Abstract
Although the exact pathophysiology of critical illness polyneuropathy (CIP) is still unknown, there are several hypotheses, some of which are increased inflammation and oxidative stress. We used rodent sepsis model in which we induced sepsis through cecal ligation followed by cecal puncture. We then administered ascorbic acid (AA) and evaluated outcomes. The levels of malondialdehyde (MDA), tumor necrosis factor α (TNF-α), interleukins (IL)-6 in the plasma, and heat shock protein-70 (HSP-70) levels in the sciatic nerve were measured, and also electromyography analyses were performed. While plasma MDA, TNF-α, and IL-6 levels were decreased significantly with AA treatment, sciatic nerve levels of HSP-70 were significantly elevated in the AA group. A significant increase in compound muscle action potential (CMAP) amplitude and a significant decrease in CMAP latency were detected in the AA group. We observed healing effects of AA on a rat model of CIP and these effects seem to be related to its anti-inflammatory and antioxidant properties.
Keywords: critical illness polyneuropathy, sepsis, ascorbic acid, malondialdehyde, compound muscle action potential
Introduction
Critical illness polyneuropathy (CIP) presents and progresses with generalized acute and primary sensory motor axonal degeneration. It is particularly seen in patients admitted to the intensive care unit who have a diagnosis of sepsis or systemic inflammatory response syndrome complicated with multiple organ dysfunction syndrome. The major symptoms of CIP are sensory defects and weakness of limbs and respiratory muscles causing difficulty in weaning from a ventilator. 1 Electrophysiological analyses of peripheral nerves and muscles are used to diagnose CIP. Reduction in the amplitudes of compound muscle action potential (CMAP) is the characteristic finding of CIP. 2
Although the exact pathophysiology of CIP is still unknown, there are several hypotheses. Latronica et al 1 claimed that sepsis-associated microcirculation disorder plays an important role in the pathogenesis of CIP. It has been established that humoral and cellular responses against sepsis significantly alter microcirculation. Cellular response includes macrophages, neutrophils, and endothelial cells which secrete proinflammatory mediators such as arachidonic acid, tumor necrosis factor α (TNF-α), reactive oxygen species (ROS), proteases, and interleukins (ILs) 1, 2, and 6. Endothelial damage (that occurs through humoral and cellular responses in association with adhesion molecules like E-selectin) increases capillary permeability; thus, leading to endoneurial edema with hypoxemia and energy depletion. 3 4
Heat shock proteins (HSPs) are a group of proteins whose production is increased by exposure of cells to high temperature (42–46°C). 5 The dramatic increase of these proteins is mostly regulated by the heat shock factor and is called the heat shock response. 5 6 Previous studies have shown that HSPs are the components of the stress response in the event of severe stress, such as sepsis. 6 7 However, in other studies, ascorbic acid (AA) has been reported to show antioxidant properties by increasing HSPs such as HSP-70, HSP-60, and HSP-90 upon damage caused by sepsis in various tissues such as liver and heart. 8
It is well known that increased ROS production causes damage to tissues and can be associated with sepsis development and progression. Previous studies have hypothesized that antioxidant supplementation with various compounds, such as AA, vitamins A and E, N-acetyl-cysteine, selenium, and melatonin, may counteract the effects of ROS; and thus, could limit the development or progression of multiple organ failure and CIP. 3 Passage et al 9 have shown that the use of AA in mouse models improved muscle function and decreased demyelination. We aimed to demonstrate the healing effects of AA on experimentally induced CIP in rats. For this purpose, electrophysiological analyses and measurements of malondialdehyde (MDA), TNF-α, and IL-6 in plasma were performed as well as the determination of HSP-70 levels in sciatic nerve tissue.
Materials and Methods
Animals
In this study, 52 male Sprague-Dawley albino mature rats at 12 weeks, weighing 200 to 220 g, were used. The experiments performed in this study were performed according to the requirements of The Guide for the Care and Use of Laboratory Animals by the National Institutes of Health (U.S.). We also received approval from the Animal Ethics Committee (Decision number: 2013–033b). The rats used in the experiment were obtained from the Experimental Animal laboratory of Demiroglu Bilim University. Rats were fed ad libitum and housed in pairs in steel cages that were kept in a temperature- and humidity-controlled environment (22 ± 2°C) with 12-hour light/dark cycles.
