Abstract
Achieving a deeper understanding of the factors controlling the defense responses of invertebrate vectors to the human-infecting pathogens they transmit will provide needed new leads to pursue for control. Consequently, we provide new genomic and transcriptomic insights regarding FReDs (containing a fibrinogen domain) and FREPs (fibrinogen domain and one or two IgSF domains) from the planorbid snail Biomphalaria glabrata, a Neotropical vector of Schistosoma mansoni, causative agent of human intestinal schistosomiasis. Using new bioinformatics approaches to improve annotation applied to both genome and RNA-Seq data, we identify 73 FReD genes, 39 of which are FREPs. We provide details of domain structure and consider relationships and homologies of B. glabrata FBG and IgSF domains. We note that schistosome-resistant (BS-90) snails mount complex FREP responses following exposure to S. mansoni infection whereas schistosome-susceptible (M line) snails do not. We also identify several coding differences between BS-90 and M line snails in three FREPs (2, 3.1 and 3.2) repeatedly implicated in other studies of anti-schistosome responses. In combination with other results, our study provides a strong impetus to pursue particular FREPs (2, 3.1, 3.2 and 4) as candidate resistance factors to be considered more broadly with respect to schistosome control efforts, including involving other Biomphalaria species vectoring S. mansoni in endemic areas in Africa.
Author summary
Just as malaria parasites depend on mosquitoes for transmission, Schistosoma trematodes that infect 200+ million people in developing countries depend on freshwater snails to support their development. New control methods targeting schistosomes in the snail host are urgently needed to help break the cycle of transmission. Identification of molecules in snails that confer or facilitate resistance to successful larval development offers one way forward. Building on previous studies and new data, we used a combination of bioinformatics tools to characterize a repertoire of molecules in B. glabrata containing fibrinogen or IgSF domains, or both (the latter molecules called FREPs, first discovered in B. glabrata). We found that snails resistant to schistosomes mount vigorous, multi-component FREP responses following exposure to schistosomes whereas responses of susceptible snails are modest by comparison. Furthermore, for four FREPs repeatedly implicated in snail responses to trematodes, three (FREPs 2, 3.1 and 3.2) exist in distinctive allelic forms in susceptible and resistant snails. We propose that the search for homologous molecules needs to move to the field, to B. glabrata in the Neotropics and to other vector species of Biomphalaria in Africa, to explore the potential of FREPs as resistance factors worthy of development as control agents.
Introduction
Human schistosomiasis, one of the most persistent and common of the neglected tropical diseases, is caused by digenetic trematodes in the genus Schistosoma [1]. Schistosoma mansoni causes intestinal schistosomiasis and infects 83 million [2] of the more than 200 million people in the world estimated to need protective treatment from the disease annually [3,4]. Transmission of S. mansoni to people depends on the presence of compatible species of the freshwater planorbid snail genus Biomphalaria which serve as obligatory intermediate hosts. Snails become infected when penetrated by miracidia hatched from S. mansoni eggs passed in human feces. Within infected snails schistosome sporocysts eventually produce thousands of cercariae which leave the snail body, contact and penetrate human skin and develop into adult worms. The most important snail host for S. mansoni in the Neotropics is B. glabrata. The combination of S. mansoni and B. glabrata has become an oft-studied model for trematode-snail interactions and for both molluscan and invertebrate immune response capabilities [5,6,15–17,7–14].
Our goal in this paper is to provide a comprehensive genomics-based overview, along with insights from their transcriptional profiles, for a group of defense-related molecules from B. glabrata known as fibrinogen-related domain containing proteins, or FReDs (Fig 1). In addition to the fibrinogen-related (FBG) domain, FReDs may or may not also contain IgSF domains. Among the FReDs from B. glabrata is a family of diverse hemolymph lectins [18,19] originally termed fibrinogen-related proteins, or FREPs [20]. FREPs have become one of the most studied groups of invertebrate defense molecules and for this reason are a particular focus of this paper. FREPs contain one or two N-terminal immunoglobulin superfamily (IgSF) domains, an interceding region (ICR without distinctive homology to other domains) and a C-terminal FBG-like domain (Fig 1). IgSF members, which share a conserved immunoglobulin domain or fold but exhibit a wide range of amino acid sequence variation, are well known for their role of ligand binding, showing high specificity and diverse recognition capacity [21]. In animals, IgSF domain-containing proteins are involved in cell-cell recognition, cell-surface receptors, muscle structure and the immune system [22,23]. Invertebrate IgSF domain-containing proteins are also capable of binding to various pathogen-associated molecular patterns [20,24–26]. IgSF domains share a common Greek key-like core with beta-sandwiches. Based on differing numbers of beta-sandwiches, IgSF domains are grouped into four types: V-set (variable), C1-set (constant-1), C2-set and I-set (intermediate) [27,28]. FREP-associated IgSF1 and IgSF2 domains both resemble V-type IgSF domains based on sequence similarity and immunoglobin loop size [26,29], but they are readily separable from one another with respect to their amino acid sequences, designated as IgSF1 and 2 [26]. In 1-IgSF FREPs, it is the IgSF2 domain that is present, and in 2-IgSF FREPs, the IgSF1 is N-terminal and the IgSF2 domain lies immediately upstream of the ICR and the relatively conserved C-terminal FBG domain [26,29–31].
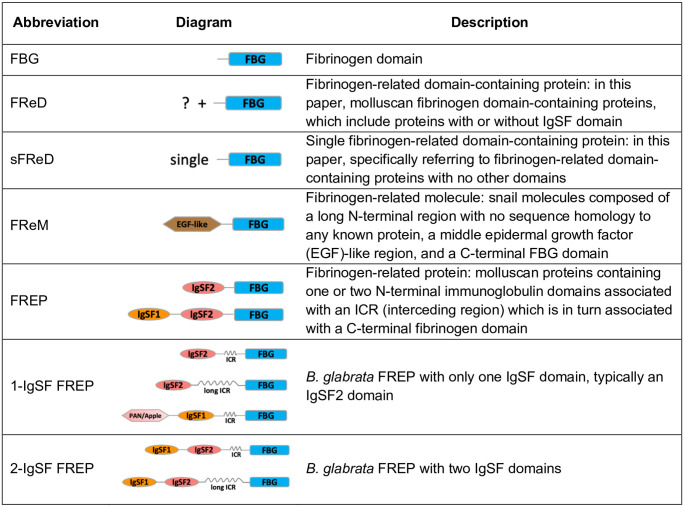
Fig 1. Some relevant abbreviations used in this paper.
Detailed domain sturcture for each abbreviation can be found in Fig 3. FBG: fibrinogen domain; EGF-like: epidermal growth factor (EGF)-like domain; IgSF1: Immunoglobulin Superfamily Member 1; IgSF2: Immunoglobulin Superfamily Member 2; ICR: interceding region (<150aa) between IgSF and FBG domains that form coiled coils; long ICR: long interceding region (150~320aa).
Starting with an initial characterization of 14 B. glabrata FREPs [29,30], the genome-based study of B. glabrata BB02 strain went on to reveal 22 FREP genes, 19 with two tandem IgSF domains and 3 with one IgSF domain [32]. In a study of the de novo assembled transcriptome of the BgBRE strain of B. glabrata, 28 transcripts covered at least IgSF and FBG domains, yielding 8 new FREPs [33], bringing the known total to 30. Also, in an RNA-Seq study of multiple strains of B. glabrata, a FREP-specific transcriptome consisting of 69 transcripts was assembled, and 24 genomic loci potentially encoding for 69 complete or partial FREP transcripts were identified [34], bringing the known number of distinct FREP loci to 32. One of the most distinctive features of FREP biology is, for a particular FREP gene such as BgFREP3.2, to recover variant forms of genomic DNA and mRNA from different cells (such as from the circulating defense cells called hemocytes) from a single snail. These variants derive from characteristic germ-line source sequences through both gene conversion and point mutation processes [35], providing additional FREP diversity [31].
FREPs have drawn attention because of their responsiveness upon exposure to relevant pathogens, especially digenetic trematodes of biomedical significance like S. mansoni. With the exception of the study by Galinier et al. [34], all of these studies pre-date the RNA-Seq era [20,36–44]. As circulating hemolymph lectins, the abundance of FREPs increases in the blood of B. glabrata following exposure to some trematodes. FREPs are also known to bind to sporocysts in a carbohydrate-inhibitable manner and can act as opsonins to facilitate phagocytosis of particles by hemocytes [35]. Microarray studies have also confirmed that a particular FREP, FREP3.2, is up-regulated in B. glabrata known to be resistant to the digenean S. mansoni [39]. Furthermore, use of RNAi to specifically knock-down FREP3.2 expression diminished the resistance shown to either echinostome or schistosome trematode larvae [38,40].
With respect to arriving at a mechanistic understanding of snail susceptibility or resistance, it is known that S. mansoni has a large repertoire of genes encoding polymorphic mucin molecules (SmPoMucs) [45]. Given that these are displayed on the surface of sporocysts, it has been argued that particular combinations of expressed FREPs and SmPoMucs act as determinants of compatibility, thus providing an allele vs. allele matching system that can also help to explain the specificity of compatibility [46]. A recent functional study of several humoral immune factors from B. glabrata revealed that an association forms between B. glabrata FREP3.2 (here confirmed as FREP3.2, AAK28656.1), thioester-containing protein 1 (TEP1), biomphalysin and FREP2. It was observed that FREP3.2 can associate with TEP1 and biomphalysin in both schistosome-susceptible M line (SUS) and resistant BS-90 (RES) snails, but only in RES snails does this immune complex uniquely recruit representatives of FREP2 and of FREP3 distinct to RES snails. One version of FREP3 specifically mentioned in this context was FREP3.3, a FREP locus not yet fully assembled in the current version of the genome [47]. From the earliest studies of FREPs to more recent studies on the binding preferences of FREPs [36,47–50], it has been of interest to learn if and how the FREPs encoded by SUS and RES snails might differ. The approach taken in our study allows us to address this issue.
In addition to FREPs, we are interested in other B. glabrata FReDs. The FBG domain is a conserved component of bioactive molecules in a wide range of taxa [51], and is well-known for its role in coagulation in vertebrates [51]. It is generally considered FBG domains in invertebrates play roles in defense responses [30,52,53]. Invertebrate FBG domains have been discovered in genes involved in 1) recognizing bacterial molecules containing acetyl groups [54–56]; 2) regulating defense-related enzymatic pathways [57,58]; 3) eye and central nervous system development [59–61]; and 4) allorecognition [62,63]. A proteomics study characterizing B. glabrata hemolymph proteins binding to proteins from S. mansoni mother sporocysts [49] found that along with FREPs, sFReDs (Fig 1) could also bind parasite molecules, again suggestive of a role in defense.
From many other studies it is also clear that several humoral factors other than FReDs and FREPs deserve additional consideration for the role they may play in influencing the degree of compatibility between schistosomes and their snail hosts [12,16,17,47,64,65]. We in no way wish to deemphasize their potential importance, but for the purposes of this study, our focus is on FReDs and FREPs. In this study, we developed a novel automated method to accurately identify IgSF domains. Together with traditional IgSF and FBG domain identification methods, we provide the first comprehensive genome-wide investigation of B. glabrata FReDs (including FREPs) structure and function. We explored relationships among B. glabrata FReDs and depict the diversity of IgSF and FBG domains. Expression profiles of FReDs in both RES and SUS strains of B. glabrata, with or without S. mansoni exposure, are documented and related to previous studies relating the role of FREPs in resistance to schistosomes. We also highlight differences in coding regions between key FREP genes between RES and SUS snails.
Methods
FBG domain searches with InterProScan
The FBG domains were identified from protein sequences derived from the B. glabrata BB02 genome reference annotation version 1.6 downloaded from VectorBase (https://www.vectorbase.org/organisms/biomphalaria-glabrata/bb02/bglab16). Initial functional domain searches were performed using InterProScan v5.34–73.0 [66] with databases including TIGRFAM (15.0), SFLD (4), SUPERFAMILY (1.75), PANTHER (14.1), Gene3D (4.2.0), Coils (2.2.1), SMART (7.1), CDD (3.16), PRINTS (42.0), ProSitePatterns (2019_01), Pfam (32.0), ProDom (2006.1) and PIRSF (3.02). Signal peptides were predicted using SignalP 4.1 [67] and trans-membrane domains were predicted using TMHMM 2.0 [68]. Genes containing an FBG domain were identified using a keyword “fibrinogen” search in InterProScan results and confirmed with FBG domain identifications in several different databases of PF00147 (Pfam), SM00186 (SMART), cd00087 (CDD) and SSF56496 (SUPERFAMILY). Domain prediction results with overlapping regions from different programs or databases were resolved based on E-values and predicted length using the tool cath-resolve-hits (https://cath-tools.readthedocs.io/en/latest/tools/cath-resolve-hits/).
IgSF domain searches with new HMM profiles
For comprehensive identification of IgSF domains in the B. glabrata genome, we employed a Hidden Markov Models (HMM) search using customized probabilistic models or HMMs profiles built with IgSF domain sequences identified from an InterProScan search [66,69]. Based on the initial predicted IgSF domain sequences extracted from InterProScan output, IgSF domains were grouped into clusters with less than 30% identity as a cutoff value. For clusters of IgSF domains containing more than two sequences, multiple sequence alignments using Clustal Omega v1.2.4 [70] were performed. Additionally, the profile HMMs using the hmmbuild command in HMMER 3.2.1 [71] was followed. The HMMs profiles were visualized to generate logos using Skylign [72]. Only those HMMs consensus profiles containing an intrachain cysteine loop of 40 aa or greater in size were selected for search against B. glabrata protein sequences using the hmmsearch command in HMMER package [73]. The 40 aa cut-off was chosen because to date all records of Ig domains in Pfam database had a minimum of 41 aa as the size of the cysteine loop. IgSF HMM profiles which hit with conditional E-values (cE-value) less than 0.001 were selected as candidate signatures for IgSF domains. IgSF domains were assigned only if a predicted IgSF signature contained a complete cysteine loop.
Identifying additional FREPs based on genomic context and transcriptome data
The current genome assembly contained some inconsistencies that required consideration of BAC-derived genomic sequence data [38,74]. Furthermore, the genome-wide prediction analysis extended to upstream and/or downstream sequences, yielding more complete sequences for 14 of the previously known FREPs.
Considering the possibility of errors in annotation in the current assembly of the B. glabrata genome, we also manually checked sequences that were identified by the InterProScan and the new IgSF HMM profiles searches against available genomic sequences of FREP genes. This avoided potential errors in automated genome annotation that might call one gene as two separate genes. For each predicted partial gene/protein in this study, the flanking gene’s domain architecture was manually checked for presence of domains that could contribute to a complete FREP structure. Additionally, assembled transcripts from available RNA-Seq studies helped to capture longer or complete versions of particular FREPs, among them transcripts obtained from 12 different B. glabrata organs [32]. The coding sequence of each predicted FReD gene in this study was blasted against the assembled transcripts from the BB02 genome [32] using BLASTn with cut-off value ≥95% identity and aligned length ≥150 nt.
FREP class assignment based on similarity to published FREPs
Protein sequences for FREP candidates were searched using BLASTp against all available FREPs proteins sequences in B. glabrata [30,32–34]. Candidate FREPs were assigned to a particular class using a cut-off value for BLASTp search of ≥90% (e.g. FREP12) or assigned as “class-like” with the cutoff value 85~90% (e.g. FREP4-like).
Genomic location of FReDs in B. glabrata
Scaffold coordinates of FReDs identified in this study were downloaded from VectorBase [75] using the integrated BioMart web tool with B. glabrata VectorBase ID. The genomic distributions of FReDs were illustrated using the R package ggplot2 [76] with genomic location specified for IgSF and FBG domains.
Relationship analysis, domain architectures and gene structures of FReDs
Full-length protein sequences of all identified FReDs were used to construct a maximum likelihood tree through IQ-TREE [77] with standard model selection followed by tree inference of 1000 bootstrap replicates. Conservative FBG domain predictions from InterProScan and of IgSF domains from new HMM profiles were merged using the cath-resolve-hits tool [78]. Domain architectures and gene structures of FReDs were drawn along the FReD gene tree using R packages ggplot2 v3.2.1 [76], ggtree v1.14.6 [79] and ggpubr v0.2.2 [80].
Search for FReDs with binding affinity to early developing larval S. mansoni proteins within susceptible and resistant B. glabrata snails
Wu et. al (2017) identified peptides from plasma of SUS (NMRI) and RES (BS-90) B. glabrata strains eluted from affinity columns enriched with S. mansoni sporocyst membrane- and larval transformation proteins [49]. All data for sporocyst-bound snail peptides from Wu et al. [49], were blasted against predicted FReD proteins from this study. Matches of full peptide sequences with 100% identity from either strain of B. glabrata were identified and highlighted on our gene structures of the FReD proteins.
