Abstract
Background
Cardiovascular complications, including myocardial infarction, ischemic stroke, and pulmonary embolism, represent an important source of adverse outcomes in coronavirus disease-2019 (COVID-19).
Objectives
To assess the frequency of arterial and venous thromboembolic disease, risk factors, prevention and management patterns, and outcomes in patients with COVID-19, the authors designed a multicenter, observational cohort study.
Methods
We analyzed a retrospective cohort of 1,114 patients with COVID-19 diagnosed through our Mass General Brigham integrated health network. The total cohort was analyzed by site of care: intensive care (n = 170); hospitalized nonintensive care (n = 229); and outpatient (n = 715). The primary study outcome was a composite of adjudicated major arterial or venous thromboembolism.
Results
Patients with COVID-19 were 22.3% Hispanic/Latinx and 44.2% non-White. Cardiovascular risk factors of hypertension (35.8%), hyperlipidemia (28.6%), and diabetes (18.0%) were common. Prophylactic anticoagulation was prescribed in 89.4% of patients with COVID-19 in the intensive care cohort and 84.7% of those in the hospitalized nonintensive care setting. Frequencies of major arterial or venous thromboembolism, major cardiovascular adverse events, and symptomatic venous thromboembolism were highest in the intensive care cohort (35.3%, 45.9%, and 27.0 %, respectively) followed by the hospitalized nonintensive care cohort (2.6%, 6.1%, and 2.2%, respectively) and the outpatient cohort (0% for all).
Conclusions
Major arterial or venous thromboembolism, major adverse cardiovascular events, and symptomatic venous thromboembolism occurred with high frequency in patients with COVID-19, especially in the intensive care setting, despite a high utilization rate of thromboprophylaxis.
Key Words: anticoagulation, cardiovascular disease, coronavirus, COVID-19, deep venous thrombosis, myocardial infarction, pulmonary embolism, stroke, thromboembolism
Abbreviations and Acronyms: ARDS, acute respiratory distress syndrome; CI, confidence interval; COVID-19, coronavirus disease-2019; DVT, deep vein thrombosis; EHR, electronic health record; ICU, intensive care unit; MI, myocardial infarction; OR, odds ratio; PCR, polymerase chain reaction; PE, pulmonary embolism; TIA, transient ischemic attack; VTE, venous thromboembolism
Central Illustration
Coronavirus disease-2019 (COVID-19), caused by severe acute respiratory syndrome-coronavirus-2, has emerged as a devastating global public health crisis. Whereas the morbidity and mortality associated with COVID-19 are usually attributed to acute respiratory distress syndrome (ARDS) and end-organ failure, cardiovascular complications, including myocardial infarction (MI), ischemic stroke, and pulmonary embolism (PE), also cause disability and death in these patients (1, 2, 3). An increased frequency of arterial and venous thrombosis was observed early in the COVID-19 pandemic and has been attributed to systemic inflammation, immobility, and a prothrombotic milieu (4,5). Venous thromboembolism (VTE) is now recognized as among the predominant cardiovascular hazards in patients with COVID-19 (4,6, 7, 8, 9, 10). The frequency of VTE is highest in the intensive care unit (ICU) setting and has ranged from 25%, when symptomatic disease is considered, to 69%, when surveillance venous ultrasonography is performed (4,6, 7, 8). A high prevalence of in situ microthrombosis suspected to be due to endothelial injury from direct viral infection has also been described (11, 12, 13). Quantifying the risk of cardiovascular complications in the heterogeneous population of patients with COVID-19 has been hampered by reports of limited sample size, restriction of assessments to the ICU setting, variable outcome definitions, and differing thromboprophylaxis patterns. Antithrombotic therapy has been recommended for hospitalized patients with COVID-19 to prevent thromboembolic cardiovascular events (1,14, 15, 16); however, a subset of patients appears to experience arterial and venous events despite standard thromboprophylaxis.
To assess frequency of arterial and venous thromboembolic disease, risk factors, prevention and management patterns, and outcomes in patients with COVID-19, we undertook an investigator-initiated, electronic health record (EHR)–guided multicenter observational cohort study (CORONA-VTE [COVID-19 Registry to Assess Frequency, Risk Factors, Management, and Outcomes of Arterial and Venous Thromboembolic Complications]).
Methods
Study design
The study was a retrospective observational cohort analysis using data abstracted through the EHR within the Mass General Brigham integrated health network. Mass General Brigham includes the Massachusetts General Hospital, Brigham and Women’s Hospital, 10 regional hospitals, and several outpatient centers. A silent computerized decision support program was embedded as a Best Practice Advisory within the EHR (EPIC, version 2015; Epic Systems Corporation, Verona, Wisconsin) to identify eligible patients. The computerized decision support identified patients with a laboratory-confirmed diagnosis of COVID-19. The program was “silent” because it provided no notification to the clinician regarding its presence or findings. The Mass General Brigham Medical Informatics team designed the program. We confirmed 100% accuracy of the program by reviewing the records of every patient in the registry. No action was required by the provider of record. The study was approved by the Institutional Review Board of Mass General Brigham. The Institutional Review Board waived the requirement of informed consent.
Study population
From March 13, 2020, to April 3, 2020, our computerized decision support program identified 1,114 consecutive patients 18 years or older and who tested positive for severe acute respiratory syndrome-coronavirus-2 infection based on polymerase chain reaction (PCR) testing (sensitivity 73.3%, specificity 98.6%, false positive rate from 1% to 1.5%) (17). All hospitalized patients were admitted for COVID-19–related respiratory symptoms, including COVID-19 pneumonia or ARDS. Patients admitted to the critical care setting required mechanical ventilation, hemodynamic support, or increased monitoring.
Data collection
Data were gathered electronically through the EPIC Best Practice Advisory and manually entered by trained, experienced research assistants (J.E.S., S.R., M.B.P.), a research nurse (R.B.M.), and a physician (O.L.) and managed using the Research Electronic Data Capture, or REDCap tool hosted at Brigham and Women’s Hospital (18). Patient demographics and baseline clinical characteristics were recorded, including age, sex, race, ethnicity, and comorbid conditions. Data were collected regarding underlying cardiovascular disease and risk factors, COVID-19 presentation and diagnosis, laboratory testing, disease-specific management, concomitant medications, thromboprophylaxis, and 30-day outcomes. Whereas no COVID-19–specific guidelines for thromboembolism prevention were in place within our system at the time of this study, guidance for thromboprophylaxis in hospitalized patients was available through a VTE guidebook accessible through the network intranet.
The study population was analyzed as a total cohort (n = 1,114). Additionally, stratified analysis was performed based on site of care: ICU (n = 170); non-ICU hospitalized (n = 229); and nonhospitalized patients (n = 715). ICU patients were defined as those who were admitted to a critical care setting at any point following diagnosis of COVID-19. Therefore, the 3 cohorts were mutually exclusive.
Thirty-day clinical outcomes of symptomatic VTE, catheter- or device-related arterial thrombosis, MI, non–MI-related coronary revascularization, ischemic stroke, transient ischemic attack (TIA), or systemic embolism; major adverse limb events; heart failure hospitalization; new atrial fibrillation; and myocarditis, including diagnosis and treatments, were extracted from the EHR. Additional outcomes of interest included 30-day all-cause mortality, major and clinically relevant nonmajor bleeding, disseminated intravascular coagulation, and thrombocytopenia. Only the first occurrence of a particular 30-day outcome was counted.
Standardized outcome definitions were adopted and used for outcome adjudication (Supplemental Appendix). Exhaustive EHR review was conducted for each subject, including review of discharge summaries, office notes, diagnostic testing reports, medical treatment summaries, and procedure reports, regardless of the facility or office location. Follow-up data for 30-day outcomes were obtained for 96% of the study cohort. Follow-up was unavailable in 40 outpatients and 5 hospitalized non-ICU patients and 1 ICU patient who all survived to hospital discharge.
