Abstract
A precisely balanced activity of canonical Wnt signaling is essential for a number of biological processes and its perturbation leads to developmental defects or diseases. Here, we demonstrate that alternative isoforms of the KDM2A and KDM2B lysine demethylases have the ability to negatively regulate canonical Wnt signaling. These KDM2A and KDM2B isoforms (KDM2A-SF and KDM2B-SF) lack the N-terminal demethylase domain, but they still have the ability to bind to CpG islands in promoters and to interact with their protein partners via their other functional domains. We have observed that KDM2A-SF and KDM2B-SF bind to the promoters of axin 2 and cyclin D1, two canonical Wnt signaling target genes, and repress their activity. Moreover, KDM2A-SF and KDM2B-SF are both able to strongly repress a Wnt-responsive luciferase reporter. The transcriptional repression mediated by KDM2A-SF and KDM2B-SF, but also by KDM2A-LF, is dependent on their DNA binding domain, while the N-terminal demethylase domain is dispensable for this process. Surprisingly, KDM2B-LF is unable to repress both the endogenous promoters and the luciferase reporter. Finally, we show that both KDM2A-SF and KDM2B-SF are able to interact with TCF7L1, one of the transcriptional mediators of canonical Wnt signaling. KDM2A-SF and KDM2B-SF are thus likely to negatively affect the transcription of canonical Wnt signaling target genes by binding to their promoters and by interacting with TCF7L1 and other co-repressors.
Introduction
KDM2A and KDM2B (KDM2A/B) are two closely related lysine demethylases with the ability to bind to non-methylated CpG islands through their CXXC DNA binding domain. After binding to non-methylated CpG islands in transcriptionally active promoters, KDM2A/B demethylate mono- and di-methylated H3K36 lysines (H3K36me1/2) using their N-terminal Jumonji-C demethylase domain [1, 2]. KDM2B is able to demethylate also H3K4me3 [3]. By demethylating H3K36me1/2 and H3K4me3 in transcriptionally active promoters [4–6], KDM2A/B function as transcriptional repressors of the promoters that contain CpG islands [1, 7–10]. Although KDM2A and KDM2B have very similar structure, they have been shown to interact with different protein partners to repress different target regions. For example, KDM2A interacts with HP1a to repress the pericentromeric heterochromatin [11–13], whereas KDM2B forms complex with the PRC1 complex to silence developmentally important genes in embryonic stem cells [10].
Interestingly, KDM2A has been shown to interact with and to demethylate also non-histone proteins such as the p65 subunit of NF-kappaB or beta-catenin [14, 15]. Beta-catenin is the key mediator of canonical Wnt signaling, which plays an essential role in a number of processes ranging from embryogenesis to aging, and whose malfunction frequently leads to various developmental defects and diseases including cancer [16–21]. After activation of the pathway by Wnt ligands, beta-catenin enters the nucleus where it teams up with TCF/LEF transcription factors (TCF7L1, TCF7L2, TCF7, and LEF1) to activate transcription of their target genes. In the absence of Wnt ligands beta-catenin is phosphorylated at its N-terminal serines and threonines by the GSK-3/CKI kinases, which subsequently leads to its ubiquitination and finally to its proteasome mediated degradation. In the absence of beta-catenin in the nucleus, TCF/LEF proteins interact with co-repressors to act as transcriptional repressors of their target genes [16, 19, 22, 23]. Interestingly, it has been demonstrated that KDM2A can displace the nuclear beta-catenin from the complex with TCF7L1, which results in transcriptional repression of the TCF7L1 target genes [14].
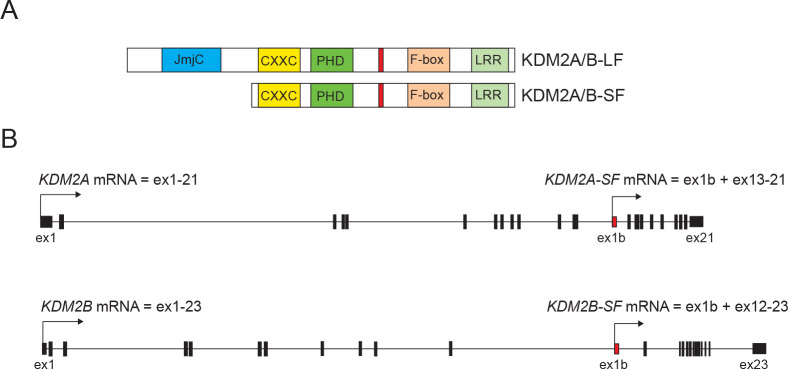
The same loci that encode the full-length KDM2A/B proteins (KDM2A/B-LF) also encode shorter KDM2A proteins that lack the N-terminal demethylase domain [1]. However, these alternative short isoforms (KDM2A/B-SF) share all the other functional domains with KDM2A/B-LF. Therefore, KDM2A/B-SF are not able to demethylate the KDM2A/B target lysines, but they still have the ability to bind to the same DNA regions as KDM2A/B-LF and to interact with the same proteins as KDM2A/B-LF (Fig 1). KDM2A/B-SF are thus likely to compete with KDM2A/B-LF or to complement their function [1]. Despite the fact that a KDM2A-SF specific knockout mutant has not been described yet and it cannot be compared to the embryonically lethal KDM2A-LF knockout phenotype [24], we previously showed that the distinct KDM2A positive nuclear structures on pericentromeric heterochromatin are formed by KDM2A-SF and not by KDM2A-LF [12]. Similarly, the fact that the KDM2B-SF specific knockout phenotype is different from that of the KDM2B-LF loss-of-function mutants also implies different functions for the short and long isoforms [25–27].