Pairwise BLAST identity analysis of IgSF1, IgSF2, or FBG domains in B. glabrata
To compare sequence diversities within IgSF1, IgSF2, or FBG domains from B. glabrata FREPs, we applied pairwise blast comparisons. Only complete FReD genes with an ATG start codon (Methionine, M), a complete FBG domain and stop codon were included in this analysis. Separate pairwise blast identity analysis of all IgSF1, IgSF2 or FBG domain protein sequences from 2-IgSF FREPs were calculated based on all-against-all BLASTp top hits. The symmetric distance of tree topology was measured [81] and calculated and visualized with webtool Phylo.io [82]. Violin plots within R [83] were generated to compare sequence diversity of FBG and IgSF domains.
Analysis of association of IgSF and FBG domains in B. glabrata
To investigate the association of IgSF domains with one another in 2-IgSF FREPs, or the associations of IgSF domains with FBG domains in FREPs, maximum likelihood trees were built using IQ-TREE with available protein sequences for IgSF1, IgSF2, and FBG domains. First, all IgSF domain sequences generated with new HMM profiles were used to build a tree to evaluate the ancestral associations among all IgSF domains in B. glabrata. The two IgSF domains from 2-IgSF FREP genes (IgSF1 and IgSF2) were highlighted in the tree. Secondly, protein sequences of FBG, IgSF1 and IgSF2 domains of 2-IgSF FREPs were used separately to build a tree of IgSF1 vs. IgSF2, matched tree of IgSF1 vs. FBG, and IgSF2 vs. FBG.
Snail infection experiments and RNA-Sequencing study
To investigate the expression levels of FReDs in B. glabrata snails with or without S. mansoni exposure, a comprehensive transcriptomics analysis was carried out. Snails and experimental treatments used in this study are described in Lu et al. [84]. Briefly, uninfected juveniles (5-8mm in diameter) of lab-reared RES (BS-90) and SUS (M line) B. glabrata were exposed individually to 20 PR1 strain S. mansoni miracidia per snail. Control snails were treated similarly but were not exposed to S. mansoni miracidia. Snails of each group were reared in aerated aquaria containing at 25–27 °C and fed with lettuce [18]. At 0.5-, 2-, 8- and 40-days post-exposure (dpe), snails were collected and individually preserved in TRIzol reagent (Invitrogen) and stored at -80 °C until extraction. Biological replicates (3~6/group) were paired-end sequenced (2x 150 base reads) for each group using an Illumina NextSeq 500 instrument (Illumina, Carlsbad CA) at the Molecular Biology Facility, Biology Department, the University of New Mexico. Each replicate represents one snail sample. There were 56 snails used in this RNA-Seq study. An overview of snail samples per group and sequencing information is summarized in S1 Table. See Lu et al. [84] for a detailed description of sample collection, RNA and DNA extraction, library preparation, and differential gene expression analyses. Genes were considered differentially expressed in comparisons with cutoff values using fold change (FC) ≥2 (absolute log2FC ≥1), and a posterior probability of differential expression (PPDE) ≥0.95 for EBSeq analysis. Although some genes are differentially expressed in comparisons (absolute log2FC ≥1), they may not have been considered DE due to variations of replicates with PPDE <0.95. The raw RNA sequencing data are available at NCBI under SRA accession: PRJNA591872.
Analysis of variant transcriptome sequences for some B. glabrata FREPs
Transcriptome variations including single nucleotide variants (SNV) were called using bcftools 1.9 (http://samtools.github.io/bcftools/) based on the pooled high quality RNA-Sequencing reads from both SUS and RES B. glabrata, followed by mapping to the B. glabrata BB02 reference genome [32]. The SNV analysis focused on coding regions of FREP genes of particular interest and used cut-off scores of quantity >10 and quality scores of filtering variants of >20. All variations were annotated and classified into categories including synonymous, nonsynonymous, splice region variants (a genetic alteration in the DNA sequence that occurs at the boundary of an exon and an intron, splice site), and stop gained variants by using Ensembl Variant Effect Predictor [85]. For SNVs within exons, homologous coding sequences for SUS and RES strains were determined mainly based on synonymous and nonsynonymous variants, and then translated to protein sequences. For each FREP gene of interest, protein sequences from the reference BB02 genome, and inferred homologous SUS and RES strain sequences were aligned together using the multiple sequence alignment program MAFFT [86] and visualized using JalView 2.11.0 [87]. Additionally, sequences of peptides of BgFREP2 and BgFREP3.3 from the plasma of SUS and RES strains obtained by liquid chromatograph-tandem mass spectrometry (LC-MS/MS) following different pull-down experiments [47], and sequences of peptides from the protomics study by Wu et al. (2017) [49] were compared to the deduced protein sequences we obtained.
Other analyses
Table summary and figure generation were performed within R [88] and Bioconductor [89], including the following packages: ggplot2 [76], reshape2 [90], and openxlsx v4.1.0.1 [91]. Intermediate data analysis was done with an in-house parallel pipeline facilitated with GNU Parallel tool [92].
Results
The definitions and abbreviations provided in Fig 1 apply below. We abbreviate the Vectorbase ID of genes to the extent possible for the sake of readability: for instance, “BGLB000152” is referred to as “Bg152”, “BGLB000011” is referred to as “Bg11”, and so on. With respect to the alternative splicing in B. glabrata FReDs, multiple proteins encoded by the same gene were differentiated by protein IDs, with “-PA”, “-PB”, “-PC”, “-PD” or “-PE” suffix following the gene ID. In this study, some gene/protein sequences extended by manual checking are referred to as, for instance, “Bg152*”/ “Bg152-PA*” to differentiate them from the original version of “Bg152”/ “Bg152-PA” in Vectorbase.
The criteria for “complete” FREP and sFReD were set differently here because whereas little is known about sFReDs and their complete structures, FREPs are known to be secreted proteins and require a signal peptide. We defined a “complete FREP” as having a signal peptide, start codon, stop codon, a complete FBG domain, and 1 or 2 IgSF domain(s) each with cysteine loop >40 aa. If any of the above components could not be verified, a FREP was considered to be a “partial” FREP. We defined a “complete sFReD” as having a start codon, stop codon and a complete FBG domain. The FBG domain begins with a SCRDV like motif, contains prosite motif PS00514 W-W-[LIVMFYW]-x(2)-C-x(2)-[GSA]-x(2)-N-G, and a EMKIRE like motif just preceding a stop codon, according to the FBG domains initially described in FREPs in B. glabrata [20]. Similary, if any of these components could not be verified, a particular sFReD was considered to be a “partial” sFReD.
Prediction of FBG and IgSF domains in B. glabrata
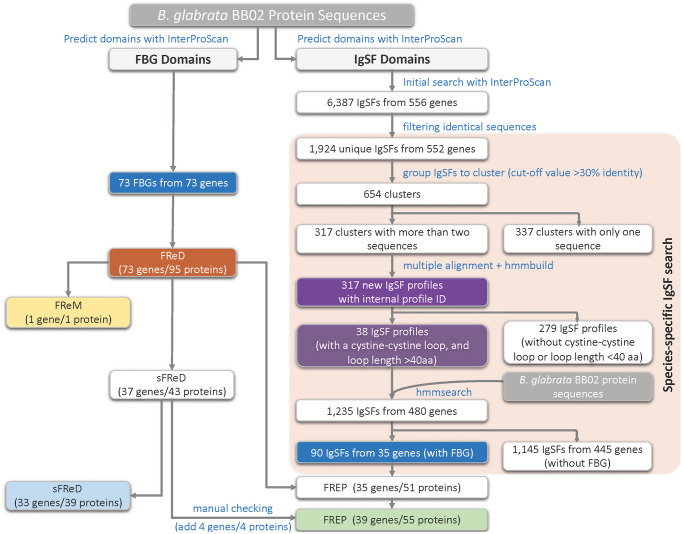
InterProScan was used to detect both FBG and IgSF domains in the genome assembly (Fig 2). As shown on the left side of the figure, a total of 73 genes with an FBG domain were found, estimated to encode 95 different proteins (through alternative splicing). Among the 73 genes, 34 did not contain an IgSF domain, of which, 33 genes were designated as sFReDs, and one as a FReM. The remaining 39 genes, including four confirmed via manual checking (S2 Table), were associated with an IgSF domain so fit the definition of being FREPs. The pipeline for identification of IgSF domains is shown on right side of Fig 2. We identified 35 FREP genes encoding 51 proteins (through alternative splicing) with this approach, again harmonized to 39 based on manual checking (green bar at bottom of Fig 2). The 39 FREPs are summarized in Table 1 including extent of completeness, number of IgSF domains, and published or newly identified in this study (S1 Fig).
Fig 2. Workflow to predict FBG and IgSF domains in the genome assembly of BB02 B. glabrata.
The colored boxes highlight some of the important steps/results in the workflow.
Table 1. Summary of 39 FREPs discussed in this study.
| Previously reported FREPs | Newly identified FREPs | Total | |||
|---|---|---|---|---|---|
| 1-IgSF | 2-IgSFs | 1-IgSF | 2-IgSFs | ||
| Complete | 6* | 19* | 1 | 0 | 26 |
| Partial | 1 | 6 | 4 | 2 | 13 |
| Total | 7 | 25 | 5 | 2 | 39 |
| 32 | 7 | ||||
*: Some previously published FREPs were extended in this study as complete FREPs.
Summary of predicted FReDs in B. glabrata genome
Additional details for the 95 proteins predicted for the 73 FReD genes (through alternative splicing) identified are provided in Table 2 and S2 Table. Genes with absence of an ATG start codon or stop codon were designated as “partial.” One partial FReM gene (Bg40437) is confirmed, which is composed of a N-terminal region, a middle epidermal growth factor (EGF)-like region, and a partial C-terminal FBG domain (missing C-terminal EMKIRE like motif of FBG,) [43].
Table 2. Summary of predicted FReD proteins in B. glabrata BB02 strain.
| ID in text | NCBI annotation | Type of FReD | Best hit to published FREP |
|---|---|---|---|
| Bg11-PA | Fibrinogen-like protein A, partial | partial 1-IgSF FREP | 11_sig_1434_BGLB000011 |
| IBg11-PB | Fibrinogen-like protein A, partial | 1-IgSF FREP | 11_sig_1434_BGLB000011 |
| Bg19-PA | Fibrinogen C domain-containing protein 1-B-like | 2-IgSF FREP | FREP5 |
| Bg19-PB | Fibrinogen C domain-containing protein 1-B-like | 2-IgSF FREP | FREP5 |
| Bg21-PA | Fibroleukin-like | partial 2-IgSF FREP | 17_tig_2702_BGLB000021 |
| Bg21-PB | Fibroleukin-like | 2-IgSF FREP | 17_tig_2702_BGLB000021 |
| Bg74-PA | Uncharacterized protein LOC106071720, partial | partial 2-IgSF FREP | FREPJ4 |
| Bg74-PB | Uncharacterized protein LOC106071720, partial | partial 2-IgSF FREP | FREPJ4 |
| Bg96-PA | Fibroleukin-like | 2-IgSF FREP | FREP3.1 |
| Bg96-PB | Fibroleukin-like | 2-IgSF FREP | FREP3.1 |
| Bg100-PA | Angiopoietin-4-like | 2-IgSF FREP | 14_tig_2094_BGLB000100 |
| Bg100-PB | Angiopoietin-4-like | 2-IgSF FREP | 14_tig_2094_BGLB000100 |
| Bg116-PA | Uncharacterized protein LOC106057350 | 2-IgSF FREP | 1_tig_99_BGLB000116 |
| Bg116-PB | Uncharacterized protein LOC106057350 | 2-IgSF FREP | 1_tig_99_BGLB000116 |
| Bg131-PA | --- | partial 2-IgSF FREP | z_tig_6870_frameshifts |
| Bg133-PA | Angiopoietin-2-like | 2-IgSF FREP | 22_tig_4104_BGLB000133 |
| Bg133-PB | Angiopoietin-2-like | 2-IgSF FREP | 22_tig_4104_BGLB000133 |
| Bg140-PA | Fibroleukin-like | 2-IgSF FREP | FREP13.1 |
| Bg140-PB | Fibroleukin-like | 2-IgSF FREP | FREP13.1 |
| Bg141-PA | Fibrinogen-like protein A | 1-IgSF FREP | FREPA |
| Bg141-PB | Fibrinogen-like protein A | 1-IgSF FREP | FREPA |
| Bg152-PA | Fibrinogen C domain-containing protein 1-like | 1-IgSF FREP | FREP4 |
| Bg152-PB | Fibrinogen C domain-containing protein 1-like | 1-IgSF FREP | FREP4 |
| Bg179-PA | Fibrinogen beta chain-like | 1-IgSF FREP | FREP1 |
| Bg179-PB | Fibrinogen beta chain-like | partial 1-IgSF FREP | FREP1 |
| Bg204-PB | Tenascin-R-like | 2-IgSF FREP | FREP3.2 |
| Bg4529-PB | Uncharacterized protein LOC106059401 | 2-IgSF FREP | FREP7-like |
| Bg4530-PB | Fibrinogen C domain-containing protein 1-B-like | 2-IgSF FREP | FREPJ2-like |
| Bg5224-PA | Uncharacterized protein LOC106061038 | partial 2-IgSF FREP | FREPJ5 |
| Bg5224-PB | Uncharacterized protein LOC106061038 | 2-IgSF FREP | FREPJ5 |
| Bg6034-PB | Uncharacterized protein LOC106062908 | partial 2-IgSF FREP | FREP12.1-as2-like |
| Bg7076-PB | Angiopoietin-related protein 2-like | 2-IgSF FREP | 4_tig_565_BGLB000073-like |
| Bg7576-PB | Uncharacterized protein LOC106066900 isoform X2 | 2-IgSF FREP | FREPM |
| Bg7576-PC | Uncharacterized protein LOC106066900 isoform X1 | 2-IgSF FREP | FREPM |
| Bg10948-PB | Uncharacterized protein LOC106074953 | partial 2-IgSF FREP | FREPK1 |
| Bg11626-PA | Angiopoietin-related protein 2-like | partial 2-IgSF FREP | FREP12-like |
| Bg11626-PB | Angiopoietin-related protein 2-like | 2-IgSF FREP | FREP12-like |
| Bg11627-PA | Angiopoietin-related protein 2-like | partial 1-IgSF FREP | 5_tig_565_BGLB011626 |
| Bg11627-PB | Angiopoietin-related protein 2-like | 2-IgSF FREP | 5_tig_565_BGLB011626 |
| Bg14428-PB | Ryncolin-4-like | 1-IgSF FREP | FREP14 |
| Bg16934-PA | Fibrinogen beta chain-like | 2-IgSF FREP | 20_tig_3569_BGLB009245b |
| Bg16935-PA | Angiopoietin-4-like | 2-IgSF FREP | 19_tig_3569_BGLB009245a |
| Bg17688-PA | Fibrinogen beta chain-like | 2-IgSF FREP | zb_tig_15796 |
| Bg17893-PA | Uncharacterized protein LOC106053933 | 2-IgSF FREP | 6_tig_990_BGLB014388 |
| Bg20516-PA* | Fibrinogen C domain-containing protein 1-like | partial 2-IgSF FREP | new partial FREP |
| Bg22646-PA | Fibrinogen C domain-containing protein 1-B-like, partial | partial 1-IgSF FREP | new partial FREP |
| Bg23857-PA | Fibrinogen C domain-containing protein 1-like, partial | partial 1-IgSF FREP | new partial FREP |
| Bg25144-PA* | Ficolin-1-like | partial 2-IgSF FREP | new partial FREP |
| Bg25334-PA | Fibrinogen C domain-containing protein 1-like | partial 1-IgSF FREP | FREPJ5 |
| Bg28617-PA | Fibrinogen-like protein A | 1-IgSF FREP | FREP2 |
| Bg29167-PA | Tenascin-like | partial 1-IgSF FREP | new partial FREP |
| Bg32905-PA* | Ficolin-2-like | partial 2-IgSF FREP | FREPJ1 |
| Bg38205-PA | Angiopoietin-4-like | partial 1-IgSF FREP | new partial FREP |
| Bg40226-PA* | Techylectin-like protein | Partial 2-IgSF FREP | FREPJ3-like |
| Bg40228-PA | Fibroleukin-like | 1-IgSF FREP | new FREP |
| Bg177-PA | Microfibril-associated glycoprotein 4-like | sFReD | |
| Bg177-PB | Microfibril-associated glycoprotein 4-like | sFReD | |
| Bg178-PA | Fibrinogen C domain-containing protein 1-like | sFReD | |
| Bg178-PB | Fibrinogen C domain-containing protein 1-like | sFReD | |
| Bg2450-PB | Ryncolin-1-like | sFReD | |
| Bg2494-PB | Ficolin-2-like isoform X1 | sFReD | |
| Bg2494-PC | Ficolin-1-like isoform X3 | sFReD | |
| Bg2494-PD | Ficolin-2-like isoform X2 | sFReD | |
| Bg2494-PE | Ficolin-1-like isoform X4 | partial sFReD | |
| Bg5196-PB | Angiopoietin-related protein 1-like | partial sFReD | |
| Bg12039-PB | Ficolin-1-like, partial | partial sFReD | |
| Bg12226-PB | Techylectin-5B-like | partial sFReD | |
| Bg12382-PB | Angiopoietin-4-like | partial sFReD | |
| Bg14428-PA | Ryncolin-4-like precursor | partial sFReD | FREP14 |
| Bg16307-PA | Ficolin-2-like | sFReD | |
| Bg16436-PA | Angiopoietin-related protein 6-like | partial sFReD | |
| Bg17041-PA | Fibrinogen C domain-containing protein 1-like | sFReD | |
| Bg17605-PA | Ficolin-2-like | sFReD | |
| Bg18336-PA | Fibrinogen-like protein A | sFReD | za_tig_4714 |
| Bg20351-PA | Ficolin-2-like | sFReD | BgMFREP4_AY012701-like |
| Bg21912-PA | Ficolin-2-like | sFReD | |
| Bg22867-PA | Fibrinogen beta chain-like, partial | partial sFReD | |
| Bg25627-PA | Ficolin-1-like | partial sFReD | |
| Bg25643-PA | Microfibril-associated glycoprotein 4-like | sFReD | |
| Bg28385-PA | Ficolin-2-like | sFReD | |
| Bg29288-PA | Uncharacterized protein LOC106051237 | partial sFReD | |
| Bg30478-PA | Fibroleukin-like, partial | partial sFReD | |
| Bg30830-PA | Ryncolin-1-like, partial | partial sFReD | |
| Bg31381-PA | Ficolin-1-like | sFReD | |
| Bg31596-PA | Angiopoietin-related protein 6-like, partial | partial sFReD | |
| Bg34525-PA | Techylectin-5A-like | partial sFReD | |
| Bg36251-PA | Ficolin-2-like | sFReD | |
| Bg37621-PA | Angiopoietin-1-like | partial sFReD | |
| Bg38117-PA | Fibrinogen C domain-containing protein 1-like, partial | partial sFReD | |
| Bg38186-PA | Ficolin-1-like | sFReD | |
| Bg38723-PA | Ficolin-1-like | sFReD | |
| Bg39918-PA | Ficolin-2-like | sFReD | |
| Bg40034-PA | Ficolin-1-like, partial | partial sFReD | 16_tig_2402_BGLB007591-like |
| Bg40339-PB | Ficolin-2-like | sFReD | |
| Bg40437-PA | Uncharacterized protein LOC106061221 | partial FReM | FReM |
For readability, the dashed lines in Table 2 separate the three types of FReDs: FREP, sFReD, or FReM.
