Abstract
Inhaled nitric oxide (iNO) may be continued during the transition from invasive to noninvasive respiratory support. Upper airway obstruction from laryngeal edema following extubation and lower airway obstruction from asthma and bronchiolitis may be managed with inhaled helium. The coadministration of helium with iNO and the impact on delivered amounts of iNO have not been extensively studied. A bench model simulating a spontaneously breathing infant received iNO at varying preset doses delivered with either helium-oxygen or nitrogen-oxygen via a Vapotherm unit. iNO levels were measured at the simulated trachea. Results from the two conditions were compared using t-tests. When nitrogen-oxygen was used, there was no difference between preset and measured iNO levels. A significant difference was present when helium-oxygen was used, with a 10-fold increase in measured iNO levels compared with preset values. The use of helium resulted in a significant increase in measured iNO at the level of the simulated trachea. Clinicians must be aware that iNO will not be delivered at prescribed doses when used with helium under the conditions used in this study.
Keywords: inhaled nitric oxide, high flow nasal cannula, high velocity nasal insufflation
Introduction
Inhaled nitric oxide (iNO) is a selective pulmonary vasodilator that is used in the management of pulmonary hypertension and acute hypoxic respiratory failure. 1 While primarily used during invasive mechanical ventilation, iNO has been increasingly delivered following liberation from invasive mechanical ventilation with noninvasive respiratory support devices, such as high-flow nasal cannula (HFNC) and high-velocity nasal insufflation (HiVNi). 2 3 4 5
Tracheal intubation can contribute to the development of subglottic edema that may result in upper airway obstruction following tracheal extubation. Postextubation stridor may occur in up to 30% of children, and upper airway obstruction following extubation is causative of one-third of extubation failures. 6 7 Lower airway obstruction may occur in conditions common to children, such as asthma and bronchiolitis. 8 9 10 Administration of helium-oxygen gas mixtures is one approach to the management of upper and lower airway obstruction. Helium-oxygen gas mixtures were successfully administered using HFNC and HiVNi. 9 10
Upper and lower airway obstruction may occur in children who require noninvasive administration of iNO, such that the coadministration of helium-oxygen gas mixtures might be considered. However, there are limited bench and clinical evaluations of the use of iNO and helium-oxygen gas mixtures in combination, and none evaluate the impact of helium-oxygen gas mixtures on the delivery of iNO. 11 12 13 Reliable delivery of iNO is critical due to the dangers of underdosing (suboptimal pulmonary vasodilation) or overdosing (hemodynamic deterioration, methemoglobinemia). We therefore evaluated the effect of using helium-oxygen gas mixtures versus nitrogen-oxygen gas mixtures on the delivery of iNO, hypothesizing that iNO in conjunction with a helium-oxygen gas mixture would result in the delivery of iNO in excess of preset levels.
Methods
A bench model was prepared to simulate a spontaneously breathing 10-kg infant patient. An infant head simulator with patent nares was interfaced with an adult and infant test lung equipped with PneuView 3.2 software (Michigan Instruments; Grand Rapids, Michigan, United States). A Servo-I ventilator (Getinge; Wayne, New Jersey, United States) was connected to the test lung to simulate spontaneous breathing, using a pressure-regulated volume control mode of ventilation with a tidal volume of 500 mL, a positive end-expiratory pressure of 5 cm water, and a frequency of 26 breaths per minute. Test lung compliance was set at 0.004 L/cm water. Resultant inhaled and exhaled tidal volume were 64 mL with achieved peak inspiratory pressures of 15.3 cm water. The infant head simulator is designed with a patent mouth, which simulates open-mouth breathing. We also simulated closed-mouth breathing by obstructing the simulated oropharynx.
Vapotherm HiVNi systems were used for this study (Vapotherm; New Hampshire, United States). Helium-oxygen gas mixtures were delivered using the Vapotherm Heliox Precision Flow with an 80%/20% helium/oxygen mixture at 6 liters per minute (lpm) flow and a temperature of 37°C. iNO was introduced into this system using a Vapotherm Nitric Oxide low-flow cartridge. Two INOmax DSir Plus units (Mallinckrodt; Hazelwood, Missouri, United States) were used in this study after completion of recommended preuse checks and low and high calibrations. INOmax DSir Plus unit 1 was used to deliver iNO and was integrated into the Vapotherm Heliox Precision Flow by attaching the injector module into the low-flow cartridge. INOmax DSir Plus unit 2 was used to measure delivered iNO by placing a sample line adaptor in the simulated trachea (between the infant head simulator and the test lung). INOmax DSir Plus unit 2 was not used to deliver iNO. The Vapotherm Heliox Precision Flow intermediate infant nasal cannula was applied directly to the infant head simulator nares. This cannula occluded an estimated 40% of the simulated nares.
