Abstract
The on-going pandemic of COVID-19 wreaked by a viral infection of SARS-CoV-2, has generated a catastrophic plight across the globe. Interestingly, one of the hallmarks of COVID-19 is the so-called ‘cytokine storm’ due to attack of SARS-Cov-2 in the lungs. Considering, mesenchymal stem cells (MSCs) therapy could contribute against SARS-CoV-2 viruses attack because of their immune modulatory and anti-inflammatory ability linked to their stemness, to the arsenal of treatments for COVID-19. Another novel therapeutic strategies include the blockade of rampant generation of pro-inflammatory mediators like acute respiratory distress syndrome (ARDS), degradation of viral protein capsids by PROTACs, composed of Ubiquitin-proteasome framework, and ubiquitination-independent pathway directing the SARS-CoV-2 nucleocapsid protein (nCoV N) and proteasome activator (PA28γ), etc. This review is consequently an endeavour to highlight the several aspects of COVID-19 with incorporation of important treatment strategies discovered to date and putting the real effort on the future directions to put them into the perspective.
Graphical abstract
Highlights
-
•
Coronavirus disease (COVID-19) emerged out as a potential global biological disaster caused by “SARS-CoV-2″
-
•
Early detection of the SARS-CoV-2 had a better prognosis for treatment strategies.
-
•
Clinical trials among repurposed molecules had progressed with several methodological limitations.
-
•
Systemic overview of various aspects in epidemiology and treatment strategies.
-
•
Challenges and the future directions have also been discussed.
1. Introduction
The COVID-19 disease has become a household name, which puts fear into everyone's heart and mind. It gained fame due to how quickly it spread around the world since its birth in Wuhan city, China. It felt like almost overnight, this disease caught on globally, bringing both business and travel to a grinding halt and the government tried to curb its spread among our citizens. At any given time, hundreds of people are rushing into an emergency room nearby looking for urgent care trying to fight for their lives. COVID- 19 manifests itself as a respiratory illness that is quite like a flu. It took doctors and scientists a few weeks to distinguish between the COVI-19 and normal flu, as both diseases have the same means of transmission and share some key symptoms. This similarity between both the diseases generated some confusion when the pandemic first broke out early this year because patients with COVID-19 mistook it as an ordinary flu.
The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has now spread quickly to 213 countries and caused a large scale COVID-19 pandemic. Presently, the newly identified SARS-CoV-2 has caused a high mortality rate with tens of thousands of positive cases across globe, posing a grave threat to public health. COVID-19 has caused severe human pneumonia since the beginning of the 21st century [1]. Initially, the world health organization (WHO) alluded the term 2019 novel coronavirus (2019-nCov) to underline the spread of this infection, which was officially turned into COVID-19 caused by SARS-CoV-2 virus [2].
Importantly, the availability of clinically approved vaccines or specific therapeutic drugs for COVID-19 is still awaited. Furthermore, to elucidate the pathogenesis, epidemiology, and to identify potential drug candidates, extensive researches is urgently needed endowing to the discovery of effective therapeutic strategies [3]. At present, various diagnostic kits to test for COVID-19 and diverse ranges of clinically effective “repurposing therapeutics” are available and accessible. Furthermore, various healthcare organizations have initiated to formulate vaccines to prevent COVID-19 [4]. A large subfamily of single-stranded positive RNA viruses enveloped under the viral coat carrying one of the biggest genome sizes of around 30–32 Kb is capable of infecting organisms in the wild as well as the humans [5]. Studies suggest that these viruses fall under the category of Coronavirinae subfamily being a part of the family Coronaviridae and the order Nidovirales [6], which splits into three distinguished genera (α-, β-, and γ-coronavirus) which are affirmed by the antigenic property and genome sequencing. Further, international committee on scientific categorization of viruses (2009) added another genus named δ-coronavirus, which was suspected to be evolved from the bird's family [7].
