Abstract
The present study was to evaluate antioxidative effect of tea extract granule (TEG) on oxidative stress induced by cyclophosphamide (Cy) in chickens. In experiment 1, chickens were randomly divided into 5 groups with 10 birds in each. Groups 3 to 5 were orally administered TEG in drinking water for 7 D at doses of 20, 40, and 80 mg/kg body weight, respectively. After that, groups 2 to 5 received intramuscular injection of Cy (100 mg/kg BW) for 3 D. Group 1 was not treated as a control. In experiment 2, chickens were grouped in the same way as in experiment 1. Groups 2 to 5 received intramuscular injection of Cy (100 mg/kg BW) for 3 D. After that, groups 3 to 5 were orally administered TEG in drinking water for 7 D at doses of 20, 40, and 80 mg/kg BW, respectively. Results showed that Cy injection induced significantly decreased body weight and oxidative stress. Oral administration of TEG before or after Cy injection increased body weight, the thymus, bursa, and spleen indices, total antioxidant capacity, and the levels of glutathione; elevated the activity of superoxide dismutase, catalase, and glutathione peroxidase; as well as decreased the protein carbonyl content, lipid peroxide, and malondialdehyde. In addition, TEG administration reduced intracellular reactive oxygen species. Therefore, TEG could be a promising agent against oxidative stress in the poultry industry.
Key words: oxidative stress, antioxidant, tea extract granule, cyclophosphamide
Introduction
In modern commercial poultry production, chickens are frequently exposed to condensed population, polluted air, and contaminated feed and drinking water, which usually result in the susceptibility of the birds to oxidative stress (Bizeray et al., 2002, Weber et al., 2007, Segato et al., 2011). Oxidative stress is defined as an imbalance of antioxidative and pro-oxidative reactions in favor of the pro-oxidants, which leads to a condition where the production of reactive oxygen species (ROS) exceeds the capacity of a biological system to readily detoxify the reactive intermediates or to repair the resulting damage (Schieber and Chandel, 2014, Furukawa et al., 2016, Cieslar-Pobuda et al., 2017). Many radicals and metabolic substances produced during oxidative stress are highly reactive and can modify several biologically cellular macromolecules, such as proteins, lipids, and nucleic acids (Davies, 1995). Large amount of ROS would activate the nuclear factor-κB pathway (Zhao et al., 2018), inducing the release of downstream inflammatory mediators and stimulating the organs, for example, spleen, and kidney, to increase the synthesis lipid peroxidation for example malondialdehyde (MDA) (Min et al., 2017a, Min et al., 2017b), lipid peroxide (LPO), and carbonyl and decrease the antioxidant enzymes for example, total antioxidant capacity (T-AOC), superoxide dismutase (SOD), and glutathione (GSH) (Ma et al., 2015, Ma et al., 2017). In turn, the overproduction of lipid peroxidation results in the substantial production of free radicals, which can reduce antioxidative capability and aggravate oxidative stress, exerting negative impact on animal production (Lushchak, 2011). Therefore, it will greatly benefit the poultry production to control the oxidative stress.
Tea as a traditional beverage has been consumed for more than 4,000 yr in China (Li et al., 2013). According to statistics, 5,800,000 don of tea is annually produced in the world, in which China produces 2,610,000 don and accounts for 45% of world production (Ding et al., 2019). The natural resource of tea is huge. Tea is made from the leaf of a plant Camellia sinensis (L) O. Ktze. The profitable health effects of green tea has been demonstrated, including the drooping of serum cholesterol, the prevention of low-density lipoprotein oxidation, and a lessened risk of cardiovascular disease and cancer (Duthie et al., 2000, Chan et al., 2007, Suliburska et al., 2012, Wang et al., 2019, Zhou et al., 2019). It is generally agreed that many of the effects of green tea are mediated by its polyphenols which include flavanols and flavonoids. The flavanols, which are also known as catechins, including (−) - epiafzelechin, (+) - catechin, (−) - epicatechin, (+) - gallocatechin, (−) - epigallocatechin, etc. (Okamoto et al., 2004, Matsuo et al., 2008). In addition, 3 flavonoids, namely kaempferol, quercetin, and myricetin, have been segregated as components of green tea (Labbe et al., 2009). The use of tea products in animal production has been found. For example, a dietary tea extract supplemented to broiler chicken diets at 0.5 to 1.0% was reported to make birds within cholesterol deposition and less fat and less oxidative sketch without acute detrimental effect on general performance (Biswas et al., 2000).
In the present study, oral administration of a tea extract granule (TEG) in drinking water was evaluated for its antioxidative stress in chickens by measuring the body weight, organ indices, antioxidant enzymes, redox products, and intracellular ROS. Cyclophosphamide (Cy) was administered to induce oxidative stress in chickens as it was previously used in chickens for the same purpose by many researchers (Reece et al., 1987, El-Abasy et al., 2004, Zanchi et al., 2015, Zheng et al., 2017).
Materials and methods
Chickens
One-day-old specific-pathogen-free (SPF) chickens (male) were purchased from Zhejiang Shennong Stock Breeding Inc. (Ningbo, China), housed in separated units. The room was set at 36°C ± 2°C for the first 3 D and then adjusted to 26°C ± 2°C. Feed and water were supplied ad libitum. Feed was purchased from Charoen Pokphand Group Co., Ltd. (Ningbo, China), and its ingredients were shown in Table 1. All the birds were treated according to the Zhejiang University Committee on Animal Care and Use.
Table 1.
Ingredients of the diet.
| Charoen Pokphand group chickens feed | |||
|---|---|---|---|
| Ingredients | Percent (%) | Ingredients | Percent (%) |
| Dry matter | ≥0.8 | Crude protein | ≥21.0 |
| Crude fat | 7.0 | Crude ash | ≤7.5 |
| Nitrogen-free extract | ≤6.5 | Crude fiber | ≤5.0 |
| Neutral fiber | ≤3.5 | Calcium | 0.7–1.4 |
| Acidic fiber | ≤9.0 | Total phosphorus | ≥0.6 |
| Nonphytic acid phosphorus | ≥30.0 | Sodium chloride | 0.3–0.8 |
| Methionine | 0.5–0.9 | Moisture | ≤13.5 |
Tea Extract Granule
The leaf of C. sinensis (L.) O. Ktze was decocted twice in water with 2 h for the first and 1.5 h for the second decoction. The soups were filtered and combined. The liquid was adjusted to pH 9.0 using saturated lime water. After 30 min, the mixture was filtered, and the precipitate was dissolved by adding dilute hydrochloric acid to adjust pH to 4.0. After filtration, the liquid was concentrated and dried in reduced air pressure at 60°C and ground into powders. The extract was then mixed with dextrin to form TEG, which was in form of light brown powder with total amount of epicatechin (C15H14O6) and epicatechin gallate (C22H18O10) > 30 mg/g and epigallocatechin gallate (C22H18O11) > 144 mg/g.
