Abstract
We conducted a randomized controlled pilot study in infants with critical bronchiolitis ( n = 63) comparing high-flow nasal cannula (HFNC, n = 35) to continuous positive airway pressure (CPAP, n = 28). The primary outcome was treatment failure, defined as the need for bilevel positive pressure ventilation or endotracheal intubation. Treatment failure occurred in 10 patients (35.7%) in the CPAP group and 13 patients (37.1%) in the HFNC group ( p = 0.88). Pediatric intensive care unit length of stay was similar between the CPAP and HFNC groups (5 [4–7] days and 5 [4–8] days, p = 0.46, respectively). In this pilot study, treatment with HFNC resulted in a rate of treatment failure similar to CPAP.
Keywords: bronchiolitis, high-flow nasal cannula, continuous positive airway pressure, respiratory syncytial virus, acute respiratory failure, infants
Introduction
Bronchiolitis is an acute infection of the lower respiratory tract and the leading cause of hospitalization among infants. 1 2 3 It is most commonly caused by the respiratory syncytial virus (RSV) and exhibits a strong seasonal pattern, with the vast majority of cases occurring during the winter months. 1 3 4 5 6 7 Bronchiolitis is a cause of substantial morbidity and health care system burden: nearly ⅔ of children will be infected by the RSV in the first year of life, and virtually all children will be infected at least once before age 2 years. 8 Although most children with bronchiolitis in the United States are managed in the outpatient setting, between 2 and 3% of all infants under 12 months of age are hospitalized, 2 and of these, nearly ¼ require care in a pediatric intensive care unit (PICU). 9 This later subset of patients is termed to have “critical bronchiolitis.” 10 11
The treatment of bronchiolitis is largely supportive, with evidence-based guidelines focusing on provision of fluids, comfort measures, and oxygen supplementation to correct hypoxemia. 12 Patients with critical bronchiolitis and acute respiratory failure, however, may require more advanced treatments, such as instrumentation with arterial or central venous catheters, inotropic and vasoactive infusions, noninvasive respiratory support, and mechanical ventilation. 9 In patients with more severe disease, the application of continuous positive airway pressure (CPAP) has been associated with rapid unloading of the respiratory muscles and decreased work of breathing. 13 14 15 Pre-emptive application of CPAP in these cases has also been associated with improved clinical outcomes and decreased cost, 16 making it the standard support modality for patients with moderate-to-severe bronchiolitis. 17
Over the last decade, the administration of heated humidified air-oxygen mixtures at high flow, commonly known as high-flow nasal cannula (HFNC), has gained widespread popularity in the treatment of patients with critical bronchiolitis for its simplicity, ease of use, and high tolerability. 12 18 The use of HFNC support has been associated with a reduced need for mechanical ventilation in retrospective studies using historical controls. 19 20 A report of 9,628 admissions for critical bronchiolitis spanning 13 years from Australia and New Zealand showed that the rise in use of HFNC was associated with a concurrent decrease in use of mechanical ventilation. 21 In a recent prospective randomized controlled trial of HFNC versus standard low flow oxygen in children with bronchiolitis of moderate severity, HFNC support resulted in significantly lower rates of treatment failure. 22 In addition, the use of HFNC as the initial support modality was associated with significantly lower need for mechanical ventilation in a database study of 6,496 children with critical bronchiolitis, compared with CPAP. 23 However, a multicenter prospective randomized controlled noninferiority trial comparing HFNC with CPAP in children with moderate-to-severe bronchiolitis showed a higher risk of failure for patients assigned to the HFNC arm, despite no differences in important clinical outcomes, such as need for invasive mechanical ventilation and PICU length of stay. 17
The global burden of acute lower respiratory infections due to RSV and its resultant morbidity and mortality disproportionately affect young children in developing countries. 24 Therefore, we conducted this randomized controlled pilot study of HFNC versus CPAP in infants with critical bronchiolitis to assess the feasibility of a larger international trial in a developing country, and to measure effect size for sample size calculations to properly power such a trial. We hypothesized that HFNC would be equivalent to CPAP for clinically meaningful outcomes, namely the need for escalation of therapy to bilevel noninvasive ventilation or endotracheal intubation with mechanical ventilation.
Methods
Study Design and Setting
We conducted this open-label, single-center, randomized controlled pilot trial in the PICU of the Hospital Infantil Sabará, a tertiary referral children's hospital located in São Paulo, Brazil. This 36-bed high acuity PICU is continuously staffed by pediatric intensivists, specialized pediatric nursing and respiratory therapists, and a multidisciplinary support team. The PICU receives patients from the institution's own emergency department, as well as referrals from other regions of Brazil requiring advanced critical care. This PICU is capable of delivering the entire spectrum of pediatric critical care, including advanced mechanical ventilation, high-frequency oscillatory ventilation, inhaled nitric oxide, renal replacement therapy, and extracorporeal life support.
This study was approved by the institution's Ethics and Research Committee, and was conducted in accordance with resolution 196/96 of the National Health Council, the agency with regulatory oversight of human research in Brazil.
