lower in WSR extract supplemented and infected group B2 as compared with group A2 from 7 to 21 days post infection (DPI). Clinical signs of E. coli infection in group A2 (control infected, nonsupplemented) started to appear at 24 h postinfection. These were inappetence, dullness, depression, closing of eyes, anorexia, listlessness, ruffled feathers, and drooping of head and neck. They were reluctant to move and huddled together near the light source. These clinical signs were more severe at 7 DPI. After 10 DPI, the severity of clinical signs declined. The birds were almost normal at 21 DPI.
Table 2.
Mean score of clinical signs of chickens of different experimental groups at different intervals (mean ± SE).
| Groups | 0 DPI (n = 35) | 7 DPI (n = 24) | 14 DPI (n = 15) | 21 DPI (n = 10) | 28 DPI (n = 5) |
|---|---|---|---|---|---|
| A1 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| A2 | 0.0 ± 0.0 | 3.21a ± 0.19 | 1.8a ± 0.17 | 0.4a ± 0.16 | 0.0 ± 0.0 |
| B1 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| B2 | 0.0 ± 0.0 | 1.33b ± 0.98 | 0.53b ± 0.133 | 0.0 ± 0.0 | 0.0 ± 0.0 |
Abbreviation: DPI, days post infection.
a,bMeans with unlike superscript in the column differ significantly (P < 0.05).
n is no. of birds evaluated at each interval.
On the other hand, the clinical signs of E. coli infection in group B2 (infected, WSR extract supplemented) appeared at 36 h postinfection. Clinical signs were almost identical to those observed in group A2, but they were less severe as compared with group A2. The birds of group B2 chicks appeared almost normal after 14 DPI.
There was no mortality in noninfected groups A1 and B1. The overall mortality in group A2 and B2 was 25.71 and 17.14%, respectively.
Body Weight
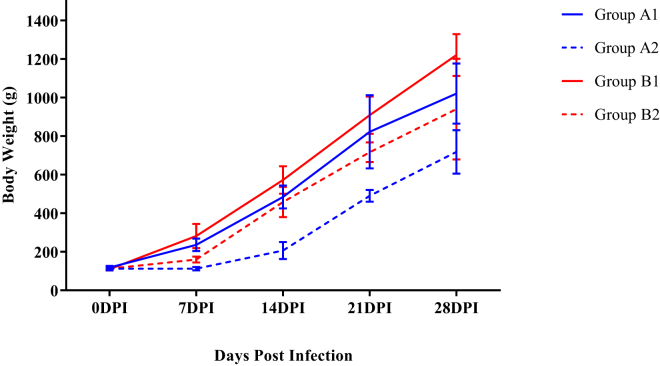
Mean body weights of different groups are illustrated in Figure 1. Mean body weight of WSR supplemented groups (B1 and B2) was significantly higher (P < 0.05) as compared with corresponding control groups (nonsupplemented) that is A1and A2 from 7 DPI onward, indicating the ameliorating effect of WSR extract on body growth.
Figure 1.
Mean body weight (g) of broiler chickens in different experimental groups at different intervals. Abbreviation: DPI, days post infection.
Biochemical Studies
Total Serum Protein Concentration
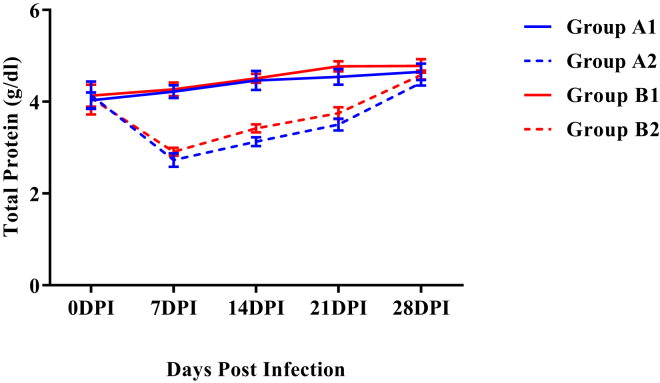
Figure 2 showed that a significant decrease (P < 0.05) in mean total serum protein concentrations was observed in both the infected groups (A2 and B2) as compared with noninfected groups from 7 to 21 DPI. This decrease was less in group B2 as compared with group A2 though the difference was nonsignificant.
Figure 2.
Mean total serum proteins concentration (g/dl) of broiler chickens in different experimental groups at different intervals. Abbreviation: DPI, days post infection.
Albumin Concentration
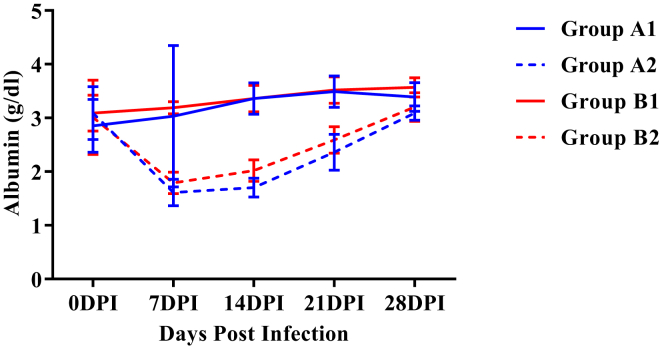
A significant decrease (P < 0.05) in mean serum albumin concentrations was observed in both the infected groups as compared with corresponding control groups from 7 DPI onward (Figure 3). However, the mean serum albumin concentration in group B2 was significantly higher (P < 0.05) as compared with group A2 on 14 DPI.
Figure 3.
Mean serum albumin concentration (g/dl) of broiler chickens in different experimental groups at different intervals. Abbreviation: DPI, days post infection.
Aspartate Transaminase
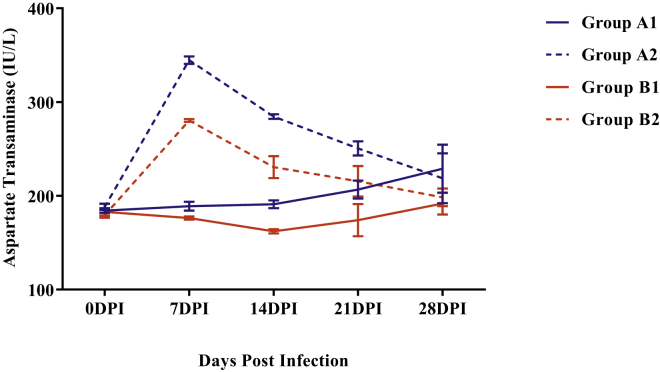
A significant increase (P < 0.05) in mean serum aspartate transaminase (AST) activity was observed in both the infected groups A2 and B2 as compared with noninfected control groups A1 and B1 respectively, from 7 to 21 DPI (Figure 4). Moreover, the serum AST activity of group B2 was significantly low (P < 0.05) as compared with group A2 at 7 and 14 DPI.
