ABSTRACT
Plant chloroplasts have complex membrane systems. Among these, thylakoids serve as the sites for photosynthesis and photosynthesis-related adaptation. In addition to the photosynthetic membrane complexes and associated molecules, lipids in the thylakoid membranes, are predominantly composed of MGDG (monogalactosyldiacylglycerol), DGDG (digalactosyldiacylglycerol), SQDG (sulfoquinovosyldiacylglycerol) and PG (phosphatidylglycerol), play essential roles in shaping the thylakoid architecture, electron transfer, and photoregulation. In this review, we discuss the effect of abiotic stress on chloroplast structure, the changes in membrane lipid composition, and the degree of unsaturation of fatty acids. Advanced understanding of the mechanisms regulating chloroplast membrane lipids and unsaturated fatty acids in response to abiotic stresses is indispensable for improving plant resistance and may inform the strategies of crop breeding.
KEYWORDS: Lipids, fatty acids, lipid metabolism, lipid transport, abiotic stress
Introduction
Plants often need to cope with a variety of stressful environments that are not conducive to growth and development, such as drought, salt and temperature stress.1, 2 Drought stress can hinder protein synthesis, while reducing the rates of plant cell division and the efficiency of photosynthesis,3,4 ultimately resulting in slower plant growth.5–7 Salt stress can alter the membrane lipid composition,8,9 inhibit seed germination,10–13 and disrupt ion homeostasis,14,15 and lead to oxidative stress.16–18 Continuous temperature stress can destroy the structure of plant cells,19,20 disturb the physiological and biochemical metabolisms,21,22 reduce crop yield,4 and limit the geographical distribution of plants.23
Chloroplasts are the special organelles executing photosynthesis in plants and eukaryotic algae, and contain a complex membrane system.19,24 The photosynthetic membranes (also called thylakoid membranes) accommodate photosynthetic pigment-protein complexes and electron transport chains.25–27 When plants are subjected to abiotic stress, photosynthetic organs are susceptible to environmental influences and undergo structural and metabolic regulations.28–31 As a result, the integrity and fluidity of the chloroplast membranes may be destroyed, and the chloroplasts in the entire plant may be deactivated.32–34 Membrane structure and fluidity are affected by lipid composition and fatty acid desaturation.35 The fluidity of the lipid membrane is determined by the variable unsaturated fatty acid content.36 Changes in unsaturated fatty acid content can improve the plant’s tolerance to environmental stresses, such as cold, high temperature and drought.37 The glycerolipids of thylakoid membranes in cyanobacteria and plant eukaryotes chloroplasts have a glycerol skeleton, where two fatty acid molecules are bonded to sn-1 and sn-2, and have phosphorus (phospholipid) or sugar at the sn-3 position (Glycolipid) molecule.38 The lipid bilayer is mainly composed of four unique lipids, including monogalactosyldiacylglycerol (MGDG), digalactosyldiacylglycerol (DGDG), sulfoquinoxayldiacylglycerol (SQDG) and phosphatidylglycerin (PG).39 MGDG and DGDG are uncharged galactolipids, which form the main body of thylakoid membrane lipids, and provide a lipid bilayer matrix as the main component for photosynthetic complexes.40 Glycolipid SQDG and phospholipid PG are anionic lipids with negatively charged head groups.41,42 This review summarizes a series of physiological changes in chloroplast membrane lipids under abiotic stress. Changes in the composition and content of chloroplast membrane lipids and unsaturated fatty acids have physiological impacts on the structure of chloroplasts and thylakoid membranes, and thereby affecting photosynthesis and plant growth.
Biosynthesis and transportation of fatty acid and membrane lipid
The production of chloroplast lipids begins with the synthesis of fatty acids in chloroplast intermediates. Figure 1 shows the whole process of membrane lipid synthesis and transport. The fatty acids are derived entirely from chloroplast FA synthase (FAS), while phosphatidic acid (PA) can be produced in both chloroplast and endoplasmic reticulum (ER), depending on the plant species.43 Fatty acid synthesis is catalyzed by acetyl-CoA carboxylase (ACC) and FAS.44 Most de novo synthesized fatty acids assemble into phospholipids and neutral lipids in the ER, so fatty acids must be transported from the plastid to the endoplasmic reticulum.45 Fatty acids are synthesized through repeated cycles of condensation, dehydration, and reduction on acyl carrier proteins.46 The acyl chain grows and attaches to the acyl carrier protein (ACP). The newly synthesized acyl-ACP (acyl-ACP) is hydrolyzed by acyl-ACP thioesterases to release free fatty acids or perform the next cycle of fatty acid chain extension.47 Free fatty acids synthesize C16 and C18 long-chain fatty acids under the action of Long-chainacyl-COA synthetase (LACS).44 40% of fatty acids are left in the plastids to synthesize plastid lipids. This pathway is called the prokaryotic pathway for lipid synthesis.48 The eukaryotic biological process is that 60% of fatty acids are transported out of the plastid in the form of acyl-COA and then transported to the endoplasmic reticulum for extension and processing.49 About half of the lipids were transported back to the plastid for further modification.50 Such as Pea (Pisum sativum) and rice (Oryza sativa L.), which only use the eukaryotic pathway for chloroplast glycolipid assembly, and these plants have a high proportion of α-linolenic acid (C18:3) in chloroplast lipids, giving rise to their designation “18:3 plant”.51 Such as Arabidopsis thaliana and spinach (Spinacia oleracea L.), these two pathways are involved in the biosynthesis of chloroplast lipids.52 Their chloroplast lipids contain a large amount of hexadecanoic acid (C16:3), so they are called “16: 3 plants”.53 The prokaryotic pathway directly generates MGDG, DGDG, SQDG and PG from newly synthesized FA.54 Diacylglycerol (DAG), as a precursor of MGDG and DGDG, can be synthesized through eukaryotic and prokaryotic pathways.55 Part of the DAG that synthesizes chloroplast lipids comes from the chloroplast, and the other part is synthesized using phospholipid synthesized by endoplasmic reticulum as a precursor.56
Figure 1.
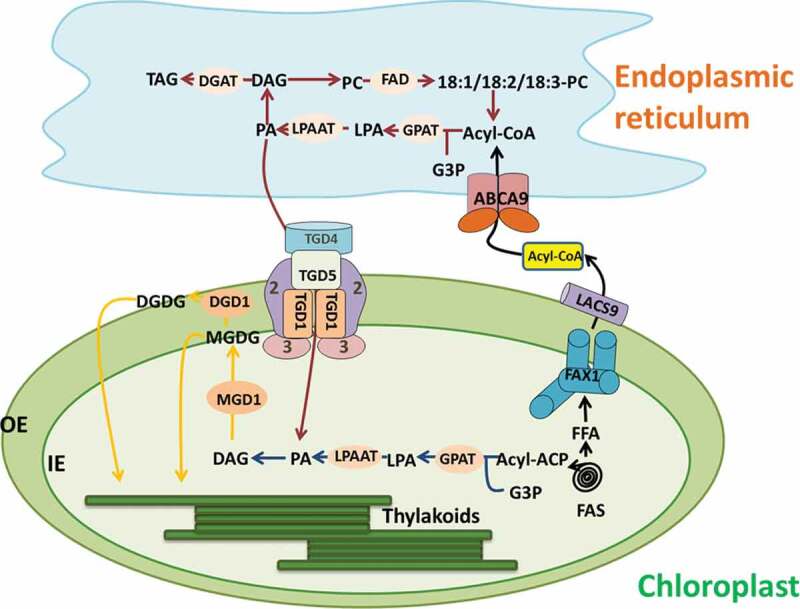
ER-chloroplast interacts in the process of lipid biosynthesis, including: exporting fatty acids from plastids, thylakoid lipid biosynthesis. Acyl-ACP is released from the fatty acid synthase complex (FAS) and hydrolyzed into free fatty acids (FFA), using the FAX1/LACS mechanism to export FFA, reactivated into the acyl-CoA in the outer membrane of the chloroplast, through the Kennedy pathway or acyl.The editing approach incorporates acyl-CoA into the ER lipid. TGD regulates the transport of lipids (mainly PA) across the inner and outer chloroplast membranes into the chloroplast. MGDG is synthesized from chloroplast-derived lipids and ER-derived lipids, which can then be desaturated by chloroplast-specific FAD. The blue arrow shows the lipid assembly reaction of the plastid pathway, the red arrow shows the ER pathway reaction, and the yellow arrow shows the common reaction, mainly the biosynthesis of MGDG and DGDG. The biosynthesis of MGDG occurs on the surface of the inner envelope, and the biosynthesis of DGDG occurs on the cytoplasmic surface of the outer envelope. FADs fatty acid desaturases, DGD1 digalactosyldiacylglycerol synthase 1, MGD1 monogalactosyldiacylglycerol synthase 1, PAP phosphatidic acid phosphatase, LPAAT lyso-phosphatidic acid acyltransferase, GPAT glycerol-phosphate acyltransferase, DGAT diac glycerol acyltransferase, TGD trigalactosyldiacylglycerol, LACS long-chain acyl-CoA synthetases, FAX1 fatty acid export 1, PA phosphatidic acid, PC phosphatidylcholine, MGDG monogalactosyldiacylglycerol, DGDG digalactosyldiacylglycerol
