Abstract
Using antibiotics as feed additives have been successively banned worldwide from 1986; therefore, it is an urgent task to finding safe and effective alternatives. As natural products of plant origin, essential oils (EOs) are an outstanding option due to their reported bioactivity. In this research, ten EOs of Labiatae species were extracted by steam distillation and its chemical constituents were identified by gas chromatography-mass spectrometry (GC-MS). A total of 123 chemical compounds, including alkenes, phenols, aldehydes and ketones, were identified. The results of antioxidant activity carried out through DPPH free radical scavenging (DPPH) and ferric reducing antioxidant power (FRAP), showing that EOs of Ocimum basilicum Linn. (ObEO), Thymus mongolicus Ronn. (TmEO), Origanum vulgare Linn. (OvEO) and Mosla chinensis Maxim. (McEO) have strong antioxidant activities. Their 50%-inhibitory concentration (IC50) value was <1.00, 1.42, 1.47 and 1.92 μg/mL, respectively; and their FRAP value was 1536.67 ± 24.22, 271.84 ± 4.93, 633.71 ± 13.14 and 480.66 ± 29.90, respectively. The results of filter paper diffusion showing that McEO, OvEO and TmEO inhibition zone diameter (IZD) are all over 30 mm. The results of two-fold dilution method showed that McEO, OvEO and TmEO have strong antibacterial activities against Staphylococcus aureus (S. aureus) and their minimal inhibitory concentrations (MIC) value was 1 μL/mL, 2 μL/mL, and 2 μL/mL, respectively. In conclusion, the results in this work demonstrate the possibility for development and application of EOs as potential feed additives.
Keywords: Labiatae species, essential oil, antioxidant, antibacterial, feed additive
1. Introduction
Antibiotics, the development of which dates back to 1929, have been widely used as feed growth-promoting additives (AGPs) for a long time. Nevertheless, antibiotics were constantly and globally banned as feed additives from 1986, due to its drug resistance and safety hazards [1]. AGPs were prohibited using as commercial feed additives from July 1, 2020, in China; therefore, the product which can replace the AGPs become a pressing need in the breeding industry. Meanwhile, the search for relevant alternatives from plants is a focal research point due to its low toxicity, no drug resistance, no residues and no pollution [2]. Plant extracts with antioxidant activity, antibacterial activity and regulating intestinal health, make it become potential alternatives of AGPs, are getting more and more attention [3,4].
Essential oils (EOs), important secondary metabolites of plants contain several chemical classes of compounds, which are volatile at normal temperature, immiscible with water and obtained by steam distillation from plants [5,6]. EOs presented more biological properties, such as antibacterial activity [7,8,9], antioxidant activity [10,11] and regulating intestinal function [12], and have been widely used in pharmaceutical [9], agriculture [13,14], food [15] and cosmetic industries [16]. EOs have also attracted increasing attention from the market and researchers in the breeding industry. The use of EOs as alternatives to antibiotics has always had great expectation, and the search for low-cost and effective essential oils from forage plants as feed additives is a hot topic of research.
Labiatae species are an important source of EOs, with 10 subfamilies, more than 220 genera, and about 3500 species distributed throughout the world. There are 99 genera and 808 species in China, and many of them have a wide range of pharmacological activities. In particular, studies have shown that EOs of the Labiatae family have significant bioactivity and have the potential to be developed as an alternative to antibiotics [17].
In this research, ten EOs of Labiatae species including Thymus mongolicus Ronn., Mosla chinensis Maxim., Origanum vulgare Linn., Rosmarinus officinalis Linn., Ocimum basilicum Linn., Mentha haplocalyx Briq., Pogostemon cablin (Blanco) Benth., Mentha spicata Linn., Salvia officinalis Linn., Perilla frutescens (Linn.) Britt. were chosen as the research objects to analysis its chemical composition and biological activity. EOs of ten Labiatae species were extracted, with the chemical constituents were analyzed and identified through GC-MS, and their antioxidant and antibacterial activities were evaluated using DPPH and ferric reducing antioxidant power (FRAP).
2. Results and Discussion
2.1. Extraction and Yield
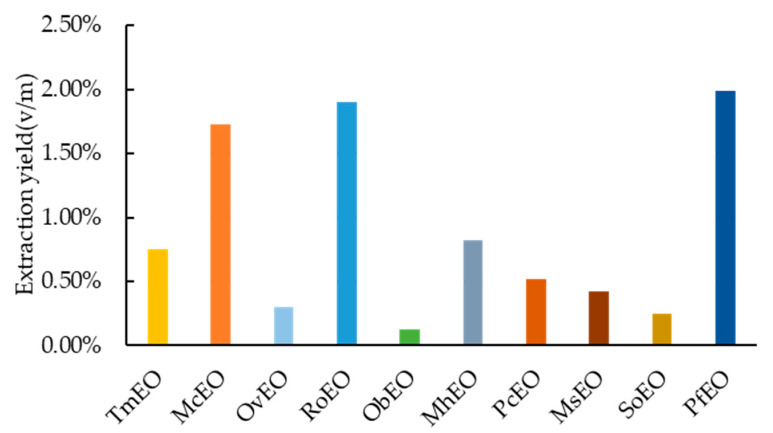
10 EOs of Labiatae species were obtained by hydrodistillation, with a yield of 0.75, 1.73, 0.30, 1.90, 0.13, 0.82, 0.52, 0.42, 0.25 and 1.99% respectively (Figure 1). The highest (1.99%) belonged to Perilla frutescens (Linn.) Britt. (PfEO), while the lowest (0.13%) was recorded for the Ocimum basilicum Linn. (ObEO).
Figure 1.
Extraction yield of ten essential oils (EOs). TmEO: Thymus mongolicus Ronn.; McEO: Mosla chinensis Maxim.; OvEO: Origanum vulgare Linn; RoEO: Rosmarinus officinalis Linn.; ObEO: Ocimum basilicum Linn.; MhEO: Mentha haplocalyx Briq.; PcEO: Pogostemon cablin (Blanco) Benth.; MsEO: Mentha spicata Linn.; SoEO: Salvia officinalis Linn.; PfEO: Perilla frutescens (Linn.) Britt.
2.2. Chemical Composition
The chemical constituents of 10 EOs were identified by GC-MS combined with the database of NIST.17 (Table 1). Regarding the chemical profile of the EOs, a total of 123 components were identified. The results show that significant differences in the chemical composition of 10 EOs. The relative content of each compound was determined using peak area normalization. The major components of each EOs displayed in Table 1, and the total compound content was also identified. The largest amounts of 10 EOs are D-carvone (71.1%) and menthol (69.05%), followed by patchouli alcohol (50.52%), perillaketone (35.56%), tanacetone (27.99%), thymol (23.7%), linalool (26.65%), α-pinene (26.46%).
Table 1.
Percentage composition of the EOs from 10 plants.