Figure 4.
Mean serum aspartate transaminase activities (IU/L) of broiler chickens in different experimental groups at different intervals. Abbreviation: DPI, days post infection.
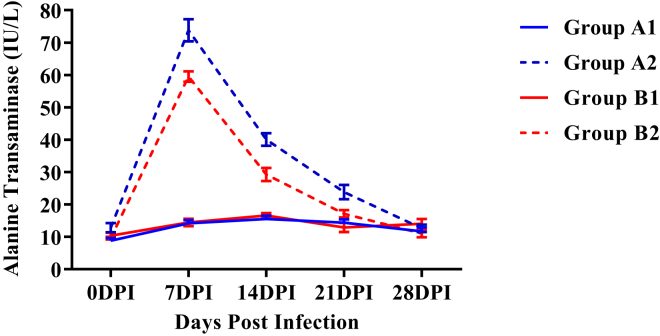
Alanine Transaminase
Mean serum alanine transaminase (ALT) activities were significantly higher (P < 0.05) in both the infected groups as compared with respective control groups from 7 DPI onward (Figure 5). This increase in serum ALT activity persisted up to 21 DPI in group A2 while only up to 14 DPI in group B2. Moreover, the mean serum ALT activity was significantly low (P < 0.05) in group B2 as compared with group A2 from 7 to 21 DPI.
Figure 5.
Mean serum alanine transaminase activities (IU/L) of broiler chickens in different experimental groups at different intervals. Abbreviation: DPI, days post infection.
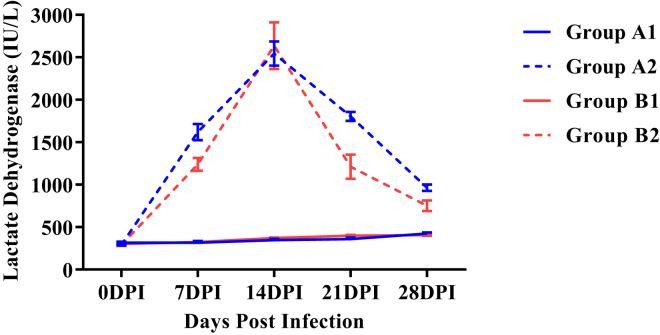
Lactate Dehydrogenase
Figure 6 revealed that mean serum lactate dehydrogenase (LDH) activities were significantly higher (P < 0.05) in both the infected groups as compared with respective control groups from 7 DPI onward. Mean serum LDH activity of group B2 was significantly lower (P < 0.05) as compared with group A2 at 7, 21 and 28 DPI.
Figure 6.
Mean serum lactate dehydrogenase activities (IU/L) of broiler chickens in different experimental groups at different intervals. Abbreviation: DPI, days post infection.
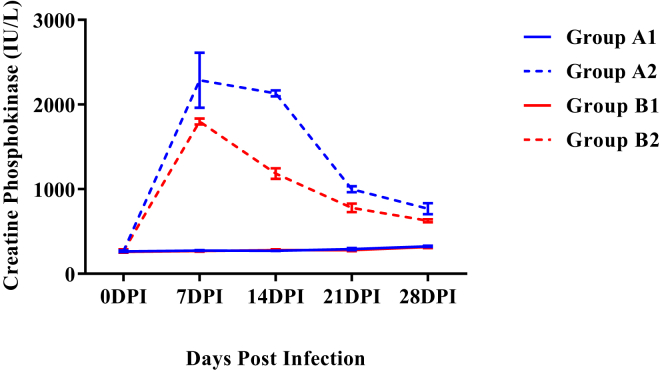
Creatine Phosphokinase
Mean serum creatine phosphokinase (CPK) activities were significantly higher (P < 0.05) in both the infected groups as compared with respective control groups from 7 DPI onward (Figure 7). Mean serum CPK activity of group B2 was significantly low (P < 0.05) as compared with group A2 from 7 DPI onward up to 28 DPI.
Figure 7.
Mean serum creatine phosphokinase activities (IU/L) of broiler chickens in different experimental groups at different intervals. Abbreviation: DPI, days post infection.
Oxidative Stress Parameters
Assay of Blood Superoxide Dismutase
Figure 8 shows that the mean SOD activities of group A2 at 7 DPI were significantly lower (P < 0.05) as compared with respective control group A1 indicating oxidative stress because of E. coli infection. However, the SOD activity of group B2 was significantly low than that of group B1 at 21 and 28 DPI. There was significantly higher activity of SOD in group B1 as compared with group A1 at 21 DPI indicating increased antioxidant scavenging activity in WSR extract–supplemented chicks.
Figure 8.
Mean superoxide dismutase levels (U/mg Hb) of broiler chickens in different experimental groups at different intervals. Abbreviations: DPI, days post infection; SOD, superoxide dismutase.
Assay of Blood Catalase
The mean catalase activities of infected groups were significantly lower as compared with respective noninfected control groups (Figure 9). Furthermore, the mean catalase activities of group B2 were considerably higher as compared with group A2 from 7 to 28 DPI, though the differences were nonsignificant.
Figure 9.
Mean catalase levels (U/mg Hb) of broiler chickens in different experimental groups at different intervals. Abbreviation: DPI, days post infection.
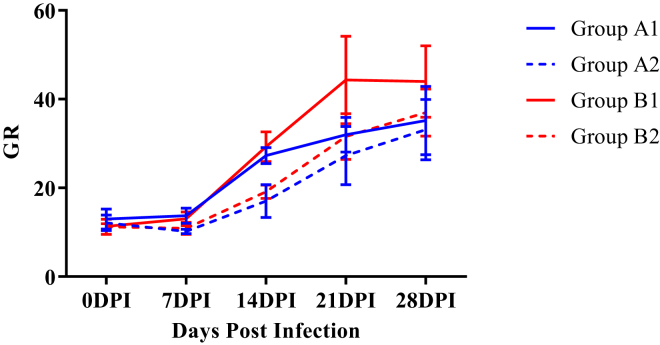
Assay of Blood Glutathione Reductase
The mean GR activities of control WSR supplemented group was significantly higher (P < 0.05) as compared with control nonsupplemented group (Figure 10). Moreover, the GR activities of infected groups A2 and B2 were significantly lower as compared with respective control groups from 7 to 21 DPI.
Figure 10.
Mean glutathione reductase levels (U/mg Hb) of broiler chickens in different experimental groups at different intervals. Abbreviations: DPI, days post infection; GR, glutathione reductase.