Despite the acknowledged delicacy of the protein coat, other curiosity that may include is coronavirus's potential natural resistance [8]. The changes in the genetic diversity and increased human-animal interaction, lead to the frequent recombination across the genomes may also lead to the generation of new strains of coronavirus [9]. To date, six variations of human coronavirus have been reported, α-CoVs (NL63 and 229E), β-CoVs (OC43 and HKU1), SARS-CoV-1&2, and middle east respiratory syndrome-CoV (MERS-CoV) [10]. Among them, SARS-CoV-2 is known to be the seventh viral category to infect the people and are serious ailment causing organisms now-a-days. To curb the current outbreak, stringent measures to mitigate person-to-person transference of COVID-19 have been executed. Globally, countries are responding differently to the COVID-19 outbreak. Overloading of the local health systems has been resulted due to delayed detection and response across globe. On the contrary, effective and strong therapeutic strategies have been put in place by several nations that led to fewer deaths since the beginning of the pandemics [11].
Despite the availability of plethora of literatures, emphasising the symptoms, epidemiology, transmission, pathogenesis, diagnosis and clinical manifestations, none of the previous state-of-the-art has been devoted the real efforts on repurposing as well as the novel therapeutic strategies at current level of the global hallmarks of COVID-19, in the best of our knowledge. This article is consequently devoted to review the explored scientific insights on our substantial attention towards cytokine storm syndrome (CSS), mesenchymal stem cells (MSCs) therapy, pro-inflammatory mediators like acute respiratory distress syndrome (ARDS), degradation of viral protein capsids by PROTACs, Ubiquitin-proteasome framework, SARS-CoV-2 nucleocapsid protein (nCoV N), proteasome activator (PA28γ), etc to the arsenal of treatments for COVID-19. Furthermore, extensive searches have been attempted to design a schematic illustration of recently discovered repurposing and novel therapeutics that blocks the different steps in the COVID-19 replication signalling pathway. Moreover, the current review also covers the current scenario of COVID-19 epidemiology, pathogenesis, symptoms, transmission, pathogenesis, diagnosis, and clinical manifestations by representing heat-map diagram showing the database of antiviral agents COVID-19. The last section of the review encompasses our creative and real effort to steal the attention of emerging scientists towards the unexplored insights into the notable therapeutic strategies in an anticipation to combat COVID-19 disease. These future directions ultimately could inform the utility of a proof-of-principle in discovery of drugs to fight against the pandemic.
2. Epidemiology
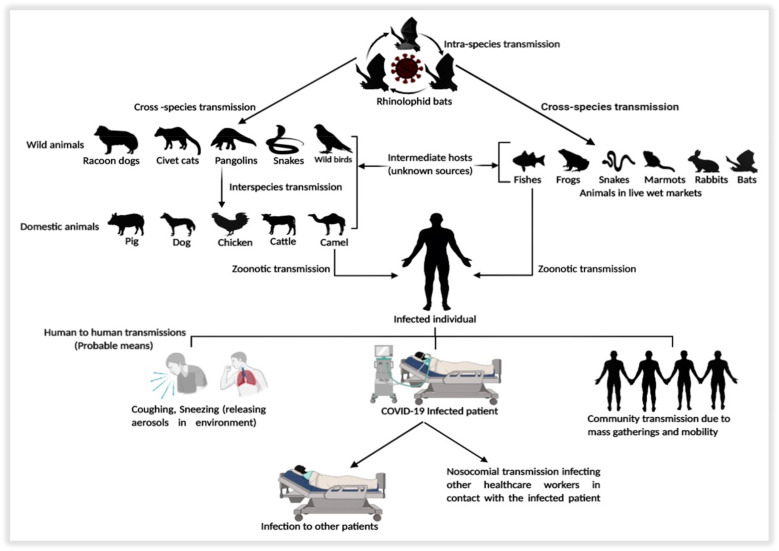
Coronavirus disease 19 (COVID-19) is an extraordinarily transmittable and pathogenic viral infection caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), which originated in Wuhan, China in December 2019 and spread across the globe [12]. The infectibility of these viruses ought to be in the wild only until the world spotted the outbreak of severe acute respiratory syndrome (SARS) in 2002 and middle east respiratory syndrome (MERS) in 2012 [12,13]. (SARS-CoV) spreading to five landmasses in 2002 and Middle-East Respiratory Syndrome Coronavirus (MERS-CoV) in Arabian Peninsula in 2012 indicated that these gathering of infections are zoonotic, which can transmit from the animals to people and from people to people [6,14]. Although the disease spread in the wild across the globe, the transmission to humans from the wild was negligible, and still unclear of the mode. However, analysis in the whole genome sequence of the bats was found with similarity index to be 96% identical with a severe acute respiratory syndrome-like (SARS-like) bat viruses and 79.5% similar with SARS-CoV, suggesting it to be the probable means of transmission to the humans [15,16]. Table 1 shows the comparative study of the epidemiological characteristics among the SARS-CoV, MERS-CoV, and 2019-nCoV. Although the standard mode of communication is still unclear due to its interspecies jumping capacity [8,12], subsequent studies have shown that snakes [17], bats and minks [18], pangolins [15] could be the intermediate hosts for the transmission to humans. Still, intermediate hosts may have multiple hosts [19]. The key reservoirs and the probable modes of transmission pathway of the disease is described in (Fig. 1 ).
