Abstract
The NOD-like receptor protein family pyrin domain containing 3 (NLRP3) inflammasome, activated in the setting of HIV, contributes to pro-atherogenic inflammation. Among antriretroviral therapy–naïve people with HIV (vs controls), levels of caspase-1—a key component of the NLRP3 inflammasome—were significantly increased. Six months of elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate significantly decreased caspase-1 levels in association with CD4+/CD8+ ratio recovery.
Trial registration. ClinicalTrials.gov NCT 01766726.
Keywords: atherosclerosis, cardiovascular disease, caspase-1, HIV, NLRP3 inflammasome, pyroptosis
Among people with HIV (PWH), heightened systemic immune activation and inflammation contribute to an increased risk for atherosclerotic cardiovascular disease (ASCVD) [1–3]. Regulation of the immune/inflammatory milieu occurs in part through intracellular multiprotein signaling complexes known as inflammasomes—particularly the NOD-like receptor protein family pyrin domain containing 3 (NLRP3) inflammasome. General population studies suggest that NLRP3 inflammasome activation fuels atherogenesis (Figure 1) [4]. Moreover, administration of a monoclonal antibody therapy targeting an NLRP3 inflammasome-modulated immune pathway was shown in the general population to prevent ASCVD events [5]. Importantly, the HIV virus promotes NLRP3 inflammasome activation within monocytes [6, 7]. Thus, understanding how HIV infection and integrase strand transfer inhibitor (INSTI)–based antiretroviral therapy (ART) affect the NLRP3 inflammasome may elucidate possible mechanisms for heightened ASCVD risk among PWH on ART.
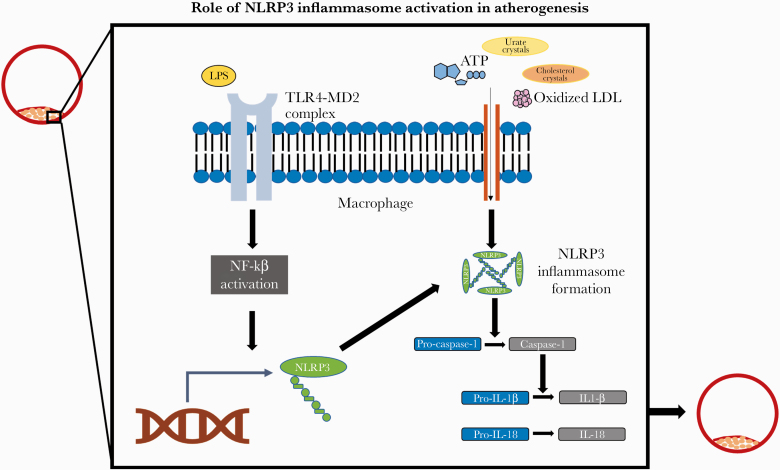
Figure 1.
Role of NLRP3 inflammasome activation in atherogenesis. NLRP3 inflammasome activation within macrophages requires 2 distinct signals. In vitro studies have demonstrated that HIV infection can serve as the first signal for inflammasome activation by stimulating NF-ĸB intracellular signaling within monocytes. NF-ĸB intracellular signaling, in turn, promotes the expression of the protein NLRP3 and the formation of the NLRP3 inflammasome. A distinct secondary signal, such as cholesterol crystals, is then required for inflammasome activation. After inflammasome activation, pro-caspase-1 is cleaved to generate caspase-1, which in turn mediates the generation of pro-inflammatory and pro-atherogenic cytokines, such as IL-18 and IL-1β. Abbreviations: ATP, adenosine triphosphate; IL-18, interleukin-18; IL-1β, interleukin-1 beta; LDL, low-density lipoprotein; LPS, lipopolysaccharide; NLRP3, NOD-like receptor protein family pyrin domain containing 3; NF-kB, nuclear factor–kB; TLR4-MD2, Toll-like receptor 4–myeloid differentiation factor 2.