Reagents
Cyclophosphamide were purchased from Dalian Meilune Biotechnology Co., Ltd. (MB1315, Dalian, China). Detection kits for SOD (A001-3), T-AOC (A015-2), glutathione peroxidase (GSH-PX) (A005), catalase (CAT) (A007-1-1), GSH (A006-2), MDA (A003-2), protein carbonyl (Carbonyl) (A087-2), and LPO (A106) were purchased from the Institute of Nanjing Jiancheng Bioengineering Co., Ltd. (Nanjing, China). Hanks balance salt solution (20180930), RPMI 1640 (20181012), and phosphate-buffered saline (201807071) were purchased from Genom Biotechnology Co., Ltd. (Hangzhou, China). Fetal calf serum were purchased from Sijiqing Co., Ltd. (201810, Hangzhou, China). Test kit for ROS was purchased from Dalian Meilune Biotechnology Co., Ltd. (MA0219).
Experimental Design
In experiment 1, 50 one-day-old male SPF chickens were randomly divided into 5 groups with 10 birds in each. Following acclimatized for 7 D, groups 3 to 5 were orally administered TEG in drinking water for 7 D at doses of 20, 40, and 80 mg/kg body weight, respectively. After that, groups 2 to 5 were exposed to intramuscular injection of Cy (100 mg/kg BW) for 3 D. Group 1 was not treated as a control. In experiment 2, 50 one-day-old male SPF chickens were randomly divided into 5 groups with 10 birds in each. Following acclimatized for 7 D, groups 2 to 5 received intramuscular injection of Cy (100 mg/kg BW) for 3 D. After that, groups 3 to 5 were orally administered TEG in drinking water for 7 D at doses of 20, 40, and 80 mg/kg BW, respectively. Group 1 was not treated as a control. According to the experimental schedule as shown in Figure 1, body weight was measured, and blood was sampled for analysis of oxidative activities splenocytes and were prepared for ROS assay, and the spleen, thymus, and bursa were collected for evaluation of organ indices. The organ index was calculated according to the formula: Organ index = organ weight (mg)/body weight (g).
Figure 1.
Schedule for experiments 1 and 2. TEG, tea extract granule; Cy, cyclophosphamide.
Biochemical Analysis
The activity of T-AOC, SOD, CAT, GSH-PX and the levels of GSH, Carbonyl, LPO, and MDA were determined by using commercial detection kits according to the protocols.
Analysis of Intracellular ROS
Intracellular ROS was determined by a ROS kit with 2, 7-dichlorodihydrofluorescein diacetate as a fluorescent probe (Jiang and Li, 2014). After blood collection, the chickens were sacrificed and immersed in 75% alcohol for 10 min. The spleen was aseptically collected into suitable amount Hanks balance salt solution and were minced and passed through a fine steel mesh to obtain the cell suspension, filtered with 200 mesh copper. The cells were centrifuged for 1,000 × g at 4°C for 10 min 3 times, the supernatant was discarded, and the cell culture medium (RPMI 1640 + 0.05 mM 2-mercaptoethanol + 100 IU/mL penicillin, 100 μg/mL streptomycin, and 10% heat inactivated fetal calf serum) were supplemented to suspend. Cell suspension was adjusted to 5.0 × 106/mL. Then cells incubated with 2, 7-dichlorodihydrofluorescein diacetate at final concentration of 8 μM for 40 min at 37°C and collected and centrifuged at 1,000 × g for 10 min. The cells were washed twice with phosphate-buffered saline. The fluorescence intensity of each sample was measured by flow cytometry, which reflect the level of ROS.
Statistical Analysis
Analysis of data was performed using SPSS software (version 20.0, SPSS Inc., Chicago, IL). One-way analysis of variance with Duncan post hoc test was used for multiple comparisons between groups. Values were expressed as the mean ± standard deviation. P-values of less than 0.05 were considered statistically significant.
Results
Body weight
Table 2 showed that there was no significant difference in body weight between groups before TEG administration. After TEG administration, groups 3 to 5 had significantly higher body weight than groups 1 and 2. Cyclophosphamide injection induced significantly decreased body weight in group 2, 3, and 5 but not in group 4. Table 3 showed that Cy injection significantly decreased in group 3 but numerically decreased in groups 2, 4, and 5 body weight when compared with the control group. Tea extract granule administration significantly increased the body weight in groups 4 and 5 but not in group 3, when compared with group 2. Throughout the experiments, chickens was not found to have abnormal behaviors and side-effects.
Table 2.
Body weight (mean ± SD gram) of chickens, oral administration of TEG before Cy injection.
| Group | n | Treatment | Pre-TEG | Post-TEG | Post-Cy |
|---|---|---|---|---|---|
| 1 | 10 | – | 55.5 ± 4.3 | 95.4 ± 8.5b | 129.2 ± 14.2a |
| 2 | 10 | Cy | 56.1 ± 3.2 | 101.4 ± 7.5b | 112.6 ± 7.4c |
| 3 | 10 | TEG (20 mg/kg) + Cy | 58.3 ± 3.1 | 113.2 ± 8.1a | 121.0 ± 6.0b,c |
| 4 | 10 | TEG (40 mg/kg) + Cy | 56.9 ± 2.8 | 115.6 ± 6.0a | 126.4 ± 7.9a,b |
| 5 | 10 | TEG (80 mg/kg) + Cy | 58.1 ± 2.0 | 112.4 ± 6.7a | 119.8 ± 5.6b,c |
a-cData with different letters statistically differ (P < 0.05).
Abbreviations: Cy, cyclophosphamide; TEG, tea extract granule.
Table 3.
Body weight (mean ± S.D. gram) of chickens, oral administration of TEG after Cy injection.
| Group | n | Treatment | Pre-Cy | Post-Cy | Post-TEG |
|---|---|---|---|---|---|
| 1 | 10 | – | 54.5 ± 5.0 | 70.7 ± 8.9a | 132.8 ± 12.5a |
| 2 | 10 | Cy | 54.7 ± 3.7 | 64.0 ± 8.2a,b | 104.9 ± 9.9d |
| 3 | 10 | Cy + TEG (20 mg/kg) | 57.7 ± 4.5 | 60.4 ± 11.8b | 107.3 ± 10.6c,d |
| 4 | 10 | Cy + TEG (40 mg/kg) | 57.9 ± 2.6 | 65.4 ± 4.8a,b | 115.4 ± 10.0b,c |
| 5 | 10 | Cy + TEG (80 mg/kg) | 56.2 ± 5.2 | 66.4 ± 10.0a,b | 118.6 ± 9.7b |
a-dData with different letters statistically differ (P < 0.05).
