Abstract
We describe a case of a 19-year-old female presenting with Mycobacterium bovis meningitis, a rarely encountered infection. We discuss the use of pyrosequencing to aid in prompt diagnosis of M. bovis infection, as well as treatment strategies and challenges given the organism’s intrinsic resistance to pyrazinamide.
Keywords: bovis, meningitis, tuberculosis
Despite advances in antituberculous chemotherapy, tuberculous meningitis (TBM) remains associated with significant morbidity and mortality [1, 2]. Although most commonly associated with Mycobacterium tuberculosis (Mtb), other members of the Mtb complex can cause central nervous system (CNS) disease. Indeed, in the early 20th century, Mycobacterium bovis (M. bovis) caused ~25% of cases of TBM [3] and was particularly a concern for young children [4]. As M. bovis infection is largely attributed to transmission from cattle and consumption of unpasteurized milk, its incidence has dramatically decreased in developed countries following implementation of milk pasteurization and bovine tuberculosis surveillance. However, these improvements have not been universal, and substantial rates of M. bovis disease have been noted in immigrant communities [5, 6]. Rarely, cases of meningitis with M. bovis BCG have been reported complicating BCG vaccination [7].
There remain significant gaps in our understanding of M. bovis disease and its management. While M. bovis has been associated with a higher proportion of extrapulmonary manifestations than Mtb [8], rates of CNS disease might be similar [5]. Published experience in the management of CNS M. bovis, however, is limited to individual case reports and small series [5, 9–12], and there are no major treatment guidelines available.
We report a case of a young female presenting with disseminated M. bovis infection complicated by meningitis and stroke secondary to TB vasculitis, managed with the addition of corticosteroids, aspirin, and levofloxacin to her TB regimen.
CASE PRESENTATION
A 19-year-old female with a medical history of cerebral palsy, developmental delay, and prior seizures presented to an outside hospital with nausea, vomiting, abdominal distension, and fevers. She was found to have bilateral tubo-ovarian abscesses (TOAs) and was treated for presumed pelvic inflammatory disease with ampicillin-sulbactam, metronidazole, and doxycycline. Following discharge, she continued to have worsening abdominal distension, nausea, anorexia, and weight loss. She presented again to an outside hospital about 6 months later.
Computed tomography (CT) imaging of the abdomen and pelvis at that time was notable for bilateral TOA, mesenteric and omental nodularity, and bilateral pulmonary nodules. Drainage from her TOA and tissue from a retroperitoneal lymph node biopsy were both smear-negative for acid fast bacilli (AFB). However, both samples were positive for M. tuberculosis complex on molecular testing (TOA fluid via real-time PCR at Quest Diagnostics, San Juan Capistrano, CA, USA, and lymph node tissue via Cepheid Xpert MTB/RIF assay). She was diagnosed with tuberculosis with peritoneal and pulmonary involvement and was transferred to our hospital for ongoing management.
Her developmental history was notable for premature birth and being nonverbal at baseline. Social history was notable for prior residence on a farm in Mexico, where she lived in close proximity to cattle. She was not known to be immunocompromised, and fourth-generation HIV enzyme-linked immunosorbent assay was negative. She had no history of treatment for active or latent tuberculosis and no known tuberculosis (TB) contacts.