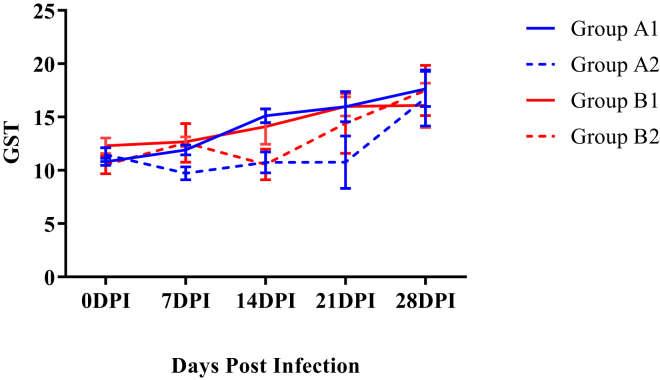
Assay of Blood Glutathione-S-Transferase
The mean GST activity of the infected group A2 was lower compared with the corresponding control group (A1) at 14 DPI (Figure 11). However, the mean GST activity of infected WSR supplemented group B2 did not decrease significantly as compared with corresponding noninfected group B1.
Figure 11.
Mean glutathione-s-transferase levels (U/mg Hb) of broiler chickens in different experimental groups at different intervals. GST, glutathione-S-transferase. Abbreviation: DPI, days post infection.
Viable Bacterial Cell Count
Table 3 shows that mean viable bacterial (E. coli) cell counts in the liver of group B2 was lower as compared with group A2 from 7 DPI onward though the difference was nonsignificant. The noninfected groups (A1 and B1) did not reveal any viable bacterial cell.
Table 3.
Mean viable bacterial cell count (X106 CFU/g of liver, mean ± SE) in different experimental groups at different intervals.
| Groups | Days postinfection |
|||
|---|---|---|---|---|
| 7 | 14 | 21 | 28 | |
| A1 | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a |
| A2 | 1.22 ± 0.32a | 3.06 ± 1.00a | 0.014 ± 0.008a | 0.002 ± 0.001a |
| B1 | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a |
| B2 | 1.08 ± 0.40a | 1.29 ± 0.32a | 0.005 ± 0.002a | 0.00 ± 0.00a |
aMeans with superscript in the column did not differ significantly (P < 0.05).
Immunological Studies
Antibody Titer
Mean reciprocal log10 antibody titer against E. coli in group B2 was higher as compared with group A2 from 7 DPI onward though significant difference (P < 0.05) was noticed only at 21 DPI (Figure 12).
Figure 12.
Mean reciprocal log10 antibody titer against E. coli infection in all the infected groups. Abbreviation: DPI, days post infection.
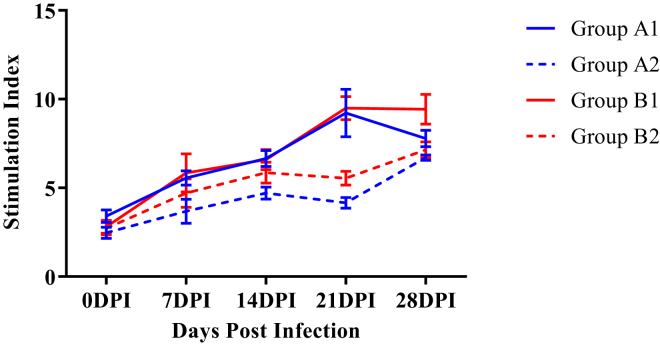
Lymphocyte Proliferation Assay
The SI means for antigen (E. coli)–driven lymphocyte proliferation responses in group B2 were found to be higher as compared with group A2 from 14 DPI onward though a significant difference was present only at 21 DPI (Figure 13).
Figure 13.
Mean stimulation index of antigen-driven lymphocyte proliferation responses of broiler chickens in different experimental groups. Abbreviation: DPI, days post infection.
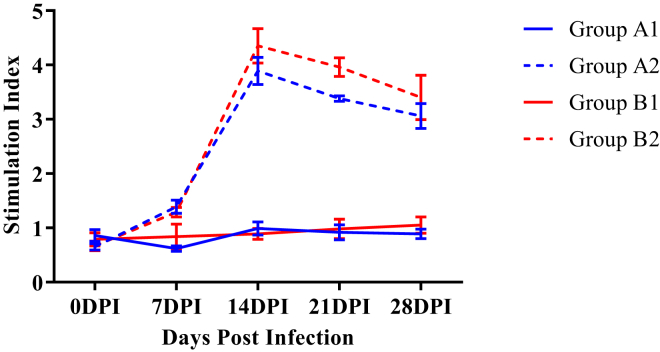
The SI means for the PHA-driven lymphocyte proliferation responses were significantly higher (P < 0.05) in groups A1 and B1 as compared with groups A2 and B2, respectively, from 7 DPI onward (Figure 14). Moreover, group B1 exhibited significantly higher (P < 0.05) values of SI as compared with group A1 at 28 DPI.
Figure 14.
Mean stimulation index of mitogen (phytohemeagglutinin)-driven lymphocyte proliferation responses of broiler chickens in different experimental groups. Abbreviation: DPI, days post infection.
Gross and Histopathological Lesions
No pathological changes could be observed in chicks from noninfected groups (A1and B1) at different intervals throughout the experiment.
Group A2 (E. coli Infected)
On 7 DPI, there was fibrinous perihepatitis associated with coagulative necrosis of hepatocytes at focal areas, fibrinous pericarditis, and myocarditis. Congestion was noticed in various visceral organs such as liver, heart, lungs, spleen, proventriculus, and kidneys. Lungs revealed bronchopneumonia along with pleuritis. In spleen, depletion of lymphocytes was noticed in the white pulp along with congestion and focal necrotic areas. Bursa of Fabricius revealed atrophy of bursal follicles, depletion of lymphocytes, and increase in inter follicular space, infiltration of heterophils, and edema. On 14 DPI, the heart, liver, and spleen lesions were more severe along with adhesions with other abdominal organs because of thick fibrin mass on the surface of the liver. In spleen, depletion of lymphocytes was noticed in the white pulp along with congestion and focal necrotic areas. On 21 DPI onward, the lesions in different organs were of mild magnitude as there was only a thin fibrin layer covering on the surface of heart and liver.
Group B2 (E. coli infected + WSR extract supplemented)
On 7 DPI, heart and liver surface revealed mild inflammatory lesions and lungs and spleen revealed only congestion. On 14 DPI onward, the lesions in different organs were of very mild degree. On 21 DPI, the bursa of Fabricius was almost normal with the prominent demarcation between the medulla and cortex maintaining the normal architecture of follicles along with presence of lymphocytes in the follicles.