Abbreviations: Cy, cyclophosphamide; TEG, tea extract granule.
Indices of Thymus, Bursa, and Spleen
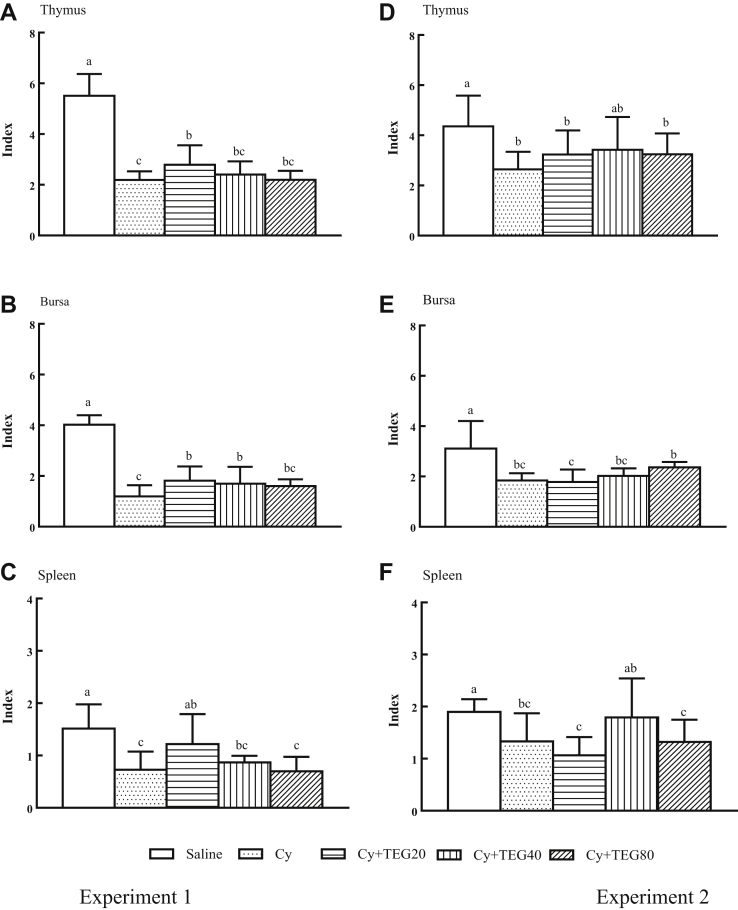
Figure 2 demonstrated that Cy injection prominently lessened the indices of thymus, bursa, and spleen, when compared with the control group. While TEG administration before (Experiment 1, Figure 2A–C) or after (Experiment 2, Figure 2D–F) Cy injection numerically or significantly increased the organ indices.
Figure 2.
Effect of TEG administration on organ indices of chickens. Birds were orally administered TEG (20, 40, 80 mg/kg BW) before (Experiment 1: A, B, and C) and after (Experiment 2: D, E, and F) Cy (100 mg/kg BW) injection. Two days after the end of administration, thymus, bursa, and spleen were collected and organ indices were evaluated. Data were expressed as mean ± SD (n = 10). Bars with different letters statistically differ (P < 0.05). TEG, tea extract granule; Cy, cyclophosphamide.
Antioxidant Enzymes
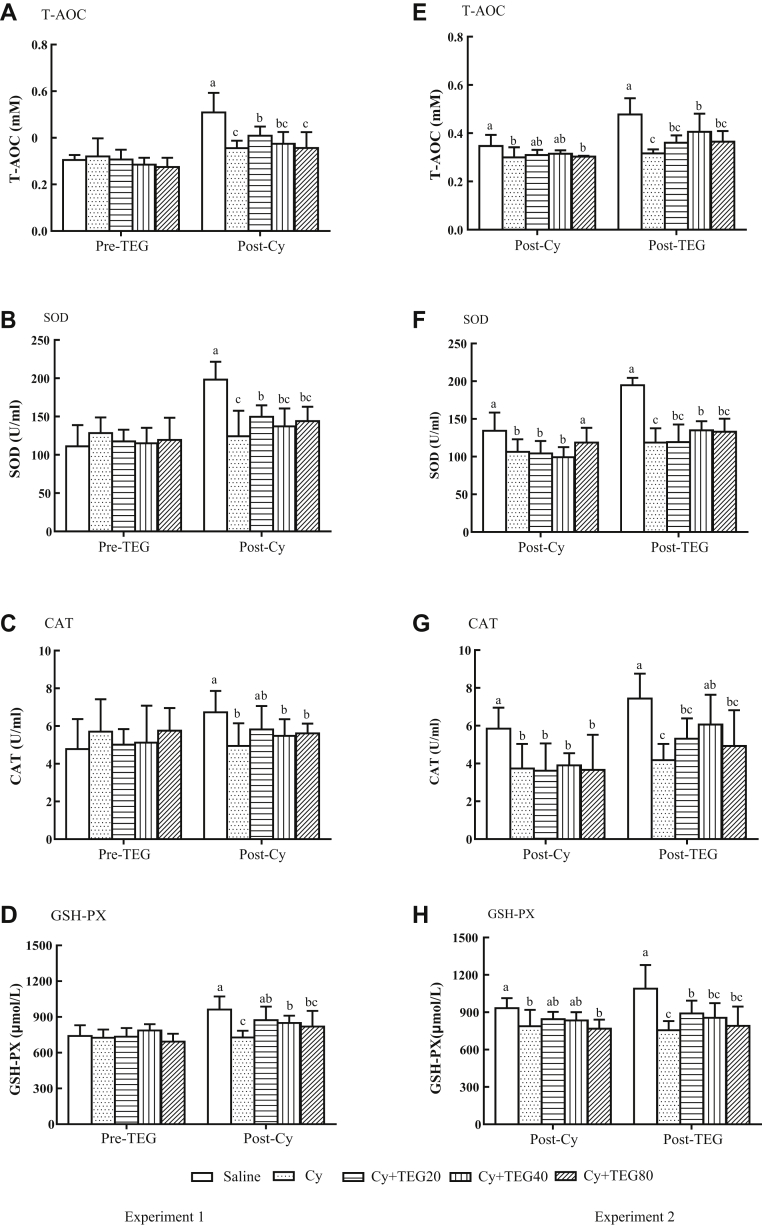
In experiment 1, no significant difference was found for T-AOC, SOD, CAT, and GSH-PX before TEG administration (Figure 3A–D). After Cy injection, chickens without TEG treatment had significantly decreased activities of antioxidant enzymes when compared with the control, whereas the chickens previously treated with TEG had significantly or numerically higher than the chickens without TEG treatment.