In the eukaryotic pathway of lipid synthesis, fatty acids synthesized in plastids are transported out of the plastids for the synthesis of phospholipids and triacylglycerols (TAG).57 FAX1 (fatty acid export1) transporter can regulate lipid transport between chloroplast and endoplasmic reticulum.58 FAX1 is a new Arabidopsis Tmemb_14 family transporter located in the inner membrane of the chloroplast, which mediates the output of free fatty acids in the chloroplast.59 The ATP binding cassette (ABC) protein located in the endoplasmic reticulum mediates the transport of cytoplasmic acyl-COA or fatty acids to the endoplasmic reticulum in Arabidopsis,60 and the protein family has a transmembrane domain and a nucleotide binding domain.61 ABCA9 regulates the transport of fatty acyl-COA or fatty acids in the cytoplasm to the endoplasmic reticulum to provide lipid raw materials for the synthesis of TAG.62
Fatty acids enter into the ER and are incorporated through the Kennedy pathway. The fatty acid in the form of fatty acyl-COA is catalyzed by glycerol-3-phosphate acyltransferase (GPAT) to esterify the fatty acid to the sn-1 position of glycerol-3-phosphate (G3P), preferably 18:1 acyl-ACP. Lyso-phosphatidic acid acyltransferase (LPAAT) esterifies the second fatty acid to the glycerol backbone at the sn-2 position. The resulting PA is phosphorylated by phosphatidic acid phosphatase (PAP) to generate DAG. DAG is incorporated into various lipids, including phosphatidylcholine (PC).63,64 The second pathway is called as “acyl-editing”. In this pathway, fatty acids are added directly to lyso-PC to regenerate PC, which is cycled back into lyso-PC.65 Lipid transport from the endoplasmic reticulum to the chloroplast requires Arabidopsis thalactosyl diglyceride trigalactosyldiacylglycerol (TGD) to regulate lipid across the chloroplast inner and outer membranes into the chloroplast. Lipids transported through the TGD protein complex can include PC, PA or DAG, each TGD protein complex specifically binds PA.66 TGD1 is the first identified protein located on the outer membrane of the chloroplast, and this protein mutation promotes the accumulation of DGDG.67 Three proteins TGD1, TGD2 and TGD3 all inhibited the transport of Arabidopsis endoplasmic reticulum lipids to the chloroplast.68 TGD4 is a transmembrane lipid transfer and plays a more direct role in lipid transfer from the ER to the outer plastid envelope.69 TGD5 facilitates lipid transfer from the outer to the inner plastid envelope by bridging TGD4 with the TGD1,2,3 transporter complex.70 LACS can not only catalyze the formation of fatty acid-CoA from free fatty acids to participate in the synthesis of long-chain fatty acid derivatives, but also regulate the transport of fatty acids from the endoplasmic reticulum to the chloroplast.71 Studies have shown that LACS9 localized on the outer membrane of chloroplasts is involved in regulating the input of chloroplast fatty acids.72 MGDG is synthesized by MGDG synthetase, which catalyzes the transfer of galactose from Uridine diphosphate galactose (UDP-Gal) to the DAG backbone. DGD synthase then transfers a second galactose from UDP-Gal to MGDG to form DGDG, where the contents of MGDG and DGDG are regulatable under stress conditions (Figure 2).73,74
Figure 2.
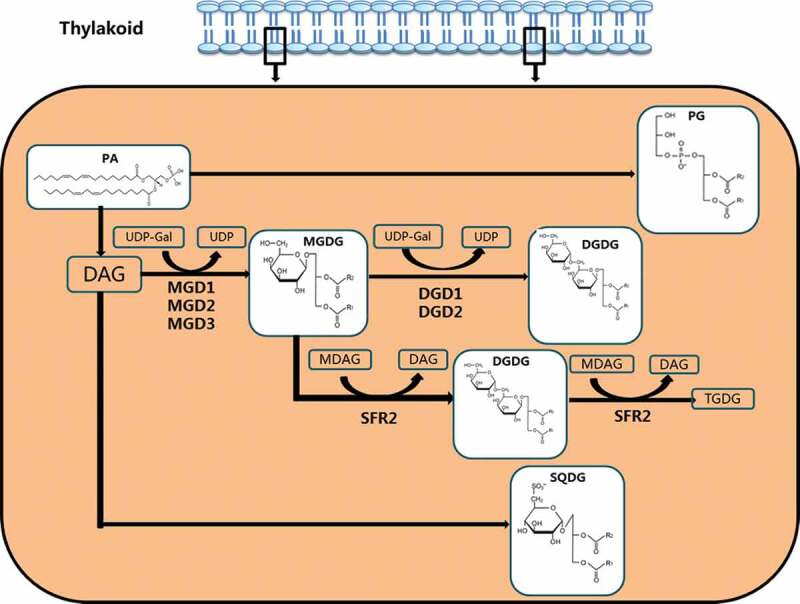
In thylakoids, MGDG, DGDG, SQDG and PG are synthesized by PA through different pathways. MGDG is synthesized by MGD synthetase, which catalyzes the transfer of galactose from UDP-Gal to the DAG. DGD synthase transfers a second galactose from UDP-Gal to MGDG to form DGDG; MGDG synthesizes DGDG through DGD1/DGD2 pathway, and then forms TGDG from SFR2
The effect of abiotic stress on chloroplast structure in cellular level
Abiotic stress can cause irreversible damage to the structure of the chloroplast. Maintaining structural stability under adverse conditions and reducing damage to chloroplasts may play an important role in improving plant stress resistance.75 The plasma membrane is considered to be the main barrier between the organism and the external environment, and is a substance that overcomes pressure damage.76
Chloroplasts are usually the earliest abiotic damage sites visible in the ultrastructure of plants. The degradation of chloroplasts in plants leads to a decrease in net photosynthetic rate and growth retardation.77 Temperature, drought and salt stress can cause irreversible damage to the structure of the chloroplast, such as the reduction of the aspect ratio and area of the chloroplast, and the phase change of the chloroplast membrane.78,79 The thylakoid membrane system is essential for photosynthesis. Once the system is disturbed, the number and size of plastid spheres change. After being treated at 4°C for 20 days, the thylakoid membrane of the sweet pepper swelled and deformed and the thylakoid of the grain split, and at the same time the starch grains increased.80 Chloroplasts gradually expanded from ellipsoids to larger spheres. Studies have shown that chloroplast swelling could lead to an increase in cell matrix permeability and low temperature could cause chloroplast degradation.81,82 When the Chinese cabbage Wucai (Brassica campestris L.) was exposed to high temperature, the chloroplast envelopes were degraded, the thylakoids were inflated, and the grana lamellae were loosely arranged. The osmiophilic particles in the chloroplasts were increased in both number and size.83 Treatment of Rice salt-sensitive (IR-29) varieties with 100mMNaCl showed that the chloroplast structure was damaged, which was manifested in the cracking of the existing grana stacks, the increase of the existing grana stacks, and the expansion of the thylakoid membranes, which ultimately led to a decrease in photosynthetic activity.84
Maintaining structural stability under adverse conditions and reducing damage to chloroplasts may play an important role in improving plant stress resistance. Changes in lipid composition and structure in the plasma membrane under ambient pressure are essential to maintain the stability and function of the membrane. When plant organelles experience stress, chloroplasts respond most rapidly and with the most sensitivity.85,86 Changes in the ultrastructure of chloroplasts result in a series of adaptive and evasive responses.87,88 Expression of the chloroplast targeting protein SlCOR413IM1 in tomato (Solanum lycopersicum L.) increased rapidly under low temperature, causing minimal damage to the chloroplast membrane system and maintaining the integrity of the chloroplast ultrastructure.89 In the dry state, AtCOR15 protein could interact with the galactose head group of chloroplast lipid MGDG in Arabidopsis thaliana. The decrease of the gel-liquid crystal transition temperature depends on the unsaturation of the fatty acyl chain and the structure of the lipid head group. FTIR (Fourier-transforminfrared) spectra from membranes containing MGDG showed evidence for increased fatty acyl chain mobility in the gel phase in the presence of the COR15 proteins.90 In cucumber (Cucumis sativus L.), exogenous spermine (Spm) can prevent chloroplast and thylakoid membrane structural changes induced by salt stress, and maintain a complete internal layering system. Spm can also prevent chlorophyll degradation in cucumber leaves caused by salt stress, and protect the light harvesting complex (LHC) and PSII from salt-induced damage.91
The functions of chloroplast membrane lipids during abiotic stress
The thylakoid membrane is the site of photo-driven photochemical reactions and electron transfer in plants, and it also plays an important role in maintaining the stability of photosynthesis (Table 1).19,92 Membrane lipids are also part of the thylakoid complex.93 For example, DGDG and PG are involved in the binding of extrinsic proteins, thereby stabilizing the manganese cluster in PSII.94 Plants resist abiotic stress and protect themselves by changing the synthesis and composition of thylakoid membrane lipids.74,95–97
Table 1.