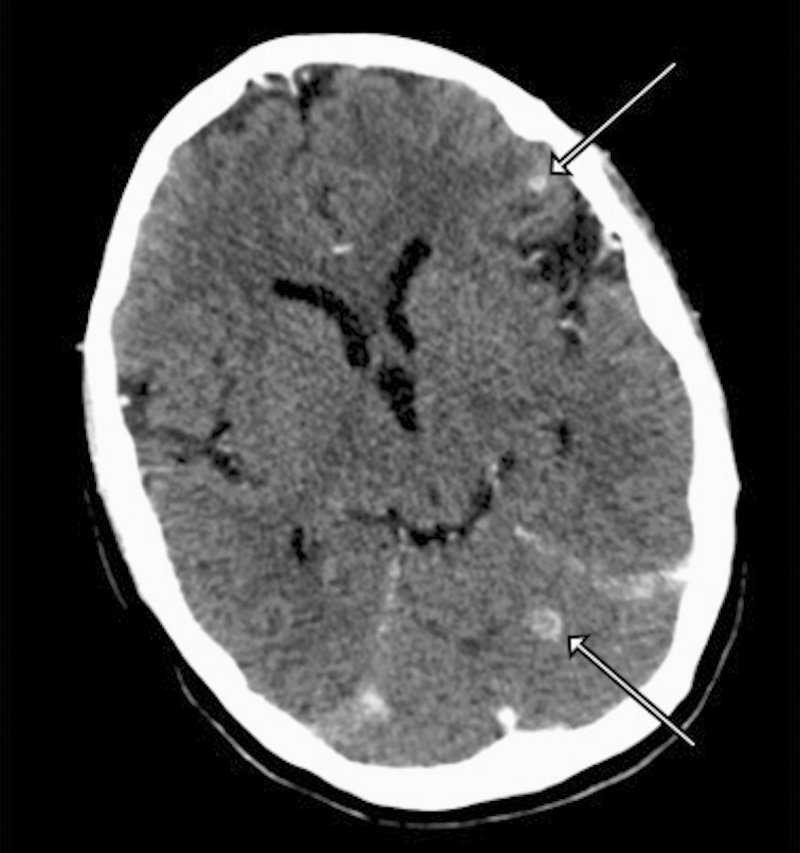
On presentation to our hospital, she was noted to have nuchal rigidity and ongoing nausea and vomiting. A head CT scan demonstrated multiple small ring-enhancing parenchymal lesions in the left frontal lobe and left cerebellar vermis, along with bilateral supratentorial sulcal enhancement concerning for CNS tuberculosis with tuberculomas and meningitis (Figure 1).
Figure 1. .
Contrast-enhanced computed tomography imaging on presentation demonstrating tuberculomas (arrows) in the left frontal lobe and left cerebellar vermis.
Treatment for tuberculosis was initiated—rifampin 600 mg/d (15 mg/kg; patient weight, 38 kg), isoniazid 300 mg/d, pyrazinamide 750 mg/d, and ethambutol 600 mg/d—and the patient was started on adjunctive dexamethasone 0.4 mg/kg intravenously daily, using the tapered regimen reported by Thwaites and colleagues [13]. Cerebrospinal fluid (CSF) testing showed a white blood cell count of 115 cells/mcL (56% lymphocytes), protein 98 mg/dL, and glucose 20 mg/dL. AFB smear was negative, but CSF cultures were ultimately positive for M. tuberculosis complex. Culture from her TOA fluid also grew M. tuberculosis complex, whereas culture from her retroperitoneal lymph node biopsy was negative.
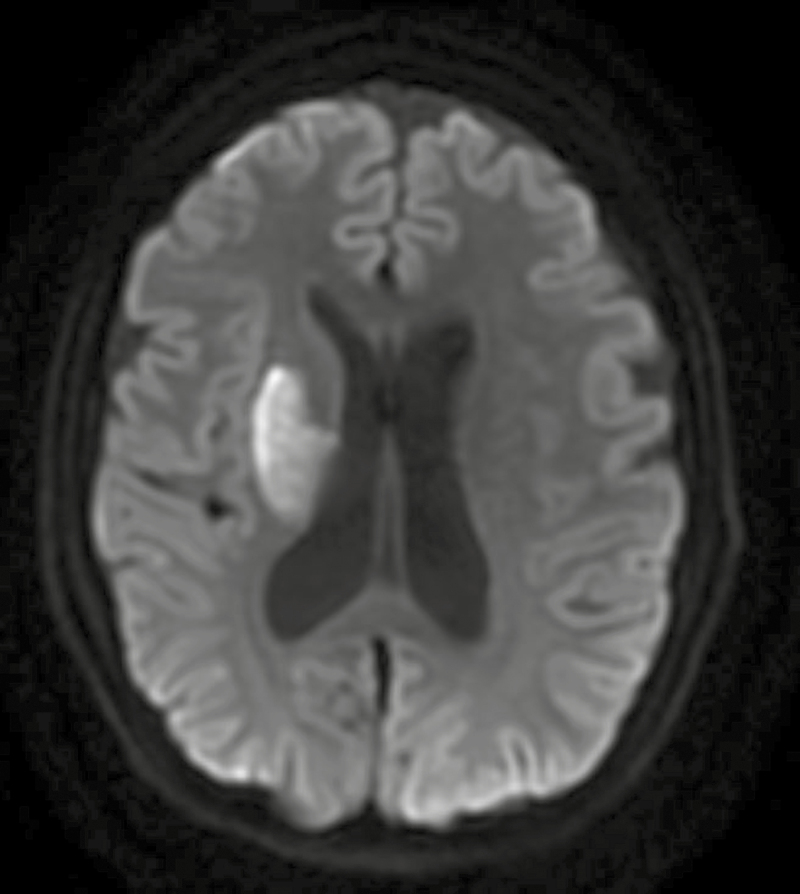
After 2 days of treatment, ethambutol was discontinued due to concern for difficulty monitoring potential ocular toxicity given her cognitive impairment, and levofloxacin 750 mg/d was started. On day 5 of hospitalization, she was noted to have new left-sided hemiplegia, and magnetic resonance imaging of the brain was consistent with acute infarct likely secondary to TB vasculitis (Figure 2). Aspirin 162 mg daily was initiated due to its potential benefit in stroke reduction in TBM [14, 15].
