ABSTRACT
Amorphophallus
has attracted tremendous interest because of its high contents of glucomannan and starch. Very few genes regulating glucomannan and starch were reported in Amorphophallus. In this study, an ADP-glucose pyrophosphorylase (AGP) gene that plays a significant role in plant starch synthesis was cloned from Amorphophallus muelleri. It was shown that it encoded a predicted protein containing a conserved plant ADP-Glucose-PP repeat domain and seven potential ligand-binding sites. The real-time quantitative PCR showed that AmAGP was most abundant in tubers, and it was positively correlated with starch content. Additionally, its influencers about temperature and exogenous plant hormone were also discussed, showing that AmAGP expressed highly in tubers under treatments using 25°C and IAA. Furthermore, starch content was closely related to AmAGP expression level, suggesting that AmAGP was involved in the regulation of starch synthesis in A. muelleri. Therefore, identifying the sequence of AmAGP and its expression pattern during tuber enlarging and the changes of its transcript levels in response to temperature and plant hormones would contribute to a better understanding of starch synthesis, and also providing a reference information for future preferable breeding for obtaining more starch or more glucomannan in Amorphophallus.
KEYWORDS: Amorphophallus muelleri, starch, ADP-glucose pyrophosphorylase, temperature, plant hormone
1. Introduction
Amorphophallus is a perennial, herbaceous monocot that is mainly distributed in countries of tropical and subtropical Asia, including China, Japan, Myanmar, Vietnam, and Indonesia. At present, more than 170 species of Amorphophallus have been identified, and 21 species have been discovered and named in China, and nine of these are endemic to China.1 Amorphophallus tuber is edible, which is rich in konjac glucomannan (KGM) and starch. KGM is a kind of dietary fiber, which has a beneficial effect on human health. Because of its non-toxicity, biocompatibility, biodegradability, and hydrophilicity, KGM has attracted people’s attention.2 At present, Amorphophallus is the only plant that can produce large amounts of KGM. In addition to KGM, starch is seemed as the major caloric source in a variety of human diets and is also a major nutrient in Amorphophallus.3 A. muelleri is a special Amorphophallus species, its leaves displayed living bulbils on the proximal petiole. Because of its high disease resistance and its apomictic characterization, it has a strong competitive advantage and a very high economic value.2 Studies have shown that A. muelleri starch can moderate the rate of digestion of foods, and can also reduce glycemic response and insulin resistance in patients with diabetes via influencing the controlled glucose release profiles.4
Starch, an important storage substance produced by photosynthesis in most higher plants, is synthesized inside the plastid stroma within plant cells.5 There have been many studies on Amorphophallus glucomannan at home and abroad, but fewer have focused specifically on Amorphophallus starch,6 especially in its biosynthesis. Biosynthesis of starch is at least regulated by four classes of enzymes, including ADP-glucose pyrophosphorylase (AGPase, EC 2.7.7.27), Starch Synthase (SS, EC 2.4.1.21), Starch branching Enzyme (BE, EC 2.4.1.18), and Starch Debranching Enzyme (DBE, EC3.2.1.68).7,8 Each of them plays a different role in starch synthesis. AGPase is the first key regulatory and rate-limiting enzyme for starch biosynthesis and has a high degree of control over starch biosynthesis.9,10 In plants, it is a heterotetramer that composed of two pairs of large and small subunits.11,12 The small subunit has both catalytic and regulatory functions, it was found in the leaf and contributes to increase plant biomass,13,14 and the large subunit has largely a regulatory function,15 it was found in the seed endosperm plays an important role in determining grain yield in cereals.13,16,17 The small subunit includes the cytosolic small subunit (SSUI) and the plastidial small subunit (SSUII), the large subunit includes the cytosolic large subunit (LSUI) and the plastidial large subunit (LSUII).18 Genes encoding SSUII, SSUII, LSUI, and LSUII have been characterized at the genome level across many plant species such as Arabidopsis,19 rice,20,21 sweet potato,22 banana,23 potato,24 wheat,25 maize,26 and cassava.27 AGPase genes are closely associated with starch synthesis in plant tissues. For example, in the OsAPL3-defective mutants of rice, starch content was reduced with the decreased AGPase activity in leaves.28 Additionally, it was also regulated by environmental factors. In potato, the expression of AGPase genes (StAGP) was regulated by low temperature;29 the expression of tomato AGPL1 and AGPS1 was up-regulated in salt-stressed developing fruits; the expression of two banana AGPase genes was induced by cold, salt, and drought;23 starch accumulation in developing rice grains was positively correlated with an increase in the AGPase and SS activity under drought.30 Additionally, phosphorylation is also found to regulate starch synthesis by controlling carbon flux into starch while simultaneously modulating expression of genes involved in starch synthesis. Over the past century, starch phosphorylation has aroused increasing interest as the only naturally occurring covalent modification in starch.31 It occurs during starch synthesis, although the rates of phosphorylation are likely to be different.32 The starch levels are in a continual state of flux during plant development; thus, starch accumulation is determined by the regulation of both synthetic and degradative enzymes.33 Research in the past decade has uncovered new and surprising information about the pathways of starch synthesis and degradation. Starch degradation in chloroplasts requires β‐amylase (BAM) activity, but in Arabidopsis, there are nine BAM proteins, five of which are thought to be catalytic.34 These discoveries also uncover complexities of starch synthesis mechanism: results from model plants (such as Arabidopsis) are not necessarily applicable to other plant species or to growth in natural.
The genome size of Amorphophallus is quite large, and it is, therefore, challenging to sequence the entire genome of Amorphophallus species. So far, due to limitations in genomic data, fewer studies have screened for suitable functional genes in Amorphophallus. Recently, transcriptome data were obtained from A. muelleri, and most of the significant genes in the sphingolipid metabolic pathway were identified.35 In the present study, AmAGP gene was isolated because of its important role in starch synthesis, and its expression patterns were explored during tuber expansion in A. muelleri. Additionally, the function of AmAGP was further characterized by examining its response to temperatures and hormones that are favorable for starch accumulation. Taken collectively, considering the relationship between starch and KGM, the assessment of AmAGP function provides a theoretical basis for the identification of starch- and glucomannan-related regulatory networks, and also a support for directional breeding in agricultural production.