Notes:
In the column of “ID in text”, multiple proteins encoded by the same gene due to alternative splicing were differentiated by different version of protein, such as “-PA”, “-PB”, “-PC”, “-PD” or “-PE” suffix following the gene ID;
In the column of “NCBI Annotation”, “---” represents no available annotation for a particular protein;
In the column of “Type of FReD”, each particular FReD was considered as complete or partial based on the criteria set in this study above; “1-IgSF FREP” and “2-IgSF FREP” represent the number of IgSF domains present in a particular FREP;
“Best hit to published FREP” represent the best hit of individual protein against published B. glabrata FREP sequences from available sources [30,32–34]: best hit of protein sequence identity ≥90% is considered as the same FREP; identity 85~90% is considered as “-like” of the corresponding FREP; identity <85% is not assigned to any known FREP.
In the “Best hit to published FREP” column, several FREPs were emphasized in this study were highlighted in yellow.
Of the 39 FREP genes (Fig 2, Table 1), 26 were considered as complete and 13 were partial. In addition to FREPs noted in previous studies [30,32–34], two new FREPs were found. The first one is a 1-IgSF FREP (Bg40228) and has an ATG start codon, a signal peptide of 19 aa, a complete IgSF1 (114 aa), a truncated IgSF2 (only 26 aa), an interceding region, and a full length FBG (203 aa) domain, which is considered as a complete “new FREP”. The second is a partial 2-IgSF FREP (Bg20516) that lacks an ATG start codon, but is otherwise complete with a 23 aa signal peptide, and complete domains of IgSF1 (114 aa), IgSF2 (120 aa), an ICR, and FBG (202 aa), which is considered as a “new partial FREP”. Five more genes with both FBG and IgSF domains were found for the first time, but no signal peptides could be identified for these genes in the current assembly. These five genes are designated as “new partial FREP” in Table 2 and await future study.
In addition, we also extended or clarified 18 previously known FREP sequences. For instance, 26aa including a signal peptide were added to the N-terminus of Bg179. The protein sequences for some previously reported FREPs were extended to include N- or C-termini. (Table 1). These improvements provided signal peptides for 10 published FREPs and FBG or IgSF2 domain sequences for 5 published FREPs (see more details in S3 Table). We note that FREP3.3 [47] is not on our list because its key portions were found on different scaffolds, precluding full annotation. The sequence and structure of this gene may be completed with future improved genome assembly.
Summary of domain architecture of FReDs in B. glabrata
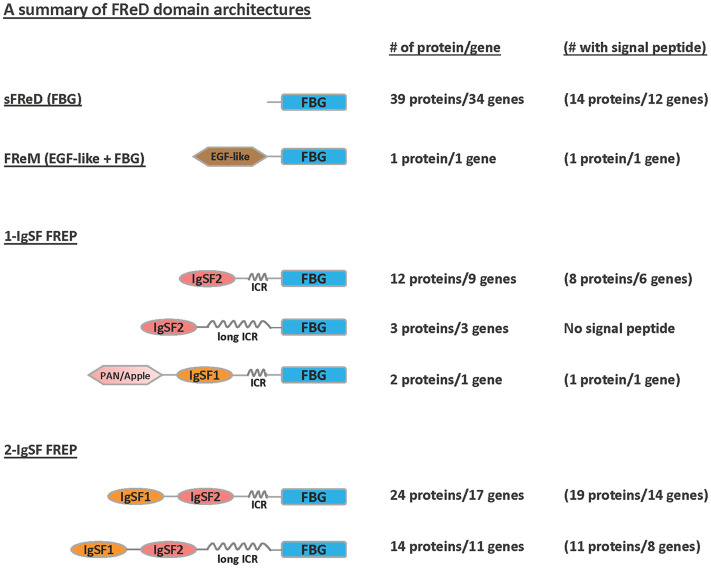
Some FReD genes may encode proteins with more than one type of domain architecture (Fig 3). The PAN/Apple domain in a 1-IgSF FREP gene (Bg179) previously identified in B. glabrata genome [32] was confirmed here. This FREP gene is unusual for containing an IgSF1 domain next to the ICR rather than the IgSF2 domain typically found in this location. It is noticed that long sequences of coiled coils (150~320aa) were detected between IgSF and FBG domains in some FREPs and are considered as long interceding regions (long ICR) in the present study.
Fig 3. Predicted domain architectures of FReD proteins in B. glabrata BB02.
Three main categories are indicated: sFReD (FBG), FReM (EGF-like + FBG), 1-IgSF FREP (1 IgSF loop + FBG), and 2-IgSF FREP (2 IgSF loops + FBG). FBG: fibrinogen domain; EGF-like: epidermal growth factor (EGF)-like domain; PAN/Apple: PAN/Apple domain; IgSF1: Immunoglobulin Superfamily Member 1; IgSF2: Immunoglobulin Superfamily Member 2; ICR: interceding region (<150aa) between IgSF and FBG domains that form coiled coils; long ICR: long interceding region (150~320aa).
Analysis of relationships among B. glabrata FReD genes
To compare the 73 FReD genes predicted in B. glabrata, a gene tree with full length sequences of the FReDs was constructed (Fig 4A). As the sequences being compared vary considerably in length, the general purpose of this tree was to show a broad overview of relationships among all FReDs (alternative version of the tree showing only FBG domains is provided in S2 Fig). For each gene, the predicted domain structure (Fig 4B) and gene exon intron structure (S4C Fig) of representative protein is also provided. Two 1-IgSF FREPs, Bg28617 (FREP2) and Bg14428 (FREP14), were both found to have only one exon (S4C Fig). Although poly-A tails were not found within the exon for either FREP, the presence of a single exon coding for both IgSF2 and FBG domain suggests a history of retrotransposition for both.
Fig 4. Gene tree and representative protein domain architecture of 73 B. glabrata FReDs.
A. Maximum likelihood tree with 1000 bootstrap tests was constructed using full length representative protein sequences of 73 FReD genes in B. glabrata. Nodes with bootstrap support of 75 or higher are marked with black squares. The gene IDs of the 73 FReDs were followed with number of IgSF domains and the best hit with any published FREP genes (BLASTp hit with at least 150 aa aligned with ≥ 90% identity was considered as the same FREP; or considered a “FREP-like” gene if identity was 85% ~ 90%; identity <85% was not assigned to any published FREP). Any FReD genes with best hit to FREPs from the genome paper [32] are labeled with a red dot at the corresponding gene ID. Complete FREP or FReD genes fitting the criteria in this study were highlighted in blue text. B. Domain architectures were predicted using InterProScan and lineage specific HMM models. “Peptide sequences” represent multiple peptide sequences extracted from a proteomics study [49]. Colored small horizontal bars above or below some protein domain structures highlight the matched location of peptide sequences with binding affinity to S. mansoni sporocyst membrane-enriched and larval transformation proteins, from either schistosome-susceptible NMRI strain (SUS in green) or -resistant BS-90 strain (RES in red) of B. glabrata. The dashed gray line was manually added to distinguish most “sFReD clades” from “FREP clades”.
As expected because of the manner of tree construction, most sFReDs grouped together (see above the dashed line which is included as a convenient reference point) as did most FREPs (below the line). We noted that 11 sFReD genes above the dashed line were located near (less than 5kb) an edge of a scaffold within the genome assembly (S3 Fig). Although some of them have a predicted signal peptide or ATG start codon, we cannot rule out the possibility that some sFReDs are actually part of complete FREP genes, which contain upstream IgSF domains located on another scaffold. One such example is sFReD gene Bg40034 which was located on a 1.12kb scaffold (S3 Fig).
A single 1-IgSF FREP (Bg141) falls above the dashed line nesting among sFReDs. This FREP is divergent from others in at least two ways. Its IgSF2 domain has only about 30% protein sequences identity to the IgSF2 domains in other FREPs, placing it in the lowest quartile (Q1 = 37.6%) of all IgSF2 pairwise comparisons in FREPs (Fig 5C, S4 Table). Similarly, its FBG domain had ~60% identity protein sequences to FBG domains in all other FREPs, also placing it in the lowest quartile (Q1 = 64%) of all FBG pairwise comparisons in FREPs (Fig 5D, S4 Table). Also, 6 sFReD genes and the FReM gene (Bg40437) fall among the FREPs (below the dashed line) and as they are not near scaffold edges, would appear not to be parts of FREPs yet to be fully resolved. Their scattered locations on the tree suggest separate origins or derivations from the FBG domains of neighboring FREPs.
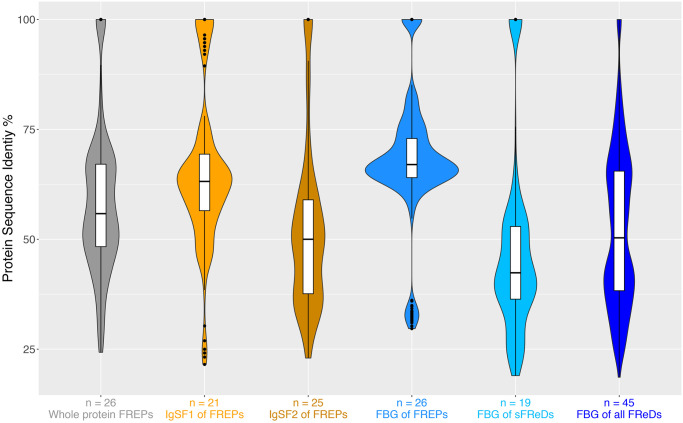
Fig 5. Violin plots of protein sequence-pairwise identity analyses of IgSF1, IgSF2, and FBG domains in B. glabrata FReD genes.
The 26 complete FREP protein sequences, and complete FBG domain sequences from all FReD genes in B. glabrata were used for pairwise blast identity analysis. Number under each Violin plot at x-axis is the number used for each assigned analysis, and y-axis represents percentage of protein sequence identity (%).
Highlighting some of the better-known FREPs, there is some tendency for those 1-IgSF FREPs to cluster together (note especially FREP1, 2 and 4). Similarly, FREP3.1, 3.2, 5, 7 and 13 (2-IgSF FREPs) formed a cluster on the tree. Noteworthy is that the remaining FREPs were scattered amongst several clusters on this tree suggesting separate orgins and functionalities.
Peptide sequences match analysis
Among the predicted protein domains in Fig 4B, 40 proteins encoded by 30 FReD genes have absolute matches to peptide sequences derived from whole plasma proteins of SUS (NMRI) or RES snails found to bind to early developing S. mansoni sporocyst tegmental membrane proteins or larval transformation proteins [49]. Here it should be noted that the peptides retrieved do not necessarily comprise the exact region of the proteins binding to the sporocysts, but are the ones successfully retrieved following their sampling protocol [49]. These peptides matched several of our predicted FBG, IgSF1, IgSF2, regular ICR, or long ICR regions, particularly so for FBG domains. Several peptides along much of the length of Bg38205 were retrieved only from SUS snails whereas in RES snails, two peptides found in the IgSF1 and IgSF2 regions of FREP3.2 were distinctive (Fig 4).
Summary of pairwise BLAST identity analyses of IgSF1, IgSF2, and FBG domains in B. glabrata
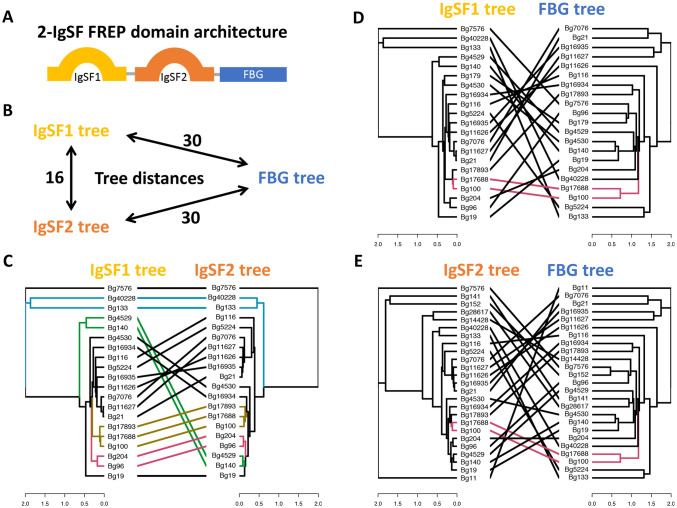
To trace the associations of IgSF1, IgSF2 and FBG domains, 19 available complete 2-IgSF FREPs (Table 1) with all three domains were selected (Fig 6A and 6B).
Fig 6. Domain tree comparisons of IgSF1, IgSF2 and FBG domains from complete 2-IgSF FREPs in B. glabrata. A.
Domain structure of 2-IgSF FREPs; B. Tree distances were measured by Robinson-Foulds or symmetric distance among trees of IgSF1, IgSF2 and FBG domains from 2-IgSF FREPs; C. Tanglegram plot of a side by side ML (Maximum Likelihood) trees for IgSF1 and IgSF2 domains from 2-IgSF FREPs; D. Tanglegram plot of ML trees for IgSF1 and FBG domains from the 2-IgSF FREPs; E. Tanglegram plot of ML trees for IgSF2 and FBG domains from the 2-IgSF FREPs. The colored connection lines in 6C, 6D and 6E highlighted some common subtrees.
The symmetric distance between the IgSF1 tree and the IgSF2 tree (16) is closer than either the distance between IgSF1 tree and FBG tree (30) or the distance between IgSF2 tree and FBG tree (30), measured by Robinson-Foulds distances. In Fig 6C, 6D, and 6E, separate trees were built based on the protein sequences of IgSF1, IgSF2, or FBG domains. Such trees for pairs of domains are shown side-by-side to highlight discordant relationships between two domains from the same FREP. More concordance is seen between IgSF1 vs. IgSF2 trees (Fig 6C) than between IgSF1 vs. FBG trees (Fig 6D) or IgSF2 vs. FBG trees (Fig 6E).