Figure 2. .
Diffusion-weighted image (DWI) magnetic resonance imaging sequence demonstrating acute ischemic infarct affecting the right basal ganglia. Areas of restricted diffusion were visualized in the right corona radiata, posterior limb of the internal capsule, and putamen, as well as in the left anterior centrum semiovale.
Once a sample of pelvic abscess fluid yielded growth of acid fast bacilli, the sample was sent by clinician request to the California Department of Public Health for pyrosequencing and showed that her disease was actually due to M. bovis, based on pncA sequencing. Pyrazinamide was discontinued given the organism’s intrinsic resistance, and she was started on linezolid 600 mg/d in order to provide an additional agent with good CNS penetration pending full drug susceptibility test results. Phenotypic susceptibility testing performed via MGIT on isolates from pelvic fluid, and CSF showed that her M. bovis was resistant to pyrazinamide but susceptible to isoniazid, rifampin, ethambutol, and streptomycin. Additional susceptibility testing for her CSF isolate was performed at National Jewish Health via the indirect proportion method on 7H11 agar and showed susceptibility to all tested drugs (amikacin, capreomycin, ethionamide, levofloxacin, moxifloxacin, para-aminosalicylic acid (PAS), and rifabutin). Pyrosequencing also did not detect any resistance-associated mutations for isoniazid, rifampin, quinolones, amikacin, kanamycin, or capreomycin. Linezolid was stopped after 1 month due to induced anemia (nadir hemoglobin 9.3 g/dL from 12.7g/dL). The patient continued treatment with isoniazid, rifampin, levofloxacin, and aspirin, and dexamethasone taper was initiated. She remained stable on this regimen, with an anticipated total treatment duration of 12 months. There has been no clinical evidence of recurrent infarcts, and there were no new neurological sequelae on follow-up several months later.
CONCLUSIONS
This case highlights some of the clinical challenges that arise in the management of M. bovis meningitis, a rarely encountered infection. There was a significant lag in diagnosis likely related to difficulties with history and exam given her developmental delay. Unfortunately, even when these communication barriers are not present, the diagnosis of TBM may not be initially considered due to nonspecific symptoms such as fever, headache, vomiting, anorexia, and failure to thrive. Delayed diagnosis in TBM remains a major concern given the poor prognosis associated with more advanced disease [1, 9, 16]. The availability of rapid molecular diagnostic tools such as the Cepheid Xpert MTB/RIF assay has the potential to allow for more timely diagnosis of TB, as in the case reported here, where initial body fluid samples were all smear-negative. However, despite the potential utility of Xpert for the diagnosis of extrapulmonary TB [17], its use on nonrespiratory specimens is considered off-label by the US Food and Drug Administration, and testing of such specimens may not be widely available due to need for laboratory validation.
While the prompt recognition of TBM is important for clinical management, working with the clinical laboratory to speciate the mycobacterium may also be helpful, particularly before phenotypic antimicrobial susceptibility results are available. M. bovis is intrinsically resistant to pyrazinamide, so its identification allows this potentially hepatotoxic therapy to be discontinued at an earlier point. In this case, we were fortunate to have access to rapid molecular testing through the California Department of Public Health’s Microbial Diseases Laboratory [18, 19]. The lab’s pyrosequencing assay screens for and reports resistance-associated mutations in multiple bacterial genes associated with drug resistance (ie, rpoB for rifampin); M. bovis is differentiated from M. tuberculosis based on pyrosequencing of the pncA gene [20].