Table 1.
Comparative study of the epidemiological characteristics among the SARS-CoV, MERS-CoV, and 2019-nCoV. DPP4 - Dipeptidyl peptidase-4; CD26 - Cluster of Differentiation 26; TMPRSS2- Transmembrane protease, serine 2; ACE-2- Angiotensin-Converting Enzyme 2; ORF –Open Reading Frame; SARS-CoV - Severe acute respiratory syndrome coronavirus; MERS-CoV – Middle East Respiratory Syndrome Coronavirus; 2019-nCoV – 2019 Novel Coronavirus.
| Characteristics | SARS-CoV | MERS-CoV | 2019-nCoV |
|---|---|---|---|
| Year | 2002 | 2012 | 2019 |
| Origin | Guangdong, China | Arabian Peninsula, Republic of Korea | Wuhan, China |
| Genus | Beta coronavirus Lineage B | Beta coronavirus Lineage C | Beta coronavirus Lineage B |
| Gene | RNA | RNA | RNA |
| Nucleotide length | 29,727 ntds | 30,119 ntds | 29,903 ntds |
| Natural Host | Bats | Bats | Bats |
| Intermediary Host | Civets, Raccoon Dogs | Dromedary Camels | Pangolins, Marmots |
| Affected Countries | 26 | 27 | 216 |
| Total Cases (Global) | 8422 (June 30, 2020) | 2494 (June 30, 2020) | 10,185,374 (June 30, 2020) |
| Total Deaths | 916 | 858 | 503,862 (June 30, 2020) |
| Total Cases (India) | 03 | – | 585,493 (June 30, 2020) |
| Total Deaths (India) | – | – | 17,400 (June 30, 2020) |
| Transmission mode | Zoonotic | Zoonotic | Zoonotic |
| Binding site | ACE-2 | DPP4/CD26 | TMPRSS2, ACE-2 |
| Susceptible cell line | Respiratory Tracts; Liver; Kidney | Respiratory; Monocytes; Lymphocytes; Intestinal; Liver; Neural; Kidney; Histiocytic cell lines | Respiratory Tracts; Neural; Kidney |
| Viral replication efficiency | Low | High | Extremely High |
| Gene order characteristics | 5′-Replicase ORF1ab, spike (S), envelope (E), membrane (M), and nucleocapsid (N)-3′ | 5′-Replicase ORF1ab, spike (S), envelope (E), membrane (M), and nucleocapsid (N)-3′ | 5′-Replicase ORF1ab, spike (S), envelope (E), membrane (M), and nucleocapsid (N)-3′ |
Fig. 1.
Key reservoirs and probable modes of transmission of SARS-CoV2.