Experimental Procedures
Rats were randomly assigned into five groups: study groups were designed as follows: Group 1: normal (nonoperative and orally fed control, n = 10); Group 2: sham-operated ( n = 10); Group 3: cecal ligation and puncture (CLP) and 1 mL/kg/day % 0.9 NaCl saline group ( n = 10); Group 4: CLP and 500 mg/kg/day AA intraperitoneally ( n = 10); Group 5: CLP and 1,000 mg/kg/day AA intraperitoneally ( n = 10). The sepsis model was induced via the CLP procedure in three of these groups (30 rats). For the surgical procedure, rats were anesthetized by intraperitoneal injection of a combination of 100 mg/kg ketamine hydrochloride and 10 mg/kg xylazine hydrochloride (final concentrations). Soon after the procedure, intraperitoneal saline was administered at 30 mL/kg in sham and CLP rats ( N = 40). Two rats that underwent CLP died during the first 96 hours following the surgical procedure and were excluded from the study. There were no mortalities in the sham-operated group. All treatments were administered for 5 days in all groups after the CLP procedures. On the 6th day, electromyography (EMG) was performed and blood samples were obtained in ethylenediaminetetraacetic acid containing tubes. The blood samples were centrifuged at 3,000 revolutions per minute (rpm) for 10 minutes and the resulting plasma was stored at –20°C until assays were performed. The study was finally terminated by sacrificing animals via cervical dislocation under high-dose anesthesia (ketamine hydrochloride 100 mg/kg and 10 mg/kg xylazine hydrochloride) and sciatic nerves were obtained.
The procedures were performed as follows: under aseptic conditions, a 3-cm midline laparotomy was performed to allow exposure of the cecum with adjoining intestine. The cecum was ligated tightly with 3–0 silk suture at its base under the ileocecal valve and it was punctured once with a 22-gauge needle. The cecum was then gently squeezed to extrude a small amount of feces from the perforation site. The cecum was returned to the peritoneal cavity, and the laparotomy incision was closed with 4–0 polyglactin 910 sutures. Following surgery, a recovery period was allowed and then they were placed in their cages. Rats that underwent CLP were accepted to be septic at postoperative 5 hours. In the sham group, under aseptic conditions, only laparotomy was performed on rats without ligation or puncturing of the cecum ( Fig. 1 ).
Fig. 1.
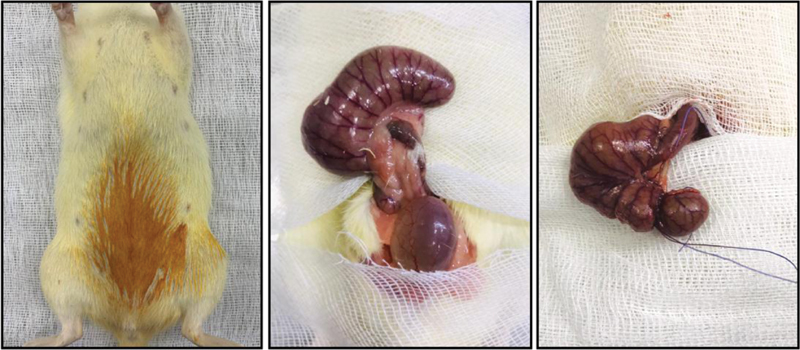
Cecal ligation and puncture (CLP) procedure: midline laparotomy, exposure of the cecum, and ligated tightly with a 3.0 silk suture.
Measurement of Plasma TNF- α and IL-6 Levels
Plasma TNF-α and IL-6 levels were measured using commercially available enzyme-linked immunosorbent assay (ELISA) kits (BD-Biosciences). The plasma samples were diluted 2× and TNF-α and IL-6 levels were determined in duplicate according to the manufacturer's guides.
