Abstract
Chronic alcohol consumption is linked to the development of alcohol-associated liver disease (ALD). This disease is characterized by a clinical spectrum ranging from steatosis to hepatocellular carcinoma. Several cell types are involved in ALD progression, including hepatic macrophages. Kupffer cells (KCs) are the resident macrophages of the liver involved in the progression of ALD by activating pathways that lead to the production of cytokines and chemokines. In addition, KCs are involved in the production of reactive oxygen species. Reactive oxygen species are linked to the induction of oxidative stress and inflammation in the liver. These events are activated by the bacterial endotoxin, lipopolysaccharide, that is released from the gastrointestinal tract through the portal vein to the liver. Lipopolysaccharide is recognized by receptors on KCs that are responsible for triggering several pathways that activate proinflammatory cytokines involved in alcohol-induced liver injury. In addition, KCs activate hepatic stellate cells that are involved in liver fibrosis. Novel strategies to treat ALD aim at targeting Kupffer cells. These interventions modulate Kupffer cell activation or macrophage polarization. Evidence from mouse models and early clinical studies in patients with ALD injury supports the notion that pathogenic macrophage subsets can be successfully translated into novel treatment options for patients with this disease.
Alcohol-associated liver disease (ALD) is a major cause of chronic liver injury,1 which has a wide clinical spectrum. This ranges from the accumulation of lipids in the liver (steatosis), steatosis with inflammation (steatohepatitis), fibrosis, cirrhosis, and an increased risk of hepatocellular carcinoma.2 Despite alcohol abuse, only 35% of heavy drinkers develop ALD.1 This suggests there are additional factors influencing ALD development, such as sex, weight, drinking patterns, as well as other genetic and metabolic factors.1 Women tend to drink less alcohol than men. However, they are more susceptible to the hepatotoxic effects of alcohol.3 Binge drinking, defined as a pattern of drinking alcohol that brings blood alcohol concentration to ≥0.08%, or ≥0.08 g of alcohol per deciliter, which corresponds to consuming of five or more alcoholic drinks for males or four or more alcoholic drinks for females on the same occasion within about 2 hours on at least 1 day in the past month by the National Institute on Alcohol Abuse and Alcoholism, and heavy drinking (≥8 drinks a week for women and ≥15 drinks a week for men) are the particularly concerning drinking patterns. They exacerbate liver injury and increase immune system activation, intestinal permeability, and oxidative stress.3 Alcohol-induced liver injury is mediated through several processes, including the generation of harmful metabolites and reactive oxygen species (ROS), increase of intestinal permeability, and an increase of endogenous mediators.4
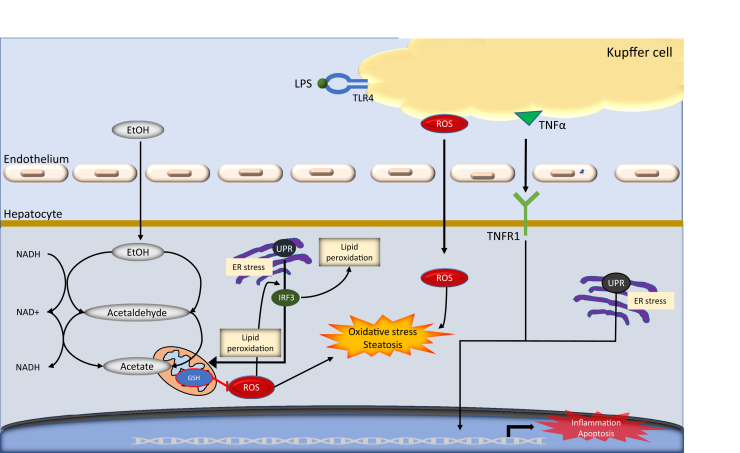
There are multiple ethanol catabolic routes leading to toxic effect in ALD: i) the oxidation of ethanol to acetate, ii) the microsomal ethanol-oxidizing system, and iii) peroxisomal catalase. The oxidation of ethanol to acetate is a two-step process performed by the enzymes alcohol dehydrogenase (ADH) and aldehyde dehydrogenase (ALDH) that use NAD+ as a cofactor (Figure 1).5 This process results in the accumulation of NADH lowering the ratio of NAD+/NADH in the mitochondria,5 resulting in a reduction of β-oxidation.5 The change in ratio results is the accumulation of lipids in the liver, resulting in fatty liver. This is the first stage of ALD, known as steatosis. Although this condition seems reversible after abstinence,2 if neglected it can progress to inflammation and liver fibrosis.5
Figure 1.
A schematic of the critical roles of Kupffer cell injury in ethanol metabolism. The oxidation of ethanol to acetate is a two-step process performed by the enzymes alcohol dehydrogenase (ADH) and aldehyde dehydrogenase (ALDH) that use NAD+ as a cofactor. During the two-step process, the ADH and ALDH reactions allow the accumulation of NADH, reducing the NAD+/NADH ratio in the mitochondria, which initiated the oxidative stress and steatosis process. ER, endoplasmic reticulum; EtOH, ethanol; GSH, glutathione; IRF, interferon regulatory factor; LPS, lipopolysaccharide; ROS, reactive oxygen species; TLR, toll-like receptor; TNF-α, tumor necrosis factor-α; TNFR1, tumor necrosis factor receptor 1; UPR, unfolded protein response.
The microsomal ethanol-oxidizing system (Figure 2) is activated after high alcohol consumption, and cytochrome P450 (CYP2E1) converts alcohol to acetaldehyde.5,6 CYP2E1 plays a major role in oxidative stress, and ethanol-induced fatty liver7 and chronic alcohol exposure can lead to CYP2E1 activation in small intestine as well as in Kupffer cells (KCs)8,9 with production of significant amounts of ROS, which is exacerbated by hypoxia, bacterial translocation, and the release of proinflammatory cytokines.10
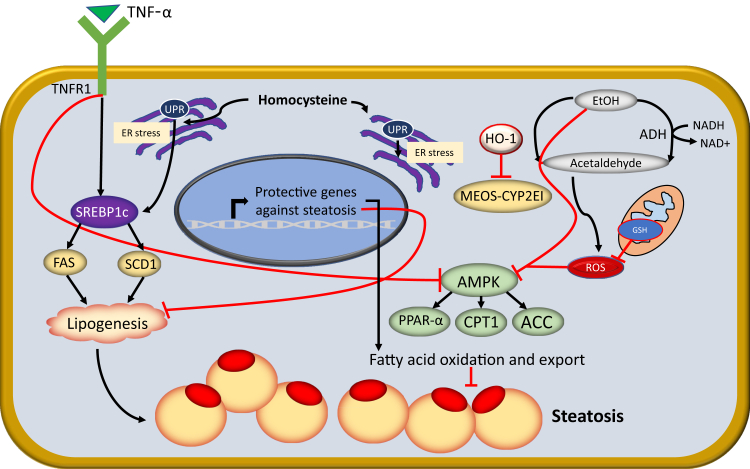
Figure 2.