What are the therapeutic implications if pyrazinamide cannot be used in the treatment of TB meningitis? Despite its potential toxicities, pyrazinamide is appealing as a part of CNS TB treatment given its good CNS penetration [21]. The impact of omitting pyrazinamide is not entirely clear in the context of 9–12 months of TB meningitis therapy, though its use has been associated with improved outcomes [22]. In our case, identification of M. bovis through pyrosequencing prompted modification of her treatment regimen to include linezolid, an alternative drug with good CNS penetration [23], albeit with limited published experience in TBM [24]. Additional TB drugs known to have good CNS penetration include isoniazid and fluoroquinolones, both of which were included in her treatment, along with the more poorly tolerated second-line drugs cycloserine and ethionamide [21]. The role for newer TB drugs such as bedaquiline and delamanid in TB meningitis remains to be defined, though CSF concentrations may be low for both [25, 26]. Despite promising initial studies, the addition of a fluoroquinolone has not been shown to confer a mortality benefit in TBM [27–29]. Whether there is a benefit to incorporating a fluoroquinolone or an alternative agent with good CNS penetration into TB treatment regimens when pyrazinamide cannot be used remains uncertain.
In addition to drug choice, drug dosing remains an important consideration in the management of TB meningitis [30]. Rifampin has garnered the most attention in this regard [31] based on animal models suggesting greater efficacy with higher rifampin dosing. Although Ruslami and colleagues found that a 2-week course of intravenous rifampin (13 mg/kg) was associated with reduced mortality compared with standard dosing of oral rifampin (10 mg/kg) [29], a similar mortality benefit was not seen with higher-dose oral rifampin (15 mg/kg) in Vietnam [27]. However, there is ongoing interest in studying even larger doses of rifampin for both pulmonary and meningeal TB [32, 33].
The optimal duration of therapy for pan-susceptible TB meningitis remains a matter of debate, with current US guidelines recommending a duration of 9–12 months [34]. This uncertainty is reflected by the variation observed in clinical practice globally; for example, nearly half of clinicians in 1 Indian study preferred to treat for 18 months [35]. It is uncertain whether treatment beyond 12 months has a role in the setting of pyrazinamide monoresistance.
Despite the availability of effective antimicrobial agents, patients with TB meningitis remain at risk for paradoxical worsening on therapy (labeled immune reconstitution inflammatory syndrome [IRIS] in HIV+ patients) [36] and severe complications such as vasculitis leading to ischemic stroke, which may be driven by a pathologic immune response [37]. Consequently, there has been considerable interest in the use of adjunctive host-directed therapies in tuberculosis [38]. Corticosteroids have been shown to improve mortality [13], whereas aspirin may reduce the risk of stroke [14, 15]. The optimal dosing of aspirin remains uncertain and is an area of ongoing study [39]; we chose to treat the case patient with 162 mg/d of aspirin to approximate the 150-mg/d dosing used by Misra and colleagues [14]. It is not clear whether corticosteroids and aspirin have similar benefit in cases of CNS M. bovis infection, though it seems reasonable to consider their use, as they are generally recommended in TB meningitis.
In 1 retrospective case series conducted in Mexico, CNS M. bovis infection was associated with worse outcomes compared with M. tuberculosis, including increased frequency of tuberculomas and neurological sequelae [9]. Whether these differences in outcomes are due to differences in species virulence or immune response, or instead reflect antimicrobial regimens of differing potency, is not clear. While our patient’s disseminated M. bovis infection was complicated by meningitis, tuberculomas, and stroke, to date she has not had additional neurologic complications in the context of a treatment strategy employing drugs with good CNS penetration such as linezolid and levofloxacin, as well as adjunctive corticosteroids and aspirin. Further study is needed to clarify optimal therapy for M. bovis and pyrazinamide-resistant TBM more generally.
Acknowledgments
Financial support. There were no contributing grants in the production of this article. Publication made possible in part by support from the University of California San Francisco Open Access Publishing Fund.
Potential conflicts of interest. No author has any potential conflict of interest related to this article. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Patient consent. Our article does not include factors necessitating written patient consent. No human subjects experiments were conducted related to this case report; therefore, approval by local ethical committees was not indicated.
Author contributions. K.C. and J.S. participated in the literature review and production of the manuscript. All authors reviewed the final manuscript and approved its contents.