Fig 1. The structure of the KDM2A and KDM2B proteins and mRNAs.
A. The protein structure of KDM2A and KDM2B is very similar. They contain the N-terminal Jumonji-C demethylase domain (JmjC), the DNA binding domain (CXXC), the zinc finger PHD domain (PHD), the F-box domain (F-box), and the C-terminal leucine rich repeats domain (LRR). The KDM2A proteins contain also the HP1a interaction motif (in red). B. The exon structures of the KDM2A and KDM2B genes are also very similar. The full length KDM2A and KDM2B mRNA isoforms contain 21 and 23 exons, respectively, and the transcription of both KDM2A-SF and KDM2B-SF mRNAs starts in the 12th intron of the KDM2A and KDM2B genes, respectively.
Here we asked whether KDM2A/B-SF also affect canonical Wnt signaling despite lacking the demethylase domain. We demonstrate that both KDM2A-SF and KDM2B-SF have the ability to negatively regulate this signaling pathway by directly binding to the CpG island containing promoters of the canonical Wnt signaling target genes axin 2 and cyclin D1. Moreover, we found that KDM2A-SF and KDM2B-SF can interact with TCF7L1, one of the TCF/LEF transcriptional mediators of the pathway, which further broadens the negative effects that KDM2A/B-SF have on the TCF/LEF target genes.
Material and methods
Cells
HEK293T cells (SIGMA 12022001-1VL) were grown in 5% CO2 at 37°C and in the high glucose DMEM medium supplemented with 10% fetal bovine serum and the PenStrep antibiotics (all ThermoFisher Scientific). To stabilize beta-catenin and to induce canonical Wnt signaling the cells were treated with 1 μM BIO (6-Bromoindirubin-3′-oxime, SIGMA B1686) for 24 hours before harvesting.
Plasmids and transfection
The coding regions of the corresponding genes were amplified by RT-PCR using the primers listed in S1 Table. The RT-PCR products were cloned in the pCS2 expression plasmids and the mutant constructs were prepared by PCR mutagenesis using the Phusion polymerase (ThermoFisher Scientific). The TOP5 and FOP5 luciferase constructs were prepared by re-cloning the promoter regions of the previously described TOPflash and FOPflash plasmids [28] into the pNL1.1 luciferase plasmid (Promega). The AXIN2 promoter luciferase constructs were prepared by PCR using the primers listed in S1 Table. The PCR products were cloned into the luciferase pNL1.1 vector (Promega) and verified by sequencing. The constructs were verified by sequencing. The expression of the wild type and mutant KDM2A/B proteins was verified by western blot (S1 Fig). The plasmids were transfected into cells using Fugene6 (Promega) or Turbofect (ThermoFisher Scientific).
RNA and Q-RT-PCR
Total RNA was prepared with TRIzol (ThermoFisher Scientific) according to the manufacturer's instructions and reverse transcribed with the LunaScript RT SuperMix kit (NEB). cDNA was analyzed by quantitative PCR using the CFX96 Touch Real-Time PCR Detection System (BIO-RAD), PowerUP SYBR Green mix (ThermoFisher Scientific), and the primers listed in S1 Table. The results were analyzed using the CFX Maestro software (BIO-RAD) and are presented as means ±SD of at least three independent experiments. The significance was determined using the student t-test.
Luciferase reporter assay
Cells were co-transfected with the pNL1.1 nano luciferase reporter constructs, expression constructs and control firefly construct using Fugene6 (Promega). The reporter assays were performed using the NanoGlo Dual luciferase system (Promega) and the Infinite 200 luminometer (Tecan). To induce the transcription of the canonical Wnt signaling target genes, the cells were treated with the pathway agonist BIO (1 μM, 24 hrs, SIGMA B1686). The results are presented as means ±SD of at least three independent experiments.
Proteins and western blot
Whole cell extracts were prepared by rotating the cell pellets for 2 hrs at + 4°C in five volumes of the high salt lysis buffer (50 mM Tris, 300 mM NaCl, 10% glycerol, 0.5% NP-40, 1x complete ULTRA protease inhibitors (Roche)). Proteins were resolved on 10% SDS-PAGE gels, transferred to the Immobilon-P/E PVDF membrane (Merck Millipore), and immunodetected using the SuperSignal West Pico PLUS Chemiluminescent Substrate (ThermoFisher Scientific) and the following antibodies: anti-FLAG M2 (Sigma, F1804, 1:1000), anti-DYKDDDDK Tag (Cell Signaling, 14793, 1:1000), anti-Myc Tag (Millipore, 05–724, 1:1000), anti-mouse-HRP (GE healthcare, NA931-1ML, 1:5000), anti-rabbit-HRP (GE healthcare, NA934-1ML, 1:5000).
Co-immunoprecipitation
HEK293T cells were transfected with the corresponding FLAG tag expression constructs using Turbofect (ThermoFisher Scientific). The whole cell extracts were prepared as described above and diluted to 150 mM NaCl and 0.1% NP-40. 500 μg of the whole cell extract was rotated overnight at + 4°C with 2.5 μg of the anti-FLAG antibody (Sigma F1804) and the protein-immunocomplexes were separated with the Dynabeads Protein G magnetic beads (ThermoFisher Scientific). Proteins were eluted by boiling the beads in the LDS sample buffer (ThermoFisher Scientific) and analyzed by western blot.