Ethanol (EtOH) and tumor necrosis factor-α (TNF-α) mediate free acid oxidation and lipogenesis during the development of liver steatosis. Human liver develops a significant innate immune response during alcohol-associated liver injury. Kupffer cells increase in number and produce inflammatory cytokines that inhibit β-oxidation of fatty acids. Elimination of Kupffer cells improves mRNA involved in the β-oxidation of fatty acids. Heavy ethanol use blocks fatty acid oxidation, through inhibition of peroxisome proliferator-activated receptor-α (PPARα) and inhibition of AMP-activated protein kinase (AMPK). With the discovery of SREBP-1 (alias SREBF1), the transcription factor regulating fatty acid, triglyceride, and cholesterol synthesis, it was proved that ethanol can cause fatty liver by acting through this transcription factor, which subsequently integrated into the newly discovered endoplasmic reticulum (ER) stress response and alcoholic steatosis. ACC, acetyl-CoA carboxylase; CPT-1, carnitine palmitoyltransferase-1; CYP2E1, cytochrome P450 2E1; FAS, fatty acid synthase; GSH, glutathione; HO-1, heme oxygenase-1; MEOS, microsomal ethanol-oxidizing system; ROS, reactive oxygen species; SCD, stearoyl-CoA desaturase; TNFR1, tumor necrosis factor receptor 1; UPR, unfolded protein response.
Several liver cell types then play a role in alcohol-induced liver injury, including hepatic stellate cells (HSCs), hepatocytes, hepatic dendritic cells, biliary epithelial cells (ie, cholangiocytes), and sinusoidal endothelial cells. However, Kupffer cells, the hepatic macrophages, play an important role in triggering the inflammatory and fibrotic processes leading to end-stage liver injury.
In this review, we describe the specific contribution of KCs in the progression of injury during ALD.
The Kupffer Cells
KCs, first described at the end of the 18th Century as cells of endothelial origin, were lately more correctly identified as liver resident macrophages.11 KCs make up approximately 80% of the total macrophages of the body.12 Their strategic localization in sinusoidal spaces allows KCs to behave not only as an important immunologic barrier against pathologic components deriving from the gut but also to provide to senescent red cells removal and iron recovery. However, their activities may undergo relevant changes comparing healthy or diseased conditions. In fact, although KCs are immunologically regarded as cells maintaining a tolerogenic status during normal circumstances,13 their response may sometimes enhance liver injury, such as during ethanol abuse.14 This observation is not surprising as KCs mirror the characteristic functional plasticity shared by the components of the macrophage family.15 Macrophages are in fact able to express several functional patterns, which may also change during time if the stimulating trigger is maintained. Finally, important changes occur according to the surrounding microenvironment so that KCs are largely different from alveolar macrophages or microglial cells.15 In the immunologic human liver environment, at least two well distinct populations of resident macrophages were identified by using single-cell RNA sequencing.16 One is supposed to participate in inflammatory response; the other has immune-modulatory properties. In a simplistic view, these two subsets give origin to a different M1 or M2 response. The details of this process, also with regard to ethanol injury, will be reviewed below (M1/M2 Kupffer Cells Unbalance during ALD).
Role of Kupffer Cells in ALD
KC contribution to alcohol-induced liver injury was clearly demonstrated in research using the selective KC blocking agent, gadolinium chloride, in rat models.6 The inactivation of KCs prevented ethanol-induced liver damage, confirming the important KC involvement in tissue injury. KC-induced secretion of proinflammatory cytokines has been largely demonstrated after ethanol exposure.17 Tumor necrosis factor (TNF), for instance, is a major mediator of alcohol-induced damage in the liver,17 it interacts with TNF receptors on hepatocytes,18 it increases free fatty acid released from peripheral adipocytes and de novo lipogenesis, and it inhibits β-oxidation.19 In this respect, the lipid accumulation in hepatocytes is the liver's first response to alcohol abuse. However, several hits are needed for the progression from fatty liver toward chronic inflammation and fibrosis during ethanol abuse. The important role of KCs in this multistep process is discussed in detail in the following paragraphs.
The Working Model Linking Kupffer Cells to the Inflammatory Response during ALD
The liver is chronically exposed to gut-derived bacteria and bacterial components, such as lipopolysaccharide (LPS).20 In this way, a gut-liver axis is established. LPS is normally circulating in blood at a low concentration, peaking around 0.45 EU/mL without significant consequences.21 However, the total LPS content in the gut is 1000 times higher than its lethal dose in blood.22
When excess LPS is presented to toll-like receptors (TLRs) on KCs, this results in the production of proinflammatory cytokines, including TNF-α, interleukins (IL-1β and IL-6), chemokines (IL-8 and CCL2), and ROS.18 TNF-α is the principal mediator of the inflammatory response in mammals, and has a role in the development of acute septic shock as well as a variety of inflammatory diseases, including ALD.23 Activated KCs trigger signaling cascades that include CD14, MyD88, MD-2, mitogen-activated protein kinases [c-Jun N-terminal kinase (JNK)], and NF-κB.18 In addition, Kupffer cells produce nitric oxide (NO) and nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, which further contributes to ALD.