Symptomatic VTE was defined as symptomatic deep vein thrombosis (DVT) (including symptomatic catheter- or device-related DVT) or PE, confirmed by imaging, within 30 days of enrollment. No systematic screening protocol for VTE in patients with COVID-19 was in place during the study. Acute ischemic stroke was defined as a new, focal neurologic deficit of sudden onset, lasting ≥24 h, not due to a readily identifiable nonvascular cause (e.g., brain tumor, trauma), as confirmed by a neurologist, imaging, or autopsy. TIA was defined as a transient episode of neurologic dysfunction lasting <24 h and caused by suspected focal cerebral, spinal cord, or retinal ischemia without evidence of acute infarction and confirmed by a neurologist. Systemic embolism was defined as sudden loss of perfusion of a limb or extracranial organ. Acute MI was defined as the detection of a rise and/or fall of cardiac biomarkers (cardiac troponin T), with at least 1 value >99th percentile upper reference limit and with at least 1 of the following: 1) symptoms of myocardial ischemia; 2) new (or presumably new) significant ST-segment/T-wave changes or left bundle branch block; 3) development of pathological Q waves; 4) new loss of viable myocardium or regional wall motion abnormality by imaging; or 5) identification of intracoronary thrombus by angiography or autopsy (19). Heart failure hospitalization was defined as symptoms and signs of heart failure with institution or adjustment of treatment and associated hospitalization or extension of length of stay. Heart failure with preserved ejection fraction and heart failure with reduced ejection fraction were included. Major adverse limb events included acute limb ischemia or critical limb threatening ischemia. Catheter- or device-related arterial thrombosis, in situ arterial thrombosis, and systemic arterial embolism were considered separately. Catheter- or device-related arterial thrombosis was not classified as a major adverse limb event.
Bleeding events were classified by the International Society of Thrombosis and Haemostasis criteria for major and clinically relevant nonmajor bleeding (20). All-cause mortality was determined by review of the EHR. Causes of death were categorized as due to PE, MI, stroke, sudden cardiac death or arrhythmia, other cardiovascular disease, infection, cancer, or other condition.
All study outcomes were adjudicated by a dedicated, independent Clinical Endpoint Committee (U.C., Z.A., and V.N.) composed of 3 cardiovascular medicine specialists (Supplemental Appendix).
Statistical analysis
The primary statistical analysis was the frequency of adjudicated, objectively confirmed major arterial and venous thromboembolic events at 30 days in patients with COVID-19. Major arterial and venous thromboembolic events comprised a composite of symptomatic VTE, catheter- or device-related arterial thrombosis, MI, non–MI-related coronary revascularization, stroke, TIA, or systemic embolism, and major adverse limb events at 30 days. The composite outcome of major adverse cardiovascular events included symptomatic VTE, catheter- or device-related arterial thrombosis, MI, non–MI-related coronary revascularization, stroke, TIA, or systemic embolism; major adverse limb events; heart failure hospitalization; new diagnosis of atrial fibrillation; and myocarditis. Secondary analyses included frequencies of adjudicated all-cause death, major and clinically relevant nonmajor bleeding, disseminated intravascular coagulation, thrombocytopenia, individual thromboembolic outcomes, and other cardiovascular events at 30 days. Means, medians, and frequency distributions were calculated for continuous variables. Number and percentages were reported for binary and categorical variables. Kaplan-Meier plots were presented for the time from COVID-19 PCR diagnosis to each of the 3 major composite outcomes for the inpatient groups. Cumulative incidence estimates and 95% confidence intervals (CIs) accounted for death as a competing risk for symptomatic VTE, major arterial and venous thromboembolism and noncardiovascular death for the major cardiovascular events outcome (21).
We utilized multivariable logistic regression modeling to identify groups of factors that may identify ICU patients with COVID-19 at greatest risk for major adverse outcomes. Variables included in the regression model were selected based on the univariable results and on a priori knowledge. They included age, sex, history of coronary artery disease, prescription of prophylactic anticoagulation, ARDS, and baseline D-dimer level by decile. A bias correction for rare events was used in regressions involving ARDS and symptomatic VTE (22). All analyses were performed using STATA version 15 (STATA Corporation, College Station, Texas) and SAS version 9 (SAS Institute Inc., Cary, North Carolina).
Results
Overall COVID-19 cohort
The overall study cohort of 1,114 patients diagnosed with COVID-19 had a mean age of 50.6 years and mean body mass index of 29.8 kg/m2 (Table 1 ). Women accounted for 54% of the overall study cohort. Patients with COVID-19 were 22.3% Hispanic/Latinx and 44.2% non-White. Cardiovascular risk factors of hypertension (35.8%), hyperlipidemia (28.6%), and diabetes (18.0%) were common (Table 2 ). The most common symptoms of COVID-19 were cough (74.0%), fever (70.7%), and myalgias (51.1%) (Table 3 ). COVID-19–associated pneumonia was the most common complication (37.8%).
Table 1.
Baseline Characteristics and Comorbid Conditions in Patients With COVID-19 by Care Setting
| Intensive Care (n = 170) | Admitted, Nonintensive Care (n = 229) | Outpatient (n = 715) | Total (N = 1,114) | |
|---|---|---|---|---|
| Age, yrs | 61.7 ± 15.8 | 60.6 ± 18.0 | 44.8 ± 16.2 | 50.6 ± 18.3 |
| Female | 64 (37.7) | 106 (46.3) | 433 (60.6) | 603 (54.1) |
| Hispanic/Latinx | 52 (30.6) | 63 (27.5) | 133 (18.6) | 248 (22.3) |
| Race | ||||
| White | 86 (50.6) | 128 (55.9) | 407 (56.9) | 621 (55.8) |
| Black | 17 (10.0) | 27 (11.8) | 113 (15.8) | 157 (14.1) |
| Asian | 6 (3.5) | 9 (3.9) | 23 (3.2) | 38 (3.4) |
| Other | 61 (35.9) | 65 (28.3) | 172 (24.1) | 298 (26.8) |
| BMI, kg/m2 | 30.3 ± 5.7 | 30.1 ± 6.9 | 29.6 ± 11.8 | 29.8 ± 10.1 |
| Active cancer | 11 (6.5) | 15 (6.6) | 14 (2.0) | 40 (3.6) |
| Serum creatinine >2.5 mg/dl | 10 (5.9) | 7 (3.1) | 4 (0.6) | 21 (1.9) |
| Hemodialysis | 7 (70.0) | 6 (85.7) | 1 (25.0) | 14 (66.7) |
| Smoking | ||||
| Current | 9 (5.3) | 12 (5.2) | 29 (4.1) | 50 (4.5) |
| Former | 59 (34.7) | 82 (35.8) | 104 (14.6) | 245 (22.0) |
| Never | 93 (54.7) | 131 (57.2) | 511 (71.5) | 735 (66.0) |
| Chronic lung disease | 35 (20.6) | 61 (26.6) | 111 (15.6) | 207 (18.6) |
| Baseline medications | ||||
| Statin | 127 (74.7) | 146 (63.8) | 91 (12.7) | 364 (32.7) |
| Aspirin | 67 (39.4) | 56 (24.5) | 38 (5.3) | 161 (14.5) |
| 81 mg | 61 (91.0) | 53 (95.0) | 34 (89.5) | 148 (91.9) |
| 325 mg | 5 (7.5) | 2 (3.6) | 3 (7.9) | 10 (6.2) |
| Immunosuppressive | 21 (12.4) | 26 (11.4) | 44 (6.2) | 91 (8.2) |
| P2Y12 inhibitor | 6 (3.5) | 8 (3.5) | 5 (0.7) | 19 (1.7) |
| Dual antiplatelet | 6 (3.5) | 6 (2.6) | 4 (0.6) | 16 (1.4) |
| Therapeutic anticoagulation | 43 (25.3) | 21 (9.2) | 16 (2.3) | 80 (7.2) |
| Unfractionated heparin | 21 (48.8) | 1 (4.8) | 1 (6.3) | 23 (28.8) |
| Enoxaparin | 7 (16.3) | 2 (9.5) | 0 (0.0) | 9 (11.3) |
| Warfarin | 6 (14.0) | 7 (33.3) | 8 (50.0) | 21 (26.3) |
| Apixaban | 7 (16.3) | 7 33.3) | 3 (18.8) | 17 (21.3) |
| Rivaroxaban | 2 (4.7) | 3 (14.3) | 4 (25.0) | 9 (11.3) |
| Thromboprophylaxis | 152 (89.4) | 194 (84.7) | 1 (0.1) | 347 (31.2) |
| LMWH | 109 (71.7) | 159 (69.4) | 1 (100.0) | 269 (77.5) |
| Unfractionated heparin | 46 (30.3) | 34 (14.9) | 0 (0.0) | 80 (23.1) |
| Rivaroxaban | 2 (1.3) | 0 (0.0) | 0 (0.0) | 2 (0.6) |
Values are mean ± SD or n (%).