2. Materials and methods
2.1. Plant materials and growth conditions
The test materials were A. muelleri which were grown in orchard in Yunnan University, Yunnan Province, China. A. muelleri is a special Amorphophallus species and widely planted in Yunnan and Sichuan Province, China.
2.2. Asexual seed evaluation of A. muelleri
To examine the formation of asexual seeds, the spathe was cut off before flower buds had opened to expose pistils to the air for pollination. The stigma was removed with a sharp blade and covered with a paper bag. Compared with the total number of decapitated flowers, the number of parthenogenetic fruits that produced seeds in the absence of fertilization is calculated as a percentage of asexual seeds.
2.3. Cloning and homology analysis of the AmAGP gene
For cloning AmAGP gene in A. muelleri, total RNA of tubers was isolated using Trizol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. First-strand cDNA was synthesized using the M-MLV system and used as a template for RT-PCR. Referring to the conserved sequences of ADP-glucose pyrophosphorylase genes from Amorphophallus konjac and Amorphophallus albus, the forward primer 5ʹ-ATGGATGCCAGCAGTATGATGGT-3ʹ and reverse primer 5ʹ-GTATGATGGTCACAAGCTTCGGT-3ʹ were designed and synthesized by the Beijing Genomics Institute (BGI), the sequences were download from NCBI and Gene number were JF727266.1 and AF316326.1, respectively. PCR conditions were as follows: 3 min at 94°C (one cycle), followed by 30 s at 94°C, 30 s at 55.5°C, and 45 s at 72°C (30 cycles) for 25 μL reaction buffer. The PCR products were run on a 1.0% (m/v) agarose gel and detected using an image analyzer (white/UV transilluminator), and the desired band was recovered and ligated to the pMD18-T cloning vector for sequencing.
For homology analysis of AmAGP gene. The sequence of the amplified AmAGP gene was used to identify its ORF by Edit sequence software, and then the nucleotide sequence was translated into an amino acid sequence by DNAMAN software. Conserved domain analysis was also used DNAMAN software. Nucleotide and amino acid sequence similarity alignments were performed by Blast in NCBI (http://blast.ncbi.nlm.nih.gov/). The ProtParam protein analysis tool (http://web.expasy.org/protparam/) was used to analyze the molecular weight, theoretical isoelectric point, and other protein properties.
2.4. Phylogenetic tree construction
Firstly, BLAST was performed on the AmAGP protein sequence, and then 17 AGP protein sequences were downloaded from NCBI (http://ncbi.nlm.nih.gov). A phylogenetic tree was constructed in the MEGA6 program using the neighbor-joining method with the ‘pairwise deletion’ option, the ‘Poisson correction’ model, and 1000 bootstrap replicates.36
2.5. Real-time quantitative PCR (qRT-PCR) and semi-quantitative PCR (sqRT-PCR) analysis
Both total RNA and first-strand cDNA of A. muelleri from tubers of different developing stages, root, petiole, petiolar base, and leaf were isolated according to 2.2 methods. For qRT-PCR, two pairs of specific primers were designed based on the AmAGP gene sequence: AmAGP-F, 5ʹ-CGGCGGCATCATAGGAGGGAG-3ʹ and AmAGP-R, 5ʹ-GACCGCAGCCACGTTCTTC-3ʹ. qRT-PCR assays were performed using the Power SYBR Green PCR Master Mix (Applied Biosystems, Foster, USA). The amplification reaction was conducted in a 20 μL reaction volume containing 10 μL SYBR Green using a BIO-RAD iQ5 instrument (USA). Thermocycling parameters were 2 min at 56°C, 10 min at 95°C, and 50 cycles of 15 s at 95°C, and 60 s at 60°C. Three technical replicates were performed for each sample. The ΔCt was calculated using the A. muelleri 18S gene (forward primer 5ʹ-GTATGATGGTCACAAGCTTCGG-3ʹ and reverse primer 5ʹ-CCTCAACAAATCCATCCCCAAA-3ʹ) as an endogenous reference gene.37 Relative quantitation values were calculated using the 2−ΔΔCt method.38 The specificity of the amplification was determined by performing a dissociation curve analysis. For sqRT-PCR, PCR conditions were 3 min at 94°C, 30 s at 94°C; 30 s at 54°C and 45 s at 72°C (32 cycles) for 25 µl reaction buffer. The PCR products were separated on a 1.0% (m/v) agarose gel and detected using an image analyzer (white/UV transilluminator).
2.5. Treatment of tubers with different temperatures and hormones in A. muelleri
A. muelleri bulbils of consistent size were used as seeds and grown under greenhouse conditions at Yunnan University (Kunming, Yunnan). Each treatment was performed using 30 number of tubers at 60 d after planting (DAP), 90 DAP, 120 DAP, and 150 DAP, respectively.
For temperature treatments, according to the preferable temperature for the development of A. muelleri, the temperature treatments were chosen from 20°C to 35°C. A. muelleri was grown at temperatures of 18°C for 8 h in the dark and 20°C, 25°C, 30°C, and 35°C for 16 h in the light. Tubers were harvested at 90 DAP, immediately frozen in liquid nitrogen, and stored at −80°C.
For hormone treatments, 1.0 mg L−1 IAA, 0.5 mg L−1 TDZ, or 25 mg L−1 SA were sprayed on the leaves of A. muelleri every 7 d, and tubers sprayed with water were used as controls. After the treatments had been repeated five times using the same hormone concentrations, 90 DAP tubers were harvested, immediately frozen in liquid nitrogen, and stored at −80°C.
2.6. Measurement of starch and glucomannan
Starch measurements were performed using an iodine-starch colorimetry assay with a minor revision.39 Firstly, three 90 DAF tubers were chopped and mixed together, and 0.5 g tubers were added into 15 ml 80% calcium nitrate solution. Secondly, 20 mL ddH2O was added to the boiling mixture, and then the mixture was centrifuged for 5 min at 5000 rpm. After removal of the supernatant, the subsidence was mixed with 50 mL ddH2O, and the solution was measured. Finally, 8 ml of the starch extract was added to a centrifuge tube containing 2 ml of 0.5% iodine solution, mixed, allowed to stand for 15 minutes, and centrifuged for 5 min at 5000 rpm. The supernatant was then discarded to make a working solution. After the color was developed, the absorbance was measured with an ultraviolet (UV) spectrophotometer at a wavelength of 590 nm. Three technical replicates were performed for each sample. Contents of starch in tubers were calculated as follows:
where C is the starch content obtained from the standard curve, V0 is the total volume of the starch extraction solution, V1 is the volume of the measured solution, and m is the weight of measured tubers.