| No | Molecular Formula | Compounds | Relative Peak Area (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TmEO | McEO | OvEO | RoEO | ObEO | MhEO | PcEO | MsEO | SoEO | PfEO | |||
| 1 | C10H16 | α-Pinene | 0.46 | 0.13 | - | 26.46 | 0.09 | 0.4 | - | 0.58 | 1.93 | - |
| 2 | C10H16 | (−)-Camphene | - | - | - | 3.53 | - | - | - | - | - | - |
| 3 | C10H16 | α-thujene | 1.14 | 0.26 | - | - | - | - | - | - | - | - |
| 4 | C10H16 | Camphene | 0.49 | 0.1 | - | - | - | - | - | 0.03 | 3.63 | - |
| 5 | C10H16 | β-Pinene | 0.2 | - | 0.4 | 1.09 | 0.22 | 0.31 | - | 0.59 | 3.65 | - |
| 6 | C10H14 | 2,4(10)-Thujadiene | - | - | - | 0.72 | - | - | - | - | - | - |
| 7 | C10H16 | Sabinene | 0.08 | - | - | - | 0.08 | 0.08 | - | 0.25 | 0.19 | - |
| 8 | C10H16 | β-Myrcene | 0.87 | 2.18 | - | 0.83 | - | 0.27 | - | 1.59 | 0.6 | - |
| 9 | C10H16 | α-terpinene | 2.44 | 3.19 | 0.84 | 0.44 | - | - | - | - | 0.11 | - |
| 10 | C15H24 | β-Patchoulene | - | - | - | - | - | - | 1.23 | - | - | - |
| 11 | C15H24 | α-Guaiene | - | - | - | - | - | - | 6.09 | - | - | - |
| 12 | C15H24 | γ-Gurjunene | - | - | - | - | - | - | 0.49 | - | - | - |
| 13 | C10H16 | d-Limonene | 0.3 | 0.26 | 0.06 | 0.85 | 0.07 | 1.53 | - | 9.64 | 0.49 | 1.38 |
| 14 | C10H18O | Eucalyptol | - | - | 0.31 | 11.31 | 4.48 | - | - | 0.64 | 5.11 | - |
| 15 | C10H16 | trans-β-Ocimene | - | - | - | - | - | - | - | 0.48 | 0.03 | - |
| 16 | C10H16 | allo-Ocimene | - | - | - | - | - | - | - | 0.16 | - | - |
| 17 | C10H16 | β-phellandrene | - | 0.25 | - | - | - | - | - | - | - | - |
| 18 | C10H16 | γ-Terpinene | 16.42 | 15.14 | 6.14 | 1.11 | 0.09 | - | - | - | 0.46 | - |
| 19 | C10H16 | β-Ocimene | - | - | - | - | 0.71 | - | - | - | - | - |
| 20 | C10H14 | p-Cymene | 21.17 | 19.52 | 14.11 | 1.76 | - | - | - | - | 0.25 | - |
| 21 | C10H16 | (+)-4-Carene | 0.09 | - | - | - | - | - | - | - | 0.23 | - |
| 22 | C10H16O | Tanacetone | - | - | - | - | - | - | - | - | 27.99 | - |
| 23 | C10H16O | Thujone | - | - | - | - | - | - | - | - | 5.24 | - |
| 24 | C10H16 | Terpinolene | - | 0.18 | - | 0.85 | 0.13 | - | - | - | - | - |
| 25 | C6H12O | 3-Hexen-1-ol | - | - | - | - | 0.33 | - | - | - | - | - |
| 26 | C8H18O | 3-Octanol | 0.27 | - | 2.05 | - | - | 0.6 | - | 0.31 | - | 0.1 |
| 27 | C10H14O | Perillene | - | - | - | - | - | - | - | - | - | 1.62 |
| 28 | C10H18O | l-Menthone | - | - | - | - | - | 12.21 | - | - | - | - |
| 29 | C10H18O | (+)-Isomenthone | - | - | - | - | - | 3.07 | - | - | - | - |
| 30 | C8H16O | 1-Octen-3-ol | 2.97 | - | 1.66 | 0.02 | 0.05 | 0.14 | 0.08 | 0.18 | ||
| 31 | C10H18O | Cis-β-Terpineol | - | - | - | - | 0.19 | - | - | - | - | - |
| 32 | C10H16O | (+)-2-Bornanone | - | - | - | 4.08 | - | - | - | - | - | - |
| 33 | C10H16O | Isopinocamphon | - | - | - | 1 | - | - | - | 0.06 | - | |
| 34 | C10H14O | Pinocarvone | - | - | - | 0.41 | - | - | - | - | - | - |
| 35 | C10H16O | Camphor | 0.1 | - | 0.57 | - | 0.67 | - | - | - | 16.21 | |
| 36 | C15H24 | (−)-β-Bourbonene | - | - | 0.23 | - | 0.14 | 0.16 | - | 1.21 | - | 0.07 |
| 37 | C12H22O2 | l-Menthyl acetate | - | - | - | - | - | 3.73 | - | - | - | - |
| 38 | C10H18O | Linalool | 2.97 | 0.06 | 0.14 | 2.99 | 26.65 | - | - | 0.49 | 0.37 | 2.97 |
| 39 | C10H16O | 2-methyl-5-(1-methylethenyl)-Cyclohexanone | - | - | - | - | - | - | - | 2.76 | - | - |
| 40 | C11H16O | 2-Isopropyl-1-methoxy-4-methylbenzene | - | - | 11.34 | - | - | - | - | - | - | - |
| 41 | C7H8O2 | 5-methyl-2-acetyl-Furan | - | - | 0.23 | - | - | - | - | - | - | - |
| 42 | C10H20O | Menthol | - | - | 0.97 | - | - | 69.05 | - | - | - | - |
| 43 | C10H12O | Estragole | - | - | 0.31 | - | 1.32 | - | - | - | - | - |
| 44 | C15H24 | cis-Muurola-4(15),5-diene | - | - | - | - | 0.29 | - | - | - | - | - |
| 45 | C15H24 | γ-Muurolene | - | - | 0.34 | - | 4.14 | - | - | - | - | - |
| 46 | C12H20O2 | Bornyl acetate | 0.26 | - | - | 1.85 | 2.28 | - | - | - | 0.73 | - |
| 47 | C11H16O | 2-Isopropyl-5-methylanisole | 3.17 | - | 5.6 | - | - | - | - | - | - | - |
| 48 | C15H24 | Caryophyllene | 0.03 | - | - | 0.64 | - | 0.44 | - | - | 1.81 | 10.21 |
| 49 | C10H14O2 | Elsholtzia ketone | - | - | - | - | - | - | - | - | - | 0.59 |
| 50 | C10H20O | Neoisomenthol | - | - | - | - | - | 1.63 | - | - | - | - |
| 51 | C10H16O | Pulegone | - | - | - | - | - | 0.75 | - | - | - | - |
| 52 | C10H18O | Lavandulol | - | - | - | - | - | 0.37 | - | - | - | - |
| 53 | C10H18O | α-Terpineol | - | - | - | - | - | 0.11 | - | - | - | - |
| 54 | C10H18O | Terpinen-4-ol | - | - | - | 1.21 | 0.76 | - | - | 0.06 | 0.63 | - |
| 55 | C10H16O | Hotrienol | - | - | - | 0.02 | 0.07 | - | - | - | - | - |
| 56 | C10H18O | p-menth-1(7)-en-8-ol | - | - | - | 0.32 | 0.27 | - | - | - | - | - |
| 57 | C10H14O | Verbenone | - | - | - | 16.56 | - | - | - | - | - | - |
| 58 | C12H20O2 | Nerol acetate | - | - | - | 0.57 | - | - | - | - | - | - |
| 59 | C10H20O | (S)-3,7-dimethyl-7-Octen-1-ol | - | - | - | 0.61 | - | - | - | - | - | - |
| 60 | C15H24 | Humulene | 0.25 | 3.74 | - | - | - | - | 0.16 | 0.05 | 6.44 | 1.14 |
| 61 | C15H24 | (E)-β-Famesene | - | - | - | - | - | - | - | 0.55 | - | 0.23 |
| 62 | C15H24 | cis-β-farnesene | 0.05 | - | - | - | 2.27 | - | - | - | - | - |
| 63 | C10H18O | Borneol | 2.33 | 0.1 | 1.34 | - | 0.03 | - | - | - | 1.89 | - |
| 64 | C15H24 | (+)-δ-Cadinene | - | - | - | - | - | - | - | - | 0.04 | 0.02 |
| 65 | C10H16O | Piperitone | - | - | 0.61 | - | - | 1.6 | - | - | - | 2.94 |
| 66 | C10H14O | d-Carvone | - | - | 0.89 | - | - | - | - | 71.1 | - | - |
| 67 | C10H18O | Dihydrocarveol | - | - | 0.34 | - | - | - | - | 1.14 | - | - |
| 68 | C12H18O2 | cis-Carvyl acetate | - | - | - | - | - | - | - | 0.87 | - | - |
| 69 | C15H22 | cis-Calamenene | - | - | - | - | - | - | - | 0.34 | - | - |
| 70 | C15H24 | Germacrene D | 0.77 | - | - | - | 4.35 | - | - | - | - | 0.76 |
| 71 | C15H24 | α-Bulnesene | - | - | - | - | 1.74 | - | 5.68 | - | - | - |
| 72 | C15H26O | Ledol | - | - | - | - | - | - | 1.22 | - | - | - |
| 73 | C10H14O | (−)-Carvone | 0.03 | - | - | - | - | - | - | - | - | 0.22 |
| 74 | C15H24 | (Z,E)-α-Farnesene | - | - | - | - | - | - | - | - | - | 4.44 |
| 75 | C15H24 | γ-Elemene | 0.03 | - | - | - | 0.08 | - | - | - | - | 0.09 |
| 76 | C15H24 | Copaene | - | - | - | - | - | - | - | - | - | 0.14 |
| 77 | C15H24 | β-bisabolene | 3.96 | - | 2.26 | - | 0.22 | - | - | - | - | - |
| 78 | C10H14O | perillaldehyde | - | - | - | - | - | - | - | - | - | 2.83 |
| 79 | C10H14O2 | Perillaketone | - | - | - | - | - | - | - | - | - | 35.56 |
| 80 | C10H16O | Myrtenol | - | - | 0.02 | 0.83 | - | - | - | - | - | - |
| 81 | C10H14O | α-α-4-trimethyl-Benzenemethanol | - | - | - | 0.11 | - | - | - | - | 0.04 | - |