To compare sequence diversity of FBG, IgSF1 and IgSF2 domains in FReD genes, violin plots (Fig 5) were generated for complete FReD genes (S3 Table) based on the criteria in this study (Fig 1). Specifically, protein sequences for IgSF1, IgSF2 and FBG domains from the 26 complete FREPs, and complete FBG domain sequences from sFReD genes were analyzed with pairwise blast identity. Median values of FBG domain pairwise blast identity is 66.99 (S4 Table), significantly higher than for IgSF1 (63.16, non-parametric Mann–Whitney U test p = 2.02−8 or IgSF2 (50.0, p = 2.99−41), consistent with earlier observations [26,29,31]. FBG domains from FREPs were significant more conserved with respect to protein sequence identity than sFReDs (p = 9.41−40). See more details in S4 Table.
It is also noteworthy that there are 9 “outliers” with high pairwise identities in the violin plot of IgSF1 (Fig 5B). Some of the “outliers” are among a subgroup of 2-IgSF FREPs with long ICRs that clustered closely on the FReD gene tree (Fig 4A), including Bg21, Bg7076, Bg11626, Bg11627, and Bg16935.
Subfamilies of IgSF domains in B. glabrata
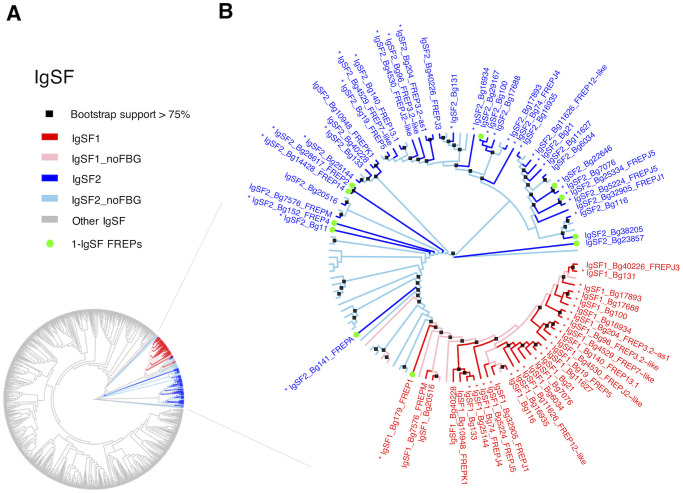
An unrooted Maximum Likelihood (ML) tree (Fig 7A) was generated based on all 38 IgSF new HMM profiles from the workflow in Fig 2. The two FREP-associated IgSFs (IgSF1 in red and IgSF2 in blue) clearly separated from the gray branches, which represent 1,145 IgSFs (445 genes) predicted from the 36 non-FREP-IgSFs HMM profiles (Fig 2).
Fig 7. Unrooted Maximum Likelihood (ML) trees of IgSF domains identified in B. glabrata with new HMM profile search.
A: ML tree with all IgSF domains from search using the 38 new IgSF HMM profiles (in Fig 2). Gray branches in panel A represent other IgSFs predicted with 36 new HMM profiles, which are not associated with FBG domain; B: An ML tree for only IgSF1 and IgSF2 domains from B. glabrata. ML trees with 1000 bootstrap tests were constructed using IQ-TREE. Nodes with bootstrap support of 75 or higher were highlighted with black squares at the nodes in panel B. Any domains associated with FBG domain are colored in red (IgSF1) or in blue (IgSF2) and followed with name of best hit to published FREPs. IgSF1 or IgSF2 domains without association with FBG domain are colored in pink (IgSF1) or in light blue (IgSF2). Gene has been identified previously in the genome paper [32] is labelled with an asterisk next to the corresponding gene ID. 1-IgSF FREPs are labeled with a green dot at the end of the branches. All FREPs identified in this study were named with IgSF domain, the Vectorbase ID and the best hit to published FREPs.
IgSF1 and IgSF2 domains were used to generate a more detailed ML tree in Fig 7B. IgSF1 and IgSF2 domains were again clearly separable. Care is required in these interpretations because some of the IgSF2 domains not associated with FBG domains may reflect incomplete or erroneous annotation, or could be from other groups of molecules like CREPs for instance [33]. Notable is the fact that the majority of extant FREP genes (27 of the 39) have both IgSF1 and IgSF2 domains. With respect to IgSF1 domains, the peculiar FREP1, the only FREP to have a single IgSF domain that is of the IgSF1 category, falls on the outside of the IgSF1 group. Most of the remaining IgSF1 domains, all coming from 2-IgSF FREPs, cluster more closely together, suggestive of a separate diversification. With respect to IgSF2 domains, a cluster suggestive of a separate diversification is observed including all those FREPs such as FREPs 3, 5 and 7 known to be 2-IgSF FREPs. The IgSF2 domains associated with those 1-IgSF FREPs (FREPs 2 and 4 for instance) fall in adjacent, separate groups. As supported by Fig 6C, it suggests IgSF1 and 2 domains may have diversified in parallel in those 2-IgSF FREPs.
Comparisons of expression levels of FReD genes in SUS (M line) or RES (BS-90) B. glabrata: Constitutive and post-exposure responses to S. mansoni
Transcriptomic profiles of B. glabrata snails not exposed to S. mansoni were considered as representative of constitutive gene expression. For such snails, using SUS snails as a baseline, there are three time-matched comparisons with RES snails: 0.5-, 2- and 40 days (Table 3). The first two time points represent juvenile snails and the third represents sexually mature snails. The overall constitutive expression profiles for the different FReDs are mixed between the two snail strains. Four FReD genes (2 FREPs and 2 sFReDs) were constitutively higher in RES than SUS snails in at least 2 of the 3 comparisons, and 6 FReD genes (5 FREPs and 1 sFReDs) were constitutively higher in SUS than RES snails in at least 2 of the 3 comparisons.
Table 3. Summary of expression levels of FReD genes in RES and SUS B. glabrata: Constitutive and post-exposure responses to S. mansoni.
| ID in text | Unexposed RES vs. Unexposed SUS | RES exposed to S. mansoni | SUS exposed to S. mansoni | Type of FReD | Best hit to known FREP | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.5d | 2d | 40d | 0.5d | 2d | 8d | 40d | 0.5d | 2d | 8d | 40d | |||
| Bg11 | ns | ns | ns | 1.31 | ns | 1 | ns | ns | ns | ns | ns | 1-IgSF FREP | 11_sig_1434_BGLB000011 |
| Bg19 | ns | ns | -2.16 | ns | ns | ns | ns | ns | ns | ns | ns | 2-IgSF FREP | FREP5 |
| Bg74 | ns | 1.51 | ns | ns | ns | ns | ns | ns | ns | ns | ns | partial 2-IgSF FREP | FREPJ4 |
| Bg96 | ns | 1.06 | ns | ns | 1.86 | 1.09 | ns | ns | ns | ns | ns | 2-IgSF FREP | FREP3.1 |
| Bg116 | ns | -2.11 | ns | 1.95 | 3.64 | ns | ns | ns | ns | ns | ns | 2-IgSF FREP | 1_tig_99_BGLB000116 |
| Bg131 | ns | -1.74 | -2.52 | ns | ns | ns | ns | ns | ns | ns | ns | partial 2-IgSF FREP | z_tig_6870_frameshifts |
| Bg133 | 1.8 | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | 2-IgSF FREP | 22_tig_4104_BGLB000133 |
| Bg141 | ns | ns | 3.18 | ns | ns | ns | ns | ns | ns | ns | ns | 1-IgSF FREP | FREPA |
| Bg152 | ns | ns | ns | 2.93 | 9.18 | 4.91 | 3.37 | -5.59 | ns | ns | ns | 1-IgSF FREP | FREP4 |
| Bg204 | -2.1 | -2.58 | -3.15 | 1.94 | 3.09 | ns | ns | ns | ns | ns | ns | 2-IgSF FREP | FREP3.2 |
| Bg4529 | ns | -1.06 | ns | ns | 1.07 | ns | ns | ns | ns | ns | ns | 2-IgSF FREP | FREP7-like |
| Bg4530 | ns | -2.79 | -3.88 | ns | 2.59 | ns | ns | ns | ns | ns | ns | 2-IgSF FREP | FREPJ2-like |
| Bg5224 | ns | 1.24 | ns | 1.13 | 1.45 | ns | ns | ns | ns | ns | ns | 2-IgSF FREP | FREPJ5 |
| Bg6034 | ns | -1.19 | ns | ns | 3.64 | ns | ns | ns | ns | ns | ns | partial 2-IgSF FREP | FREP12.1-as2-like |
| Bg14428 | ns | ns | -1.11 | 1.05 | ns | ns | ns | ns | ns | ns | ns | 1-IgSF FREP | FREP14 |
| Bg16934 | ns | ns | -5.41 | ns | ns | ns | ns | ns | ns | ns | -6.42 | 2-IgSF FREP | 20_tig_3569_BGLB009245b |
| Bg16935 | 1.04 | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | 2-IgSF FREP | 19_tig_3569_BGLB009245a |
| Bg17688 | ns | ns | -2.74 | ns | ns | ns | ns | ns | ns | ns | ns | 2-IgSF FREP | zb_tig_15796 |
| Bg20516* | 8.96 | ns | ns | ns | 6.79 | 4.84 | ns | ns | ns | ns | ns | partial 2-IgSF FREP | new partial FREP |
| Bg22646 | ns | ns | ns | ns | 1.1 | ns | ns | ns | ns | ns | ns | partial 1-IgSF FREP | new partial FREP |
| Bg23857 | ns | -2.33 | -2.53 | 1.4 | 2.42 | ns | ns | ns | ns | ns | ns | partial 1-IgSF FREP | new partial FREP |
| Bg25144* | ns | ns | ns | ns | 1.44 | ns | ns | ns | ns | ns | ns | partial 2-IgSF FREP | new partial FREP |
| Bg25334 | 4.4 | 4.34 | 2.93 | ns | 1.4 | ns | ns | 1.78 | ns | ns | ns | partial 1-IgSF FREP | FREPJ5 |
| Bg28617 | ns | ns | 1.8 | 2.29 | 3.92 | ns | ns | ns | ns | ns | 2.48 | 1-IgSF FREP | FREP2 |
| Bg29167 | ns | ns | -2.3 | ns | ns | ns | ns | ns | ns | ns | ns | partial 1-IgSF FREP | new partial FREP |
| Bg32905* | 6.74 | 5.68 | ns | ns | ns | 1 | ns | ns | ns | 2.75 | ns | partial 2-IgSF FREP | FREPJ1 |
| Bg38205 | ns | -3.62 | -3.69 | 1.28 | 2.62 | 2.29 | ns | ns | ns | ns | ns | partial 1-IgSF FREP | new partial FREP |
| Bg177 | ns | ns | 3.86 | ns | ns | ns | ns | ns | ns | ns | ns | sFReD | |
| Bg178 | ns | ns | 2.79 | ns | ns | ns | ns | ns | ns | ns | ns | sFReD | |
| Bg2450 | ns | 3.1 | ns | ns | ns | ns | ns | ns | ns | ns | ns | sFReD | |
| Bg2494 | ns | ns | -5.03 | ns | ns | ns | ns | ns | ns | ns | -5.69 | sFReD | |
| Bg12039 | -3.93 | -3.43 | ns | ns | ns | ns | ns | ns | ns | ns | ns | partial sFReD | |
| Bg16307 | ns | 4.38 | -4.36 | ns | ns | ns | ns | ns | ns | 4.12 | -7.23 | sFReD | |
| Bg16436 | ns | 1.23 | ns | ns | ns | ns | ns | ns | ns | ns | ns | partial sFReD | |
| Bg17605 | ns | ns | -1.68 | ns | ns | ns | ns | ns | ns | ns | ns | sFReD | |
| Bg18336 | ns | ns | ns | 1.14 | ns | ns | ns | ns | ns | ns | ns | sFReD | za_tig_4714 |
| Bg20351 | ns | ns | ns | 3.46 | 6.59 | 2.98 | ns | ns | ns | ns | ns | sFReD | BgMFREP4_AY012701-like |
| Bg25643 | ns | 1.24 | ns | 1.64 | ns | ns | ns | ns | ns | ns | ns | sFReD | |
| Bg28385 | 5.85 | 5.88 | ns | 2.55 | ns | ns | ns | 3.36 | 3.82 | ns | ns | sFReD | |
| Bg34525 | ns | ns | ns | 1.6 | ns | ns | ns | ns | ns | ns | ns | partial sFReD | |
| Bg37621 | ns | ns | ns | 2.83 | ns | ns | ns | ns | ns | ns | ns | partial sFReD | |
| Bg38723 | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | -5.61 | sFReD | |
| Bg40034 | 4.55 | 3.82 | 2.06 | ns | 1.11 | ns | ns | ns | ns | ns | ns | partial sFReD | 16_tig_2402_BGLB007591-like |
| Bg40437 | ns | ns | -4.14 | ns | ns | ns | ns | ns | ns | ns | -5.16 | partial FReM | FReM |
Notes:
Value in cells represents the value of log2(fold change) or log2(FC). Any up-regulated gene with log2(FC) ≥1 is highlighted in red, and any down-regulated gene with log2(FC) ≤-1 is highlighted in blue.
0.5d, 2d, 8d, 40d represent the 4 sampling time points. They include time-matched unexposed controls and exposed snails for valid comparisons.
ns: no significant expression difference detected. Gene with PPDE ≥0.95 and FC ≥2 in EBSeq analysis is considered to be differentially expressed in this study.
In the column of “Type of FReD”, B. glabrata FReD proteins were considered “partial” based on the criteria set in this study. “1-IgSF FREP” and “2-IgSF FREP” represent numbers of IgSF domains in a particular FREP.
“Best hit to published FREP” in the last column represents the best hit of the protein against published B. glabrata FREP sequences from available sources [30,32–34]. Any protein sequence identity ≥90% is considered as the same FREP; identity 85~90% is considered as the corresponding FREP “-like”; identity <85% is not assigned to any published FREP. Several FREPs were highlighted in yellow to emphasize in this study.
In the differential expression analysis, SUS unexposed snails were set as baseline for the corresponding time-matched RES unexposed snails at constitutive level; RES unexposed snails were used as baseline for the corresponding time-matched RES snails exposed to S. mansoni; similarly, SUS unexposed snails were used as baseline for the corresponding time-matched SUS snails exposed to S. mansoni.
With respect to snails exposed to S. mansoni, within strain comparisons were made using unexposed snails as a baseline. For RES snails, S. mansoni miracidia penetrate and transform into mother sporocysts but are typically attacked by host hemocytes and destroyed within 8 days post-exposure (dpe). Many FReD genes (18 FREPs and 7 sFReDs) were responsive to exposure to S. mansoni in RES snails, all of which were up-regulated, and most were responsive at 0.5–8 days dpe (Table 3).
In comparison, in SUS snails, S. mansoni miracidia penetrate, transform into mother sporocyst which produce daughter sporocysts which grow and eventually successfully produce massive numbers of cercariae beginning about 5 weeks after infection. Three FREPs and 3 sFReDs were transiently and weakly up-regulated and two FREPs and 4 sFReDs were strongly down-regulated. Down-regulated expression was notably strong later in infection when the snail hosts were producing cercariae. The disparate FREP4 responses of snails of the two strains to S. mansoni was particularly striking.
With respect to the normalized read counts (S5 Table) and the raw read counts (S6 Table), the expression level of some FREPs and sFReDs are consistently high in SUS snails. For instance, the normalized read counts of FREP3.2 (Bg204) in SUS are high (>1,900 reads) with or without exposure to S. mansoni during the 4 sampling time points. Similarly, Bg23857 (newly found partial FREP) also has consistent high and stable read counts (250+ reads) in SUS snails regardless of exposure to S. mansoni. Bg20516, one of the new partial 2-IgSF FREPs found, is expressed in RES strain with average normalized counts of 156 in RES samples, while the average normalized read counts was less than 1 (<1) in SUS strain.
We also directly compared RES and SUS snails exposed to S. mansoni (S7 Table). Caution in interpretation is required here as we have noted the two strains have quite different baseline representations of FReDs, and they respond so differently to S. mansoni. Interestingly, the expression pattern noted accentuates the differences we have already noted for the within strain comparisons noted above: RES snails show many up-regulated FReDs especially shortly following exposure and SUS mostly show strong patterns of down-regulation later in the course of infection.
Additionally, the following observations for S4 Table were noted: 1) Compared to FREPs, the read counts numbers of sFReDs in either strain were modest and did not respond as dramatically to S. mansoni; 2) The normalized average constitutive read counts among FREPs from SUS snails range from 0–3,500 with FREP3.2 being the most expressed FREP (>3,500), and from 0–3,366 for RES snails with FREP2 being the highest expressed (for one snail was >6,400). A greater variety of FREPs were expressed at high constitutive levels and post-exposure levels for RES than SUS snails (with cut-off >100 normalized reads counts) in S5 Fig; 3) The overall changes in read counts were modest for FREPs from SUS snails exposed to S. mansoni, even for the constitutively highly expressed FREP3.2. By contrast, RES snails had much more pronounced responses especially for FREP2, 3.1, 3.2, 4, J1, J5, 14, and one of the new FREPs (Bg20516*); 4) Of the 4 most abundant FREPs in RES snails responding to S. mansoni (2, 3.1, 3.2 and 4), only read counts for one of them, FREP 3.2, were as common in SUS snails. Although lack of a fully annotated sequence prevented a complete expression analysis for the presumptive FREP3.3 locus, our preliminary read count values for it mirror those of FREP3.1 and 3.2 in showing a strong up-regulation in RES but not SUS snails following exposure to S. mansoni.