Figure 3.
Effect of TEG administration on the serum antioxidant enzymes of chickens. Birds were orally administered TEG (20, 40, 80 mg/kg BW) before (Experiment 1: A, B, C, and D) and after (Experiment 2: E, F, G, and H) Cy (100 mg/kg BW) injection. One day before and 2 D after the end of administration, blood samples were collected to determine serum T-AOC, SOD, CAT, and GSH-PX. Data were expressed as mean ± SD (n = 10). Bars with different letters statistically differ (P < 0.05). TEG, tea extract granule; Cy, cyclophosphamide; T-AOC, total antioxidant capacity; SOD, superoxide dismutase; glutathione; GSH-PX, glutathione peroxidase; CAT, catalase.
In experiment 2, Cy injection significantly decreased activities of antioxidant enzymes (Figure 3E–H). Tea extract granule administration significantly or numerically increased activities of antioxidant enzymes, whereas the enzymes in chickens without TEG treatment remained suppressed.
Redox Products
In experiment 1, no significant difference was found for GSH, carbonyl, LPO, and MDA before TEG administration (Figure 4A–D). After Cy injection, chickens without TEG treatment had significantly decreased GSH and increased carbonyl, LPO, and MDA, when compared with the control while the chickens previously treated with TEG had significantly or numerically higher GSH and lower carbonyl, LPO, and MDA than the chickens without TEG treatment.
Figure 4.
Effect of TEG administration on the serum redox products of chickens. Birds were orally administered TEG (20, 40 and 80 mg/kg BW) before (Experiment 1: A, B, C, and D) and after (Experiment 2: E, F, G, and H) Cy (100 mg/kg BW) injection. One day before and 2 D after the end of administration, blood samples were collected to determine serum GSH, carbonyl, LPO and MDA. Data were expressed as mean ± SD (n = 10). Bars with different letters statistically differ (P < 0.05). TEG, tea extract granule; Cy, cyclophosphamide; MDA, malondialdehyde; LPO, lipid peroxide; GSH, glutathione.
In experiment 2, Cy injection significantly or numerically increased carbonyl, LPO, and MDA (Figure 4F–H). Tea extract granule administration significantly or numerically decreased carbonyl, LPO, and MDA, whereas the products in chickens without TEG treatment remained at high levels.
Intracellular ROS
Figure 5 showed that splenocytes isolated from chickens treated differently had different intracellular 2,7-dichlorodihydrofluorescein diacetate fluorescence intensities. In experiment 1, Cy injection significantly increased the intracellular ROS level in group 1 compared with the control group. However, treatment with TEG significantly reduced intracellular ROS production (Figure 5A–B).
Figure 5.
Effect of TEG administration on the intracellular ROS in lymphocytes of chickens. Birds were orally administered TEG (20, 40 and 80 mg/kg BW) before (Experiment 1: A and B) and after (Experiment 2: C and D) Cy (100 mg/kg BW) injection. Two days after the end of administration, splenocytes were prepared from the chickens to evaluate intracellular ROS by detecting DCFH-DA as described in the text. Data were expressed as mean ± SD (n = 10). Bars with different letters are statistically different (P < 0.05). TEG, tea extract granule; Cy, cyclophosphamide; ROS, reactive oxygen species; DCFH-DA, 2, 7-dichlorodihydrofluorescein diacetate.
In experiment 2, Cy injection induced a significantly higher intracellular ROS level in the splenocytes in group 2 than the cells from chickens without Cy treatment in group 1, whereas TEG administration in groups 3 to 5 significantly decreased intracellular ROS level (Figure 5C–D).
Discussion
Antioxidant property of TEG was demonstrated in chickens. When orally administered before or after Cy-induced oxidative stress in a chicken's model, TEG significantly increased weight gain, the organ indices, T-AOC, and the levels of GSH, elevated the activity of SOD, CAT, and GSH-PX, as well as decreased the protein carbonyl content, MDA, LPO, and intracellular ROS.
Cyclophosphamide is an alkylating agent, served as an antineoplastic for the treatment of various cancers and disorders such as advanced epithelial ovarian cancer, rheumatic arthritis, acquired factor VIII inhibitors, and refractory antibody-mediated autoimmune disorders (Nishino et al., 2001, Arzoo et al., 2002, Samaritani et al., 2007). Cyclophosphamide is metabolized by P-450 enzymes in the liver by 2 pathways. First, Cy is catalyzed by cytochrome P-450 2B and P-450 2C to generate the DNA cross-linking agent, phosphoramide mustard, and the toxic metabolite, acrolein (Zhang et al., 2009), producing excessive ROS (Sudharsan et al., 2006). Second, in trans is accomplished the CYP3A4-mediated N-dechloroethylation of Cy to 3-dechloroethyl cyclophosphamide and the toxic by-product, chloroacetaldehyde (Levova et al., 2011). It has been demonstrated that manage of Cy in poultry brings about to generation of oxidative stress with a resulting decrease in GSH, T-AOC, the indices of spleen and thymus, and the activities of antioxidant enzymes such as lysozyme, CAT, and SOD, as well as increase in the levels of MDA and the activity of xanthine oxidase (El-Abasy et al., 2004, He et al., 2007, Zhang et al., 2009, Cheng et al., 2017, Bi et al., 2018). Induction of oxidative stress by administration of Cy were also found in the present study (Figure 3).
Oxidative stress is an important mechanism of biological damage in animals, and it is regarded as the cause of several pathologies that affect poultry growth (Fuhrmann and Sallmann, 1995, Avanzo et al., 2001). Studies carried out in chicks demonstrated that exposure to multiple stressors resulted in decreased BW (Mcfarlane et al., 1989a, Mcfarlane et al., 1989b). Dietary antioxidants have been reported to decease oxidative stress and encounter against the inhibitory effect of oxidative stress on growth of chickens. Ethoxyquin (ETOX) is a synthetic antioxidant nonphenolic structure and served in animal diets such as in the preservation of aviary foods (Bailey et al., 1996). Wang et al., (1997) reported that addition of ETOX to the diet produced a slight increase (6–7%) in the body weight after 3 wk of treatment, however not after 7 wk (Wang et al., 1997). Waldroup et al., (1960) found that dietary supplementation with ETOX produced remarkably higher weight gain after 4 wk in 1 experiment, but they discovered only a slight increase in weight gain after 8 wk (Waldroup et al., 1960). Shakeri et al., (2019) observed that dietary betaine increased final weight and breast weight in heat stress chickens (Shakeri et al., 2019). The present study showed that injection of Cy suppressed body weight gain in chickens, but oral administration of TEG reduced the inhibitory effect on the weight gain (Tables 2 and 3).