Content of glucomannan was determined by Huang’s method.40 The absorbance of working solutions of glucomannan was measured at 550 nm with a UV spectrophotometer, and the glucomannan content was calculated as follows:
where ε = 0.9, T is the starch content obtained from standard curves, and M is the weight of purified powder (mg).
3. Results
3.1. Molecular cloning and phylogenetic analysis of AmAGP
A. muelleri is a special Amorphophallus species, its leaves displayed living bulbils on the proximal petiole (Figure S1A). Leaf bulbils were easily separated from the leaf after it was withered. Besides, A. muelleri displayed a special growth type in which one to three seedlings all supported the same underground tuber development, thereby making full use of photosynthesis to promote tuber expansion. The inflorescence might eventually reach up to 1 m or more in height, and it was composed of a colorful outer spathe and an inner spadix with stamen and pistil born all-around upper and down, respectively (Figure S1C, D). Interestingly, fruit could develop spontaneously after removing its stigma, and a mature fruit contains one or two seeds at least (Figure S1E–G), suggesting that A. muelleri displays apomictic seed formation (Figure S1H). Because of its high disease resistance and its apomictic characterization, it has a strong competitive advantage and a very high economic value.
To identify the function of AGPase genes of A. muelleri, an AmAGP gene was identified according to sequence similarity with AGPase genes from Oryza sativa (AAA33891.1), Triticum aestivum (CAA46879), Hordeum vulgare (ABX72229.1), and A. konjac (AEH27531.1). AmAGP had a long open reading frame that encoded a predicted protein containing 552 amino acid residues, with a calculated molecular mass of 61,091 Da, and an isoelectric point of 8.63. The predicted AmAGP protein contained a conserved plant ADP-Glucose-PP repeat domain (CD02508) and seven potential ligand-binding sites including the ‘LGGG’ motif (Figure 1), which were similar to those of AGPase proteins from other plant species, suggesting that it was conserved among different plants.
Figure 1.
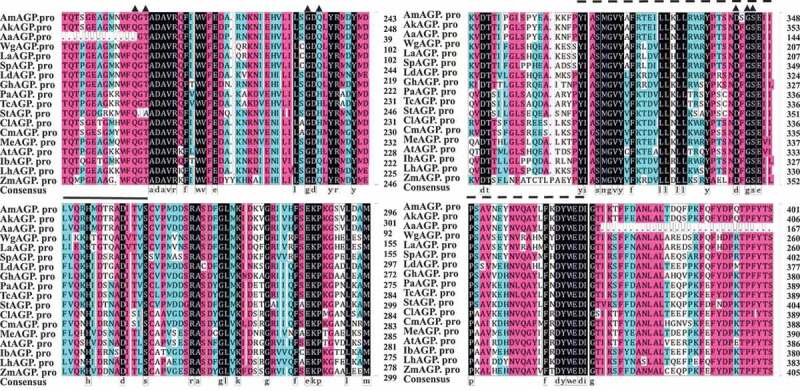
Sequence analysis of the predicted AmAGP protein and comparison of its sequence with other AGP protein sequences. Ak, Amorphophallus konjac (JF727266.1); Aa, Amorphophallus albus (AF316326.1); Wg, Wolffia globosa (KR263899.1); La, Lemna aequinoctialis (KR263896.1); Sp, Spirodela polyrhiza (JN180633.1); Ld, Lilium davidii var. unicolor (AJG44462.1); Gh, Gladiolus hybrid cultivar (AIO11223.1); Pa, Populus alba (TKS10148.1); Tc, Theobroma cacao (EOY14815.1); St, Solanum tuberosum (CAA52917.1); Cl, Citrullus lanatus (AF032472.1); Cm, Cucumis melo (NM_001297522.2); Me, Manihot esculenta (KU243122.1); At, Arabidopsis thaliana (AY096657.1); Ib, Ipomoea batatas (JQ797692.1); Lh, Lycopersicon hirsutum (AF184345.1); and Zm, Zea mays (CAA 86227.1). Two ADP-Glucose-PP repeat domains are highlighted with solid and dashed lines, respectively. The potential ligand-binding sites are highlighted with triangle
To further clarify the evolutionary relationships of AmAGP in plants, phylogenetic analysis of AGPase proteins was performed using MEGA6.0. Eighteen AGPase proteins were obtained from different plant species. The results showed that AGPase proteins were classified into two groups, group I and group II, respectively. Interestingly, all of the monocots were belonged to group I and most of the dicots were fell into group II with the exception of Zea mays (Figure 2).
Figure 2.