Chromatin immunoprecipitation
HEK293T cells were transfected with the pCS2-FLAG empty and protein coding constructs using Turbofect (ThermoFisher Scientific). After 48 hrs the cells were crosslinked with 1% formaldehyde (ThermoFisher Scientific) for 15 minutes at room temperature. The crosslinking reaction was stopped by 0.125M glycine and the samples were processed using the MAGnify Chromatin Immunoprecipitation System (ThermoFisher Scientific), Bioruptor (Diagenode), the anti-FLAG antibody (Sigma, F1804, 1:35), anti-H3K4me3 (Cell Signaling, 9751, 1:40), and the control IgG (Sigma, 12–371, 1:50). The immunoprecipiated DNA was analyzed using the CFX96 Touch Real-Time PCR Detection System (BIO-RAD), Luna Universal qPCR Master Mix (NEB), and the primers listed in S1 Table. The HEK293T cells transfected on the same day with the same constructs were used to verify the expression of the FLAG-tagged proteins by western blot.
Results
KDM2A-SF and KDM2B-SF repress a Wnt-responsive luciferase reporter
Since KDM2A-LF has been previously shown to strongly repress the Wnt-responsive luciferase Topflash reporter activated by elevated levels of beta-catenin [14], we set out to test whether the KDM2A-SF and KDM2B-SF isoforms are also able to repress this reporter despite lacking the demethylase domain. We used the TCF/LEF luciferase reporter TOP5, in which the luciferase gene is under the control of five TCF/LEF consensus binding sites and which thus reflects the activity of canonical Wnt signaling (Fig 2A). We activated the canonical Wnt pathway in HEK293T cells with the pathway agonist BIO (6-Bromoindirubin-3′-oxime). BIO blocks the function of glycogen synthase kinase-3 (GSK3), whose role is to phosphorylate beta-catenin in the absence of a Wnt ligand and by doing so to prevent it from entering the nucleus [16, 18]. Blocking the function of GSK3 results in accumulation of non-phosphorylated beta-catenin, its nuclear deposition and consequently in activation of TCF/LEF target genes including the above-mentioned TCF/LEF responsive TOP5 luciferase reporter [14, 29–34]. Our luciferase reporter experiments confirmed that elevated levels of KDM2A-LF lead to a repression of the activated reporter, but they further showed that both KDM2A-SF or KDM2B-SF are also able to strongly repress this Wnt-responsive reporter despite lacking the N-terminal demethylase domain (Fig 2B and 2C). Interestingly, the full-length KDM2B-LF protein was not able to repress the activated TOP5 reporter (Fig 2C).
Fig 2. KDM2A-SF and KDM2B-SF repress the canonical Wnt signaling luciferase reporter.
A. The wild type TCF/LEF reporter (TOP5) contains five TCF/LEF consensus binding sites (CTTTGAT) that drive the expression of the luciferase gene. The FOP5 construct contains five mutant TCF/LEF binding sites (CTTTGCC) instead and serves as the background activity control. B. Both KDM2A-LF and KDM2A-SF strongly repressed the TOP5 reporter activated with BIO. The reporter activity is expressed as the fold change ratio between the normalized luciferase signal of TOP5 and FOP5. C. KDM2B-SF, but not KDM2B-LF, also strongly repressed the activated reporter. D. Repression of the TOP5 reporter by KDM2A-LF is not dependent on the activity of its JmjC demethylase domain, but on the CXXC DNA binding domain, PHD domain and HP1a interaction motif. The HP1a motif of KDM2A-SF is not necessary for the repressive effect. E. KDM2B-LF was not able to repress the activated TOP5 reporter, whereas KDM2B-SF strongly repressed it in a DNA binding domain dependent manner independently of the PHD domain. (*p < 0.05; **p < 0.01).
These results imply that the N-terminal demethylase domain of KDM2A is not behind the transcriptional repressive effect that KDM2A has on the TOP5 reporter. To analyze that the activity of the JmjC demethylase domain is not required for the repressive effect, we performed the same reporter experiment with the KDM2A protein bearing the mutations previously shown to disrupt the function of the KDM2A demethylase domain, H212A and D214A [35]. This experiment confirmed that the activity of the JmjC demethylase domain is dispensable for the KDM2A-mediated repression of the TOP5 reporter (Fig 2D). Surprisingly, the K601A mutation that disrupts the CXXC DNA binding domain of KDM2A not only reverted the repressive effect of both KDM2A-LF and KDM2A-SF, but it had a positive regulatory effect on the TOP5 reporter (Fig 2D, mutJmjC) [35]. Similarly, disruption of the PHD domain by the C620A/C623A mutations abolished the repressive abilities of both KDM2A-LF and KDM2A-SF (Fig 2D, mutPHD) [11]. Unlike KDM2B, KDM2A contains a short aminoacid motif that is important for the interaction with the heterochromatin protein HP1 [11]. Disruption of this HP1 motif by the V801A and V803A substitutions reverted the repressive effect of KDM2A-LF, but not that of KDM2A-SF (Fig 2D, mutHP1a). As already stated above, KDM2B-LF did not repress the TOP5 reporter and mutating its functional domains had no significant effect in this regard (Fig 2E). On the other hand, the strong repression of the TOP5 reporter by KDM2B-SF was also dependent on its DNA-binding domain, whereas its PHD domain seems dispensable for this repression (Fig 2E).