References
- 1. Thwaites GE, van Toorn R, Schoeman J. Tuberculous meningitis: more questions, still too few answers. Lancet Neurol 2013; 12:999–1010. [DOI] [PubMed] [Google Scholar]
- 2. Stadelman AM, Ellis J, Samuels THA, et al. Treatment outcomes in adult tuberculous meningitis: a systematic review and meta-analysis. Open Forum Infect Dis 2020; 7:ofaa257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Griffith AS. Bovine tuberculosis in man. Tuberculosis 1937; 18:529–43. [Google Scholar]
- 4. Novick N. The incidence of bovine infection in tuberculous meningitis. J Med Res 1920; 41:239–46. [PMC free article] [PubMed] [Google Scholar]
- 5. Dankner WM, Waecker NJ, Essey MA, et al. Mycobacterium bovis infections in San Diego: a clinicoepidemiologic study of 73 patients and a historical review of a forgotten pathogen. Medicine (Baltimore) 1993; 72:11–37. [PubMed] [Google Scholar]
- 6. Gallivan M, Shah N, Flood J. Epidemiology of human Mycobacterium bovis disease, California, USA, 2003–2011. Emerg Infect Dis 2015; 21:435–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tardieu M, Truffot-Pernot C, Carriere JP, et al. Tuberculous meningitis due to BCG in two previously healthy children. Lancet 1988; 1:440–1. [DOI] [PubMed] [Google Scholar]
- 8. Dürr S, Müller B, Alonso S, et al. Differences in primary sites of infection between zoonotic and human tuberculosis: results from a worldwide systematic review. Phillips RO, ed. PLoS Negl Trop Dis 2013; 7:e2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gonzàlez-Duarte A, Ponce de Leon A, Sifuentes Osornio J. Importance of differentiating Mycobaterium bovis in tuberculous meningitis. Neurol Int 2011; 3:e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jones PG, Joseph S. Mycobacterium bovis meningitis. JAMA 1982; 247:2270–1. [PubMed] [Google Scholar]
- 11. Wilkins EG, Griffiths RJ, Roberts C, Green HT. Tuberculous meningitis due to Mycobacterium bovis: a report of two cases. Postgrad Med J 1986; 62:653–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Albrecht H, Stellbrink HJ, Eggers C, et al. A case of disseminated Mycobacterium boris infection in an AIDS patient. Eur J Clin Microbiol Infect Dis 1995; 14:226–9. [DOI] [PubMed] [Google Scholar]
- 13. Thwaites GE, Nguyen DB, Nguyen HD, et al. Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N Engl J Med 2004; 351:1741–51. [DOI] [PubMed] [Google Scholar]
- 14. Misra UK, Kalita J, Nair PP. Role of aspirin in tuberculous meningitis: a randomized open label placebo controlled trial. J Neurol Sci 2010; 293:12–7. [DOI] [PubMed] [Google Scholar]
- 15. Mai NT, Dobbs N, Phu NH, et al. A randomised double blind placebo controlled phase 2 trial of adjunctive aspirin for tuberculous meningitis in HIV-uninfected adults. eLife 2018; 7:e33478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lincoln EM, Sordillo SVR, Davies PA. Tuberculous meningitis in children: a review of 167 untreated and 74 treated patients with special reference to early diagnosis. J Pediatr 1960; 57:807–23. [DOI] [PubMed] [Google Scholar]
- 17. Denkinger CM, Schumacher SG, Boehme CC, et al. Xpert MTB/RIF assay for the diagnosis of extrapulmonary tuberculosis: a systematic review and meta-analysis. Eur Respir J 2014; 44:435–46. [DOI] [PubMed] [Google Scholar]
- 18. Lin SY, Rodwell TC, Victor TC, et al. Pyrosequencing for rapid detection of extensively drug-resistant Mycobacterium tuberculosis in clinical isolates and clinical specimens. J Clin Microbiol 2014; 52:475–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lowenthal P, Lin SG, Desmond E, et al. Evaluation of the impact of a sequencing assay for detection of drug resistance on the clinical management of tuberculosis. Clin Infect Dis 2019; 69:668–75. [DOI] [PubMed] [Google Scholar]
- 20. Scorpio A, Collins D, Whipple D, et al. Rapid differentiation of bovine and human tubercle bacilli based on a characteristic mutation in the bovine pyrazinamidase gene. J Clin Microbiol 1997; 35:106–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Donald PR. Cerebrospinal fluid concentrations of antituberculosis agents in adults and children. Tuberculosis (Edinb) 2010; 90:279–92. [DOI] [PubMed] [Google Scholar]