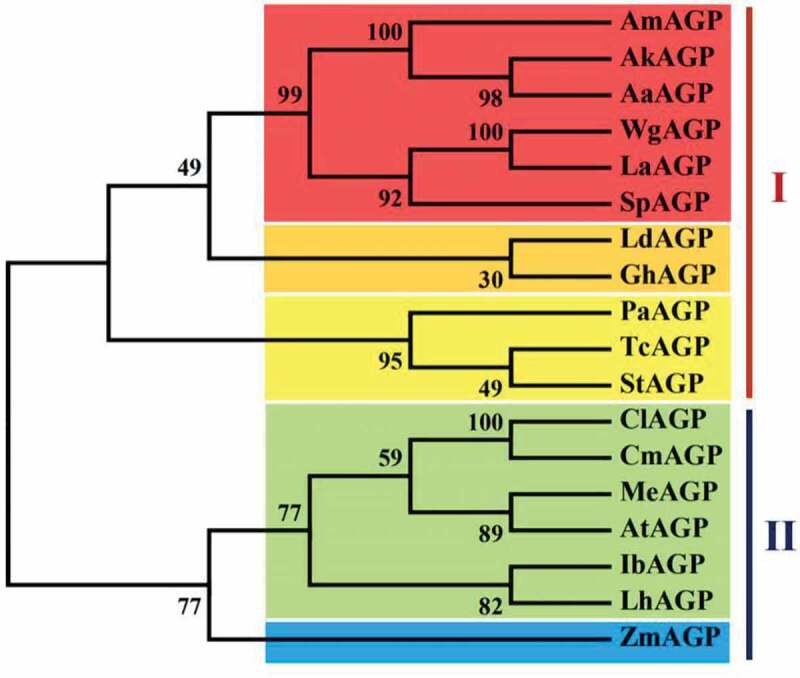
Phylogenetic analysis of the AmAGP protein and other homologous proteins, including Ak, Amorphophallus konjac (JF727266.1); Aa, Amorphophallus albus (AF316326.1); Wg, Wolffia globosa (KR263899.1); La, Lemna aequinoctialis (KR263896.1); Sp, Spirodela polyrhiza (JN180633.1); Ld, Lilium davidii var. unicolor (AJG44462.1); Gh, Gladiolus hybrid cultivar (AIO11223.1); Pa, Populus alba (TKS10148.1); Tc, Theobroma cacao (EOY14815.1); St, Solanum tuberosum (CAA52917.1); Cl, Citrullus lanatus (AF032472.1); Cm, Cucumis melo (NM_001297522.2); Me, Manihot esculenta (KU243122.1); At, Arabidopsis thaliana (AY096657.1); Ib, Ipomoea batatas (JQ797692.1); Lh, Lycopersicon hirsutum (AF184345.1); and Zm, Zea mays (CAA 86227.1). Multiple sequence alignment of AGP proteins was performed using ClustalX2 with default parameters. The unrooted phylogenetic tree was constructed in MEGA 6 with the neighbor-joining (NJ) method and 1000 bootstrap replicates
3.2. AmAGP was expressed highly in tubers
To characterize the potential function of AmAGP in different A. muelleri tissues, the temporal and spatial expression patterns were analyzed using qRT-PCR assays (Figure 3(a)) and sqRT-PCR (Figure 3(b)). The results showed that AmAGP was expressed not only in reproductive organs but also in vegetative ones. It was expressed constitutively in all tissues tested, although its expression levels varied among tissues. Its transcript level was the lowest in the base of petiole and the highest in the tuber, respectively. The tuber is the economically valuable part of the Amorphophallus plant and which produces abundant starch, and its high level of AmAGP expression suggested that it might be probably important for the synthesis of starch in tuber.
Figure 3.
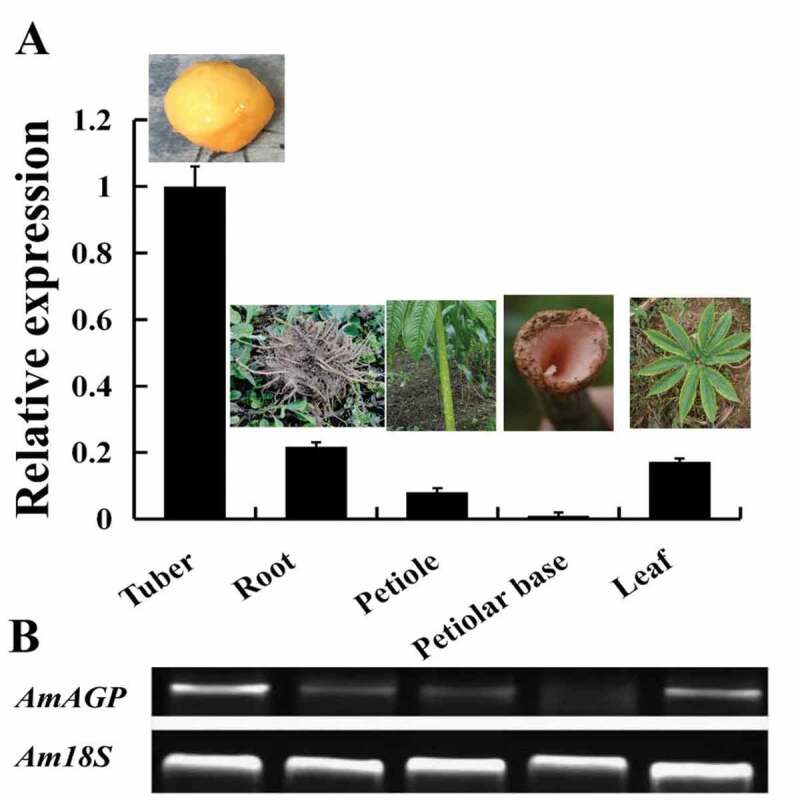
qRT-PCR (a) and sqRT-PCR (b) analysis of AmAGP in different tissues. Am18S was used as a loading reference
3.3. AmAGP was induced in tubers under 25°C treatment and was positively correlated with starch synthesis in A. muelleri
As commercial products, A. muelleri tubers can be divided into two types, the starch type and the glucomannan type, respectively. To document the preferred characteristics and the relationship between starch and glucomannan in tubers, both of them were assessed during tuber elongation. Tubers were collected at 60, 90, 120, and 150 DAP for measurement of starch and glucomannan content. The results showed that the starch content increased significantly over time and reached a maximum at 120 DAP, it then decreased at 150 DAP, indicating that the starch had transformed into other monosaccharides gradually. In contrast, glucomannan accumulation increased throughout the development period (Figure 4(a)). Our results showed that both starch and glucomannan were significantly increased at 90 DAP, indicating that 90 DAP was the essential period for tuber elongation. Additionally, to better identify the relationship of transcript level of AmAGP and starch synthesis, the expression level of AmAGP was detected in 60, 90, 120, and 150 DAP tubers using qRT-PCR. The results showed that AmAGP expressed highly in 120 DAP tuber, correspondingly, starch content increased significantly over time and reached a maximum at 120 DAP, it then decreased at 150 DAP (Figure 4(b)), indicating that the starch had transformed into other monosaccharides gradually.
Figure 4.