Subjects and Randomization
Children up to 9 months of age admitted to the PICU between September 1, 2016 and July 31, 2017 with a primary diagnosis of critical bronchiolitis of moderate severity or greater (a modified Wood–Downes score 25 of at least 4) and preserved respiratory drive were eligible for inclusion. Patients were excluded if they had one of the following conditions: congenital or acquired heart disease, neuromuscular disease, chronic lung disease, pulmonary malformations, or the presence of a tracheostomy.
Following determination of inclusion criteria, and in the absence of exclusion criteria, parents were asked for signed permission to enroll their children after a detailed discussion of the study methods and procedures, aided by the approved structured informed consent document. Once enrolled in the study, children were randomized to one of the experimental groups by a member of the PICU nursing staff not involved in the study, in the presence of at least one of the investigators. Randomization was accomplished by means of selecting an opaque envelope containing the experimental group allocation, with each patient having equal chance of entering the HFNC or CPAP group (serial 1:1 allocation).
Experimental Procedure
Patients were connected to multisignal cardiorespiratory monitors (Infinity Delta XL; Dräger do Brasil, SP, Brazil) for continuous measurement of heart rate, respiratory rate, pulse oximetry (SpO 2 ), and arterial blood pressure (when applicable). Standard PICU care was provided to study patients at the discretion of the care team, with exception of the respiratory management described below. The Pediatric Index of Mortality 2 (PIM2) 26 was calculated for every child at the time of treatment allocation.
Children allocated to the CPAP group were fitted with properly sized soft anatomically curved nasal prongs (Hudson RCI/Teleflex; Morrisville, North Carolina, United States). CPAP was generated through a Dräger Evita 4 ventilator (Dräger do Brasil, SP, Brazil) outfitted with a heated humidifier. CPAP was set at 6 cm H 2 O for all patients.
Children allocated to the HFNC group were fitted with a nasal cannula sized to occlude no more than 50% of the cross-sectional area of the nostrils. HFNC support was provided through a dedicated hollow fiber heated humidified system (Precision Flow; Vapotherm, Exceter, New Hampshire, United States) with a disposable circuit. Flow was titrated up to a maximum of 1.5 L/kg/min, as needed, based on clinical assessment.
For both experimental groups, fraction of inspired oxygen (FiO 2 ) was adjusted to achieve a SpO 2 >93%. Patients continued on the assigned treatment until successfully weaned to a simple oxygen cannula by the care team, or upon reaching treatment failure criteria. Treatment failure was determined by the care team and was defined as the need to escalate support to noninvasive bi-level pressure ventilation, or endotracheal intubation. The study protocol did not allow for crossover between the experimental groups.
Outcomes
The primary outcome was the rate of treatment failure, defined as the need to escalate support to noninvasive bilevel pressure ventilation, or endotracheal intubation. Predetermined secondary outcomes included duration of the primary treatment, PICU and hospital length of stay, and the development of apnea.
Statistical Analyses
Prospectively acquired data were abstracted into Excel spreadsheets (Microsoft Corporation; Redmond, Washington, United States) for subsequent analysis. Categorical data are presented as counts ( n ) and percentages (%). Continuous data are presented as means and standard deviations (if normally distributed) or medians and interquartile ranges (if non-normally distributed). Groups were compared using the Student's t -test, Mann–Whitney U Rank Sum test, Fisher's exact test, and log rank survival analysis.
Primary outcome data obtained from this study were used to perform a sample size calculation to assess the feasibility of a larger international trial of HFNC versus CPAP in critical bronchiolitis. These calculations considered a 1:1 treatment allocation, 80% power, and a relative noninferiority margin of 15% (as in the TRAMONTANE study 17 ). We also conducted a second sample size calculation using a more conservative relative noninferiority margin of 10%. For both calculations, the sample size estimate was inflated by 5% to account for the possibility of missing or unusable data in the final analysis.
All statistical analyses were performed using SigmaPlot version 13 (Systat Software Inc.; San Jose, California, United States).
Results
A total of 63 children with critical bronchiolitis were enrolled into this pilot study, with 28 allocated to the CPAP arm and 35 to the HFNC arm. The median age and weight at enrollment for the entire cohort was 2.69 months and 5.72 kg, respectively. A total of 56 patients (88.9%) tested positive for RSV. The median modified Wood–Downes score and PIM2 score was 5 and 0.9, respectively. The mean respiratory rate was 48 breaths/min, and heart rate was 150 beats/min at baseline. The median SpO 2 and FiO 2 was 98% and 0.4, respectively. There were no differences in baseline characteristics between the CPAP and HFNC groups ( Table 1 ).