Typical functions of membrane lipids
| Lipid species | Description | Related references |
|---|---|---|
| MGDG | In the mgd1 mutant, the electrical conductivity of the thylakoid membrane increased, thereby weakening the photoprotective effect of the thylakoid membrane. | 100–102 |
| DGDG | DGDG confers thermotolerance to plants due to its bilayer-stabilizing properties as demonstrated by the failure of DGDG-defi cient dgd1 mutant plants to adapt to high growth temperatures. | 103,104 |
| SQDG | SQDG is a negatively charged glycolipid, composed of more saturated fatty acids, and contains different numbers of eukaryotic and prokaryotic species according to plant species. | 106–109 |
| PG | PGs are the major phospholipid in thylakoid membranes of higher plants and can be used as a precursor of cardiolipins located on the inner mitochondrial membrane that are required for proper functioning of the oxidative phosphorylation enzymes. | 115,118 |
Abbreviations: MGDG, monogalactosyldiacylglycerol; DGDG, digalactosyldiacylglycerol; SQDG, sulfoquinovosyldiacylglycerol; PG, phosphatidylglycerol.
Variations in the DGDG/MGDG ratio could modify the stability of chloroplast membranes.98,99 When plants were subjected to drought stress, MGDG was most sensitive to drought.100 In the MGDG synthetic gene knockout Arabidopsis mutant mgd1, the expression level of MGDG was reduced and had no effect on PSII activity.101 However, in the mgd1 mutant, the electrical conductivity of the thylakoid membrane increased, thereby weakening the photoprotective effect of the thylakoid membrane.102 Studies have shown that drought stress increases the ratio of DGDG/MGDG in spring wheat, and a decrease in PG content is observed. The author believes that it may be that PC or PC-derived lipids are directly or indirectly transported to galactolipid biosynthetic plastids, or that DAG is phosphorylated into PA for synthesis of DGDG.103,104 In the process of drying and recovery, the content of chloroplast membrane lipid and the expression of related genes of desiccation-tolerant plants (Craterostigma plantagineum and Lindernia brevidens) and desiccation-sensitive plants (Lindernia subracemosa) must change. In desiccation-tolerant plants, the total lipid content remains constant, but the membrane lipid composition changes and the MGDG content decreases. One of the ways to reduce MGDG is the synthesis of phospholipids by DAG, and the other is the conversion of MGDG to the DGD1/DGD2 pathway, followed by the formation of oligogalactolipids from SFR2 (Figure 2).105 The reduced MGDG/DGDG ratio helps maintain the bimolecular conformation of membrane lipids and greatly improves the stability of the chloroplast membrane.105
In the halophyte Thellungiella, increasing the content of PG and SQDG in membrane lipids and the ratio of MGDG/DGDG under salt stress could alleviate PSII photoinhibition.106 Under salt stress, there are decreases in the content of SQDG, the ratio of MGDG/DGDG in the chloroplast membranes of peanut (Arachis hypogaea L.), the expression of ω-3 FAD gene, and unsaturated fatty acid content. Increasing the unsaturated fatty acid content of peanut leaf membrane lipid reduced the photoinhibition of PSII and PSI and improved salt tolerance.107,108 By contrast, Arabidopsis and rice have different lipid synthesis pathways. Arabidopsis is a “16:3 plant” with both eukaryotic and prokaryotic lipid synthesis pathways, while rice is an “18:3 plant” with only a eukaryotic lipid synthesis pathway. Under low temperature, Arabidopsis contains higher levels of galactolipid than those in rice. The higher double bond index and lower average acyl chain length make Arabidopsis chloroplast membranes more fluidic at low temperatures.13 Two varieties of Fabaceae: Sulla carnosa and Sulla coronaria, treated with 200 mM NaCl for 20 days. The experimental results show that (a) maintaining a constant MGDG/DGDG ratio and fatty acids unsaturation level, (b) increasing unsaturation level in MGDG, DGDG and PG may contribute to some degree in the adaptation to salt stress and could protect chloroplast membrane integrity against salt stress effects.109
Roles of fatty acid composition in abiotic stress response
Plants can adjust the fluidity of membrane lipids, by changing the degree of saturation of polyunsaturated fatty acids, to cope with stress conditions.110,111 The levels of the unsaturated FAs (those that carry double bonds between carbons) 18:1, 18:2, and 18:3 are particularly important in plant defense.112 Analysis of fatty acids in thylakoid membrane lipids revealed the presence of the saturated fatty acids palmitic acid and stearic acid, and unsaturated fatty acids palmitoleic acid and oleic acid (18:1), linoleic acid (18:2) and linolenic acid (18:3).113 In the neutral membrane lipids (MGDG and DGDG) of the photosynthetic membrane, the two fatty acyl chains are mostly unsaturated linolenic acid.114 The negatively charged DGDG is mainly unsaturated linolenic acid and saturated palmitic acid, or a mixture of PG.115 Fatty acid desaturase (FAD) is an important enzyme that introduces double bonds into fatty acids during the synthesis of glycerolipids.116 For example, the ω-3 FAD is based on the first carbon atom at the methyl terminus being the ω-1 position, with a C = C double bond at the ω-3 position, and consists of at least two C = C double bonds.117 According to different electron donors, there is one type of omega-3 FAD in the endoplasmic reticulum, which mainly acts on PG or other phospholipids, while the other type exists in the plastid and acts on phosphatidylglycerol or galactosyl.118ω-3 FAD and two plastid enzymes, FAD7 and FAD8, are the key enzymes that catalyze the conversion of 16:2 or 18:2 into 16:3 or 18:3, respectively (Figure 3a).119 The increase of unsaturated fatty acids can enhance plant resistance to stress.120,121 Therefore, the regulation of fatty acid saturation by FAD is an important way for plants to adapt to abiotic stress.
Figure 3.
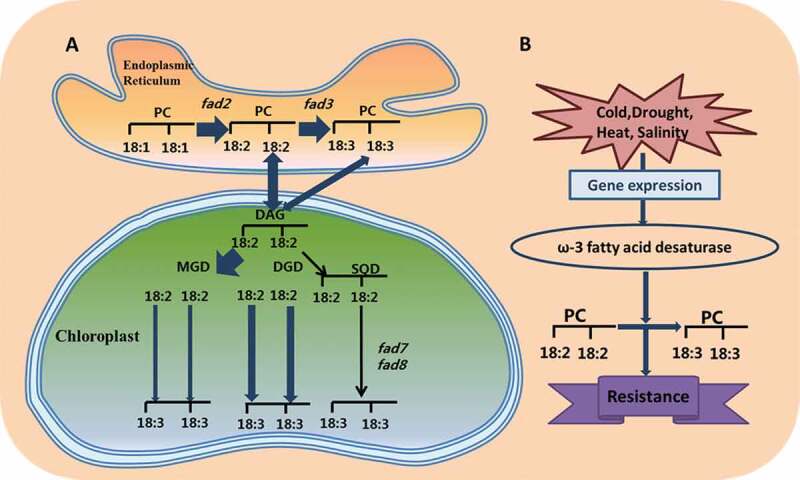
(a) Fatty acid biosynthetic pathway and regulating mechanism of fatty acid desaturases in response to stress. (b) Under abiotic stress, the ω-3 fatty acid desaturase gene FAD3 catalyzes 18:2 to 18:3 in phospholipids, giving plants resistance to stress
Under low temperature stress, the PSII D1 protein is the target of photoinhibition. Fatty acids in PG through over-expression of LeGPAT can alleviate PSII photoinhibition.122 The increase of unsaturated fatty acids in PG reduces the formation of ROS and damage to photosynthetic complexes, thereby improving the low-temperature tolerance of tomato plants.123 At lower temperatures, an increase of unsaturated fatty acid content was observed in the transgenic lines. The CaHSP26 protein protects PSII by reducing photooxidation, maintaining antioxidant enzyme activity and increasing the fluidity of the thylakoid membrane.124 Under heat stress, the relative amount of one triacylglycerol species (54:9) containing α-linolenic acid (18:3) increased. Heat stress could induce an increase in TAG levels in Arabidopsis leaves, which acts as an intermediate in lipid turnover and leads to a reduction in membrane polyunsaturated fatty acids.125
Sui et al.122 found that the increase of unsaturated fatty acids in the membrane lipids of Suaeda salsa increases the protection of PSII under high salinity, and that unsaturated fatty acids in membrane lipids can protect PS from NaCl stress. Under salt treatment of the halophyte Thellungiella, 18:3 unsaturated fatty acids increased significantly, whereas 18:1, 18:2, and 18:3 decreased greatly in the non-halophyte Arabidopsis.106 This may be due to the ion channel or Na+/H+ reverse transport system that are located on the plasma membrane. The increased unsaturated fatty acids in the membrane lipids could improve the fluidity of the membrane, thereby activating the ion channel and protecting the photosystem.106,126 In tomato, LeFAD3 overexpression can enhance the tolerance of early seedlings to salt stress. It could increase the level of 18:3 in plants to remove excess active oxygen, and promote the repair of PSII, finally reduce the damage to membrane lipids (Figure 3b)127–129 Under drought stress, the proportion of saturated fatty acids in thylakoid membranes increased, and mature leaves elevated the heat tolerance of plants by increasing the levels of saturated fatty acids, thereby increasing the melting temperature of the plasma membrane.127 A smaller reduction in the index of unsaturated fatty acids under drought stress is beneficial to thylakoid membrane stability.130 In rice LYPJ varieties, linoleic acid (18:2) increased significantly at 28 days.131 The increase in linoleic acid can enhance the fluidity of thylakoid membranes, thus improving the PSII repair rate in crops under severe drought stress.131 High temperature causes changes in the lipid profile of wheat, and plants respond to high temperature stress by remodeling lipids and reducing the level of lipid unsaturation.132 The lower lipid unsaturation level under high temperature stress is mainly due to lower levels of 18:3 fatty acyl chains and higher levels of 18:1 and 16:0 fatty acyl chains.133
Conclusions and perspectives
The structure and composition of chloroplast membrane lipids are vital for maintaining the normal physiological activities in plants. Abiotic stress could induce changes in the content and ratio of the components of chloroplast membrane lipids. The regulation of the corresponding genes has become a hot topic in molecular biology. As transcriptome sequencing and gene editing technologies become increasing popular, we are now able to analyze more comprehensively the key genes that are involved in regulating membrane lipid biosynthesis under abiotic stress, to provide new insight into the expression and regulatory mechanism of these genes.