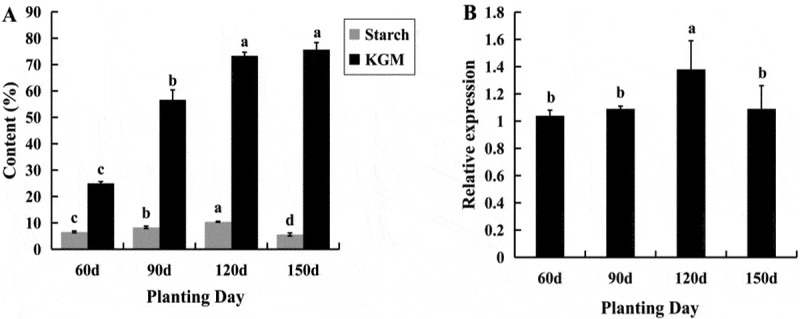
Starch and glucomannan content (a) and AmAGP transcript level (b) during tuber expansion in A. muelleri. For a given substance, values that share a letter do not differ significantly (P < .05, N = 3 biological replicates). Error bars represent standard errors
To assess the effect of temperature on starch and glucomannan synthesis, 90 DAP tubers were treated using different temperatures of 20°C, 25°C, 30°C, and 35°C, respectively. As treating temperature increased, the starch content reached 13.64% at 30°C, which was not significantly different from the 25°C treatment. However, when the treatment temperature was 35°C, the starch content decreased significantly to 8.85%. The glucomannan content was increased gradually with temperature changes from 20°C to 35°C, and reached a maximum of 65.98% at 35°C in tubers (Figure 5(a)). The differences in starch and glucomannan content using different temperature treatments were significant, and the optimal temperatures for starch and glucomannan accumulation were 25°C and 35°C, respectively. The results showed that a temperature of 25°C was most conducive to the accumulation of starch in A. muelleri.
Figure 5.
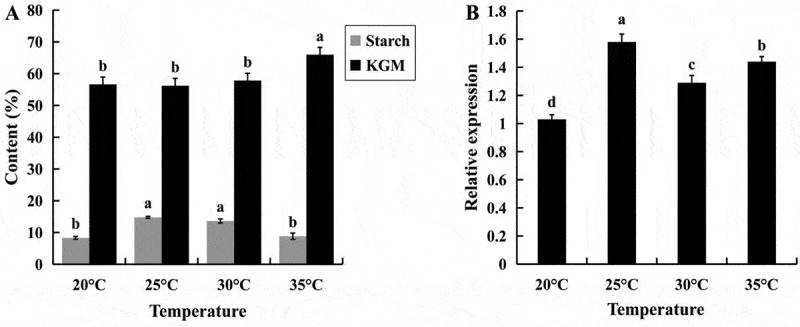
Analysis of starch and glucomannan content (a) and AmAGP transcript levels (b) in 90 DAP tubers under different temperature treatments. Values that share a letter do not differ significantly (P < .05, N = 3 biological replicates). Error bars represent standard errors
To further confirm the regulation of AmAGP in starch synthesis in A. muelleri, its expression levels were measured in 90 DAP tubers using different temperature treatments. The results showed that 90 DAP tubers which grown at 25°C produced higher AmAGP transcript levels than any other treatment groups (Figure 5(b)), indicating that AmAGP expression level was positively correlated with starch content in A. muelleri.
3.4. Both starch synthesis and AmAGP expression were induced by IAA
Starch could be induced by hormones, to assess which hormones were involved in starch synthesis, three different hormones (IAA, TDZ and SA) were used to spray the leaves of A. muelleri. Compared with control tubers, all the tubers treated using 1.0 mg L−1 Indole-3-acetic acid (IAA), 0.5 mg L−1 Thidiazuron (TDZ), and 25 mg L−1 Salicylic acid (SA) displayed higher contents of both starch and glucomannan. Additionally, they were significantly higher (8.23% and 68.2%, respectively) in response to IAA treatment than those in response to other treatments (Figure 6(a)). Therefore, both starch and glucomannan were promoted by IAA in A. muelleri.
Figure 6.
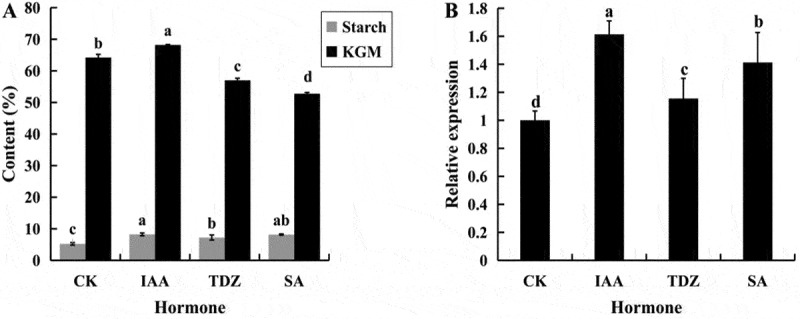
Analysis of starch and glucomannan content (a) and AmAGP transcript levels (b) in 90 DAP tubers under different hormone treatments. Tubers sprayed with water were used as controls. Values that share a letter do not differ significantly (P < .05, N = 3 biological replicates). Error bars represent standard errors
In order to further identify the involvement of AmAGP into starch synthesis, the abundance of AmAGP was detected in 90 DAP tubers that treated using different hormones as above. It was showed that AmAGP expression was lowest in the control tubers and highest in the tubers treated with 1.0 mg L−1 IAA, followed by 25 mg L−1 SA and 0.5 mg L−1 TDZ (Figure 6(b)). These findings further confirmed that AmAGP was induced by IAA and acted as a positive regulator for starch synthesis.
4. Discussion
A. muelleri is an Amorphophallus species with a high competitive ability and economic value. Both the bulbs and tubers of Amorphophallus are rich in the biopolymers starch and glucomannan, at levels that differ among species. In recent years, these biomaterials have received considerable attention as potential components of drug delivery systems for the controlled release of drugs at specific targeted delivery sites.41 Among the natural polymers, cassava starch KGM is regarded as a potential carrier for a site-specific bioactive protein drug delivery system.42 In view of this potential function, an increased understanding of starch and glucomannan biosynthesis and metabolism may help to increase the abundance of this relatively easily digestible and fermentable polymer.