Overall severity of lesions in different organs in WSR extract–fed infected group B2 was significantly less than control infected group A2. On 28 DPI, there were no lesions in any of the organs.
Lesion Score
The colibacillosis-specific mean GLS and HLS in various organs (heart, liver, spleen, bursa of Fabricius, lungs, and intestines) in different experimental groups are shown in Table 4 and Table 5. The mean GLS was considerably lower in WSR extract–supplemented infected group B2 as compared with group A2. Similarly, HLS was also lower in group B2 in comparison to group A2. The overall percent mean GLS and HLS in different organs irrespective of postinfection period and organs in different experimental groups (Table 6) were also lower in supplemented infected groups as compared with the nonsupplemented infected group.
Table 4.
Mean gross lesion score in various organs of chickens of different experimental groups.
| Organs | Groups | Days postinfection |
||||
|---|---|---|---|---|---|---|
| 0 | 7 | 14 | 21 | 28 | ||
| Heart | A2 | 0.00 ± 0.00 | 3.20 ± 0.20a | 3.20 ± 0.37a | 1.20 ± 0.37a | 0.00 ± 0.00 |
| B2 | 0.00 ± 0.00 | 3.00 ± 0.32a | 2.80 ± 0.37a | 0.40 ± 0.24a | 0.00 ± 0.00 | |
| Liver | A2 | 0.00 ± 0.00 | 3.40 ± 0.24a | 3.20 ± 0.37a | 0.60 ± 0.24a | 0.00 ± 0.00 |
| B2 | 0.00 ± 0.00 | 2.60 ± 0.51a | 2.40 ± 0.24a | 0.40 ± 0.24a | 0.00 ± 0.00 | |
| Lungs | A2 | 0.00 ± 0.00 | 2.40 ± 0.24a | 2.40 ± 0.51a | 0.60 ± 0.24a | 0.00 ± 0.00 |
| B2 | 0.00 ± 0.00 | 2.20 ± 0.37a | 1.80 ± 0.37a | 0.40 ± 0.24a | 0.00 ± 0.00 | |
| Spleen | A2 | 0.00 ± 0.00 | 2.40 ± 0.24a | 2.00 ± 0.32a | 0.80 ± 0.37a | 0.00 ± 0.00 |
| B2 | 0.00 ± 0.00 | 1.60 ± 0.40a | 1.40 ± 0.24a | 0.20 ± 0.20a | 0.00 ± 0.00 | |
| Bursa | A2 | 0.00 ± 0.00 | 2.20 ± 0.20b | 2.20 ± 0.37b | 0.40 ± 0.24a | 0.00 ± 0.00 |
| B2 | 0.00 ± 0.00 | 1.00 ± 0.32a | 1.20 ± 0.20a | 0.00 ± 0.00a | 0.00 ± 0.00 | |
| Intestine | A2 | 0.00 ± 0.00 | 1.00 ± 0.32a | 1.00 ± 0.32a | 0.20 ± 0.20a | 0.00 ± 0.00 |
| B2 | 0.00 ± 0.00 | 0.20 ± 0.20a | 0.40 ± 0.24a | 0.00 ± 0.00a | 0.00 ± 0.00 | |
a,bMeans with unlike superscript in the column differ significantly (P < 0.05).
Table 5.
Mean histopathological lesion score in various organs of chickens of different experimental groups.
| Organs | Groups | Days postinfection |
||||
|---|---|---|---|---|---|---|
| 0 | 7 | 14 | 21 | 28 | ||
| Heart | A2 | 0.00 ± 0.00 | 3.00 ± 0.32a | 2.20 ± 0.49a | 1.00 ± 0.45a | 0.20 ± 0.20a |
| B2 | 0.00 ± 0.00 | 3.20 ± 0.37a | 1.80 ± 0.37a | 0.20 ± 0.20a | 0.00 ± 0.00a | |
| Liver | A2 | 0.00 ± 0.00 | 3.00 ± 0.45a | 1.80 ± 0.37a | 1.00 ± 0.45a | 0.20 ± 0.20a |
| B2 | 0.00 ± 0.00 | 2.00 ± 0.32a | 1.80 ± 0.20a | 0.60 ± 0.24a | 0.20 ± 0.20a | |
| Lungs | A2 | 0.00 ± 0.00 | 2.60 ± 0.24a | 2.40 ± 0.51a | 1.00 ± 0.32a | 0.40 ± 0.24a |
| B2 | 0.00 ± 0.00 | 1.60 ± 0.40a | 1.60 ± 0.24a | 0.20 ± 0.20a | 0.00 ± 0.00a | |
| Spleen | A2 | 0.00 ± 0.00 | 2.20 ± 0.20a | 2.40 ± 0.24b | 0.60 ± 0.24a | 0.00 ± 0.00 |
| B2 | 0.00 ± 0.00 | 1.80 ± 0.58a | 1.20 ± 0.37a | 0.20 ± 0.20a | 0.00 ± 0.00 | |
| Bursa | A2 | 0.00 ± 0.00 | 3.00 ± 0.32b | 2.80 ± 0.58b | 1.20 ± 0.20b | 0.00 ± 0.00 |
| B2 | 0.00 ± 0.00 | 1.80 ± 0.20a | 1.00 ± 0.32a | 0.20 ± 0.20a | 0.00 ± 0.00 | |
| Intestine | A2 | 0.00 ± 0.00 | 0.40 ± 0.24a | 0.60 ± 0.40a | 0.00 ± 0.00a | 0.00 ± 0.00 |
| B2 | 0.00 ± 0.00 | 0.80 ± 0.37a | 0.60 ± 0.40a | 0.20 ± 0.20a | 0.00 ± 0.00 | |
a,bMeans with unlike superscript in the column differ significantly (P < 0.05).
Table 6.
Overall percent mean lesion scores irrespective of postinfection period and organs in different experimental groups.
| Lesion score | Groups | Overall percent mean lesion scores irrespective of postinfection period and organs |
|---|---|---|
| GLS | Group A2 | 27.00 ± 3.95 |
| Group B2 | 18.50 ± 4.33 | |
| HLS | Group A2 | 26.67 ± 4.50 |
| Group B2 | 17.50 ± 2.6 |
Abbreviations: GLS, gross lesion score; HLS, histopathological lesion score.
Percent protection due to WSR extract against E. coli infection on the basis of gross and histopathological lesions (Table 7) was 31.48 and 34.38%, respectively.
Table 7.
Percent protection due to W. somnifera extract against E. coli infection in broiler chicken.
| Group | Percent protection |
|
|---|---|---|
| GLS | HLS | |
| B2 (WSR extract supplemented and infected) | 31.48 | 34.38 |
Abbreviations: GLS, gross lesion score; HLS, histopathological lesion score.