BMI = body mass index; COVID-19 = coronavirus disease-2019; LMWH = low-molecular weight heparin.
Table 2.
Baseline Cardiovascular Disease and Risk Factors in Patients With COVID-19 by Care Setting
| Intensive Care (n = 170) | Admitted, Nonintensive Care (n = 229) | Outpatient (n = 715) | Total (N = 1,114) | |
|---|---|---|---|---|
| Coronary artery disease | 29 (17.1) | 39 (17.0) | 22 (3.1) | 90 (8.1) |
| MI | 9 (5.3) | 18 (17.9) | 8 (1.1) | 35 (38.9) |
| Prior PCI | 7 (4.1) | 16 (7.0) | 7 (1.0) | 30 (33.3) |
| Stable angina | 4 (2.4) | 5 (2.2) | 5 (0.7) | 14 (15.5) |
| Prior CABG | 4 (2.4) | 6 (2.6) | 1 (0.1) | 11 (12.2) |
| Unstable angina | 0 (0.0) | 1 (0.4) | 1 (0.1) | 2 (2.2) |
| Cardiomyopathy | 5 (2.9) | 10 (4.4) | 6 (0.8) | 21 (1.9) |
| LVEF, % | 40.2 ± 15.6 | 49.1 ± 16.2 | 38.5 ± 12.5 | 44.0 ± 15.2 |
| HFrEF | 4 (2.4) | 12 (5.2) | 3 (0.4) | 19 (1.7) |
| HFpEF | 8 (4.7) | 15 (6.6) | 6 (0.8) | 29 (2.6) |
| Prior atrial fibrillation | 16 (9.4) | 21 (9.2) | 15 (2.1) | 52 (4.7) |
| Prior stroke/TIA | 11 (6.5) | 16 (7.0) | 14 (2.0) | 41 (3.7) |
| Hypertension | 95 (55.9) | 129 (56.3) | 175 (24.5) | 399 (35.8) |
| Hyperlipidemia | 73 (42.9) | 97 (42.4) | 149 (20.8) | 319 (28.6) |
| Diabetes | 66 (38.8) | 68 (29.7) | 67 (9.4) | 201 (18.0) |
| Prior VTE | 9 (5.3) | 10 (4.4) | 19 (2.7) | 38 (3.4) |
Values are n (%) or mean ± SD.
CABG = coronary artery bypass grafting; COVID-19 = coronavirus disease-2019; HFpEF = heart failure preserved ejection fraction; HFrEF = heart failure reduced ejection fraction; LVEF = left ventricular ejection fraction; MI = myocardial infarction; PCI = percutaneous coronary intervention; TIA = transient ischemic attack; VTE = venous thromboembolism.
Table 3.
Characteristics of COVID-19 and Treatment by Care Setting
| Intensive Care (n = 170) | Admitted, Nonintensive Care (n = 229) | Outpatient (n = 715) | Total (N = 1,114) | |
|---|---|---|---|---|
| Symptoms | ||||
| Cough | 124 (72.9) | 184 (80.4) | 516 (72.2) | 824 (74.0) |
| Fever/chills | 138 (81.2) | 189 (82.5) | 460 (64.3) | 781 (70.7) |
| Myalgias | 71 (41.8) | 107 (46.7) | 391 (54.7) | 569 (51.1) |
| Shortness of breath | 133 (78.2) | 137 (59.8) | 228 (31.9) | 498 (44.7) |
| Sore throat | 33 (19.4) | 46 (20.1) | 261 (36.5) | 340 (30.5) |
| Malaise/fatigue | 81 (47.7) | 104 (45.4) | 145 (20.3) | 330 (29.6) |
| Gastrointestinal | 62 (36.5) | 90 (39.3) | 137 (19.2) | 289 (25.9) |
| Coryza | 19 (11.2) | 34 (14.9) | 232 (32.5) | 285 (25.6) |
| Headache | 28 (16.5) | 44 (19.2) | 126 (17.6) | 198 (17.8) |
| Loss of smell/taste | 12 (7.1) | 21 (9.2) | 163 (22.8) | 196 (17.6) |
| Chest pain | 16 (9.4) | 42 (18.3) | 48 (6.7) | 106 (9.5) |
| Length of hospital stay, days | 16 (8–24) | 5 (3–8) | — | — |
| Still hospitalized by 30 days | 49 (28.8) | 4 (1.8) | — | — |
| COVID-19 pneumonia | 164 (96.5) | 189 (82.5) | 68 (9.5) | 421 (37.8) |
| ARDS | 134 (78.8) | 13 (5.7) | 1 (0.1)∗ | 148 (13.3) |
| Mechanical ventilation | 128 (75.3) | 1 (0.4)† | — | 129 (11.6) |
| Multisystem organ failure | 56 (32.9) | 9 (3.9) | 0 (0.0) | 65 (5.8) |
| Sepsis/septic shock | 97 (57.1) | 8 (3.5) | 0 (0.0) | 105 (9.4) |
| Systemic arterial hypotension | 133 (78.2) | 37 (16.2) | 1 (0.1)‡ | 171 (15.3) |
| Laboratory assessment§ | 170 (100.0) | 228 (99.6) | 108 (15.1) | 506 (45.4) |
| LDH, U/l | 404.2 ± 226.4 | 315.7 ± 183.2 | 234.7 ± 97.1 | 343.4 ± 202.9 |
| Lactate, mmol/l | 1.7 ± 1.1 | 1.4 ± 0.7 | 1.3 ± 0.6 | 1.6 ± 0.9 |
| NT-proBNP, pg/ml | 2,124.1 ± 7,937.8 | 1,613.4 ± 7,492.1 | 343.4 ± 983.2 | 1,788 ± 7,473.9 |
| High-sensitivity troponin T, ng/l | 69.0 ± 305.4 | 24.3 ± 71.1 | 10.4 ± 15.3 | 40.5 ± 199.6 |
| D-dimer, ng/ml | 2,515.6 ± 7,913.3 | 1,156.5 ± 1,410.2 | 757.4 ± 994.7 | 1,692.3 ± 5,254.5 |
| High-sensitivity CRP, mg/l | 120.3 ± 83.6 | 72.7 ± 56.7 | — | 97.0 ± 75.8 |
| High-sensitivity IL-6, pg/ml | 327.4 ± 648.8 | 29.2 ± 24.3 | — | 208.1 ± 507.7 |
| COVID-19 specific therapies | ||||
| Azithromycin | 149 (87.7) | 151 (65.9) | 51 (7.1) | 351 (31.5) |
| Hydroxychloroquine | 148 (87.1) | 131 (57.2) | 7 (1.0) | 286 (25.7) |
| Remdesivir | 27 (15.9) | 24 (10.5) | 0 (0.0) | 51 (4.6) |
| IL-6 receptor antagonist | 22 (12.9) | 4 (1.8) | 0 (0.0) | 26 (2.3) |
| Lopinavir/ritonavir | 13 (7.7) | 5 (2.2) | 0 (0.0) | 18 (1.6) |
Values are n (%), median (interquartile range), or mean ± SD. Dashes indicate data were not available.