Variant FREP transcripts from RES and SUS snails
Protein sequences for four FREPs indicated in previous literature to be pivotal to the B. glabrata response to trematodes, FREPs 2, 3.1, 3.2 and 4, were generated from available transcripts (Fig 8) and aligned with corresponding proteins from the BB02 genome. Note that BB02 snails are also susceptible to schistosome infection. The SNV analysis focused on coding regions of these FREP genes and used cut-off scores of quantity >10 and quality scores of filtering variants of >20. The single nucleotide variants (SNVs) shown are only those that result in actual coding change in amino acids, at the positions shown in Fig 8. Other SNVs were identified in Fig 8 but did not differentiate RES from SUS snails, so are not discussed further.
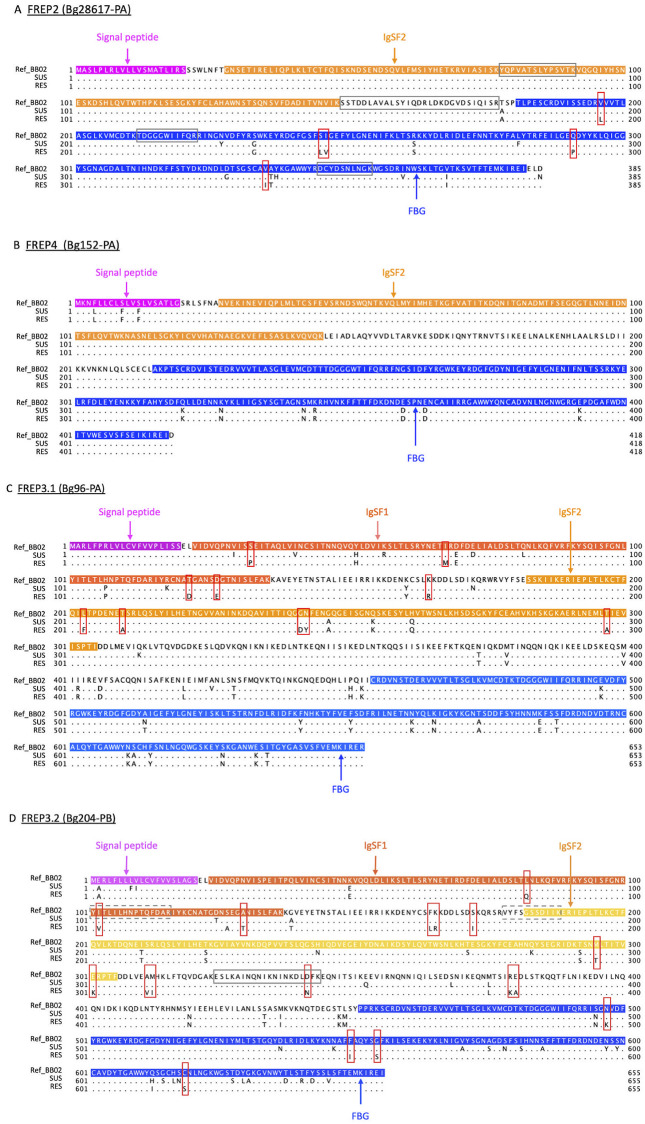
Fig 8. Single nucleotide variant (SNV) analysis in B. glabrata FREPs between SUS and RES with RNA-Seq sequences.
SNV analysis of (A) FREP2 (Bg28617-PA); (B) FREP4 (Bg125-PA); (C) FREP3.1 (Bg96-PA); and (D) FREP3.2 (Bg204-PB). “Ref_BB02”, “SUS”, and “RES” are protein sequences of corresponding FREPs; in which RES and SUS are regenerated based on SNVs in their transcriptome data with BB02 as reference. Four featured sequences of “Signal peptide”, “IgSF1”, “IgSF2” and “FBG” are highlighted in colors. Each SNVs in RES from the two susceptible strains of BB02 and SUS are boxed in red. Any absolute matched sequences to identified peptides from FREP2, FREP3.2 of the plasma of SUS or RES from the pull-down experiments by the Li et al. [47] are framed in gray boxes with solid line. Sequences in FREP3.2 absolutely matched to the two RES-specific peptides from the pull-down experiments by Wu et al. [49] are framed in gray boxes with dash line.
For FREP2 (Fig 8A), all SNVs unique to RES snails were in the FBG domain. For FREP 3.1 (Fig 8C), RES-specific SNVs were found in both IgSF1 and IgSF2 domains. For FREP 3.2 (Fig 8D), one of the most common FREPs in both SUS and RES snails, RES-specific SNVs were found in IgSF1, IgSF2, ICR and FBG domains. The RES-specific SNV at position #102 aa (within the IgSF1 domain, Fig 8D) overlaps with the peptide (#101-115aa: YITLILHNPTQFDAR) from the RES-specific sporocyst-binding protein identified by Wu et al. [49]. Although FREP 3.1 and FREP 3.2 are similar, there are no SNVs in common between them (Fig 8C and 8D). Interestingly, no SNVs were found in FREP4 (Fig 8B).
Some amino acid changes due to SNVs between SUS and RES strains result in changes of hydropathy score, charge, and biochemical properties [93,94] (S8 Table). For instance, at position 245 in the FREP2 protein sequence, a serine (S) in both BB02 and SUS is replaced by a leucine (L) in RES snails. This amino acid change increases the hydropathy score from -0.8 (S) to 3.8 (L). More details are found in S8 Table. As we pieced together FREP sequences from transcriptomic data, our SNV calls will benefit from verification from genomic sequence data for SUS and RES snails.
Additionally, four absolute matches with the FREP peptide sequences identified by Li et al. [47] were found in FREP2 (Fig 8A). No SNVs were located among these four matched sequences. Similarly, one absolute match with the FREP peptide sequence was found in FREP3.2, and one SNV was located in this matched sequence (Fig 8D).
Discussion
The freshwater gastropod B. glabrata and the larval stages of the human-infecting digenetic trematode S. mansoni together comprise the most prominent model system for exploring snail-trematode interactions. Given that over 18,000 species of digenetic trematodes exist [95], almost all of which require a molluscan intermediate host to complete their life cycles, it is important to gain a deeper understanding of their associations. Proteins containing FBG domains called FReDs, particularly when associated with IgSF domains as in FREPs, have become the most studied group of B. glabrata molecules, particularly in the context of their responsiveness to trematode infection and involvement in resistance. Many questions remain with respect to FReD biology, including overviews of their genomic representation, domain structures, evolutionary relationships, responsiveness to infection, and their variability among strains of B. glabrata differing in schistosome susceptibility. We add to our knowledge of FReDs by addressing these issues here.
We found 73 FBG domains encoded in the BB02 B. glabrata genome using our InterProScan search: a single FReM associated with an EGF-like domain, 39 associated with one or two IgSF domains to comprise the FREPs, and 33 comprised only of an FBG domain and designated as sFReDs.
In searching out the IgSF domains so prominently found in FREPs, customized new HMM search profiles were used. Such search profiles are justified by the fact that IgSF domain sequences are highly diversified in both vertebrates [96,97] and in invertebrates like B. glabrata [35]. Furthermore, records of vertebrate IgSF sequences predominate (as of February 2020, nearly 80% of the 434 thousand entries for metazoan immunoglobulin-like folds) relative to invertebrate IgSF sequences.
The customized new HMM search allowed us to identify more B. glabrata IgSF domains than did a conventional search (Table 1 and S2 Table). Consequently, the expanded number of 39 FREPs noted above includes one complete and six partial, previously undocumented new FREP-encoding genes, all of which lack signal peptides that might yet be located with more complete genome assemblies. The automated IgSF and FBG identification workflow presented in Fig 2 is in effect a “FREP finder,” and should facilitate discovery of variable immunoglobulin and lectin domain containing molecules (VIgLs) in other molluscs or invertebrates, at proteome or transcriptome level.
Interestingly, only 2 of the 38 B. glabrata IgSF HMM profiles we generated are associated with FBG domains to form FREPs. Some of the remaining identified IgSF domains found on isolated contigs may also associate with FBG domains but this too awaits improved genome assemblies.
With respect to unusual features of some of the FReDs examined, one contains an accociation of FREP1 with a PAN/Apple domain, previously discovered in B. glabrata [32]. PAN/Apple domains are found from proteobacteria to mammals and function in protein-protein or protein-carbohydrate interactions [98–100]. In molluscs, they are known from 4 Crassostrea gigas genes [101], and from some Lottia gigantea FReDs [56]. FREP1 is remarkable as the only 1-IgSF FREP to possess an IgSF1 instead of an IgSF2 domain. FREP1 is not expressed significantly in RES or SUS snails exposed to S. mansoni, so it may function in some other unknown context.
Another unusual feature revealed by the genome-wide domain predictions for FREPs pertains to the ICR region, which is usually rather variable and short in length (14–133 aa) but was found to be >100aa in length (and up to 320aa long) in 15 of the 39 complete and partial FREPs. Coiled-coils are prominent within ICR regions and may play a role in multimerization of FREPs [53]. InterProScan scanning results showed that coiled coils 35 aa in length occur in the ICR of FREP4 but were not identified in the ICR of FREP2, in line with previous observations that FREP4 forms multimers, while FREP2 does not [50].
We found the two IgSF domains in 2-IgSF FREPs, IgSF1 and IgSF2, cluster separately from all non-FREP IgSF (branches in gray, Fig 7A) known from B. glabrata, and can also be differentiated from one another (Fig 7B), as also noted for FREP 2, 3, 4, 7 by Zhang et al. [35]. Fig 7B also suggests that the IgSF2 domain in 1-IgSF FREPs like FREP2 and 4 are divergent from the IgSF2 domains found in 2-IgSF FREPs which show a pattern of diversification from a common source. A similar pattern was found for the IgSF1 domains. FREP1 with its divergent IgSF1 domain grouped separately from the rest of the IgSF1 domains, all of which are found in 2-IgSF FREPs. The latter IgSF1 domains also show a pattern of diversification from a common source. Along with the results from Fig 6C, the pattern suggests that the two IgSF domains have diverged in parallel.
The plots of distributions of protein sequence diversity (Fig 5) suggest for FREPs that the IgSF2 domain is significantly more variable than the IgSF1 domain (p = 7.82−22). They also show that the FBG domains associated with FREPs are relatively conserved, compared both to the IgSF domains [30], and to FBG domains found in sFReDs. The FBG domain in FREPs may be under selection to conserve an essential function whereas the greater diversity of FBG domains in sFReDs may reflect a broader range of functional roles, or a broader collective pathogen carbohydrate recognition capacity. The role of sFReDs in defense in B. glabrata, however, remains somewhat conjectural. Parts of two sFReDs were found by [49] to bind S. mansoni sporocysts. Relatively few of the sFReDs we recovered had homology to the sporocyst-binding peptides identified by Wu et al. (2017) [49]. Furthermore, as indicated in Table 3, we saw but little evidence of sFReDs responding to trematode infection. Four complete sFReDs (Bg18336, Bg20351, Bg25643, and Bg28385) were upregulated in RES strains at 12 hours post-exposure to S. mansoni; two partial sFReDs (Bg34525 and Bg37621) were also upregulated in RES strains (Table 3). These six sFReDs deserve additional study in anti-trematode contexts.
One of the goals of this study was to investigate how the FReDs of SUS and RES snails differ with respect to their expression before and after exposure to S. mansoni. As our first approach, several peptide sequences from the proteomic study of Wu et al. [49] were matched to the FREP sequences identified in the current study (Fig 4), and in some cases strain differences were noted among the sporocyst-bound plasma peptides identified [49]. For instance, 3 peptide sequences fully matched on some portion of RES FREP3.2, one each in IgSF1, IgSF2 and FBG domains. Only one peptide sequence matched SUS FREP3.2. Additionally, only the first RES-specific peptide in IgSF1(#101-115aa: YITLILHNPTQFDAR) overlaps with one RES-specific SNV (red box highlighted, at #102aa) in FREP3.2 (Fig 8D), which may be relevant to the role of FREP3.2 in resistant to S. mansoni. There were 11 peptide sequences from SUS snails that fully matched portions of a new partial FREP (Bg38205), representing IgSF2, long ICR and FBG regions of the molecule. In contrast, only two peptide sequences from RES snails matched sequences of this FREP, both in the FBG domain. From our expression studies, this same FREP shows higher constitutive expression in unexposed SUS relative to RES snails, but following exposure to S. mansoni, it is up-regulated in RES but not SUS snails (Table 3 and S4 Table). It is one of several FREPs we discuss worthy of further study, in this case because it is clearly capable of binding to sporocysts.
Our second approach to looking at strain differences in responses to S. mansoni was to interrogate our RNA-Seq data with respect to the comprehensive list of FReDs we gleaned from the B. glabrata genome and from assembled transcripts (Table 3 and S4 Table). With respect to constitutive levels of FReD expression, the overall comparison between SUS and RES snails is mixed. For instance, RES snails do not have dramatically higher resting levels of FREPs than SUS snails. This is somewhat in contrast to our recent study of GTPase IMAP family members (GIMAPs) showing that over 20 members of this gene family are constitutively expressed at significantly higher levels in RES than in SUS snails, in both juvenile and adult stages [84]. Although normalized read counts may not provide the best indicator of true relative abundance among various FReD transcripts, it is clear that some FREPs are expressed at higher constitutive levels than others. Especially noteworthy is FREP 3.2 in SUS snails with a consistently high read count level across all samples.
For FREPs in particular, we found a greater variety were expressed in RES than SUS snails both before and after S. mansoni exposure (S4 Fig). This observation is consistent with a transcriptome study on multistrain compatibility comparisons in B. glabrata [34]. In their study, 4 strains of B. glabrata snails were compared, BgVEN, BgGUA, BgBRE and BgBAR, of which BgBAR is the least susceptible to S. mansoni. They used only unexposed snails and found BgBAR had the highest level of FREPs expression among their four strains [34].
What we found to be particularly remarkable is how responsive the RES FREP repertoire was relative to SUS snails following S. mansoni exposure, especially during the 0.5–8 dpe interval when RES snails are actively rejecting infection (Table 3 and S4 Table). FREPs 2 and 4 are noteworthy for the extent of their early up-regulation in RES snails in comparison to SUS snails which are either unresponsive or show sharp-down-regulation, confirming the previous qPCR results of Hertel et al. (2005) [41]. We note that previous studies based on microarray results [39] found FREP4 in particular to be more responsive in SUS snails, so much so as to be a marker of infection, but our RNA-Seq studies using different methods and snail groups did not confirm this.
Both FREP3.1 and FREP3.2 (and tentatively FREP3.3) were both also strongly up-regulated in RES snails shortly following exposure to infection, lending support to a role for FREP3.2 in the resistance response of snails to trematode parasites [38,40,47]. SUS snails consistently showed high levels of expression of FREP 3.2, but neither this FREP nor any other showed pronounced up-regulation following exposure to S. mansoni, except FREP2. This FREP showed upregulation only in snails actively shedding cercariae, but most other FReDs showed no response or down-regulation in shedding snails. A transcriptome study of Kenyan field-derived Biomphalaria pfeifferi, a snail species highly susceptible to S. mansoni, found that FREP2 was consistently up-regulated during infection, FREPs 5, 7,14 were upregulated in early and shedding stages [102], while FREP12 was consistently down-regulated in B. pfeifferi in early infections (1- and 3-day post-infection). FREP3 was minimally responsive in this Kenyan B. pfeifferi study. It was modestly up-regulated only at 1d, in the snail with fewest S. mansoni reads, and the FREP4 homolog was not expressed in B. pfeifferi during the infection.
Some other comparisons with past studies are as follows: 1) for FREP5, we noted no changes in its expression whereas down-regulation in SUS snails after 4 dpe to S. mansoni was noted in a microarray study [39]; 2) FREP7 was noted to be modestly upregulated in RES snails and unresponsive in SUS snails whereas [41] found it to be highly upregulated in SUS snails by qPCR, and Adema et al. (2010) found its response to bacterial exposure to be mixed; 3) for FREP14, we noted modest up-regulation in RES snails following exposure to S. mansoni although no significant responses were observed using qPCR in expression for either RES or SUS snails after exposure to S. mansoni [43].
To summarize this aspect of our study, RES snails showed strong upregulated FREP responses following exposure to S. mansoni whereas SUS snails did not. Some FREPs worthy of additional consideration include FREPs A, J1 and J2 as well as Bg38205 and Bg20516. In addition, four FREPs that persistently turn up of being of interest in previous studies [20], [26,29,31,35,38–44,47,49] were again prominent players: FREPs 2, 4 and FREPs 3.1 and 3.2. These FREPs are responsive to infection and expressed at relatively high levels. Also, relative to FREPs, sFReDs seem to be expressed at lower levels and did not show conspicuous patterns of response though Bg20351, Bg28385 and Bg37621 from RES snails should not be discounted and some modest changes in sFReDs were noted in SUS snails.
