Abstract
Objective
To determine if there are distinct developmental trajectories of medical responsibility in youth with spina bifida (SB) across ages 8–17 years and to identify condition-related, parental, and family systems predictors of membership in these trajectory groups.
Methods
Participants were 140 youth with SB and their parents who participated in four waves of a longitudinal study across 6 years (ages 8–15 years at Time 1). Multi-method (questionnaires and observed family interactions) and multi-respondent assessments were conducted during home visits.
Results
Findings revealed that there were two distinct developmental trajectories that characterized this sample, with one being labeled “high increasing” (two thirds of the sample) and one labeled “low increasing” (one third of the sample). Most predictor variables were significantly associated with trajectory group membership, with the exception of ethnicity, SES, and measures of conflict. When all significant univariate predictors were included in the same model, only intelligence quotient (IQ), family stress, and gender were retained as significant.
Conclusions
Most youth exhibited relatively rapid increases in responsibility over the course of late childhood and adolescence, but there was a smaller portion of the sample that did not exhibit this type of developmental trajectory. The magnitude of the IQ effect on group differentiation appeared to attenuate the effects of most other predictors. It will be important for clinicians working with youth with SB to recognize that the transfer of medical responsibility from parent to child cannot be expected to unfold in the same manner for all families of youth with SB.
Keywords: family, longitudinal, medical responsibility, parenting, self-management, spina bifida, trajectory
Introduction
Spina bifida (SB) is caused by an incomplete closure of the neural tube during the early stages of pregnancy and is the most common, permanent, congenital birth defect that affects the central nervous system (3 of every 10,000 live births; Copp et al., 2015). Incidence rates vary geographically and across ethnic and racial groups, with Latinx families having the highest rates among all racial groups (4.2 per 10,000 live births; Boulet et al., 2008). Common manifestations of SB include motor and sensory deficits, neurogenic bladder and bowel leading to infections or incontinence, hydrocephalus requiring shunting, orthopedic conditions, pressure ulcers, seizures, and obesity (Copp et al., 2015). Individuals with SB also experience cognitive and academic difficulties, including executive dysfunction and attention problems (Dennis & Barnes, 2010; Tarazi et al., 2008). Psychologically, youth with SB are at-risk for depressive symptoms and social difficulties, tend to be more dependent on adults for guidance, and have a reduced health-related quality of life (Holmbeck et al., 2002, 2003, 2010; Murray et al., 2015; Sawin & Bellin, 2010). During early adulthood, they are less likely to be employed, go to college, and be in a romantic relationship (Zukerman et al., 2011). In short, individuals with SB face significant challenges and obstacles to self-management. They must adhere to an extremely complex multi-component treatment regimen, while at the same time managing a unique array of cognitive and psychosocial comorbidities that hinder both self-management and adherence.
For typically developing youth, the adolescent developmental period is characterized by significant physical, cognitive, and social changes, including increases in autonomy and decision-making capacity (Williams et al., 2002). For youth with chronic health conditions, such normative autonomy-related strivings are often at odds with the daily demands of a complex medical regimen. In fact, a key developmental challenge for youth with chronic health conditions such as SB involves the unfolding of self-management and the successful transfer of responsibility for certain medical tasks from parent to child (Psihogios et al., 2015). It has even been argued that the successful transfer of medical responsibility during the adolescent period is a “necessary prerequisite” for a successful transition from pediatric to adult health care (Reed-Knight et al., 2014, p. 219). Although there are several recent models of self-management that: (a) define the components of the self-management process, (b) explain the process through which individuals with pediatric conditions develop self-management skills, and (c) identify modifiable factors that influence the development of self-management (Grey et al., 2015; Modi et al., 2012; Reed-Knight et al., 2014; Sawin, 2017), very few studies have examined the transfer of medical responsibility over time. Thus, the purpose of this research was to identify distinct trajectories of youth medical responsibility from ages 8 to 17 years, as well as factors that differentiate between these varying longitudinal patterns of change in medical responsibility.
To our knowledge, there are no studies that have identified distinct trajectories of medical responsibility in samples of youth with SB. Indeed, most research on self-management and medical responsibility in this population is cross-sectional (e.g., O’Hara & Holmbeck, 2013; Psihogios & Holmbeck, 2013) and, although there has been some longitudinal research, none has identified distinct trajectories of medical responsibility (e.g., Psihogios et al., 2015, 2017; Stern et al., 2018). On the other hand, research has sought to examine trajectories of medically relevant variables in other pediatric populations. When conducting such studies, researchers typically employ one of the following data analytic approaches: (a) latent growth curve analyses, which examine predictors of the intercept and slope over time for an outcome of interest (e.g., medical responsibility; e.g., Miller & Jawad, 2019; Wiebe et al., 2014), and (b) latent class growth analyses (also known as growth mixture modeling or group-based trajectory modeling) where two or more distinct trajectories are identified and membership in these trajectory classes is predicted (e.g., Kayle et al., 2019; Rassart et al., 2017). It is this latter type of analysis that is the focus of this report. In this study, the use of such modeling allowed for the: (a) identification of latent but distinct groups of youth who follow a similar pattern on medical responsibility across multiple time points, and (b) determination of the shape of the trajectory for each of these groups (Nagin, 2005).
In addition to isolating distinct trajectories of medical responsibility, we also sought to examine factors that are associated with membership in these trajectory groups. Examining significant, early predictors of subsequent trajectories could aid in the identification of modifiable targets. For example, if we determine that high levels of family conflict attenuate the pace at which youth acquire medical responsibility, future interventions could target such conflicts to promote the transfer of responsibility. Importantly, all recent models of self-management have included family factors as a primary domain that influences the unfolding of self-management (Grey et al., 2015; Modi et al., 2012; Reed-Knight et al., 2014; Sawin, 2017). Specifically, parenting behaviors and parental symptoms and perceptions as well as the functioning of the family system as a whole have all been identified as likely contributors (Reed-Knight et al., 2014).
With respect to the family system, the selection of predictors was guided by work that identifies conflict and cohesion as central dimensions of family functioning (Cox & Brooks-Gunn, 1999; Holmbeck, Coakley et al., 2002). Indeed, these two factors would be expected to undermine or facilitate, respectively, the transfer of medical responsibility in youth with SB. Moreover, family-level stress is a commonly studied and modifiable family construct that likely has implications for the development of self-management (Bakula & Mullins, 2018; Holmbeck, Coakley et al., 2002). With respect to parenting behaviors, the degree to which the parent is intrusive or overprotective appears to undermine the development of decision-making autonomy in youth with SB (Holmbeck, Johnson et al., 2002). With respect to parental attitudes and beliefs, parents who perceive their children as medically vulnerable and have lower expectations for their children’s future accomplishments may be less likely to grant increasing medical responsibility to their children over time (Driscoll et al., 2020).