The observations of the ultrastructures of chloroplasts and thylakoids enable us to study the organ damage under environmental stress. However, the development of molecular probes is needed to decipher the accurate positions of individual lipid molecules in membranes and membrane integral complexes, so that changes in chloroplasts and thylakoids can be seen more intuitively and dynamically.104 Given that the regulation of membrane lipid fatty acids in chloroplasts depends largely on FAD activity, it is imperative to seek how to regulate the genes in other organelles and tissues. Under abiotic stress, changes in membrane lipids may be accompanied by the effects of plant hormones or some signal proteins. A profound understanding of their mutual regulatory relationship will pave the way for improving plant resistance.
Funding Statement
We are grateful for financial support from the National Natural Science Research Foundation of China (U1906204, 31871538), Shandong Province Key Research and Development Program (2019GSF107079), the Development Plan for Youth Innovation Team of Shandong Provincial (2019KJE012), the Science and Technology Demonstration Project of “Bohai Granary” of Shandong Province (2019BHLC002), the Royal Society University Research Fellowship (UF120411 and URF\R\180030, L.-N.L.), Biotechnology and Biological Sciences Research Council Grant (BB/R003890/1, L.-N.L.).
Author contributions
Jinlu Li prepared the manuscript. Na Sui, Hai Fan, Qingwei Meng and Lu-Ning Liu conceptualized the idea and revised the manuscript. All authors read and approved the final manuscript.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Guo J, Suo S, Wang B.. Sodium chloride improves seed vigour of the euhalophyte Suaeda salsa. Seed Sci Res. 2015;25:1–9. doi: 10.1017/S0960258515000239. [DOI] [Google Scholar]
- 2.An J, Yao J, Xu R, You C, Wang X, Hao Y.. An apple NAC transcription factor enhances salt stress tolerance by modulating the ethylene response. Physiol Planta. 2018;164:279–289. doi: 10.1111/ppl.12724. [DOI] [PubMed] [Google Scholar]
- 3.Li M, Guo S, Xu Y, Meng Q, Li G, Yang X.. Glycine betaine-mediated potentiation of HSP gene expression involves calcium signaling pathways in tobacco exposed to NaCl stress. Physiol Planta. 2014;150:63–75. doi: 10.1111/ppl.1206. [DOI] [PubMed] [Google Scholar]
- 4.Sui N, Yang Z, Liu M, Wang B. Identification and transcriptomic profiling of genes involved in increasing sugar content during salt stress in sweet sorghum leaves. BMC Genomics. 2015;16:534. doi: 10.1186/s12864-015-1760-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feng Z, Deng Y, Fan H, Sun QJ, Sui N, Wang BS. Effects of NaCl stress on the growth and photosynthetic characteristics of Ulmus pumila L. seedlings in sand culture. Photosynthetica. 2014;52:313–320. doi: 10.1007/s11099-014-0032-y. [DOI] [Google Scholar]
- 6.Ma X, Wang G, Zhao W, Yang M, Ma N, Kong F, Dong X, Meng Q. SlCOR413IM1: A novel cold-regulation gene from tomato, enhances drought stress tolerance in tobacco. J Plant Physiol. 2017;216:88–99. doi: 10.1016/j.jplph.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 7.You L, Song Q, Wu Y, Li S, Jiang C, Chang L, Yang X, Zhang J. Accumulation of glycine betaine in transplastomic potato plants expressing choline oxidase confers improved drought tolerance. Planta. 2019;249:1963–1975. doi: 10.1007/s00425-019-03132-3. [DOI] [PubMed] [Google Scholar]
- 8.Cui F, Sui N, Duan G, Liu Y, Han Y, Liu S, Wan S, Li G. Identification of metabolites and transcripts involved in salt stress and recovery in peanut. Front Plant Sci. 2018;9:217. doi: 10.3389/fpls.2018.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tao LI, Liu R, Xinhua HE, Wang B. Enhancement of superoxide dismutase and catalase activities and salt tolerance of euhalophyte Suaeda salsa L. by mycorrhizal fungus glomus mosseae. Pedosphere. 2012;22:217–224. doi: 10.1016/S1002-0160(12)60008-3. [DOI] [Google Scholar]
- 10.Feng Z, Sun Q, Deng Y, Sun S, Zhang J, Wang B. Study on pathway and characteristics of ion secretion of salt glands of Limonium bicolor. Acta Physiol Planta. 2014;36:2729–2741. doi: 10.1007/s11738-014-1644-3. [DOI] [Google Scholar]
- 11.Guo YY, Tian SS, Liu S, Wang W, Sui N. Energy dissipation and antioxidant enzyme system protect photosystem II of sweet sorghum under drought stress. Photosynthetica. 2018;56:861–872. doi: 10.1007/s11099-017-0741-0. [DOI] [Google Scholar]
- 12.Liu Q, Liu R, Ma Y, Song J. Physiological and molecular evidence for Na+ and Cl− exclusion in the roots of two Suaeda salsa populations. Aquat Bot. 2018;146:1–7. doi: 10.1016/j.aquabot.2018.01.001. [DOI] [Google Scholar]
- 13.Zheng G, Li L, Li W. Glycerolipidome responses to freezing- and chilling-induced injuries: examples in Arabidopsis and rice. BMC Plant Biol. 2016;16:70. doi: 10.1186/s12870-016-0758-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng ZT, Deng YQ, Zhang SC, Liang X, Yuan F, Hao JL, Zhang JC, Sun SF, Wang BS. K(+) accumulation in the cytoplasm and nucleus of the salt gland cells of Limonium bicolor accompanies increased rates of salt secretion under NaCl treatment using NanoSIMS. Plant Sci. 2015;238:286–296. doi: 10.1016/j.plantsci.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 15.Shaheen HL, Iqbal M, Azeem M, Shahbaz M, Shehzadi M. K-priming positively modulates growth and nutrient status of salt-stressed cotton (Gossypium hirsutum) seedlings. Arch Agron Soil Sci. 2016;62:759–768. doi: 10.1080/03650340.2015.1095292. [DOI] [Google Scholar]
- 16.Duan M, Feng H, Wang L, Li D, Meng Q. Overexpression of thylakoidal ascorbate peroxidase shows enhanced resistance to chilling stress in tomato. J Plant Physiol. 2012;169:867–877. doi: 10.1016/j.plantsci.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 17.Duan M, Ma NN, Li D, Deng YS, Kong FY, Lv W, Meng QW. Antisense-mediated suppression of tomato thylakoidal ascorbate peroxidase influences anti-oxidant network during chilling stress. Plant Physiol Biochem. 2012;58:37–45. doi: 10.1016/j.plaphy.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 18.Song Y, Li J, Liu M, Meng Z, Liu K, Sui N. Nitrogen increases drought tolerance in maize seedlings. Funct Plant Biol. 2019;46:350–359. doi: 10.1016/j.plantsci.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 19.Yang J, Li M, Xie X, Han G, Sui N, Wang B. Deficiency of phytochrome B alleviates chilling-induced photoinhibition in rice. Am J Bot. 2013;100:1860–1870. doi: 10.3732/ajb.1200574. [DOI] [PubMed] [Google Scholar]
- 20.Liu Z, Yue M, Yang D, Zhu S, Ma N, Meng Q. Over-expression of SlJA2 decreased heat tolerance of transgenic tobacco plants via salicylic acid pathway. Plant Cell Rep. 2017;36:529–542. doi: 10.1007/s00299-017-2100-9. [DOI] [PubMed] [Google Scholar]
- 21.Cheng S, Yang Z, Wang M, Song J, Sui N, Fan H. Salinity improves chilling resistance in Suaeda salsa. Acta Physiol Planta. 2014;36:1823–1830. doi: 10.1007/s11738-014-1555-3. [DOI] [Google Scholar]
- 22.Zhuang K, Gao Y, Liu Z, Diao P, Sui N, Meng Q, Meng C, Kong F. WHIRLY1 regulates HSP21.5A expression to promote thermotolerance in tomato. Plant Cell Physiol. 2020;61:169–177. doi: 10.1093/pcp/pcz189. [DOI] [PubMed] [Google Scholar]
- 23.Zhou B, Deng Y, Kong F, Li B, Meng Q. Overexpression of a tomato carotenoid ɛ-hydroxylase gene alleviates sensitivity to chilling stress in transgenic tobacco. Plant Physiol Biochem. 2013;70:235–245. doi: 10.1016/j.plaphy.2013.05.035. [DOI] [PubMed] [Google Scholar]