Table 1. Baseline characteristics.
| All ( n = 63) | CPAP ( n = 28) | HFNC ( n = 35) | p- Value | |
|---|---|---|---|---|
| Age, mo (IQR) | 2.69 (1.29–4.67) | 2.43 (0.92–3.28) | 3.37 (1.4–5.38) | 0.12 |
| Weight, kg (SD) | 5.72 (1.63) | 5.49 (1.49) | 5.9 (1.75) | 0.33 |
| RSV positive, n (%) | 56 (88.9%) | 26 (92.9%) | 30 (85.7%) | 0.45 |
| mWDS (IQR) | 5 (4–6) | 5 (4–5.75) | 5 (4–6) | 0.49 |
| PIM-2 score (IQR) | 0.9 (0.7–1.1) | 0.91 (0.7–1.1) | 0.8 (0.5–1.1) | 0.18 |
| RR, breaths/min (SD) | 48.23 (12.64) | 47 (14.79) | 49.23 (10.73) | 0.49 |
| HR, beats/min (SD) | 149.76 (20.69) | 152.43 (18.1) | 147.63 (22.62) | 0.37 |
| SpO 2 , % (IQR) | 98 (96–99) | 98 (96 - 99) | 97.5 (95–99) | 0.68 |
| FiO 2 (IQR) | 0.4 (0.35–0.5) | 0.4 (0.35–0.5) | 0.38 (0.3–0.46) | 0.26 |
Abbreviations: CPAP, continuous positive airway pressure; FiO 2 , fraction of inspired oxygen; HFNC high-flow nasal cannula; HR, heart rate; mWDS, modified Wood–Downes score; RR, respiratory rate; RSV, respiratory syncytial virus; SpO 2 , peripheral capillary oxygen saturation.
Note: Data are mean and standard deviation (SD), median and interquartile range (IQR), or count ( n ) and percentage (%).
The primary end point of treatment failure occurred in 10/28 patients (36%) in the CPAP group and 13/35 patients (37%) in the HFNC group ( Fig. 1 ). Intubation was necessary in 3/10 patients in the CPAP group and 7/13 patients in the HFNC group.
Fig. 1.
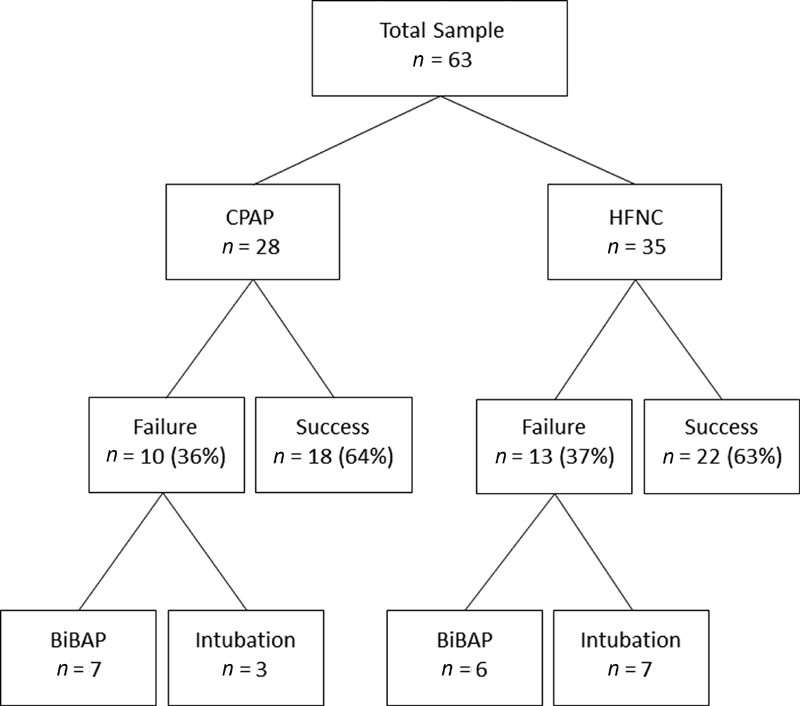
Treatment group allocation and primary outcomes. CPAP, continuous positive airway pressure; HFNC, high flow nasal cannula; BiPAP, bi-level positive airway pressure.
The median PICU and hospital length of stay for the entire cohort were 5 and 8 days, respectively. There were no statistically significant differences between groups relative to secondary outcomes ( Table 2 ).
Table 2. Primary and secondary outcomes.
| All ( n = 63) | CPAP ( n = 28) | HFNC ( n = 35) | p- Value | |
|---|---|---|---|---|
| Primary treatment failure, n (%) | 23 (36.5%) | 10 (35.7%) | 13 (37.1%) | 0.88 |
| Primary treatment duration, h (IQR) | 58.17 (22–80.83) | 56.12 (24.88–72.06) | 67 (15.17–82.5) | 0.41 |
| Bi-level positive airway pressure, n (%) | 13 (20.6%) | 7 (25%) | 6 (17.1%) | 0.65 |
| Intubation, n (%) | 10 (15.9%) | 3 (10.7%) | 7 (20%) | 0.49 |
| PICU LOS, d (IQR) | 5 (4–7) | 5 (4–7) | 5 (4–8) | 0.46 |
| Hospital LOS, d (IQR) | 8 (7–12) | 8 (7–11) | 9 (7–12) | 0.95 |
Abbreviations: CPAP, continuous positive airway pressure; HFNC high-flow nasal cannula; LOS, length of stay; PICU, pediatric intensive care unit.
Note: Data are mean and standard deviation (SD), median and interquartile range (IQR), or count ( n ) and percentage (%).