Discussion
The present experiment was undertaken to evaluate the in vivo effect of aqueous WSR extract on E. coli infection in broiler chicken with respect to biochemical, immunological, and pathological parameters. The clinical signs of colibacillosis in WSR extract–supplemented group appeared later, comparatively of less intensity and persisted for a shorter period as compared with control infected group. Mortality was also reduced. These findings suggest that the ameliorating effect of the WSR extract on severity of colibacillosis may reduce the economic losses associated with the disease. These findings are in accordance with those of Kumari et al. (2015b) and Owais et al. (2005) who observed an increase in the survival rate in Balb/C mice and Salmonella-infected broiler chicks, respectively, when they were treated orally with WSR. The protective effect of WSR on the disease manifestations might be because of immunomodulatory and antimicrobial effects as observed in the present study and literature (Akotkar et al., 2007, Gupta and Rana, 2007). Body weight gain in WSR extract supplemented groups (infected as well as noninfected) was significantly higher as compared with their respective control groups indicating an accelerated growth response in the WSR extract supplemented birds. The enhanced growth rate because of WSR supplementation might be attributed to improved feed conversation ratio and free radical scavenging effect of WSR supplementation (Akotkar et al., 2007, Vasanthakumar et al., 2015). The significant reduction in serum total protein concentration in all the infected groups was due to hypoalbuminemia. More or less similar results have been reported by other workers because of E. coli infection (Jindal et al., 2003, Zaki et al., 2012, Kumari et al., 2014). This might be because of severe hepatic lesions observed in the infected groups in the present study. According to Kaneko (1997), during inflammation, the synthesis of albumin (negative acute phase protein) in the liver is inhibited. Serum albumin concentration in WSR extract–supplemented infected group which might be attributed to variance in the severity of hepatic lesions of both the infected groups. Dhenge et al., (2009) also reported a significant increase in serum total protein in broilers supplemented with W. somnifera. According to Udayakumar et al., (2009), the phytochemicals present in WSR extract might have contributed to the reversal of changes in serum total protein and albumin concentration.
The significantly higher activities of serum aspartate transaminase and alanine transaminase observed in E. coli–infected groups as compared with control have also been reported by other workers (Eleiwa et al., 2011, Zaki et al., 2012, Verma and Swamy, 2013, Kumari et al., 2014). The restoration of the normal activity of these enzymes was evident in WSR extract supplemented infected group as compared with control infected group indicating the hepatoprotective effect of WSR supplementation. Nevertheless, pathological changes in liver due to E. coli infection in WSR supplemented group was of lower magnitude as compared with nonsupplemented infected group of the present study. More or less, similar ameliorating effects of WSR supplementation have been reported in rats (Harikrishnan et al., 2008, Udayakumar et al., 2009), which could be due to presence of alkaloids, flavonoids, and free radical scavenging activity of WSR.
The significant increase in serum lactate dehydrogenase activity observed in both the infected groups as compared with control in the present study might be because of injury to cardiac lesions. The restoration of LDH activity in WSR supplemented group may indicate the cardiac and hepatoprotective activity of WSR. These findings are in agreement to the observations of Mohanty et al. (2004) in rats.
The results of CPK activity revealed that the WSR supplementation had contributed to significant restoration of cardiac lesions caused by E. coli infection. Mohanty et al. (2004) reported that hydroalcoholic extract of W. somnifera restored myocardial damage in rats.
A number of serum antioxidant enzymes were analyzed to assess the role of WSR extract in the prevention of oxidative stress due to E. coli infection. A significant reduction in mean SOD level in control infected group A2 as compared with noninfected control group A1 indicates overwhelmed free radical production and less free radical scavenging activity of SOD, resulting in oxidative stress due to E. coli infection in the birds. However, B1 revealed significantly higher SOD levels as compared with group A1 suggesting an increase in the free radical scavenging activity of SOD in WSR extract–fed chicken. Other workers have also reported the antioxidant effect of W. somnifera in mice (Panda and Kar, 1997), rats (Mohanty et al., 2004, Hosny and Farouk, 2012), and broiler chicken (Gupta et al., 2008, Bharavi et al., 2010, Vasanthakumar et al., 2015).
The mean catalase activity of WSR extract supplemented infected group B2 was higher than control infected group A2, though the difference was nonsignificant. Panda and Kar (1997) reported an increase in catalase activity in mice because of supplementation of WSR powder. Other workers have also reported increase in catalase activity in rats (Bhattacharya et al., 2001, Mohanty et al., 2004, Hosny and Farouk, 2012) and broilers (Gupta et al., 2008, Bharavi et al., 2010, Vasanthakumar et al., 2015) because of supplementation of W. somnifera.
The higher mean GR activity of the WSR extract supplemented and infected group as compared with control infected group is in accordance with the findings of Malik et al. (2013), who reported enhanced GR activities in mice with the administration of aqueous root extract of W. somnifera.
The mean GST activities of control infected group decreased significantly as compared with control placebo, but WSR extract supplemented infected group did not reveal significant decrease in GST activity as compared with control indicating antioxidant activity of WSR extract. Enhanced activity of GST because of the administration of W. somnifera has also been reported in rats (Sabina et al., 2013).
Antioxidant activity of WSR may be contributed to its active principles such as glycowithanolides, sitoindoside VII-K, and withaferin A (Bhattacharya et al., 2000; Mishra et al., 2000).
The lower viable E. coli count observed in the liver of the supplemented infected group as compared with the nonsupplemented group may reflect the antibacterial activity of WSR (Sonwane et al., 2017). Nevertheless our in vitro experimental results have also demonstrated a remarkable antibacterial activity of aqueous extract of WSR against E. coli (Kumari and Gupta, 2014). Iuvone et al., (2003) have reported that that WSR extract enhanced nitric oxide synthetase activity of the macrophages, which in turn increased the microbial killing power of these immune cells. The significantly higher mean E. coli-specific antibody titer in WSR extract supplemented infected group suggests enhancement of humoral immune response due to supplementation of aqueous WSR extract. Bhardwaj et al. (2012) reported significantly higher immunoglobulins in Japanese quails supplemented with 1.5% W. somnifera as compared with the control group. Arivuchelvan et al. (2013) reported reversal of enrofloxacin-induced immunosuppression in broilers because of supplementation of crude extract of W. somnifera. Similarly, enhanced humoral immune response in Salmonella gallinarum–infected broiler chicks because of the administration of WSR has also been reported (Kumari et al., 2015a). Ghosal et al. (1989) suggested that the increase in humoral immune response might be because of the immunomodulatory activity of glycowithanolides found in W. somnifera.