KDM2A-SF and KDM2B-SF repress the canonical Wnt signaling target genes axin 2 and cyclin D1
To complement our luciferase reporter data and to analyze whether KDM2A-SF and KDM2B-SF are able to repress also endogenous canonical Wnt signaling target genes, we focused on the axin 2 (AXIN2) and cyclin D1 (CCND1) genes. AXIN2 and CCND1 are two widely studied direct target genes of canonical Wnt signaling and as such their promoters can be activated with BIO [36–40]. We stimulated their expression with BIO and analyzed their transcriptional activity by Q-RT-PCR in the presence of the above described KDM2A and KDM2B protein variants. Consistently with our luciferase assays, both KDM2A-LF and KDM2A-SF strongly repressed activated axin 2 and cyclin D1 (Fig 3A and 3B). While the transcriptional repression of axin 2 by both KDM2A-LF and KDM2A-SF is dependent on their CXXC DNA binding domain, disruption of this domain did not completely revert the repressive effect on cyclin D1 (y3A and 3B, mutCXXC). However, the KDM2A-LF mediated repression of the cyclin D1 gene seems to be dependent on the HP1 motif, whose disruption reverted the repressive effect (Fig 3B, mutHP1a). This implies that the nature of the transcriptional regulation of cyclin D1 by the KDM2A isoforms is different from that of axin 2. Consistently with the results of our luciferase assay, KDM2B-LF failed to repress both axin 2 and cyclin D1 (Fig 3C and 3D). On the other hand, KDM2B-SF efficiently repressed both axin 2 and cyclin D1 in a DNA binding domain dependent manner, since disruption of its CXXC domain by the C586/589/592A mutations reverted the repression (Fig 3C and 3D) [41]. The C661/664A mutations that disrupt the KDM2B PHD domain had no significant effect and these KDM2B mutant proteins still repressed both axin 2 and cyclin D1 (Fig 3C and 3D) [42].
Fig 3. KDM2A-SF and KDM2B-SF repress axin 2 and cyclin D1.
A. Both KDM2A-LF and KDM2A-SF repressed axin 2 (AXIN2) in a DNA binding domain dependent manner. B. When activated with BIO, cyclin D1 (CCND1) is also repressed by both KDM2A-LF and KDM2A-SF, but independently of the DNA binding domain. C. Similarly to KDM2A-SF, KDM2B-SF repressed activated axin 2, whereas KDM2B-LF had no effect on its transcription. D. cyclin D1 was also repressed by KDM2B-SF, but not by KDM2B-LF. The mRNA levels were determined by Q-RT-PCR and are related to GAPDH. Similar patterns were obtained also with HPRT and RPL32 (*p < 0.05; **p < 0.01).
KDM2A-SF and KDM2B-SF repress the axin 2 promoter
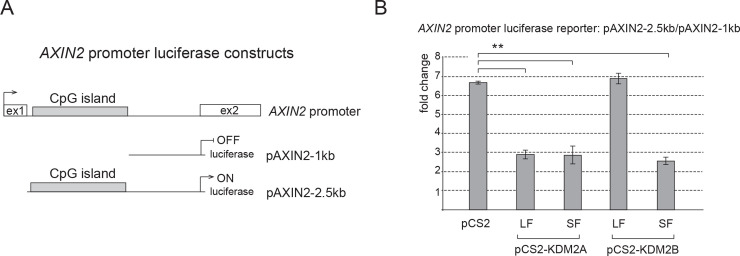
Transcriptional repression of axin 2 and cyclin D1 by KDM2A-LF, KDM2A-SF and KDM2B-SF is well consistent with the fact that KDM2A/B act as CpG island binding transcriptional repressors [1]. Since the axin 2 and cyclin D1 promoters contain CpG islands, the KDM2A/B-SF mediated repression of these promoters is likely to be direct. To investigate whether KDM2A-SF and KDM2B-SF directly repress the axin 2 promoter, we focused on the axin 2 promoter region in its first intron (Fig 4A). This intronic promoter region contains a CpG island and it has been shown to act as an important regulatory promoter element of axin 2 [38]. The ENCODE ChIP-seq data that are publicly available via the UCSC genome browser further show that this region is bound by RNA-POL II and various transcription factors in multiple cell lines, which further confirms its importance for the transcriptional regulation of axin 2 [43]. Consistently with the above-mentioned facts, this approximately 2.5 kb axin 2 intron 1 region exhibited a high transcription inducing activity in a luciferase assay as opposed to a shorter approximately 1 kb axin 2 intron 1 region that lacks the CpG island (Fig 4A and 4B). Consistently with our TOP5 reporter and Q-RT-PCR results, the luciferase activity driven by this axin 2 promoter region was repressed by KDM2A-LF, KDM2A-SF, and KDM2B-SF, whereas KDM2B-LF was not able to repress this region (Fig 4B).
Fig 4. KDM2A-SF and KDM2B-SF repress the axin 2 promoter.
A. The axin 2 promoter luciferase reporter constructs contain either a 1kb axin 2 intron 1 region (pAXIN2-1kb) or a 2.5kb axin 2 intron 1 region (pAXIN2-2.5kb). B. KDM2A-LF, KDM2A-SF and KDM2B-SF all repressed the pAXIN2-2.5kb reporter, whereas KDM2B-LF did not. The activity of the pAXIN2-2.5kb reporter is expressed as the fold change ratio between the activity of pAXIN2-2.5kb and that of pAXIN2-1kb. Similar pattern was observed when the results were expressed as the fold change between the activity of pAXIN2-2.5kb and the empty luciferase plasmid. (*p < 0.05; **p < 0.01).