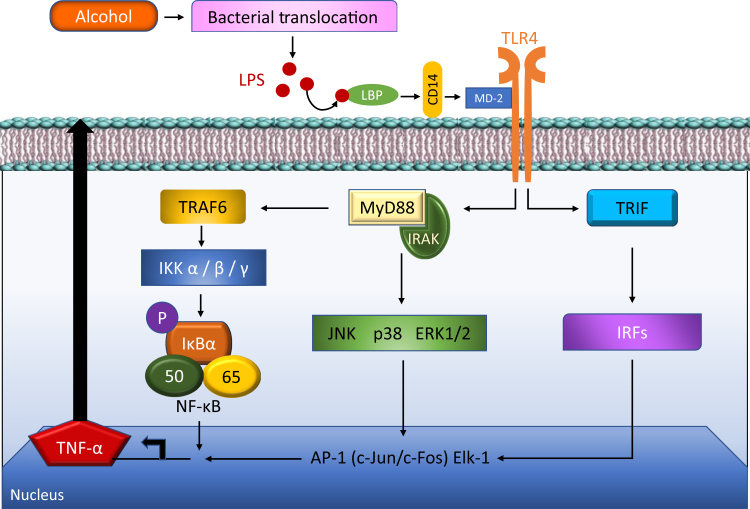
When the canonical cascade of events following the interaction between LPS and KCs is examined in detail, the first step is represented by the interaction of LPS with TLR4, and with its coreceptors, CD14 and MD-2. TLRs are involved in cytotoxicity and effector responses,19 which recognize signature motifs, often referred to as pathogen-associated molecular patterns.24 CD14 facilitates the transfer of LPS to the TLR4/MD2 receptor complex and modulates LPS recognition25 (Figure 3). MD2 is noncovalently associated with TLR4 and binds LPS directly also in the absence of TLRs.25 LPS binding protein (LBP), in this system, facilitates the association between LPS and CD14.25 The TLR4/CD14 receptor complex recognition of LPS then originates the following molecular steps. The LPS/TLR4 transduction pathway may in general progress through a MyD88-dependent or MyD88-independent [TIR domain-containing adapter-inducing interferon-β (TRIF)–mediated] route.26 Because reduction of inflammatory alcoholic damage was not observed in rodents after MyD88 disruption,27 this type of liver injury is thought to mainly progress through the TRIF/MyD88-undependent pathway. The three, TRIF-regulated, downstream inflammatory activators are finally represented by NF-κB, MAPK, and IRF3,28 responsible for the cascade of events characterizing the inflammatory immune response to alcoholic damage.
Figure 3.
Overview of lipopolysaccharide (LPS)/CD14/toll-like receptor 4 (TLR4) signaling pathway in alcohol-associated liver disease. Chronic ethanol exposure leads to bacterial translocation and increased leaking of the gut barrier and translocation of LPS from gut to liver. In liver, lipopolysaccharide binding protein (LBP; the shuttle protein) transfers LPS to CD14, which facilitates the binding of LPS to TLR-4/MD-2/IRAK complex. TLR4 undergoes dimerization and transduces signal by MyD88-dependent and TRIF-dependent pathways. The subsequent downstream signaling included the recruitment of IRAK4, IRAK1, and TRAF-6, which ultimately leads to the production of proinflammatory cytokines by the activation of NF-κB, JUNK, Erk1/2, and p38 MAPK pathways. IKK2, inhibitor of nuclear factor kappa-B kinase 2; IRAK, IL-1R–associated kinase; IRF, interferon regulatory factor; JNK1, c-Jun N-terminal kinase 1; MD-2, myeloid differentiation protein-2; TNF-α, tumor necrosis factor-α; TRAF, TNF receptor associated factor; TRIF, TIR-domain-containing adapter-inducing interferon-β.
However, the increased concentration of LPS in blood (so-called endotoxemia) is a crucial step in eliciting liver inflammation, during ethanol abuse.25 In fact, since the late 1980s, data on rats under alcoholic diet demonstrated that for progression from simple steatosis to liver inflammation, administration of LPS was required.29 Moreover, a linear relationship between plasma endotoxin levels and histologic liver necrosis and inflammation was also demonstrated in rats under ethanol administration.30 These observations recall our attention on the possible failure of the physiological mechanisms limiting or preventing endotoxemia in the course of chronic alcohol exposure. Conditions that have been linked to LPS blood increase in this setting include defective removal of gut-derived products by KCs31 and intestinal bacteria dysbiosis/overgrowth.32 However, the evidence of a so-called leaky gut in humans, affected by ALD, supports the important role of an impaired intestinal barrier at the base of endotoxemia during alcohol abuse.33,34 Increased gut permeability in this setting is likely related to cellular adherens and tight junction damage by acetaldehyde.35 Leaky gut may occur and support injury also in other liver and nonliver diseases; however, possible treatments for this condition have not been identified so far.36
Specific Pathologic Aspects
M1/M2 Kupffer Cells Unbalance during ALD
Macrophages, including KCs, can widely modulate their phenotypic properties according to environmental immunologic signals.37 In this perspective, a categorical classification of these cells denotes important limits as this pool may evolve in a continuum of phenotypes, switching one in the other according to environmental condition and stimuli.38
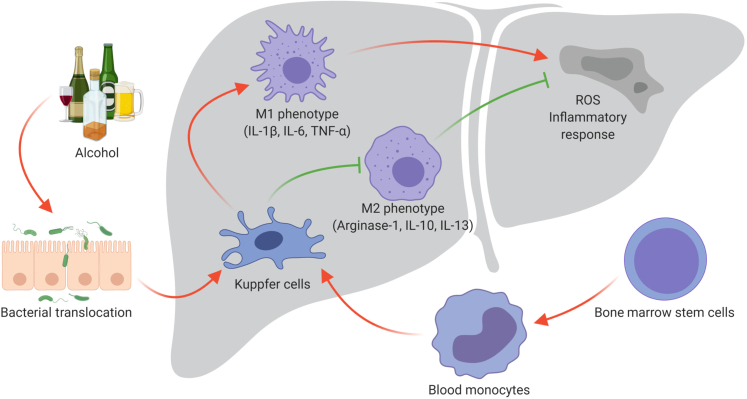
However, to enhance comprehension on the role of macrophages during inflammatory processes, a simplistic M1 or M2 functional classification has been adopted, and the possible switching between these two phenotypes has been described.39 M1 subtype expansion/activation (generally elicited by LPS/TLR interaction) is thought to be the first step in acute inflammatory response (Figure 4), enhancing phagocytic activities, type 1 helper T-cell (Th1) response, and release of proinflammatory cytokines, such as TNF-α, IL-6, and others.40 On the other hand, M2 phenotype seems to be linked to Th2 response, showing modest phagocytic and proinflammatory activity and instead releasing TGF-β and IL-10. The latter are mainly considered as important anti-inflammatory cytokines, currently investigated as possible homeostatic/therapeutic factors for immunologic treatment of autoimmune diseases.41
Figure 4.
M1/M2 Kupffer cells (KCs) unbalance during alcohol-associated liver disease (ALD). Kupffer cells, the resident macrophages in the liver, originate from the precursor cells in the bone marrow, which further develop to blood monocytes. Blood monocytes migrate into liver and produce liver macrophages, namely Kupffer cells. In the liver, during alcoholic liver damage and bacterial translocation, Kupffer cells can polarize in two ways: classic activation/M1 polarization and alternative activation/M2 polarization, which exhibit proinflammatory and anti-inflammatory effects, respectively. The imbalance between M1 and M2 polarization of KCs contributes to the pathogenesis of ALD. ROS, reactive oxygen species; TNF-α, tumor necrosis factor-α.