The thymus, bursa of Fabricius, and spleen are the immune organs in poultry, and normal development of them is important for the animal's healthy. Cyclophosphamide treatment has been reported to cause a significant decrease in the lymphoid organs or disappearance in lymphoid organs such as the bursa, spleen, and thymus (He et al., 2007, Fu et al., 2012, Fan et al., 2013, Kim et al., 2014). In the present study, similar results were found that thymus, bursa, and spleen indices were significantly decreased by injection of Cy. However, oral administration of TEG at least numerically decreased the inhibitory effect of Cy on the organs (Figure 2).
The poultry antioxidant system comprises enzymatic and nonenzymatic defenses (Surai, 1999, Surai, 2000). Enzymatic defenses include SOD, GSH-PX, and CAT. And the latter are represented by macromolecules, such as albumin, ceruloplasmin, and ferritin as well as an array of small molecules, such as vitamin C, GSH, uric acid, and minerals (Fang et al., 2002, Valko et al., 2007). The present data revealed that Cy administration produced a marked oxidative impact, as evidenced by the significant increase in MDA (Figure 4D) and carbonyl (Figure 4B) levels as well as decrease in enzymatic (Figure 3) and nonenzymatic defenses (Figure 4) levels.
Lipid and protein are the targets of ROS and can be induced by free radicals into the process of LPO and protein peroxidation, respectively. Lipid peroxidation is generated by conversion of polyunsaturated fatty acid into lipid peroxides or MDA. As a major product of LPO, MDA is supposed to be a marker of tissue damage (Zhang, 2005, Eroglu et al., 2013). Protein carbonyl is one of the final products of protein peroxidation, which is formed by oxidation via either an increase of ROS or the attack of reactive aldehydes such as MDA formed during lipid peroxidation (Haribabu et al., 2013), namely in a way that LPO aggravates protein peroxidation. Accordingly, the protein carbonyl level is considered a surrogate marker of protein peroxidation (Negi et al., 2014). Our study clearly showed a significant increase in serum MDA and protein carbonyl content post Cy injection, but TEG counteracted the increase of carbonyl, LPO, and MDA (Figure 4), indicating that TEG may effectively protect the tissue from damage by eliminating or diminishing the production of carbonyl, LPO, or MDA.
Total antioxidant capacity includes a series of antioxidant enzymes and the related biomolecules complicated in scavenging free radicals (Wei et al., 2012). It is a sensitive and reliable marker to detect changes of in vivo oxidative stress, which may not be detectable through the measure of single specific antioxidant. Because the antioxidants do not work alone, the cooperation among the different antioxidants may provide greater protection against ROS attack. Thus, the overall antioxidant capacity (T-AOC) may give more biologically relevant information than that obtained from individual antioxidants (Ghiselli et al., 2000). The present study elucidated that Cy injection significantly depressed the activities of T-AOC, and oral administration of TEG before or after TEG administration neutralized the inhibitory effect of Cy on T-AOC as indicated in Figure 3A and E. Antioxidant enzymes are considered to be a primary defense against cell oxidant damage. The antioxidant enzymes in the tissues can effectively scavenge the free radicals that are generated during the oxidative stress (Cui et al., 2010). Superoxide dismutase is an intracellular antioxidant that works on the superoxide anion. It can catalyze the conversion of superoxide radicals to hydrogen peroxide and maintain a low superoxide concentration (Eroglu et al., 2013). Catalase is the most important cellular antioxidant. It can degrade O2 and decompose H2O2 and result in a decrease in oxidative stress, which is the effective way of protection from ROS attack (Ince et al., 2014). Glutathione peroxidase is an enzyme involved in scavenging the ROS and protecting the body from LPO by reducing both lipidic and nonlipidic hydroperoxides as H2O2 (Avanzo et al., 2001). In the present study, the activities of SOD, CAT, and GSH-PX were significantly suppressed by injection of Cy. But oral administration of TEG partially recovered the activities of the 3 enzymes (Figure 3).
Cellular nonenzymatic antioxiadant defense involves GSH, vitamin C, and VE. Glutathione was analyzed in this study as it plays a vital role in protection of cells against oxidative injury. The depletion of GSH by Cy could be because of the direct conjugation metabolites of Cy with GSH. Therefore, reduction of GSH may lead to oxidative stress (Zarei and Shivanandappa, 2013, Wang et al., 2014). Our findings showed that Cy injection significantly decreased the levels of GSH (Figure 4A and E). Oral administration of TEG suppressed the inhibitory effect of Cy on GSH.
Cells possess many unsaturated fatty acids in their plasma membranes and are particularly vulnerable to ROS (Jang et al., 2006). In animals, redox signaling pathways use ROS to transfer signals from different sources to the nucleus to regulate a number of various functions including growth, differentiation, proliferation, and apoptosis. Figure 5 showed that TEG administration decreased the intracellular ROS level induced by Cy injection. Indicating that TEG could be used as an antioxidant to reduce oxidative stress.
After observing the antioxidative effect of TEG on the oxidative stress induced by Cy in a bird model, we conclude that oral administration of TEG in drinking water had antioxidative property, as evidenced by increasing body weight gain, the organ indices, T-AOC, and the levels of GSH, elevating the activity of SOD, CAT, and GSH-PX, as well as decreasing the protein carbonyl content, LPO, and MDA. In addition, TEG administration reduced intracellular oxygen species (ROS). Therefore, TEG could be a promising agent against oxidative stress in the poultry industry.
Acknowledgments
This study was supported by the National Key R&D Program of China (project no.: 2017YFD0502200). The authors wish to thank the students in the Laboratory of Traditional Chinese Veterinary Medicine (TCVM Lab) for their assistance in sample collection.
Conflict of Interest: The authors did not provide any conflict of interest statement.