Activation of the NLRP3 inflammasome results in the generation of caspase-1, a proteolytic enzyme that generates the pro-inflammatory cytokines interleukin-18 (IL-18) and interleukin-1β (1L-1β) [8–10]. Thus, caspase-1 plays a central role in the inflammatory response generated by NLRP3 inflammasome activation. Recent studies have shown that caspase-1 activation also triggers a highly inflammatory form of programmed cell death, pyroptosis, contributing to CD4+ T-cell depletion in HIV infection [11]. Given the influence of HIV on the NLRP3 inflammasome [6, 7, 12], we hypothesized that suppression of HIV viremia with INSTI-based ART among ART-naïve PWH would reduce NLRP3 inflammasome activation, reflected in reduced circulating levels of caspase-1. Moreover, given the role of caspase-1 in mediating CD4+ T-cell depletion in HIV, we further hypothesized that ART-induced changes in caspase-1 levels may relate to concomitant changes reflecting immune recovery.
METHODS
Study Design
Twelve ART-naïve individuals who were newly diagnosed with HIV and who were being started on elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate (E/C/F/TDF) by their infectious disease provider were recruited and enrolled [13]. These participants underwent study assessments at baseline and 6 months after E/C/F/TDF. Twelve participants without HIV—matched based upon cardiovascular disease (CVD) risk scores—were recruited via flyers and online advertisements and also underwent study assessments at baseline [13]. Eligibility criteria were similar for participants in both groups and included no history of coronary artery disease or diabetes. We previously reported the effects of E/C/F/TDF on arterial inflammation [13] and high-density lipoprotein (HDL) cholesterol efflux capacity [14], but the effects of E/C/F/TDF on NLRP3 inflammasome activation have not been previously published.
Patient Consent Statement
The Partners Institutional Review Board approved this study, and written informed consent was obtained from study participants.
Laboratory Assessments
Plasma caspase-1 levels were assessed using an enzyme-linked immunosorbent assay (R&D Systems; Minneapolis, MN, USA; mean minimum detectable limit, 0.68 pg/mL; intra-assay coefficient of variability, 4.9%–6.1%; interassay coefficient of variability, 8.3%–9.4%). HIV viral load was assessed using ultrasensitive reverse transcription polymerase chain reaction (Cobas Ampliprep, Roche Molecular Diagnostics; Pleasanton, CA, USA; lower limit of detection, 20 copies/mL), and CD4+ and CD8+ T-cell counts were assessed using standard techniques.
Statistical Analysis
The primary end point for this analysis was the change in caspase-1 levels among participants with HIV. Between-group comparisons were performed using the Student t test, Wilcoxon rank-sum, or Fisher exact test, as appropriate. The change in caspase-1 in response to INSTI-based ART among participants with HIV was assessed using a Wilcoxon signed-rank test. Bivariate analyses were performed using the Spearman correlation coefficient. All statistical analyses were performed using SAS JMP software, version 12.0 (SAS Institute).