KDM2A-SF and KDM2B-SF bind to the axin 2 and cyclin D1 promoters
Our results imply that KDM2A-SF and KDM2B-SF directly repress the transcription of axin 2 and cyclin D1. To test whether KDM2A/B-SF isoforms directly bind to the promoters of these genes we performed a series of chromatin immunoprecipitation (ChIP) assays. The amino acid sequence of the alternative KDM2A/B-SF isoforms is identical to that of the corresponding region of the canonical full-length KDM2A/B-LF proteins [1]. Therefore, it is not possible to prepare antibodies specific for KDM2A-SF and KDM2B-SF. To discriminate between binding of the long and short isoforms of KDM2A/B, we overexpressed their N-terminally FLAG-tagged versions in HEK293T cells and tested the selected regions for their presence by ChIP. In our ChIP assay we focused on the promoter regions that have been previously shown to be important for transcriptional regulation of axin 2 and cyclin D1 [38–40]. Furthermore, our in silico analysis of the publicly available data showed that these regions contain CpG islands, which makes them potential targets of the KDM2A/B CpG island binding proteins, and that they are bound by multiple transcription factors in various cell lines, which further confirms their role in transcriptional regulation of the associated genes [43]. Consistently with the results of our Q-RT-PCR and luciferase experiments, the ChIP assay showed that KDM2A-LF, KDM2A-SF and KDM2B-SF bind to the promoter of axin 2, whereas KDM2B-LF does not (Fig 5A and 5B). Moreover, we tested the same axin 2 promoter region for the levels of H3K4me3, the histone lysine methylation associated with transcriptionally active regions [4, 5]. This ChIP experiment revealed that the H3K4me3 levels on the axin 2 promoter expectedly rise after the treatment with BIO, whereas they fall back in the presence of KDM2A-LF or KDM2A-SF (Fig 5C). These changes in the H3K4me3 levels are consistent with the transcriptional activation of the axin 2 promoter with BIO and with the KDM2A/B-SF mediated repression of this promoter, respectively. Similarly, the cyclin D1 promoter was bound by KDM2A-LF, KDM2A-SF and KDM2B-SF, but not by KDM2B-LF (Fig 5D and 5E), and its H3K4me3 levels are also lower in the presence of KDM2A-LF or KDM2A-SF (Fig 5F).
Fig 5. KDM2A-SF and KDM2B-SF bind to the repressed axin 2 and cyclin D1 promoter regions.
A. KDM2A-LF and KDM2A-SF both bind to the AXIN2 promoter. B. Only KDM2B-SF, but not KDM2B-LF, binds to the AXIN2 promoter. C. The levels of H3K4me3 on the AXIN2 promoter rose after the treatment with BIO and then decreased in the presence of the KDM2A isoforms. D. KDM2A-SF binds to the AXIN2 promoter with a higher efficiency than KDM2A-LF. E. KDM2B-SF, but not KDM2B-LF, binds to the cyclin D1 (CCND1) promoter. F. The KDM2A isoforms negatively affected the H3K4me3 levels also on the cyclin D1 promoter. G. The negative control region did not co-immunoprecipitate with the KDM2A/B isoforms. (*p < 0.05; **p < 0.01).
KDM2A-SF and KDM2B-SF interact with TCF7L1
Since KDM2A-LF has been shown to form a complex with TCF7L1 [14], we set out to investigate whether KDM2A-SF and KDM2B-SF are also able to interact with this transcriptional mediator of canonical Wnt signaling. Our co-immunoprecipitation (Co-IP) assays confirmed that tagged KDM2A-LF and TCF7L1 overexpressed in HEK293T interact (Fig 6). In addition, our Co-IP experiments revealed that both short isoforms, KDM2A-SF and KDM2B-SF, and the canonical full-length KDM2B-LF isoform are also able to form complex with TCF7L1 (Fig 6). These results imply that the N-terminal demethylase domain of the KDM2A/B demethylases is not necessary for the interaction with TCF7L1. Furthermore, these interactions help explain why KDM2A-SF and KDM2B-SF can repress the TCF/LEF-responsive TOP5 luciferase reporter (Fig 2), although the promoter of this reporter does not contain any CpG island.
Fig 6. Both the canonical and the demethylase domain deficient isoforms of KDM2A and KDM2B interact with TCF7L1.
The myc-tagged TCF7L1 was overexpressed in HEK293T cells together with the FLAG-tagged KDM2A/B isoforms and the proteins immunoprecipitated with anti-FLAG antibody were analyzed by western blot. All the four FLAG-tagged KDM2A/B isoforms co-immunoprecipitated with TCF7L1. The input corresponds to 5% of the whole cell extract that was used for each immunoprecipitation reaction.