SARS-CoV's pandemic origin believed in assimilating by people from feral wild predators, such as civet cats and raccoon dogs, who, in turn, thought to have caused by rhinolophid bats infection [20]. Although the transmission is not by natural vectors, however, relying upon the disease, species can transmit through fomites or aerosols as well as fecal-oral courses [11]. One of the essential zoonotic reservoirs for the virus could be the domestic animals that facilitate transmission to humans [21]. Nonetheless, the human-to-human transmission has been the essential mode of spreading pandemics, which has happened in working environments, homes, and open transportation. The most significant course of human-human spread seems, by all accounts, to be directly or indirectly through contact of the mucosae with irresistible respiratory droplets or fomites [22]. However, reports have suggested that the SARS-CoV found in tears, but the infection through conjunctival or oral means is still unclear [23]. SARS-CoV2 spreads for the most part by air-droplets created because of the coughing or sneezing of an infected individual. One can get the disease by being in close contact with the infected patient, mainly if they do not cover their face when coughing or sneezing. Another means of the transmission is contacting any such contaminated surface or fabric. Afterward, communicating one's mouth, nose or eyes can transmit the disease as the droplets reside on surfaces and garments for a long time [24]. However, some individuals with the disease, yet with no genuine symptoms, can likewise spread the infection [25]. Still, a significant threat prevails due to asymptomatic and pre-symptomatic virus transmission to infection prevention. In comparison, people with moderate and unspecific signs and isolation are often challenging to classify [26]. It is essential to know the exact incubation time of any disease to control its spread as it gives the establishment for epidemiological counteraction, clinical activities, and medication revelation. This period is the time from infection to the phase where the disease symptoms are seen [27]. The incubation period of SARS-CoV2 in the host ranges from 2 to 14 days as estimated by WHO, contagious during that period. After that, the infection may transmit during the incubation time wherein the asymptomatic carriers of SARS-CoV2 accounting for 1% confirmed by the laboratory testing [28]. They ought to infect the people with immunosuppression, older people, and young neonates, responsible for upper respiratory infections, common cold, and nosocomial viral infections as a significant symptom with the fatality emergence in humans. In severe cases, the disease can lead to the condition of pneumonia, respiratory syndrome, chest and muscle pain, kidney failure, and even death [15].
2.1. Pathogenesis
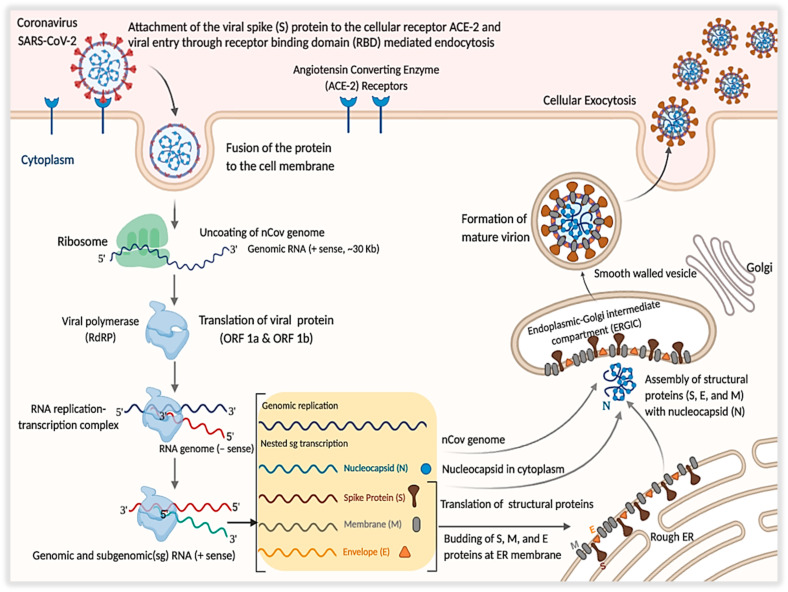
The immune response in the host is associated with the infiltration and pathogenesis SARS-CoV-2. The trimeric transmembrane spike (S) glycoprotein mediates the entry of the virus into the host cells. S is class I viral fusion protein synthesized as a chain of ~1300 amino acids forming a crown-like structure, which leads the role in neutralizing the antibodies [29]. The infection initiates when the virus enters the host cell attaching the “Viral Spike Protein” to the cellular receptors. Attachment of the virus, the host cell's proteolytic enzymes cleaves and activates the spike macromolecule attached to the receptor [30], accompanied by cell membrane fusion, the primary target being the lung epithelial cells [31]. In this context, the inter-human transmission of the disease ought to have caused by the binding of the receptor-binding domain (RBD) of the viral spike protein to a cellular receptor known as the angiotensin-converting-receptor-2 (ACE-2) [32, 33] and the transmembrane protease serine-2 (TMPRSS2) [34]. Alveolar cells in the lung contain plenty of ACE2, enabling COVID-19 to be harbored within the alveoli [35]. The binding protein of the SARS-CoV2 to the ACE-2 receptor possesses almost 10–20 times higher affinity than the SARS, indicating that the efficiency of SARS-CoV2 transmitted to the others is readily higher [21]. TMPRSS2 is an alveolar protease and human airway, which colocalize captured by the immunoprecipitation of ACE-2 [36]. ACE-2 is a type I membrane protein consisting of N-terminal peptidase domain (P.D.) and C-terminal collection-like domain (CLD) ending with ~40 residues intercellular segment and forming single transmembrane helix [37], expressed in heart, lungs, intestine, and kidneys and associated with cardiovascular disorders [38].