Measurement of Lipid Peroxidation
Lipid peroxidation was determined in plasma samples by measuring MDA levels as thiobarbituric acid reactive substances (TBARS). Briefly, trichloroacetic acid and TBARS were added to the plasma samples, vortexed, and incubated at 100°C for 60 minutes. After cooling on ice, the samples were centrifuged at 3,000 rpm for 20 minutes and the absorbance of the supernatant was read at 535 nm. MDA levels were expressed as nanometer, and tetraethoxypropane was used for calibration.
Sciatic Nerve Biochemical Analysis
After decapitation, sciatic nerves were rapidly removed and stored at −20°C until biochemical analyses were performed. For tissue analysis, whole nerve tissues were homogenized with a glass homogenizer in 5 volume of phosphate-buffered saline (PBS) that was five times the volume of the obtained tissue (pH 7.4) and centrifuged at 5,000 × g for 15 minutes. The supernatant was collected and total protein concentration in nerve homogenates was determined according to Bradford's method using bovine serum albumin as standard. 10
The sciatic nerve levels of HSP-70 in the tissue supernatants were measured using commercially available rat ELISA kits. All samples obtained from animals were measured in duplicate according to the manufacturer's guidelines.
Electrophysiological Recordings
All EMG studies were performed on the 6th day after surgery. EMG was obtained three times from the right sciatic nerve that was stimulated supramaximally (intensity 10 V, duration 0.05 milliseconds, frequency 1 Hz, in the range of 0.5–5,000 Hz, 40 kHz/s with a sampling rate) by a Biopac bipolar subcutaneous needle stimulation electrode (BIOPAC Systems, Inc; Santa Barbara, California, United States) from the sciatic notch. CMAPs were recorded from 2 to 3 interosseous muscles by unipolar platinum electrodes. Data were evaluated using Biopac Student Laboratory Pro version 3.6.7 software (BIOPAC Systems, Inc), with distal latency and amplitude of CMAP as the parameters. During EMG recordings, the rectal temperatures of rats were monitored by a rectal probe (HP Viridia 24-C; Hewlett-Packard Company, Palo Alto, California, United States) and the temperature of each rat was kept at approximately 36 to 37°C by using a heating pad. Following EMG recordings, animals were sacrificed and blood samples were collected by cardiac puncture for biochemical measurements.
Statistical Analysis
Statistical evaluation was performed using SPSS version 15.0 for Windows. The Shapiro–Wilk test was used to determine whether quantitative variables conformed to normal distribution. Parametric variables were compared with regard to groups using the Student's t -test and analysis of variance, while nonparametric variables were compared using the Mann–Whitney U test. Results are presented as mean + standard error of mean. The determination of p < 0.05 was accepted to demonstrate statistical significance.
Results
Evaluation of Plasma MDA Levels
Plasma MDA levels were measured as a marker of lipid peroxidation. 11 Plasma MDA level in the saline-treated sepsis group was significantly higher than that of controls and also the AA 500 mg/kg ( p < 0.05) and 1,000 mg/kg AA ( p < 0.0001) groups. We found that, as the AA dose increased, plasma MDA levels decreased significantly ( Table 1 ).
Table 1. Comparison of plasma MDA, TNF-α, IL-6, and sciatic nerve HSP-70 levels of the groups.
| Normal control | Sham operated | CLP and saline | CLP and 500 mg/kg ascorbic acid | CLP and 1,000 mg/kg ascorbic acid | |
|---|---|---|---|---|---|
| MDA (nM) | 88.4 ± 16.3 | 89.6 ± 13.08 | 295.3 ± 11.6 a | 165.4 ± 18.1 c | 105.8 ± 11.5 d |
| TNF-α (pg/mL) | 22.9 ± 3.08 | 25.6 ± 4.9 | 341.9 ± 19.2 b | 211.9 ± 15.9 d | 65.2 ± 4.4 d |
| IL-6 (pg/mL) | 24.3 ± 1.4 | 32.5 ± 7.2 | 85.3 ± 12.4 b | 54.9 ± 7.08 c | 45.3 ± 10.1 d |
| Sciatic nerve HSP-70 (mcg/mg protein) | 8.5 ± 1.1 | 9.2 ± 2.5 | 14.2 ± 4.8 | 21.6 ± 7.2 c | 28.5 ± 8.3 d |
Abbreviations: ANOVA, analysis of variance; CLP, cecal ligation and puncture; HSP-70, heat shock protein-70; IL, interleukin; MDA, malondialdehyde; SEM, standard error of mean; TNF-α, tumor necrosis factor α.