As the third component of our examination of FReD responses, given the relative abundance and prominence of FREPs 2, 3.1, 3.2 and 4 responses in RES relative to SUS snails, we looked for interstrain amino acid coding differences among these FREPs (Fig 8). No interstrain amino acid substitutions occurred for FREP4, but substitutions were found for FREPs 2, 3.1 and 3.2. For FREP2, all inter-strain coding differences were found in the FBG domain whereas for FREP3.2 coding differences were found in IgSF1, IgSF2 and FBG domains. Interestingly, FREP3.1 and FREP3.2 do not share any SNVs. Most SNVs in FREP3.1 are in IgSF1 and 2 domains, and SNVs in FREP3.2 are mainly in the IgSF1 and FBG domains. Although we have been unable to do a full analysis on FREP3.3, preliminary sequence analysis suggests this FREP also shows interstrain differences. Although these results need to be confirmed with genomic sequence data for these two snail strains, they suggest that along with considerable differences in expression profiles, resistant BS-90 and susceptible M line snails have different alleles at these key loci. Accordingly, these results fall in line with the interaction model proposed by Li et al. [47] from the specific standpoint that for both the various forms of FREP 3 (3.1, 3.2 and likely 3.3) and for FREP2, it was postulated that the forms involved in responding to S. mansoni sporocysts are different between SUS and RES snails.
We emphasize we are not claiming that differences in FREP expression or allelic composition represent the sole basis of resistance in B. glabrata to S. mansoni as several other hypotheses are in play [17,65,103]. Our genome-wide and inter-strain comparative approach focused on a group of molecules of long-standing interest to us, B. glabrata FREPs and sFReDs, and is the first to provide a comprehensive overview of transcriptional activity involving molecules with identified amino acid coding differences between schistosome-susceptible and -resistant B. glabrata strains. In developing models for how resistance is manifested at the molecular level, both expression changes and allelic differences among snails in key loci clearly need to be considered.
Also, with respect to applying our results to real world situations where S. mansoni is being transmitted, we need to determine what FREP loci are present in local Biomphalaria sp. vector populations, whether allelic versions of key FREPs may exist in such populations as well, and monitor what alleles are present in individual snails that are able to reject S. mansoni infections or not. It will be of particular interest to learn if certain FREP genotypes might vary in time relative to extant infectivity genes in S. mansoni in accordance with matching alleles models of compatibility [104–106] or whether a different, more generalized anti-trematode strategy, possibly based on FREPs as well, also exists in snail populations experiencing heavy burdens of parasitism from a variety of trematode species [107].
Supporting information
Notes: nd: not done, *: The 2 dpe unexposed group was used for the 8 dpe comparison as well, because they did not differ in size. dpe: day post-exposure to S. mansoni. Unexposed: control snails not exposed to S. mansoni and were set to be time-matched to a corresponding exposed group that was.
(XLSX)
(XLSX)
Notes: 1. In the column of “ID in text”, multiple proteins encoded by the same gene due to alternative splicing were differentiated by different version of protein, such as “-PA”, “-PB”, “-PC”, “-PD” or “-PE” suffix following the gene ID. 2. In the column of “NCBI Annotation”, “—” represents no available annotation for a particular protein; 3. In the column of “Type of FReD”, each particular FReD was considered as complete or partial based on the criteria set in this study above; “1-IgSF FREP” and “2-IgSF FREP” represent the number of IgSF domains present in a particular FREP; 4. “Best hit to published FREP” represent the best hit of individual protein against published B. glabrata FREP sequences from available sources [30,32–34]: best hit of protein sequence identity ≥90% is considered as the same FREP; identity 85~90% is considered as “-like” of the corresponding FREP; identity <85% is not assigned to any known FREP. Several FREPs were highlighted in yellow to emphasize in this study. 5. In the column of "IgSF2 length (aa)", texts in red indicate truncated domains, which are possible to be extended to complete domains with improved assembly or annotation; text in blue indicates a residue of IgSF domain, which is not likely to be extended to a complete domain due to the location between two complete domains; 6. Asterisk marked 4 FREPs IDs in the first two columns were extended sequences after manual checking. 7. The highlighted gene "Bg6034" is a partial 2-IgSF FREP with disrupted architecture: the partial FBG domain was not located at C terminal but inserted into the IgSF2 domain, which was broken into two parts. From the VectorBase genome browser view, there is a gene with 7 exons spans over two contigs APKA01042331.1 (exon 4–7) and APKA01042332.1 (exon 1–3). This unusual gene sequence is most likely a genome assembly error; no matching sequences of transcripts were found among the transcripts from 12 different B. glabrata organs [32].
(XLSX)
(XLSX)
Normalized average read counts for each group in RES and SUS with or without exposure to S. mansoni Notes: 1. Values in cells represent the average normalized read counts for each group at different sampling time points. 2. “0.5dpe, 2dpe, 8dpe, 40dpe” represent the 4 sampling time points. They include time-matched unexposed controls and exposed snails for valid comparisons. 3. In the column of “Type of FReD”, B. glabrata FReD proteins were considered “partial” based on the criteria set in this study. “1-IgSF FREP” and “2-IgSF FREP” represent numbers of IgSF domains in a particular FREP. 4. In the column of “ID used in text”, IDs highlighted in gray represent the differentially expressed (DE) FReD genes in any comparison applied. 5. In the column of “Best hit to published FREP”, the best hit of the protein against published B. glabrata FREP sequences from available sources [30,32–34] were listed. Any protein sequence identity ≥90% is considered as the same FREP; identity 85~90% is considered as the corresponding FREP “-like”; identity <85% is not assigned to any published FREP. Several FREPs were highlighted in yellow to emphasize in this study. 6. In the differential expression analysis, SUS unexposed snails were set as baseline for the corresponding time-matched RES unexposed snails at constitutive level; RES unexposed snails were used as baseline for the corresponding time-matched RES snails exposed to S. mansoni; similarly, SUS unexposed snails were used as baseline for the corresponding time-matched SUS snails exposed to S. mansoni.
(XLSX)
Raw read counts for each replicate snail sample in RES and SUS with or without exposure to S. mansoni Notes: 1. Values in cells represent raw read counts for each snail sample/each replicate at different sampling time points. 2. “R1, R2, R3, R4, R5, R6” represent the number of replicates for each particular group (unexposed or exposed to S. mansoni) 3. “0.5dpe, 2dpe, 8dpe, 40dpe” represent the 4 sampling time points. They include time-matched unexposed controls and exposed snails for valid comparisons. 4. In the column of “Type of FReD”, B. glabrata FReD proteins were considered “partial” based on the criteria set in this study. “1-IgSF FREP” and “2-IgSF FREP” represent numbers of IgSF domains in a particular FREP. 5. In the column of “ID used in text”, IDs highlighted in gray represent the differentially expressed (DE) FReD genes in any comparison applied. 6. In the column of “Best hit to published FREP”, the best hit of the protein against published B. glabrata FREP sequences from available sources [30,32–34] were listed. Any protein sequence identity ≥90% is considered as the same FREP; identity 85~90% is considered as the corresponding FREP “-like”; identity <85% is not assigned to any published FREP. Several FREPs were highlighted in yellow to emphasize in this study. 7. In the differential expression analysis, SUS unexposed snails were set as baseline for the corresponding time-matched RES unexposed snails at constitutive level; RES unexposed snails were used as baseline for the corresponding time-matched RES snails exposed to S. mansoni; similarly, SUS unexposed snails were used as baseline for the corresponding time-matched SUS snails exposed to S. mansoni.
(XLSX)
Differential expression analysis of B. glabrata FReD genes between RES-exposed and SUS-exposed to S. mansoni at different time points Notes: 1. Value in cells represents the value of log2(fold change) or log2(FC). Any up-regulated gene with log2(FC) ≥1 is highlighted in red, and any down-regulated gene with log2(FC) ≤-1 is highlighted in blue. 2. “0.5dpe, 2dpe, 8dpe, 40dpe” represent the 4 sampling time points. They include time-matched unexposed controls and exposed snails for valid comparisons. 3. ns: no significant expression difference detected. Gene with PPDE ≥0.95 and FC ≥2 in EBSeq analysis is considered to be differentially expressed in this study. 4. In the column of “Type of FReD”, B. glabrata FReD proteins were considered “partial” based on the criteria set in this study. “1-IgSF FREP” and “2-IgSF FREP” represent numbers of IgSF domains in a particular FREP. 5. “Best hit to published FREP” in the last column represents the best hit of the protein against published B. glabrata FREP sequences from available sources [30,32–34]. Any protein sequence identity ≥90% is considered as the same FREP; identity 85~90% is considered as the corresponding FREP “-like”; identity <85% is not assigned to any published FREP. Several FREPs were highlighted in yellow to emphasize in this study. 6. In the differential expression analysis, SUS unexposed snails were set as baseline for the corresponding time-matched RES unexposed snails at constitutive level; RES unexposed snails were used as baseline for the corresponding time-matched RES snails exposed to S. mansoni; similarly, SUS unexposed snails were used as baseline for the corresponding time-matched SUS snails exposed to S. mansoni.
(XLSX)
Summary of amino acid change in coding region of interested FREPs between B. glabrata susceptible strains (BB02 and SUS) and resistant strain (RES) Notes: 1. The “Hydropathy Score” is a quantitative analysis of the degree of hydrophobicity or hydrophilicity of amino acids of a protein [93]. 2. pKa is the negative of the logarithm of the dissociation constant for the -COOH group; pKb is the negative of the logarithm of the dissociation constant for the -NH3 group; pKx is the negative of the logarithm of the dissociation constant for any other group in the molecule; pI is the pH at the isoelectric point [92].
(XLSX)
(TIFF)
A. Maximum likelihood tree with 1000 bootstrap test was constructed based on FBG domain sequences of 73 FReD genes in B. glabrata. Nodes with bootstrap support of 75 or higher are marked with black squares. The gene IDs of the 73 FReDs were followed with number of IgSF domains and the best hit with any published FREP genes (BLASTp hit with at least 150 aa aligned with ≥90% identity was considered as the same FREP; or considered a “FREP-like” gene if identity was 85% ~ 90%; identity <85% was not assigned to any published FREP). Any FReD genes with best hit to FREPs from the genome paper [32] are labeled with a red dot at the corresponding gene ID. Complete FREP or sFReD genes fitting the criteria in this study were highlighted in blue text. B. Domain architectures were predicted using InterProScan and lineage specific HMM models. “Peptide sequences” represent multiple peptide sequences extracted from the proteomics study [49]. Colored small horizontal bars above or below some protein domain structures highlight the matched location of peptide sequences with binding affinity to S. mansoni sporocyst membrane-enriched and larval transformation proteins, from either schistosome-susceptible NMRI strain (SUS in green) or -resistant BS-90 strain (RES in red) of B. glabrata. The dashed gray line was manually added to distinguish most “sFReD clades” from “FREP clades”.
(TIFF)
(TIFF)
A. Maximum likelihood tree with 1000 bootstrap test was constructed using full length representative protein sequences of 73 FReD genes in B. glabrata. Nodes with bootstrap support of 75 or higher are marked with black squares. The gene IDs of the 73 FReDs were followed with number of IgSF domains and the best hit with any published FREP genes (BLASTp hit with at least 150 aa aligned with ≥90% identity was considered as the same FREP; or considered a “FREP-like” gene if identity was 85% ~ 90%; identity <85% was not assigned to any published FREP). Any FReD genes with best hit to FREPs from the genome paper [32] are labeled with a red dot at the corresponding gene ID. Complete FREP or sFReD genes fitting the criteria in this study were highlighted in blue text. B. Domain architectures were predicted using InterProScan and lineage specific HMM models. “Peptide sequences” represent multiple peptide sequences extracted from the proteomics study [49]. Colored small horizontal bars above or below some protein domain structures highlight the matched location of peptide sequences with binding affinity to S. mansoni sporocyst membrane-enriched and larval transformation proteins, from either schistosome-susceptible NMRI strain (SUS in green) or -resistant BS-90 strain (RES in red) of B. glabrata. The dashed gray line was manually added to distinguish most “sFReD clades” from “FREP clades”. C. Gene structures of the 73 B. glabrata FReD genes.
(TIFF)
With the cut-off >100 normalized read counts, number of expressed FREPs in RES or SUS strains with or without exposure to S. mansoni were summarized.