Regarding the cell-mediated immune response, the significantly higher SI of PHA-driven lymphocyte proliferation responses in control WSR extract–supplemented group B1 as compared with control placebo A1 revealed enhancement of cell-mediated immune response. Furthermore, the significantly higher mean SI of E. coli antigen-driven lymphocyte proliferation responses in group B2 as compared with group A2 suggests that the immune system was less compromised because of E. coli infection in group B2. Enhanced cell-mediated immune response due to supplementation of WSR powder has also been reported by other workers in the broiler chicken (Akotkar et al., 2007, Kumari et al., 2015a) and rats (Gupta et al., 2006). Raghavan et al. (2011) also reported that supplementation of W. somnifera to broilers improved the immune status and reduced stress by improving the total leukocyte count and reducing the heterophil:lymphocyte ratio. The role of interferon gamma, interleukin-2, and granulocyte macrophage colony-stimulating factor in immunopotentiating effect of WSR has been reported in mice (Iuvone et al., 2003). The gross and histopathological lesions in WSR extract supplemented infected group at different intervals were of lower magnitude as compared with control infected group (nonsupplemented), which indicates less injury to the visceral organs of chicks supplemented with WSR extract.
The results of lesion scores revealed that the protective effect of WSR extract on E. coli infection was 31.48% in gross lesions and 34.38% in histopathological lesions. The differences in the severity of hepatic and cardiac lesions between the control infected and WSR supplemented infected group might be because of antioxidant principles of W. somnifera such as withanolides, which reduces lipid peroxidation, improves antioxidant status (Bharavi et al., 2010), antilipoperoxidative activity (Bhattacharya et al., 2001, Hosny and Farouk, 2012), and antiinflammatory activity (Mahmoud et al., 2012), which work together to reduce the cellular injury in these organs. The hepatoprotective and cardioprotective potential of WSR has also been demonstrated by different workers in rats/mice (Mohanty et al., 2004, Harikrishnan et al., 2008, Kaithwas et al., 2011) and chickens (Waihenya et al., 2002, Kumari et al., 2015a). The recovery of the lesions in the spleen and bursa of Fabricius in WSR–supplemented infected group indicates an immunomodulatory effect of WSR. W. somnifera has also been reported to provide immunoprotection against E. coli infection in Guinea pigs (Teixeira et al., 2006) and S. gallinarum infection in broilers (Kumari et al., 2015a).
The overall pathological findings in different organs suggested that WSR extract resulted in considerable reduction in inflammation and injury to various organs due to colibacillosis. Rasool and Varalakshmi (2006) reported that W. somnifera activated and mobilized macrophages for rendering increased phagocytic activity.
It is concluded that administration of 20% aqueous WSR extract @ 20 ml/liter of water to broiler chicken caused a significant reduction in the severity and course of E. coli infection and enhanced humoral and cellular immune responses, suggesting a protective effect on the pathology and pathogenesis of E. coli infection.
Acknowledgments
The authors gratefully acknowledge the authorities of the Lala Lajpat Rai University of Veterinary and Animal Sciences and the Department of Science and Technology, New Delhi, India for providing financial support to conduct this study under Grant No. IF130095.
References
- Aebi H.E. Catalase. In: Bergmeyer H.O., editor. Methods of Enzymatic Analysis. Acad. Press; New York, NY: 1983. pp. 273–286. [Google Scholar]
- Akotkar N.S., Sarag A.N., Rekhate D.H., Dhok A.P. Effect of supplementation of Ashwagandha (Withania somnifera) on the performance of broilers. Indian J. Poult. Sci. 2007;42:92–94. [Google Scholar]
- Arivuchelvan A., Murugesan S., Mekala P. Immunomodulatory effect of Withania somnifera in broilers treated with high doses of enrofloxacin. Indian J. Drugs Dis. 2013;2:276–279. [Google Scholar]
- Arora S., Dhillon S., Rani G., Nagpal A. The in vitro antibacterial/synergistic activities of Withania somnifera extracts. Fitoterapia. 2004;75:385–388. doi: 10.1016/j.fitote.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Aviagen T. Aviagen Group; Huntsville, AL: 2014. Ross- Broiler management handbook. Accessed Oct. 2018. https://mafiadoc.com/ross-broiler-handbook_59c1f11f1723ddc052bf1a81.html. [Google Scholar]
- Bass L., Liebert C.A., Lee M.D., Summers A.O., White D.G., Thayer S.G., Maurer J.J. Incidence and characterization of integrons, genetic elements mediating multiple-drug resistance in avian Escherichia coli. Antimicrob. Agents Chemother. 1999;43:2925–2929. doi: 10.1128/aac.43.12.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin M.M. Outline of Veterinary Clinical Pathology. Kalyani Publishers; New Delhi, India: 1985. pp. 25–48, 60. [Google Scholar]
- Bharavi K., Gopala Reddy A., Rao G.S., Rajasekhara Reddy A., Rama Rao S.V. Reversal of cadmium-induced oxidative stress in chicken by herbal adaptogens Withania somnifera and Ocimum sanctum. Toxicol. Int. 2010;17:59–63. doi: 10.4103/0971-6580.72671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj R.K., Bhardwaj A., Gangwar S.K. Efficacy of Ashwagandha (Withania somnifera) supplementation on haematological and immunological parameters of Japanese Quails. Int. J. Sci. Nat. 2012;3:476–478. [Google Scholar]
- Bhattacharya A., Majumdar P., Dutta M.K. Isolation, characterization and antibiotic spectra of S. Gallinarum from an outbreak of fowl typhoid in adult broiler parent flock in Tripura. Indian J. Comp. Microbiol. Immunol. Infect. Dis. 2001;22:56–58. [Google Scholar]