Note: Results were presented as mean ± SEM. Statistical analyses were performed by one-way ANOVA.
p < 0.001.
p < 0.0001 different from normal and sham-operated groups.
p < 0.05.
p < 0.0001 different from CLP and saline group.
Measurement of Sciatic Nerve Levels of HSP-70
The HSP-70 levels of rats in both groups receiving AA at 500 and 1,000 mg/kg were significantly higher than that of the saline-treated sepsis group ( p < 0.05 and p < 0.0001, respectively). The increase in HSP-70 levels was more pronounced in the group receiving 1,000 mg/kg AA compared with the group receiving a dose of 500 mg/kg ( p < 0.05) ( Table 1 ).
Evaluation of Plasma TNF-α Levels
Plasma TNF-α levels in the saline-treated sepsis group was significantly higher than that of both the normal control group and the sham-operated group ( p < 0.0001 for both). Plasma TNF-α levels of the sepsis group treated with 500 and 1000 mg/kg AA were significantly lower than that of the saline-treated sepsis group ( p < 0.0001). As the AA dose increased, plasma TNF-α levels decreased significantly ( Table 1 ).
Evaluation of Plasma IL-6 Levels
Plasma IL-6 levels of the saline-treated sepsis group were significantly higher than that of both normal controls and the sham-operated group ( p < 0.0001). Plasma IL-6 levels in the sepsis groups treated with 500 mg/kg AA ( p < 0.05) and 1,000 mg/kg AA ( p < 0.0001) were significantly lower than IL-6 levels in the saline-treated sepsis group. As the AA dose increased, plasma IL-6 levels decreased significantly ( Table 1 ).
Evaluation of the Amplitudes of CMAP
The CMAP amplitude of the saline-treated sepsis group was significantly lower than that of both the normal control and sham-operated groups ( p < 0.00001). CMAP amplitudes of sepsis groups treated with 500 and 1000 mg/kg AA were significantly higher compared with the saline-treated sepsis group ( p < 0.00001). As the AA dose increased, CMAP amplitudes also increased significantly ( Table 2 ; Fig. 2 ).
Table 2. Comparison of CMAP latency and amplitude values of the groups.
| Normal control | Sham operated | CLP and saline | CLP and 500 mg/kg ascorbic acid | CLP and 1,000 mg/kg ascorbic acid | |
|---|---|---|---|---|---|
| CMAP latency (ms) | 2.41 ± 0.05 | 2.48 ± 0.11 | 3.25 ± 0.07 a | 2.8 ± 0.13 b | 2.65 ± 0.2 c |
| CMAP amplitude (mV) | 13.9 ± 0.46 | 13.5 ± 0.68 | 4.8 ± 0.5 a | 8.56 ± 0.6 c | 12.05 ± 0.91 c |
Abbreviations: ANOVA, analysis of variance; CLP, cecal ligation and puncture; CMAP, compound muscle action potential; SEM, standard error of mean.
Note: Results were presented as mean ± SEM. Statistical analyses were performed by one-way ANOVA and post hoc Bonferroni test.
p < 0.00001, different from normal and sham-operated groups.
p < 0.01.
p < 0.00001 different from CLP group.
Fig. 2.