What is the optimal process by which families transfer medical responsibility from parent to child? Past research highlights the importance of gradually transferring condition-related tasks in response to the adolescent’s early successes with medical self-management (Wysocki et al., 1996) rather than abruptly transferring responsibilities, such as when the child reaches a particular age (e.g., age 12) or stage of development (e.g., adolescence; Reed-Knight et al., 2014). Research also suggests that high levels of medical adherence during adolescence require sustained parental involvement (Helgeson et al., 2008; Psihogios & Holmbeck, 2013). On the other hand, parental involvement could become excessive; indeed, withholding medical responsibility from individuals who are both older and developmentally ready to assume such responsibility may undermine the self-management process (Campbell et al., 2019). With respect to different types of responsibility trajectories, we expected that there would be one subgroup that takes on responsibility fairly quickly with other subgroups either taking on responsibility at a slower rate or not taking on any new responsibilities after a specific age (i.e., it is common for researchers in related content areas to find three trajectory types; e.g., Rassart et al., 2017). With respect to predictors of these trajectories, it might simply be the case that more adaptive parenting and family relationships would be associated with a relatively rapid transfer of responsibility from parent to child (i.e., steeper trajectories). On the other hand, and given a more nuanced view of the transfer process (where the transfer process could occur too abruptly or be unnecessarily delayed), we might expect that a moderate (as opposed to a steeper or less steep) trajectory group would be associated with the most adaptive levels of parenting and family functioning.
Thus, this study aimed to identify distinct developmental trajectories of medical responsibility in youth with SB across ages 8–17 as well as predictors of membership in these trajectory groups with multi-method (observational and self-report) and multi-respondent (mother, father, youth, and trained coders) assessments. It was hypothesized that there would be three trajectory groups: (a) a rapidly increasing trajectory, (b) a moderately increasing trajectory, and (c) a lower level, relatively flat, trajectory. It was also anticipated that families high in cohesion, low in conflict, and low in SB-related family stress would have children in the more rapidly increasing trajectory group. Similarly, it was also expected that youth in this group would have parents who are less intrusive, have more positive views of their children’s future, and perceive their children as less vulnerable. An alternative hypothesis would be that the optimal responsibility trajectory is one that has a more moderate slope and that this trajectory group would be predicted by the most adaptive levels of parenting and family relationships. Finally, we included demographic (i.e., gender, socioeconomic status [SES], and ethnicity) and condition-related severity factors (i.e., type of SB, shunt status, number of shunt revisions, lesion level, and intelligence quotient [IQ]) in the models. Although we had no specific predictions for the demographics, we expected that youth in the most rapidly increasing trajectory group would have higher IQs, fewer shunt revisions (or no shunt), lower lesion levels, and a less severe type of SB.
Materials and Methods
Participants
Participants were recruited for a longitudinal study of neuropsychological functioning, social relationships, and psychosocial adjustment in youth with SB (e.g., Driscoll et al., 2020). This study used data from 4 time points (i.e., Times 1–4) from this larger longitudinal study, each spaced 2 years apart. Families of youth with SB were recruited from four hospitals and a statewide SB association in the Midwest. Inclusionary criteria were: (a) a diagnosis of SB (myelomeningocele, lipomeningocele, or myelocystocele); (b) age 8–15 years; (c) proficiency in English or Spanish; (d) involvement of at least one primary caregiver; and (e) residence within 300 mi of the laboratory. Participants with any additional major medical conditions or a major psychiatric condition were not recruited.
During recruitment, 246 families were approached for possible participation. Out of the 163 families who agreed to participate initially, 21 could not be contacted or later declined, and two did not meet the inclusion criteria. Thus, the final sample included 140 families of children with SB at Time 1 (see Table I for demographic and condition-related data). Children of families who declined participation did not differ from those who agreed to participate with respect to type of SB (e.g., myelomeningocele vs. other), χ2 (1) = .0002, p > .05, shunt status, χ2 (1) = .003, p > .05, or occurrence of shunt infections, χ2 (1) = 1.08, p > .05.
Table I.
Youth Demographic and Medical Information at Time 1
| Youth (N = 140) M (SD) or N (%) | |
|---|---|
| Gender: female | 76 (54.3%) |
| Age | 11.43 (2.46) |
| Race | |
| Caucasian | 78 (55.7%) |
| African-American/Black | 21 (15.0%) |
| Hispanic | 34 (24.3%) |
| Other | 7 (5.5%) |
| Family Hollingshead SES | 39.12 (16.09) |
| IQ | 85.75 (19.54) |
| Spina bifida type | |
| Myelomeningocele | 122 (87.1%) |
| Non-myelomeningocele | 17 (12.1%) |
| Not sure/not reported | 1 (0.7%) |
| Lesion level | |
| Thoracic | 23 (16.4%) |
| Lumbar | 69 (49.3%) |
| Sacral | 41 (29.3%) |
| Unknown/not reported | 7 (5.0%) |
| Shunt: present | 109 (77.9%) |
Although many families participated at all four time points (N = 76; 54%), not all families who participated at Time 1 participated at each of the subsequent time points. Of the 140 families that were included at Time 1, 110 (79%) participated at Time 2, 102 (73%) participated at Time 3, and 93 (66%) participated at Time 4. It should also be noted that we continued to contact participants at later time points if they were unable to participate at an earlier time point. For example, of the 30 who did not participate at Time 2, 11 participants re-entered the study at Time 3 and 3 participants re-entered the study at Time 4, thus raising the cross-time retention rate to 124/140 (89%). Analyses revealed no significant differences across demographic and youth condition-related factors between those who participated at all four time points vs those who only participated at one, two, or three time points.
In terms of the household composition, 112 of the 140 participants (80.0%) came from two-parent homes, with 94 (67.1%) participants living with both biological parents. For all two-parent households, both parents were invited to participate, and the final sample included 128 mothers (91% of 140) and 102 fathers (73% of 140) at Time 1, with both parents participating for 95 families (67.9%). Importantly, beginning at Time 3, parents of participants who had turned 18 (roughly 25% of the sample at Time 3 and 50% of the sample at Time 4) no longer participated in the home visit data collections (i.e., only young adults completed questionnaires/interviews once they turned 18). Thus, participation rates of parents at Times 2–4 were as follows: Time 2: 102 mothers (93% of 110) and 79 fathers (72% of 110); Time 3: 71 mothers (91% of 78 families with participants who were < 18) and 51 fathers (65% of 78 families with participants who were < 18); and Time 4: 42 mothers (98% of 43 families with participants who were < 18) and 29 fathers (67% of 43 families with participants who were < 18). In summary, maternal data were collected from 91%-98% of families with youth < 18 years old from Times 1–4 and paternal data were collected from 65% to 73% of such families from Times 1–4.
Procedure
This study was approved by university and hospital IRBs and used a multi-method, multi-informant longitudinal research design. Data were collected by trained research assistants (RAs) during two 3-h home visits at Time 1 and one 3-h home visit at Times 2–4. For home visits with families who primarily spoke Spanish at home, at least one RA was bilingual. Informed consent from parents and assent from youth were obtained via written signatures. Parents also signed releases of information for teacher, health professional, and medical chart data. Youth completed questionnaires and neuropsychological assessments independently from their parents. RAs were available to assist youth with the completion of questionnaires (e.g., reading questions aloud) as needed. Mothers and fathers completed identical questionnaires separately. Questionnaires that were only available in English were translated into Spanish using forward and back translation strategies by a trained translation team. Also, parents and children participated in a series of videotaped triadic interaction tasks; if there was only one parent available to participate, the videotaped interaction only included the parent–youth dyad. Families received monetary compensation and small gifts (e.g., logo t-shirts, water bottles) for participation at each time point.
Measures
Demographics and Condition-Related Severity Variables
At Time 1, parents reported on demographic information, including child age, child gender, and child race/ethnicity. Parents also reported on their own ethnicity, education, employment, income, and relationship to the child. The Hollingshead Index of SES was computed to assess SES based on parents’ education and occupation, with higher scores indicating higher SES (Hollingshead, 1975). Youth’s condition-specific medical information, including lesion level, type of SB, shunt status, and number of shunt revisions, was collected from medical charts (or was based on parent report if medical chart data were unavailable).