- 24.Kong F, Deng Y, Wang G, Wang J, Liang X, Meng Q. LeCDJ1, a chloroplast DnaJ protein, facilitates heat tolerance in transgenic tomatoes. J Integr Plant Biol. 2014;56:63–74. doi: 10.1111/jipb.12119. [DOI] [PubMed] [Google Scholar]
- 25.Liu L. Distribution and dynamics of electron transport complexes in cyanobacterial thylakoid membranes. Biochim Biophys Acta. 2016;1857:256–265. doi: 10.1016/j.bbabio.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shu DF, Wang LY, Duan M, Deng YS, Meng QW. Antisense-mediated depletion of tomato chloroplast glutathione reductase enhances susceptibility to chilling stress. Plant Physiol Biochem. 2011;49:1228–1237. doi: 10.1016/j.plaphy.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 27.Wang LF. Physiological and molecular responses to drought stress in rubber tree (Hevea brasiliensis Muell. Arg.) Plant Physiol Biochem. 2014;83:243–249. doi: 10.1016/j.plaphy.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 28.Ivanov AG, Velitchkova MY, Allakhverdiev SI, Huner NPA. Heat stress-induced effects of photosystem I: an overview of structural and functional responses. Photosynth Res. 2017;133:17–30. doi: 10.1007/s11120-017-0383-x. [DOI] [PubMed] [Google Scholar]
- 29.Chaves MM, Flexas J, Pinheiro C. Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot. 2009;103:551–560. doi: 10.1093/aob/mcn125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen S, Jia H, Wang X, Shi C, Wang X, Ma P, Wang J, Ren M, Li J. Hydrogen sulfide positively regulates abscisic acid signaling through persulfidation of SnRK2.6 in guard cells. Mol Plant. 2020. doi: 10.1016/j.molp.2020.01.004. [DOI] [PubMed] [Google Scholar]
- 31.Ferreyroa GV, Lagorio MG, Trinelli MA, Lavado RS, Molina FV. Lead effects on Brassica napus photosynthetic organs. Ecotoxicol Environ Saf. 2017;140:123–130. doi: 10.1016/j.ecoenv.2017.02.031. [DOI] [PubMed] [Google Scholar]
- 32.Han H, Gao S, Li B, Dong XC, Feng HL, Meng QW. Overexpression of violaxanthin de-epoxidase gene alleviates photoinhibition of PSII and PSI in tomato during high light and chilling stress. J Plant Physiol. 2010;167:176–183. doi: 10.1016/j.jplph.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 33.Peng X, Teng L, Yan X, Zhao M, Shen S. The cold responsive mechanism of the paper mulberry: decreased photosynthesis capacity and increased starch accumulation. BMC Genomics. 2015;16:898. doi: 10.1186/s12864-015-2047-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Upchurch RG. Fatty acid unsaturation, mobilization, and regulation in the response of plants to stress. Biotechnol Lett. 2008;30:967–977. doi: 10.1007/s10529-008-9639-z. [DOI] [PubMed] [Google Scholar]
- 35.Mikami K, Murata N. Membrane fluidity and the perception of environmental signals in cyanobacteria and plants. Prog Lipid Res. 2003;42:527–543. doi: 10.1016/S0163-7827(03)00036-5. [DOI] [PubMed] [Google Scholar]
- 36.Kachroo A, Kachroo P. Fatty acid-derived signals in plant defense. Ann Rev Phytopathol. 2009;47:153–176. doi: 10.1146/annurev-phyto-080508-081820. [DOI] [PubMed] [Google Scholar]
- 37.ScottiCampos P, PhamThi A. Correlation between total lipids, linolenic acid and membrane injury under PEG-induced dehydration in leaves of Vigna genotypes differing in drought resistance. Emirates J Food Agr. 2016;28:485. doi: 10.9755/ejfa.2016-04-342. [DOI] [Google Scholar]
- 38.Guo Q, Liu L, Barkla BJ. Membrane lipid remodeling in response to salinity. Int J Mol Sci. 2019;20:4264. doi: 10.3390/ijms20174264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moellering ER, Benning C. Galactoglycerolipid metabolism under stress: a time for remodeling. Trends Plant Sci. 2011;16:98–107. doi: 10.1016/j.tplants.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 40.Shimojima M, Madoka Y, Fujiwara R, Murakawa M, Yoshitake Y, Ikeda K, Koizumi R, Endo K, Ozaki K, Ohta H. An engineered lipid remodeling system using a galactolipid synthase promoter during phosphate starvation enhances oil accumulation in plants. Front Plant Sci. 2015;6:664. doi: 10.3389/fpls.2015.00664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mizusawa N, Wada H. The role of lipids in photosystem II. Biochim Biophys Acta. 2012;1817:194–208. doi: 10.1016/j.bbabio.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 42.Siegenthaler PA. Molecular organization of acyl lipids in photosynthetic membranes of higher plants. Springer, Dordrecht. 1998;6:119–144. doi: 10.1007/0-306-48087-5_7. [DOI] [Google Scholar]
- 43.Benning C. Mechanisms of lipid transport involved in organelle biogenesis in plant cells. Annu Rev Cell Dev Biol. 2009;25:71–91. doi: 10.1146/annurev.cellbio.042308.113414. [DOI] [PubMed] [Google Scholar]
- 44.Troncosoponce MA, Nikovics K, Marchive C, Lepiniec L, Baud S. New insights on the organization and regulation of the fatty acid biosynthetic network in the model higher plant Arabidopsis thaliana. Biochimie. 2016;120:3–8. doi: 10.1016/j.biochi.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 45.Libeisson Y, Shorrosh BS, Beisson F, Andersson MX, Arondel V, Bates PD, Welti R. Acyl-Lipid metabolism. The Arabidopsis Book. 2010;11:1–70. doi: 10.1199/tab.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holzl G, Dormann P. Chloroplast lipids and their biosynthesis. Annu Rev Plant Biol. 2019;70:51–81. doi: 10.1146/annurev-arplant-050718-100202. [DOI] [PubMed] [Google Scholar]
- 47.Li N, Xu C, Li-Beisson Y, Philippar K. Fatty acid and lipid transport in plant cells. Trends Plant Sci. 2016;21:145–158. doi: 10.1016/j.tplants.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 48.Boudiere L, Michaud M, Petroutsos D, Rebeille F, Falconet D, Bastien O, Roy S, Finazzi G, Rolland N, Jouhet J, et al. Glycerolipids in photosynthesis: composition, synthesis and trafficking. Biochim Biophys Acta. 2014;1837:470–480. doi: 10.1016/j.bbabio.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 49.Joyard J, Ferro M, Masselon CD, Seigneurinberny D, Salvi D, Garin J, Rollanda N. Chloroplast proteomics highlights the subcellular compartmentation of lipid metabolism. Prog Lipid Res. 2010;49:128–158. doi: 10.1016/j.plipres.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 50.Browse J, Warwick N, Somerville C, Slack CR. Fluxes through the prokaryotic and eukaryotic pathways of lipid synthesis in the ‘16:3ʹ plant Arabidopsis thaliana. Bioche J. 1986;235:25–31. doi: 10.1042/bj2350025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Awai K, Marechal E, Block MA, Brun D, Masuda T, Shimada H, Takamiya K, Ohta H, Joyard J. Two types of MGDG synthase genes, found widely in both 16:3 and 18:3 plants, differentially mediate galactolipid syntheses in photosynthetic and nonphotosynthetic tissues in Arabidopsis thaliana. Proc Natl Acad Sci USA. 2001;98:10960–10965. doi: 10.1073/pnas.181331498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Z, Benning C. Chloroplast lipid synthesis and lipid trafficking through ER-plastid membrane contact sites. Biochem Soc Trans. 2012;40:457–463. doi: 10.1042/BST20110752. [DOI] [PubMed] [Google Scholar]
- 53.Frentzen M, Heinz E, Mckeon TA, Stumpf PK. Specificities and selectivities of glycerol-3-phosphate acyltransferase and monoacylglycerol-3-phosphate acyltransferase from pea and spinach chloroplasts. Febs J. 2005;129:629–636. doi: 10.1111/j.1432-1033.1983.tb07096.x. [DOI] [PubMed] [Google Scholar]
