Abstract
We report a case of acute weight gain after switching from emtricitabine/tenofovir disoproxil to emtricitabine/tenofovir alafenamide for human immunodeficiency virus pre-exposure prophylaxis.
Keywords: emtricitabine/tenofovir alafenamide, pre-exposure prophylaxis, weight gain
An estimated 1.2 million people in the United States are at high risk of human immunodeficiency virus (HIV) acquisition whereas approximately 40 000 are newly infected annually [1]. Human immunodeficiency virus pre-exposure prophylaxis (PrEP) is a safe and effective intervention to prevent HIV acquisition among patients at high risk [2–4]. Daily emtricitabine/tenofovir disoproxil fumurate (F/TDF) became the first regimen to receive US Food and Drug Administration (FDA) approval for HIV PrEP in 2012 [5]. Yet, concerns regarding TDF-related renal and bone toxicity remained [6, 7]. The DISCOVER trial recently demonstrated noninferiority of emtricitabine/tenofovir alafenamide (F/TAF) compared with F/TDF for PrEP among men who have sex with men and transgender women resulting in the FDA approval of F/TAF for PrEP in 2019. Significant improvement in renal and bone biomarkers were observed with F/TAF in comparison to F/TDF in DISCOVER [8]. Thus, some clinicians may prefer F/TAF among patients with higher risk for renal and bone toxicity. A growing concern regarding weight gain associated with F/TAF, particularly when in combination with other antiretroviral agents, has emerged from HIV treatment trials [9–11]. However, only small increases in weight were observed among patients randomized to F/TAF in the DISCOVER trial at 96 weeks [8]. In this study, we present a case of rapid weight gain after switching from F/TDF to F/TAF for PrEP.
CASE REPORT
A 61-year-old white, cisgender male with a past medical history of obesity, hypertension, hyperlipidemia, benign prostatic hypertrophy, and allergic rhinitis presented for PrEP initiation in December 2017. His concomitant medications included the following: cetirizine-pseudoephedrine, finasteride, meclizine, olmesartan, and tadalafil. All comorbid conditions were well controlled with his current pharmacotherapy. Before initial PrEP evaluation, he had reported significant intentional weight loss through strict management of diet and regular exercise with the help of a personal trainer and nutritionist. Given his history of obesity, the patient self-monitors his weight on a daily basis to evaluate trends. His reported risk for HIV acquisition was unprotected anal intercourse with multiple male sexual partners. He reported minimal alcohol use (~2–4 drinks/month) and denied tobacco or illicit drug use.
At baseline, his weight and body mass index (BMI) were 99.1 kg and 30.6 kg/m2, respectively. He was initiated on F/TDF for PrEP. The patient denied any tolerability or adherence issues while taking F/TDF. Furthermore, his weight was stable while on F/TDF (±1 kg, per patient report). Approximately 21 months after PrEP initiation, he was switched to F/TAF after its FDA approval to reduce his long-term risk of renal and bone toxicity. Five weeks after the switch to F/TAF, he reported an acute 7.3-kg weight gain representing approximately a 7.4% increase from baseline. At that time, he denied any newly prescribed medications or changes in medications. In addition, he denied any reported changes in his dietary or exercise habits postswitch to F/TAF. He chose to continue with F/TAF despite the weight gain.
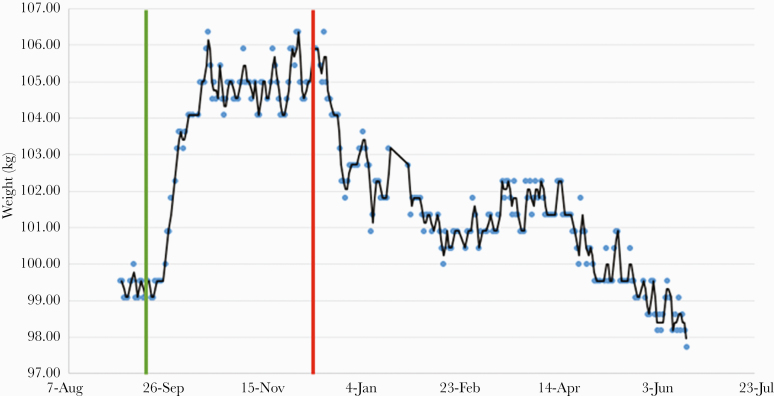
The patient presented to his next scheduled visit 12 weeks after the switch to F/TAF. His weight had plateaued at 105.9 kg. The decision was made to transition back to F/TDF due to his persistent weight gain. Over the following 12 weeks, his weight decreased to 100.5 kg (−5.9 kg), near his baseline weight, representing a decrease of 5.5% from his peak weight while on F/TAF. Again, he denied any newly prescribed or changes in medications or changes in his dietary or exercise habits after his switch back to F/TDF. The patient’s weight continued to decrease over the following weeks and reached his baseline weight 20 weeks after his switch back to F/TDF (Figure 1).