Youth IQ
Youth were administered the Vocabulary and Matrix Reasoning subtests of the Wechsler Abbreviated Scale of Intelligence at Time 1 (WASI; Wechsler, 1999). An estimated Full Scale IQ was computed; this Full Scale IQ was employed as a proxy for general intellectual functioning.
Family System Factors
At Time 1, parents completed the Family Stress Scale (FSS, Quittner et al., 1990), a 19-item scale assessing common family stressors in families of a child with SB. This scale assesses the level of stress (on a scale from 1 to 5) that an individual experiences as a result of parenting a child with SB (e.g., stress due to performing condition-related tasks). This study utilized only the six condition-specific items on the FSS. A total score (ranging from 1 to 5) was computed, with higher scores indicating higher levels of SB-specific family stress. In this study, internal consistency was high for mother-report (α = .88) and father-report (α = .92).
Family-level conflict was assessed with both questionnaires and observational procedures and family-level cohesion was assessed with an observational procedure. With respect to the questionnaire measure of family conflict, youth and parents responded to the 31-item Parent–Adolescent Conflict (PAC) Scale, a modified, brief version of the Issues Checklist (Prinz et al., 1979). The PAC is composed of a list of potential conflicts often discussed in families with a preadolescent or adolescent child (e.g., whether or not the child does chores around the house) as well as items that tap conflicts about SB-related activities (e.g., catheterization, bowel program). Each item requires three responses. The family member first responds “yes” or “no” according to whether or not the issue was discussed during the past 2 weeks. If yes, the family member indicates the number of times the issue was discussed as well as the average intensity of the discussion. Intensity is rated on a 5-point Likert scale (ranging from “calm” to “angry”). Average intensity scores were computed for each respondent and were included in the analyses of this study. Because items deemed “not applicable” by respondents are considered missing data, internal consistency alphas were not computed for this scale because reliability software programs only include participants who respond to all items.
To assess observed family conflict and cohesion, families completed a set of videotaped interaction tasks designed to generate family interaction, discussion, and collaborative problem-solving. These structured tasks were counter-balanced and included a warm-up game, a discussion of two age-appropriate socially relevant vignettes, a discussion of how the family will transfer condition-specific responsibilities to the child, and a discussion of conflict issues that were previously identified as relevant in self-report questionnaires. These four videotaped interactions were coded with a global coding method, the Family Interaction Macro-Coding System (FIMS; Holmbeck et al., 2007; Kaugars et al., 2011). FIMS includes codes that assess interaction style, conflict, affect, control, problem-solving, and the general functioning of the family system using 5-point Likert-type ratings. Two raters coded all videotapes; raters were undergraduate and graduate students who were trained to reliability during 10 h of training sessions. Composite Family Conflict and Family Cohesion scales were computed for the family interaction as a whole, collapsing across the four tasks and two raters. For the Family Conflict scale, the following FIMS codes were included: (a) Level of conflict within dyads (coded for the Mother–Youth [M-Y], Father–Youth [F-Y], and Mother–Father [M-F] dyads separately), and (b) Attempted resolution of issues (reverse-scored; coded for M, F, and Y, separately). The following FIMS codes were included in the Family Cohesion scale: (a) Requests input from other family members (coded for M-Y, Y-M, F-Y, Y-F, M-F, and F-M, separately), (b) Involvement in the task (M, F, and Y), (c) Parents present as a united front, (d) Parental promotion of dialogue and collaboration, (M and F), (e) General family atmosphere: Disengaged (reverse-scored), (f) General family atmosphere: Openness, comfortableness, and optimism, and (g) General family atmosphere: Able to reach an agreement/solution. Interrater (α = .61–.78) and scale (α = .65–90) reliabilities were acceptable for both scales.
Parental Attitudes Toward and Beliefs About Child with SB
Parental perceptions of child vulnerability (PPCV) was measured at Time 1 using parent report on the Vulnerable Child Scale (VCS; Perrin et al., 1989). A total score was calculated for this 15-item scale (one item related to worry about child death was dropped from the original 16-item scale), with higher scores indicating higher PPCV. Internal consistency was high (α = .82–.84).
Parental expectations about the future were assessed with the Questions about the Future questionnaire that was developed for a previous study on youth with SB by the same investigative team (Holbein et al., 2017). The measure asks the respondent to rate statements about the child’s future (future employment, educational achievement, transportation, living independently, relationships, and the ability to have and raise children) on a 4-point scale, from 1 (very unlikely) to 4 (very likely). Internal consistency was high (α = .93–.94).
Parenting Behavior
Maternal and paternal overprotection was assessed in two ways. First, mothers and fathers completed the Parent Protection Scale (PPS; Thomasgard et al., 1995) at Time 1. This measure consists of 25 items, and a total score was computed, with higher scores representing greater levels of parental protection. Internal consistency for this scale was acceptable (α = .69–.79).
Maternal and paternal overprotection was also assessed observationally at Time 1 using the FIMS. A composite Overprotection scale score was computed for each parent participant (M and F) by combining two specific items on the FIMS across the four tasks and two raters: (a) active catering to the child, and (b) parent behavior that infantilizes the child. Interrater (α = .58–.63) and scale (α = .90–.92) reliabilities were acceptable.
Medical Responsibility
Parent-reported medical responsibility was the outcome of interest in this study. Parents completed the Sharing of Spina Bifida Management Responsibilities Scale (SOSBMR), an adaptation of the Diabetes Family Responsibility Questionnaire (Anderson et al., 1990), at all four time points (as long as the child was <18; see Procedure section above). The DRFQ is a frequently used measure in diabetes research and has demonstrated adequate reliability and validity (e.g., Sand et al., 2013); our adaptation retained the response format of the DRFQ but changed the content of the items to make them relevant to SB. Past findings support the validity of the SOSBMR insofar as associations between the SOSBMR (when used as a primary outcome) and PPCV, executive functioning, medical adherence, medical regimen skills, impaired gross motor abilities, and depressive symptoms were consistent with hypotheses in past studies (e.g., Driscoll et al., 2020; O’Hara & Holmbeck, 2013; Psihogios & Holmbeck, 2013; Psihogios et al., 2015, 2017; Stern et al., 2018). The SOSBMR assesses responsibility for SB tasks across several domains (e.g., catheterization, health appointments, communication about SB, medications). Parent participants indicated who was responsible for 34 SB-related tasks (1 = parent, 2 = shared, 3 = youth; or “not applicable”). Mean total scores were computed, with higher scores indicating greater youth responsibility (i.e., total scores ranged from 1.0 to 3.0). Some sample items, in response to the probe “Who has responsibility?,” included “Remembering to catheterize regularly, every 2–4 h,” “Maintaining a regular bowel toileting time,” and “Conducting daily skin checks.” As was the case with the conflict measure above, because items deemed “not applicable” by respondents were considered missing data, alphas for this scale could not computed.
Data Reduction
To reduce the number of variables included in the analyses, composite scores were created across parent and youth respondents if associations between them met the following criteria: when Pearson correlation coefficients were ≥ .40 between two reporters/measures, and when Cronbach alpha’s were ≥ .60 among three or more reporters/measures. Based on these criteria, mother and father data were aggregated for the following variables: SB-related family stress, questionnaire reports of family conflict, perceptions of child vulnerability, expectations about the future, questionnaire reports of overprotectiveness, observed overprotectiveness, and medical responsibility. Youth report of family conflict was not aggregated with parent report because the associations did not meet our aggregation criterion.