Discussion
In this study, we demonstrate that KDM2A-SF and KDM2B-SF, the two alternative isoforms of the lysine demethylases KDM2A and KDM2B that lack the demethylase domain, are able to negatively affect canonical Wnt signaling at the transcriptional level. We show that KDM2A-SF and KDM2B-SF bind to the promoters of the canonical Wnt signaling target genes axin 2 and cyclin D1, and repress the transcription of these two genes. The axin 2 and cyclin D1 promoter regions we focused on here have been previously studied and shown to be important for transcriptional regulation of the two genes [38–40]. The regulatory function of these regions is further supported by the publicly available ENCODE ChIP-seq data, which indicate that these regions are bound by RNA-POL II and multiple transcription factors in various cell lines [43]. Moreover, we found that these regulatory regions contain CpG islands, which makes them potential targets of KDM2A and KDM2B, and also of KDM2A-SF and KDM2B-SF, since all these protein isoforms are CpG island binding proteins. The binding of KDM2A and KDM2B to these CpG islands in mouse embryonic stem cells have already been experimentally verified by ChIP-seq [10, 35]. However, pan-antibodies that recognize both the canonical long (KDM2A/B-LF) and the short (KDM2A/B-SF) isoforms were used in these studies and they are thus not informative as to what isoforms are bound to these promoter regions.
Since KDM2A-SF and KDM2B-SF have the identical aminoacid sequence as the corresponding regions of the long KDM2A/B-LF isoforms [1], it is not possible to prepare antibodies specific just for KDM2A/B-SF. To circumvent this obstacle, we used FLAG-tagged versions of KDM2A/B-SF and KDM2A/B-LF proteins to immunoprecipitate the chromatin regions bound by them by ChIP. Our ChIP assay demonstrated that both isoforms of KDM2A, KDM2A-LF and KDM2A-SF, bind to the CpG island containing promoter region of both axin 2 and cyclin D1 (Fig 5A and 5D), which is consistent with the ability of these protein isoforms to repress the transcription driven by these promoters (Fig 3)., and to repress the axin 2 promoter luciferase construct (Fig 4). Using ChIP, we also analyzed the selected regions for the levels of H3K4me3, a mark of transcriptionally active promoter regions [4, 5]. Consistently with the transcriptional activation of axin 2 and cyclin D1 upon stimulation of canonical Wnt signaling with BIO (Fig 3), the H3K4me3 levels in the tested promoter regions rose after the treatment with BIO (Fig 5C and 5F). The ChIP assay showed that the presence of either KDM2A-LF or KDM2A-SF results in statistically lower H3K4me3 levels (Fig 5C and 5F), which corresponds to the transcriptionally repressive effect of these proteins on the tested promoters (Fig 3). Surprisingly, KDM2B-LF was unable to bind to the tested promoter regions, which is consistent with the fact that this canonical long KDM2B protein isoform failed to repress the transcription of axin 2 and cyclin D1 (Fig 3C and 3D). The direct repressive effect of KDM2A-LF, KDM2A-SF, and KDM2B-SF on the axin 2 promoter is further supported by our luciferase assays, which show that the axin 2 promoter region is repressed by these protein isoforms, whereas KDM2B-LF failed to show any transcriptionally repressive properties (Fig 4B).
These results are further consistent with the fact that KDM2A-LF, KDM2A-SF, and KDM2B-SF, but not KDM2B-LF, are able to repress the stimulated TOP5 luciferase reporter (Fig 2). However, the TOP5 reporter does not contain any CpG island and so the repressive effect is either not dependent on the CpG island binding domain or it is mediated by some auxiliary protein. Our luciferase data demonstrate that KDM2A-SF and KDM2B-SF need their DNA binding domain to repress the reporter (Fig 2). Based on the previously described interaction of KDM2A-LF with TCF7L1 [14], and on the fact that the luciferase gene is driven from the TOP5 plasmid by five TCF/LEF binding sites, we hypothesized that the repression of the TOP5 reporter by KDM2A/B-SF might be mediated by TCF7L1. Therefore, we tested whether KDM2A/B-SF are also able to interact with TCF7L1 by Co-IP. Our Co-IP results indeed show that all the four KDM2A/B isoforms can interact with TCF7L1 (Fig 6). KDM2A/B-SF are thus likely to repress the TCF/LEF reporter, but also endogenous TCF/LEF target promoters, by interacting with TCF7L1. Our Co-IP results further indicate that the N-terminal region of KDM2A/B is not necessary for the interaction with TCF7L1.
In this study, we present a mechanism that regulates the canonical Wnt signaling activity at the transcriptional level through KDM2A-SF and KDM2B-SF. KDM2A-SF and KDM2B-SF have the ability to negatively affect canonical Wnt signaling by binding to the promoters of canonical Wnt signaling target genes such as axin 2 or cyclin D1, and by attracting co-repressors such as TCF7L1 to these regions.
Supporting information
(DOCX)
The nuclear extracts from the HEK293T cells transfected with the wild type and mutant pCS2-KDM2A/B constructs were analyzed by western blot using the KDM2A or KDM2B antibodies.
(TIF)
A. The inputs (50 μg) of the whole cell extracts analyzed by western blot using the anti-myc tag antibody to show the expression of the myc-tagged TCF7L1 protein in the transfected HEK293T cells. B. The inputs (50 μg) of the whole cell extracts analyzed with the anti-FLAG antibody to show the expression of the FLAG-tagged KDM2A/B proteins. C. One third of the immunoprecipitate analyzed with the anti-myc antibody to show co-immunoprecipitation of the myc-tagged TCF7L1 protein with the KDM2A/B proteins. D. One third of the immunoprecipitate analyzed with the anti-FLAG antibody to show the immunoprecipitated KDM2A/B proteins.