RESULTS
Baseline Characteristics
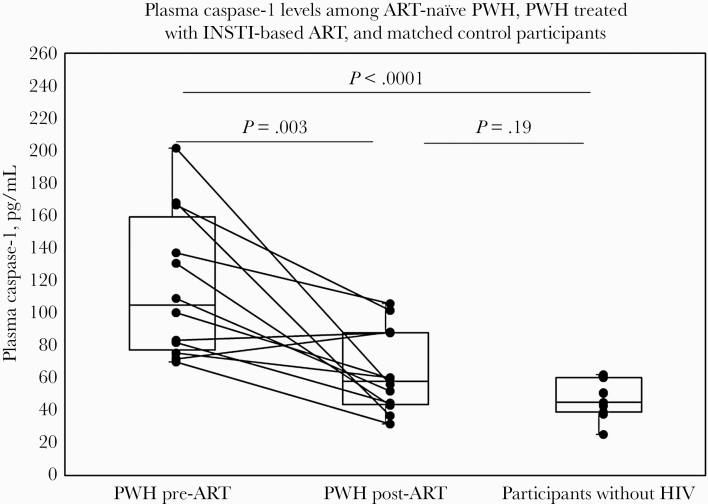
Comparing ART-naïve PWH and participants without HIV, the median age tended to be higher among participants without HIV (median [interquartile range {IQR}], 29 [26–44] years vs 48 [27–51] years; P = .31) (Supplementary Table 1) [13]. Baseline 10-year ASCVD Risk Score was similar between groups (median [IQR], 2.1 [0.7–3.6] vs 1.8 [0.7–5.3]; P = .91). Among PWH, the median duration of HIV infection (IQR) was 0.9 (0.2–1.7) years, baseline CD4+ T-cell count was 483 ± 166 cells/mm3, and baseline log viral load was 4.3 ± 0.6 copies/mL. Baseline plasma caspase-1 was significantly higher among PWH compared with participants without HIV (104.7 [77.2–159.3] vs 45.2 [39.0–60.3] pg/mL; P < .0001) (Figure 2).
Figure 2.
Plasma caspase-1 levels among ART-naïve PWH, PWH treated with INSTI-based ART, and matched control participants. Box plots are used to demonstrate non–normally distributed data. Whiskers represent minimum and maximum data values. Newly diagnosed ART-naïve PWH demonstrated significantly higher plasma caspase-1 levels at baseline as compared with matched participants without HIV. Among PWH, 6 months of treatment with INSTI-based ART (E/C/F/TDF) resulted in a significant reduction in plasma caspase-1 levels. However, among PWH, post-treatment plasma caspase-1 levels tended to remain higher than levels observed among matched participants without HIV. Abbreviations: ART, antiretroviral therapy; E/C/F/TDF, elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate; INSTI, integrase strand transfer inhibitor; PWH, people with HIV.
Effects of Integrase Inhibitor–Based ART on Plasma Caspase-1 Levels and HIV-Specific Parameters
Among PWH, levels of plasma caspase-1 were significantly reduced after 6 months of E/C/F/TDF (104.7 [77.2–159.3] to 57.9 [43.7–88.1] pg/mL; P = .003) (Figure 2). Caspase-1 levels among PWH treated with E/C/F/TDF tended to remain higher than levels among participants without HIV, but this difference was not statistically significant (57.9 [43.7–88.1] pg/mL vs 45.2 [39.0–60.3] pg/mL; P = .19). As expected, among PWH, 6 months of E/C/F/TDF resulted in a reduction in log viral load (4.3 ± 0.6 to 1.3 ± 0.03 copies/mL; P < .0001), an increase in CD4+ T-cell count (483 ± 166 to 698 ± 197 cells/mm3; P = .0004), and an increase in the CD4+/CD8+ ratio (0.6 ± 0.3 to 0.9 ± 0.4; P = .0003) [13].
Relationship Between ART-Induced Changes in Plasma Caspase-1 Levels and ART-Induced Changes in HIV-Specific Parameters
Among PWH, following 6 months of E/C/F/TDF, change in plasma caspase-1 level was inversely related to change in CD4+/CD8+ ratio (ρ = –0.66; P = .02). The change in plasma caspase-1 levels did not relate to changes in log viral load, CD4+ T-cell count, or CD8+ T-cell count (data not shown).
DISCUSSION
In our study, ART-naïve individuals newly diagnosed with HIV (vs matched controls) had significantly higher systemic levels of caspase-1, reflective of increased NLRP3 inflammasome activation. Among ART-naïve PWH, INSTI-based ART with E/C/F/TDF resulted in a significant reduction in caspase-1 levels. However, post-ART levels of caspase-1 still tended to be higher than those among matched participants without HIV. We also found a significant inverse relationship between the change in caspase-1 and the change in a key marker of immune recovery in HIV— the CD4+/CD8+ ratio. These novel findings demonstrate a possible difference in an important inflammatory pathway in atherogenesis—the NLRP3 inflammasome—among participants with vs without HIV.