(TIFF)
Acknowledgments
We would like to thank the University of New Mexico Center for Advanced Research Computing, for hosting the high-performance computing and large-scale storage resources used in this work. We also would like to thank Bioinformatics internship provided by New Mexico National Center for Genome Resources.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This study was supported by National Institutes of Health (www.nih.gov), grant: R37AI101438 (ESL), and Centers of Biomedical Research Excellence (COBRE), National Institutes of Health (www.nigms.nih.gov/Research/DRCB/IDeA/pages/COBRE.aspx), grant 5P30GM110907-05 (ESL). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Huppatz C, Durrheim DN. Control of neglected tropical diseases. N Engl J Med. 2007;357: 1018–27. 10.1056/NEJMra064142 [DOI] [PubMed] [Google Scholar]
- 2.Bizimana P, Ortu G, Van Geertruyden JP, Nsabiyumva F, Nkeshimana A, Muhimpundu E, et al. Integration of schistosomiasis control activities within the primary health care system: a critical review. Parasites and Vectors. 2019;12: 1–11. 10.1186/s13071-018-3256-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colley DG, Bustinduy AL, Secor WE, King CH. Human schistosomiasis. Lancet. 2014;383: 2253–2264. 10.1016/S0140-6736(13)61949-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hotez PJ, Alvarado M, Basáñez MG, Bolliger I, Bourne R, Boussinesq M, et al. The global burden of disease study 2010: interpretation and implications for the neglected tropical diseases. PLoS Negl Trop Dis. 2014;8 10.1371/journal.pntd.0002865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bayne CJ. Successful parasitism of vector snail Biomphalaria glabrata by the human blood fluke (trematode) Schistosoma mansoni: A 2009 assessment. Mol Biochem Parasitol. 2009;165: 8–18. 10.1016/j.molbiopara.2009.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coustau C, Gourbal B, Duval D, Yoshino TP, Adema CM, Mitta G. Advances in gastropod immunity from the study of the interaction between the snail Biomphalaria glabrata and its parasites: A review of research progress over the last decade. Fish Shellfish Immunol. 2015;46: 5–16. 10.1016/j.fsi.2015.01.036 [DOI] [PubMed] [Google Scholar]
- 7.Lewis FA, Patterson CN, Knight M, Richards CS. The relationship between Schistosoma mansoni and Biomphalaria glabrata: genetic and molecular approaches. Parasitology. 2001;123 Suppl: S169–79. Available: http://www.ncbi.nlm.nih.gov/pubmed/11769281 [DOI] [PubMed] [Google Scholar]
- 8.Morgan JAT, Dejong RJ, Snyder SD, Mkoji GM, Loker ES. Schistosoma mansoni and Biomphalaria: past history and future trends. Parasitology. 2001;123: S211–28. 10.1017/s0031182001007703 [DOI] [PubMed] [Google Scholar]
- 9.Knight M, Arican-Goktas HD, Ittiprasert W, Odoemelam EC, Miller AN, Bridger JM. Schistosomes and snails: A molecular encounter. Front Genet. 2014;5: 1–7. 10.3389/fgene.2014.00230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knight M, Ongele E, Lewis FA. Molecular studies of Biomphalaria glabrata, an intermediate host of Schistosoma mansoni. Int J Parasitol. 2000;30: 535–541. 10.1016/s0020-7519(99)00182-4 [DOI] [PubMed] [Google Scholar]
- 11.Pan SC-T. Schistosoma mansoni: the ultrastructure of larval morphogenesis in Biomphalaria glabrata and of associated host-parasite interactions. Japanese J Med Sci Biol. 1996;49: 129–149. 10.7883/yoken1952.49.129 [DOI] [PubMed] [Google Scholar]
- 12.Mitta G, Gourbal B, Grunau C, Knight M, Bridger JM, Theron A. The compatibility between Biomphalaria glabrata snails and Schistosoma mansoni: an increasingly complex puzzle. Advances in Parasitology. Elsevier Ltd; 2017. 10.1016/bs.apar.2016.08.006 [DOI] [PubMed] [Google Scholar]
- 13.Knight M, Ittiprasert W, Arican-Goktas HD, Bridger JM. Epigenetic modulation, stress and plasticity in susceptibility of the snail host, Biomphalaria glabrata, to Schistosoma mansoni infection. International Journal for Parasitology. Australian Society for Parasitology; 2016. pp. 389–394. 10.1016/j.ijpara.2016.03.003 [DOI] [PubMed] [Google Scholar]
- 14.Portet A, Pinaud S, Tetreau G, Galinier R, Cosseau C, Duval D, et al. Integrated multi-omic analyses in Biomphalaria-Schistosoma dialogue reveal the immunobiological significance of FREP-SmPoMuc interaction. Dev Comp Immunol. 2017;75: 16–27. 10.1016/j.dci.2017.02.025 [DOI] [PubMed] [Google Scholar]
- 15.Richards CS, Shade PC. The genetic variation of compatibility in Biomphalaria glabrata and Schistosoma mansoni. J Parasitol. 1987;73: 1146–1151. [PubMed] [Google Scholar]
- 16.Adema CM, Loker ES. Digenean-gastropod host associations inform on aspects of specific immunity in snails. Dev Comp Immunol. 2015;48: 275–283. 10.1016/j.dci.2014.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pila EA, Li H, Hambrook JR, Wu X, Hanington PC. Schistosomiasis from a snail’s perspective: advances in snail immunity. Trends Parasitol. 2017;33: 845–857. 10.1016/j.pt.2017.07.006 [DOI] [PubMed] [Google Scholar]
- 18.Loker ES, Hertel LA. Alterations in Biomphalaria glabrata plasma induced by infection with the digenetic trematode Echinostoma paraensei. J Parasitol. 1987;73: 503–13. [PubMed] [Google Scholar]
- 19.Monroy FP, Loker ES. Production of heterogeneous carbohydrate-binding proteins by the host snail Biomphalaria glabrata following exposure to Echinostoma paraensei and Schistosoma mansoni. J Parasitol. 1993;79: 416–23. [PubMed] [Google Scholar]
- 20.Adema CM, Hertel LA, Miller RD, Loker ES. A family of fibrinogen-related proteins that precipitates parasite-derived molecules is produced by an invertebrate after infection. Proc Natl Acad Sci. 1997;94: 8691–6. 10.1073/pnas.94.16.8691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams AF, Barclay AN. The immunoglobulin superfamily—Domains for cell surface recognition. Annu Rev Immunol. 1988;6: 381–405. 10.1146/annurev.iy.06.040188.002121 [DOI] [PubMed] [Google Scholar]
- 22.Teichmann SA, Chothia C. Immunoglobulin superfamily proteins in Caenorhabditis elegans. J Mol Biol. 2000;296: 1367–1383. 10.1006/jmbi.1999.3497 [DOI] [PubMed] [Google Scholar]
- 23.Schroeder HW, Cavacini L. Structure and function of immunoglobulins. J Allergy Clin Immunol. 2010;125: S41–S52. 10.1016/j.jaci.2009.09.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun SC, Lindström I, Boman HG, Faye I, Schmidt O. Hemolin: An insect-immune protein belonging to the immunoglobulin superfamily. Science (80-). 1990. 10.1126/science.2270488 [DOI] [PubMed] [Google Scholar]
- 25.Dong Y, Taylor HE, Dimopoulos G. AgDscam, a hypervariable immunoglobulin domain-containing receptor of the Anopheles gambiae innate immune system. PLoS Biol. 2006;4: 1137–1146. 10.1371/journal.pbio.0040229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang S-M, Léonard PM, Adema CM, Loker ES. Parasite-responsive IgSF members in the snail Biomphalaria glabrata: characterization of novel genes with tandemly arranged IgSF domains and a fibrinogen domain. Immunogenetics. 2001;53: 684–94. 10.1007/s00251-001-0386-8 [DOI] [PubMed] [Google Scholar]
- 27.Smith DK, Xue H. Sequence profiles of immunoglobulin and immunoglobulin-like domains. J Mol Biol. 1997;274: 530–545. 10.1006/jmbi.1997.1432 [DOI] [PubMed] [Google Scholar]
- 28.Potapov V, Sobolev V, Edelman M, Kister A, Gelfand I. Protein-protein recognition: Juxtaposition of domain and interface cores in immunoglobulins and other sandwich-like proteins. J Mol Biol. 2004;342: 665–679. 10.1016/j.jmb.2004.06.072 [DOI] [PubMed] [Google Scholar]
- 29.Léonard PM, Adema CM, Zhang SM, Loker ES. Structure of two FREP genes that combine IgSF and fibrinogen domains, with comments on diversity of the FREP gene family in the snail Biomphalaria glabrata. Gene. 2001;269: 155–65. Available: http://www.ncbi.nlm.nih.gov/pubmed/11376947 10.1016/s0378-1119(01)00444-9 [DOI] [PubMed] [Google Scholar]
- 30.Hanington PC, Zhang S-M. The primary role of fibrinogen-related proteins in invertebrates is defense, not coagulation. J Innate Immun. 2011;3: 17–27. 10.1159/000321882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang S-M, Loker ES. The FREP gene family in the snail Biomphalaria glabrata: additional members, and evidence consistent with alternative splicing and FREP retrosequences. Dev Comp Immunol. 2003;27: 175–187. 10.1016/s0145-305x(02)00091-5 [DOI] [PubMed] [Google Scholar]
- 32.Adema CM, Hillier LW, Jones CS, Loker ES, Knight M, Minx P, et al. Whole genome analysis of a schistosomiasis-transmitting freshwater snail. Nat Commun. 2017;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dheilly NM, Duval D, Mouahid G, Emans R, Allienne JF, Galinier R, et al. A family of variable immunoglobulin and lectin domain containing molecules in the snail Biomphalaria glabrata. Dev Comp Immunol. 2015;48: 234–243. 10.1016/j.dci.2014.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galinier R, Roger E, Moné Y, Duval D, Portet A, Pinaud S, et al. A multistrain approach to studying the mechanisms underlying compatibility in the interaction between Biomphalaria glabrata and Schistosoma mansoni. PLoS Negl Trop Dis. 2017;11: e0005398 10.1371/journal.pntd.0005398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang S-M, Adema CM, Kepler TB, Loker ES. Diversification of Ig superfamily genes in an invertebrate. Science. 2004;305: 251–4. 10.1126/science.1088069 [DOI] [PubMed] [Google Scholar]
- 36.Adema CM, Hertel LA, Loker ES. Evidence from two planorbid snails of a complex and dedicated response to digenean (echinostome) infection. Parasitology. 1999;119: 395–404. 10.1017/s0031182099004850 [DOI] [PubMed] [Google Scholar]
- 37.Adema CM, Hanington PC, Lun CM, Rosenberg GH, Aragon AD, Stout BA, et al. Differential transcriptomic responses of Biomphalaria glabrata (Gastropoda, Mollusca) to bacteria and metazoan parasites, Schistosoma mansoni and Echinostoma paraensei (Digenea, Platyhelminthes). Mol Immunol. 2010;47: 849–860. 10.1016/j.molimm.2009.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hanington PC, Forys MA, Dragoo JW, Zhang S-M, Adema CM, Loker ES. Role for a somatically diversified lectin in resistance of an invertebrate to parasite infection. Proc Natl Acad Sci U S A. 2010;107: 21087–92. 10.1073/pnas.1011242107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hanington PC, Lun C-M, Adema CM, Loker ES. Time series analysis of the transcriptional responses of Biomphalaria glabrata throughout the course of intramolluscan development of Schistosoma mansoni and Echinostoma paraensei. Int J Parasitol. 2010;40: 819–831. 10.1016/j.ijpara.2009.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hanington PC, Forys MA, Loker ES. A somatically diversified defense factor, FREP3, is a determinant of snail resistance to schistosome infection. PLoS Negl Trop Dis. 2012;6 10.1371/journal.pntd.0001591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hertel LA, Adema CM, Loker ES. Differential expression of FREP genes in two strains of Biomphalaria glabrata following exposure to the digenetic trematodes Schistosoma mansoni and Echinostoma paraensei. Dev Comp Immunol. 2005;29: 295–303. 10.1016/j.dci.2004.08.003 [DOI] [PubMed] [Google Scholar]
- 42.Jiang Y, Loker ES, Zhang S-MM. In vivo and in vitro knockdown of FREP2 gene expression in the snail Biomphalaria glabrata using RNA interference. Dev Comp Immunol. 2006;30: 855–66. 10.1016/j.dci.2005.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang S-M, Nian H, Zeng Y, DeJong RJ. Fibrinogen-bearing protein genes in the snail Biomphalaria glabrata: characterization of two novel genes and expression studies during ontogenesis and trematode infection. Dev Comp Immunol. 2008;32: 1119–1130. 10.1016/j.dci.2008.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang S-M, Loker ES. Representation of an immune responsive gene family encoding fibrinogen-related proteins in the freshwater mollusc Biomphalaria glabrata, an intermediate host for Schistosoma mansoni. Gene. 2004;341: 255–266. 10.1016/j.gene.2004.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gourbal B, Théron A, Grunau C, Duval D, Mitta G. Polymorphic mucin-like proteins in Schistosoma mansoni, a variable antigen and a key component of the compatibility between the schistosome and its snail host. Results and Problems in Cell Differentiation. 2015. 10.1007/978-3-319-20819-0_4 [DOI] [PubMed] [Google Scholar]
- 46.Moné Y, Gourbal B, Duval D, du Pasquier L, Kieffer-Jaquinod S, Mitta G. A large repertoire of parasite epitopes matched by a large repertoire of host immune receptors in an invertebrate host/parasite model. PLoS Negl Trop Dis. 2010;4 10.1371/journal.pntd.0000813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li H, Hambrook JR, Pila EA, Gharamah AA, Fang J, Wu X, et al. Coordination of humoral immune factors dictates compatibility between Schistosoma mansoni and Biomphalaria glabrata. Elife. 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoshino TP, Wu X-J, Gonzalez L, Hokke CH. Circulating Biomphalaria glabrata hemocyte subpopulations possess shared schistosome glycans and receptors capable of binding larval glycoconjugates. Exp Parasitol. 2013;133: 28–36. Toll-like 10.1016/j.exppara.2012.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu XJ, Dinguirard N, Sabat G, Lui H Di, Gonzalez L, Gehring M, et al. Proteomic analysis of Biomphalaria glabrata plasma proteins with binding affinity to those expressed by early developing larval Schistosoma mansoni. PLoS Pathog. 2017;13: 1–30. 10.1371/journal.ppat.1006081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang S-M, Zeng Y, Loker ES. Expression profiling and binding properties of fibrinogen-related proteins (FREPs), plasma proteins from the schistosome snail host Biomphalaria glabrata. Innate Immun. 2008;14: 175–89. 10.1177/1753425908093800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Doolittle RF. Structural and Functional Diversity of Fibrinogen-Related Domains. The Evolution of the Immune System: Conservation and Diversification. 2016. pp. 275–294. 10.1016/B978-0-12-801975-7.00011-6 [DOI] [Google Scholar]
- 52.Pieters M, Wolberg AS. Fibrinogen and fibrin: an illustrated review. Res Pract Thromb Haemost. 2019;3: 161–172. 10.1002/rth2.12191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gorbushin AM. Derivatives of the lectin complement pathway in Lophotrochozoa. Dev Comp Immunol. 2019;94: 35–58. 10.1016/j.dci.2019.01.010 [DOI] [PubMed] [Google Scholar]
- 54.Gokudan S, Muta T, Tsuda R, Koori K, Kawahara T, Seki N, et al. Horseshoe crab acetyl group-recognizing lectins involved in innate immunity are structurally related to fibrinogen. Proc Natl Acad Sci U S A. 1999;96: 10086–10091. 10.1073/pnas.96.18.10086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang X, Rocheleau TA, Fuchs JF, Hillyer JF, Chen CC, Christensen BM. A novel lectin with a fibrinogen-like domain and its potential involvement in the innate immune response of Armigeres subalbatus against bacteria. Insect Mol Biol. 2004;13: 273–282. 10.1111/j.0962-1075.2004.00484.x [DOI] [PubMed] [Google Scholar]
- 56.Gorbushin AM, Panchin Y V., Iakovleva N V. In search of the origin of FREPs: sharacterization of Aplysia californica fibrinogen-related proteins. Dev Comp Immunol. 2010;34: 465–473. 10.1016/j.dci.2009.12.007 [DOI] [PubMed] [Google Scholar]
- 57.Li D, Graham LD. Epiphragmin, the major protein of epiphragm mucus from the vineyard snail, Cernuella virgata. Comp Biochem Physiol—B Biochem Mol Biol. 2007;148: 192–200. 10.1016/j.cbpb.2007.05.009 [DOI] [PubMed] [Google Scholar]
- 58.Söderhäll I, Wu C, Novotny M, Lee BL, Söderhäll K. A novel protein acts as a negative regulator of prophenoloxidase activation and melanization in the freshwater crayfish Pacifastacus leniusculus. J Biol Chem. 2009;284: 6301–6310. 10.1074/jbc.M806764200 [DOI] [PubMed] [Google Scholar]
- 59.Lee EC, Yu SY, Hu X, Mlodzik M, Baker NE. Functional analysis of the fibrinogen-related scabrous gene from Drosophila melanogaster identifies potential effector and stimulatory protein domains. Genetics. 1998;150: 663–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mlodzik M, Baker NE, Rubin GM. Isolation and expression of scabrous, a gene regulating neurogenesis in Drosophila. Genes Dev. 1990;4: 1848–1861. 10.1101/gad.4.11.1848 [DOI] [PubMed] [Google Scholar]
- 61.Yamada S, Hotta K, Yamamoto TS, Ueno N, Satoh N, Takahashi H. Interaction of notochord-derived fibrinogen-like protein with Notch regulates the patterning of the central nervous system of Ciona intestinalis embryos. Dev Biol. 2009;328: 1–12. 10.1016/j.ydbio.2008.12.037 [DOI] [PubMed] [Google Scholar]
- 62.Harada Y, Takagaki Y, Sunagawa M, Saito T, Yamada L, Taniguchi H, et al. Mechanism of self-sterility in a hermaphroditic chordate. Science (80-). 2008;320: 548–550. [DOI] [PubMed] [Google Scholar]
- 63.Yamada L, Saito T, Taniguchi H, Sawada H, Harada Y. Comprehensive egg coat proteome of the ascidian Ciona intestinalis reveals gamete recognition molecules involved in self-sterility. J Biol Chem. 2009;284: 9402–9410. 10.1074/jbc.M809672200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sapp KK, Loker ES. Mechanisms underlying digenean-snail specificity: role of miracidial attachment and host plasma factors. J Parasitol. 2000;86: 1012–9. 10.1645/0022-3395(2000)086[1012:MUDSSR]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 65.Allan ERO, Tennessen JA, Bollmann SR, Hanington PC, Bayne CJ, Blouin MS. Schistosome infectivity in the snail, Biomphalaria glabrata, is partially dependent on the expression of Grctm6, a Guadeloupe Resistance Complex protein. PLoS Negl Trop Dis. 2017;11: 1–15. 10.1371/journal.pntd.0005362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jones P, Binns D, Chang H, Fraser M, Li W, Mcanulla C, et al. InterProScan 5: genome-scale protein function classification. Bioinformatics. 2014;30: 1236–1240. 10.1093/bioinformatics/btu031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brunak S, Petersen TN, Brunak S, von Heijne G, Nielsen H. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods. 2011;8: 785–786. 10.1038/nmeth.1701 [DOI] [PubMed] [Google Scholar]
- 68.Krogh A, Larsson È, von Heijne G, Sonnhammer ELL. Predicting transmembrane protein topology with a Hidden Markov Model: application to complete genomes. J Mol Biol. 2001;305: 567–580. 10.1006/jmbi.2000.4315 [DOI] [PubMed] [Google Scholar]
- 69.Vogel C, Teichmann SA, Chothia C. The immunoglobulin superfamily in Drosophila melanogaster and Caenorhabditis elegans and the evolution of complexity. Development. 2003;130: 6317–6328. 10.1242/dev.00848 [DOI] [PubMed] [Google Scholar]
- 70.Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol. 2011;7: 539 10.1038/msb.2011.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Potter SC, Luciani A, Eddy SR, Park Y, Lopez R, Finn RD. HMMER web server: 2018 update. Nucleic Acids Res. 2018;46: W200–W204. 10.1093/nar/gky448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wheeler TJ, Clements J, Finn RD. Skylign: A tool for creating informative, interactive logos representing sequence alignments and profile hidden Markov models. BMC Bioinformatics. 2014;15: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Eddy SR. A new generation of homology search tools based on probabilistic inference. Genome Informatics 2009. 2009. pp. 205–211. [PubMed] [Google Scholar]
- 74.Adema CM. Fibrinogen-related proteins (FREPs) in mollusks. Results Probl Cell Differ. 2015;57: 111–29. 10.1007/978-3-319-20819-0_5 [DOI] [PubMed] [Google Scholar]
- 75.Giraldo-Calderón GI, Emrich SJ, MacCallum RM, Maslen G, Dialynas E, Topalis P, et al. VectorBase: an updated bioinformatics resource for invertebrate vectors and other organisms related with human diseases. Nucleic Acids Res. 2015;43: D707–D713. 10.1093/nar/gku1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wickham H. ggplot2: elegant graphics for data analysis. Springer; 2016. [Google Scholar]
- 77.Nguyen LT, Schmidt HA, Von Haeseler A, Minh BQ. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol. 2014;32: 268–274. 10.1093/molbev/msu300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lewis TE, Sillitoe I, Lees JG. Cath-resolve-hits: A new tool that resolves domain matches suspiciously quickly. Bioinformatics. 2019;35: 1766–1767. 10.1093/bioinformatics/bty863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yu G, Smith DK, Zhu H, Guan Y, Lam TTY. GGTREE: an R package for visualization and annotation of phylogenetic trees with their covariates and other associated data. Methods Ecol Evol. 2017;8: 28–36. 10.1111/2041-210X.12628 [DOI] [Google Scholar]
- 80.Kassambara A. ggpubr:“ggplot2” based publication ready plots. R package version 0.1, 6. 2017.