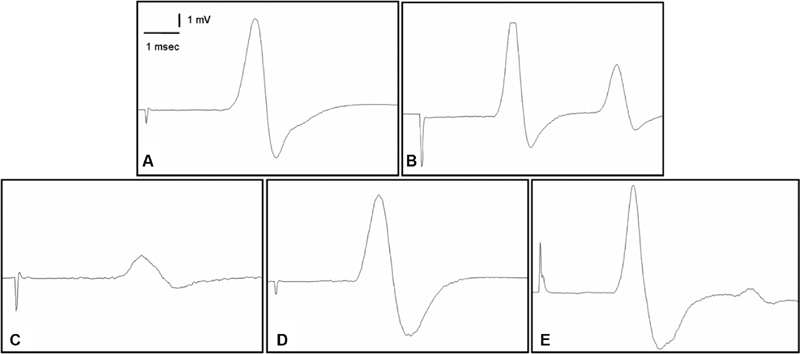
Samples of compound muscle action potential (CMAP) recorded from ( A ) normal group, ( B ) sham group, ( C ) cecal ligation and puncture (CLP) and saline group, ( D ) CLP and 500 mg/kg ascorbic acid, ( E ) CLP and 1,000 mg/kg ascorbic acid.
Evaluation of Latency Periods for CMAP
The CMAP latency of the saline-treated sepsis group was significantly higher than that of both normal controls and the sham-operated group ( p < 0.00001). Latency periods for CMAP of the sepsis groups treated with 500 mg/kg AA ( p < 0.01) and 1,000 mg/kg AA ( p < 0.00001) were significantly lower compared with the saline-treated sepsis group. As the AA dose increased, CMAP latencies decreased significantly ( Table 2 ; Fig. 2 ).
Discussion
The results of this study demonstrated that AA has ameliorative effects on sepsis-induced neuropathy in rat model. We associated these effects with the suppression of lipid peroxidation due to the antioxidant and anti-inflammatory properties of AA. To examine the role of oxidative stress in CIP and the antioxidant effect of AA, we measured plasma levels of MDA, an oxidative stress marker that results from lipid peroxidation of polyunsaturated fatty acids. 12 According to our results in this study, while the plasma MDA level was found to be significantly higher in the CIP model, its level decreased significantly with AA treatment. There are no studies in the literature investigating the effects of AA on sepsis-induced neuropathy. However, there are some studies investigating the effects of AA on different experimental peripheral nerve injury models in different studies. Apostolopoulou et al 13 showed that AA induces neuroprotection by suppressing superoxide and reactive oxygen radicals on sciatic nerve damage caused by ischemia-reperfusion injury in rats. Riffel et al 14 reported that AA given at 30 mg/kg dose in chronic constriction injury model in rats showed antioxidant properties by decreasing reactive oxygen radicals on the sciatic nerve. Li et al 15 claimed that AA can promote the morphological and functional recovery of injured peripheral nerves and this effect is potentially due to AA's bioeffects on neurons, Schwann cells, and macrophages, three of most important types of cells involved in nerve injury and regeneration, based on their study with rats in the sciatic nerve crush injury model. In accordance with these data, in our study, the decrease in MDA levels in the AA group suggested that AA showed antioxidant properties by suppressing lipid peroxidation on CIP.
Moreover, the fact that HSP-70 levels were significantly higher in the group receiving AA revealed that this agent increased the HSPs and showed neuroprotective properties in the CIP model. Although, to the best of our knowledge, there are no studies showing the neuroprotective properties of AA on the central nervous system by increasing HSPs, there are many studies reporting that this agent shows protective effects by increasing HSP levels in other organs such as liver in case of cellular stress. For example, Yun et al 16 suggested that AA shows antioxidant character by increasing HSP-70 levels in case of cyclic heat stress. Similarly, Yin et al 17 reported the protective effects of this agent in H9C2 cells through HSPs (HSP-70, HSP-60, HSP-27) in case of heat damage. In our study, we think it is important to show the possible neuroprotective effects of AA over HSP in the CIP model for the first time in the literature.