References
- Arzoo K., Sadeghi S., Liebman H.A. Treatment of refractory antibody mediated autoimmune disorders with an anti-CD20 monoclonal antibody (rituximab) Ann. Rheum. Dis. 2002;61(10):922–924. doi: 10.1136/ard.61.10.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avanzo J.L., de Mendonca C.X., Pugine S., Cesar M.D. Effect of vitamin E and selenium on resistance to oxidative stress in chicken superficial pectoralis muscle. Comp. Biochem. Phys. C. 2001;129(2):163–173. doi: 10.1016/s1532-0456(01)00197-1. [DOI] [PubMed] [Google Scholar]
- Bailey D.W., Gross J.E., Laca E.A., Rittenhouse L.R., Coughenour M.B., Swift D.M., Sims P.L. Mechanisms that result in large herbivore grazing distribution patterns. J. Ran. Mana. 1996;49(5):386–400. [Google Scholar]
- Bi S., Chi X., Zhang Y., Ma X., Liang S., Wang Y., Hu S.H. Ginsenoside Rg1 enhanced immune responses to infectious bursal disease vaccine in chickens with oxidative stress induced by cyclophosphamide. Poult. Sci. 2018;97(8):2698–2707. doi: 10.3382/ps/pey132. [DOI] [PubMed] [Google Scholar]
- Biswas M.A., Miyazaki Y., Nomura K., Wakita M. Influences of long-term feeding of Japanese green tea powder on laying performance and egg quality in hens. Asian Austral. J. Anim. 2000;13(7):980–985. [Google Scholar]
- Bizeray D., Leterrier C., Constantin P., Picard M., Faure J.M. Sequential feeding can increase activity and improve gait score in meat-type chickens. Poult. Sci. 2002;81(12):1798–1806. doi: 10.1093/ps/81.12.1798. [DOI] [PubMed] [Google Scholar]
- Chan E.W.C., Lim Y.Y., Chew Y.L. Antioxidant activity of Camellia sinensis leaves and tea from a lowland plantation in Malaysia. Food Chem. 2007;102(4):1214–1222. [Google Scholar]
- Cheng K., Song Z.H., Zheng X.C., Zhang H., Zhang J.F., Zhang L.L., Zhou Y.M., Wang T. Effects of dietary vitamin E type on the growth performance and antioxidant capacity in cyclophosphamide immunosuppressed broilers. Poult. Sci. 2017;96(5):1159–1166. doi: 10.3382/ps/pew336. [DOI] [PubMed] [Google Scholar]
- Cieslar-Pobuda A., Yue J., Lee H.C., Skonieczna M., Wei Y.H. ROS and oxidative stress in stem cells. Oxid. Med. Cell. Longev. 2017;2017:5047168. doi: 10.1155/2017/5047168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y., Du Y., Lu M., Qiang C. Antioxidant responses of Chilo suppressalis (Lepidoptera: Pyralidae) larvae exposed to thermal stress. J. Therm. Biol. 2010;36(5):292–297. [Google Scholar]
- Davies K.J. Oxidative stress: the paradox of aerobic life. Biochem. Soc. Symp. 1995;61:1–31. doi: 10.1042/bss0610001. [DOI] [PubMed] [Google Scholar]
- Ding Q., Zheng W., Zhang B., Chen X., Zhang J., Pang X., Zhang Y., Jia D., Pei S., Dong Y., Ma B. Comparison of hypoglycemic effects of ripened pu-erh tea and raw pu-erh tea in streptozotocin-induced diabetic rats. Rsc. Adv. 2019;9(6):2967–2977. doi: 10.1039/c8ra09259a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duthie G.G., Duthie S.J., Kyle J. Plant polyphenols in cancer and heart disease: implications as nutritional antioxidants. Nutr. Res. Rev. 2000;13(1):79–106. doi: 10.1079/095442200108729016. [DOI] [PubMed] [Google Scholar]
- El-Abasy M., Motobu M., Nakamura K., Koge K., Onodera T., Vainio O., Toivanen P., Hirota Y. Preventive and therapeutic effects of sugar cane extract on cyclophosphamide-induced immunosuppression in chickens. Int. Immunopharmacol. 2004;4(8):983–990. doi: 10.1016/j.intimp.2004.01.019. [DOI] [PubMed] [Google Scholar]
- Eroglu S., Pandir D., Uzun F.G., Bas H. Protective role of vitamins C and E in diclorvos-induced oxidative stress in human erythrocytes in vitro. Biol. Res. 2013;46(1):33–38. doi: 10.4067/S0716-97602013000100005. [DOI] [PubMed] [Google Scholar]
- Fan Y., Lu Y., Wang D., Liu J., Song X., Zhang W., Zhao X., Luong N., Hu Y. Effect of epimedium polysaccharide-propolis flavone immunopotentiator on immunosuppression induced by cyclophosphamide in chickens. Cell. Immunol. 2013;281(1):37–43. doi: 10.1016/j.cellimm.2013.01.008. [DOI] [PubMed] [Google Scholar]
- Fang Y.Z., Yang S., Wu G.Y. Free radicals, antioxidants, and nutrition. Nutrition. 2002;18:872–879. doi: 10.1016/s0899-9007(02)00916-4. [DOI] [PubMed] [Google Scholar]
- Fu W., Delasalle K., Wang J., Song S., Hou J., Alexanian R., Wang M. Bortezomib-cyclophosphamide-dexamethasone for Relapsing multiple Myeloma. Am. J. Clin. Oncol-canc. 2012;35(6):562–565. doi: 10.1097/COC.0b013e31822043f6. [DOI] [PubMed] [Google Scholar]
- Fuhrmann H., Sallmann H.P. The influence of dietary fatty-acids and vitamn-E on plasma prostanoids and liver microsomal alkane production in broiler-chickens with regard to nutritional encephalomalacia. J. Nutr. Sci. Vitaminol. 1995;41(5):553–561. doi: 10.3177/jnsv.41.553. [DOI] [PubMed] [Google Scholar]
- Furukawa K., Kikusato M., Kamizono T., Toyomizu M. Time-course changes in muscle protein degradation in heat-stressed chickens: Possible involvement of corticosterone and mitochondrial reactive oxygen species generation in induction of the ubiquitin-proteasome system. Gen. Comp. Endocrinol. 2016;228:105–110. doi: 10.1016/j.ygcen.2016.02.007. [DOI] [PubMed] [Google Scholar]
- Ghiselli A., Serafini M., Natella F., Scaccini C. Total antioxidant capacity as a tool to assess redox status: Critical view and experimental data. Free Radic. Bio Med. 2000;29(11):1106–1114. doi: 10.1016/s0891-5849(00)00394-4. [DOI] [PubMed] [Google Scholar]
- Haribabu A., Reddy V.S., Pallavi C., Bitla A.R., Sachan A., Pullaiah P., Suresh V., Rao P.V.L.N., Suchitra M.M. Evaluation of protein oxidation and its association with lipid peroxidation and thyrotropin levels in overt and subclinical hypothyroidism. Endocrine. 2013;44(1):152–157. doi: 10.1007/s12020-012-9849-y. [DOI] [PubMed] [Google Scholar]