In our study, we demonstrated that 6 months of an INSTI-based ART regimen significantly lowers plasma caspase-1 levels among ART-naïve PWH. In a prior study, Cai et al. demonstrated that newly initiated ART reduced the percentage of circulating CD4+ T cells expressing caspase-1 [15]. This study differs from our study in several key ways. First, our study assessed the effects of ART on systemic levels of plasma caspase-1. Second, 95% of the participants in the prior study were initiated on a non-nucleoside reverse transcriptase inhibitor (NNRTI)–based ART regimen. Of note, INSTI-based ART regimens have been shown to more potently reduce systemic markers of immune activation, such as soluble CD14 and high-sensitivity C-reactive protein, compared with NNRTI regimens [16]. Such studies highlight the importance of evaluating regimen-specific effects on inflammation and immune activation in HIV. Our study is the first to assess the effects of an INSTI-based ART regimen on NLRP3 inflammasome activation among PWH.
Our study is also the first to show that among newly diagnosed PWH, initiation of an INSTI-based regimen reduces plasma caspase-1 levels in association with bolstering a measure of immune recovery—the CD4+/CD8+ratio [17]. In a study by Serrano-Villar et al., INSTI-based ART regimens (as compared with protease inhibitor– and NNRTI-based regimens) resulted in a greater percentage of PWH achieving CD4+/CD8+ normalization [18]. The effect of INSTI-based ART regimens to improve CD4+/CD8+ ratios may be physiologically related to the role of caspase-1 in CD4+ T-cell depletion in the setting of HIV infection. Caspase-1 has been shown to trigger a highly inflammatory form of programmed cell death, pyroptosis, contributing to CD4+ T-cell depletion among PWH [11]. The destruction of a CD4+ T cell through pyroptosis, in turn, results in the release of pro-inflammatory cytokines that trigger pyroptosis of other nearby CD4+ T cells, resulting in a self-propagating cycle of CD4+ T-cell depletion. Thus, caspase-1-mediated pyroptosis contributes to a hallmark of HIV infection—concomitant immunodeficiency and immune activation [11]. Future studies aimed at investigating how INSTI-based regimens affect caspase-1-mediated pyroptosis may inform our understanding of immune recovery among PWH treated with ART.
Although this study had a small sample size, it focused on an ideal population—ART-naïve PWH—to investigate the effects of ART on an important inflammatory pathway in atherogenesis. The results of this study may not be broadly generalizable to other regimens, and additional studies comparing the regimen-specific effects of newly initiated ART on NLRP3 inflammasome activation are needed. Moreover, as this study included only male participants, the findings will need to be replicated among women with HIV. Lastly, additional studies among PWH are needed to determine how cell-specific inflammasome activation contributes to increased circulating levels of inflammasome proteins and how both processes contribute to atherogenesis—both before and after ART initiation.
In conclusion, we demonstrate for the first time that among newly diagnosed PWH, initiation of an INSTI-based ART regimen significantly reduced NLRP3 inflammasome activation in association with a marker of immune recovery. Given the key role of the NLRP3 inflammasome in atherogenesis, NLRP3 inflammasome activation may serve as a future target to mitigate CVD risk among PWH. One potential immunomodulatory CVD-preventive strategy being explored involves IL-1β antagonism [19]. Statin therapy may represent a second potential immunomodulatory CVD-preventive strategy. Select statins, such as atorvastatin, have been shown to reduce NLRP3 inflammasome activation in the general population [20] and thus may serve to reduce residual NLRP3 inflammasome activation among PWH on ART. The ongoing 7700-participant Randomized Trial to Prevent Vascular Events in HIV (REPRIEVE) [21, 22] will demonstrate whether statin therapy affords ART-treated PWH cardio-protection via effects to dampen key indices of systemic immune activation—including those triggered by NLRP3 inflammasome activation.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank the participants in this study and the Nursing Staff of the Massachusetts General Hospital (MGH) Translation and Clinical Research Center.