The iNO dose was set on INOmax DSir Plus unit 1 and allowed to stabilize for 2 minutes before obtaining measured gas delivery values from INOmax DSir Plus unit 2. iNO doses tested ranged from 0.1 to 20 ppm. Following measurements at each iNO dose, iNO was discontinued and 100% oxygen was delivered through INOmax DSir Plus unit 1 for three minutes. Prior to initiating a new dose of iNO, measurements from INOmax DSir Plus unit 2 confirmed the absence of iNO. Measurements were performed using two conditions: “open mouth” (simulated mouth kept open) and “closed mouth” (simulated mouth obstructed).
A control analysis was performed duplicating this design with the exception that a nitrogen-oxygen gas mixture (80%/20%) instead of a helium-oxygen gas mixture was delivered through the Vapotherm unit. Only open mouth measurements were obtained, as this reflects usual clinical conditions.
Set iNO and measured iNO, as well as measured iNO from the different experimental conditions, were compared using paired t -tests. A p -value of ≤ 0.05 was considered statistically significant. Statistical analysis was performed by using Stata IC version 13.1 (Stata Corp; College Station, Texas, United States).
Results
Set iNO and measured iNO levels in the different experimental conditions are shown in Fig. 1 . Under control conditions, where nitrogen-oxygen gas mixtures were delivered in conjunction with iNO, measured iNO was not statistically different from the set iNO levels ( p = 0.54). When helium-oxygen gas mixtures were used in conjunction with iNO, the measured levels of iNO were significantly higher than the prescribed doses. Statistically significant differences between set iNO and measured iNO were seen for both the open mouth experimental condition ( p = 0.0002) and the closed mouth experimental condition ( p = 0.0001). Statistically significant differences were also demonstrated when comparing measured iNO between the control open mouth condition and the helium-oxygen open mouth condition ( p = 0.0002), as well as the control open mouth condition and the helium-oxygen closed mouth condition ( p = 0.0001). No statistically significant difference was present when comparing measured iNO between the helium-oxygen open mouth condition and the helium-oxygen closed mouth condition ( p = 0.1095).
Fig. 1.
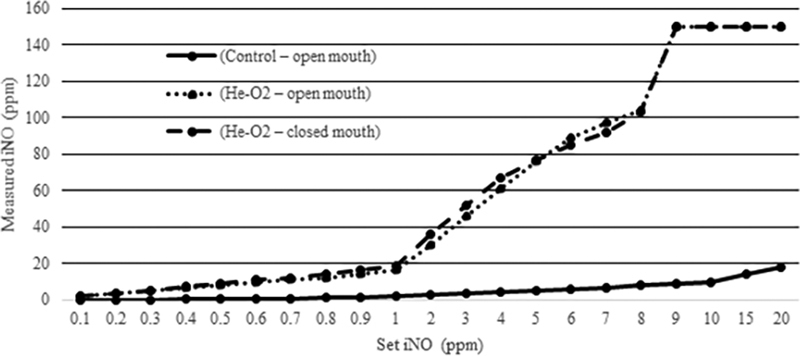
Inhaled nitric oxide measurements. PPM, parts per million.
Automated safety shutdown of iNO delivery due to high iNO readings, capping measured iNO at 150 ppm, occurred at iNO doses > 8 ppm in both the open mouth and closed mouth helium-oxygen conditions.
Discussion
iNO and helium-oxygen gas mixtures are both useful therapies in the management of critically ill neonates and children. While iNO has been used primarily during invasive ventilatory support for the management of pulmonary vascular resistance, there has been increasing application of iNO noninvasively. 3 DiBlasi et al 2 used a neonatal bench model to evaluate iNO delivery in conjunction with various noninvasive respiratory support devices and found that the accuracy of iNO delivery was compromised when HFNC was used as compared with nasal continuous positive airway pressure, sigh-positive airway pressure, and nasal intermittent mandatory ventilation. These inaccuracies in delivery, however, were more pronounced at lower gas flows (< 6 lpm). Despite these findings, noninvasive delivery of iNO showed to result in physiologic change. In adult patients with right ventricular dysfunction, Tremblay et al 4 demonstrated that delivery of iNO via low-flow nasal cannula and HFNC reduced measured pulmonary vascular resistance, mean pulmonary arterial pressure, and central venous pressure, while increasing cardiac index. Tominaga et al 5 evaluated children following Fontan procedure and found that those continued on iNO using HFNC following tracheal extubation had a shorter duration of tracheal intubation, pleural drainage, and postoperative hospitalization compared with those who did not receive noninvasive iNO.