So, in this simplistic model, linking respectively M1 or M2 activation to Th1 or Th2 response, the M1 subtype would be involved in initiating and promoting the inflammatory process, whereas M2 would contribute to resolution of injury and tissue repair. Finally, in the presence of inflammation, the predominance of an M1 or M2 response would be dependent by the balance between STAT1 and STAT3/6.42,43
Because M1 depletion and/or M2 expansion may promote healing and tissue repair during significant inflammatory processes, modulation of the M1/M2 macrophage phenotype has recently gained more attention. In this perspective, a study conducted on human samples of patients with ALD and an animal model of ethanol-fed mice gave interesting results.43 In a group of heavy alcohol drinkers, the hepatic expression of M2-associated genes [CD-206 (MRC1) and CD163] was reduced in subjects with significant liver damage compared with those with minimal tissue injury. In parallel with this finding, an increased M2/M1 ratio was associated with reduced damage and fatty infiltration of the liver in ethanol-fed C57BL6/J and BALB/c mice. Finally, in BALB/c mice strain (that showed a significant M2-mediated resistance to ethanol injury), a mechanism was identified that was characterized by M2 Kupffer cell induction of apoptosis on the M1 subset. This effect was determined by an enhanced IL-10 expression. In keeping with this study, a previous study observed increased ethanol-induced liver damage and LPS-stimulated inflammatory response in IL-10 (Il10−/−) knockout mice.44 It has been demonstrated that knocking down an inflammation-associated miRNA, miR-21 (MIR21), can inhibit cytokine production and inflammatory responses during ALD injury.45 Taken all together, it becomes evident that strategies aiming to regulate, rather than delete, Kupffer cell response may be beneficial in the course of ALD, as in other human liver afflictions.46 In this perspective, nanoparticle-driven delivery of drugs, immunomodulators, or siRNAs has been proposed and tested.47
Kupffer Cells, miRNAs, and Liver Damage during ALD
miRNAs act as the important regulators to Kupffer cell activation at different stages of acute and chronic liver diseases, including ALD (Table 148, 49, 50, 51, 52, 53, 54, 55, 56, 57). miRNAs are endogenous, small, noncoding, highly conserved, single-stranded RNAs that modulate mRNA levels through decreased transcription or by post-transcriptionally induced mRNA decay.58 Some of the miRNAs that play a role in ALD include miR-212, miR-155, mir-146a (MIR146A), and miR-217 (MIR217). During alcohol ingestion, miR-212 expression is increased within the gut epithelial cells. miR-212 suppresses zonula occludens-1 (ZO-1), which is a major component of tight junctions, causing disruption of gut integrity and permeability, resulting in transport of LPS to the liver.59 miR-155 regulates inflammatory cytokine production via TLR4 signaling, and increases KC sensitivity to alcohol and LPS. Knocking out miR-155 protects against alcohol-induced inflammation and lipid accumulation. The up-regulation of miR-155 stabilizes the production of TNF-α in Kupffer cells.48,57 miR-146a, a negative regulator of TRL signaling, is anti-inflammatory and is up-regulated in ALD.60 miR-217 expression is increased as a result of alcohol consumption, which down-regulates sirtuin-1–Lipin-1, leading to increased hepatic inflammation.61
Table 1.
Relationship between miRNA and Cytokines Related to Kupffer Cells
| miRNAs | Cytokines | Function | References |
|---|---|---|---|
| miR-155, miR-125b, miR-146a | TNF-α∗ | Positive regulation on the release of TNF-α and mRNA stabilization; miR-125b acts as a post-transcriptional repressor of TNF-α; miR-146a acts as a negative regulator | 48, 49, 50 |
| miR-146a | IL-6∗ | Suppresses IL-6 production, targeting IRAK1, IRAK2, and TRAF6 during LPS tolerance | 51 |
| miR-16, miR-142-3p, miR-223, miR-365 | IL-6∗ | Reduction of endotoxin-induced mortality by restricting TLR signaling through a feedback mechanism. | 52, 53, 54 |
| miR-146a | IL-1β∗ | Suppresses IL-1β production, targeting IRAK1, IRAK2, and TRAF6 during LPS tolerance | 55 |
| miR-223 | IL-1β | Involved in inflammatory response of Kupffer cells by regulating the production of IL-1β during acute liver failure† | 56 |
| miR-155 | IL-10 | IL-10 acts, inhibiting Ets2 mRNA and protein, both basally and in response to LPS stimulation | 57 |
IRAK, IL-1R–associated kinase; LPS, lipopolysaccharide; TLR, toll-like receptor; TNF-α, tumor necrosis factor-α; TRAF, TNF receptor associated factor.
TLR is the principal target.
Need further research. Different studies not related with alcoholic liver injury.
Kupffer Cell Contribution to Liver Fibrosis during ALD
Activated KCs produce transforming growth factor-β (TGF-β) and platelet-derived growth factor (PDGF), which are profibrogenic factors.36 These factors, along with the production of ROS, inflammatory cytokines, and lipid peroxidation, activate HSCs to transdifferentiate into myofibroblasts and contribute to liver fibrosis (Figure 3).37 The HSCs reside in the space of Disse and store vitamin A–containing lipid droplets in a healthy liver.62 When stimulated, these cells lose their vitamin A lipid droplets and play a role in liver fibrosis, which is characterized by the accumulation of extracellular matrix proteins, such as collagen.63 When TGF-β is secreted by KCs, it produces cytokines and chemokines that contribute to liver fibrogenesis.57 However, quiescent HSCs are TGF-β activation resistant because of their expression of high levels of Bambi (bone morphogenetic protein and activin membrane-bound inhibitor) that inhibits TGF-β receptor signaling.57 But Bambi is down-regulated when HSCs are activated by TLR4 recognition of LPS. This allows TGF-β signaling in HSCs,57 where they stimulate the extracellular matrix by promoting the expression of extracellular matrix proteins, such as collagen type I. The expression of collagen type I in HSCs is regulated post-transcriptionally by multiple stimuli and pathways, including TGF-β, which stimulates other matrix components, such as cellular fibronectin and proteoglycans.57 These factors, including the production of ROS, inflammatory cytokines, and lipid peroxidation, activate the hepatic stellate cells to transdifferentiate into myofibroblasts and contribute to liver fibrosis (Figure 3). The end-stage manifestation of hepatic fibrosis is cirrhosis. This is characterized histologically by the formation of regeneration parenchymal nodules, separated by fibrotic septa and associated with major distortion of liver architecture.64 Repeated inflammation occurs along with fibrogenesis and predisposes the liver to dysplasia and subsequently malignant transformation.65 Cirrhosis is considered a risk factor for hepatocellular carcinoma. Other risk factors include hepatic oxidative stress and elevated TGF-β and PDGF.66 Hepatocellular carcinoma can be considered an important severe and late-stage evolution, after prolonged alcohol abuse.