- 54.Petroutsos D, Amiar S, Abida H, Dolch L, Bastien O, Rebeille F, Jouhet J, Falconet BMA, Mcfadden GI, Bowler C, et al. Evolution of galactoglycerolipid biosynthetic pathways-From cyanobacteria to primary plastids and from primary to secondary plastids. Prog Lipid Res. 2014;54:68–85. doi: 10.1016/j.plipres.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 55.Kobayashi K. Role of membrane glycerolipids in photosynthesis, thylakoid biogenesis and chloroplast development. J Plant Res. 2016;129:565–580. doi: 10.1007/s10265-016-0827-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hurlock AK, Roston RL, Wang K, Benning C. Lipid trafficking in plant cells. Traffic. 2014;15:915–932. doi: 10.1111/tra.12187. [DOI] [PubMed] [Google Scholar]
- 57.Li N, Zhang Y, Meng H, Li S, Wang S, Xiao Z, Chang P, Zhang X, Li Q, Guo L, et al. Characterization of fatty acid exporters involved in fatty acid transport for oil accumulation in the green alga Chlamydomonas reinhardtii. Biotechnol Biofuels. 2019;12:14. doi: 10.1186/s13068-018-1332-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Botella C, Jouhet J, Block MA. Importance of phosphatidylcholine on the chloroplast surface. Prog Lipid Res. 2017;65:12–23. doi: 10.1016/j.plipres.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 59.Li N, Gugel IL, Giavalisco P, Zeisler V, Schreiber L, Soll J, Philippar K. FAX1, a novel membrane protein mediating plastid fatty acid export. PLoS Biol. 2015;13:e1002053. doi: 10.1371/journal.pbio.1002053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim S, Yamaoka Y, Ono H, Kim H, Shim D, Maeshima M, Martinoia E, Cahoon EB, Nishida I, Lee Y. AtABCA9 transporter supplies fatty acids for lipid synthesis to the endoplasmic reticulum. Proc Natl Acad Sci USA. 2013;110:773–778. doi: 10.1073/pnas.1214159110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lousa CDM, Van Roermund CWT, Postis VLG, Dietrich D, Kerr ID, Wanders RJA, Stephen A, Baldwin SA, Baker A, Theodoulou FL. Intrinsic acyl-CoA thioesterase activity of a peroxisomal ATP binding cassette transporter is required for transport and metabolism of fatty acids. Proc Natl Acad Sci USA. 2013;110:1279–1284. doi: 10.1073/pnas.1218034110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Theodoulou FL, Carrier DJ, Schaedler TA, Baldwin SA, Baker A. How to move an amphipathic molecule across a lipid bilayer: different mechanisms for different ABC transporters? Biochem Soc Trans. 2016;44:774–782. doi: 10.1042/BST20160040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.LaBrant E, Barnes AC, Roston RL. Lipid transport required to make lipids of photosynthetic membranes. Photosynth Res. 2018;138:345–360. doi: 10.1007/s11120-018-0545-5. [DOI] [PubMed] [Google Scholar]
- 64.Chapman KD, Ohlrogge JB. Compartmentation of Triacylglycerol Accumulation in Plants. J Biol Chem. 2012;287:2288–2294. doi: 10.1074/jbc.R111.290072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bates PD, Ohlrogge JB, Pollard M. Incorporation of newly synthesized fatty acids into cytosolic glycerolipids in pea leaves occurs via acyl editing. J Biological Chem. 2007;282:31206–31216. doi: 10.1074/jbc.M705447200. [DOI] [PubMed] [Google Scholar]
- 66.Xu C, Moellering ER, Muthan B, Fan J, Benning C. Lipid transport mediated by arabidopsis TGD proteins is unidirectional from the endoplasmic reticulum to the plastid. Plant Cell Physiol. 2010;51:1019–1028. doi: 10.1093/pcp/pcq053. [DOI] [PubMed] [Google Scholar]
- 67.Yang Y, Zienkiewicz A, Lavell A, Benning C. Coevolution of domain interactions in the chloroplast TGD1, 2, 3 lipid transfer complex specific to brassicaceae and poaceae plants. Plant Cell. 2017;29:1500–1515. doi: 10.1105/tpc.17.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Roston RL, Gao J, Murcha MW, Whelan J, Benning C. TGD1, −2, and −3 proteins involved in lipid trafficking form ATP-binding cassette (ABC) transporter with multiple substrate-binding proteins. J Biol Chem. 2012;287:21406–21415. doi: 10.1074/jbc.M112.370213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xu C, Fan J, Cornish AJ, Benning C. Lipid trafficking between the endoplasmic reticulum and the plastid in Arabidopsis requires the extraplastidic TGD4 protein. Plant Cell. 2008;20:2190–2204. doi: 10.1105/tpc.108.061176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fan J, Zhai Z, Yan C, Xu C. Arabidopsis TRIGALACTOSYLDIACYLGLYCEROL5 interacts with TGD1, TGD2, and TGD4 to facilitate lipid transfer from the endoplasmic reticulum to plastids. Plant Cell. 2015;27:2941–2955. doi: 10.1105/tpc.15.00394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhao L, Katavic V, Li F, Haughn GW, Kunst L. Insertional mutant analysis reveals that long-chain acyl-CoA synthetase 1 (LACS1), but not LACS8, functionally overlaps with LACS9 in Arabidopsis seed oil biosynthesis. Plant J. 2010;64:1048–1058. doi: 10.1111/j.1365-313X.2010.04396.x. [DOI] [PubMed] [Google Scholar]
- 72.Jessen D, Roth C, Wiermer M, Fulda M. Two activities of long-chain Acyl-Coenzyme a synthetase are involved in lipid trafficking between the endoplasmic reticulum and the plastid in Arabidopsis. Plant Physiol. 2015;167:351–366. doi: 10.1104/pp.114.250365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gigon A, Matos AR, Laffray D, Zuilyfodil Y, Phamthi A. Effect of drought stress on lipid metabolism in the leaves of Arabidopsis thaliana (Ecotype Columbia). Ann Bot. 2004;94:345–351. doi: 10.1093/aob/mch150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lin YT, Chen LJ, Herrfurth C, Feussner I, Li HM. Reduced biosynthesis of digalactosyldiacylglycerol, a major chloroplast membrane lipid, leads to oxylipin overproduction and phloem Cap lignification in Arabidopsis. Plant Cell. 2016;28:219–232. doi: 10.1105/tpc.15.01002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shao RX, Xin LF, Zheng HF, Li LL, Ran WL, Mao J, Yang QH. Changes in chloroplast ultrastructure in leaves of drought-stressed maize inbred lines. Photosynthetica. 2016;54:74–80. doi: 10.1007/s11099-015-0158-6. [DOI] [Google Scholar]
- 76.Janik E, Bednarska J, Zubik M, Puzio M, Luchowski R, Grudzinski W, Mazur R, Garstka M, Maksymiec W, Kulik A, et al. Molecular architecture of plant thylakoids under physiological and light stress conditions: a study of lipid-light-harvesting complex ii model membranes. Plant Cell. 2013;25:2155–2170. doi: 10.2307/23482454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Varone L, Ribas-Carbo M, Cardona C, Gallé A, Medrano H, Gratani L, Flexasb J. Stomatal and non-stomatal limitations to photosynthesis in seedlings and saplings of Mediterranean species pre-conditioned and aged in nurseries: different response to water stress. Environ Exp Bot. 2012;75:235–247. doi: 10.1016/j.envexpbot.2011.07.007. [DOI] [Google Scholar]
- 78.Zhang FJ, Zhang KK, Du CZ, Li J, Xing YX, Yang LT, Li YR. Effect of drought stress on anatomical structure and chloroplast ultrastructure in leaves of sugarcane. Sugar Tech. 2014;17:41–48. doi: 10.1007/s12355-014-0337-y. [DOI] [Google Scholar]
- 79.Grigorova B, Vassileva V, Klimchuk D, Vaseva I, Demirevska K, Feller U. Drought, high temperature, and their combination affect ultrastructure of chloroplasts and mitochondria in wheat (Triticum aestivum L.) leaves. J Plant Interactions. 2012;7:204–213. doi: 10.1080/17429145.2011.654134. [DOI] [Google Scholar]
- 80.Kong X, Wei B, Gao Z, Zhou Y, Shi F, Zhou X, Zhou Q, Ji S. Changes in membrane lipid composition and function accompanying chilling injury in bell peppers. Plant Cell Physiol. 2018;59:167–178. doi: 10.1093/pcp/pcx171. [DOI] [PubMed] [Google Scholar]