- 22. Jacobs RF, Sunakorn P, Chotpitayasunonah T, et al. Intensive short course chemotherapy for tuberculous meningitis. Pediatr Infect Dis J 1992; 11:194–8. [DOI] [PubMed] [Google Scholar]
- 23. Cresswell FV, Te Brake L, Atherton R, et al. Intensified antibiotic treatment of tuberculosis meningitis. Expert Rev Clin Pharmacol 2019; 12:267–88. [DOI] [PubMed] [Google Scholar]
- 24. Sun F, Ruan Q, Wang J, et al. Linezolid manifests a rapid and dramatic therapeutic effect for patients with life-threatening tuberculous meningitis. Antimicrob Agents Chemother 2014; 58:6297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Akkerman OW, Odish OF, Bolhuis MS, et al. Pharmacokinetics of bedaquiline in cerebrospinal fluid and serum in multidrug-resistant tuberculous meningitis. Clin Infect Dis 2016; 62:523–4. [DOI] [PubMed] [Google Scholar]
- 26. Tucker EW, Pieterse L, Zimmerman MD, et al. Delamanid central nervous system pharmacokinetics in tuberculous meningitis in rabbits and humans. Antimicrob Agents Chemother 2019; 63:e00913-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Heemskerk AD, Bang ND, Mai NT, et al. Intensified antituberculosis therapy in adults with tuberculous meningitis. N Engl J Med 2016; 374:124–34. [DOI] [PubMed] [Google Scholar]
- 28. Thwaites GE, Bhavnani SM, Chau TT, et al. Randomized pharmacokinetic and pharmacodynamic comparison of fluoroquinolones for tuberculous meningitis. Antimicrob Agents Chemother 2011; 55:3244–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ruslami R, Ganiem AR, Dian S, et al. Intensified regimen containing rifampicin and moxifloxacin for tuberculous meningitis: an open-label, randomised controlled phase 2 trial. Lancet Infect Dis 2013; 13:27–35. [DOI] [PubMed] [Google Scholar]
- 30. Davis A, Meintjes G, Wilkinson RJ. Treatment of tuberculous meningitis and its complications in adults. Curr Treat Options Neurol 2018; 20:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Svensson EM, Svensson RJ, Te Brake LHM, et al. The potential for treatment shortening with higher rifampicin doses: relating drug exposure to treatment response in patients with pulmonary tuberculosis. Clin Infect Dis 2018; 67:34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dian S, Yunivita V, Ganiem AR, et al. Double-blind, randomized, placebo-controlled phase II dose-finding study to evaluate high-dose rifampin for tuberculous meningitis. Antimicrob Agents Chemother 2018; 62:e01014–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Boeree MJ, Heinrich N, Aarnoutse R, et al. ; PanACEA consortium High-dose rifampicin, moxifloxacin, and SQ109 for treating tuberculosis: a multi-arm, multi-stage randomised controlled trial. Lancet Infect Dis 2017; 17:39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nahid P, Dorman SE, Alipanah N, et al. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America Clinical Practice Guidelines: treatment of drug-susceptible tuberculosis. Clin Infect Dis 2016; 63:e147–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vibha D, Prasad K. Prevailing practices in the treatment of tuberculous meningitis (TBM): a cross-sectional study. Postgrad Med J 2019; 95:348–9. [DOI] [PubMed] [Google Scholar]
- 36. Singh AK, Malhotra HS, Garg RK, et al. Paradoxical reaction in tuberculous meningitis: presentation, predictors and impact on prognosis. BMC Infect Dis 2016; 16:306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tobin DM, Roca FJ, Oh SF, et al. Host genotype-specific therapies can optimize the inflammatory response to mycobacterial infections. Cell 2012; 148:434–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Frank DJ, Horne DJ, Dutta NK, et al. Remembering the host in tuberculosis drug development. J Infect Dis 2019; 219:1518–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Donovan J, Thwaites GE, Huynh J. Tuberculous meningitis: where to from here? Curr Opin Infect Dis 2020; 33:259–66. [DOI] [PMC free article] [PubMed] [Google Scholar]