As it is likely that iNO will continue to be used noninvasively, patients who might benefit from continued iNO treatment may concurrently experience disease processes leading to upper and lower airway obstruction. Helium-oxygen gas mixtures have been utilized successfully in the management of postextubation stridor, bronchiolitis, and status asthmaticus 8 and can be delivered successfully via HFNC. 9 10 Thus, it can be expected that there may be clinical situations where patients receiving iNO noninvasively may be considered for treatment with inhaled helium-oxygen mixtures concurrently.
There is minimal information available on the concurrent use of iNO and helium-oxygen mixtures. In a laboratory model using intubated dogs where pulmonary vasoconstriction was induced by hypoxia, Nie et al 11 administered iNO 5 ppm in both a helium-oxygen mixture and a nitrogen-oxygen mixture and measured standard pulmonary hemodynamic parameters using a pulmonary artery catheter. They found that pulmonary vasodilation was enhanced when the helium-oxygen gas mixture was used compared with the nitrogen-oxygen gas mixture and attributed this finding to facilitated diffusion of iNO in the presence of helium. Phatak et al 12 presented a case report of an infant with localized interstitial pulmonary emphysema and pulmonary hypertension. In an effort to decrease ventilator pressures by improved ventilation and potentially to enhance absorption of trapped interstitial gas, a helium-oxygen gas mixture was used. iNO also was applied at a set amount of 6 ppm because of hypoxemia and the presence of pulmonary hypertension. Following these interventions, mechanical ventilatory support was able to be decreased with improvement in oxygenation and ventilation, and a decrease in interstitial emphysema was noted. Petros et al 13 evaluated 9 children following cardiac surgery who were receiving iNO invasively for management of increased pulmonary vascular resistance. Using a crossover study design, iNO was delivered with either helium-oxygen or nitrogen-oxygen gas mixtures. When helium-oxygen and iNO combinations were used, there was an increase in oxygenation, minute volume, and tidal volume as compared with when nitrogen-oxygen and iNO combinations were used. Improvements were attributed to increased NO and fresh gas delivery to the lung.
While these three studies demonstrate physiological changes consistent with a decrease in pulmonary vascular resistance, the mechanism is unclear. Helium has a lower viscosity and density than nitrogen, which results in a decrease in turbulent gas flow and conversion to laminar gas flow, as well as reducing the inspiratory pressure required (by a patient or a ventilator) for a given gas flow. 14 This could result in improved delivery of iNO to lung units with pulmonary vasoconstriction. It must also be remembered that the lower density of helium will result in inaccurately low gas flow readings in devices with density-dependent flow transducers. 15 As a result, gases provided in conjunction with helium, such as iNO, whose delivery is controlled by a density-dependent flow transducer (as was the case in this study 16 ), may be delivered at concentrations in excess of what is prescribed. This might also provide an explanation for the increased physiological responses noted in the three previously discussed studies.
Our study is unique in that it allowed for the measurement of iNO at the level of the simulated trachea. When delivered in conjunction with nitrogen-oxygen, there was no statistically significant difference between prescribed and measured iNO levels. When delivered in conjunction with helium-oxygen, there was a statistically significant increase in measured iNO levels. This difference was present when both open and closed mouth conditions were simulated. Comparing measured iNO levels when iNO was delivered with nitrogen-oxygen versus helium-oxygen confirmed these statistically significant differences.
The unrecognized delivery of high iNO may result in exaggerated physiologic effects, methemoglobinemia, and toxicity from nitrogen dioxide. Clinicians who are interested in delivering iNO in combination with helium-oxygen mixtures must be aware that the delivered amount of iNO will greatly exceed that prescribed when the delivery systems use density-dependent flow transducers that are not calibrated for use with helium-oxygen.
Acknowledgments
This study was presented, in part, at the American Association for Respiratory Care International Congress, Las Vegas, NV, on December 2018.
Conflict of Interest K.B. is associated as a Patient Trainer, Hill-Rom. No conflicts of interest, financial or otherwise, exist for J. H. H., A. S., J. M. B., S. P., and K. M.