Author contributions. M.T. was involved in study concept, study design, data acquisition, data analysis, data interpretation, table and figure preparation, and manuscript writing and revision. T.H.B. was involved in study concept, data acquisition, data interpretation, and manuscript revision. E.S.F. and M.C. were involved in study concept, table and figure preparation, and manuscript revision. S.M.C. was involved in study concept and manuscript revision. M.N.F. was involved in study concept, study recruitment, performing study visits, data acquisition, and manuscript revision. G.K.R. was involved in study concept, study recruitment, and manuscript revision. T.G.N. and K.M. were involved in study concept and manuscript revision. S.K.G. was involved in study concept, study design, and manuscript revision. M.V.Z. was involved in study concept, study design, study recruitment, performing study visits, data acquisition, data interpretation, and manuscript writing and revision.
Financial support. This work was funded using institutional funds and an investigator-initiated grant from Gilead Sciences to S.K.G. M.T. is supported by the National Institutes of Health/National Heart, Lung, and Blood Institute (NIH/NHLBI; grant 1K23HL147799-01) and the American Heart Association–Harold Amos Medical Research Faculty Development Program, which is supported by the Robert Wood Johnson Foundation. T.H.B. has received support from NIH/NHLBI grant R01HL141132. This work was also supported by the Nutrition Obesity Research Center at Harvard (DK040561) and by grants to the Harvard Clinical and Translational Science Center from the NIH/National Center for Research Resources (8 UL 1TR000170 and 1 UL 1TR001102).
Role of sponsor. The sponsor funded the study but had no role in the analysis of the data or the decision to publish the data.
Potential conflicts of interest. M.T., E.S.F., M.A., S.M.C., M.N.F., and G.K.R. have no disclosures to report. T.H.B. has equity in Excision BioTherapeutics, unrelated to the present project. T.G.N. has been a consultant to and received fees from Parexel Imaging, Bristol Myers Squibb, AbbVie, Intrinsic Imaging, and H3-Biomedicine, unrelated to the present project. K.M. is an employee of Gilead Sciences, Inc., and owns stock at Gilead Sciences, Inc. S.K.G. has received research support to his institution from KOWA Pharmaceuticals, Inc., Gilead Sciences, Inc., and ViiV Healthcare and consulting fees from Theratechnologies and ViiV Healthcare, unrelated to the present project. M.V.Z. is Principal Investigator of an Industry-Sponsored Research Grant from Gilead Sciences, Inc., to her institution, unrelated to the present project. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Kearns A, Gordon J, Burdo TH, Qin X. HIV-1-associated atherosclerosis: unraveling the missing link. J Am Coll Cardiol 2017; 69:3084–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zanni MV, Schouten J, Grinspoon SK, Reiss P. Risk of coronary heart disease in patients with HIV infection. Nat Rev Cardiol 2014; 11:728–41. [DOI] [PubMed] [Google Scholar]
- 3. Hsue PY, Deeks SG, Hunt PW. Immunologic basis of cardiovascular disease in HIV-infected adults. J Infect Dis 2012; 205(Suppl 3):S375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grebe A, Hoss F, Latz E. NLRP3 Inflammasome and the IL-1 pathway in atherosclerosis. Circ Res 2018; 122:1722–40. [DOI] [PubMed] [Google Scholar]
- 5. Ridker PM, Everett BM, Thuren T, et al. ; CANTOS Trial Group Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017; 377:1119–31. [DOI] [PubMed] [Google Scholar]
- 6. Hernandez JC, Latz E, Urcuqui-Inchima S. HIV-1 induces the first signal to activate the NLRP3 inflammasome in monocyte-derived macrophages. Intervirology 2014; 57:36–42. [DOI] [PubMed] [Google Scholar]