Data Analyses
To isolate distinct trajectories of medical responsibility, group-based trajectory modeling with SAS PROC TRAJ (Jones, 2018) was conducted. The youth’s age at the time of each data collection was employed as the “time” variable. Because data were collected every 2 years and to have adequate sample sizes at each age level, we operationalized age in two-year increments, as follows: age group 1 = 8, 9 years (N = 39); age group 2 = 10, 11 years (N = 58); age group 3 = 12, 13 years (N = 90); age group 4 = 14, 15 years (N = 109); and age group 5 = 16, 17 years (N = 67) which ensured that at least 30 participants were included in each age group. To clarify, and given that the sample ranged in age from 8 to 15 at Time 1, an individual’s Time 1 score on medical responsibility could be placed into any of the age groups 1–4 depending on the age of the participant. For example, if a child was 12 years old at Time 1, this child’s parent-reported medical responsibility score would be placed in age group 3. Then, the child’s scores at ages 14 and 16 would be placed in age groups 4 and 5, respectively. This method of structuring the data permits a longer term 10-year longitudinal view (ages 8–17 years) with only 6 years of data on each participant (Miller & Jawad, 2019).
Because the outcome of interest (parent-reported medical responsibility) was a scale with a possible ceiling and floor effect, we used a Censored Normal Model to analyze the conditional distribution of medical responsibility over age (Jones et al., 2001; Nagin, 1999). An extension of the group-based trajectory modeling using 95% CI on trajectories was also used (Jones, 2018). Because SAS PROC TRAJ handles missing data on longitudinal outcomes by using the maximum likelihood estimation when estimating parameters for the model as do regular mixed models (Nagin, 2005), no additional imputation of missing data on the longitudinal outcomes was needed for this step in the analysis. Model selection occurred in two stages. In stage 1, the number of trajectory groups that best described the data was determined by fitting models with a predetermined number of groups (two to four) using the quadratic order of the polynomial and comparing the fit indices of the Bayesian information criteria (BIC; Nagin, 2005). The model with the smallest BIC score was selected. In stage 2, the preferred order of the polynomial specifying the shape of each trajectory was determined based on different iterations and the model with the smallest BIC score was again selected. Whenever two models had close BIC scores, Jeffreys’ scale of evidence for Bayes factors (approximated by calculating the exponential of the difference between the two BICs) was used to determine which model was better supported (Nagin, 2005). The adequacy of the final selected model was tested by examining the posterior probabilities of group membership and setting the limit to ≥ 0.7 for all groups (Nagin, 2005).
Next, we conducted multiple imputation (five imputations) to impute missing data for the Time 1 predictors. The pooled estimates were used to describe subgroup differences on Time 1 predictors between youth assigned to different medical responsibility trajectory groups based on chi-squares and between-groups t-tests. Time 1 predictor variables explored in these univariate analyses included demographics, condition-related severity, youth IQ, family system factors, parental attitudes toward and beliefs about the child with SB, and parenting behavior variables. Variables found to be statistically significant (p ≤ .1) in the univariate analyses were selected as candidates and, then, entered into a logistic regression model using the multiply imputed data. The pooled estimates were used to explore the association between these variables and membership in the medical responsibility trajectory groups. Backward selection in the logistic regression was used and the significance level was set at .05 to achieve the final model. In other words, all independent variables that met the significance criterion in the univariate analyses (p < .10) were entered as a block of predictors into the logistic regression (with the trajectory group variable as the outcome variable) and then predictors were removed one step at a time, based on which one had the highest p-value at a given step, until only variables with p < .05 remained. The multiple imputation and all analyses based on the multiply imputed data with pooled estimates were conducted in IBM SPSS Version 26 (IBM Corp, 2019).
Results
Trajectories of Medical Responsibility
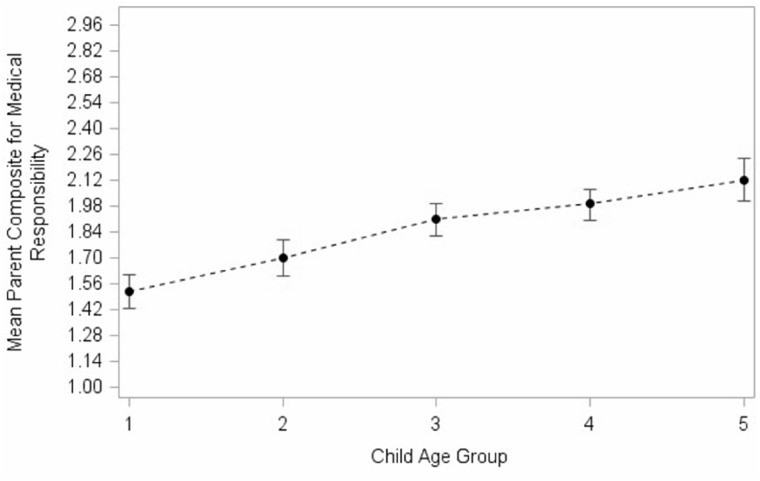
The mean parent composite scores for medical responsibility ranged from 1.52 (1.43, 1.61 95% CI) for age group 1 to 2.12 (2.01, 2.24 95% CI) for age group 5, with all means increasing as a function of age group (Figure 1), indicating that, based on parent report, youth with SB assumed more medical responsibility as they grew older.
Figure 1.
Mean parent composite score for medical responsibility by child age group. Child age group 1 = 8, 9 years; child age group 2 = 10, 11 years; child age group 3 = 12, 13 years; child age group 4 = 14, 15 years; child age group 5 = 16, 17.
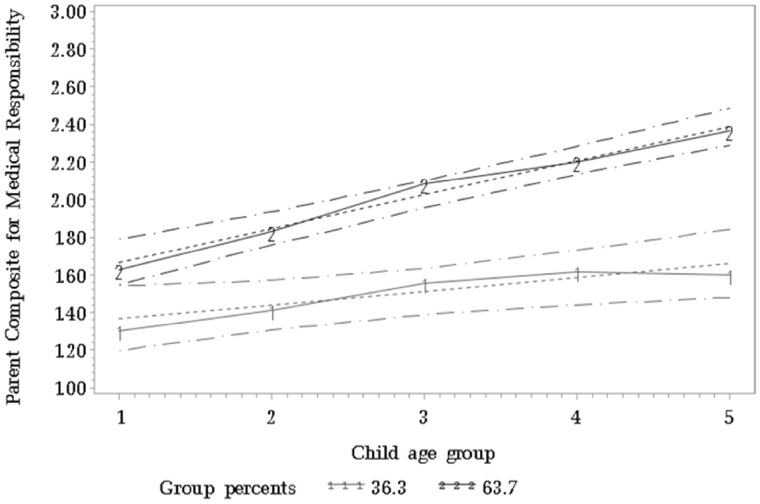
Group-based trajectory analysis revealed that a two-group model was the best fit for the data with group 1 demonstrating a low increasing trajectory and group 2 demonstrating a high increasing trajectory of medical responsibility (Figure 2). The low increasing medical responsibility trajectory group included 36% of participants (group 1); the trajectory for this group increased for age groups 2 and 3, and remained relatively stable for age groups 4 and 5 (intercept = 1.28, SE = 0.09; slope = 0.08, SE = 0.03, p < .05). The high increasing medical responsibility trajectory group included 64% of participants (group 2); this trajectory started at a higher score for age group 1, and remained consistently higher for each age group compared with the low increasing group (intercept = 1.48, SE = 0.07; slope = 0.18, SE = 0.02, p < .05). The high increasing group also demonstrated a consistent increase in medical responsibility scores with increasing age group compared with the low increasing group, who demonstrated relative stability in medical responsibility scores for age groups 3, 4, and 5. The difference in mean scores on medical responsibility between the two trajectory groups for each age group ranged between 0.30 (age group 1) and 0.79 (age group 5), a difference that increased with increasing age (Figure 2).