Thirty five percent of treatment failures (8/23) occurred in the first 12 hours after treatment initiation. The median time to treatment failure was 18.8 (9.1–41) hours in the CPAP group and 15.2 (12.5–25) hours in the HFNC group. There was no statistical difference between the two groups in the log rank survival analysis curve for probability of treatment failure over time ( Fig. 2 ). Two patients in the CPAP group developed apnea and required escalation of support. There were no instances of skin injury at the cannula or mask interface, abdominal distension requiring intervention, air leak (pneumothorax, pneumopericardium, or pneumomediastinum), or cardiorespiratory arrest.
Fig. 2.
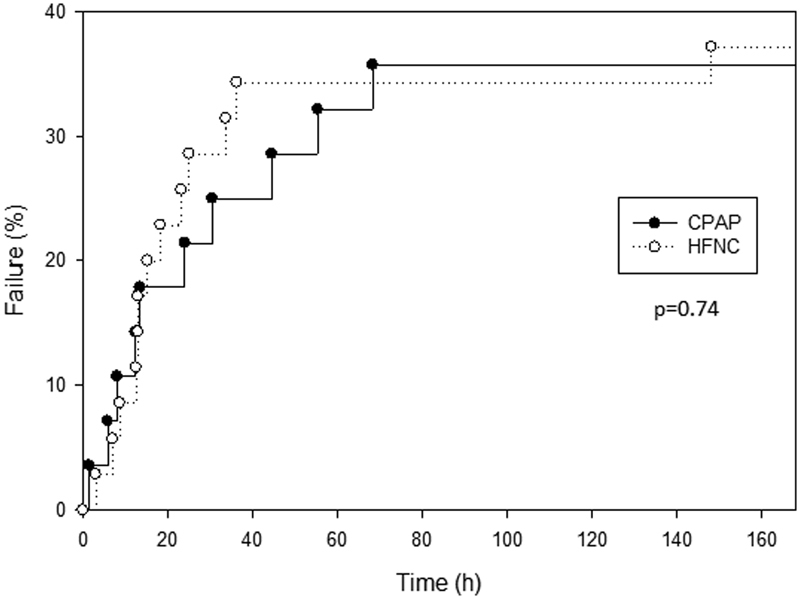
Log rank survival analysis for the occurrence of treatment failure (percentage) over time (hours) in the CPAP (solid line) and HFNC (dashed line) groups. CPAP, continuous positive airway pressure; HFNC, high flow nasal cannula.
A comparison of patient characteristics at enrollment stratified by treatment group and primary outcome is shown in Table 3 . Baseline characteristics such as age, weight, modified Wood–Downes score, PIM-2 score, heart rate, respiratory rate, oxygen saturation, and FiO 2 were not able to discern between success and failure at study entry. Not surprisingly, patients who failed the primary support modality and required escalation of support had a longer PICU and hospital length of stay ( Table 3 ).
Table 3. Patient characteristics by primary outcome and group.
| Success ( n = 40) | Failure ( n = 23) | p -Value | ||
|---|---|---|---|---|
| Age, mo (IQR) | All | 2.79 (1.29–5.78) | 2.62 (1.14–3.76) | 0.63 |
| CPAP | 2.43 (1.04–3.18) | 2.15 (0.69–4.47) | 0.98 | |
| HFNC | 4.26 (1.38–6.76) | 2.93 (1.40–4.26) | 0.21 | |
| Weight, kg (SD) | All | 5.73 (1.69) | 5.69 (1.55) | 0.94 |
| CPAP | 5.24 (1.3) | 5.94 (1.76) | 0.24 | |
| HFNC | 6.13 (1.9) | 5.51 (1.40) | 0.31 | |
| mWDS (IQR) | All | 5 (4–6) | 5 (4–6) | 0.58 |
| CPAP | 4.5 (4–5) | 5 (4–6.25) | 0.34 | |
| HFNC | 5 (4–6) | 5 (4–6.5) | 0.93 | |
| PIM-2 score, % (IQR) | All | 0.7 (0.3–1.1) | 0.9 (0.8–1.1) | 0.06 |
| CPAP | 0.65 (0.28–1.13) | 0.95 (0.80–1.32) | 0.05 | |
| HFNC | 0.7 (0.3–1.1) | 0.9 (0.7–1.05) | 0.45 | |
| RR, breaths/min (SD) | All | 46.95 (12.42) | 50.48 (12.97) | 0.15 |
| CPAP | 45.22 (13.93) | 50.2 (16.49) | 0.40 | |
| HFNC | 48.36 (11.17) | 50.69 (10.22) | 0.54 | |
| HR, beats/min (SD) | All | 149.68 (20.05) | 149.91 (22.25) | 0.48 |
| CPAP | 155.78 (18.99) | 146.4 (15.33) | 0.19 | |
| HFNC | 144.68 (19.91) | 152.62 (26.7) | 0.32 | |
| SpO 2 , % (IQR) | All | 97 (96–98) | 99 (96.5–99.25) | 0.11 |
| CPAP | 97 (96–98.25) | 99 (98.5–99.5) | 0.05 | |
| HFNC | 97 (95–98.5) | 98 (94.5–99.5) | 0.57 | |
| FiO 2 (IQR) | All | 0.4 (0.35–0.5) | 0.38 (0.3–0.44) | 0.32 |
| CPAP | 0.4 (0.35–0.5) | 0.35 (0.3–0.5) | 0.33 | |
| HFNC | 0.4 (0.38–0.5) | 0.35 (0.3–0.4) | 0.28 | |
| PICU LOS, d (IQR) | All | 5 (4–6) | 7 (5–11) | 0.01 |
| CPAP | 4.5 (3.75–6.25) | 5.4 (4.75–11.25) | 0.04 | |
| HFNC | 5 (3.75–6.25) | 8 (4.5–12.5) | 0.03 | |
| Hosp LOS, d (IQR) | All | 8 (6.25–10) | 12 (8–17) | <0.001 |
| CPAP | 8 (7–10) | 10.5 (7.75–14) | 0.09 | |
| HFNC | 7 (6–9.25) | 12 (9–17.5) | 0.002 |
Abbreviations: CPAP, continuous positive airway pressure; FiO2, fraction of inspired oxygen; HFNC high-flow nasal cannula; Hosp; hospital; HR, heart rate; LOS, length of stay; mWDS, modified Wood–Downes score; PICU, pediatric intensive care unit; RR, respiratory rate; SpO2, peripheral capillary oxygen saturation.