- He X., Yang X., Guo Y. Effects of different dietary oil sources on immune function in cyclophosphamide immunosuppressed chickens. Anim. Feed. Sci. Tech. 2007;139(3-4):186–200. [Google Scholar]
- Ince S., Avdatek F., Demirel H.H., Arslan-Acaroz D., Goksel E., Kucukkurt I. Ameliorative effect of polydatin on oxidative stress-mediated testicular damage by chronic arsenic exposure in rats. Andrologia. 2014;48(5):518–524. doi: 10.1111/and.12472. [DOI] [PubMed] [Google Scholar]
- Jang H.Y., Kong H.S., Park C.K., Oh J.D., Lee S.G., Cheong H.T., Kim J.T., Lee S.J., Yang B.K., Lee H.K. Effects of taurine on sperm characteristics during in vitro storage of boar semen. Asian Austral. J. Anim. 2006;19(11):1561–1565. [Google Scholar]
- Jiang G.Z., Li J.C. Protective effects of ginsenoside Rg1 against colistin sulfate-induced neurotoxicity in PC12 cells. Cell. Mol. Neurobiol. 2014;34(2):167–172. doi: 10.1007/s10571-013-9998-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Kwon C., Nakano I. Detoxification of oxidative stress in Glioma stem cells: mechanism, Clinical relevance, and therapeutic development. J. Neurosci. Res. 2014;92(11):1419–1424. doi: 10.1002/jnr.23431. [DOI] [PubMed] [Google Scholar]
- Labbe D., Provencal M., Lamy S., Boivin D., Gingras D., Beliveau R. The Flavonols quercetin, kaempferol, and myricetin Inhibit Hepatocyte growth factor-induced Medulloblastoma cell Migration. J. Nutr. 2009;139(4):646–652. doi: 10.3945/jn.108.102616. [DOI] [PubMed] [Google Scholar]
- Levova K., Moserova M., Kotrbova V., Sulc M., Henderson C.J., Wolf C.R., Phillips D.H., Frei E., Schmeiser H.H., Mares J., Arlt V.M., Stiborova M. Role of cytochromes P450 1A1/2 in Detoxication and activation of Carcinogenic Aristolochic acid I: studies with the Hepatic NADPH:cytochrome P450 Reductase Null (HRN) mouse model. Toxicol. Sci. 2011;121(1):43–56. doi: 10.1093/toxsci/kfr050. [DOI] [PubMed] [Google Scholar]
- Li L., Xu L.J., Ma G.Z., Dong Y.M., Peng Y., Xiao P.G. The large-leaved Kudingcha (Ilex latifolia Thunb and Ilex kudingcha CJ Tseng): a traditional Chinese tea with plentiful secondary metabolites and potential biological activities. J. Nat. Med-Tokyo. 2013;67(3):425–437. doi: 10.1007/s11418-013-0758-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lushchak V.I. Environmentally induced oxidative stress in aquatic animals. Aquat. Toxicol. 2011;101(1):13–30. doi: 10.1016/j.aquatox.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Ma X., Dang C., Kang H., Dai Z., Lin S., Guan H., Liu X., Wang X., Hui W. Saikosaponin-D reduces cisplatin-induced nephrotoxicity by repressing ROS-mediated activation of MAPK and NF-kappaB signalling pathways. Int. Immunopharmacol. 2015;28(1):399–408. doi: 10.1016/j.intimp.2015.06.020. [DOI] [PubMed] [Google Scholar]
- Ma Z.N., Liu Z., Wang Z., Ren S., Tang S., Wang Y.P., Xiao S.Y., Chen C., Li W. Supplementation of American ginseng berry extract mitigated cisplatin-evoked nephrotoxicity by suppressing ROS-mediated activation of MAPK and NF-kappaB signaling pathways. Food Chem. Toxicol. 2017;110:62–73. doi: 10.1016/j.fct.2017.10.006. [DOI] [PubMed] [Google Scholar]
- Matsuo Y., Yamada Y., Tanaka T., Kouno I. Enzymatic oxidation of gallocatechin and epigallocatechin: effects of C-ring configuration on the reaction products. Phytochemistry. 2008;69(18SI):3054–3061. doi: 10.1016/j.phytochem.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Mcfarlane J.M., Curtis S.E., Simon J., Izquierdo O.A. Multiple concurrent stressors in chicks .2. Effects on hematologic, body-composition, and pathologic traits. Poult. Sci. 1989;68(4):510–521. doi: 10.3382/ps.0680510. [DOI] [PubMed] [Google Scholar]
- Mcfarlane J.M., Curtis S.E., Shanks R.D., Carmer S.G. Multiple concurrent stressors in chicks .1. Effect on weight-gain, feee-intake, and behavior. Poult. Sci. 1989;68(4):501–509. doi: 10.3382/ps.0680501. [DOI] [PubMed] [Google Scholar]
- Min Y.N., Liu S.G., Qu Z.X., Meng G.H., Gao Y.P. Effects of dietary threonine levels on growth performance, serum biochemical indexes, antioxidant capacities, and gut morphology in broiler chickens. Poult. Sci. 2017;96(5):1290–1297. doi: 10.3382/ps/pew393. [DOI] [PubMed] [Google Scholar]
- Min Y.N., Niu Z.Y., Sun T.T., Wang Z.P., Jiao P.X., Zi B.B., Chen P.P., Tian D.L., Liu F.Z. Vitamin E and vitamin C supplementation improves antioxidant status and immune function in oxidative-stressed breeder roosters by up-regulating expression of GSH-Px gene. Poult. Sci. 2017;97(4):1238–1244. doi: 10.3382/ps/pex417. [DOI] [PubMed] [Google Scholar]
- Negi R., Pande D., Karki K., Kumar A., Khanna R.S., Khanna H.D. Association of oxidative DNA damage, protein oxidation and antioxidant function with oxidative stress induced cellular injury in pre-eclamptic/eclamptic mothers during fetal circulation. Chem-Biol Interact. 2014;208:77–83. doi: 10.1016/j.cbi.2013.11.010. [DOI] [PubMed] [Google Scholar]
- Nishino Y., Ueki K., Suto M., Uchiumi H., Ota F., Tamura S., Kaneko Y., Kuroiwa T., Tsukada Y., Maezawa A., Nojima Y. Successful treatment of patients with rheumatic disorders and acquired factor VIII inhibitors with cyclophosphamide and prednisolone combination therapy: two case reports. J. Int. Med. Res. 2001;29(5):432–436. doi: 10.1177/147323000102900508. [DOI] [PubMed] [Google Scholar]
- Okamoto M., Sugimoto A., Leung K.P., Nakayama K., Kamaguchi A., Maeda N. Inhibitory effect of green tea catechins on cysteine proteinases in Porphyromonas gingivalis. Oral Micro. A. Immun. 2004;19(2):118–120. doi: 10.1046/j.0902-0055.2003.00112.x. [DOI] [PubMed] [Google Scholar]
- Reece R.L., Barr D.A., Grix D.C. Pathogenicity studies with a strain of fowl adenovirus serotype 8 (VRI-33) in chickens. Aust. Vet. J. 1987;64(12):365–367. doi: 10.1111/j.1751-0813.1987.tb09604.x. [DOI] [PubMed] [Google Scholar]