Figure 2.
Group-based trajectory analysis of parent composite score for medical responsibility (range 1–3 with 1 = parent-only, 2 = shared responsibility, and 3 = child-only responsibility). Child age group 1 = 8, 9 years; child age group 2 = 10, 11 years; child age group 3 = 12, 13; child age group 4 = 14, 15; child age group 5 = 16, 17.
Variables Associated With the Medical Responsibility Trajectory Groups
Results of the univariate analyses examining differences between the low and high increasing trajectory groups, as a function of the Time 1 predictors, are presented in Table 2. The high increasing trajectory group had a higher percentage of female youth compared with the low increasing trajectory group (60% vs. 43%, respectively). Ethnicity/race and SES were not different between the two groups. The high increasing group had lower SB severity compared with the low increasing group. Specifically, the high increasing group had fewer myelomeningocele diagnoses (84% vs. 96%), fewer thoracic (11% vs. 28%), and more sacral (37% vs. 21%) lesions, lower prevalence of shunt placements (72% vs. 91%), and a lower average number of shunt revision surgeries (1.5 vs. 4.2) as compared with the low increasing trajectory group. The high increasing group also scored higher on the WASI IQ (mean = 90.2, SE = 2.03) compared with the low increasing group (mean = 74.2, SE = 2.81).
Table II.
Univariate Analyses by Medical Responsibility Trajectory Group
| Low increasing group |
High increasing group |
||||
|---|---|---|---|---|---|
| Variable | N | Mean (SE) or % | N | Mean (SE) or % | t- or chi-square valueª |
| Total N | 47 | 93 | |||
| Demographic variables | |||||
| Gender | 3.93* | ||||
| Male | 27 | 57% | 37 | 40% | |
| Female | 20 | 43% | 56 | 60% | |
| Ethnicity/race | 0.183 | ||||
| Caucasian | 25 | 53% | 53 | 57% | |
| Non-Caucasian | 22 | 47% | 40 | 43% | |
| Hollingshead SES status | 47 | 35.7 (2.38) | 93 | 40.6 (1.63) | −1.71 |
| Condition-related severity variables | |||||
| SB type | 3.98* | ||||
| Myelomeningocele | 44 | 96% | 78 | 84% | |
| Other | 2 | 4% | 15 | 16% | |
| Lesion level | 9.08* | ||||
| Thoracic | 13 | 28% | 10 | 11% | |
| Lumbar | 24 | 51% | 48 | 52% | |
| Sacral | 10 | 21% | 35 | 37% | |
| Shunt present | 6.38* | ||||
| Yes | 43 | 91% | 67 | 72% | |
| No | 4 | 9% | 26 | 28% | |
| Number of shunt revision surgeries | 47 | 4.2 (0.92) | 93 | 1.5 (0.40) | 2.65* |
| Youth IQ | |||||
| WASI IQ score | 47 | 74.2 (2.81) | 93 | 90.2 (2.03) | −4.50* |
| Family system variables | |||||
| Family Stress Scale | 47 | 2.2 (0.1) | 93 | 1.9 (0.06) | 2.12* |
| Parent–Adolescent Conflict (Y) | 47 | 1.7 (1.0) | 93 | 1.6 (0.06) | 0.85 |
| Parent–Adolescent Conflict (P) | 47 | 1.7 (0.08) | 93 | 1.8 (0.06) | −1.06 |
| Observed Family Conflict | 47 | 2.0 (0.06) | 93 | 2.1 (0.05) | −0.62 |
| Observed Family Cohesion | 47 | 3.3 (0.06) | 93 | 3.4 (0.04) | −2.1* |
| Parental attitudes variables | |||||
| Vulnerable Child Scale | 47 | 2.1 (0.07) | 93 | 1.9 (0.05) | 3.00* |
| Questions about the Future | 47 | 2.9 (0.12) | 93 | 3.4 (0.06) | −4.32* |
| Parenting behavior variables | |||||
| Parent Protection Scale | 47 | 1.3 (0.05) | 93 | 1.1 (0.03) | 3.17* |
| Observed Overprotection | 47 | 1.6 (0.07) | 93 | 1.4 (0.03) | 3.26* |
Note. Y = youth report; P = parent report.
Group comparisons on continuous variables conducted with between-groups t-tests (imputed data); Categorical variables compared with chi-square tests.
p < .1.
In terms of family system variables, the high increasing group had lower scores on parent-reported family stress (mean = 1.9, SE = 0.06 vs. mean = 2.2, SE = 0.1) and somewhat higher observed family cohesion (mean = 3.4, SE = 0.04 vs. mean = 3.3, SE = 0.06) compared with the low increasing group. Youth and parent report on the PAC Scale and observed family conflict were not associated with trajectory group. In terms of parenting attitudes, the high increasing group scored lower on the parent-reported VCS (mean = 1.9, SE = 0.05 vs. mean = 2.1, SE = 0.07) but higher on the Questions about the Future scale (mean = 3.4, SE = 0.06 vs. mean = 2.9, SE = 0.12) compared with the low increasing group. In terms of parenting behaviors, the high increasing trajectory group had lower scores on the parent-reported PPS (mean = 1.1, SE = 0.03 vs. mean = 1.3, SE = 0.05) and on observed parent overprotection (mean= 1.4, SE = 0.03 vs. mean= 1.6, SE = 0.07) compared with the low increasing group.
Results of the logistic regression analysis with medical responsibility trajectory group as the outcome are presented in Table 3. Only gender, IQ, and the FSS were retained in the final model. Female youth with SB were 2.65 times more likely to be in the high increasing medical responsibility group compared with males. An increase of 10 units in IQ was associated with 1.70 greater odds of being in the high increasing trajectory group. A one unit increase in the level of SB-specific family stress was associated with 0.35 times the odds (i.e., lower odds) of being in the high increasing trajectory group.
Table III.
Multivariate Logistic Regression Analysis Predicting Medical Responsibility Trajectory Group Membership (High vs. Low Increasing)
| Variable | Odds ratio | 95% CI | p-value |
|---|---|---|---|
| Female gender | 2.65 | 1.14, 6.17 | .02 |
| IQ (per 10 U) | 1.70 | 1.31, 2.20 | <.001 |
| Family Stress Scale | 0.35 | 0.15,0.79 | .01 |
Discussion
The purpose of this study was to determine if there are distinct developmental trajectories of medical responsibility in youth with SB across ages 8–17 years. We also sought to identify condition-related, parental, and family systems predictors of membership in these trajectory groups. The findings revealed that there were two distinct developmental trajectories that characterized this sample, with one being labeled “high increasing” (two thirds of the sample). This trajectory began in late childhood at a relatively high level (i.e., the intercept was 1.48 on a scale that ranges from 1.0 to 3.0) and increased over the adolescent period. The other trajectory was labeled “low increasing” (one third of the sample). This trajectory group began at a lower level than the high increasing group (i.e., the intercept was 1.28) and was characterized by a slope that rose less rapidly; thus, the two trajectories diverged (i.e., with a widening gap) over the course of adolescence. With respect to the predictors of group membership, most of the predictor variables were significantly associated with trajectory group, with the exception of ethnicity, SES, and the measures of conflict. When compared with those in the low increasing group, participants in the high increasing group were more likely to be female and have less severe forms of SB, higher IQs, less parent-reported vulnerability, higher parental expectations for the future, less parental overprotectiveness, less family stress, and more observed family cohesion. When all significant univariate predictors were allowed to enter the same model, only IQ, family stress, and gender were retained as significant.