Similarities between Nonalcoholic and Alcoholic Steatohepatitis Associated with Kupffer Cells and Insulin Resistance
Several inflammatory cytokines, including TNF-α, have been linked to both insulin resistance and progression of steatosis in non-ALD.67,68 As described previously, TNF-α correlates with inflammation and steatosis in ALD, and this inflammation has been related to non-ALD liver disease as well. Several studies on obese and insulin-resistant patients demonstrated elevated levels of IL-6, IL-18, and TNF-α.68 These elevated levels of TNF-α and IL-6 negatively affect the insulin signaling cascade, resulting in meta inflammation and the development of insulin resistance.69 Insulin resistance is described as an excessive production of insulin, in which the body does not use the insulin effectively, resulting in an increase in blood glucose levels instead of glucose absorption by cells. This condition is related to the development of type 2 diabetes.69 In high-fat diet studies, KCs were depleted to understand their role in modulating insulin sensitivity.70 Lanthier et al67 demonstrated that selective ablation of KCs significantly improved high-fat diet-induced hepatic insulin resistance and alterations of hepatic insulin signaling. This confirms that KCs have an important role in the initiation mechanism of high-fat diet-induced hepatic insulin resistance, besides or irrespectively of inflammatory changes occurring in the adipose tissue.70 KCs release prostaglandin E2 (PGE2), which is involved in the modulation of hepatic glucose output, regulation of cytokine production, and induction of insulin resistance in hepatocytes in collaboration of IL-6.69 In nonalcohol liver disease, PGE2 could act indirectly on hepatocytes by inducing the production of oncostatin M in KCs.71
Finally, the clinical evidence of the association between alcohol abuse, insulin resistance, metabolic syndrome, and type 2 diabetes72, 73, 74 suggests that the mechanisms described in nonalcoholic steatohepatitis and the link between KCs and metabolic impairment may be also present during ALD. However, further comparative studies between nonalcoholic steatohepatitis and ALD on this specific issue would be needed to clarify the differential role of KCs in these diseases.
Conclusion
Alcohol abuse increases the risk of liver injury and developing ALD. Ethanol consumption causes increased gut permeability, resulting in increased LPS presentation to the liver. Kupffer cells express TLR4 receptors that recognize LPS, which induce signaling pathways responsible for the production of the inflammatory response and HSC activation. TNF-α is the principal proinflammatory cytokine involved in inflammation and steatosis in ALD, albeit playing an important role in non-ALD disease as well. miRNAs have been shown to modulate inflammatory mediators in ALD, including TNF-α. Targeting miRNAs could be a new approach to inactivate KCs and inhibit the TNF-α production, and to improve or establish techniques to understand the role of KCs in other metabolic conditions, including insulin resistance. The relationship between Kupffer cells and hepatic stellate cells can point to a new approach to attenuate alcohol liver injury.
Footnotes
Supported by the US Department of Veteran’s Affairs Biomedical Laboratory Research and Development Service VA Merit awards 2I01BX001724 (F.M.), 1I01BX003031 (H.F.), and 5I01BX000574 (G.A.); NIH National Institute of Diabetes and Digestive and Kidney Diseases grants DK108959, DK119421, DK115184, DK054811, DK076898, DK107310, DK110035, and DK062975 (all to F.M., S.G., H.F., and G.A.); NIH National Institute on Alcohol Abuse and Alcoholism grants AA025997 and AA025157 (F.M., S.G., and G.A.); The Hickam Endowed Chair, Division of Gastroenterology and Hepatology, Department of Medicine, Indiana University School of Medicine; the Indiana University Health–Indiana University School of Medicine Strategic Research Initiative; PSC Partners Seeking a Cure (F.M. and G.A.); the Central Texas Veterans Health Care System (Temple, TX); Richard L. Roudebush VA Medical Center (Indianapolis, IN); and Department of Medical Physiology, College of Medicine, Texas A&M University (College Station, TX).
The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
Disclosures: None declared.
E.S. and L.B. contributed equally to this work.
Author Contributions
E.S., L.B., S.L.L., and F.M. performed the search, performed the experiments, and wrote the manuscript; E.L. made the original figures; N.W., B.E., K.S., H.F., L.C., L.X.C., W.X., K.K., V.M., T.Z., D.K., Y.H., L.K., and S.G. contributed to manuscript writing; and G.A. and F.M. supervised the work and wrote the manuscript; all authors read and approved the final manuscript.