- 7. Guo H, Gao J, Taxman DJ, et al. HIV-1 infection induces interleukin-1β production via TLR8 protein-dependent and NLRP3 inflammasome mechanisms in human monocytes. J Biol Chem 2014; 289:21716–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guo H, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med 2015; 21:677–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhou W, Chen C, Chen Z, et al. NLRP3: a novel mediator in cardiovascular disease. J Immunol Res 2018; 2018:5702103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baldrighi M, Mallat Z, Li X. NLRP3 inflammasome pathways in atherosclerosis. Atherosclerosis 2017; 267:127–38. [DOI] [PubMed] [Google Scholar]
- 11. Doitsh G, Galloway NL, Geng X, et al. Corrigendum: cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature 2017; 544:124. [DOI] [PubMed] [Google Scholar]
- 12. Kearns AC, Liu F, Dai S, et al. Caspase-1 activation is related with HIV-associated atherosclerosis in an HIV transgenic mouse model and HIV patient cohort. Arterioscler Thromb Vasc Biol 2019; 39:1762–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zanni MV, Toribio M, Robbins GK, et al. Effects of antiretroviral therapy on immune function and arterial inflammation in treatment-naive patients with human immunodeficiency virus infection. JAMA Cardiol 2016; 1:474–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Toribio M, Park MH, Zanni MV, et al. HDL cholesterol efflux capacity in newly diagnosed HIV and effects of antiretroviral therapy. J Clin Endocrinol Metab 2017; 102:4250–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cai R, Liu L, Luo B, et al. Caspase-1 activity in CD4 T cells is downregulated following antiretroviral therapy for HIV-1 infection. AIDS Res Hum Retroviruses 2017; 33:164–71. [DOI] [PubMed] [Google Scholar]
- 16. Hileman CO, Kinley B, Scharen-Guivel V, et al. Differential reduction in monocyte activation and vascular inflammation with integrase inhibitor-based initial antiretroviral therapy among HIV-infected individuals. J Infect Dis 2015; 212:345–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mussini C, Lorenzini P, Cozzi-Lepri A, et al. ; Icona Foundation Study Group CD4/CD8 ratio normalisation and non-AIDS-related events in individuals with HIV who achieve viral load suppression with antiretroviral therapy: an observational cohort study. Lancet HIV 2015; 2:e98–106. [DOI] [PubMed] [Google Scholar]
- 18. Serrano-Villar S, MarMnez-Sanz J, Ron R, et al. CD4/CD8 recovery and first-line ART: greatest improvement with integrase inhibitors. Paper presented at: Conference on Retroviruses and Opportunistic Infections; March 8–11, 2020; Boston, MA. [Google Scholar]
- 19. Hsue PY, Li D, Ma Y, et al. IL-1β inhibition reduces atherosclerotic inflammation in HIV infection. J Am Coll Cardiol 2018; 72:2809–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Satoh M, Tabuchi T, Itoh T, Nakamura M. NLRP3 inflammasome activation in coronary artery disease: results from prospective and randomized study of treatment with atorvastatin or rosuvastatin. Clin Sci (Lond) 2014; 126:233–41. [DOI] [PubMed] [Google Scholar]
- 21. Grinspoon SK, Fitch KV, Overton ET, et al. ; REPRIEVE Investigators Rationale and design of the randomized trial to prevent vascular events in HIV (REPRIEVE). Am Heart J 2019; 212:23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Grinspoon SK, Douglas PS, Hoffmann U, Ribaudo HJ. Leveraging a landmark trial of primary cardiovascular disease prevention in human immunodeficiency virus: introduction from the REPRIEVE coprincipal investigators. J Infect Dis 2020; 222:S1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.