Although we predicted that there would be three distinct trajectory groups, analyses revealed that a two-group solution best fit the data. A “flat” trajectory group did not emerge, indicating that nearly all youth in our sample were granted at least some responsibility for their medical care over the first four data collections. Although most of the youth in this sample showed relatively rapid increases in the acquisition of medical responsibility (i.e., the “high increasing” group), there is a smaller, but significant, portion of the sample that did not exhibit this type of developmental trajectory (i.e., the “low increasing” group).
The intercept for the high increasing group was higher (1.48) than the intercept for the low increasing group (1.28) and the slope for the high increasing group (0.18) was over two times the slope for the low increasing group (0.08), thus producing the widening gap between the two groups over time. Put another way, and given that the scale for the medical responsibility measure ranges from 1.0 to 3.0, a change of 0.72 (4 * slope value of 0.18) in the high increasing group from ages 8–9 to 16–17 represents an increase of 48% from the intercept of 1.48, which appears to be a clinically significant level of change in medical responsibility. The corresponding change in the low increasing group was 25%. Moreover, a fine-grained analysis of the level of each trajectory group across age periods is also revealing. Specifically, a mean score of 2.0 (“shared responsibility”) is the pivot point between parental control of more responsibilities (i.e., < 2.0) and child control of more responsibilities (i.e., > 2.0). The low increasing trajectory group never rose above 2.0, indicating that parents in this group tend to retain control over more responsibilities than the child over the course of the adolescent developmental period. The high increasing group crossed this threshold of 2.0 roughly at ages 12–13 years, indicating that the child is in control of more medical responsibilities than the parents during the bulk of the adolescent period (although it is important to note that scores near 2.0 may indicate that the majority of responsibilities are shared). Given these findings, the advantages of conducting the group-based trajectory analyses are clear (Nagin, 2005). Specifically, if we were to only report the average trajectory for the entire sample (Figure 1), we would have missed the observation that there is a subset of youth with SB who do not acquire significant levels of medical responsibility at any point during adolescence (Figure 2).
The levels of most variables that were tested as predictors of group trajectory membership differed significantly across the two groups. On the one hand, it appears that youth in the low increasing group were more likely to be male, have more severe forms of SB and lower IQs, and come from less cohesive and more highly stressed families where parents are more intrusive and perceive their child as more vulnerable. On the other hand, the story changes when we examine the results of the logistic regression. Specifically, the magnitude of the IQ effect on group differentiation appeared to attenuate (i.e., reduce to non-significance) the effects of most of the other predictors. Importantly, the mean IQ for the low increasing group (74.2) was one standard deviation lower than the mean IQ for the high increasing group (90.2). It may be that when a child with SB also has a low IQ, parents are more likely to reduce their expectations, view their child as more vulnerable, become more intrusive, and their families become less cohesive. Moreover, IQ attenuated the results of the SB severity variables as well. In other words, many of the significant condition related, familial, and parental differences between the two trajectory groups appear to be secondary to differences in IQ between the two groups.
Despite the attenuating impact of IQ, two predictors uniquely differentiated the two trajectory groups above and beyond the effect of IQ. Specifically, SB-related family stress was associated with the pace at which a child acquires medical responsibilities, thus making such stress an important target for future interventions. Simply put, by reducing family stress, we may be able to enhance a family’s ability to transfer responsibilities to the child. In addition, gender was also related to trajectory group differentiation after controlling for IQ. It is not entirely clear why males are less likely than females to manage their own health care regimen, although the finding that males lag developmentally behind females fits well with the larger literature on typically developing youth (Taylor, 1985). It is worth noting that similar findings for gender and IQ emerged in a different study conducted by our research team with a different sample and with a general measure of autonomy development (Friedman et al., 2009).
It is of interest that SES, ethnicity, and all of the reported and observed family conflict variables were not associated with trajectory group. The findings for SES were surprising given our past findings that decision-making trajectories are less steep for youth from lower SES households than those from higher SES households (Devine et al., 2011). On the other hand, the analyses from that earlier study focused on decision making across typical areas of family life, such as who decides when the child does homework. The findings of this study reveal that SES appears to have less of an impact on responsibilities related to SB-related self-management. The family conflict findings were also somewhat surprising given findings with other chronic illness populations regarding associations between family conflict and medical responsibility (Campbell et al., 2019). But, it is also important to note that family conflict does not increase during adolescence in families of youth with SB in the same manner that it does in families of typically developing youth (Jandasek et al., 2009); thus, level of conflict may be a less salient factor during familial negotiations of medical responsibility.
It is often stated that the transfer of medical responsibility from parent to child is a normative process in families of youth with chronic health conditions and that a premature transfer or, alternatively, an inappropriately constrained transfer may be problematic (Reed-Knight et al., 2014; Wysocki et al., 1996). Could it be that the youth in the low increasing group were inappropriately constrained with respect to their acquisition of responsibilities and that their parents should be encouraged to grant more responsibility to them in the future? Although speculative, we would argue that the answer to this question would probably be “no,” particularly given the predictive correlates of this trajectory. In other words, although we seem to be observing different processes across these two trajectory groups, both processes could be adaptive. That is, the transfer of responsibility from parent to child is probably not a one-size-fits-all process, where the same pace at which responsibilities are transferred is equally adaptive for all youth. Instead, the transfer process will probably advance more adaptively if it is tailored to the needs of each individual with SB. For example, when a child has a low IQ and high SB severity, the transfer process should probably advance more slowly. In this study, it appears that parents of such youth “put the brakes on” the process (appropriately) by becoming more protective, viewing the child as more vulnerable, and reducing their expectations (Wysocki et al., 1996).
Although this longitudinal study had several strengths, including the collection of multi-method and multi-respondent data and the use of group-based trajectory analyses, the study also had several limitations that should be taken into account when interpreting the findings. First, the sample size was relatively small, thus limiting the power of the analyses, particularly when including multiple predictors in the logistic regression analysis. Second, we do not yet know how these trajectories will unfold across young adulthood or whether a two trajectory solution will fit well for young adults, given that the sample did not include youth > 18 years old. Third, parent report of medical responsibility was employed rather than youth report; the absence of youth report in this study is a limitation. Related to this is a concern about common method variance since some of the predictors were also based on parent report. Fourth, the measure of medical responsibility used in this study (SOSBMR) has not been the focus of a thorough validation study; on the other hand, it has been associated, in the directions hypothesized, with measures of numerous other individual- and family-level constructs. Fifth, only Time 1 scores on the predictors (e.g., family stress) were included; thus, changes in these variables over time were not accounted for in the analyses. Finally, other types of variables (e.g., youth psychosocial adjustment, executive functioning) were not included as predictors. Thus, this study was not entirely comprehensive with respect to factors that may have an impact on the transfer of medical responsibilities.