- Bhattacharya A., Ramanathan M., Ghosal S., Bhattacharya S.K. Effect of Withania somnifera Glycowithanolides on iron-induced hepatotoxicity in rats. Phytotherapy Res. 2000;14:568–570. doi: 10.1002/1099-1573(200011)14:7<568::aid-ptr663>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Blanco J.E., Blanco M., Mora A., Blanco J. Prevalence of bacterial resistance to quinolones and other antimicrobials among avian Escherichia coli strains isolated from septicemic and healthy chickens in Spain. J. Clin. Microbiol. 1997;35:2184–2185. doi: 10.1128/jcm.35.8.2184-2185.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlberg I., Mannervik B. Methods in Enzymology. Acad. Press; New York, NY: 1985. Glutathione reductase; pp. 484–490. [Google Scholar]
- Cruickshank R., Duguid J.P., Marsion B.P., Swain R.H.A. Churchill Livingstone; Edinburgh, London and New York, NY: 1975. Medical-Microbiology. [Google Scholar]
- Davis L., Kuttan G. Immunomodulatory activity of Withania somnifera. J. Ethnopharmacol. 2000;71:193–200. doi: 10.1016/s0378-8741(99)00206-8. [DOI] [PubMed] [Google Scholar]
- Denizot F., Lang R. Rapid colorimetric assay for cell growth and survival modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods. 1986;89:271–277. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- Dhenge S.A., Shirbhate R.N., Bahiram K.B., Wankar A.K., Khandait V.N., Patankar R.B. Haemato-biochemical profile of broilers supplemented with Withania somnifera (Ashwagandha) and Andrographis paniculata (Bhuineem) Indian J. Field Vet. 2009;5:124–127. [Google Scholar]
- Dhuley J.N. Effect of some Indian herbs on macrophage functions in ochratoxin A treated mice. J. Ethnopharmacol. 1997;58:15–20. doi: 10.1016/s0378-8741(97)00072-x. [DOI] [PubMed] [Google Scholar]
- Eleiwa N.Z.H., Sayed E.M.E., Nazim A.A. Prophylactic and therapeutic evaluation of the phytobiotic (Orego-stim)® in chicken experimentally infected with E. coli. J. Am. Sci. 2011;7:91–102. [Google Scholar]
- Ghosal S., Lal J., Srivastava R., Bhattacharya S.K., Upadhyay S.N., Jaiswal A.K., Chattopadhyay U. Immunomodulatory and CNS effects of sitoindosides IX and X, two new glycowithanolides from Withania somnifera. Phytotherapy Res. 1989;39:201–206. [Google Scholar]
- Girdhari L., Rana A. Withania somnifera (Ashwagandha): a review. Pharmacog. Rev. 2007;1:129–136. [Google Scholar]
- Gulati S., Madan V.K., Singh S., Singh I., Dushyant Chemical and phytochemical composition of Ashwagandha (Withania somnifera L.) roots. Asian J. Chem. 2017;29:1683–1686. [Google Scholar]
- Gupta G.L., Rana A.C. Protective effect of Withania somnifera Dunal root extract against protracted social isolation induced behavior in rats. Indian J. Physiol. Pharmacol. 2007;51:345–353. [PubMed] [Google Scholar]
- Gupta M.S., Shivaprasad H.N., Kharya M.D., Rana A.C. Immunomodulatory activity of the ayurvedic formulation “Ashwagandha Churna”. Pharmaceut. Biol. 2006;44:263–265. [Google Scholar]
- Gupta S., Singh S.P., Ahmad A.H., Hore S.K. Reversal of oxidative stress by Withania somnifera monocrotophos intoxicated broiler. J. Vet. Pharmacol. Toxicol. 2008;7:2173–2174. [Google Scholar]
- Habig W.H., Pabst M.J., Jakoby W.B. Glutathione S-Transferases-The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- Harikrishnan B., Subramanian P., Subash S. Effect of Withania somnifera root powder on the levels of circulatory lipid peroxidation and liver marker enzymes in chronic hyperammonemia. Eur. J. Chem. 2008;5:872–877. [Google Scholar]
- Hosny M.H., Farouk H.H. Protective effect of Withania somnifera against radiation-induced hepatotoxicity in rats. Ecotoxicol. Environ. Saf. 2012;80:14–19. doi: 10.1016/j.ecoenv.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Iuvone T., Esposito G., Capasso F., Izzo A.A. Induction of nitric oxide synthase expression by Withania somnifera in macrophages. Life Sci. 2003;72:1617–1625. doi: 10.1016/s0024-3205(02)02472-4. [DOI] [PubMed] [Google Scholar]
- Jindal N., Kumar A., Shukla C.L., Pal Y., Ledoux D.R., Rottinghaus G.E. Effect of ochratoxin A on Escherichia coli challenged broiler chicks. Avian Dis. 2003;47:415–424. doi: 10.1637/0005-2086(2003)047[0415:EOOAOE]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Kabir S.M.L. Avian colibacillosis and salmonellosis: a closer look at epidemiology, pathogenesis, diagnosis, control and public health concerns. Int. J. Environ. Res. Public Health. 2010;7:89–114. doi: 10.3390/ijerph7010089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaithwas G., Dubey K., Pillai K.K. Effect of Aloe vera (Aloe barbadensis Miller) gel on doxorubicin-induced myocardial oxidative stress and calcium overload in albino rats. Indian J. Exp. Biol. 2011;49:260–268. [PubMed] [Google Scholar]
- Kaneko J.J. Serum proteins and the dysproteinemias. In: Kaneko J.J., Harvey J.W., Bruss M.L., editors. Clinical Biochemistry of Domestic Animals. Acad. Press; New York, NY: 1997. pp. 117–138. [Google Scholar]
- Kumari D., Mishra S.K., Lather D., Kumari M., Sharma R. Effect of Ashwagandha (Withania somnifera) supplementation in Salmonella Gallinarum infected broilers. Indian J. Vet. Pathol. 2015;39:142–148. [Google Scholar]
- Kumari D., Mishra S.K., Lather D. Effect of supplementation of ashwagandha (Withania somnifera) on haemato-biochemical parameters of Salmonella Gallinarum infected broiler chickens. Haryana Vet. 2015;54:1–6. [Google Scholar]
- Kumari M., Gupta R.P. Sequential pathological studies of experimental Escherichia coli infection in broiler chickens. Vet. Pract. 2014;15:299–302. [Google Scholar]
- Kumari M., Gupta R.P. In vitro antibacterial effect of Withania somnifera root extract on Escherichia coli. Vet. World. 2015;8:57–60. doi: 10.14202/vetworld.2015.57-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari M., Gupta R.P., Sharma R. Biochemical and immunological response of Ocimum sanctum in chickens experimentally infected with Escherichia coli. Indian J. Vet. Pathol. 2014;38:98–102. [Google Scholar]
- Lau G.L., Sieo C.C., Tan W.S., Hair-Bejo M., Jalila A., Ho Y.W. Efficacy of a bacteriophage isolated from chickens as a therapeutic agent for colibacillosis in broiler chickens. Poult. Sci. 2010;89:2589–2596. doi: 10.3382/ps.2010-00904. [DOI] [PubMed] [Google Scholar]