| 82 | C10H16O | trans-Carveol | - | - | 0.08 | - | - | - | - | 2.1 | 0.04 | - |
| 83 | C10H16O | cis-Carveol | - | - | - | - | - | - | - | 0.68 | - | - |
| 84 | C13H2O | trans-α-bergamotene | - | 1.9 | - | - | 14.16 | - | - | - | - | - |
| 85 | C15H24 | β-sesquiphellandrene | 0.06 | 0.4 | 0.05 | - | 0.15 | - | - | - | - | - |
| 86 | C10H18O | Nerol | 0.06 | - | - | - | 0.17 | - | - | - | - | 0.07 |
| 87 | C10H12O2 | Dehydroelsholtzia ketone | - | - | - | - | - | - | - | - | - | 0.59 |
| 88 | C10H12O2 | Isoegomaketone | - | - | - | - | - | - | - | - | - | 20.4 |
| 89 | C12H16O2 | Thymol acetate | 2.22 | 11.95 | - | - | - | - | - | - | - | - |
| 90 | C10H18O | Geraniol | 0.06 | - | - | 5.91 | 0.52 | - | - | - | - | - |
| 91 | C11H14O2 | Methyleugenol | - | - | - | - | 1.53 | - | - | - | - | 0.27 |
| 92 | C15H26O | Epicubenol | - | - | - | - | 1.34 | - | - | 0.33 | - | - |
| 93 | C15H26O | trans-Nerolidol | - | - | - | - | 0.34 | - | 0.24 | - | - | - |
| 94 | C15H26O | Viridiflorol | - | - | - | - | 0.13 | - | 0.08 | 0.05 | 7.85 | - |
| 95 | C14H22O | Norpatchoulenol | - | - | - | - | - | - | 1.14 | - | - | - |
| 96 | C15H24O | Caryophyllene Oxide | 0.33 | 0.06 | 0.64 | 0.15 | - | 0.07 | 1.86 | - | 0.48 | 0.87 |
| 97 | C15H26O | γ-Eudesmol | 0.4 | - | - | - | - | - | - | - | - | - |
| 98 | C15H24O | Espatulenol | 0.03 | - | 0.53 | - | - | 0.03 | 0.03 | 0.01 | 0.04 | 0.22 |
| 99 | C15H26O | Farnesol | - | - | - | - | - | - | 0.9 | - | - | - |
| 100 | C12H16O4 | Pogostone | - | - | - | - | - | - | 5.45 | - | - | - |
| 101 | C15H24O | Humulene epoxide II | - | 0.27 | - | 0.04 | - | - | 0.36 | - | 1.32 | |
| 102 | C10H12O2 | Eugenol | 0.11 | 0.26 | 0.06 | - | 10.27 | - | - | 0.17 | - | 0.33 |
| 103 | C15H26O | τ-Cadinol | - | - | - | - | 7.98 | 0.02 | - | 0.07 | - | 0.03 |
| 104 | C12H16O3 | Elemicin | - | - | - | - | - | - | - | - | - | 0.84 |
| 105 | C11H12O3 | Myristicin | - | - | - | - | - | - | - | - | - | 0.11 |
| 106 | C12H16O3 | Isoelemicin | - | - | - | - | - | - | - | - | - | 3.29 |
| 107 | C12H16O3 | Asarone | - | - | - | - | - | - | - | - | - | 1.71 |
| 108 | C15H26O | β-Eudesmol | - | - | - | - | 0.45 | - | - | - | - | - |
| 109 | C15H26O | Patchouli alcohol | - | - | 0.01 | - | - | 0.4 | 50.52 | - | - | - |
| 110 | C15H26O | Pogostol | - | - | - | - | - | - | 5.43 | - | - | - |
| 111 | C10H14O | Thymol | 23.7 | 26.59 | 14.64 | 0.15 | - | - | - | - | 0.27 | - |
| 112 | C15H26O | Neointermedeol | - | - | 0.09 | - | - | - | 0.38 | - | - | - |
| 113 | C15H24O | Longifolenaldehyde | - | - | - | - | - | - | 0.27 | - | - | - |
| 114 | C12H16O2 | Carvacryl acetate | - | - | 0.36 | - | - | - | - | - | - | - |
| 115 | C10H14O | Pulespenone | - | - | 1.75 | 0.15 | - | - | - | - | - | - |
| 116 | C11H16O | cis-Jasmone | - | - | 0.05 | 0.01 | - | - | - | - | - | - |
| 117 | C10H14O | Carvacrol | 2.48 | 7.66 | 20.82 | 0.07 | - | 0.16 | - | 0.14 | 0.05 | - |
| 118 | C15H26O | α-Cadinol | 0.03 | - | 0.03 | - | 0.26 | - | - | 0.32 | - | - |
| 119 | C12H14O3 | Eugenol acetate | - | - | - | - | 0.36 | - | - | - | - | - |
| 120 | C14H22O | 2,4-Di-tert-butylphenol | 0.07 | 0.05 | - | 0.03 | 0.04 | 0.03 | - | - | 0.04 | - |
| 121 | C20H40O | Phytol | 0.11 | - | - | - | 2.17 | - | 0.05 | - | 0.03 | - |
| 122 | C20H34O | 13-Epimanool | - | - | - | - | - | - | - | - | 8.91 | - |
| 123 | C9H11Cl3NO3PS | Chlorpyrifos | - | - | - | - | 0.47 | - | - | - | - | - |
| Total Content (%) | 90.51 | 94.25 | 89.87 | 86.68 | 92.06 | 97.02 | 81.58 | 96.85 | 97.24 | 94.22 | ||
38 compounds were identified in TmEO, accounting for 90.51% of the total EO, The main chemical constituents of TmEO are alkenes and phenols, such as γ-terpinene (16.42%), p-cymene (21.17%), thymol (23.70%). 22 compounds were identified in McEO, accounting for 94.25% of the total EO, with γ-terpinene (15.14%), p-cymene (19.52%), thymol (26.59%), alkenes and phenols compounds were dominated. 36 compounds were identified in OvEO, accounting for 89.87% of the total EO, the main chemical constituents are thymol (14.64%) and carvacrol (20.82%), which belonged to phenols. Thymol was the common component of TmEO, McEO and OvEO, this is consistent with the literature reported previous [18,19,20]. 34 compounds were identified in RoEO, accounting for 86.68% of the total EO, the main chemical components are eucalyptol (11.31%), verbenone (16.56%) and α-pinene (26.46%), monoterpenoid ketones and alkenes were dominated. While the research of Bouyahya A [21] showed the main compounds were α-pinene, 1, 8-eucalyptol and menthol, which is a little bit different from our results. It is probably due to different origins and extraction methods. 43 compounds were identified in ObEO, accounting for 92.06% of the total EO, linalool (26.65%), trans-α-bergamotene (14.16%), eugenol (10.27%), which belonged to alcohols, alkenes and phenols are main chemical consitituents. Because of different origins, the reported by Kathirvel [22] displayed that methyl cinnamate and Linalool were the main components. 23 compounds were identified in MhEO, accounting for 97.02% of the total EO, menthol (69.05%) was the most abundant in MhEO. 19 compounds were identified in PcEO, accounting for 81.58% of the total EO, the main component of PcEO was the patchouli alcohol (50.52%). 30 compounds were identified in MsEO, accounting for 96.85% of the total EO, the main chemical components are D-carvone (71.10%), which is consistent with previous reports [23,24,25]. 35 compounds were identified in SoEO, accounting for 97.24% of the total EO, tanacetone (27.99%), camphor (16.21%) are main chemical consitituents, ketones and terpene were dominated. 31 compounds were identified in PfEO, accounting for 94.22% of the total EO, the main chemical components are perillaketone (35.56%), isoegomaketone (20.40%), which belonged to ketones. However, the results were different from previous reports [26,27], which may be caused by different habitats of plants.
Statistical analysis of the chemical composition of 10 EOs shows that alkenes, phenols, aldehydes and ketones were main chemical composition, with a small number of alcohols and their oxides. Monoterpene hydrocarbons and phenolic acid compounds, which are closely related to the biological activities of EO, are dominant.
2.3. Antioxidant Activity
Antioxidant activity is a complex process usually occurring through several mechanisms, the evaluation of the antioxidant activity should be carried out by more than one test method [28]. In this study, two antioxidant assays, 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging activity and ferric reducing antioxidant power (FRAP) were applied to accurately evaluate the antioxidant properties of 10 EOs.