Note
This study was conducted at Nemours/Alfred I. duPont Hospital for Children, Wilmington, DE.
References
- 1.Dalton H J. Inhaled nitric oxide: medical miracle or passing fad? Respir Care. 1998;43:978–987. [Google Scholar]
- 2.DiBlasi R M, Dupras D, Kearney C, Costa E, Jr, Griebel J L. Nitric oxide delivery by neonatal noninvasive respiratory support devices. Respir Care. 2015;60(02):219–230. doi: 10.4187/respcare.03278. [DOI] [PubMed] [Google Scholar]
- 3.Baudin F, Gagnon S, Crulli B, Proulx F, Jouvet P, Emeriaud G. Modalities and complications associated with the use of high-flow nasal cannula: experience in a pediatric ICU. Respir Care. 2016;61(10):1305–1310. doi: 10.4187/respcare.04452. [DOI] [PubMed] [Google Scholar]
- 4.Tremblay J A, Couture E J, Albert M. Noninvasive administration of inhaled nitric oxide and its hemodynamic effects in patients with acute right ventricular dysfunction. J Cardiothorac Vasc Anesth. 2019;33(03):642–647. doi: 10.1053/j.jvca.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Tominaga Y, Iwai S, Yamauchi S. Post-extubation inhaled nitric oxide therapy via high-flow nasal cannula after Fontan procedure. Pediatr Cardiol. 2019;40(05):1064–1071. doi: 10.1007/s00246-019-02122-2. [DOI] [PubMed] [Google Scholar]
- 6.Nascimento M S, Prado C, Troster E J, Valério N, Alith M B, Almeida J F. Risk factors for post-extubation stridor in children: the role of orotracheal cannula. Einstein (Sao Paulo) 2015;13(02):226–231. doi: 10.1590/S1679-45082015AO3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khemani R G, Hotz J, Morzov R. Evaluating risk factors for pediatric post-extubation upper airway obstruction using a physiology-based tool. Am J Respir Crit Care Med. 2016;193(02):198–209. doi: 10.1164/rccm.201506-1064OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGarvey J M, Pollack C V.Heliox in airway management Emerg Med Clin North Am 20082604905–920., viii [DOI] [PubMed] [Google Scholar]
- 9.Morgan S E, Vukin K, Mosakowski S. Use of heliox delivered via high-flow nasal cannula to treat an infant with coronavirus-related respiratory infection and severe acute air-flow obstruction. Respir Care. 2014;59(11):e166–e170. doi: 10.4187/respcare.02728. [DOI] [PubMed] [Google Scholar]
- 10.Seliem W, Sultan A M. Heliox delivered by high flow nasal cannula improves oxygenation in infants with respiratory syncytial virus acute bronchiolitis. J Pediatr (Rio J) 2018;94(01):56–61. doi: 10.1016/j.jped.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Nie M, Kobayashi H, Sugawara M, Tomita T, Ohara K, Yoshimura H. Helium inhalation enhances vasodilator effect of inhaled nitric oxide on pulmonary vessels in hypoxic dogs. Am J Physiol Heart Circ Physiol. 2001;280(04):H1875–H1881. doi: 10.1152/ajpheart.2001.280.4.H1875. [DOI] [PubMed] [Google Scholar]
- 12.Phatak R S, Pairaudeau C F, Smith C J, Pairaudeau P W, Klonin H. Heliox with inhaled nitric oxide: a novel strategy for severe localized interstitial pulmonary emphysema in preterm neonatal ventilation. Respir Care. 2008;53(12):1731–1738. [PubMed] [Google Scholar]
- 13.Petros A J, Tulloh R M, Wheatley E. Heli-NO: enhanced gas exchange with nitric oxide in helium. Anesth Analg. 1996;83(04):888–889. doi: 10.1097/00000539-199610000-00052. [DOI] [PubMed] [Google Scholar]
- 14.Gentile M A.Inhaled medical gases: more to breathe than oxygen Respir Care 201156091341–1357., discussion 1357–1359 [DOI] [PubMed] [Google Scholar]
- 15.Venkataraman S T. Heliox during mechanical ventilation. Respir Care. 2006;51(06):632–639. [PubMed] [Google Scholar]
- 16.Mallinckrodt Pharmaceuticals INOmax DSir ® Plus © Operation Manual. Series 3. Available at: https://ikariaaust.com/pdfs/INOmax%20DSIR%20Plus%20CE%20Operational%20Manual%20English.pdf. Accessed April 6, 2020