- 81.Robinson DF, Foulds LR. Comparison of phylogenetic trees. Math Biosci. 1981;53: 131–147. [Google Scholar]
- 82.Robinson O, Dylus D, Dessimoz C. Phylo.io: interactive viewing and comparison of large phylogenetic trees on the web. Mol Biol Evol. 2016;33: 2163–2166. 10.1093/molbev/msw080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hintze JL, Nelson RD. Violin plots: a box plot-density trace synergism. Am Stat. 1998;52: 181–184. Available: http://www.jstor.org/stable/2685478%5Cnhttp://www.jstor.org/%5Cnhttp://www.jstor.org/action/showPublisher?publisherCode=astata.%5Cnhttp://www.jstor.org [Google Scholar]
- 84.Lu L, Loker ES, Zhang S-M, Buddenborg SK, Bu L. Genome-wide discovery, and computational and transcriptional characterization of an AIG gene family in the freshwater snail Biomphalaria glabrata, a vector for Schistosoma mansoni. BMC Genomics. 2020;21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McLaren W, Gil L, Hunt SE, Riat HS, Ritchie GRS, Thormann A, et al. The Ensembl Variant Effect Predictor. Genome Biol. 2016;17: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol Biol Evol. 2013;30: 772–780. 10.1093/molbev/mst010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Waterhouse AM, Procter JB, Martin DMA, Clamp M, Barton GJ. Jalview Version 2-A multiple sequence alignment editor and analysis workbench. Bioinformatics. 2009;25: 1189–1191. 10.1093/bioinformatics/btp033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.R Core Team. R: a language and environment for statistical computing. Vienna: R Foudation Stat Comput; 2013. [Google Scholar]
- 89.Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, et al. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 2004;5: R80 10.1186/gb-2004-5-10-r80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wickham H. Reshape2: a reboot of the reshape package. R package version. 2015. https://github.com/hadley/reshape
- 91.Walker A, Braglia L. openxlsx: read, write and edit XLSX files. R package version 3.0.0. 2015.
- 92.Tange O. GNU parallel: the command-line power tool. USENIX Mag. 2011;36: 42–47. [Google Scholar]
- 93.Lide DR. CRC Handbook of Chemistry and Physics, 88th Taylor Fr; 2007. 10.1021/ja077011d [DOI] [Google Scholar]
- 94.Kyte J, Doolittle RF. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157: 105–32. 10.1016/0022-2836(82)90515-0 [DOI] [PubMed] [Google Scholar]
- 95.Littlewood DTJ, Bray RA. The Digenea 1 ed Interrelationships of the Platyhelminthes Systematics Association Special Volume. 1 ed. CRC; 2000. pp. 168–185. [Google Scholar]
- 96.Sun Y, Liu Z, Ren L, Wei Z, Wang P, Li N, et al. Immunoglobulin genes and diversity: what we have learned from domestic animals. J Anim Sci Biotechnol. 2012;3: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hunkapiller T, Hood L. Diversity of the immunoglobulin gene superfamily. Adv Immunol. 1989;44: 1–63. 10.1016/s0065-2776(08)60639-2 [DOI] [PubMed] [Google Scholar]
- 98.Brown PJ, Mulvey D, Potts JR, Tomley FM, Campbell ID. Solution structure of a PAN module from the apicomplexan parasite Eimeria tenella. J Struct Funct Genomics. 2003;4: 227–234. 10.1023/b:jsfg.0000016118.87333.03 [DOI] [PubMed] [Google Scholar]
- 99.Tordai H, Bányai L, Patthy L. The PAN module: the N-terminal domains of plasminogen and hepatocyte growth factor are homologous with the apple domains of the prekallikrein family and with a novel domain found in numerous nematode proteins. FEBS Lett. 1999;461: 63–67. 10.1016/s0014-5793(99)01416-7 [DOI] [PubMed] [Google Scholar]
- 100.Zhou H, Casas-Finet JR, Coats RH, Kaufman JD, Stahl SJ, Wingfield PT, et al. Identification and dynamics of a heparin-binding site in hepatocyte growth factor. Biochemistry. 1999;38: 14793–14802. 10.1021/bi9908641 [DOI] [PubMed] [Google Scholar]
- 101.Huang B, Zhang L, Li L, Tang X, Zhang G. Highly diverse fibrinogen-related proteins in the Pacific oyster Crassostrea gigas. Fish Shellfish Immunol. 2015;43: 485–490. 10.1016/j.fsi.2015.01.021 [DOI] [PubMed] [Google Scholar]
- 102.Buddenborg SK, Bu L, Zhang S-M, Schilkey FD, Mkoji GM, Loker ES. Transcriptomic responses of Biomphalaria pfeifferi to Schistosoma mansoni: Investigation of a neglected African snail that supports more S. mansoni transmission than any other snail species. PLoS Negl Trop Dis. 2017;11: e0005984 10.1371/journal.pntd.0005984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Allan ERO, Yang L, Tennessen JA, Blouin MS. Allelic variation in a single genomic region alters the hemolymph proteome in the snail Biomphalaria glabrata. Fish Shellfish Immunol. 2019;88: 301–307. 10.1016/j.fsi.2019.02.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Theron A, Coustau C, Rogunon A, Gourbière S, Blouin MS. Effects of laboratory culture on compatibility between snails and schistosomes. Parasitology. 2008;135: 1179–1188. 10.1017/S0031182008004745 [DOI] [PubMed] [Google Scholar]
- 105.Mitta G, Adema CM, Gourbal B, Loker ES, Theron A. Compatibility polymorphism in snail/schistosome interactions: from field to theory to molecular mechanisms. Dev Comp Immunol. 2012;37: 1–8. 10.1016/j.dci.2011.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Webster JP, Woolhouse MEJ. Selection and strain specificity of compatibility between snail intermediate hosts and their parasitic schistosomes. Evolution (N Y). 1998;52: 1627. [DOI] [PubMed] [Google Scholar]
- 107.Laidemitt MR, Anderson LC, Wearing HJ, Mutuku MW, Mkoji GM, Loker ES. Antagonism between parasites within snail hosts impacts the transmission of human schistosomiasis. Elife. 2019;8: 1–17. 10.7554/eLife.50095 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Notes: nd: not done, *: The 2 dpe unexposed group was used for the 8 dpe comparison as well, because they did not differ in size. dpe: day post-exposure to S. mansoni. Unexposed: control snails not exposed to S. mansoni and were set to be time-matched to a corresponding exposed group that was.
(XLSX)
(XLSX)
Notes: 1. In the column of “ID in text”, multiple proteins encoded by the same gene due to alternative splicing were differentiated by different version of protein, such as “-PA”, “-PB”, “-PC”, “-PD” or “-PE” suffix following the gene ID. 2. In the column of “NCBI Annotation”, “—” represents no available annotation for a particular protein; 3. In the column of “Type of FReD”, each particular FReD was considered as complete or partial based on the criteria set in this study above; “1-IgSF FREP” and “2-IgSF FREP” represent the number of IgSF domains present in a particular FREP; 4. “Best hit to published FREP” represent the best hit of individual protein against published B. glabrata FREP sequences from available sources [30,32–34]: best hit of protein sequence identity ≥90% is considered as the same FREP; identity 85~90% is considered as “-like” of the corresponding FREP; identity <85% is not assigned to any known FREP. Several FREPs were highlighted in yellow to emphasize in this study. 5. In the column of "IgSF2 length (aa)", texts in red indicate truncated domains, which are possible to be extended to complete domains with improved assembly or annotation; text in blue indicates a residue of IgSF domain, which is not likely to be extended to a complete domain due to the location between two complete domains; 6. Asterisk marked 4 FREPs IDs in the first two columns were extended sequences after manual checking. 7. The highlighted gene "Bg6034" is a partial 2-IgSF FREP with disrupted architecture: the partial FBG domain was not located at C terminal but inserted into the IgSF2 domain, which was broken into two parts. From the VectorBase genome browser view, there is a gene with 7 exons spans over two contigs APKA01042331.1 (exon 4–7) and APKA01042332.1 (exon 1–3). This unusual gene sequence is most likely a genome assembly error; no matching sequences of transcripts were found among the transcripts from 12 different B. glabrata organs [32].
(XLSX)
(XLSX)
Normalized average read counts for each group in RES and SUS with or without exposure to S. mansoni Notes: 1. Values in cells represent the average normalized read counts for each group at different sampling time points. 2. “0.5dpe, 2dpe, 8dpe, 40dpe” represent the 4 sampling time points. They include time-matched unexposed controls and exposed snails for valid comparisons. 3. In the column of “Type of FReD”, B. glabrata FReD proteins were considered “partial” based on the criteria set in this study. “1-IgSF FREP” and “2-IgSF FREP” represent numbers of IgSF domains in a particular FREP. 4. In the column of “ID used in text”, IDs highlighted in gray represent the differentially expressed (DE) FReD genes in any comparison applied. 5. In the column of “Best hit to published FREP”, the best hit of the protein against published B. glabrata FREP sequences from available sources [30,32–34] were listed. Any protein sequence identity ≥90% is considered as the same FREP; identity 85~90% is considered as the corresponding FREP “-like”; identity <85% is not assigned to any published FREP. Several FREPs were highlighted in yellow to emphasize in this study. 6. In the differential expression analysis, SUS unexposed snails were set as baseline for the corresponding time-matched RES unexposed snails at constitutive level; RES unexposed snails were used as baseline for the corresponding time-matched RES snails exposed to S. mansoni; similarly, SUS unexposed snails were used as baseline for the corresponding time-matched SUS snails exposed to S. mansoni.
(XLSX)
Raw read counts for each replicate snail sample in RES and SUS with or without exposure to S. mansoni Notes: 1. Values in cells represent raw read counts for each snail sample/each replicate at different sampling time points. 2. “R1, R2, R3, R4, R5, R6” represent the number of replicates for each particular group (unexposed or exposed to S. mansoni) 3. “0.5dpe, 2dpe, 8dpe, 40dpe” represent the 4 sampling time points. They include time-matched unexposed controls and exposed snails for valid comparisons. 4. In the column of “Type of FReD”, B. glabrata FReD proteins were considered “partial” based on the criteria set in this study. “1-IgSF FREP” and “2-IgSF FREP” represent numbers of IgSF domains in a particular FREP. 5. In the column of “ID used in text”, IDs highlighted in gray represent the differentially expressed (DE) FReD genes in any comparison applied. 6. In the column of “Best hit to published FREP”, the best hit of the protein against published B. glabrata FREP sequences from available sources [30,32–34] were listed. Any protein sequence identity ≥90% is considered as the same FREP; identity 85~90% is considered as the corresponding FREP “-like”; identity <85% is not assigned to any published FREP. Several FREPs were highlighted in yellow to emphasize in this study. 7. In the differential expression analysis, SUS unexposed snails were set as baseline for the corresponding time-matched RES unexposed snails at constitutive level; RES unexposed snails were used as baseline for the corresponding time-matched RES snails exposed to S. mansoni; similarly, SUS unexposed snails were used as baseline for the corresponding time-matched SUS snails exposed to S. mansoni.
(XLSX)
Differential expression analysis of B. glabrata FReD genes between RES-exposed and SUS-exposed to S. mansoni at different time points Notes: 1. Value in cells represents the value of log2(fold change) or log2(FC). Any up-regulated gene with log2(FC) ≥1 is highlighted in red, and any down-regulated gene with log2(FC) ≤-1 is highlighted in blue. 2. “0.5dpe, 2dpe, 8dpe, 40dpe” represent the 4 sampling time points. They include time-matched unexposed controls and exposed snails for valid comparisons. 3. ns: no significant expression difference detected. Gene with PPDE ≥0.95 and FC ≥2 in EBSeq analysis is considered to be differentially expressed in this study. 4. In the column of “Type of FReD”, B. glabrata FReD proteins were considered “partial” based on the criteria set in this study. “1-IgSF FREP” and “2-IgSF FREP” represent numbers of IgSF domains in a particular FREP. 5. “Best hit to published FREP” in the last column represents the best hit of the protein against published B. glabrata FREP sequences from available sources [30,32–34]. Any protein sequence identity ≥90% is considered as the same FREP; identity 85~90% is considered as the corresponding FREP “-like”; identity <85% is not assigned to any published FREP. Several FREPs were highlighted in yellow to emphasize in this study. 6. In the differential expression analysis, SUS unexposed snails were set as baseline for the corresponding time-matched RES unexposed snails at constitutive level; RES unexposed snails were used as baseline for the corresponding time-matched RES snails exposed to S. mansoni; similarly, SUS unexposed snails were used as baseline for the corresponding time-matched SUS snails exposed to S. mansoni.
(XLSX)
Summary of amino acid change in coding region of interested FREPs between B. glabrata susceptible strains (BB02 and SUS) and resistant strain (RES) Notes: 1. The “Hydropathy Score” is a quantitative analysis of the degree of hydrophobicity or hydrophilicity of amino acids of a protein [93]. 2. pKa is the negative of the logarithm of the dissociation constant for the -COOH group; pKb is the negative of the logarithm of the dissociation constant for the -NH3 group; pKx is the negative of the logarithm of the dissociation constant for any other group in the molecule; pI is the pH at the isoelectric point [92].
(XLSX)
(TIFF)
A. Maximum likelihood tree with 1000 bootstrap test was constructed based on FBG domain sequences of 73 FReD genes in B. glabrata. Nodes with bootstrap support of 75 or higher are marked with black squares. The gene IDs of the 73 FReDs were followed with number of IgSF domains and the best hit with any published FREP genes (BLASTp hit with at least 150 aa aligned with ≥90% identity was considered as the same FREP; or considered a “FREP-like” gene if identity was 85% ~ 90%; identity <85% was not assigned to any published FREP). Any FReD genes with best hit to FREPs from the genome paper [32] are labeled with a red dot at the corresponding gene ID. Complete FREP or sFReD genes fitting the criteria in this study were highlighted in blue text. B. Domain architectures were predicted using InterProScan and lineage specific HMM models. “Peptide sequences” represent multiple peptide sequences extracted from the proteomics study [49]. Colored small horizontal bars above or below some protein domain structures highlight the matched location of peptide sequences with binding affinity to S. mansoni sporocyst membrane-enriched and larval transformation proteins, from either schistosome-susceptible NMRI strain (SUS in green) or -resistant BS-90 strain (RES in red) of B. glabrata. The dashed gray line was manually added to distinguish most “sFReD clades” from “FREP clades”.
(TIFF)
(TIFF)
A. Maximum likelihood tree with 1000 bootstrap test was constructed using full length representative protein sequences of 73 FReD genes in B. glabrata. Nodes with bootstrap support of 75 or higher are marked with black squares. The gene IDs of the 73 FReDs were followed with number of IgSF domains and the best hit with any published FREP genes (BLASTp hit with at least 150 aa aligned with ≥90% identity was considered as the same FREP; or considered a “FREP-like” gene if identity was 85% ~ 90%; identity <85% was not assigned to any published FREP). Any FReD genes with best hit to FREPs from the genome paper [32] are labeled with a red dot at the corresponding gene ID. Complete FREP or sFReD genes fitting the criteria in this study were highlighted in blue text. B. Domain architectures were predicted using InterProScan and lineage specific HMM models. “Peptide sequences” represent multiple peptide sequences extracted from the proteomics study [49]. Colored small horizontal bars above or below some protein domain structures highlight the matched location of peptide sequences with binding affinity to S. mansoni sporocyst membrane-enriched and larval transformation proteins, from either schistosome-susceptible NMRI strain (SUS in green) or -resistant BS-90 strain (RES in red) of B. glabrata. The dashed gray line was manually added to distinguish most “sFReD clades” from “FREP clades”. C. Gene structures of the 73 B. glabrata FReD genes.
(TIFF)
With the cut-off >100 normalized read counts, number of expressed FREPs in RES or SUS strains with or without exposure to S. mansoni were summarized.
(TIFF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.