2.3.1. DPPH Free Radical Scavenging Activity
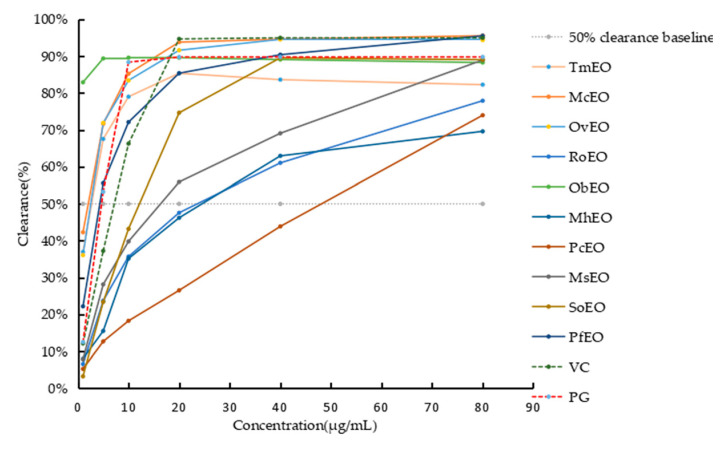
The antioxidant activity results of 10 EOs were determined by DPPH (Figure 2), which shows that there is an obvious dose-effect relationship between DPPH radical clearance rate and concentration of 10 EOs. The IC50 values were range from 0 to 49.74 μg/mL, with ObEO (<1 μg/mL), TmEO (1.42 μg/mL), OvEO (1.47 μg/mL), McEO (1.92 μg/mL), PfEO (3.77 μg/mL), SoEO (11.86 μg/mL), MsEO (13.34 μg/mL), RoEO (20.36 μg/mL), MhEO (23.95 μg/mL) and PcEO (49.74 μg/mL) (Table 2).
Figure 2.
Clearing-concentration relationship of 10 EOs, ascorbic acid (VC) and N-phenylacetyl-l-glutamine (PG).
Table 2.
FRAP and DPPH values of 10 EOs (n = 6).
| EOs | FRAP | DPPH (IC50, μg/mL) |
|---|---|---|
| TmEO | 271.84 ± 4.93 | 1.42 |
| McEO | 480.66 ± 29.90 | 1.92 |
| OvEO | 633.71 ± 13.14 | 1.47 |
| RoEO | 22.14 ± 0.63 | 20.36 |
| ObEO | 1536.67 ± 24.22 | <1 |
| MhEO | 22.32 ± 1.33 | 23.95 |
| PcEO | 30.35 ± 10.65 | 49.74 |
| MsEO | 24.54 ± 0.69 | 13.34 |
| SoEO | 17.22 ± 2.58 | 11.86 |
| PfEO | 67.14 ± 1.84 | 3.77 |
| Trolox | 2.02 ± 0.02 | - |
| VC | - | 6.04 |
| PG | - | 5.47 |
2.3.2. Ferric Reducing Antioxidant Power (FRAP)
The determination of total antioxidant activity with the FRAP method is the antioxidant can turn Fe3+-TPTZ complex to blue-purple Fe2+-TPTZ under acidic conditions, with the absorbance of Fe2+-TPTZ determined by Elisa. The change of absorbance is proportional to the content of the reduced substance, and the maximum absorption is reached at 593 nm, so it can be used as an indicator when evaluating the total antioxidant activity of sample [29]. The FRAP values of 10 EOs were calculated according to the standard curve (Figure S1), and the results are showed in Table 2 followed the crescent order as ObEO > OvEO > McEO > TmEO > PfEO > PcEO >MsEO > MhEO > RoEO > SoEO.
The results of DPPH and FRAP showed that 10 EOs of Labiatae species had strong antioxidant activity. Among the Labiatae species, ObEO with IC50 < 1 μg/mL and the highest FRAP value illustrate the strongest antioxidant activity, and the McEO, TmEO and OvEO own better antioxidant activity than other EOs. ObEO contains a large amount of linalool and eugenol, which may correspond to the antioxidant effect, is consistent with the previous reports [30]. Because of the thymol, which is the common component of TmEO, McEO and OvEO among the remaining nine EOs, has a certain amount of antibacterial and antioxidant activities, and even have been widely used in the breeding industry. Therefore, TmEO, McEO and OvEO show better antibacterial and antioxidant activities.
In a word, the results indicate that ten EOs of Labiatae species, especially for ObEO, could be potential sources of natural antioxidant feed additives, and expected to be used as feed additives in breeding industry.
2.4. Antibacterial Activity
The antibacterial activity of 10 EOs against four microorganisms was examined qualitatively (inhibition zone diameter, IZD), quantitatively (minimal inhibitory concentrations (MIC) and with minimal bactericidal concentration (MBC)). IZD tests carried out by the filter paper diffusion method, MIC and MBC were calculated using the two-fold dilution method.
Results of IZD, MIC and MBC
McEO, OvEO, TmEO had the most apparent antibacterial effect on Staphylococcus aureus (S. aureus), with the IZD values are more than 30 mm (Table 3). They also showed strong inhibitory effects on Escherichia coli (E. coli), Bacillus subtilis (B. subtilis), and Salmonella enteritidis (S. enteritidis). TmEO presented better antibacterial effect on S. aureus, compared with the results from Zhang et al. [31], in which the IZD value was 19.1 mm, although worse on B. subtilis and E. coli, with the IZD value of 34.5 and 15.0 mm respectively. The results of McEO is better than Li et al. [19], which the IZD value was 14.7 mm (S. aureus) and 8.0 mm (E. coli). Compared to the work of Assiri et al. [32], the IZD value of OvEO against S. aureus is consistent, while the inhibitory effect of OvEO against E. coli. and S. enteritidis is unsatisfactory.
Table 3.
Inhibition zone diameter (IZD, mm), MIC and MBC (μL/mL) for 10 EOs and positive control against four microorganisms (n = 6).
| EOs | Activities | S. aureus | E. coli | B. subtilis | S. enteriditis |
|---|---|---|---|---|---|
| TmEO | IZD | 30.77 ± 0.06 | 12.35 ± 0.73 | 11.59 ± 0.49 | 11.11 ± 0.19 |
| MIC | 2 | 1 | 2 | 1 | |
| MBC | 4 | 2 | 2 | 1 | |
| McEO | IZD | 32.32 ± 0.07 | 13.26 ± 0.45 | 14.72 ± 0.19 | 14.81 ± 1.55 |
| MIC | 1 | 2 | 1 | 1 | |
| MBC | 2 | 4 | 1 | 2 | |
| OvEO | IZD | 31.82 ± 0.03 | 10.78 ± 0.49 | 10.52 ± 0.29 | 10.71 ± 0.17 |
| MIC | 2 | 4 | 2 | 1 | |
| MBC | 4 | 4 | 2 | 2 | |
| RoEO | IZD | 6.00 | 6.65 ± 0.14 | 6.46 ± 0.12 | 6.67 ± 0.21 |
| MIC | 4 | 8 | 8 | 4 | |
| MBC | 16 | 16 | 16 | 4 | |
| ObEO | IZD | 12.98 ± 0.01 | 8.25 ± 0.17 | 6.82 ± 0.31 | 7.19 ± 0.50 |
| MIC | 2 | 4 | 8 | 8 | |
| MBC | 16 | 16 | 16 | 16 | |
| MhEO | IZD | 7.96 ± 0.34 | 8.20 ± 0.36 | 7.75 ± 0.20 | 7.94 ± 0.04 |
| MIC | 16 | NT | 8 | 4 | |
| MBC | 16 | NT | 8 | 4 | |
| PcEO | IZD | 12.02 ± 0.02 | 6.00 | 6.00 | 6.00 |
| MIC | NT | NT | NT | NT | |
| MBC | NT | NT | NT | NT | |
| MsEO | IZD | 9.78 ± 0.16 | 7.10 ± 0.08 | 6.93 ± 0.07 | 7.22 ± 0.04 |
| MIC | 16 | 8 | NT | NT | |
| MBC | 32 | 8 | NT | NT | |
| SoEO | IZD | 7.68 ± 0.39 | 6.00 | 6.00 | 6.00 |
| MIC | NT | NT | 16 | 4 | |
| MBC | NT | NT | 32 | 8 | |
| PfEO | IZD | 7.81 ± 0.46 | 6.00 | 6.00 | 6.44 ± 0.04 |
| MIC | 32 | NT | 8 | 4 | |
| MBC | NT | NT | 16 | 8 | |
| CTC | IZD | 16.12 ± 0.24 | 6.00 | 9.68 ± 0.12 | 6.00 |
| MIC | 8 | NT | NT | NT | |
| MBC | 8 | NT | NT | NT |
NT: not tested; CTC: chlortetracycline hydrochloride. Filter paper diameter (6 mm) is included in the test results.