In our results, while both plasma TNF-α and IL-6 levels were found to be significantly higher in the CIP model, their levels decreased significantly with AA treatment. Furthermore, increased AA dosage was associated with even lower levels of plasma TNF-α and IL-6 levels. Similar to our results, in a study by Zhou et al, 18 the TNF-α and IL-6 levels that were elevated in type 2 diabetic neuropathy models, were found to be significantly reduced with the use of berberine (a plant alkaloid that has antioxidant properties). Son et al 19 reported that vitamin C blocked TNF-α-induced nuclear factor kappa B activation and intercellular adhesion molecule 1 expression in neuroblastoma cell line. When our findings and mentioned literature data are evaluated together, AA might have anti-inflammatory properties that are prominent in the presence of increased inflammatory activity such as that caused by sepsis. 20
As expected, a significant decrease in CMAP amplitude and a significant increase in CMAP latency were detected in models of CIP. With AA treatment, a significant increase in CMAP amplitude and a significant decrease in CMAP latency were detected, indicating that this treatment was effective in countering the effects of CIP. As the dose of AA increased, these significant changes continued. We evaluated these electrophysiological findings as clinical results of the neuroprotective effect of AA. 21
In conclusion, in this study, the use of AA exhibited anti-inflammatory and antioxidant properties and demonstrated ameliorative effects in a rat model of CIP. On the other hand, it was the most important limitation of our study that we could not determine other mechanisms except from we detected which cause anti-inflammatory and antioxidant properties of AA. If further clinical trials also provide similar results and the causal relationship can be elucidated, adding AA to the treatment of patients with CIP may be possible. Additionally, the potential use of AA for sepsis-induced neuropathies would direct possible future researches.
Practical Applications
CIP is particularly seen in patients who have a diagnosis of sepsis or systemic inflammatory response syndrome (SIRS), increased inflammation, and oxidative stress causes peripheral nerve damage. The results of this study demonstrate that AA has ameliorative effects on sepsis-induced neuropathy rat model. We associate these effects with the antioxidant and anti-inflammatory properties of AA via suppression of lipid peroxidation and inflammation, also via heat shock protein 70 (HSP-70) levels elevation. If further clinical trials also provide similar results and the causal relationship can be elucidated, adding AA to the treatment of patients with CIP may be possible; additionally, the potential use of AA for sepsis-induced neuropathies would direct possible future researches.
Funding Statement
Funding None.
Footnotes
Conflict of Interest None declared.
References
- 1.Latronico N, Bolton C F. Critical illness polyneuropathy and myopathy: a major cause of muscle weakness and paralysis. Lancet Neurol. 2011;10(10):931–941. doi: 10.1016/S1474-4422(11)70178-8. [DOI] [PubMed] [Google Scholar]
- 2.Erbaş O, Yeniel A O, Akdemir A. The beneficial effects of levetiracetam on polyneuropathy in the early stage of sepsis in rats: electrophysiological and biochemical evidence. J Invest Surg. 2013;26(06):312–318. doi: 10.3109/08941939.2013.797056. [DOI] [PubMed] [Google Scholar]
- 3.Erbaş O, Ergenoglu A M, Akdemir A, Yeniel A O, Taskiran D. Comparison of melatonin and oxytocin in the prevention of critical illness polyneuropathy in rats with experimentally induced sepsis. J Surg Res. 2013;183(01):313–320. doi: 10.1016/j.jss.2012.11.043. [DOI] [PubMed] [Google Scholar]
- 4.Solmaz V, Aksoy D, Yılmaz M, Eser E, Erbas O. Demonstration of ameliorative effect of lacosamide: in a rat model of sepsis-induced critical illness polyneuropathy. Neurol Res. 2015;37(09):797–802. doi: 10.1179/1743132815Y.0000000040. [DOI] [PubMed] [Google Scholar]