In plants, both starch and KGM are derived from carbon products accumulated during photosynthesis in leaves.43 In their synthesis pathway, the first key enzyme is AGP, which catalyzes the production of ADP-glucose from glucose-1-phosphate. At the same time, glucose-1-phosphate is acted upon by GGP to produce GDP-glucose (or to further produce GDP-mannose), and glucomannan is synthesized under the action of CSLA3. AGP and GGP catalyze the production of ADP-glucose and GDP-glucose from glucose-1-phosphate,43 and these sugar phosphates then participate in starch or glucomannan synthesis, respectively. Therefore, the starch content would affect the glucomannan content in Amorphophallus. High AmAGP expression in tubers might lead to high contents of starch, which providing genetic improvement and potential utilization in oriented breeding of A. muelleri. To date, the traditional view of starch metabolism has focused on the multiplicity of enzymes and enzyme isoforms contributing to the production of the constituent polymers.44 Among these enzyme complexes, AGPase is the well studied.45 Regulation of the AGPase gene has been shown to change starch content and to accelerate or reduce the synthesis of reducing sugars. In potato, antisense-AGP transgenic plants exhibited significantly reduced starch, whereas sucrose and glucose were increased in the tubers.46 AGP was not only involved in the regulation of starch production, but it also indirectly regulated sugar formation. There are seven MeAGPs in cassava, which are the same as rice AGPase genes,20,21 but it obviously differs in number from AGPase genes of Arabidopsis,19 potato,24 maize,26 banana,23 and sweet potato.22 There might be some AGPase genes in A. muelleri. In this study, a starch synthesis-related gene AmAGP was isolated from A. muelleri, and its predicted protein was shown to have the highest homology with AkAGP and AaAGP. There were seven binding sites of substrates in AmAGP, which showed some differences from other AGPs, i.g. the binding site of 116 amino acid showed that it was ‘Q’ in most of monocots but was ‘H’ in most of dicots, suggesting that AmAGP might have some differences from other AGPs proteins in both substrate binding and enzyme activity regulation. According to protein homology, AGPs proteins could be divided into dicot and monocot types. Interestingly, maize was differed from other monocots, it might be due to its high contents of starch, suggesting that AGPs genes showed a species specific evolution among monocots and dicots.
AGP is differentially expressed not only among different plant organs but also within the same organ at different developmental stages, as shown in wheat,47 barley,48 maize,49 etc. As for wheat, the cytosolic AGP gene is expressed in the late stages of endosperm development, while the plastidial AGP gene is expressed in young endosperm and leaves.47 In hull-less barley, AGP gene had higher expression in grain than in leaves and stems.48 In A. muelleri, AmAGP was highly expressed in tubers with high starch content and low expressed in petioles and base of petioles with low starch content. Furthermore, the expression level of AmAGP was consistent with the change of starch content during tuber enlarging, suggesting that AmAGP expression was positively correlated with starch content. Interestingly, the expression level of AmAGP was decreased in 150 DAP tuber, it might be induced by the feedback regulatory mechanism of AmAGP in A.muelleri. Plants such as potato, rice, and tomato also have shown tissue-specific expression patterns similar to those documented here in A. muelleri. Additionally, AGP transcript levels changed in different tissues during different growth stages of barley.48 By contrast, maize has three genes that encode AGP large subunits, and one does not show obvious tissue-specific expression.49 The tissue-specific expression of the AGP large subunit, therefore, appears to vary among species.
Additionally, the expression of the AGPs genes was greatly affected by the environmental factors,23,29,50 suggesting that environmental conditions have an impact on AGPase activity,27,52 such as hormone, temperature, and other conditions. Specifically, IAA, SA, TDZ affect the bulbous expansion and the accumulation of inclusions, and appropriate exogenous hormone treatments can increase starch content. Both endogenous and exogenous hormones such as IAA, SA, and TDZ influence metabolites and tuber expansion in plants.51-53 In A. muelleri, the accumulation of starch was increased significantly at 90 DAP, and AmAGP transcript levels were highest at 25°C and in response to IAA treatment. It has been suggested that AmAGP is involved in the regulation of starch formation in A. muelleri. Regulation of AGP during starch synthesis is complex, and our study contributes to a better genetic improvement of A. muelleri.
Supplementary Material
Funding Statement
This work was supported by the National Natural Science Foundation of China under Grant (No. 31660566).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Liu PY. Konjac. 1st ed. Beijing (China): China Agriculture Press; 2004. p. 1–8. [Google Scholar]
- 2.Zhang DH, Zhao JR, Zhou F, Wang QP.. Promising kind of konjac, Amorphophallus Bulbifer with great potentiality from the areas between China and Burma. Resour Dev Market. 2005;21:136–138. [Google Scholar]
- 3.Zhai K, Qin HB, Hong Y.. Study on physico–chemical properties of konjac starch. Food Sci. 2008;29:59–61. [Google Scholar]
- 4.Behera SS, Ray RC.. Konjac glucomannan, a promising polysaccharide of Amorphophallus konjac K. Koch in health care. Int J Biol Macromol. 2016;92:942–956. doi: 10.1016/j.ijbiomac.2016.07.098. [DOI] [PubMed] [Google Scholar]
- 5.Goren A, Ashlock D, Tetlow IJ. Starch formation inside plastids of higher plants. Protoplasma. 2018;255(6):1855–1876. doi: 10.1007/s00709-018-1259-4. [DOI] [PubMed] [Google Scholar]
- 6.Xia Y, Deng XL, Zhou WH, Lin QL. Research progress of konjac starch. Cereals Oils. 2013;26:9–11. [Google Scholar]