ARDS = acute respiratory distress syndrome; COVID-19 = coronavirus disease-2019; CRP = C-reactive protein; IL = interleukin; LDH = lactate dehydrogenase; NT-proBNP = N-terminal pro–B-type natriuretic peptide.
Patient was a resident at an assisted living facility and was diagnosed with COVID-19 as an outpatient. When she developed ARDS, she was transitioned to palliative care without hospitalization.
Patient experienced acute respiratory failure while hospitalized in the non–intensive care setting, was initiated on mechanical ventilation, but was ultimately transitioned to comfort measures.
Patient presented to the emergency department with hypotension that resolved with intravenous fluid.
Laboratory reference values: LDH = 110 to 210 U/l; lactate = 0.5 to 2.0 mmol/l; NT-proBNP = 0 to 1,800 pg/ml; high-sensitivity cardiac troponin T = 0 to 9 ng/l; D-dimer < 500 ng/ml; high-sensitivity CRP = 0 to 3 mg/l; high-sensitivity IL-6 < 5.00 pg/ml.
At 30 days from diagnosis, major arterial or VTE events, major adverse cardiovascular events, and symptomatic VTE occurred in 5.9%, 8.3%, and 4.6%, respectively (Table 4 , Figure 1 ). All-cause 30-day mortality was 5.7% in the overall cohort of patients with COVID-19. The most frequent cause of death was sepsis (89.5%) followed by cardiovascular disease (7%).
Table 4.
30-Day Cardiovascular Outcomes of COVID-19 by Care Setting
| Intensive Care (n = 170) | Admitted, Nonintensive Care (n = 229) | Outpatient (n = 715) | Total (N = 1,114) | |
|---|---|---|---|---|
| Major arterial or venous thromboembolic event | 60 (35.3) | 6 (2.6) | 0 (0.0) | 66 (5.9) |
| Major cardiovascular events | 78 (45.9) | 14 (6.1) | 0 (0.0) | 92 (8.3) |
| Symptomatic VTE | 46 (27.0) | 5 (2.2) | 0 (0.0) | 51 (4.6) |
| Symptomatic DVT∗ | 39 (22.9) | 0 (0.0) | 0 (0.0) | 39 (3.5) |
| Upper extremity | 6 (12.5) | — | — | 6 (12.5) |
| Proximal lower extremity | 4 (8.3) | — | — | 4 (8.3) |
| Isolated calf | 3 (6.3) | — | — | 3 (6.3) |
| Catheter-/device-related | 30 (76.9) | — | — | 30 (76.9) |
| Symptomatic PE | 3 (1.8) | 5 (2.2) | 0 (0.0) | 8 (0.7) |
| High-risk | 2 (66.7) | 1 (20.0) | — | 3 (37.5) |
| Intermediate-high-risk | 0 (0.0) | 1 (20.0) | — | 1 (12.5) |
| Intermediate-low-risk | 0 (0.0) | 1 (20.0) | — | 1 (12.5) |
| Low-risk | 1 (33.3) | 2 (40.0) | — | 3 (37.5) |
| Catheter-/device-related arterial thrombosis | 11 (6.5) | 0 (0.0) | 0 (0.0) | 11 (1.0) |
| Disseminated intravascular coagulation | 10 (5.9) | 4 (1.7) | 0 (0.0) | 14 (1.3) |
| Death | 40 (23.5) | 15 (6.7) | 2 (0.3) | 57 (5.1) |
| In-hospital | 39 (97.5) | 15 (100.0) | — | 54 (94.7) |
| Outpatient | 1 (2.5) | 0 (0.0) | 2 (100.0) | 3 (5.3) |
| Thrombocytopenia | 79 (46.5) | 67 (30.6) | 15 (2.1) | 161 (14.5) |
| Platelet nadir, /μl | 105.3 ± 36.7 | 112.9 ± 26.3 | 122.6 ± 23.8 | 110.0 ± 32.0 |
| Myocardial infarction | 13 (7.7) | 1 (0.5) | 0 (0.0) | 14 (1.3) |
| ST-segment elevation | 0 (0.0) | 1 (100.0) | — | 1 (7.1) |
| Non–ST-segment elevation | 13 (100.0) | 0 (0.0) | — | 13 (92.9) |
| Fatal | 1 (7.7) | 0 (0.0) | — | 1 (7.1) |
| Percutaneous intervention | 0 (0.0) | 1 (100.0) | — | 1 (7.1) |
| Medical therapy only | 13 (100.0) | 0 (0.0) | — | 13 (92.9) |
| Stroke | 1 (0.6) | 0 (0.0) | 0 (0.0) | 1 (0.1) |
| Fatal | 1 (100.0) | 1 (100.0) | ||
| Heart failure hospitalization | 5 (2.9) | 1 (0.5) | 0 (0.0) | 6 (0.6) |
| New atrial fibrillation | 21 (12.4) | 6 (2.7) | 0 (0.0) | 27 (2.6) |
| Myocarditis | 7 (4.1) | 0 (0.0) | 0 (0.0) | 7 (0.7) |
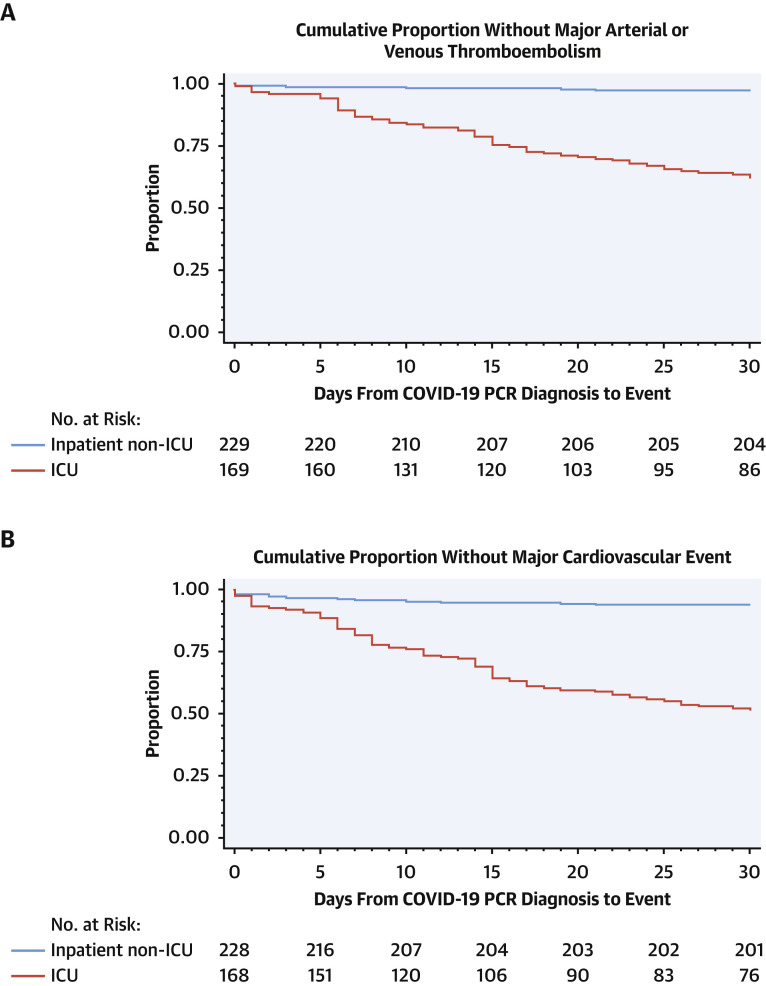
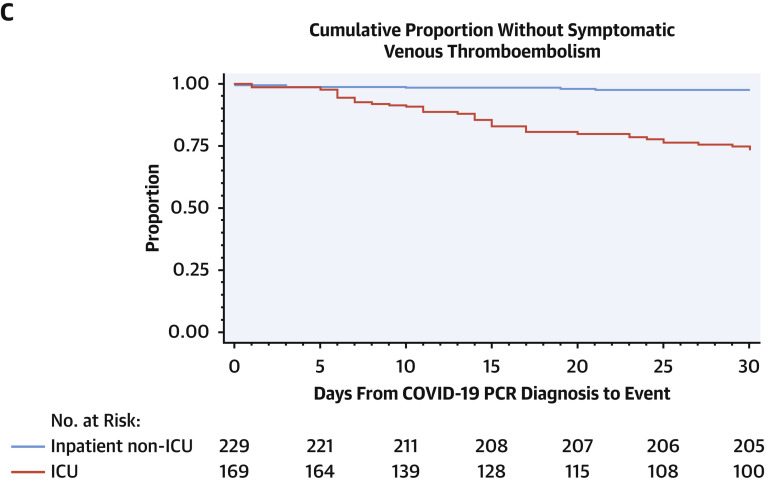
Figure 1.