(TIF)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
YES: This work was supported by the Grant Agency of Czech Republic (19-19779S, 17-07164S) and the project of Charles University (Progres Q28).
References
- 1.Vacik T, Ladinovic D, Raska I. KDM2A/B lysine demethylases and their alternative isoforms in development and disease. Nucleus. 2018;9(1):431–41. 10.1080/19491034.2018.1498707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsukada Y, Fang J, Erdjument-Bromage H, Warren ME, Borchers CH, Tempst P, et al. Histone demethylation by a family of JmjC domain-containing proteins. Nature. 2006;439(7078):811–6. 10.1038/nature04433 [DOI] [PubMed] [Google Scholar]
- 3.Janzer A, Stamm K, Becker A, Zimmer A, Buettner R, Kirfel J. The H3K4me3 histone demethylase Fbxl10 is a regulator of chemokine expression, cellular morphology, and the metabolome of fibroblasts. J Biol Chem. 2012;287(37):30984–92. 10.1074/jbc.M112.341040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gates LA, Foulds CE, O'Malley BW. Histone Marks in the 'Driver's Seat': Functional Roles in Steering the Transcription Cycle. Trends Biochem Sci. 2017;42(12):977–89. 10.1016/j.tibs.2017.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Black JC, Van Rechem C, Whetstine JR. Histone lysine methylation dynamics: establishment, regulation, and biological impact. Mol Cell. 2012;48(4):491–507. 10.1016/j.molcel.2012.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zaghi M, Broccoli V, Sessa A. H3K36 Methylation in Neural Development and Associated Diseases. Front Genet. 2019;10:1291 10.3389/fgene.2019.01291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dhar SS, Alam H, Li N, Wagner KW, Chung J, Ahn YW, et al. Transcriptional repression of histone deacetylase 3 by the histone demethylase KDM2A is coupled to tumorigenicity of lung cancer cells. J Biol Chem. 2014;289(11):7483–96. 10.1074/jbc.M113.521625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wagner KW, Alam H, Dhar SS, Giri U, Li N, Wei Y, et al. KDM2A promotes lung tumorigenesis by epigenetically enhancing ERK1/2 signaling. J Clin Invest. 2013;123(12):5231–46. 10.1172/JCI68642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He J, Kallin EM, Tsukada Y, Zhang Y. The H3K36 demethylase Jhdm1b/Kdm2b regulates cell proliferation and senescence through p15(Ink4b). Nat Struct Mol Biol. 2008;15(11):1169–75. 10.1038/nsmb.1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farcas AM, Blackledge NP, Sudbery I, Long HK, McGouran JF, Rose NR, et al. KDM2B links the Polycomb Repressive Complex 1 (PRC1) to recognition of CpG islands. eLife. 2012;1:e00205 10.7554/eLife.00205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borgel J, Tyl M, Schiller K, Pusztai Z, Dooley CM, Deng W, et al. KDM2A integrates DNA and histone modification signals through a CXXC/PHD module and direct interaction with HP1. Nucleic Acids Res. 2017;45(3):1114–29. 10.1093/nar/gkw979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ladinovic D, Novotna J, Jaksova S, Raska I, Vacik T. A demethylation deficient isoform of the lysine demethylase KDM2A interacts with pericentromeric heterochromatin in an HP1a-dependent manner. Nucleus. 2017;8(5):563–72. 10.1080/19491034.2017.1342915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frescas D, Guardavaccaro D, Kuchay SM, Kato H, Poleshko A, Basrur V, et al. KDM2A represses transcription of centromeric satellite repeats and maintains the heterochromatic state. Cell Cycle. 2008;7(22):3539–47. 10.4161/cc.7.22.7062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu L, Gao Y, Zhang Z, Cao Q, Zhang X, Zou J, et al. Kdm2a/b Lysine Demethylases Regulate Canonical Wnt Signaling by Modulating the Stability of Nuclear beta-Catenin. Dev Cell. 2015;33(6):660–74. 10.1016/j.devcel.2015.04.006 [DOI] [PubMed] [Google Scholar]
- 15.Lu T, Jackson MW, Wang B, Yang M, Chance MR, Miyagi M, et al. Regulation of NF-kappaB by NSD1/FBXL11-dependent reversible lysine methylation of p65. Proc Natl Acad Sci U S A. 2010;107(1):46–51. 10.1073/pnas.0912493107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nusse R, Clevers H. Wnt/beta-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell. 2017;169(6):985–99. 10.1016/j.cell.2017.05.016 [DOI] [PubMed] [Google Scholar]
- 17.Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer. 2013;13(1):11–26. 10.1038/nrc3419 [DOI] [PubMed] [Google Scholar]
- 18.Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149(6):1192–205. 10.1016/j.cell.2012.05.012 [DOI] [PubMed] [Google Scholar]
- 19.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17(1):9–26. 10.1016/j.devcel.2009.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sokol SY. Maintaining embryonic stem cell pluripotency with Wnt signaling. Development. 2011;138(20):4341–50. 10.1242/dev.066209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cadigan KM, Peifer M. Wnt signaling from development to disease: insights from model systems. Cold Spring Harbor perspectives in biology. 2009;1(2):a002881 10.1101/cshperspect.a002881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stamos JL, Weis WI. The beta-catenin destruction complex. Cold Spring Harbor perspectives in biology. 2013;5(1):a007898 10.1101/cshperspect.a007898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fagotto F. Looking beyond the Wnt pathway for the deep nature of beta-catenin. EMBO Rep. 2013;14(5):422–33. 10.1038/embor.2013.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawakami E, Tokunaga A, Ozawa M, Sakamoto R, Yoshida N. The histone demethylase Fbxl11/Kdm2a plays an essential role in embryonic development by repressing cell-cycle regulators. Mech Dev. 2015;135:31–42. 10.1016/j.mod.2014.10.001 [DOI] [PubMed] [Google Scholar]