- 5.Sarge K D, Murphy S P, Morimoto R I. Activation of heat shock gene transcription by heat shock factor 1 involves oligomerization, acquisition of DNA-binding activity, and nuclear localization and can occur in the absence of stress. Mol Cell Biol. 1993;13(03):1392–1407. doi: 10.1128/mcb.13.3.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aschkenasy G, Bromberg Z, Raj N, Deutschman C S, Weiss Y G. Enhanced Hsp70 expression protects against acute lung injury by modulating apoptotic pathways. PLoS One. 2011;6(11):e26956. doi: 10.1371/journal.pone.0026956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lyons M M, Raj N N, Chittams J L, Kilpatrick L, Deutschman C S. TAT-HSP70 attenuates experimental lung injury. Shock. 2015;43(06):582–588. doi: 10.1097/SHK.0000000000000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Albokhadaim I F, Althnaian T A, El-Bahr S M. Gene expression of heat shock proteins/factors (HSP60, HSP70, HSP90, HSF-1, HSF-3) and antioxidant enzyme activities in heat stressed broilers treated with vitamin C. Pol J Vet Sci. 2019;22(03):565–572. doi: 10.24425/pjvs.2019.129965. [DOI] [PubMed] [Google Scholar]
- 9.Passage E, Norreel J C, Noack-Fraissignes P. Ascorbic acid treatment corrects the phenotype of a mouse model of Charcot-Marie-Tooth disease. Nat Med. 2004;10(04):396–401. doi: 10.1038/nm1023. [DOI] [PubMed] [Google Scholar]
- 10.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 11.Tsikas D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: analytical and biological challenges. Anal Biochem. 2017;524:13–30. doi: 10.1016/j.ab.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 12.Davey M W, Stals E, Panis B, Keulemans J, Swennen R L. High-throughput determination of malondialdehyde in plant tissues. Anal Biochem. 2005;347(02):201–207. doi: 10.1016/j.ab.2005.09.041. [DOI] [PubMed] [Google Scholar]
- 13.Apostolopoulou K, Konstantinou D, Alataki R. Ischemia-reperfusion injury of sciatic nerve in rats: protective role of combination of vitamin C with E and tissue plasminogen activator. Neurochem Res. 2018;43(03):650–658. doi: 10.1007/s11064-017-2465-8. [DOI] [PubMed] [Google Scholar]
- 14.Riffel A PK, Santos M CQ, de Souza J A. Treatment with ascorbic acid and α-tocopherol modulates oxidative-stress markers in the spinal cord of rats with neuropathic pain. Braz J Med Biol Res. 2018;51(04):e7097. doi: 10.1590/1414-431X20177097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li L, Li Y, Fan Z. Ascorbic acid facilitates neural regeneration after sciatic nerve crush injury. Front Cell Neurosci. 2019;13:108. doi: 10.3389/fncel.2019.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yun S H, Moon Y S, Sohn S H, Jang I S. Effects of cyclic heat stress or vitamin C supplementation during cyclic heat stress on HSP70, inflammatory cytokines, and the antioxidant defense system in Sprague Dawley rats. Exp Anim. 2012;61(05):543–553. doi: 10.1538/expanim.61.543. [DOI] [PubMed] [Google Scholar]
- 17.Yin B, Tang S, Sun J. Vitamin C and sodium bicarbonate enhance the antioxidant ability of H9C2 cells and induce HSPs to relieve heat stress. Cell Stress Chaperones. 2018;23(04):735–748. doi: 10.1007/s12192-018-0885-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou G, Yan M, Guo G, Tong N. Ameliorative effect of berberine on neonatally induced type 2 diabetic neuropathy via modulation of BDNF, IGF-1, PPAR-γ, and AMPK expressions. Dose Response. 2019;17(03):1.559325819862449E15. doi: 10.1177/1559325819862449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Son E W, Mo S J, Rhee D K, Pyo S. Vitamin C blocks TNF-alpha-induced NF-kappaB activation and ICAM-1 expression in human neuroblastoma cells. Arch Pharm Res. 2004;27(10):1073–1079. [PubMed] [Google Scholar]
- 20.Kawade N, Tokuda Y, Tsujino S. Dietary intake of ascorbic acid attenuates lipopolysaccharide-induced sepsis and septic inflammation in ODS rats. J Nutr Sci Vitaminol (Tokyo) 2018;64(06):404–411. doi: 10.3177/jnsv.64.404. [DOI] [PubMed] [Google Scholar]
- 21.Gess B, Baets J, De Jonghe P, Reilly M M, Pareyson D, Young P. Ascorbic acid for the treatment of Charcot-Marie-Tooth disease. Cochrane Database Syst Rev. 2015;(12):CD011952. doi: 10.1002/14651858.CD011952. [DOI] [PMC free article] [PubMed] [Google Scholar]