- 7.Abe N, Asai H, Yago H, Oitome NF, Itoh R, Crofts N, Nakamura Y, Fujita N. Relationships between starch synthase I and branching enzyme isozymes determined using double mutant rice lines. BMC Plant Biol. 2014;14:80. doi: 10.1186/1471-2229-14-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu B, Lin R, Jiang Y, Jiang S, Xiong Y, Lian H, Zeng Q, Liu X, Liu Z, Chen S. Transcriptome analysis and identification of genes associated with starch metabolism in Castanea henryi seed (Fagaceae). Int J Mol Sci. 2020;21:1431. doi: 10.3390/ijms21041431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mugford ST, Fernandez O, Brinton J, Flis A, Krohn N, Encke B, Feil R, Sulpice R, Lunn JE, Stitt M, et al. Regulatory properties of ADP glucose pyrophosphorylase are required for adjustment of leaf starch synthesis in different photoperiods. Plant Physiol. 2014;166(4):1733–1877. doi: 10.1104/pp.114.247759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dong S, Beckles DM. Dynamic changes in the starch–sugar interconversion within plant source and sink tissues promote a better abiotic stress response. J Plant Physiol. 2019;234–235:80–93. doi: 10.1016/j.jplph.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 11.Singh S, Choi SB, Modi MK, Okita TW. Isolation and characterization of cDNA clones encoding ADP–glucose pyrophosphorylase (AGPase) large and small subunits from chickpea (Cicer arietinum L.). Phytochemistry. 2002;59:261–268. doi: 10.1016/S0031-9422(01)00457-5. [DOI] [PubMed] [Google Scholar]
- 12.Zheng QK, Wei F, Luo SQ. Cloning of one full–length AGPase large subunit cDNA sequence and expression analysis. J Trop Cro. 2014;35:1523–1527. [Google Scholar]
- 13.Li N, Zhang S, Zhao Y, Li B, Zhang J. Overexpression of AGPase genes enhances seed weight and starch content in transgenic maize. Planta. 2011;233:241–250. doi: 10.1007/s00425-010-1296-5. [DOI] [PubMed] [Google Scholar]
- 14.Schlossar AJ, Martin JM, Beecher BS, Giroux MJ. Enhanced rice growth is conferred by increased leaf ADP–glucose pyrophosphorylase activity. J Plant Physiol Pathol. 2014;2:4. [Google Scholar]
- 15.Johnson PE, Patron NJ, Bottrill AR, Dinges JR, Fahy BF, Parker ML, Waite DN, Denyer K. A low–starch barley mutant, ris 16, lacking the cytosolic small subunit of ADP–glucose pyrophosphorylase, reveals the importance of the cytosolic isoform and the identity of the plastidial small subunit. Plant Physiol. 2003;131:684–696. doi: 10.1104/pp.013094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee SK, Hwang SK, Han M, Eom JS, Kang HG, Han Y, Choi SB, Cho MH, Bhoo SH, An G, et al. Identification of the ADP–glucose pyrophosphorylase isoforms essential for starch synthesis in the leaf and seed endosperm of rice (Oryza sativa L.). Plant Mol Biol. 2007;65:531–546. doi: 10.1007/s11103-007-9153-z. [DOI] [PubMed] [Google Scholar]
- 17.Kang G, Liu G, Peng X, Wei L, Wang C, Zhu Y, Ma Y, Jiang Y, Guo T. Increasing the starch content and grain weight of common wheat by overexpression of the cytosolic AGPase large subunit gene. Plant Physiol Biochem. 2013;73:93–98. doi: 10.1016/j.plaphy.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Tetlow IJ, Morell MK, Emes MJ. Recent developments in understanding the regulation of starch metabolism in higher plants. J Exp Bot. 2004;55:2131–2145. doi: 10.1093/jxb/erh248. [DOI] [PubMed] [Google Scholar]
- 19.Crevillen P, Ventriglia T, Pinto F, Orea A, Merida A, Romero JM. Differential pattern of expression and sugar regulation of Arabidopsis thaliana ADP–glucose pyrophosphorylase–encoding genes. J Biol Chem. 2005;280:8143–8149. doi: 10.1074/jbc.M411713200. [DOI] [PubMed] [Google Scholar]
- 20.Lu FH, Park YJ. Sequence variations in OsAGPase significantly associated with amylose content and viscosity properties in rice (Oryza sativa L.). Genet Res. 2012;94:179–189. doi: 10.1017/S0016672312000390. [DOI] [PubMed] [Google Scholar]
- 21.Tang XJ, Peng C, Zhang J, Cai Y, You XM, Kong F, Yan HG, Wang GX, Wang L, Jin J, et al. ADP–glucose pyrophosphorylase large subunit 2 is essential for storage substance accumulation and subunit interactions in rice endosperm. Plant Sci. 2016;249:70–83. doi: 10.1016/j.plantsci.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 22.Zhou YX, Chen YX, Tao X, Cheng XJ, Wang HY. Isolation and characterization of cDNAs and genomic DNAs encoding ADP-glucose pyrophosphorylase large and small subunits from sweet potato. Mol Genet Genomics. 2016;291:609–620. doi: 10.1007/s00438-015-1134-3. [DOI] [PubMed] [Google Scholar]
- 23.Miao H, Sun P, Liu Q, Liu J, Xu B, Jin Z. The AGPase family proteins in banana: genome–wide identification, phylogeny, and expression analyses reveal their involvement in the development, ripening, and abiotic/biotic stress responses. Int J Mol Sci. 2017;18:1581. doi: 10.3390/ijms18081581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Harsselaar JK, Lorenz J, Senning M, Sonnewald U, Sonnewald S. Genome–wide analysis of starch metabolism genes in potato (Solanum tuberosum L.). BMC Genomics. 2017;18:37. doi: 10.1186/s12864-016-3381-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Batra R, Saripalli G, Mohan A, Gupta S, Gill K, Varadwaj P, Balyan HS, Gupta PK. Comparative analysis of AGPase genes and encoded proteins in eight monocots and three dicots with emphasis on wheat. Front Plant Sci. 2017;8:19. doi: 10.3389/fpls.2017.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qu J, Xu S, Zhang Z, Chen G, Zhong Y, Liu L, Zhang R, Xue J, Guo D. Evolutionary, structural and expression analysis of core genes involved in starch synthesis. Sci Rep. 2018;8:12736. doi: 10.1038/s41598-018-30411-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dong MY, Fan XW, Li YZ. Cassava AGPase genes and their encoded proteins are different from those of other plants. Planta. 2019;250:1621–1635. doi: 10.1007/s00425-019-03247-7. [DOI] [PubMed] [Google Scholar]