- 81.Moellering ER, Muthan B, Benning C. Freezing tolerance in plants requires lipid remodeling at the outer chloroplast membrane. Science. 2010;330:226–228. doi: 10.1126/science.1191803. [DOI] [PubMed] [Google Scholar]
- 82.Yang S, Tang XF, Ma NN, Wang LY, Meng QW. Heterology expression of the sweet pepper CBF3 gene confers elevated tolerance to chilling stress in transgenic tobacco. J Plant Physiol. 2011;168:1804–1812. doi: 10.1016/j.jplph.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 83.Zou M, Yuan L, Zhu S, Liu S, Ge J. Effects of heat stress on photosynthetic characteristics and chloroplast ultrastructure of a heat-sensitive and heat-tolerant cultivar of wucai (Brassica campestris L.). Acta Physiol Plant. 2016. doi: 10.1007/s11738-016-2319-z. [DOI] [Google Scholar]
- 84.Lee MH, Cho EJ, Wi SG, Bae H, Kim J, Cho J, Lee SB, Kim JH, Chung BY. Divergences in morphological changes and antioxidant responses in salt-tolerant and salt-sensitive rice seedlings after salt stress. Plant Physiol Biochem. 2013;70:325–335. doi: 10.1016/j.plaphy.2013.05.047. [DOI] [PubMed] [Google Scholar]
- 85.Feng L, Wu QY, Sun YL, Wang LY, Yang XH, Meng QW. Overexpression of chloroplastic monodehydroascorbate reductase enhanced tolerance to temperature and methyl viologen-mediated oxidative stresses. Physiologia Plantarum. 2010;139:421–434. doi: 10.1111/j.1399-3054.2010.01369.x. [DOI] [PubMed] [Google Scholar]
- 86.Velikova V, Muller C, Ghirardo A, Rock TM, Aichler M, Walch A, Schmittkopplin P, Schnitzler J. Knocking down of isoprene emission modifies the lipid matrix of thylakoid membranes and influences the chloroplast ultrastructure in poplar. Plant Physiol. 2015;168:859–870. doi: 10.1104/pp.15.00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tarazona P, Feussner K, Feussner I. An enhanced plant lipidomics method based on multiplexed liquid chromatography-mass spectrometry reveals additional insights into cold-and drought-induced membrane remodeling. Plant J. 2015;84:621–633. doi: 10.1111/tpj.13013. [DOI] [PubMed] [Google Scholar]
- 88.Wang G, Kong F, Zhang S, Meng X, Wang Y, Meng Q. A tomato chloroplast-targeted DnaJ protein protects rubisco activity under heat stress. J Exp Bot. 2015;66:3027–3040. doi: 10.1093/jxb/erv102. [DOI] [PubMed] [Google Scholar]
- 89.Ma X, Chen C, Yang M, Dong X, Lv W, Meng Q. Cold-regulated protein (SlCOR413IM1) confers chilling stress tolerance in tomato plants. Plant Physiol Biochem. 2018;124:29–39. doi: 10.1016/j.plaphy.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 90.Thalhammer A, Hundertmark M, Popova AV, Seckler R, Hincha DK. Interaction of two intrinsically disordered plant stress proteins (COR15A and COR15B) with lipid membranes in the dry state. Biochim Biophys Acta. 2010;1798:1812–1820. doi: 10.1016/j.bbamem.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 91.Shu S, Yuan L, Guo S, Sun J, Yuan Y. Effects of exogenous spermine on chlorophyll fluorescence, antioxidant system and ultrastructure of chloroplasts in Cucumis sativus L. under salt stress. Plant Physiol Biochem. 2013;63:209–216. doi: 10.1016/j.plaphy.2012.11.028. [DOI] [PubMed] [Google Scholar]
- 92.Szymanski J, Brotman Y, Willmitzer L, Cuadrosinostroza A. Linking gene expression and membrane lipid composition of Arabidopsis. Plant Cell. 2014;26:915–928. doi: 10.1105/tpc.113.118919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fujii S, Kobayashi K, Nakamura Y, Wada H. Inducible knockdown of MONOGALACTOSYLDIACYLGLYCEROL SYNTHASE1 reveals roles of galactolipids in organelle differentiation in Arabidopsis Cotyledons. Plant Physiol. 2014;166:1436–1449. doi: 10.1104/pp.114.250050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Aronsson H, Schottler MA, Kelly AA, Sundqvist C, Dormann P, Karim S, Jarvis P. Monogalactosyldiacylglycerol deficiency in Arabidopsis affects pigment composition in the prolamellar body and impairs thylakoid membrane energization and photoprotection in leaves. Plant Physiol. 2008;148:580–592. doi: 10.1104/pp.108.123372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Testerink C, Munnik T. Molecular, cellular, and physiological responses to phosphatidic acid formation in plants. J Exp Bot. 2011;62:2349–2361. doi: 10.1093/jxb/err079. [DOI] [PubMed] [Google Scholar]
- 96.Djanaguiraman M, Prasad PVV, Kumari J, Rengel Z. Root length and root lipid composition contribute to drought tolerance of winter and spring wheat. Plant Soil. 2018;439:57–73. doi: 10.1007/s11104-018-3794-3. [DOI] [Google Scholar]
- 97.Sui N, Han G. Salt-induced photoinhibition of PSII is alleviated in halophyte Thellungiella halophila by increases of unsaturated fatty acids in membrane lipids. Acta Physiol Plant. 2014;36:983–992. doi: 10.1007/s11738-013-1477-5. [DOI] [Google Scholar]
- 98.Liu S, Wang W, Li M, Wan S, Sui N. Antioxidants and unsaturated fatty acids are involved in salt tolerance in peanut. Acta Physiol Plant. 2017:39. doi: 10.1007/s11738-017-2501-y. [DOI] [Google Scholar]
- 99.Sui N, Tian S, Wang W, Wang M, Fan H. Overexpression of glycerol-3-phosphate acyltransferase from suaeda salsa improves salt tolerance in Arabidopsis. Front Plant Sci. 2017;8:1337. doi: 10.3389/fpls.2017.01337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kalisch B, Dormann P, Holzl G. DGDG and glycolipids in plants and algae. Subcell Biochem. 2016;86:51–83. doi: 10.1007/978-3-319-25979-6_3. [DOI] [PubMed] [Google Scholar]
- 101.Hernandez ML, Sicardo MD, Martinezrivas JM. Differential Contribution of endoplasmic reticulum and chloroplast ω-3 fatty acid desaturase genes to the linolenic acid content of olive (Olea europaea) fruit. Plant Cell Physiol. 2016;57:138–151. doi: 10.1093/pcp/pcv159. [DOI] [PubMed] [Google Scholar]
- 102.Cao B, Ma Q, Zhao Q, Wang L, Xu K. Effects of silicon on absorbed light allocation, antioxidant enzymes and ultrastructure of chloroplasts in tomato leaves under simulated drought stress. Scientia Horticulturae. 2015;194:53–62. doi: 10.1016/j.scienta.2015.07.037. [DOI] [Google Scholar]
- 103.Kobayashi K, Fujii S, Sasaki D, Baba S, Ohta H, Masuda T, Wada H. Transcriptional regulation of thylakoid galactolipid biosynthesis coordinated with chlorophyll biosynthesis during the development of chloroplasts in Arabidopsis. Front Plant Sci. 2014;5:272. doi: 10.3389/fpls.2014.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kobayashi K, Endo K, Wada H. Roles of Lipids in Photosynthesis. Sub-cellular Biochemistry. 2016;86:21–49. doi: 10.1007/978-3-319-25979-6_2. [DOI] [PubMed] [Google Scholar]
- 105.Nakajima Y, Umena Y, Nagao R, Endo K, Kobayashi K, Akita F, Suga M, Wada H, Noguchi T, Shen JR. Thylakoid membrane lipid sulfoquinovosyl-diacylglycerol (SQDG) is required for full functioning of photosystem II inThermosynechococcus elongatus. J Biol Chem. 2018;293:14786–14797. doi: 10.1074/jbc.RA118.004304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rottet S, Besagni C, Kessler F. The role of plastoglobules in thylakoid lipid remodeling during plant development. Biochim Biophys Acta. 2015;1847:889–899. doi: 10.1016/j.bbabio.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 107.Sebastiana M, Duarte B, Monteiro F, Malho R, Cacador I, Matos AR. The leaf lipid composition of ectomycorrhizal oak plants shows a drought-tolerance signature. Plant Physiol Biochem. 2019;144:157–165. doi: 10.1016/j.plaphy.2019.09.032. [DOI] [PubMed] [Google Scholar]