Studies from the electron microscopy suggested that the ACE-2 receptor exists in the dimeric form, able to be in both “open” as well as “closed” confirmations [39]. Together, ACE-2 and TMPRSS2 facilitate the virus's entry into the cells [36]. The hypothesis suggests that the renin-angiotensin-aldosterone system (RAAS) inhibition boosts the regulation of ACE-2 expression, thereby facilitating the entry of virus and replication [40]. Coronavirus RNA has a 5′ methyl group and 3′ poly-A tail, attaching to the host's free ribosomal unit contributing to translation and the chain formation of polypeptides [30]. Binding of the receptor is regulated via the receptor-binding domain (RBD) of the subunit S1. Proteolytic activation of the S2 subunit after binding to the ACE2 receptor mediates the interaction between the virus and the cellular membranes (Fig. 2 ). Because of S glycoprotein's important function in cellular infection, antibodies attachment to S1 and S2 prevents infection [41]. ACE2 generally shows high articulation in alveolar type 2 cells, hence permitting the virus to enter and duplicate in enormous numbers. SARS-CoV-2 gets into alveolar cells and quickly multiplies, which cause quick and massive production of various types of cytokines, including interleukins (I.L.s), interferons (IFNs), colony-stimulating factors (CSFs), chemokines and tumor necrosis factors (TNFs) in body fluids. Such cytokines activate the immune cells continuously and accumulate at inflammation site leading to “cytokine storm,” resulting in causing up edema, fever, respiratory congestion, injury in lung tissues, eventually leading up to the situation of acute respiratory distress syndrome and respiratory failure [42]. ACE2 is not only found in the respiratory tract organs but also duodenum, small intestine, rein, and the testis. Spread of the infection in target cells can cause bowel dysfunction, kidney failure, reduced fertility, and other disorders [42]. COVID-19's pathological results showed that over activation of T cells, evidenced by an increase in Th17 and high cytotoxicity of CD8 T cells, partly accounts for the extreme immune injury [43].
Fig. 2.
The SARS-CoV-2 pathogenesis mechanism.
Insights on the pathogenesis of SARS are studied through quantitative analyses of viral load. In the lower respiratory tract, the viral load is higher than in the upper airways. During the first 4 days, the viral load in the upper respiratory tract and stool is low and peaks at about day 7–10 of the disease. In strong comparison, influenza virus loads peaks immediately after the onset of clinical symptoms. This rare SARS-CoV infection trait explains its poor transmissibility early in the epidemic [24]. Studies suggested that no substantial correlation was observed between viral load and health effects, including oxygen support, or survival. Interestingly, some publications have reported extreme cases of COVID-19 with high morbidity that arise late in the disease phase, meaning that severe symptoms are unlikely to be associated with high viral load. In short, evidence indicates that SARS-CoV-2 RNA can be identified in individuals 1–3 days before their onset of symptoms, with the highest viral loads observed about the day of symptom onset as measured by RT-PCR, accompanied by a steady decline over time [44].