Time From COVID-19 PCR Diagnosis to Major Composite Outcomes
Kaplan-Meier plots demonstrating time from coronavirus disease-2019 (COVID-19) polymerase chain reaction (PCR) diagnosis to each of the 3 major composite outcomes. Major arterial and venous thromboembolism (A), major cardiovascular events (B), and symptomatic venous thromboembolism (VTE) (C) were more frequent in intensive care unit (ICU) patients than in hospitalized non-ICU patients. Adjusting for competing risk of death, estimated cumulative incidences at 30 days for major arterial and venous thromboembolism, major cardiovascular events, and symptomatic VTE in the ICU cohort were 0.34 (95% confidence interval [CI]: 0.28 to 0.42), 0.44 (95% CI: 0.38 to 0.52), and 0.24 (95% CI: 0.19 to 0.31), respectively. The estimated cumulative incidences at 30 days for major arterial and venous thromboembolism, major cardiovascular events, and symptomatic VTE in the hospitalized non-ICU cohort were 0.03 (95% CI: 0.01 to 0.06), 0.06 (95% CI: 0.03 to 0.1), and 0.02 (95% CI: 0.01 to 0.05), respectively. Three patients had outcomes prior to PCR diagnosis and were excluded from the plots.
ICU patients with COVID-19
The cohort of 170 patients with COVID-19 admitted at any point to an ICU had a mean age of 61.7 years, was predominantly male (62.3%), and was obese (mean body mass index 30.3 kg/m2) (Table 1). Fifty percent of ICU patients with COVID-19 were non-White and 30.6% were Hispanic/Latinx. A history of coronary artery disease (17.1%) and cardiovascular risk factors of hypertension (55.9%), hyperlipidemia (42.9%), and diabetes (38.8%) were particularly common in the ICU cohort (Table 2). Statins, aspirin, and antithrombotic therapy were prescribed at baseline in 74.7%, 32.9%, and 25.3% of patients with COVID-19 in the ICU cohort, respectively. Prophylactic anticoagulation was prescribed in 89.4% of patients with COVID-19 in the ICU cohort. No patients who survived to hospital discharge were prescribed post-discharge thromboprophylaxis for primary prevention.
Fever (81.2%), cough (72.9%), and shortness of breath (78.2%) were the most frequent symptoms of COVID-19 in patients in the ICU cohort (Table 3). Patients with COVID-19 in the ICU cohort had a median length of hospital stay of 16 days and were still hospitalized at 30 days 28.8% of the time. Admission to the ICU was associated with a high frequency of COVID-19–related complications of pneumonia (96.5%), ARDS (78.8%), sepsis (57.1%), and hypotension (78.2%).
At 30 days from diagnosis, major arterial or venous thromboembolic events, major adverse cardiovascular events, and symptomatic VTE occurred in 35.3%, 45.9%, and 27.0% of patients with COVID-19 in the ICU cohort, respectively (Table 4, Figure 1, Central Illustration ). Nearly all occurred during hospitalization (98.3%, 97.4%, and 97.6%, respectively). The estimated cumulative incidences at 30 days for major arterial and venous thromboembolism, major cardiovascular events, and symptomatic VTE in the ICU cohort were 0.34 (95% CI: 0.28 to 0.42), 0.44 (95% CI: 0.38 to 0.52), and 0.24 (95% CI: 0.19 to 0.31), respectively.
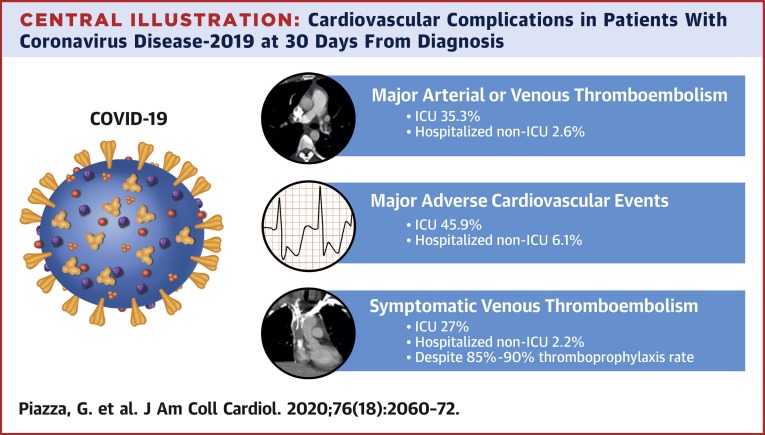
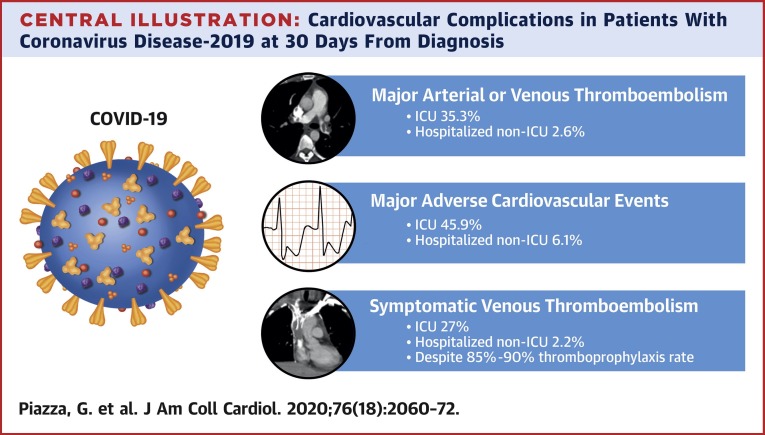
Central Illustration.