The findings also have several important clinical and research implications. Two of the most robust predictors of trajectory group were variables that are not amendable to interventions, namely, IQ and gender, but the presence of a low IQ and being male can alert the clinician that responsibilities might be transferred (normatively) more slowly for such individuals. For IQ, a 10- to 15-min screening such as the one that was employed in this study (i.e., the two subtest WASI), could be employed; if the child scores below a predetermined level, more comprehensive services may be needed to facilitate the successful acquisition of self-management skills. On the other hand, the 6-item FSS that was employed in this study is a modifiable predictor, but it too could be used as a clinical screener since high levels of such stress appear to undermine the transfer process. More generally, when working with families of youth with SB, pediatric psychologists can use these non-modifiable and modifiable predictors of trajectory group to inform other health professionals about possible expectations for the transfer of medical responsibilities.
It will be important for clinicians working with youth with SB to recognize that the transfer of medical responsibility from parent to child cannot be expected to unfold in the same manner or at the same pace for all families of youth with SB. Clinically, we might work with families in the “low increasing” group on other ways to maximize opportunities for mastery and self-efficacy (e.g., a child with low IQ may not be able to manage their entire bowel program, but may be able to decide which fiber-rich foods to eat). For those clinicians who are developing interventions to support self-management in SB, our results suggest that interventions which involve the entire family may be the most helpful in facilitating the transfer of responsibility.
Finally, with respect to future research, these findings show that it will be important to consider both individual and contextual factors beyond age as an indicator of development. Future work could explore mechanisms by which male gender, low IQ, and family stress impact medical responsibility. For example, increased family stress may lead to poorer quality parent–child relationships, which may lead to lower medical responsibility. Future work could also use the trajectories identified in this study to predict other indicators of independence, such as living independently or obtaining employment or post-secondary education.
Acknowledgments
The authors thank the Illinois Spina Bifida Association as well as staff of the spina bifida clinics at Ann & Robert H. Lurie Children’s Hospital of Chicago, Shriners Hospital for Children-Chicago, and Loyola University Medical Center. They also thank the numerous undergraduate and graduate research assistants who helped with data collection and data entry. Finally, they would like to thank the parents, children, teachers, and health professionals who participated in this study.
Funding
This work was supported in part by research grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (No. R01 HD048629), the March of Dimes Birth Defects Foundation (No. 12-FY13-271), and the National Institute of Nursing Research (No. R01 NR016235).
Conflicts of interest: None declared.
References
- Anderson B. J., Auslander W. F., Jung K. C., Miller J. P., Santiago J. V. (1990). Assessing family sharing of diabetes responsibilities. Journal of Pediatric Psychology, 15, 477–492. [DOI] [PubMed] [Google Scholar]
- Bakula D. M., Mullins L. L. (2018). Stress, conflict, and the family system in pediatric cancer. Journal of Pediatric Psychology, 43, 599–500. [DOI] [PubMed] [Google Scholar]
- Boulet S. L., Yang Q., Mai C., Kirby R. S., Collins J. S., Robbins J. M., Meyer R., Canfield M. A., Mulinare J., Mulinare J, for the National Birth Defects Prevention Network (2008). Trends in the postfortification prevalence of spina bifida and anencephaly in the United States. Birth Defects Research, 82, 527–532. https://doi.510.1002/bdra.20468 [DOI] [PubMed] [Google Scholar]
- Campbell M. S., Wang J., Cheng Y., Cogen F. R., Streisand R., Monaghan M. (2019). Diabetes-specific family conflict and responsibility among emerging adults with type 1 diabetes. Journal of Family Psychology, 33, 788–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copp A. J., Adzick N. S., Chitty L. S., Fletcher J. M., Holmbeck G. N., Shaw G. M. (2015). Spina bifida. Nature Reviews Disease Primers, 1, 155007 https://doi.155010.151038/nrdp.152015.155007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox M. J., Brooks-Gunn J. (Eds.) (1999). Conflict and cohesion in families: causes and consequences. Erlbaum. [Google Scholar]
- Dennis M., Barnes M. A. (2010). The cognitive phenotype of spina bifida meningomyelocele. Developmental Disabilities Research Reviews, 16, 31–39. https://doi.10.1002/ddrr.1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine K. A., Wasserman R. M., Gershenson L. S., Holmbeck G. N., Essner B. (2011). Mother-child agreement regarding decision-making autonomy: A longitudinal comparison study of families of adolescents with and without spina bifida. Journal of Pediatric Psychology, 36, 277–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll C. F. B., Ohanian D. M., Ridosh M. M., Stern A., Wartman E. C., Starnes M., Holmbeck G. N. (2020). Pathways by which maternal factors are associated with youth spina bifida-related responsibility. Journal of Pediatric Psychology, 45, 610–621. https://doi.10.1093/jpepsy/jsaa020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman D., Holmbeck G. N., DeLucia C., Jandasek B., Zebracki K. (2009). Trajectories of autonomy development across the adolescent transition in children with spina bifida. Rehabilitation Psychology, 54, 16–27. [DOI] [PubMed] [Google Scholar]
- Grey M., Schulman-Green D., Knafl K., Reynolds N. R. (2015). A revised self- and family management framework. Nursing Outlook, 63, 162–170. [DOI] [PubMed] [Google Scholar]
- Helgeson V. S., Reynolds K. A., Siminerio L., Escobar O., Becker D. (2008). Parent and adolescent distribution of responsibility for diabetes self-care: Links to health outcomes. Journal of Pediatric Psychology, 33, 497–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbein C., Zebracki K., Bechtel C., Papadakis J., Bruno E., Holmbeck G. (2017). Milestone achievement in emerging adulthood in spina bifida: A longitudinal investigation of parental expectations. Developmental Medicine and Child Neurology, 59, 311–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead A. B. (1975). Four factor index of social status. Yale University. [Google Scholar]
- Holmbeck G. N., Coakley R. M., Hommeyer J., Shapera W. E., Westhoven V. (2002). Observed and perceived dyadic and systemic functioning in families of preadolescents with a physical disability. Journal of Pediatric Psychology, 27, 177–189. [DOI] [PubMed] [Google Scholar]
- Holmbeck G. N., DeLucia C., Essner B. S., Kelly L., Zebracki K., Friedman D., Jandasek B. (2010). Trajectories of psychosocial adjustment in adolescents with spina bifida: A 6-year, four-wave longitudinal follow-up. Journal of Consulting and Clinical Psychology, 78, 511–525. https://doi.510.1037/a0019599 [DOI] [PubMed] [Google Scholar]
- Holmbeck G. N., Johnson S. Z., Wills K. E., McKernon W., Rose B., Erklin S., Kemper T. (2002). Observed and perceived parental overprotection in relation to psychosocial adjustment in preadolescents with a physical disability: The mediational role of behavioral autonomy. Journal of Consulting and Clinical Psychology, 70, 96–110. [DOI] [PubMed] [Google Scholar]
- Holmbeck G. N., Westhoven V. C., Phillips W. S., Bowers R., Gruse C., Nikolopoulos T., Wienke C. M., Davison K. (2003). A multimethod, multi-informant, and multidimensional perspective on psychosocial adjustment in preadolescents with spina bifida. Journal of Consulting and Clinical Psychology, 71, 782–796. [DOI] [PubMed] [Google Scholar]
- Holmbeck G. N., Zebracki K., Johnson S. Z., Belvedere M., Schneider J. (2007). Parent-Child Interaction Macro-Coding Manual. Unpublished manual, Loyola University of Chicago. [Google Scholar]
- IBM Corp. (2019). IBM SPSS Regression 26 IBM Corp Downloaded July, 3, 2020. ftp://public.dhe.ibm.com/software/analytics/spss/documentation/statistics/26.0/en/client/Manuals/IBM_SPSS_Regression.pdf.