- 28.Rösti S, Denyer K. Two paralogous genes encoding small subunits of ADP–glucose pyrophosphorylase in maize, Bt2 and L2, replace the single alternatively spliced gene found in other cereal species. J Mol Evol. 2007;65:316–327. doi: 10.1007/s00239-007-9013-0. [DOI] [PubMed] [Google Scholar]
- 29.Wiberley–Bradford AE, Busse JS, Jiang J, Bethke PC. Sugar metabolism, chip color, invertase activity, and gene expression during long–term cold storage of potato (Solanum tuberosum) tubers from wild–type and vacuolar invertase silencing lines of Katahdin. BMC Res Notes. 2014;7:801. doi: 10.1186/1756-0500-7-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prathap V, Ali K, Singh A, Vishwakarma C, Krishnan V, Chinnusamy V, Tyagia A. Starch accumulation in rice grains subjected to drought during grain filling stage. Plant Physiol Biochem. 2019;142:440–451. doi: 10.1016/j.plaphy.2019.07.027. [DOI] [PubMed] [Google Scholar]
- 31.You YX, Zhang MY, Yang W, Li C, Liu YT, Li CM, He JL, Wu WJ. Starch phosphorylation and the in vivo regulation of starch metabolism and characteristics. Int J Biol Macromol. 2020;159:823–831. doi: 10.1016/j.ijbiomac.2020.05.156. [DOI] [PubMed] [Google Scholar]
- 32.Ritte G, Scharf A, Eckermann N, Haebel S, Steup M. Phosphorylation of transitory starch is increased during degradation. Plant Physiol. 2004;135:2068–2077. doi: 10.1104/pp.104.041301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orawetz T, Malinova I, Orzechowski S, Fettke J. Reduction of the plastidial phosphorylase in potato (Solanum tuberosum L.) reveals impact on storage starch structure during growth at low temperature. Plant Physiol Bioch. 2016;100:141–149. doi: 10.1016/j.plaphy.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 34.Smith AM, Zeeman SC. Starch: A Flexible, Adaptable Carbon Store Coupled to Plant Growth. Annu Rev Plant Biol. 2020;71:217–245. doi: 10.1146/annurev-arplant-050718-100241. [DOI] [PubMed] [Google Scholar]
- 35.Liu E, Yang CZ, Liu JD, Jin SR, Harijati N, Hu ZL, Diao Y, Zhao LL. Comparative analysis of complete chloroplast genome sequences of four major Amorphophallus species. Sci Rep. 2019;9:809. doi: 10.1038/s41598-018-37456-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. Mega6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kozera B, Rapacz M. Reference genes in real–time PCR. J Appl Genet. 2013;54(4):391–406. doi: 10.1007/s13353-013-0173-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real–time quantitative PCR and the 2–ΔΔCt method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 39.Xie BX, Chen X. Woody starch plants of China. 1st ed. Beijing (China): Science Press; 2008. p. 333–334. [Google Scholar]
- 40.Huang R. Experimental studies on the extraction and relieving constipation of Amorphallus glucomannan. Sichuan (China): Sichuan Normal University; 2010. p. 26–27. [Google Scholar]
- 41.Chen LG, Liu ZL, Zhuo RX. Synthesis and properties of degradable hydrogels of konjac glucomannan grafted acrylic acid for colon–specific drug delivery. Polymer. 2005;46:6274–6281. doi: 10.1016/j.polymer.2005.05.041. [DOI] [Google Scholar]
- 42.Nair SB, Jyothi AN. Cassava starch–konjac glucomannan biodegradable blend films: in vitro study as a matrix for controlled drug delivery. Starch–Starke. 2013;65:273–284. doi: 10.1002/star.201200070. [DOI] [Google Scholar]
- 43.Gille S, Cheng K, Skinner ME, Liepman AH, Wilkerson CG, Pauly M. Deep sequencing of voodoo lily (Amorphophallus konjac): an approach to identify relevant genes involved in the synthesis of the hemicellulose glucomannan. Planta. 2011;234:515–526. doi: 10.1007/s00425-011-1422-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Diao Y, Yang C, Yan M, Zheng X, Jin S, Wang Y, Hu Z. De novo transcriptome and small RNA analyses of two Amorphophallus species. PLoS One. 2014;9:e95428. doi: 10.1371/journal.pone.0095428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abt MR, Zeeman SC. Evolutionary innovations in starch metabolism. Curr Opin Plant Biol. 2020;55:109–117. doi: 10.1016/j.pbi.2020.03.001. [DOI] [PubMed] [Google Scholar]
- 46.Baroja–Fernández E, Muñoz FJ, Montero M, Etxeberria E, Sesma MT, Ovecka M, Bahaji A, Ezquer I, Li J, Prat S, et al. Enhancing sucrose synthase activity in transgenic potato (Solanum tuberosum L.) tubers results in increased levels of starch, ADPglucose and UDPglucose and total yield. Plant Cell Physiol. 2009;50:1651–1662. doi: 10.1093/pcp/pcp108. [DOI] [PubMed] [Google Scholar]
- 47.Burton RA, Johnson PE, Beckles DM, Fincher GB, Jenner HL, Naldrett MJ, Denyer K. Characterization of the genes encoding the cytosolic and plastidial forms of ADP–glucose pyrophosphorylase in wheat endosperm. Plant Physiol. 2002;130:1464–1475. doi: 10.1104/pp.010363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li DM, Yang ZM, Liu XC, Song Z, Feng ZY, He Y. Cloning and expression analysis of cDNAs encoding ADP–glucose pyrophosphorylase large and small subunits from hulless barley (Hordeum vulgare L. var. nudum). Z Naturforsch C. 2018;73:191–197. doi: 10.1515/znc-2017-0154. [DOI] [PubMed] [Google Scholar]
- 49.Xie B. Expression analysis of AGPase genes in source and organ of maize. Sichuan (China): Sichuan Agricultural University; 2010. p. 19. [Google Scholar]
- 50.Yin YG, Kobayashi Y, Sanuki A, Kondo S, Fukuda N, Ezura H, Sugaya S, Matsukura C. Salinity induces carbohydrate accumulation and sugar–regulated starch biosynthetic genes in tomato (Solanum lycopersicum L. cv. ‘MicroTom’) fruits in an ABA– and osmotic stress–independent manner. J Exp Bot. 2010;61:563–574. doi: 10.1093/jxb/erp333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang DM, Li L, Zhong FL. The application of indole–3–acetic acid in vegetables. Modern Agr Sci Tech. 2011;18:204–205,209. [Google Scholar]
- 52.Shan SM, Liu GJ, Li SH, Miao PF. Effects of IAA, GA3 and 6–BA applied in autumn on plant quality of strawberry. J Fruit Sci. 2007;24:545–548. [Google Scholar]
- 53.Liu F, Zhou YW. Effects of salicylic acid on bulb development and relation to endogenous hormone contents in two species of Lily. Plant Physiol Commun. 2009;45:1085–1088. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