References
- 1.Xu J., Liu X., Gao B., Karin M., Tsukamoto H., Brenner D., Kisseleva T. New approaches for studying alcoholic liver disease. Curr Pathobiol Rep. 2014;2:171–183. doi: 10.1007/s40139-014-0053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ohashi K., Pimienta M., Seki E. Alcoholic liver disease: a current molecular and clinical perspective. Liver Res. 2018;2:161–172. doi: 10.1016/j.livres.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ajakaiye M., Jacob A., Wu R., Nicastro J.M., Coppa G.F., Wang P. Alcohol and hepatocyte-Kupffer cell interaction (review) Mol Med Rep. 2011;4:597–602. doi: 10.3892/mmr.2011.471. [DOI] [PubMed] [Google Scholar]
- 4.Nagy L.E., Ding W.X., Cresci G., Saikia P., Shah V.H. Linking pathogenic mechanisms of alcoholic liver disease with clinical phenotypes. Gastroenterology. 2016;150:1756–1768. doi: 10.1053/j.gastro.2016.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang L., Wu D., Wang X., Cederbaum A.I. Cytochrome P4502E1, oxidative stress, JNK, and autophagy in acute alcohol-induced fatty liver. Free Radic Biol Med. 2012;53:1170–1180. doi: 10.1016/j.freeradbiomed.2012.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lautt W.W. Morgan & Claypool Life Sciences; San Rafael, CA: 2009. Hepatic Circulation: Physiology and Pathophysiology. [PubMed] [Google Scholar]
- 7.Louvet A., Mathurin P. Alcoholic liver disease: mechanisms of injury and targeted treatment. Nat Rev Gastroenterol Hepatol. 2015;12:231–242. doi: 10.1038/nrgastro.2015.35. [DOI] [PubMed] [Google Scholar]
- 8.Forsyth C.B., Voigt R.M., Shaikh M., Tang Y., Cederbaum A.I., Turek F.W., Keshavarzian A. Role for intestinal CYP2E1 in alcohol-induced circadian gene-mediated intestinal hyperpermeability. Am J Physiol Gastrointest Liver Physiol. 2013;305:G185–G195. doi: 10.1152/ajpgi.00354.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jarvelainen H.A., Fang C., Ingelman-Sundberg M., Lukkari T.A., Sippel H., Lindros K.O. Kupffer cell inactivation alleviates ethanol-induced steatosis and CYP2E1 induction but not inflammatory responses in rat liver. J Hepatol. 2000;32:900–910. doi: 10.1016/s0168-8278(00)80094-x. [DOI] [PubMed] [Google Scholar]
- 10.Paik Y.-H., Kim J., Aoyama T., De Minicis S., Bataller R., Brenner D.A. Role of NADPH oxidases in liver fibrosis. Antioxid Redox Signaling. 2014;20:2854–2872. doi: 10.1089/ars.2013.5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naito M., Hasegawa G., Takahashi K. Development, differentiation, and maturation of Kupffer cells. Microsc Res Tech. 1997;39:350–364. doi: 10.1002/(SICI)1097-0029(19971115)39:4<350::AID-JEMT5>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 12.Choi W.-M., Kim M.-H., Jeong W.-I. Functions of hepatic non-parenchymal cells in alcoholic liver disease. Liver Res. 2019;3:80–87. [Google Scholar]
- 13.Thomson A.W., Knolle P.A. Antigen-presenting cell function in the tolerogenic liver environment. Nat Rev Immunol. 2010;10:753–766. doi: 10.1038/nri2858. [DOI] [PubMed] [Google Scholar]
- 14.Nagy L.E. Recent insights into the role of the innate immune system in the development of alcoholic liver disease. Exp Biol Med (Maywood) 2003;228:882–890. doi: 10.1177/153537020322800803. [DOI] [PubMed] [Google Scholar]
- 15.Stout R.D., Suttles J. Functional plasticity of macrophages: reversible adaptation to changing microenvironments. J Leukoc Biol. 2004;76:509–513. doi: 10.1189/jlb.0504272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacParland S.A., Liu J.C., Ma X.Z., Innes B.T., Bartczak A.M., Gage B.K., Manuel J., Khuu N., Echeverri J., Linares I., Gupta R., Cheng M.L., Liu L.Y., Camat D., Chung S.W., Seliga R.K., Shao Z., Lee E., Ogawa S., Ogawa M., Wilson M.D., Fish J.E., Selzner M., Ghanekar A., Grant D., Greig P., Sapisochin G., Selzner N., Winegarden N., Adeyi O., Keller G., Bader G.D., McGilvray I.D. Single cell RNA sequencing of human liver reveals distinct intrahepatic macrophage populations. Nat Commun. 2018;9:4383. doi: 10.1038/s41467-018-06318-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Massey V.L., Arteel G.E. Acute alcohol-induced liver injury. Front Physiol. 2012;3:193. doi: 10.3389/fphys.2012.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roy S., Benz F., Luedde T., Roderburg C. The role of miRNAs in the regulation of inflammatory processes during hepatofibrogenesis. Hepatobiliary Surg Nutr. 2015;4:24–33. doi: 10.3978/j.issn.2304-3881.2015.01.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mandrekar P., Szabo G. Signalling pathways in alcohol-induced liver inflammation. J Hepatol. 2009;50:1258–1266. doi: 10.1016/j.jhep.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou Z., Zhong W. Targeting the gut barrier for the treatment of alcoholic liver disease. Liver Res. 2017;1:197–207. doi: 10.1016/j.livres.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jayashree B., Bibin Y.S., Prabhu D., Shanthirani C.S., Gokulakrishnan K., Lakshmi B.S., Mohan V., Balasubramanyam M. Increased circulatory levels of lipopolysaccharide (LPS) and zonulin signify novel biomarkers of proinflammation in patients with type 2 diabetes. Mol Cell Biochem. 2014;388:203–210. doi: 10.1007/s11010-013-1911-4. [DOI] [PubMed] [Google Scholar]
- 22.Wassenaar T.M., Zimmermann K. Lipopolysaccharides in food, food supplements, and probiotics: should we be worried? Eur J Microbiol Immunol (Bp) 2018;8:63–69. doi: 10.1556/1886.2018.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ajakaiye M.A., Jacob A., Wu R., Zhou M., Ji Y., Dong W., Wang Z., Qiang X., Chaung W.W., Nicastro J., Coppa G.F., Wang P. Upregulation of Kupffer cell alpha2A-adrenoceptors and downregulation of MKP-1 mediate hepatic injury in chronic alcohol exposure. Biochem Biophys Res Commun. 2011;409:406–411. doi: 10.1016/j.bbrc.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soares J.B., Pimentel-Nunes P., Roncon-Albuquerque R., Leite-Moreira A. The role of lipopolysaccharide/toll-like receptor 4 signaling in chronic liver diseases. Hepatol Int. 2010;4:659–672. doi: 10.1007/s12072-010-9219-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seki E., Schnabl B. Role of innate immunity and the microbiota in liver fibrosis: crosstalk between the liver and gut. J Physiol. 2012;590:447–458. doi: 10.1113/jphysiol.2011.219691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawai T., Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 27.Hritz I., Mandrekar P., Velayudham A., Catalano D., Dolganiuc A., Kodys K., Kurt-Jones E., Szabo G. The critical role of toll-like receptor (TLR) 4 in alcoholic liver disease is independent of the common TLR adapter MyD88. Hepatology. 2008;48:1224–1231. doi: 10.1002/hep.22470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang H.J., Gao B., Zakhari S., Nagy L.E. Inflammation in alcoholic liver disease. Annu Rev Nutr. 2012;32:343–368. doi: 10.1146/annurev-nutr-072610-145138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhagwandeen B.S., Apte M., Manwarring L., Dickeson J. Endotoxin induced hepatic necrosis in rats on an alcohol diet. J Pathol. 1987;152:47–53. doi: 10.1002/path.1711520107. [DOI] [PubMed] [Google Scholar]