- Jandasek B., Holmbeck G. N., DeLucia C., Zebracki K., Friedman D. (2009). Trajectories of family processes across the adolescent transition in youth with spina bifida. Journal of Family Psychology, 23, 726–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B. L. (2018). Traj group-based modeling of longitudinal data, available at https://www.andrew.cmu.edu/user/bjones/index.htm, accessed October 18, 2019.
- Jones B. L., Nagin D. S., Roeder K. (2001). A SAS procedure based on mixture models for estimating developmental trajectories. Sociological Methods & Research, 29, 374–393. [Google Scholar]
- Kaugars A. S., Zebracki K., Kichler J. C., Fitzgerald C. J., Greenley R. N., Alemzadeh R., Holmbeck G. N. (2011). Use of the Family Interaction Macro-coding System with families of adolescents: Psychometric properties among pediatric and healthy populations. Journal of Pediatric Psychology, 36, 539–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayle M., Docherty S. L., Sloane R., Tanabe P., Maslow G., Pan W., Shah N. (2019). Transition to adult care in sickle cell disease: a longitudinal study of clinical characteristics and disease severity. Pediatric Blood & Cancer, 66, e27463 https://doi.0.1002/pbc.27463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V. A., Jawad A. F. (2019). Decision-making involvement and prediction of adherence in youth with type 1 diabetes: A cohort sequential study. Journal of Pediatric Psychology, 44, 61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modi A. C., Pai A. L., Hommel K. A., Hood K. K., Cortina S., Hilliard M. E., Guilfoyle S. M., Gray W. N., Drotar D. (2012). Pediatric self-management: a framework for research, practice, and policy. Pediatrics, 129, e473–e485. https://doi.410.1542/peds.2011-1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray C. B., Holmbeck G. N., Ros A. M., Flores D. M., Mir S. A., Varni J. W. (2015). A longitudinal examination of health-related quality of life in children and adolescents with spina bifida. Journal of Pediatric Psychology, 40, 419–430. https://doi.10.1093/jpepsy/jsu1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagin D. S. (1999). Analyzing developmental trajectories: a semiparametric, group-based approach. Psychological Methods, 4, 139–157. [DOI] [PubMed] [Google Scholar]
- Nagin D. S. (2005). Group-based modeling of development. Harvard University Press. [Google Scholar]
- O’Hara L. K., Holmbeck G. N. (2013). Executive functions and parenting behaviors in association with medical adherence and autonomy among youth with spina bifida. Journal of Pediatric Psychology, 38, 675–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin E. C., West P. D., Culley B. S. (1989). Is my child normal yet? Correlates of vulnerability. Pediatrics, 83, 355–363. [PubMed] [Google Scholar]
- Prinz R. J., Foster S., Kent R. N., O’Leary K. D. (1979). Multivariate assessment of conflict in distressed and non-distressed mother-adolescent dyads. Journal of Applied Behavior Analysis, 12, 691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psihogios A. M., Holmbeck G. N. (2013). Discrepancies in mother and child perceptions of spina bifida medical responsibilities during the transition to adolescence: Associations with family conflict and medical adherence. Journal of Pediatric Psychology, 38, 859–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psihogios A. M., Kolbuck V., Holmbeck G. N. (2015). Disease self-management in pediatric spina bifida: A longitudinal investigation of medical adherence, responsibility-sharing, and independence skills. Journal of Pediatric Psychology, 40, 790–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psihogios A. M., Murray C. B., Zebracki K., Acevedo L., Holmbeck G. N. (2017). Testing the utility of a bio-neuropsychosocial model for predicting medical adherence and responsibility during early adolescence in youth with spina bifida. Journal of Pediatric Psychology, 42, 910–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quittner A. L., Glueckauf R. L., Jackson D. N. (1990). Chronic parenting stress: Moderating versus mediating effects of social support. Journal of Personality and Social Psychology, 59, 1266–1278. [DOI] [PubMed] [Google Scholar]
- Rassart J., Luyckx K., Berg C. A., Oris L., Wiebe D. J. (2017). Longitudinal trajectories of benefit finding in adolescents with type 1 diabetes. Health Psychology, 36, 977–986. [DOI] [PubMed] [Google Scholar]
- Reed-Knight B., Blount R. L., Gilleland J. (2014). The transition of health care responsibility from parents to youth diagnosed with chronic illness: a developmental systems perspective. Families, Systems, & Health, 32, 219–234. https://doi.210.1037/fsh0000039 [DOI] [PubMed] [Google Scholar]
- Sand P., Kleiberg A. N., Forsander G. (2013). The reliability and validity of the Diabetes Family Responsibility Questionnaire, in a sample of Swedish children with Type 1 diabetes and their parents. Journal of Nursing Education and Practice, 3, 165–171. [Google Scholar]
- Sawin K. J. (2017). Definitions, frameworks, and theoretical issues in self-management. Journal of Pediatric Rehabilitation, 10, 169–176. [Google Scholar]
- Sawin K. J., Bellin M. H. (2010). Quality of life in individuals with spina bifida: A research update. Developmental Disabilities Research Reviews, 16, 47–59. https://doi.10.1002/ddrr.1096 [DOI] [PubMed] [Google Scholar]
- Stern A., Driscoll C. F. B., Ohanian D., Holmbeck G. (2018). A longitudinal study of depressive symptoms, neuropsychological functioning, and medical responsibility in youth with spina bifida: Examining direct and mediating pathways. Journal of Pediatric Psychology, 43, 895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarazi R. A., Zabel T. A., Mahone E. M. (2008). Age-related differences in executive function among children with spina bifida/hydrocephalus based on parent behavior ratings. The Clinical Neuropsychologist, 22, 585–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. (1985). Developmental rate is the major differentiator between the sexes. Behavior and Brain Sciences, 8, 459–460. [Google Scholar]
- Thomasgard M., Metz W. P., Edelbrock C., Shonkoff J. P. (1995). Parent-child relationship disorders: I. Parental overprotection and the development of the Parent Protection Scale. Journal of Developmental and Behavioral Pediatrics, 16, 244–250. [PubMed] [Google Scholar]
- Wechsler D. (1999). Manual for the Wechsler abbreviated intelligence scale (WASI). The Psychological Corporation. [Google Scholar]
- Wiebe D. J., Chow C. M., Palmer D. L., Butner J., Butler J. M., Osborn P., Berg C. A. (2014). Developmental processes associated with longitudinal declines in parental responsibility and adherence to type 1 diabetes management across adolescence. Journal of Pediatric Psychology, 39, 532–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P., Holmbeck G. N., Neff R. (2002). Adolescent health psychology. Journal of Consulting and Clinical Psychology, 70, 828–842. [PubMed] [Google Scholar]
- Wysocki T., Taylor A., Hough B. S., Linscheid T. R., Yeates K. O., Naglieri J. A. (1996). Deviation from developmentally appropriate self-care autonomy. Association with diabetes outcomes. Diabetes Care, 19, 119–125. [DOI] [PubMed] [Google Scholar]
- Zukerman J. M., Devine K. A., Holmbeck G. N. (2011). Adolescent predictors of emerging adulthood milestones in youth with spina bifida. Journal of Pediatric Psychology, 36, 265–276. https://doi.210.1093/jpepsy/jsq1075 [DOI] [PMC free article] [PubMed] [Google Scholar]