Figure 1.
Weight change after switch to emtricitabine/tenofovir alafenamide (F/TAF). Plot of the patient’s weight diary from September 2, 2019 to June 18, 2020. Daily weights before September 2, 2019 were unavailable (discarded previously). At the time of the switch to F/TAF, he had been on emtricitabine/tenofovir disoproxil fumurate (F/TDF) for 20.3 months. Green line represents the switch from F/TDF to F/TAF. Red line represents the switch from F/TAF back to F/TDF.
Blood pressure readings and lipid markers have remained controlled throughout the entirety of the patient’s course of PrEP. All HIV screening tests have remained negative, and all safety laboratory testing has revealed unremarkable values.
Patient Consent Statement
The patient provided written informed consent for the publication of this case report.
DISCUSSION
This case report documents an episode of rapid weight gain after a switch from F/TDF to F/TAF for PrEP with subsequent weight loss after a transition back to F/TDF. After transition to F/TAF, the patient gained a total of 7.3 kg over a 5-week period, representing a 7.4% increase from baseline. The patient’s weight returned to baseline 20 weeks after he was transitioned back to F/TDF. Notable risk factors for weight gain in this patient included older age and elevated baseline BMI (≥30 kg/m2). Although we cannot attribute causality to F/TAF in this case report, no other contributory etiology was soundly identified.
Several findings from our case report should be considered. First, the patient’s age may have contributed to his weight gain. Studies of weight gain among people with HIV (PWH) initiating and switching antiretroviral therapy (ART) have found an association with demographic factors such as older age (>60 years), female sex, and black race [6, 11]. Although the magnitude of weight gain in this case was greater than that observed in participants receiving F/TAF in the DISCOVER trial (difference, 1.2 kg; F/TAF, 1.7 kg; F/TDF, 0.5 kg), the population included in the DISCOVER trial was overall quite young (F/TAF arm: median age, 34 years; range, 18–72). No other reports of significant weight gain among patients on F/TAF for PrEP have been described.
Our patient’s baseline weight before PrEP initiation may be an important consideration. More importantly, only an estimated 20% of people achieving significant weight loss have been able to maintain their reduced weight [12]. Reasons for weight regain remain unclear, but suggested metabolic mechanisms after significant weight loss may induce an obesogenic environment [13]. However, our case report seemingly demonstrates a single variable (F/TAF) for weight changes.
Controversy currently exists around the impact of antiretroviral agents on body weight among PWH. Much attention has been focused on the anchor agents within ART regimens, particularly with regard to integrase strand transfer inhibitors and more specifically with dolutegravir [10, 14]. However, more recent data from both naive and switch studies highlight the role of the nucleoside/nucleotide reverse-transcriptase inhibitor backbone (specifically F/TAF) in the observed weight gain [6, 9–11]. Our case demonstrates similar weight gains (7.3 kg) yet within a considerably shorter period (5 weeks).
Another important take away from this case is the weight loss that followed the switch back to F/TDF because this may have important implications for the clinician struggling to manage the patient who experiences weight gain after a switch from F/TDF to F/TAF for PrEP. Although the mechanism is unclear, F/TDF was associated with suppression of weight and body fat accumulation compared with placebo in the iPrEX trial [15] and highlights the role F/TDF may have contributed in the patient’s ability to lose weight after his switch back to this regimen.
There are several limitations contained in this case report. Most notably, the case report relies on the subjective report from the patient. Additional information on the patient’s exercise and food patterns during this time period would have strengthened the report. Furthermore, although the patient anecdotally reported most fat accumulation in the truncal area, we do not have quantifiable diagnostic testing to confirm changes in weight attributed to limb or truncal fat gain or changes in metabolic rate.