Cardiovascular Complications in Patients With Coronavirus Disease-2019 at 30 Days From Diagnosis
Cardiovascular complications, including major arterial or venous thromboembolism, in 1,114 patients with coronavirus disease-2019 (COVID-19) at 30 days from diagnosis. Adjudicated major arterial (including myocardial infarction, stroke/transient ischemic attack, systemic embolism, and major adverse limb events) or venous thromboembolism, major adverse cardiovascular events, and symptomatic venous thromboembolism (VTE) (including catheter- and device-related deep vein thrombosis [DVT]) were frequent in patients with COVID-19 admitted to the intensive care unit (ICU) setting (n = 170). Among those admitted to the non-ICU setting (n = 229), the frequency of major arterial or venous thromboembolism, major adverse cardiovascular events, and symptomatic VTE was also elevated but lower than for those with critical illness. The increased frequency of thromboembolic complications occurred in the context of a relative high rate of thromboprophylaxis prescription. Outpatients (n = 715) were considered to be low risk for major arterial or venous thromboembolism, major adverse cardiovascular events, and symptomatic VTE.
Symptomatic DVT was largely composed of catheter- or device-related thrombosis (76.9%). Symptomatic PE occurred in only 3 ICU patients with COVID-19, but 2 of these were hemodynamically unstable (high-risk) PE. Catheter- or device-related arterial thrombosis occurred in 6.5% of ICU patients. MI occurred in 7.7% of ICU patients and was exclusively non–ST-segment elevation. Among patients with COVID-19 in the ICU cohort, 23.5% were deceased at 30 days, with 97.5% dying while in the hospital and 92.5% of those due to sepsis. Patients with COVID-19 in the ICU cohort who were receiving prophylactic anticoagulation were more likely to have major arterial or VTE events (15.9% vs. 0.7%; p < 0.0001), major adverse cardiovascular events (20.2% vs. 1.1%; p < 0.0001), and symptomatic VTE (11.5% vs. 0.1%; p < 0.0001) than those not receiving thromboprophylaxis.
In patients with COVID-19 in the ICU cohort, ARDS was associated with a 7-fold increased odds of major arterial or VTE events (adjusted odds ratio [OR]: 6.69; 95% CI: 1.85 to 24.14), 6-fold increased odds of major adverse cardiovascular events (adjusted OR: 5.79; 95% CI: 2.01 to 16.69), and 24-fold increased odds of symptomatic VTE (adjusted OR: 24.39; 95% CI: 1.50 to 398.00) (Table 5 ).
Table 5.
Univariate and Multivariate Analysis of Factors Associated With Increased Odds of Adverse Events at 30 Days in Patients With COVID-19 in the Critical Care Setting
| Univariate |
Multivariate |
|||
|---|---|---|---|---|
| OR | 95% CI | Adjusted OR | 95% CI | |
| Major arterial or venous thromboembolic event | ||||
| Age | 0.98 | 0.96–1.00 | 0.97 | 0.95–0.99 |
| Male | 1.33 | 0.69–2.58 | 1.31 | 0.64–2.71 |
| VTE prophylaxis | 1.47 | 0.5–4.35 | 0.76 | 0.22–2.57 |
| ARDS | 8.14 | 2.38–27.87 | 6.69 | 1.85–24.14 |
| D-dimer (decile) | 1.13 | 1.0–1.29 | 1.17 | 1.03–1.33 |
| Major cardiovascular event | ||||
| Age | 1.01 | 0.99–1.03 | 1.00 | 0.98–1.03 |
| Male | 1.92 | 1.02–3.64 | 2.07 | 1.03–4.16 |
| VTE prophylaxis | 1.07 | 0.4–2.85 | 0.58 | 0.18–1.90 |
| ARDS | 7.42 | 2.72–20.25 | 5.79 | 2.01–16.69 |
| D-dimer (decile) | 1.13 | 1.01–1.27 | 1.12 | 0.99–1.27 |
| History of CAD | 0.68 | 0.3–1.53 | 0.71 | 0.27–1.85 |
| Symptomatic VTE | ||||
| Age | 0.98 | 0.96–1.01 | 0.97 | 0.95–1.0 |
| Male | 1.63 | 0.76–3.49 | 1.54 | 0.69–3.44 |
| VTE prophylaxis | 6.07 | 0.78–47.1 | 2.51 | 0.41–15.44 |
| ARDS | 32.4 | 1.87–562.29 | 24.39 | 1.50–398.00 |
| D-dimer (decile) | 1.10 | 0.97–1.25 | 1.11 | 0.97–1.27 |
| Death | ||||
| Age | 1.08 | 1.04–1.11 | 1.08 | 1.05–1.12 |
| Male | 1.82 | 0.83–3.95 | 1.91 | 0.78–4.64 |
| VTE prophylaxis | 0.44 | 0.16–1.21 | 0.63 | 0.18–2.22 |
| ARDS | 2.94 | 0.97–8.89 | 3.23 | 0.87–12.06 |
| D-dimer (decile) | 1.04 | 0.91–1.18 | 0.93 | 0.80–1.09 |
| History of CAD | 1.95 | 0.82–4.63 | 1.09 | 0.38–3.16 |
Non-ICU hospitalized patients with COVID-19
There were 229 hospitalized patients with COVID-19 who were never admitted to the ICU; this cohort had a mean age of 60.6 years, was obese (mean body mass index: 30.1 kg/m2), and was more likely to be male (53.7%) (Table 1). Forty-four percent were non-White and 27.5% were Hispanic/Latinx. Histories of coronary artery disease (17.0%) and cardiovascular risk factors of hypertension (56.3%), hyperlipidemia (42.4%), and diabetes (29.7%) were common in non-ICU hospitalized patients with COVID-19 (Table 2). Statins, aspirin, and antithrombotic therapy were prescribed at baseline in 63.8%, 24.5%, and 9.2% of patients with COVID-19 in the hospitalized, non-ICU cohort, respectively. Prophylactic anticoagulation was prescribed in 84.7% of these patients. No patients were prescribed post-discharge anticoagulation for primary prevention of thromboembolic complications.
Fever (82.5%), cough (80.4%), and shortness of breath (59.8%) were the most frequent symptoms in patients with COVID-19 in the hospitalized non-ICU setting (Table 3). Patients with COVID-19 in the hospitalized non-ICU cohort had a median length of hospital stay of 5 days. COVID-19–associated pneumonia was the most common complication (96.5%).
At 30 days from COVID-19 diagnosis, major arterial or VTE events, major adverse cardiovascular events, and symptomatic VTE occurred in 2.6%, 6.1%, and 2.2%, respectively of patients in the hospitalized non-ICU cohort (Table 4, Figure 1). The estimated cumulative incidences at 30 days for major arterial and venous thromboembolism, major cardiovascular events, and symptomatic VTE in the hospitalized non-ICU cohort were 0.03 (95% CI: 0.01 to 0.06), 0.06 (95% CI: 0.03 to 0.1), and 0.02 (95% CI: 0.01 to 0.05), respectively. Major arterial or venous thromboembolic events, major adverse cardiovascular events, and symptomatic VTE occurred during hospitalization in 50%, 78.6%, and 40% of this cohort, respectively. Those receiving prophylactic anticoagulation were more likely to have major arterial or VTE events (1.4% vs. 0.1%; p = 0.01), major adverse cardiovascular events (3.8% vs. 0.1%; p < 0.0001), and symptomatic VTE (1.4% vs. 0.0%; p<0.0001) than those not receiving thromboprophylaxis.
Among patients with COVID-19 in the hospitalized non-ICU cohort, the frequency of death at 30 days was 6.7%. All deaths occurred during the hospitalization, and 80% were due to sepsis.