- 30.Nanji A.A., Khettry U., Sadrzadeh S.M., Yamanaka T. Severity of liver injury in experimental alcoholic liver disease. Correlation with plasma endotoxin, prostaglandin E2, leukotriene B4, and thromboxane B2. Am J Pathol. 1993;142:367–373. [PMC free article] [PubMed] [Google Scholar]
- 31.Fukui H., Kitano H., Okamoto Y., Kikuchi E., Matsumoto M., Kikukawa M., Morimura M., Tsujita S., Nagamoto I., Nakatani T., Tsujii T. Interaction of Kupffer cells to splenic macrophages and hepatocytes in endotoxin clearance: effect of alcohol. J Gastroenterol Hepatol. 1995;10(Suppl 1):S31–S34. doi: 10.1111/j.1440-1746.1995.tb01793.x. [DOI] [PubMed] [Google Scholar]
- 32.Hartmann P., Chu H., Duan Y., Schnabl B. Gut microbiota in liver disease: too much is harmful, nothing at all is not helpful either. Am J Physiol Gastrointest Liver Physiol. 2019;316:G563–G573. doi: 10.1152/ajpgi.00370.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rao R.K., Seth A., Sheth P. Recent advances in alcoholic liver disease I: role of intestinal permeability and endotoxemia in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2004;286:G881–G884. doi: 10.1152/ajpgi.00006.2004. [DOI] [PubMed] [Google Scholar]
- 34.Keshavarzian A., Holmes E.W., Patel M., Iber F., Fields J.Z., Pethkar S. Leaky gut in alcoholic cirrhosis: a possible mechanism for alcohol-induced liver damage. Am J Gastroenterol. 1999;94:200–207. doi: 10.1111/j.1572-0241.1999.00797.x. [DOI] [PubMed] [Google Scholar]
- 35.Rao R. Endotoxemia and gut barrier dysfunction in alcoholic liver disease. Hepatology. 2009;50:638–644. doi: 10.1002/hep.23009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Odenwald M.A., Turner J.R. Intestinal permeability defects: is it time to treat? Clin Gastroenterol Hepatol. 2013;11:1075–1083. doi: 10.1016/j.cgh.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martinez F.O., Helming L., Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 38.Xu W., Zhao X., Daha M.R., van Kooten C. Reversible differentiation of pro- and anti-inflammatory macrophages. Mol Immunol. 2013;53:179–186. doi: 10.1016/j.molimm.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 39.Chistiakov D.A., Myasoedova V.A., Revin V.V., Orekhov A.N., Bobryshev Y.V. The impact of interferon-regulatory factors to macrophage differentiation and polarization into M1 and M2. Immunobiology. 2018;223:101–111. doi: 10.1016/j.imbio.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 40.Atri C., Guerfali F.Z., Laouini D. Role of human macrophage polarization in inflammation during infectious diseases. Int J Mol Sci. 2018;19:1801. doi: 10.3390/ijms19061801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Komai T., Inoue M., Okamura T., Morita K., Iwasaki Y., Sumitomo S., Shoda H., Yamamoto K., Fujio K. Transforming growth factor-beta and interleukin-10 synergistically regulate humoral immunity via modulating metabolic signals. Front Immunol. 2018;9:1364. doi: 10.3389/fimmu.2018.01364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sica A., Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wan J., Benkdane M., Teixeira-Clerc F., Bonnafous S., Louvet A., Lafdil F., Pecker F., Tran A., Gual P., Mallat A., Lotersztajn S., Pavoine C. M2 Kupffer cells promote M1 Kupffer cell apoptosis: a protective mechanism against alcoholic and nonalcoholic fatty liver disease. Hepatology. 2014;59:130–142. doi: 10.1002/hep.26607. [DOI] [PubMed] [Google Scholar]
- 44.Hill D.B., D'Souza N.B., Lee E.Y., Burikhanov R., Deaciuc I.V., de Villiers W.J. A role for interleukin-10 in alcohol-induced liver sensitization to bacterial lipopolysaccharide. Alcohol Clin Exp Res. 2002;26:74–82. [PubMed] [Google Scholar]
- 45.Wu N., McDaniel K., Zhou T., Ramos-Lorenzo S., Wu C., Huang L., Chen D., Annable T., Francis H., Glaser S., Alpini G., Meng F. Knockout of microRNA-21 attenuates alcoholic hepatitis through the VHL/NF-kappaB signaling pathway in hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2018;315:G385–G398. doi: 10.1152/ajpgi.00111.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sica A., Invernizzi P., Mantovani A. Macrophage plasticity and polarization in liver homeostasis and pathology. Hepatology. 2014;59:2034–2042. doi: 10.1002/hep.26754. [DOI] [PubMed] [Google Scholar]
- 47.van der Heide D., Weiskirchen R., Bansal R. Therapeutic targeting of hepatic macrophages for the treatment of liver diseases. Front Immunol. 2019;10:2852. doi: 10.3389/fimmu.2019.02852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bala S., Csak T., Kodys K., Catalano D., Ambade A., Furi I., Lowe P., Cho Y., Iracheta-Vellve A., Szabo G. Alcohol-induced miR-155 and HDAC11 inhibit negative regulators of the TLR4 pathway and lead to increased LPS responsiveness of Kupffer cells in alcoholic liver disease. J Leukoc Biol. 2017;102:487–498. doi: 10.1189/jlb.3A0716-310R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murphy A.J., Guyre P.M., Pioli P.A. Estradiol suppresses NF-kappa B activation through coordinated regulation of let-7a and miR-125b in primary human macrophages. J Immunol. 2010;184:5029–5037. doi: 10.4049/jimmunol.0903463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Javidan A., Jiang W., Okuyama M., Thiagarajan D., Yang L., Moorleghen J.J., Muniappan L., Subramanian V. miR-146a deficiency accelerates hepatic inflammation without influencing diet-induced obesity in mice. Sci Rep. 2019;9:12626. doi: 10.1038/s41598-019-49090-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park H., Huang X., Lu C., Cairo M.S., Zhou X. MicroRNA-146a and microRNA-146b regulate human dendritic cell apoptosis and cytokine production by targeting TRAF6 and IRAK1 proteins. J Biol Chem. 2015;290:2831–2841. doi: 10.1074/jbc.M114.591420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Servais F.A., Kirchmeyer M., Hamdorf M., Minoungou N.W.E., Rose-John S., Kreis S., Haan C., Behrmann I. Modulation of the IL-6-signaling pathway in liver cells by miRNAs targeting gp130, JAK1, and/or STAT3. Mol Ther Nucleic Acids. 2019;16:419–433. doi: 10.1016/j.omtn.2019.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Song Q., Li H., Shao H., Li C., Lu X. MicroRNA-365 in macrophages regulates Mycobacterium tuberculosis-induced active pulmonary tuberculosis via interleukin-6. Int J Clin Exp Med. 2015;8:15458–15465. [PMC free article] [PubMed] [Google Scholar]