CONCLUSIONS
This case report highlights an abrupt weight gain in a patient switching from F/TDF to F/TAF for PrEP and a subsequent weight loss after transition back to F/TDF. Weight gain and obesity may be of important consideration when either initiating or switching to F/TAF for PrEP, especially among older patients and those with higher baseline BMI and/or risk for metabolic comorbidities. Important differences with regard to the metabolic impacts of TDF and TAF may exist and should be further explored. Finally, further studies are needed to properly evaluate weight changes when initiating and discontinuing F/TAF for PrEP.
Acknowledgments
We thank the patient for allowing publication of the manuscript.
Potential conflicts of interest. J. P. H. and S. H. B. report grants from Gilead Sciences, Inc., outside of the submitted work. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Smith DK, Van Handel M, Wolitski RJ, et al. Vital signs: estimated percentages and numbers of adults with indications for preexposure prophylaxis to prevent HIV acquisition–United States, 2015. MMWR Morb Mortal Wkly Rep 2015; 64:1291–5. [DOI] [PubMed] [Google Scholar]
- 2. Celum C, Baeten JM. Tenofovir-based pre-exposure prophylaxis for HIV prevention: evolving evidence. Curr Opin Infect Dis 2012; 25:51–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Choopanya K, Martin M, Suntharasamai P, et al. ; Bangkok Tenofovir Study Group Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2013; 381:2083–90. [DOI] [PubMed] [Google Scholar]
- 4. Grant RM, Lama JR, Anderson PL, et al. ; iPrEx Study Team Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010; 363:2587–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ogbuagu O, Podzamczer D, Salazar L, et al. Longer-term safety of F/TAF and F/TDF for HIV PrEP: DISCOVER trial week-96 results. Conference of Retroviruses and Opportunisitc Infections (Boston, MA). March 8–11, 2020. [Google Scholar]
- 6. Mulligan K, Glidden DV, Anderson PL, et al. ; Preexposure Prophylaxis Initiative Study Team Effects of emtricitabine/tenofovir on bone mineral density in HIV-negative persons in a randomized, double-blind, placebo-controlled trial. Clin Infect Dis 2015; 61:572–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Solomon MM, Lama JR, Glidden DV, et al. ; iPrEx Study Team Changes in renal function associated with oral emtricitabine/tenofovir disoproxil fumarate use for HIV pre-exposure prophylaxis. AIDS 2014; 28:851–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. US Food and Drug Administration. Background package for NDA 21-752/Supplement 30, 2012 Available at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/021752Orig1s030MedR.pdf. Accessed 2 July 2020.
- 9. Taramasso L, Berruti M, Briano F, Di Biagio A. The switch from tenofovir disoproxil fumarate to tenofovir alafenamide determines weight gain in patients on rilpivirine-based regimen. AIDS 2020; 34:877–81. [DOI] [PubMed] [Google Scholar]
- 10. Venter WDF, Moorhouse M, Sokhela S, et al. Dolutegravir plus two different prodrugs of tenofovir to treat HIV. N Engl J Med 2019; 381:803–15. [DOI] [PubMed] [Google Scholar]
- 11. Sax PE, Erlandson KM, Lake JE, et al. Weight gain following initiation of antiretroviral therapy: risk factors in randomized comparative clinical trials. Clin Infect Dis 2020; 71:1379–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wing RR, Phelan S. Long-term weight loss maintenance. Am J Clin Nutr 2005; 82:222–5S. [DOI] [PubMed] [Google Scholar]
- 13. Melby CL, Paris HL, Foright RM, Peth J. attenuating the biologic drive for weight regain following weight loss: must what goes down always go back up? Nutrients 2017; 9:468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bourgi K, Rebeiro PF, Turner M, et al. Greater weight gain in treatment-naive persons starting dolutegravir-based antiretroviral therapy. Clin Infect Dis 2020; 70:1267–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Glidden DV, Mulligan K, McMahan V, et al. Metabolic effects of preexposure prophylaxis with coformulated tenofovir disoproxil fumarate and emtricitabine. Clin Infect Dis 2018; 67:411–9. [DOI] [PMC free article] [PubMed] [Google Scholar]