Outpatients with COVID-19
The mean age was 44.8 years in 715 patients with COVID-19 who were not hospitalized (Table 1). Patients with COVID-19 in the outpatient cohort were more likely to be female (60.6%), and were 18.6% Hispanic/Latinx and 43.1% non-White. Cardiovascular risk factors of hypertension (24.5%), hyperlipidemia (20.8%), and diabetes (9.4%) were common (Table 2). The most common symptoms of COVID-19 were cough (72.2%), fever (64.3%), and myalgias (54.7%) (Table 3). COVID-19–associated pneumonia was the most common complication (37.8%). Prophylactic anticoagulation was rare in the outpatient cohort. The outpatient cohort had a low risk of major arterial or VTE events, major adverse cardiovascular events, and symptomatic VTE (Table 4). Two outpatients died by 30 days due to sepsis without hospitalization, after being designated as hospice care.
Discussion
Major arterial or VTE events, major adverse cardiovascular events, and symptomatic VTE occurred with high frequency over 30 days in patients with COVID-19 in the ICU cohort, despite a nearly 90% prescription of thromboprophylaxis. ARDS complicating COVID-19 was strongly associated with an increased odds of major arterial or venous thromboembolism, major adverse cardiovascular events, and symptomatic VTE. The 30-day mortality rate in the ICU cohort was 23.5% or 1 in 4 patients. Despite a nearly 85% thromboprophylaxis rate, hospitalized non-ICU patients with COVID-19 were also susceptible to these cardiovascular complications, although not to the extent of those in the critical care setting. The outpatient cohort had a low risk of adverse events.
The global experience of the COVID-19 pandemic has identified an increased risk of arterial and venous thromboembolism and other major adverse cardiovascular events, especially among ICU patients (4,7,8,23, 24, 25). Thromboembolic events, in particular DVT and PE, complicating COVID-19 have been estimated to occur in 20% to 40% of patients requiring admission to the ICU and have been associated with increased mortality (4,6, 7, 8,26). Similarly, myocardial injury, whether due to ischemic insult or inflammation, has also been linked to adverse outcomes, including increased in-hospital mortality, in patients with COVID-19 (23,25). With respect to cardiovascular complications, we observed that non–ST-segment elevation MI predominated in our ICU cohort. This was likely due to a high prevalence of underlying heart disease, cardiovascular risk factors, and clinical courses complicated by systemic arterial hypotension and hypoxemia.
In the current study, we found comparable 30-day frequencies of thromboembolism (35.3%) and major adverse cardiovascular events (45.9%) in the ICU cohort. All events underwent rigorous adjudication and included only symptomatic VTE. VTE occurred despite a high rate of prophylactic anticoagulation. Counterintuitively, we observed a higher frequency of adverse events in patients with COVID-19 in the ICU and hospitalized non-ICU cohorts who were receiving thromboprophylaxis compared with those who were not. Possible explanations for this observation include confounding by indication and failure of standard thromboprophylactic dosing (26).
A prothrombotic state due to immobility, a high frequency of thromboembolic risk factors, severe systemic inflammation, virus-mediated hypercoagulability, bacterial coinfection, and indwelling catheters and devices may explain the increased frequency of arterial and venous thromboembolism despite thromboprophylaxis (5,16,27). Catheter- and device-associated DVT accounted for 76.9% of the DVTs observed in our study. Our finding of high frequency of catheter-associated DVT supports the judicious use of central venous catheters that have been widely implemented especially in the ICU to minimize recurrent health care team exposure and facilitate monitoring.
ARDS may play a critical role in the prothrombotic state of COVID-19 (28). Patients with COVID-19 and ARDS have elevated D-dimer, fibrinogen, and interleukin-6 levels (28). The prothrombotic state associated with elevated D-dimer and fibrin degradation product levels has correlated with increased mortality in COVID-19 (5). In our analysis of 170 patients with COVID-19 in the ICU cohort, we observed similar increases in D-dimer and inflammatory biomarkers of high-sensitivity C-reactive protein and high-sensitivity interleukin-6. However, we found that presence of ARDS had the strongest association with adverse outcomes, including major arterial or venous thromboembolism, major adverse cardiovascular events, symptomatic VTE, and death. The severe inflammatory state associated with ARDS and other complications of COVID-19 and its resultant hypercoagulability may explain, at least in part, the high frequency of thromboembolic events. Improved risk stratification, utilizing biochemical markers of inflammation and activated coagulation as well as clinical indicators, such as ARDS, may play an important role in the early identification of patients with an increased likelihood of developing symptomatic VTE or arterial thrombosis. They may benefit from full- or intermediate-intensity antithrombotic therapy rather than prophylactic anticoagulation. An enhanced understanding of the interplay between infection, inflammation, and thrombosis may yield opportunities for therapeutic intervention to reduce mortality.
Whereas the highest burden of thromboembolism and major adverse cardiovascular events rests on those in the ICU, patients with COVID-19 admitted to the non-ICU setting still bear an important risk of these complications, especially VTE (8,29). The frequency of VTE in patients with COVID-19 admitted to the non-ICU setting has varied from 5% to 15% depending on whether systematic screening for asymptomatic DVT was performed (8,29). We observed a lower 30-day frequency of thromboembolism (2.6%) in the hospitalized non-ICU cohort, likely due to high baseline rate of utilization of thromboprophylaxis (85%), decision support for thromboembolism prevention (30,31), and rigorous event adjudication.
The health and economic implications of these frequent cardiovascular complications, including arterial and venous thromboembolism, myocardial injury, and heart failure, are unlikely to be fully realized until the pandemic abates, social distancing measures are relaxed, medical centers reopen for routine care, and large population datasets are analyzed. In the current study, cardiovascular complications accounted for 7% of deaths at 30 days in patients with COVID-19 compared with sepsis in nearly 90%. The consequences associated with the high frequency of cardiovascular events are likely to be substantial, given the high prevalence of cardiovascular disease and risk factors in the COVID-19 population. As documented in other studies, we observed an ethnically and racially diverse population infected with COVID-19 (32). The impact of cardiovascular complications of COVID-19 on pre-existing health care disparities could be devastating in underserved communities.
Our study provides a cross-sectional view of the cardiovascular complications of COVID-19 in a large health care network, consisting of 2 academic medical centers serving the Greater Boston area, several community hospitals, and numerous outpatient care sites. The study incorporates a wide scope of clinically meaningful cardiovascular endpoints and utilizes a rigorous process of event adjudication. Although data on patients with COVID-19 in the ICU have been the subject of most reports, our study provides insights into the broad spectrum of all hospitalized and outpatient populations.
Study limitations
The current study must also be interpreted within the limitations of its retrospective design. Although the clinical significance of asymptomatic VTE remains a matter of debate, we did not perform surveillance screening, and, therefore, we probably underestimated the frequency of VTE. Catheter- and device-related DVT composed a large proportion of VTE in the ICU cohort and may have inflated the estimate. Because we designated the study cohorts as mutually exclusive, we may have underestimated the frequency of events in the hospitalized, non-ICU setting. For example, a hospitalized patient in the noncritical care setting with a DVT who subsequently is admitted to the ICU would be counted as an event in the ICU cohort. Because we did not study a comparator group without COVID-19 but with other medical illness of similar severity, we were unable to determine the extent to which COVID-19 itself increases the risk of thromboembolic events. A U.K.-based study of 1,877 hospital discharges related to COVID-19 and 18,159 related to non-COVID-19 medical illness did not show a difference in the rate of hospitalization-associated VTE (OR: 1.6; 95% CI: 0.77 to 3.1) (33).