Note: Data are mean and standard deviation (SD), median and interquartile range (IQR), or count ( n ) and percentage (%).
Considering our finding of treatment failure in 10 out of 28 infants in the CPAP group (35.7%, 95% confidence interval: 18.0–53.5%), a noninferiority study comparing HFNC to CPAP that assumes a 1:1 treatment allocation, 80% power, and a relative noninferiority margin of 15% (as in the TRAMONTANE study) 17 would require a total of 3,015 patients, or 1,583 patients per group. The application of a more conservative relative noninferiority margin of 10% under these same assumptions would require a total of 6,475 patients, or 3,400 patients in each group.
Discussion
In this single-center pilot study comparing HFNC therapy and CPAP in 63 infants with critical bronchiolitis, we found that both modalities had similar rates of treatment failure, as well as PICU and hospital lengths of stay.
Although bronchiolitis typically affects children under 2 years of age, we elected to limit enrolment in this study to infants up to 9 months of age, as these younger patients tend to be more severely affected by the disease. 9 24 Our final sample consisted of younger critically-ill children with a median age of 2.7 months, a high rate of confirmed infection by the RSV(88.9%), and modified Wood–Downes scores in the moderate-to-severe range, indicating a clinically enriched sample that would be more likely to meet criteria for treatment failure. As expected, the median PIM2 score was low (0.9), and in line with the low expected mortality in patients with critical bronchiolitis. 9 It should be noted that although the PIM2 score is useful in grading disease severity and predicting mortality in critically ill children, 26 27 it is likely not a robust measure of illness severity in infants with bronchiolitis. 28 Nevertheless, the similar PIM2 scores between the experimental groups at baseline suggest that these were comparable and not unbalanced by misallocation of comorbid conditions, like malignancies or immune deficiencies. We did not observe significant differences in other important baseline characteristics between the experimental groups, like age, weight, viral etiology, modified Wood–Downes score, vital signs, and oxygen requirement. Therefore, we believe the HFNC and CPAP groups were comparable at baseline and a true sample of the critical bronchiolitis population we aimed to study.
For clarity and simplicity, we elected to apply the same level of end-expiratory pressure (6 cm H 2 O) to all patients allocated to the CPAP group. This level of CPAP has been shown to rapidly unload respiratory muscle work and decrease respiratory distress in infants with severe bronchiolitis, and is in line with the 5 to 8 cm H 2 O CPAP level employed in comparable studies. 14 17 29 Patients allocated to HFNC received a flow of up to 1.5 L/kg/min. This flow setting has been shown to significantly attenuate work of breathing in children with acute respiratory failure, and to be equivalent to 2 L/kg/min. 30 It is unlikely that additional uptitration of flow would have significantly changed our findings, especially considering that flows in excess of 2 L/kg/min have not been shown to yield additional clinical benefit in this population. 31
In retrospective observational cohort studies, the introduction of HFNC has been associated with a decrease in the need for mechanical ventilation in children with bronchiolitis. 19 20 McKiernan et al studied 115 children with bronchiolitis admitted to the PICU over two respiratory seasons; 57 children before and 58 children after the implementation of HFNC therapy in their hospital. 19 In that study, the use of HFNC was associated with a decrease in intubation rates from 23 to 9% and a shorter length of stay. 19 Similarly, Schibler et al observed a decrease in the need for mechanical ventilation from 37 to 7% that corresponded with an increase in use of HFNC in 298 critically ill infants with acute respiratory failure, 56% of whom had bronchiolitis. 20 Although compelling, these studies can only suggest an association but not causation between HFNC use and a decrease in the need for mechanical ventilation.