- 54.Li M., He Y., Zhou Z., Ramirez T., Gao Y., Ross R.A., Cao H., Cai Y., Xu M., Feng D., Zhang P., Liangpunsakul S., Gao B. MicroRNA-223 ameliorates alcoholic liver injury by inhibiting the IL-6-p47(phox)-oxidative stress pathway in neutrophils. Gut. 2017;66:705–715. doi: 10.1136/gutjnl-2016-311861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nahid M.A., Satoh M., Chan E.K. Interleukin 1beta-responsive microRNA-146a is critical for the cytokine-induced tolerance and cross-tolerance to toll-like receptor ligands. J Innate Immun. 2015;7:428–440. doi: 10.1159/000371517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jimenez Calvente C., Del Pilar H., Tameda M., Johnson C.D., Feldstein A.E. MicroRNA 223 3p negatively regulates the NLRP3 inflammasome in acute and chronic liver injury. Mol Ther. 2020;28:653–663. doi: 10.1016/j.ymthe.2019.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Quinn S.R., Mangan N.E., Caffrey B.E., Gantier M.P., Williams B.R., Hertzog P.J., McCoy C.E., O'Neill L.A. The role of Ets2 transcription factor in the induction of microRNA-155 (miR-155) by lipopolysaccharide and its targeting by interleukin-10. J Biol Chem. 2014;289:4316–4325. doi: 10.1074/jbc.M113.522730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bala S., Marcos M., Kodys K., Csak T., Catalano D., Mandrekar P., Szabo G. Up-regulation of microRNA-155 in macrophages contributes to increased tumor necrosis factor α (TNFα) production via increased mRNA half-life in alcoholic liver disease. J Biol Chem. 2011;286:1436–1444. doi: 10.1074/jbc.M110.145870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kumar V., Mansfield J., Fan R., MacLean A., Li J., Mohan M. miR-130a and miR-212 disrupt the intestinal epithelial barrier through modulation of PPARgamma and occludin expression in chronic simian immunodeficiency virus-infected rhesus macaques. J Immunol. 2018;200:2677–2689. doi: 10.4049/jimmunol.1701148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bala S., Petrasek J., Mundkur S., Catalano D., Levin I., Ward J., Alao H., Kodys K., Szabo G. Circulating microRNAs in exosomes indicate hepatocyte injury and inflammation in alcoholic, drug-induced, and inflammatory liver diseases. Hepatology. 2012;56:1946–1957. doi: 10.1002/hep.25873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jager J., Aparicio-Vergara M., Aouadi M. Liver innate immune cells and insulin resistance: the multiple facets of Kupffer cells. J Intern Med. 2016;280:209–220. doi: 10.1111/joim.12483. [DOI] [PubMed] [Google Scholar]
- 62.Liu J. Ethanol and liver: recent insights into the mechanisms of ethanol-induced fatty liver. World J Gastroenterol. 2014;20:14672–14685. doi: 10.3748/wjg.v20.i40.14672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gao B., Seki E., Brenner D.A., Friedman S., Cohen J.I., Nagy L., Szabo G., Zakhari S. Innate immunity in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2011;300:G516–G525. doi: 10.1152/ajpgi.00537.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tsochatzis E.A., Bosch J., Burroughs A.K. Liver cirrhosis. Lancet. 2014;383:1749–1761. doi: 10.1016/S0140-6736(14)60121-5. [DOI] [PubMed] [Google Scholar]
- 65.Capece D., Fischietti M., Verzella D., Gaggiano A., Cicciarelli G., Tessitore A., Zazzeroni F., Alesse E. The inflammatory microenvironment in hepatocellular carcinoma: a pivotal role for tumor-associated macrophages. Biomed Res Int. 2013;2013:1–15. doi: 10.1155/2013/187204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Natarajan S., Pachunka J., Mott J. Role of microRNAs in alcohol-induced multi-organ injury. Biomolecules. 2015;5:3309–3338. doi: 10.3390/biom5043309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lanthier N., Molendi-Coste O., Horsmans Y., van Rooijen N., Cani P.D., Leclercq I.A. Kupffer cell activation is a causal factor for hepatic insulin resistance. Am J Physiol Gastrointest Liver Physiol. 2010;298:G107–G116. doi: 10.1152/ajpgi.00391.2009. [DOI] [PubMed] [Google Scholar]
- 68.Lauterbach M.A.R., Wunderlich F.T. Macrophage function in obesity-induced inflammation and insulin resistance. Pflügers Arch. 2017;469:385–396. doi: 10.1007/s00424-017-1955-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Henkel J., Gärtner D., Dorn C., Hellerbrand C., Schanze N., Elz S.R., Püschel G.P. Oncostatin M produced in Kupffer cells in response to PGE2: possible contributor to hepatic insulin resistance and steatosis. Lab Invest. 2011;91:1107–1117. doi: 10.1038/labinvest.2011.47. [DOI] [PubMed] [Google Scholar]
- 70.He X., Jing Z., Cheng G. MicroRNAs: new regulators of toll-like receptor signalling pathways. Biomed Res Int. 2014;2014:1–14. doi: 10.1155/2014/945169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu Z., Xiao S.-B., Xu P., Xie Q., Cao L., Wang D., Luo R., Zhong Y., Chen H.-C., Fang L.-R. miR-365, a novel negative regulator of interleukin-6 gene expression, is cooperatively regulated by Sp1 and NF-κB. J Biol Chem. 2011;286:21401–21412. doi: 10.1074/jbc.M110.198630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Carlsson S., Hammar N., Grill V., Kaprio J. Alcohol consumption and the incidence of type 2 diabetes: a 20-year follow-up of the Finnish twin cohort study. Diabetes Care. 2003;26:2785–2790. doi: 10.2337/diacare.26.10.2785. [DOI] [PubMed] [Google Scholar]
- 73.Fan A.Z., Russell M., Naimi T., Li Y., Liao Y., Jiles R., Mokdad A.H. Patterns of alcohol consumption and the metabolic syndrome. J Clin Endocrinol Metab. 2008;93:3833–3838. doi: 10.1210/jc.2007-2788. [DOI] [PubMed] [Google Scholar]
- 74.Lee K. Gender-specific relationships between alcohol drinking patterns and metabolic syndrome: the Korea National Health and Nutrition Examination Survey 2008. Public Health Nutr. 2012;15:1917–1924. doi: 10.1017/S136898001100365X. [DOI] [PubMed] [Google Scholar]