Although we obtained 30-day follow-up data on 96% of subjects in the total study population, we were unable to obtain follow-up for 40 outpatients, and, therefore, we are likely to have underestimated 30-day outcomes in this cohort. We were also unable to assess microvascular thrombosis, particularly in the pulmonary circulation, which has emerged as an important concern in COVID-19. We were not able to obtain data regarding medication adherence to antiplatelet, antithrombotic, statin, and insulin therapy prior to the diagnosis of COVID-19. We were also unable to determine the rationale for omission of thromboprophylaxis. Finally, a limited sample size precluded more extensive statistical assessment of less frequent cardiovascular complications and development of larger multivariable models. We anticipate a greater ability to discern important associations with subsequent analyses of this rapidly growing registry.
Conclusions
Arterial or venous thromboembolism and major adverse cardiovascular events were common over 30 days in ICU patients with COVID-19. ARDS was strongly associated with cardiovascular complications. Patients with COVID-19 in the hospitalized non-ICU cohort were also susceptible to cardiovascular complications, although not to the extent of those in the critical care setting. The high frequency of arterial or venous thromboembolism in hospitalized patients despite routine thromboprophylaxis suggests the need for improved risk stratification and enhanced preventive efforts.
Perspectives.
COMPETENCY IN PATIENT CARE AND PROCEDURAL SKILLS: Arterial and venous thromboembolism and other major adverse cardiovascular events represent critical hazards to patients with COVID-19 despite routine thromboprophylaxis, especially among those in hospital intensive care units.
TRANSLATIONAL OUTLOOK: The high frequency of arterial and venous thromboembolism in hospitalized patients with COVID-19 warrants further investigation to refine risk stratification and establish safe and effective antithrombotic management.
Footnotes
This study was funded, in part, by a research grant from Janssen Pharmaceuticals. Dr. Piazza has received research grant support from EKOS Corporation, Bayer, Bristol Myers Squibb/Pfizer, Portola Pharmaceuticals, and Janssen Pharmaceuticals; and has received consulting fees from Amgen, Pfizer, Boston Scientific, Agile, and Thrombolex. Mr. Fanikos has received research support from AstraZeneca and Boehringer Ingelheim; and has received consulting fees from Portola Pharmaceuticals. Dr. Goldhaber has received research support from Boehringer Ingelheim, Boston Scientific EKOS Corporation, Bristol Myers Squibb/Pfizer, Portola Pharmaceuticals, Janssen Pharmaceuticals, and the National Heart, Lung, and Blood Institute; and has received consulting fees from Boehringer Ingelheim, Bayer, and Agile. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACCauthor instructions page.
Appendix
For supplemental material about the CORONA-VTE, please see the online version of this paper.
Appendix
References
- 1.Bikdeli B., Madhavan M.V., Jimenez D., for the Global COVID-19 Thrombosis Collaborative Group COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonow R.O., Fonarow G.C., O'Gara P.T., Yancy C.W. Association of coronavirus disease 2019 (COVID-19) with myocardial injury and mortality. JAMA Cardiol. 2020;5:751–753. doi: 10.1001/jamacardio.2020.1105. [DOI] [PubMed] [Google Scholar]
- 3.Driggin E., Madhavan M.V., Bikdeli B. Cardiovascular considerations for patients, health care workers, and health systems during the coronavirus disease 2019 (COVID-19) pandemic. J Am Coll Cardiol. 2020;75:2352–2371. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klok F.A., Kruip M., van der Meer N.J.M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cui S., Chen S., Li X., Liu S., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18:1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Llitjos J.F., Leclerc M., Chochois C. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J Thromb Haemost. 2020;18:1743–1746. doi: 10.1111/jth.14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lodigiani C., Iapichino G., Carenzo L., for the Humanitas COVID-19 Task Force Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marietta M., Ageno W., Artoni A. COVID-19 and haemostasis: a position paper from Italian Society on Thrombosis and Haemostasis (SISET) Blood Transfus. 2020;18:167–169. doi: 10.2450/2020.0083-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Middeldorp S., Coppens M., van Haaps T.F. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18:1995–2002. doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ackermann M., Verleden S.E., Kuehnel M. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varga Z., Flammer A.J., Steiger P. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wichmann D., Sperhake J.P., Lutgehetmann M. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med. 2020;173:268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moores L.K., Tritschler T., Brosnahan S. Prevention, diagnosis and treatment of VTE in patients with coronavirus disease 2019: CHEST guideline and expert panel report. Chest. 2020;158:1143–1163. doi: 10.1016/j.chest.2020.05.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spyropoulos A.C., Levy J.H., Ageno W. Scientific and Standardization Committee communication: clinical guidance on the diagnosis, prevention and treatment of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18:1859–1865. doi: 10.1111/jth.14929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boger B., Fachi M.M., Vilhena R.O., Cobre A.F., Tonin F.S., Pontarolo R. Systematic review with meta-analysis of the accuracy of diagnostic tests for COVID-19. Am J Infect Control. 2020 Jul 10 doi: 10.1016/j.ajic.2020.07.011. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thygesen K., Alpert J.S., Jaffe A.S. Third universal definition of myocardial infarction. Circulation. 2012;126:2020–2035. doi: 10.1161/CIR.0b013e31826e1058. [DOI] [PubMed] [Google Scholar]
- 20.Schulman S., Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3:692–694. doi: 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 21.Fine J.P., Gray R.J. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 22.Firth D. Bias reduction of maximum likelihood estimates. Biometrika. 1993;80:27–38. [Google Scholar]
- 23.Li X., Guan B., Su T. Impact of cardiovascular disease and cardiac injury on in-hospital mortality in patients with COVID-19: a systematic review and meta-analysis. Heart. 2020;106:1142–1147. doi: 10.1136/heartjnl-2020-317062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poyiadji N., Cormier P., Patel P.Y. Acute pulmonary embolism and COVID-19. Radiology. 2020 May 14 doi: 10.1148/radiol.2020201955. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi S., Qin M., Cai Y. Characteristics and clinical significance of myocardial injury in patients with severe coronavirus disease 2019. Eur Heart J. 2020;41:2070–2079. doi: 10.1093/eurheartj/ehaa408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bikdeli B., Madhavan M.V., Gupta A. Pharmacological agents targeting thromboinflammation in COVID-19: review and implications for future research. Thromb Haemost. 2020;120:1004–1024. doi: 10.1055/s-0040-1713152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ranucci M., Ballotta A., Di Dedda U. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemost. 2020;18:1747–1751. doi: 10.1111/jth.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Demelo-Rodriguez P., Cervilla-Munoz E., Ordieres-Ortega L. Incidence of asymptomatic deep vein thrombosis in patients with COVID-19 pneumonia and elevated D-dimer levels. Thromb Res. 2020;192:23–26. doi: 10.1016/j.thromres.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kucher N., Koo S., Quiroz R. Electronic alerts to prevent venous thromboembolism among hospitalized patients. N Engl J Med. 2005;352:969–977. doi: 10.1056/NEJMoa041533. [DOI] [PubMed] [Google Scholar]
- 31.Piazza G., Hurwitz S., Galvin C.E. Alert-based computerized decision support for high-risk hospitalized patients with atrial fibrillation not prescribed anticoagulation: a randomized, controlled trial (AF-ALERT) Eur Heart J. 2020;41:1086–1096. doi: 10.1093/eurheartj/ehz385. [DOI] [PubMed] [Google Scholar]
- 32.Khatana S.A.M., Groeneveld P.W. Health disparities and the coronavirus disease 2019 (COVID-19) pandemic in the USA. J Gen Intern Med. 2020;35:2431–2432. doi: 10.1007/s11606-020-05916-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roberts L.N., Whyte M.B., Georgiou L. Post-discharge venous thromboembolism following hospital admission with COVID-19. Blood. 2020 Aug 3 doi: 10.1182/blood.2020008086. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.