- 108.Shimojima M, Ohta H. Critical regulation of galactolipid synthesis controls membrane differentiation and remodeling in distinct plant organs and following environmental changes. Prog Lipid Res. 2011;50:258–266. doi: 10.1016/j.plipres.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 109.Hori K, Nobusawa T, Watanabe T, Madoka Y, Suzuki H, Shibata D, Shimojima M, Ohta H. Tangled evolutionary processes with commonality and diversity in plastidial glycolipid synthesis in photosynthetic organisms. Biochim Biophys Acta. 2016;1861:1294–1308. doi: 10.1016/j.bbalip.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 110.Gasulla F, Vom Dorp K, Dombrink I, Zahringer U, Gisch N, Dormann P, Bartels D. The role of lipid metabolism in the acquisition of desiccation tolerance in Craterostigma plantagineum: a comparative approach. Plant J. 2013;75:726–741. doi: 10.1111/tpj.12241. [DOI] [PubMed] [Google Scholar]
- 111.Bejaoui F, Salas JJ, Nouairi I, Smaoui A, Abdelly C, Martinezforce E, Youssef NB. Changes in chloroplast lipid contents and chloroplast ultrastructure in Sulla carnosa and Sulla coronaria leaves under salt stress. J Plant Physiol. 2016;198:32–38. doi: 10.1016/j.jplph.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 112.Gondor OK, Szalai G, Kovacs V, Janda T, Pal M. Impact of UV-B on drought- or cadmium-induced changes in the fatty acid composition of membrane lipid fractions in wheat. Ecotoxicol Environ Saf. 2014;108:129–134. doi: 10.1016/j.ecoenv.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 113.Botella C, Sautron E, Boudiere L, Michaud M, Dubots E, Yamaryo-Botte Y, Albrieux C, Marechal E, Block MA, Jouhet J. ALA10, a phospholipid flippase, controls FAD2/FAD3 desaturation of phosphatidylcholine in the ER and affects chloroplast lipid composition in Arabidopsis thaliana. Plant Physiol. 2016;170:1300–1314. doi: 10.1104/pp.15.01557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lim GH, Singhal R, Kachroo A, Kachroo P. Fatty acid- and lipid-mediated signaling in plant defense. Annu Rev Phytopathol. 2017;55:505–536. doi: 10.1146/annurev-phyto-080516-035406. [DOI] [PubMed] [Google Scholar]
- 115.Zhong D, Du H, Wang Z, Huang B. Genotypic variation in fatty acid composition and unsaturation levels in bermudagrass associated with leaf dehydration tolerance. J Ame Society Horticultural Sci. 2011;136:35–40. doi: 10.2503/jjshs1.80.113. [DOI] [Google Scholar]
- 116.Gao Q, Yu K, Xia Y, Shine MB, Wang C, Navarre DA, Kachroo A, Kachroo P. Mono-and digalactosyldiacylglycerol lipids function nonredundantly to regulate systemic acquired resistance in plants. Cell Rep. 2014;9:1681–1691. doi: 10.1016/j.celrep.2014.10.069. [DOI] [PubMed] [Google Scholar]
- 117.Karabudak T, Bor M, Ozdemir F, Turkan I. Glycine betaine protects tomato (Solanum lycopersicum) plants at low temperature by inducing fatty acid desaturase7 and lipoxygenase gene expression. Mol Biol Rep. 2014;41:1401–1410. doi: 10.1007/s11033-013-2984-6. [DOI] [PubMed] [Google Scholar]
- 118.Wang G, Cai G, Kong F, Deng Y, Ma N, Meng Q. Overexpression of tomato chloroplast-targeted DnaJ protein enhances tolerance to drought stress and resistance to Pseudomonas solanacearum in transgenic tobacco. Plant Physiol Biochem. 2014;82:95–104. doi: 10.1016/j.plaphy.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 119.Avila CA, Arevalo-Soliz LM, Jia L, Navarre DA, Chen Z, Howe GA, Meng QW, Smith JE, Goggin F. Loss of function of FATTY ACID DESATURASE7 in tomato enhances basal aphid resistance in a salicylate-dependent manner. Plant Physiol. 2012;158:2028–2041. doi: 10.1104/pp.111.191262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang QY, Wang LY, Kong FY, Deng YS, Li B, Meng QW. Constitutive accumulation of zeaxanthin in tomato alleviates salt stress-induced photoinhibition and photooxidation. Physiol Planta. 2012;146:363–373. doi: 10.1111/j.1399-3054.2012.01645.x. [DOI] [PubMed] [Google Scholar]
- 121.Sun X, Lin L, Sui N. Regulation mechanism of microRNA in plant response to abiotic stress and breeding. Mol Biol Rep. 2019;46:1447–1457. doi: 10.1007/s11033-018-4511-2. [DOI] [PubMed] [Google Scholar]
- 122.Sui N, Li M, Li K, Song J, Wang BS. Increase in unsaturated fatty acids in membrane lipids of Suaeda salsa L. enhances protection of photosystem II under high salinity. Photosynthetica. 2010;48:623–629. doi: 10.1007/s11099-010-0080-x. [DOI] [Google Scholar]
- 123.Sun XL, Yang S, Wang LY, Zhang QY, Zhao SJ, Meng QW. The unsaturation of phosphatidylglycerol in thylakoid membrane alleviates PSII photoinhibition under chilling stress. Plant Cell Rep. 2011;30:1939–1947. doi: 10.1007/s00299-011-1102-2. [DOI] [PubMed] [Google Scholar]
- 124.Li M, Ji L, Yang X, Meng Q, Guo S. The protective mechanisms of CaHSP26 in transgenic tobacco to alleviate photoinhibition of PSII during chilling stress. Plant Cell Rep. 2012;31:1969–1979. doi: 10.1007/s00299-012-1309-x. [DOI] [PubMed] [Google Scholar]
- 125.Higashi Y, Okazaki Y, Myouga F, Shinozaki K, Saito K. Landscape of the lipidome and transcriptome under heat stress in Arabidopsis thaliana. Sci Rep. 2015;5:10533. doi: 10.1038/srep10533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Allakhverdiev SI, Kinoshita M, Inaba M, Suzuki I, Murata N. Unsaturated fatty acids in membrane lipids protect the photosynthetic machinery against salt-induced damage in Synechococcus. Plant Physiol. 2001;125:1842–1853. doi: 10.1104/pp.125.4.1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang HS, Yu C, Tang XF, Zhu ZJ, Ma NN, Meng QW. A tomato endoplasmic reticulum (ER)-type omega-3 fatty acid desaturase (LeFAD3) functions in early seedling tolerance to salinity stress. Plant Cell Rep. 2013;33:131–142. doi: 10.1007/s00299-013-1517-z. [DOI] [PubMed] [Google Scholar]
- 128.Yu C, Wang H, Yang S, Tang X, Duan M, Meng QW. Overexpression of endoplasmic reticulum omega-3 fatty acid desaturase gene improves chilling tolerance in tomato. Plant Physiol Biochem. 2009;47:1102–1112. doi: 10.1016/j.plaphy.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 129.Wang HS, Yu C, Tang XF, Wang LY, Dong XC, Meng QW. Antisense-mediated depletion of tomato endoplasmic reticulum omega-3 fatty acid desaturase enhances thermal tolerance. J Integr Plant Biol. 2010;52:568–577. doi: 10.1111/j.1744-7909.2010.00957.x. [DOI] [PubMed] [Google Scholar]
- 130.Yurchenko O, Park S, Ilut DC, Inmon JJ, Millhollon JC, Liechty ZS, Page JT, Jenks MA, Chapman KD, Udall JA, et al. Genome-wide analysis of the omega-3 fatty acid desaturase gene family in Gossypium. BMC Plant Biol. 2014;14:312. doi: 10.1186/s12870-014-0312-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang YW, Jiang DX, Chen JJHGX. Physiological characterization and thylakoid ultrastructure analysis in super high-yield hybrid rice leaves under drought stress. Photosynthetica. 2019;57:890–896. doi: 10.32615/ps.2019.106. [DOI] [Google Scholar]
- 132.Narayanan S, Tamura PJ, Roth MR, Prasad PV, Welti R. Wheat leaf lipids during heat stress: I. High day and night temperatures result in major lipid alterations. Plant Cell Environ. 2016;39:787–803. doi: 10.1111/pce.12649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Narayanan S, Prasad PV, Welti R. Wheat leaf lipids during heat stress: II. Lipids experiencing coordinated metabolism are detected by analysis of lipid co-occurrence. Plant Cell Environ. 2016;39:608–617. doi: 10.1111/pce.12648. [DOI] [PMC free article] [PubMed] [Google Scholar]