The MIC values of 10 EOs are different on the tested microorganisms (Table 3).This indicates that the EOs’ antibacterial activity on four microorganisms is not regular, such as PfEO and MhEO to E. coli, MsEO to B. subtilis and S. enteriditis, SoEO against S. aureus and E. coli. PcEO even had no MIC on all four tested microorganisms. Nevertheless, McEO, OvEO and TmEO had obvious antibacterial effect on S. aureus, with the MIC value were 1, 2, 2 μL/mL respectively, is consistent with the IZD results. The MIC value for TmEO to B. subtilis, S. aureus and E. coli is higher than the results of Zhang et al. [31], while nearly the same with the results of Niu et al. [33]. The differences of thyme phenol content which was the main ingredient in TmEO maybe the main reason. McEO’s MIC value is much better than Li et al. [19], of which the MIC value is 62.5 μg/mL (S. aureus) and 250 μg/mL (E. coli). Dutra et al. [34], in their research, evaluated the antibacterial activity of OvEO against S. aureus and E. coli with the both MIC value of 12.5 μg/mL.
The inhibitory effect of other seven EOs on the four microorganisms are weak, SoEO and PfEO had no obvious inhibitory effect on the four tested microorganisms. The RoEO’s antibacterial activity, evaluated by Hussain et al. [35], is more desirable relative to our work. Similarly, it is the same situation to the ObEO compared to the results of Saha et al. [36] Though Delamare et al. [37] have evaluated the antibacterial activity of SoEO, there is no practical significance of the MIC values against E. coli, B. subtilis and S. aureus, due to the results were 5–10 mg/mL. In addition, there were very few reports on antibacterial activity of MhEO, PcEO and PfEO.
According to the results of MBC (Table 3), it is obvious that McEO, OvEO, TmEO own strong inhibitory effects to the four microorganisms, with disappointing results of the rest seven EOs. It is interesting that most of the reports on antibacterial activity of EOs focus on the determination of IZD and MIC, without MBC. Research on antibacterial activity focus more on inhibition than on sterilization may probably the reason. Meanwhile, plant essential oils are a potentially useful source of antibacterial compounds. The results of different studies are difficult to compare, most probably because of the different test methods, bacterial strains and sources of antibacterial samples used.
EOs have been used as an alternative to antibiotic growth promoter on intestinal health, immune response and antioxidant status in broiler chickens Chowdhury et al. [38], due to its antibacterial activity. Essential oil and aromatic plants as feed additives in non-ruminant nutrition is also well-known for a long time Zeng et al. [39]. However, the quality of EOs from different origin is uneven during the practical applications, and it is difficult to identify the EOs products which replaced by synthetic product. Therefore, the quality of EOs is out of control, and we suggest utilizing the specific chromatogram technology to identify the different EOs.
2.5. Antibacterial Stability of Essential Oils
The antibacterial experiments were tested under different temperature, pH and UV irradiation time, and the antibacterial results were compared with those under normal conditions.
2.5.1. Effects of Different pH on Antibacterial Stability of EOs
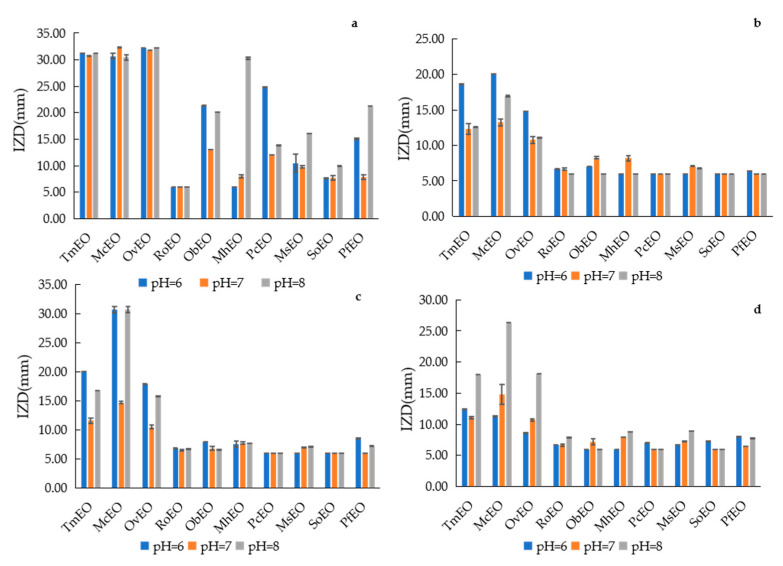
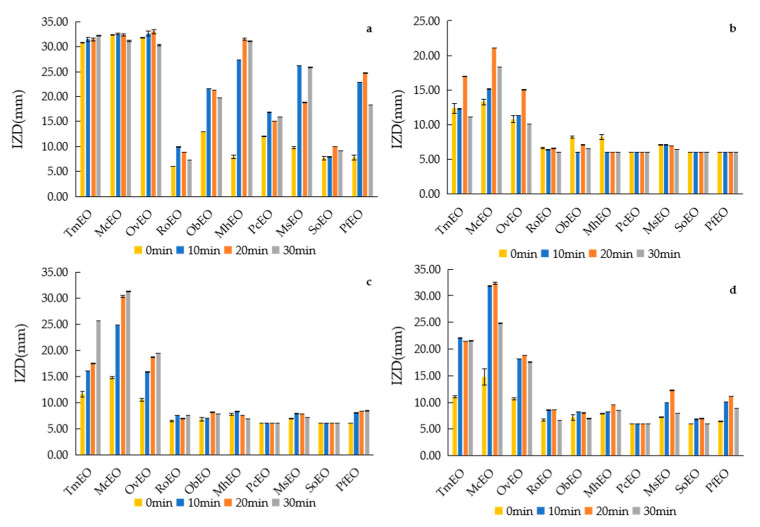
EOs had different effects on the antibacterial activities of four tested microorganisms under different pH (Figure 3 and Table S1). The results showed that TmEO, McEO and OvEO of these ten EOs had stronger inhibitory effect on the four tested microorganisms than the other seven, and there were no significant differences on the inhibitory effect of the other seven EOs on E. coli, B. subtilis, and S. enteriditis within different pH. The effect of pH on the inhibitory effect of essential oils against S. aureus is diverse, pH = 8 was the best condition to MhEO, MsEO and PfEO, while ObEO and PcEO performed best at pH = 6, and three conditions is equally effective for RoEO and SoEO. According to the reported literature, the evaluation of EOs’ pH on antibacterial activity is rarely reported. Most of the reports usually combined EOs with other substance as products, such as gum arabic (Niu et al. [33]), alginate (Sarengaowa et al. [40]) and so on.
Figure 3.
IZD of EOs under different pH. (a) S. aureus, (b) E. coli, (c) B. subtilis, (d) S. enteriditis.
2.5.2. Effects of Different Temperature on Antibacterial Stability of EOs
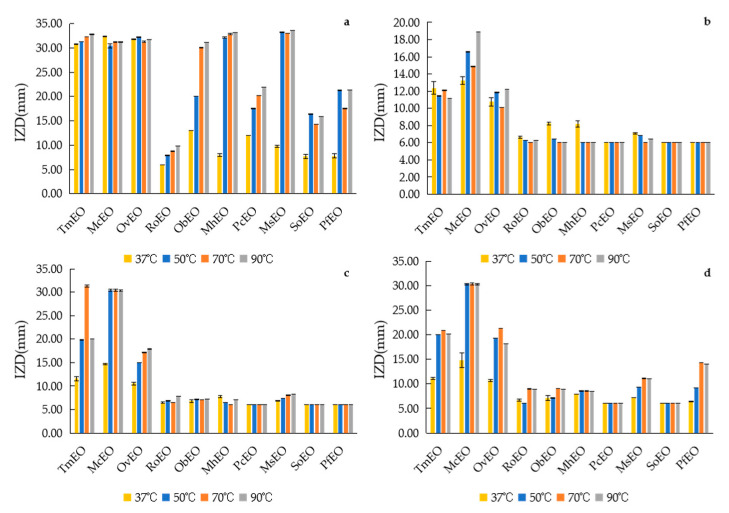
The inhibitory effects on S. aureus of 10 EOs can be divided into two categories, one is stable team consist of TmEO, McEO and OvEO, which the inhibitory effects was stable with the temperature changing, and the rest EOs are the other team, which the inhibitory effects improved as the increasing of temperature (Figure 4 and Table S2). Conversely, the inhibitory effects on E. coli, B. subtilis and S. enteriditis is opposite to S. aureus. The temperature only obviously affects TmEO, McEO and OvEO, with no significant fluctuations on the rest seven EOs. Though the results shown that the EOs own thermal stability, lots of reported work have directly choose 37 °C as experimental temperature [19,31,32,33].
Figure 4.
IZD of EOs at different temperature. (a) S. aureus, (b) E. coli, (c) B. subtilis, (d) S. enteriditis.