The early administration of HFNC to children with bronchiolitis outside the PICU is associated with lower rates of treatment failure or need to escalate care, compared with standard oxygen therapy. 22 32 However, data comparing HFNC to CPAP in critical bronchiolitis are less clear. We found a similar rate of treatment failure between HFNC and CPAP (37 and 36%, respectively), and intubation was eventually needed in 7 out of 35 patients allocated to HFNC and 3 out of 28 patients allocated to CPAP. At first glance, our findings seem to contrast those of the TRAMONTANE study conducted by Milési et al, 17 which found a significantly higher treatment failure rate in patients initially randomized to HFNC compared with CPAP (51 vs. 31%, respectively). The main difference between that study and ours lies in the definition of failure. While our study employed what we believe to be clinically relevant definition of treatment failure—namely, the need for noninvasive bilevel pressure ventilation or endotracheal intubation—the TRAMONTANE study 17 defined failure as the occurrence of at least one of four criteria (1 point increase in a modified clinical asthma score, or in a neonatal pain and discomfort score; an increase in respiratory rate by more than 10 breaths per minute; or the presence of more than 2 episodes of severe apnea). This lower threshold for failure in the TRAMONTANE study was deliberate, likely due to concern that the low occurrence of intubation in the current era would require a prohibitively large sample to properly power that study. 17 Another important difference between the TRAMONTANE study and ours is the experimental design: while patients who failed initial treatment allocation in our study were immediately escalated to bi-level noninvasive ventilation or intubation, those in the TRAMONTANE study crossed over to the other treatment arm. It was only after failure at the secondary treatment allocation that patients were escalated to bi-level noninvasive ventilation or intubation. Assuming that HFNC was indeed inferior to CPAP, it is interesting to note that 18 of the 22 (82%) patients who failed CPAP in the TRAMONTANE study were actually rescued by HFNC following crossover. 17
More recently, another study comparing HFNC to CPAP in 31 infants with critical bronchiolitis found similar rates of clinical improvement for physiologic variables and clinical scores between the two experimental groups. 29 In that small study, HFNC appears to have been better tolerated than CPAP (lower incidence of nasal injury), and the rate of intubation was similar (1 patient in each group). 29 A recent systematic review and meta-analysis 33 pooling data from four studies on HFNC and CPAP in 264 children with bronchiolitis also found no significant difference in the incidence of intubation between the two experimental groups.
In our study, the median time to treatment failure was 15.2 hours in the HFNC group and 18.5 hours in the CPAP group, both considerably longer than the ∼6 hours for both groups in the TRAMONTANE study. 17 Although most of our patients experienced treatment failure within the first day, some met failure criteria well into the third day, underscoring the need for continued close vigilance in this setting.
The main purpose of our study was to obtain effect size data to assess the feasibility of a larger international trial of HFNC versus CPAP in critical bronchiolitis. We estimated that such a trial would require at least 3,000 patients, or 1,500 patients per group. Nearly twice as many patients would be necessary if a more conservative noninferiority margin was chosen. Considering these large sample size estimates and the rapid adoption of HFNC throughout the world leading to potential loss of clinical equipoise, it is unlikely that such a trial would be successfully completed.
Our study has several limitations. First, it is a relatively small pilot study that precludes us from drawing more definitive conclusions regarding the equivalence of HFNC compared with CPAP as first-line treatment of critical bronchiolitis. Second, we did not employ a crossover design, so it is impossible to determine how many patients who failed CPAP could have been rescued by HFNC, and vice versa. Third, we did not include a true control group (i.e., standard oxygen therapy), choosing instead to compare what many consider standard therapy (CPAP) with a newer modality (HNFC). Considering the various studies showing superiority of HFNC compared with standard oxygen therapy in reducing clinical deterioration and treatment failure, we lacked equipoise to include this true control group (standard oxygen therapy). Fourth, we selected, by design, a somewhat enriched sample with a higher risk phenotype (young infants with moderate to severe critical bronchiolitis, predominantly caused by RSV), so our results might not be completely generalizable to a broader critical bronchiolitis population. Fifth, this study was planned and executed as “open label,” so the investigators and clinicians were aware of the treatment group allocation. Considering the relatively high threshold in our definition of treatment failure, we find it unlikely that knowledge of group allocation could have biased our results. Furthermore, all other studies on this subject have employed a similar open design due to the impracticality of masking HFNC or CPAP support. Lastly, this study was conducted in a PICU with extensive experience in the use of CPAP in critical bronchiolitis but relatively new to the application of HFNC. Although the lack of a crossover option prevented clinicians from declaring failure of HFNC so as to gravitate toward the more familiar CPAP arm, it is impossible to estimate whether the timing of failure in the HFNC group could have been influenced by clinician experience or comfort with this treatment modality. It is possible that the rate of failure in the HFNC group could have dropped with increasing familiarity and experience by the care team. This was the case in the TRAMONTANE 2 study 31 where HFNC had an overall failure rate of 38.5% (in contrast to the 50.7% failure rate in the original TRAMONTANE study 17 ) that was not significantly different from the 31% CPAP failure rate.
Conclusion
The conduct of a noninferiority trial comparing HFNC and CPAP under similar conditions to those in this pilot study, although feasible, would require at least 3,000 patients, depending on the noninferiority margin selected. In this single-center randomized controlled pilot study comparing HFNC therapy and CPAP in infants with critical bronchiolitis, we found that both modalities had similar rates of treatment failure, as well as PICU and hospital lengths of stay.