- Samaritani R., Corrado G., Vizza E., Sbiroli C. Cyclophosphamide "metronomic" chemotherapy for palliative treatment of a young patient with advanced epithelial ovarian cancer. Bmc Cancer. 2007;7(65):1–5. doi: 10.1186/1471-2407-7-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieber M., Chandel N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014;24(10):R453–R462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segato G., Benetti C., Angeletti R., Montesissa C., Biancotto G. Doxycycline and sulfadimethoxine transfer from cross-contaminated feed to chicken tissues. Food Addit. Contam. Par.T A. Chem. Anal. Control Expo. Risk Assess. 2011;28(7):860–868. doi: 10.1080/19440049.2011.569574. [DOI] [PubMed] [Google Scholar]
- Shakeri M., Cottrell J.J., Wilkinson S., Le H.H., Suleria H.A., Warner R.D., Dunshea F.R. Growth performance and Characterization of meat quality of broiler chickens supplemented with betaine and antioxidants under cyclic heat stress. Antioxidants. 2019;8(9):336. doi: 10.3390/antiox8090336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudharsan P.T., Mythili Y., Selvakumar E., Varalakshimi P. Lupeol and its ester exhibit protective role against cyclophosphamide-induced cardiac mitochondrial toxicity. J. Cardiovasc. Pharm. 2006;47(2):205–210. doi: 10.1097/01.fjc.0000200658.89629.ba. [DOI] [PubMed] [Google Scholar]
- Suliburska J., Bogdanski P., Szulinska M., Stepien M., Pupek-Musialik D., Jablecka A. Effects of green tea supplementation on Elements, total antioxidants, lipids, and Glucose values in the serum of obese patients. Biol. Trace. Elem. Res. 2012;149(3):315–322. doi: 10.1007/s12011-012-9448-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surai P.F. Vitamin E in avian reproduction. Poult. Avian Biology Reviews. 1999;10(1):1–60. [Google Scholar]
- Surai P.F. Effect of selenium and vitamin E content of the maternal diet on the antioxidant system of the yolk and the developing chick. Br. Poult. Sci. 2000;41(2):235–243. doi: 10.1080/713654909. [DOI] [PubMed] [Google Scholar]
- Valko M., Leibfritz D., Moncol J., Cronin M.T.D., Mazur M., Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell B. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Waidroup P.W., Douglas C.R., Mccall J.T., Harms R.H. The effects of santoquin on the performance of broilers. Poult. Sci. 1960;39(6):1313–1317. [Google Scholar]
- Wang D., Zhang M., Wang T., Cai M., Qian F., Sun Y., Wang Y. Green tea polyphenols prevent lipopolysaccharide-induced inflammatory liver injury in mice by inhibiting NLRP3 inflammasome activation. Food Funct. 2019;10(7):3898–3908. doi: 10.1039/c9fo00572b. [DOI] [PubMed] [Google Scholar]
- Wang L., Zhang L., Niu Y., Sitia R., Wang C.C. Glutathione peroxidase 7 utilizes hydrogen peroxide generated by Ero1α to promote oxidative protein folding. Antioxid. Redox Sign. 2014;20(4):545–556. doi: 10.1089/ars.2013.5236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.Y., Bottje W., Maynard P., Dibner J., Shermer W. Effect of santoquin(R) and oxidized fat on liver and intestinal glutathione in broilers. Poult. Sci. 1997;76(7):961–967. doi: 10.1093/ps/76.7.961. [DOI] [PubMed] [Google Scholar]
- Weber M., Stiller S., Balogh K., Wagner L., Erdelyi M., Mezes M. Effect of feeding T-2 toxin contaminated feed on the utilisation of vitamin E in chickens. Acta Vet. Hung. 2007;55(1):21–27. doi: 10.1556/AVet.55.2007.1.3. [DOI] [PubMed] [Google Scholar]
- Wei X.J., Hu T.J., Chen J.R., Wei Y.Y. Inhibitory effect of carboxymethylpachymaran on cyclophosphamide-induced oxidative stress in mice. Int. J. Biol. Macromol. 2012;49(4):801–805. doi: 10.1016/j.ijbiomac.2011.07.017. [DOI] [PubMed] [Google Scholar]
- Zanchi M.M., Manfredini V., Brum D., Vargas L.M., Spiazzi C.C., Soares M.B., Izaguirry A.P., Santos F.W. Green tea infusion improves cyclophosphamide-induced damage on male mice reproductive system. Toxicol. Rep. 2015;2:252–260. doi: 10.1016/j.toxrep.2014.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarei M., Shivanandappa T. Amelioration of cyclophosphamide-induced hepatotoxicity by the root extract of Decalepis hamiltonii in mice. Food Chem. Toxicol. 2013;57:179–184. doi: 10.1016/j.fct.2013.03.028. [DOI] [PubMed] [Google Scholar]
- Zhang Q.H., Wu C.F., Duan L., Yang J.Y. Protective effects of total saponins from stem and leaf of Panax ginseng against cyclophosphamide-induced genotoxicity and apoptosis in mouse bone marrow cells and peripheral lymphocyte cells. Food Chem. Toxicol. 2009;46(1):293–302. doi: 10.1016/j.fct.2007.08.025. [DOI] [PubMed] [Google Scholar]
- Zhang Y.M. Protective effect of quercetin on aroclor 1254-induced oxidative damage in cultured chicken spermatogonial cells. Toxicol. Sci. 2005;88(2):545–550. doi: 10.1093/toxsci/kfi333. [DOI] [PubMed] [Google Scholar]
- Zhao H., Wang Y., Shao Y., Liu J., Wang S., Xing M. Oxidative stress-induced skeletal muscle injury involves in NF-kappaB/p53-activated immunosuppression and apoptosis response in copper (II) or/and arsenite-exposed chicken. Chemosphere. 2018;210:76–84. doi: 10.1016/j.chemosphere.2018.06.165. [DOI] [PubMed] [Google Scholar]
- Zheng Y., Zong Z.M., Chen S.L., Chen A.H., Wei X.Y. Ameliorative effect of Trametes orientalis polysaccharide against immunosuppression and oxidative stress in cyclophosphamide-treated mice. Int. J. Biol. Macromol. 2017;95:1216–1222. doi: 10.1016/j.ijbiomac.2016.11.013. [DOI] [PubMed] [Google Scholar]
- Zhou F., Jongberg S., Zhao M., Sun W., Skibsted L.H. Antioxidant efficiency and mechanisms of green tea, rosemary or mate extracts in porcine Longissimus dorsi subjected to iron-induced oxidative stress. Food Chem. 2019;298:125030. doi: 10.1016/j.foodchem.2019.125030. [DOI] [PubMed] [Google Scholar]