2.5.3. Effects of UV Irradiation Time on Antibacterial Stability of EOs
The antibacterial activity of SoEO and ObEO to the four tested microorganisms had no significant effect under UV irradiation. Nevertheless, UV irradiation enhanced the antibacterial activity of PfEO, MhEO, MsEO and PcEO to S. aureus, with no apparent impact on the other three tested microorganisms. The enhancement of antibacterial activity of the other 6 EOs under UV irradiation is evident (Figure 5 and Table S3). The composition of EOs may change under UV irradiation, which may be one of the factors that lead to the enhancement of the antibacterial activity of EOs.
Figure 5.
IZD of EOs under different UV irradiation time. (a) S. aureus, (b) E. coli, (c) B. subtilis, (d) S. enteriditis.
In a word, part of Eos’ antibacterial activity was greatly diminished under UV irradiation, while rest of them were mainly enhanced. Therefore, plant EOs can also achieve a good antibacterial effect under this condition.
The results show that the antibacterial activity of 10 EOs has small amplitude fluctuation under different temperature range (50~90 °C), different pH (6~8) and different UV irradiation time (10~30 min), but there is no significant difference compared with the normal control group. The antibacterial activity of 10 EOs generally remained stable under different temperature, pH and UV irradiation time.
3. Materials and Methods
3.1. Plant Materials Collection and EOs Extraction
Thymus mongolicus Ronn. (Shandong, Qingdao), Mosla chinensis Maxim. (Hunan, Xinning), Origanum vulgare Linn. (Shandong, Qingdao), Rosmarinus officinalis Linn. (Hunan, Changsha), Ocimum basilicum Linn. (Shandong, Qingdao), Mentha haplocalyx Briq. (Shandong, Qingdao), Pogostemon cablin (Blanco) Benth. (Shandong, Qingdao), Mentha spicata Linn. (Shandong, Qingdao), Salvia officinalis Linn. (Shandong, Qingdao), Perilla frutescens (Linn.) Britt. (Shandong, Qingdao), were collected from different regions of China. They were unambiguously authenticated by Prof Guangmin Yang (Hunan University of Chinese Medicine, Hunan, China).
The EOs of 10 plants were extracted using the steam distillation [41]. The samples were dried in a ventilated place, and cut into 1 cm pieces and separately hydro-distilled for 4 h (200 g samples in 1200 to 1600 mL of distilled water) in a round-bottom flask equipped an EO tester and condensation reflux tube, of which filled with distilled water. The EOs dried with anhydrous sodium sulfate and collected in a brown bottle, stored at 4 °C before analysis. The yields of the EOs were calculated by the Equation (1).
| (1) |
3.2. Identification of the Chemical Components of the EOs
The GC/MS analysis was carried out using splitless injection mode on a QP2010 gas chromatograph-mass spectrometer instrument (Shimadzu, Yubinbang, Japan) equipped with a CD-WAX chromatographic column (30 m, 0.25 mm, 0.25 μm film thicknesses, ANPEL, Shanghai, China), and data analysis through GC/MS solution chromatography workstation and NIST.17 mass spectrometry database (Shimadzu, Yubinbang, Japan). Helium was used as carrier gas at a flow rate of 1.0 mL/min, splitless. The oven temperature was programmed from low to high designed at a specified speed (Table S4) and injector heater 240 °C. The mass-spectrometer was accomplished in the range of 28–500 m/z in the EI-mode at 70 eV and ion source temperature was set to 200 °C. The components of EOs were identified by matching their recorded mass spectra with the data bank mass spectra (NIST 17). For each compound on the gas chromatogram, the percentage of peak area relative to the total peak area of all compounds was determined and reported.
3.3. Determination of Antioxidant Activity
3.3.1. Antioxidant Activity Determined by DPPH
The ability to scavenge DPPH free radicals was evaluated according to the procedure reported with some modifications [42,43]. At first, 2.0 mL Eos (Homemade, Changsha, China) were blended at chosen concentrations (1, 5, 10, 20, 40 and 80 μg/mL) with 0.2 mM ethanol DPPH solution (2 mL, Beyotime, Shanghai, China). While the reaction mixture without any sample was used as a negative control (A0). Reaction mixtures were incubated in the dark at room temperature for 30 min, the absorbance at 517 nm was recorded using a microplate reader (NanoQuant infinite M200Pro TECAN, Tecan, Hombrechtikon, Switzerland). The inhibition percentage was plotted versus the sample concentration and 50% of the inhibitory concentration (IC50) of the DPPH values was defined by linear regression analysis. Ascorbic acid (VC) of different concentrations (1–80 μg/mL) and N-phenylacetyl-l-glutamine (PG) were used as a positive control, The scavenging activity percentage against DPPH radicals was calculated as follows the Equation (2).
| SR% = (1 − (Ai − Aj)/A0) × 100 % | (2) |
where, Ai is the absorbance of the sample at 30 min; A0 is the absorbance of reaction mixture without any sample at 30 min; Aj is the absorbance of the ethanol (100%) and EOs.
3.3.2. Antioxidant Activity Determined by FRAP
The FRAP assay was evaluated according to Chen with some modifications [44]. FRAP reagent (a kind of rapid test kits, Shanghai Beyotime Biotechnolgy Co., LTD, Shanghai, China). According to the operating instructions, 180 μL FRAP reagent was mixed at 37 °C with each test sample solution (5 μL) of different concentration (TmEO (2 μg/mL), McEO (2 μg/mL), OvEO (2 μg/mL), RoEO (20 μg/mL), ObEO (1 μg/mL), MhEO (20 μg/mL), PcEO (20 μg/mL), MsEO (20 μg/mL), SoEO (20 μg/mL), PfEO (20 μg/mL)) in 96-well plates. After 3–5 min, the colored products were then taken at 593 nm. Results were expressed as trolox equivalent antioxidant capacity. The standard curve was determined by the FeSO4 standard solution. Trolox as the positive control the total antioxidant capacity of the sample was expressed by the FRAP value. The value of 1 FRAP is equivalent to 1 mM FeSO4.
3.4. Determination of Antibacterial Activity
3.4.1. Test Microorganisms
The microbial strains applied in this investigation were Escherichia coli (E. coli), Staphylococcus aureus (S. aureus), Salmonella enteritidis (S. enteriditis), Bacillus subtilis (B. subtilis), which were provided by China Center for Type Culture Collection (CCTCC, Wuhan, China). The strains were inoculated in liquid lysogeny broth (LB) medium and cultured (37 °C) for 24 h after activated, then diluted with new liquid LB mediums to a suspension liquid (107 CFU/mL).
3.4.2. Inhibition Zone Diameter
The filter paper diffusion method was used to obtain the inhibition zone [45]. EOs (2 μL) were absorbed on the filter paper (d = 6 mm), meanwhile, applied to the Muller Hinton Agar plates (pH = 7) with the proper bacteria solution (100 μL, 106 CFU/mL, manufacturer, city, country). Paper contained EOs in duplicate and 1 piece of paper contained sterile water were put on a plate, Chlortetracycline Hydrochloride was used as a positive control, then the plates were inoculated at 37 °C for each strain for 12 h. Crossing method was used to determine the inhibition zone diameter [46].
3.4.3. Minimal Inhibitory Concentrations (MIC) and Minimal Bactericidal Concentration (MBC)
The MIC and MBC values of ten EOs and CTC against two Gram-positive bacteria and two Gram-negative bacteria were evaluated using two-fold dilution method as reported earlier [47,48]. Ten EOs were diluted into 320 μL/mL with 1% tween 80 aqueous solution. Take 11 sterile tubes numbered 1–11, 1.6 mL LB medium was added to the tube NO.1, while NO. 2–11 were added with 1.0 mL, respectively. Then 0.4 mL EOs was added into tube NO.1, following aspirate 1.0 mL of the mixed solution to the tube NO.2. Similarly, aspirate 1.0 mL of the mixed solution in tube NO.2 to the tube NO.3, and the same operation until NO.9. The tube NO.10 and NO.11 were used as sample blank and matrix blank, with no addition of mixed solution from previous tube. To keep the volume of solution consistent in all tubes, discard 1.0 mL of the mixed solution from tube NO.9.
Then, 1.0 mL bacterial suspension (107 CFU/mL) was added to the tube NO.1-9, make the concentration of EOs was 0.125 to 32 μL/mL and a concentration of bacterial was 106 CFU/mL. The tubes were inoculated at 37 °C for each strain for 24 h, and then each tube (100 μL) was pipetted to the agar plate, inoculated at 37 °C for 12 h. The MIC of EOs were specified as the lowest concentration showing no perceptible microbial growth. Chlortetracycline Hydrochloride was used as a positive control. MBC value was determined by plates that showed no growth and incubating at 37 °C for another 12 h. The lowest concentration that disclosed no visible growth of bacteria was deemed as MBC.