Funding Statement
Funding This study was supported by Hospital Infantil Sabará and Instituto PENSI. High-flow devices and circuits were provided by Vapotherm, Inc. at no cost to the investigators. Vapotherm was not involved in the planning, execution, data analysis, data interpretation, or writing of the manuscript, and was not privy to its results.
Footnotes
Conflict of Interest A.T.R. is a scientific advisory board member for Breas Medical U.S., received honoraria for lecturing and developing educational materials for Vapotherm, Inc., and continues to receive royalties from Elsevier for editorial work on a pediatric critical care textbook. The other authors have no potential conflicts of interest to disclose.
References
- 1.Hall C B, Weinberg G A, Iwane M K. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360(06):588–598. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meissner H C. Viral bronchiolitis in children. N Engl J Med. 2016;374(01):62–72. doi: 10.1056/NEJMra1413456. [DOI] [PubMed] [Google Scholar]
- 3.Zorc J J, Hall C B. Bronchiolitis: recent evidence on diagnosis and management. Pediatrics. 2010;125(02):342–349. doi: 10.1542/peds.2009-2092. [DOI] [PubMed] [Google Scholar]
- 4.Øymar K, Skjerven H O, Mikalsen I B. Acute bronchiolitis in infants, a review. Scand J Trauma Resusc Emerg Med. 2014;22:23. doi: 10.1186/1757-7241-22-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin A J, Gardner P S, McQuillin J.Epidemiology of respiratory viral infection among paediatric inpatients over a six-year period in north-east England Lancet 19782(8098):1035–1038. [DOI] [PubMed] [Google Scholar]
- 6.Everard M L. Acute bronchiolitis and croup. Pediatr Clin North Am. 2009;56(01):119–133. doi: 10.1016/j.pcl.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention (CDC) . Respiratory syncytial virus activity - United States, July 2008-December 2009. MMWR Morb Mortal Wkly Rep. 2010;59(08):230–233. [PubMed] [Google Scholar]
- 8.Glezen W P, Taber L H, Frank A L, Kasel J A. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child. 1986;140(06):543–546. doi: 10.1001/archpedi.1986.02140200053026. [DOI] [PubMed] [Google Scholar]
- 9.Gupta P, Beam B W, Rettiganti M. Temporal trends of respiratory syncytial virus-associated hospital and ICU admissions across the United States. Pediatr Crit Care Med. 2016;17(08):e343–e351. doi: 10.1097/PCC.0000000000000850. [DOI] [PubMed] [Google Scholar]
- 10.Slain K N, Shein S L, Stormorken A G, Broberg M CG, Rotta A T. Outcomes of children with critical bronchiolitis living in poor communities. Clin Pediatr (Phila) 2018;57(09):1027–1032. doi: 10.1177/0009922817740666. [DOI] [PubMed] [Google Scholar]
- 11.Slain K N, Rotta A T, Martinez-Schlurmann N, Stormorken A G, Shein S L. Outcomes of children with critical bronchiolitis meeting at risk for pediatric acute respiratory distress syndrome criteria. Pediatr Crit Care Med. 2019;20(02):e70–e76. doi: 10.1097/PCC.0000000000001812. [DOI] [PubMed] [Google Scholar]
- 12.American Academy of Pediatrics . Ralston S L, Lieberthal A S, Meissner H C. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(05):e1474–e1502. doi: 10.1542/peds.2014-2742. [DOI] [PubMed] [Google Scholar]
- 13.Cambonie G, Milési C, Jaber S. Nasal continuous positive airway pressure decreases respiratory muscles overload in young infants with severe acute viral bronchiolitis. Intensive Care Med. 2008;34(10):1865–1872. doi: 10.1007/s00134-008-1201-x. [DOI] [PubMed] [Google Scholar]
- 14.Essouri S, Durand P, Chevret L. Optimal level of nasal continuous positive airway pressure in severe viral bronchiolitis. Intensive Care Med. 2011;37(12):2002–2007. doi: 10.1007/s00134-011-2372-4. [DOI] [PubMed] [Google Scholar]
- 15.Milési C, Matecki S, Jaber S. 6 cmH2O continuous positive airway pressure versus conventional oxygen therapy in severe viral bronchiolitis: a randomized trial. Pediatr Pulmonol. 2013;48(01):45–51. doi: 10.1002/ppul.22533. [DOI] [PubMed] [Google Scholar]
- 16.Essouri S, Laurent M, Chevret L. Improved clinical and economic outcomes in severe bronchiolitis with pre-emptive nCPAP ventilatory strategy. Intensive Care Med. 2014;40(01):84–91. doi: 10.1007/s00134-013-3129-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Groupe Francophone de Réanimation et d'Urgences Pédiatriques (GFRUP) . Milési C, Essouri S, Pouyau R. High flow nasal cannula (HFNC) versus nasal continuous positive airway pressure (nCPAP) for the initial respiratory management of acute viral bronchiolitis in young infants: a multicenter randomized controlled trial (TRAMONTANE study) Intensive Care Med. 2017;43(02):209–216. doi: 10.1007/s00134-016-4617-8. [DOI] [PubMed] [Google Scholar]