- Li X.S., Wang G.Q., Du X.D., Cui B.A., Zhang S.M., Shen J.Z. Antimicrobial susceptibility and molecular detection of chloramphenicol and florfenicol resistance among Escherichia coli isolates from diseased chickens. J. Vet. Sci. 2007;8:243–247. doi: 10.4142/jvs.2007.8.3.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna L.G. 3rd ed. Mc Graw Hill Book Company; New York: 1968. Manual of Histologic Staining Method of Armed Forces Institute of Pathology. [Google Scholar]
- Mahmoud M.E., Khaled M., Hassanein A. Prevention of tri-nitrobenzene of sulfonic acid-induced colitis in chicken by using extract of Aloe vera. Vet. World. 2012;5:469–476. [Google Scholar]
- Malik T., Pandey D.K., Dogra N. Ameliorative potential of aqueous root extract of Withania somnifera against paracetamol induced liver damage in mice. Pharmacologia. 2013;4:89–94. [Google Scholar]
- Marklund S., Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- Mirjalilli M.,H., Moyano E., Bonfil M. Steroidal lactones from Withania somnifera, an ancient plant for novel medicine. Molecules. 2009;14:2373–2393. doi: 10.3390/molecules14072373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra L.C., Singh B.B., Dagenias S. Scientific basis for the therapeutic use of Withania somnifera (Ashwagandha): a review. Altern. Med. Rev. 2000;5:334–336. [PubMed] [Google Scholar]
- Mohanty I., Arya D.S., Dinda A., Talwar K.K., Joshi S., Gupta S.K. Mechanisms of cardioprotective effect of Withania somnifera in experimentally induced myocardial infarction. Basic Clin. Pharmacol. Toxicol. 2004;94:184–190. doi: 10.1111/j.1742-7843.2004.pto940405.x. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Oberholzer H.M., Pretorius E., Smit E. Investigating the effect of Withania somnifera, selenium and hydrocortisone on blood count and bronchial lavage of experimental asthamatic BALB/C mice. Scand. J. Lab. Anim. Sci. 2008;35:239–248. [Google Scholar]
- Owais M., Sharad K.S., Shehbaz A., Saleemuddin M. Antibacterial efficacy of Withania somnifera (Ashwagandha) an indigenous medicinal plant against experimental murine salmonellosis. Phytomedicine. 2005;12:229–235. doi: 10.1016/j.phymed.2003.07.012. [DOI] [PubMed] [Google Scholar]
- Panda S., Kar A. Evidence for free radical scavenging activity of ashwagandha root powder in mice. Indian J. Physiol. Pharmacol. 1997;41:424–426. [PubMed] [Google Scholar]
- Pujari S.A., Gandhi M.B. Studies on effect of root extracts of Withania somnifera on some clinically isolated bacterial pathogens. J. Environ. Res. Develop. 2012;7:1032–1035. [Google Scholar]
- Raghavan R.P., Sreekumar K.P., Zarina A. Effect of Ashwagandha on growth performance haematology and gastrointestinal enzymes in broiler chicken. Indian J. Poult. Sci. 2011;46:52–55. [Google Scholar]
- Rasool M., Varalakshmi P. Immunomodulatory role of Withania somnifera root powder on experimental induced inflammation: an in vivo and in vitro study. Vasc. Pharmacol. 2006;44:406–410. doi: 10.1016/j.vph.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Rastogi R.P., Mehrotra B.N. Vol. 6. Central Drug Research Institute, National Institute of Science Communication, Council of Scientific and Industrial Research; New Delhi, India: 1998. pp. 225–230. (Compendium of Indian Medicinal Plants). [Google Scholar]
- Roth N., Kasbohrer A., Mayrhofer S., Zitz U., Hofacre C., Domig K.J. The application of antibiotics in broiler production and the resulting antibiotic resistance in Escherichia coli: a global overview. Poult. Sci. 2019;98:1791–1804. doi: 10.3382/ps/pey539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabina E.P., Rasool M., Vedi M., Navaneethan D., Ravichander M., Parthasarthy P., Thella S.R. Hepatoprotective and antioxidant potential of Withania somnifera against paracetamol-induced liver damage in rats. Int. J. Pharm. Pharm. Sci. 2013;5:648–651. [Google Scholar]
- Sonwane S., Ingole R.S., Hedau M., Rathod P.R., Hajare S.W., Ingawale M.V. Ameliorative effect of Andrographis paniculata on hematobiochemical parameters in Escherichia coli induced broilers. J. Pharmacogn. Phytochem. 2017;6:1284–1288. [Google Scholar]
- Teixeira S.T., Valadares M.C., Goncalves S.A., de Melo A., Queiroz M.L.S. Prophylactic administration of Withania somnifera extract increases host resistance in Listeria monocytogenes infected mice. Int. Immunopharmacol. 2006;6:1535–1542. doi: 10.1016/j.intimp.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Udayakumar R., Kasthurirengan S., Mariashibu T.S. Hypoglycaemic and hypolipidaemic effects of Withania somnifera root and leaf extracts on alloxan-induced diabetic rats. Int. J. Mol. Sci. 2009;10:2367–2382. doi: 10.3390/ijms10052367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma M., Sharma D.P., Paaneri S., Mishra A., Sinha A.R.S. Potential clinical benefits of garlic (Allium sativum) J. Environ. Res. Develop. 2011;5:652–655. [Google Scholar]
- Vasanthakumar P., Pangayarselvi B., Sasikumar P., Chandrasekaran D., Doraisamy K.A., Purushothaman M.R. Performance of broilers fed Ashwagandha (Withania somnifera) incorporated diets during summer season for alleviating heat stress. Indian J. Anim. Res. 2015;49:333–335. [Google Scholar]
- Verma Y., Swamy M. Experimental Escherchia coli infection in broilers. Indian J. Poult. Sci. 2013;48:352–356. [Google Scholar]
- Waihenya R.K., Mtambo M.M., Nkwengulila G., Minga U.M. Efficacy of crude extract of Aloe secundiflora against Salmonella Gallinarum in experimentally infected free-range chickens in Tanzania. J. Ethnopharmacol. 2002;79:317–323. doi: 10.1016/s0378-8741(01)00397-x. [DOI] [PubMed] [Google Scholar]
- Witter R.L. Protection by attenuated and polyvalent vaccines against highly virulent strains of Marek’s disease virus. Avian Pathol. 1982;11:49–62. doi: 10.1080/03079458208436081. [DOI] [PubMed] [Google Scholar]
- Zaki M.S., Fawzy O., Osfor M.H. Effect of E. coli OH157 on baladi broiler chicken and some biochemical studies. Life Sci. J. 2012;9:91–94. [Google Scholar]
- Ziauddin M., Phansalkar N., Patki P., Diwanay S., Patwardhan B. Studies on the immunomodulatory effects of Ashwagandha. J. Ethnopharmacol. 1996;50:69–76. doi: 10.1016/0378-8741(95)01318-0. [DOI] [PubMed] [Google Scholar]