3.4.4. Antibacterial Stability of EOs
The experiment used 0.1 mol/L NaOH and 0.1 mol/L HCl to adjust the pH of the medium to 6.0, 7.0 and 8.0, respectively, then test the antibacterial activities of EOs under different pH. Ten EOs were placed in 1.5 mL tube and irradiated by UV light for 0, 10, 20 and 30 min on the benchtop respectively, then test the antibacterial activities of EOs. Ten EOs were put into 10 amber bottles respectively, and then placed in water bath pot with different temperatures (37, 50, 70 and 90 °C), then test the antibacterial activities of EOs after 30 min.
3.5. Statistical Analysis
All analyses were performed in triplicate and the results expressed as the mean standard deviation (SD). For the data with normal distribution, an analysis of variance (ANOVA) was performed, considering the compound and its concentration as fixed factors, while the inhibition percentage as the dependent variable. The significance criterion was set to p < 0.05.
4. Conclusions
Chemical composition and bioactivity of essential oil of ten Labiatae species have been investigated in this research. The yield of these EOs is satisfactory, which make it possible to develop into products in economic value. A range of chemical compounds have been identified from these ten EOs, with alkenes, phenols, aldehydes and ketones were major categories. The results of DPPH and FRAP have proved that ObEO own the best antioxidant activity, and followed OvEO, McEO and TmEO. Meanwhile, OvEO, McEO and TmEO also presented desirable antibacterial activity. ObEO, McEO, TmEO, OvEO get the most potential of developing into a new kind of feed additives, and also should have broad development potential in food, feed, cosmetics and other industries.
Supplementary Materials
The following are available online.
Author Contributions
Conceptualization, M.L. and F.L.; methodology, F.L.; software, F.L.; validation, Z.Q., X.L.; formal analysis, M.L.; investigation, M.L.; resources, J.Z.; data curation, H.Y. and F.L.; writing—original draft preparation, M.L.; writing—review and editing, M.L.; visualization, J.Z.; supervision, J.Z.; project administration, Z.Y. and J.Z.; funding acquisition, J.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Key Research and development program of China [2017YED0501500].
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability: Samples of the compounds are available from the authors.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bjornerot L., Franklin A., Tysen E. Usage of antibacterial and antiparasitic drugs in animals in Sweden between 1988 and 1993. Veter- Rec. 1996;139:282–286. doi: 10.1136/vr.139.12.282. [DOI] [PubMed] [Google Scholar]
- 2.Sui Q.W., Zhang J.Y., Wei Y.S., Chen M.X., Dong H.M., Xiong J.H. Veterinary antibiotics use, occurrence of antibiotic resistance pathogen and its antibiotic resistance genes in animal production: An overview. Asian J. Ecotoxicol. 2015;10:20–34. doi: 10.7524/AJE.1673-5897.20150922001. [DOI] [Google Scholar]
- 3.Chen X., Zhao X., Deng Y., Bu X., Ye H., Guo N. Antimicrobial potential of myristic acid against Listeria monocytogenes in milk. J. Antibiot. 2019;72:298–305. doi: 10.1038/s41429-019-0152-5. [DOI] [PubMed] [Google Scholar]
- 4.Guo S., Ma J., Xing Y., Xu Y., Jin X., Yan S., Shi B. Artemisia annua L. aqueous extract as an alternative to antibiotics improving growth performance and antioxidant function in broilers. Ital. J. Anim. Sci. 2020;19:399–409. doi: 10.1080/1828051X.2020.1745696. [DOI] [Google Scholar]
- 5.Bakkali F., Averbeck S., Idaomar M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008;46:446–475. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- 6.Burt S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004;94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 7.Baker D.H.A., Al-Moghazy M., Elsayed A. The in vitro cytotoxicity, antioxidant and antibacterial potential of Satureja hortensis L. essential oil cultivated in Egypt. Bioorganic Chem. 2020;95:103559. doi: 10.1016/j.bioorg.2019.103559. [DOI] [PubMed] [Google Scholar]
- 8.Al-Mariri A., Safi M. The Antibacterial Activity of Selected Labiatae (Lamiaceae) Essential Oils against Brucella melitensis. Iran. J. Med Sci. 2013;38:44–50. [PMC free article] [PubMed] [Google Scholar]
- 9.Nabavi S.M., Marchese A., Izadi M., Curti V., Daglia M. Plants belonging to the genus Thymus as antibacterial agents: From farm to pharmacy. Food Chem. 2015;173:339–347. doi: 10.1016/j.foodchem.2014.10.042. [DOI] [PubMed] [Google Scholar]
- 10.Xie Q., Liu Z., Li Z. Chemical Composition and Antioxidant Activity of Essential Oil of Six Pinus Taxa Native to China. Molecules. 2015;20:9380–9392. doi: 10.3390/molecules20059380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Della Cuna F.S.R., Calevo J., Bari E., Caser M., Boselli C., Tava A., Della Cuna F.R. Characterization and Antioxidant Activity of Essential Oil of Four Sympatric Orchid Species. Molecules. 2019;24:3878. doi: 10.3390/molecules24213878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Z., Shen P., Liu J., Gu C., Lu X., Li Y., Cao Y., Liu B., Fu Y., Zhang N. In Vivo Study of the Efficacy of the Essential Oil of Zanthoxylum bungeanum Pericarp in Dextran Sulfate Sodium-Induced Murine Experimental Colitis. J. Agric. Food Chem. 2017;65:3311–3319. doi: 10.1021/acs.jafc.7b01323. [DOI] [PubMed] [Google Scholar]
- 13.Lai F., Wissing S.A., Müller R.H., Fadda A.M. Artemisia arborescens L essential oil-loaded solid lipid nanoparticles for potential agricultural application: Preparation and characterization. AAPS PharmSciTech. 2006;7:E10–E18. doi: 10.1208/pt070102. [DOI] [PubMed] [Google Scholar]
- 14.González J.O.W., Gutiérrez M.M., Murray A.P., Ferrero A. Composition and biological activity of essential oils from Labiatae against Nezara viridula (Hemiptera: Pentatomidae) soybean pest. Pest Manag. Sci. 2011;67:948–955. doi: 10.1002/ps.2138. [DOI] [PubMed] [Google Scholar]
- 15.Ju J., Xie Y., Guo Y., Cheng Y., Qian H., Yao W. Application of edible coating with essential oil in food preservation. Crit. Rev. Food Sci. Nutr. 2018;59:2467–2480. doi: 10.1080/10408398.2018.1456402. [DOI] [PubMed] [Google Scholar]
- 16.Andrys D., Adaszyńska-Skwirzyńska M., Kulpa D. Essential oil obtained from micropropagated lavender, its effect on HSF cells and application in cosmetic emulsion as a natural protective substance. Nat. Prod. Res. 2017;32:849–853. doi: 10.1080/14786419.2017.1361950. [DOI] [PubMed] [Google Scholar]
- 17.Li G.P., Dudai N., Li J.J., Lin L.J. Comparison of volatile composition of essential oils from three different Lamiaceae plants. Chin. J. Trop. Crops. 2018;39:1644–1650. doi: 10.3969/j.issn.1000-2561.2018.08.026. [DOI] [Google Scholar]
- 18.Wang D., Cheng B., Ding L., Ren M.S. Extraction Technology and Chemical Composition Analysis of Thyme Essential Oil. China Condiment. 2019;44:76–80. doi: 10.3969/j.issn.1000-9973.2019.07.016. [DOI] [Google Scholar]
- 19.Cao L., Si J.Y., Liu Y., Sun H., Jin W., Li Z., Zhao X.H., Le Pan R. Essential oil composition, antimicrobial and antioxidant properties of Mosla chinensis Maxim. Food Chem. 2009;115:801–805. doi: 10.1016/j.foodchem.2008.12.064. [DOI] [Google Scholar]
- 20.La Pergola A., Restuccia C., Napoli E., Bella S., Brighina S., Russo A., Suma P. Commercial and wild SicilianOriganum vulgareessential oils: Chemical composition, antimicrobial activity and repellent effects. J. Essent. Oil Res. 2017;29:451–460. doi: 10.1080/10412905.2017.1353448. [DOI] [Google Scholar]