- 18.Lee J H, Rehder K J, Williford L, Cheifetz I M, Turner D A. Use of high flow nasal cannula in critically ill infants, children, and adults: a critical review of the literature. Intensive Care Med. 2013;39(02):247–257. doi: 10.1007/s00134-012-2743-5. [DOI] [PubMed] [Google Scholar]
- 19.McKiernan C, Chua L C, Visintainer P F, Allen H. High flow nasal cannulae therapy in infants with bronchiolitis. J Pediatr. 2010;156(04):634–638. doi: 10.1016/j.jpeds.2009.10.039. [DOI] [PubMed] [Google Scholar]
- 20.Schibler A, Pham T M, Dunster K R. Reduced intubation rates for infants after introduction of high-flow nasal prong oxygen delivery. Intensive Care Med. 2011;37(05):847–852. doi: 10.1007/s00134-011-2177-5. [DOI] [PubMed] [Google Scholar]
- 21.Australian & New Zealand Intensive Care Society (ANZICS) Centre for Outcomes & Resource Evaluation (CORE) and the Australian & New Zealand Intensive Care Society (ANZICS) Paediatric Study Group . Schlapbach L J, Straney L, Gelbart B. Burden of disease and change in practice in critically ill infants with bronchiolitis. Eur Respir J. 2017;49(06):1.601648E6. doi: 10.1183/13993003.01648-2016. [DOI] [PubMed] [Google Scholar]
- 22.Kepreotes E, Whitehead B, Attia J.High-flow warm humidified oxygen versus standard low-flow nasal cannula oxygen for moderate bronchiolitis (HFWHO RCT): an open, phase 4, randomised controlled trial Lancet 2017389(10072):930–939. [DOI] [PubMed] [Google Scholar]
- 23.Clayton J A, McKee B, Slain K N, Rotta A T, Shein S L. Outcomes of children with bronchiolitis treated with high-flow nasal cannula or noninvasive positive pressure ventilation. Pediatr Crit Care Med. 2019;20(02):128–135. doi: 10.1097/PCC.0000000000001798. [DOI] [PubMed] [Google Scholar]
- 24.Nair H, Nokes D J, Gessner B D.Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis Lancet 2010375(9725):1545–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferrés Mataró J, Mangues Bafalluy M A, Farré Riba R, Juliá Brugues A, Bonal de Falgas J. [Subcutaneous adrenaline versus inhaled salbutamol in the treatment of childhood asthmatic crisis] An Esp Pediatr. 1987;27(01):37–40. [PubMed] [Google Scholar]
- 26.Paediatric Index of Mortality (PIM) Study Group . Slater A, Shann F, Pearson G. PIM2: a revised version of the Paediatric Index of Mortality. Intensive Care Med. 2003;29(02):278–285. doi: 10.1007/s00134-002-1601-2. [DOI] [PubMed] [Google Scholar]
- 27.Tibby S M, Taylor D, Festa M. A comparison of three scoring systems for mortality risk among retrieved intensive care patients. Arch Dis Child. 2002;87(05):421–425. doi: 10.1136/adc.87.5.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Modesto I Alapont V, Pons-Òdena M, Medina A. High-flow nasal cannula versus noninvasive ventilation: a matter of confusion. Pediatr Crit Care Med. 2019;20(12):1210–1211. doi: 10.1097/PCC.0000000000002110. [DOI] [PubMed] [Google Scholar]
- 29.Sarkar M, Sinha R, Roychowdhoury S. Comparative study between noninvasive continuous positive airway pressure and hot humidified high-flow nasal cannulae as a mode of respiratory support in infants with acute bronchiolitis in pediatric intensive care unit of a tertiary care hospital. Indian J Crit Care Med. 2018;22(02):85–90. doi: 10.4103/ijccm.IJCCM_274_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weiler T, Kamerkar A, Hotz J, Ross P A, Newth C JL, Khemani R G. The Relationship between high flow nasal cannula flow rate and effort of breathing in children. J Pediatr. 2017;189:66–71000. doi: 10.1016/j.jpeds.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 31.GFRUP Respiratory Study Group . Milési C, Pierre A F, Deho A. A multicenter randomized controlled trial of a 3-L/kg/min versus 2-L/kg/min high-flow nasal cannula flow rate in young infants with severe viral bronchiolitis (TRAMONTANE 2) Intensive Care Med. 2018;44(11):1870–1878. doi: 10.1007/s00134-018-5343-1. [DOI] [PubMed] [Google Scholar]
- 32.Franklin D, Babl F E, Schlapbach L J. A randomized trial of high-flow oxygen therapy in infants with bronchiolitis. N Engl J Med. 2018;378(12):1121–1131. doi: 10.1056/NEJMoa1714855. [DOI] [PubMed] [Google Scholar]
- 33.Lin J, Zhang Y, Xiong L, Liu S, Gong C, Dai J. High-flow nasal cannula therapy for children with bronchiolitis: a systematic review and meta-analysis. Arch Dis Child. 2019;104(06):564–576. doi: 10.1136/archdischild-2018-315846. [DOI] [PubMed] [Google Scholar]