- 25.Boulard M, Edwards JR, Bestor TH. Abnormal X chromosome inactivation and sex-specific gene dysregulation after ablation of FBXL10. Epigenetics & chromatin. 2016;9(22):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andricovich J, Kai Y, Peng W, Foudi A, Tzatsos A. Histone demethylase KDM2B regulates lineage commitment in normal and malignant hematopoiesis. J Clin Invest. 2016;126(3):905–20. 10.1172/JCI84014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fukuda T, Tokunaga A, Sakamoto R, Yoshida N. Fbxl10/Kdm2b deficiency accelerates neural progenitor cell death and leads to exencephaly. Mol Cell Neurosci. 2011;46(3):614–24. 10.1016/j.mcn.2011.01.001 [DOI] [PubMed] [Google Scholar]
- 28.Vacik T, Stubbs JL, Lemke G. A novel mechanism for the transcriptional regulation of Wnt signaling in development. Genes Dev. 2011;25(17):1783–95. 10.1101/gad.17227011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tseng AS, Engel FB, Keating MT. The GSK-3 inhibitor BIO promotes proliferation in mammalian cardiomyocytes. Chemistry & biology. 2006;13(9):957–63. [DOI] [PubMed] [Google Scholar]
- 30.Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nature medicine. 2004;10(1):55–63. 10.1038/nm979 [DOI] [PubMed] [Google Scholar]
- 31.Meijer L, Skaltsounis AL, Magiatis P, Polychronopoulos P, Knockaert M, Leost M, et al. GSK-3-selective inhibitors derived from Tyrian purple indirubins. Chemistry & biology. 2003;10(12):1255–66. [DOI] [PubMed] [Google Scholar]
- 32.Cohen P, Goedert M. GSK3 inhibitors: development and therapeutic potential. Nat Rev Drug Discov. 2004;3(6):479–87. 10.1038/nrd1415 [DOI] [PubMed] [Google Scholar]
- 33.Patel P, Woodgett JR. Glycogen Synthase Kinase 3: A Kinase for All Pathways? Curr Top Dev Biol. 2017;123:277–302. 10.1016/bs.ctdb.2016.11.011 [DOI] [PubMed] [Google Scholar]
- 34.Hur EM, Zhou FQ. GSK3 signalling in neural development. Nat Rev Neurosci. 2010;11(8):539–51. 10.1038/nrn2870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blackledge NP, Zhou JC, Tolstorukov MY, Farcas AM, Park PJ, Klose RJ. CpG islands recruit a histone H3 lysine 36 demethylase. Mol Cell. 2010;38(2):179–90. 10.1016/j.molcel.2010.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, et al. Identification of c-MYC as a target of the APC pathway. Science. 1998;281(5382):1509–12. 10.1126/science.281.5382.1509 [DOI] [PubMed] [Google Scholar]
- 37.Lustig B, Jerchow B, Sachs M, Weiler S, Pietsch T, Karsten U, et al. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol Cell Biol. 2002;22(4):1184–93. 10.1128/mcb.22.4.1184-1193.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22(4):1172–83. 10.1128/mcb.22.4.1172-1183.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398(6726):422–6. 10.1038/18884 [DOI] [PubMed] [Google Scholar]
- 40.Shtutman M, Zhurinsky J, Simcha I, Albanese C, D'Amico M, Pestell R, et al. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci U S A. 1999;96(10):5522–7. 10.1073/pnas.96.10.5522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou Z, Yang X, He J, Liu J, Wu F, Yu S, et al. Kdm2b Regulates Somatic Reprogramming through Variant PRC1 Complex-Dependent Function. Cell reports. 2017;21(8):2160–70. 10.1016/j.celrep.2017.10.091 [DOI] [PubMed] [Google Scholar]
- 42.He J, Shen L, Wan M, Taranova O, Wu H, Zhang Y. Kdm2b maintains murine embryonic stem cell status by recruiting PRC1 complex to CpG islands of developmental genes. Nat Cell Biol. 2013;15(4):373–84. 10.1038/ncb2702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, Zahler AM, et al. The human genome browser at UCSC. Genome Res. 2002;12(6):996–1006. 10.1101/gr.229102 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
The nuclear extracts from the HEK293T cells transfected with the wild type and mutant pCS2-KDM2A/B constructs were analyzed by western blot using the KDM2A or KDM2B antibodies.
(TIF)
A. The inputs (50 μg) of the whole cell extracts analyzed by western blot using the anti-myc tag antibody to show the expression of the myc-tagged TCF7L1 protein in the transfected HEK293T cells. B. The inputs (50 μg) of the whole cell extracts analyzed with the anti-FLAG antibody to show the expression of the FLAG-tagged KDM2A/B proteins. C. One third of the immunoprecipitate analyzed with the anti-myc antibody to show co-immunoprecipitation of the myc-tagged TCF7L1 protein with the KDM2A/B proteins. D. One third of the immunoprecipitate analyzed with the anti-FLAG antibody to show the immunoprecipitated KDM2A/B proteins.
(TIF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.