- 21.Bouyahya A., Et-Touys A., Bakri Y., Talbaui A., Fellah H., Abrini J., Dakka N. Chemical composition of Mentha pulegium and Rosmarinus officinalis essential oils and their antileishmanial, antibacterial and antioxidant activities. Microb. Pathog. 2017;111:41–49. doi: 10.1016/j.micpath.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 22.Kathirvel P., Ravi S. Chemical composition of the essential oil from basil (Ocimum basilicumLinn.) and itsin vitrocytotoxicity against HeLa and HEp-2 human cancer cell lines and NIH 3T3 mouse embryonic fibroblasts. Nat. Prod. Res. 2012;26:1112–1118. doi: 10.1080/14786419.2010.545357. [DOI] [PubMed] [Google Scholar]
- 23.Li G.M., Li S.L., Bai Y.B., Hu Y.L. Analysis of Chemical Components of Essential Oil from Mentha piperita Grown in Ruilli by GC-MS. Chin. J. Trop. Agric. 2017;37:84–88. doi: 10.12008/j.issn.1009-2196.2017.10.018. [DOI] [Google Scholar]
- 24.Pang Y.X., Zhang Y.B., Wu M., Yuan Y., Yu F.L., Chen X.L., Hu X. Comparative Analysis of Patchouli Essential Oils with Singlecropping and Intercropping with Rubber Tree. Biomass Chem. Eng. 2014;48:15–18. doi: 10.3969/j.issn.1673-5854.2014.05.004. [DOI] [Google Scholar]
- 25.Almeida P.P., Mezzomo N., Ferreira S.R. Extraction of Mentha spicata L. Volatile Compounds: Evaluation of Process Parameters and Extract Composition. Food Bioprocess. Technol. 2010;5:548–559. doi: 10.1007/s11947-010-0356-y. [DOI] [Google Scholar]
- 26.Kulak M., Gul F., Sekeroglu N. Changes in growth parameter and essential oil composition of sage (Salvia officinalis L.) leaves in response to various salt stresses. Ind. Crop. Prod. 2020;145:112078. doi: 10.1016/j.indcrop.2019.112078. [DOI] [Google Scholar]
- 27.You C.X., Yang K., Wu Y., Zhang W.J., Wang Y., Geng Z.F., Chen H.P., Jiang H.Y., Du S.S., Deng Z.W., et al. Chemical composition and insecticidal activities of the essential oil of Perilla frutescens (L.) Britt. aerial parts against two stored product insects. Eur. Food Res. Technol. 2014;239:481–490. doi: 10.1007/s00217-014-2242-8. [DOI] [Google Scholar]
- 28.Aruoma O.I. Methodological considerations for characterizing potential antioxidant actions of bioactive components in plant foods. Mutat. Res. Mol. Mech. Mutagen. 2003:9–20. doi: 10.1016/S0027-5107(02)00317-2. [DOI] [PubMed] [Google Scholar]
- 29.Baharfar R., Azimi R., Mohseni M. Antioxidant and antibacterial activity of flavonoid-, polyphenol- and anthocyanin-rich extracts from Thymus kotschyanus boiss & hohen aerial parts. J. Food Sci. Technol. 2015;52:6777–6783. doi: 10.1007/s13197-015-1752-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skrypnik L., Novikova A., Tokupova E. Improvement of Phenolic Compounds, Essential Oil Content and Antioxidant Properties of Sweet Basil (Ocimum basilicum L.) Depending on Type and Concentration of Selenium Application. Plants. 2019;8:458. doi: 10.3390/plants8110458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Z., Zhang S., Su R., Xiong D., Feng W., Chen J. Controlled Release Mechanism and Antibacterial Effect of Layer-By-Layer Self-Assembly Thyme Oil Microcapsule. J. Food Sci. 2019;84:1427–1438. doi: 10.1111/1750-3841.14610. [DOI] [PubMed] [Google Scholar]
- 32.Assiri A.M.A., Elbanna K., Al-Thubiani A., Ramadan M.F. Cold-pressed oregano (Origanum vulgare) oil: A rich source of bioactive lipids with novel antioxidant and antimicrobial properties. Eur. Food Res. Technol. 2015;242:1013–1023. doi: 10.1007/s00217-015-2607-7. [DOI] [Google Scholar]
- 33.Niu F., Pan W., Su Y., Yang Y. Physical and antimicrobial properties of thyme oil emulsions stabilized by ovalbumin and gum arabic. Food Chem. 2016;212:138–145. doi: 10.1016/j.foodchem.2016.05.172. [DOI] [PubMed] [Google Scholar]
- 34.Dutra T.V., Castro J.C., Menezes J.L., Ramos T.R., Prado I.N.D., Machinski M., Mikcha J.M.G., Filho B.A.D.A. Bioactivity of oregano (Origanum vulgare) essential oil against Alicyclobacillus spp. Ind. Crop. Prod. 2019;129:345–349. doi: 10.1016/j.indcrop.2018.12.025. [DOI] [Google Scholar]
- 35.Hussain A.I., Anwar F., Chatha S.A.S., Jabbar A., Mahboob S., Nigam P.S. Rosmarinus officinalis essential oil: Antiproliferative, antioxidant and antibacterial activities. Braz. J. Microbiol. 2010;41:1070–1078. doi: 10.1590/S1517-83822010000400027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saha S., Dhar T., Sengupta C., Ghosh P. Biological activities of essential oils and methanol extracts of five Ocimum species against pathogenic bacteria. Czech., J. Food Sci. 2013;31:195–202. doi: 10.17221/234/2012-CJFS. [DOI] [Google Scholar]
- 37.Delamare A.P.L., Moschen-Pistorello I.T., Artico L., Atti-Serafini L., Echeverrigaray S. Antibacterial activity of the essential oils of Salvia officinalis L. and Salvia triloba L. cultivated in South Brazil. Food Chem. 2007;100:603–608. doi: 10.1016/j.foodchem.2005.09.078. [DOI] [Google Scholar]
- 38.Chowdhury S., Mandal G.P., Patra A.K., Kumar P., Samanta I., Pradhan S., Samanta A.K. Different essential oils in diets of broiler chickens: 2. Gut microbes and morphology, immune response, and some blood profile and antioxidant enzymes. Anim. Feed. Sci. Technol. 2018;236:39–47. doi: 10.1016/j.anifeedsci.2017.12.003. [DOI] [Google Scholar]
- 39.Zeng Z., Zhang S., Wang H., Piao X. Correction to: Essential oil and aromatic plants as feed additives in non-ruminant nutrition: A review. J. Anim. Sci. Biotechnol. 2020;11:1. doi: 10.1186/s40104-020-00467-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu W., Feng K., Xiu Z., Jiang A., Lao Y. Efficacy of thyme oil-alginate-based coating in reducing foodborne pathogens on fresh-cut apples. Int. J. Food Sci. Technol. 2019;54:3128–3137. doi: 10.1111/ijfs.14229. [DOI] [Google Scholar]
- 41.Wang S.P. Study on Antimicrobial Activity of Several Kinds of Aromatic Essential Oil. Anhui Agric. Bull. 2018;24:20–24. doi: 10.16377/j.cnki.issn1007-7731.2018.02.007. [DOI] [Google Scholar]
- 42.Polatoğlu K., Sen A., Kandemir A., Gören N. Essential Oil Composition and DPPH Scavenging Activity of EndemicTanacetum mucroniferumHub.—Mor. & Grierson from Turkey. J. Essent. Oil Bear. Plants. 2012;15:66–74. doi: 10.1080/0972060x.2012.10644021. [DOI] [Google Scholar]
- 43.Thaipong K., Boonprakob U., Crosby K., Cisneros-Zevallos L., Byrne D.H. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006;19:669–675. doi: 10.1016/j.jfca.2006.01.003. [DOI] [Google Scholar]
- 44.Chen Y.X., Liu J.H., Lin F., Du X.D. Determination of Antioxidative Activity of 41 Kinds of Chinese Herbal Medicines by Using DPPH and FRAP Methods. Res. Explor. Lab. 2011;30:11–14. [Google Scholar]
- 45.Deng R.N., Zhang D.Q. Comparative Study on Determination Methods of Bacteriostasis of Lavender Essential Oil in Field. Environ. Environ. Sci. Manag. 2017;42:102–105. [Google Scholar]
- 46.Ding W.L., Wang R., Hao D.L., Zhang X.Y., Li Y. Evaluation of Antagonistic Activity of Biocontrol Microbial Agents against Ginseng Diseases. Mod. Chin. Med. 2018;20:1122–1125. [Google Scholar]
- 47.Yang Y.D., Lu D.H., Yang D.M., Yuan X.J., Qin H.Y. Antibacterial Activity and Mechanism of Essential Oil from Cinamomum camphora on Staphylococcus aureus. Mod. Chin. Med. 2017;19:372–376. doi: 10.13313/j.issn.1673-4890.2017.3.013. [DOI] [Google Scholar]
- 48.Duan W.L., Liu Y.Q., Bao Y.H. Study on Antimicrobial Activities and Stability of Essential Oil from Artemisia argyi. J. Food Sci. Biotechnol. 2015;34:1332–1337. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.