2.2. Clinical manifestations
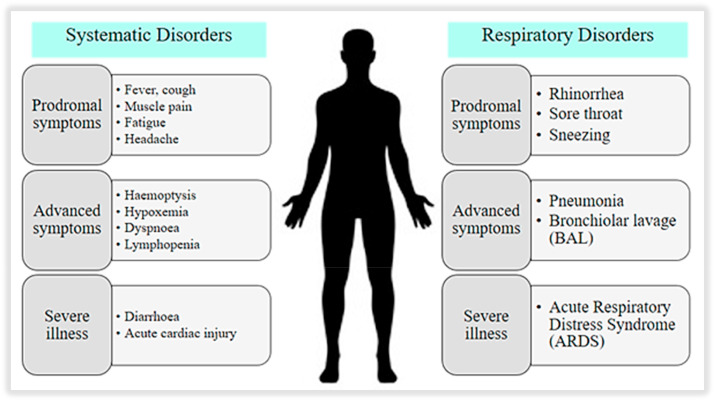
The mean period of incubation is approximately 3–9 days, ranging from 0 to 24 days [1]. The mean interval is around 3–8 days, which shows up earlier than the end of incubation, suggesting that one becomes contagious before symptoms are present (about 2.5 days before symptoms start) [45]. It is estimated that around 44% of transmission occurs before symptoms develop, which are asymptomatic, thereby, increasing the chance of transmission [46]. The initial clinical manifestations of the viral disease begin to occur after some 4–6 days of incubation. The time from the onset of COVID-19 symptoms to death ranged from 6 to 40 days, depending on the status of the patient's age and immune system [48]. Patients with pre-existing medical conditions and those with age >70 years were likely to have a shorter duration in showing symptoms [47]. Most people at the onset show symptoms like fever (88%), dry cough (68%), fatigue with muscle pain (38%), and shortness of breath with some minor respiratory disorders like rhinorrhoea, sore throat etc. (Fig. 3 ). In contrast, other clinical signs are nausea, diarrhoea, headache, sputum production, dyspnoea, lymphopenia, and haemoptysis [48]. Patients diagnosed with COVID-19 displayed irregular breathing counts, and elevated pro-inflammatory cytokines in plasma [31]. Some of the patients may show variation in the counts of leukocytes and drop in the lymphocyte's levels [49]. Mainly if they are elderly or have some pre-existing health condition, patients may experience breathing difficulties. In such cases, the situation may need the patient to hospitalize. In worsening cases, the person may experience a condition where the pleural cavity of the lungs is filled with fluids, leading to a situation of acute respiratory distress syndrome (ARDS). Further, the development of the disease ends in the worsening of the symptoms, and at this factor, the patient is shifted to ICU. Patients with milder symptoms probably have more abdominal pain and lack of appetite [30]. However, the signs in the earlier days of the infection are hard to find out. Symptoms reported ranged from mild to severe disease and death due to confirmed coronavirus disease 2019 [50]. The overview of systemic progression of the COVID-19 symptoms from the early stage to the fatality stage with the recovering phase is summarized in Table 2 .
Fig. 3.
The systematic and respiratory symptoms of COVID-19 infection.
Table 2.
Systematic progression of symptoms in COVID-19 patients and their recovery stages.
| Case Types | Day | Symptoms | Recovery Stages | Source |
|---|---|---|---|---|
| Mild | 1 | Common cold | [30] | |
| 2 | Mild sore throat | [51] | ||
| 3 | Throat pain, Rise in body temperature, vomiting, nausea. | [51] | ||
| 4 | Severe throat pain, weakness, joint pains | [51] | ||
| 5 | Mild fever, dry cough, dyspnoea | [30] | ||
| 6 | Breathing difficulty, Tiredness | Mild Cases Recovery | [52] | |
| Moderate | 7 | Severe coughs, breathing difficulties, high fever, headache, body pain, worsening diarrhoea, and pneumonia. Patients need to admit to the hospital. | [30,51,53] | |
| 8 | Symptoms worsen for patients with pre-existing medical conditions. | [30] | ||
| 9 | Frequent breathing, Average Sepsis infection starts (affects 40% of patients) | [51] | ||
| 10 | Chest Diagnosis for Respiratory Distress | Moderate Cases Recovery | [54] | |
| Severe | 11 | Loss of appetite, abdominal pain | [53] | |
| 12 | Fever relaxes slowly, breathing difficulties ceases. | [52,55] | ||
| 13 | ||||
| 14 | Mouth cough persists, even after hospital discharge. | [52] | ||
| 15 | Possible Cardiac Injury or Kidney Injury | Severe Cases Recovery | [56] | |
| 16 | ||||
| Critical | 17 | Vulnerable patients develop a secondary infection in the lower respiratory tract, ARDS. | [30,54] | |
| 18 | Cases Become Fatal requiring treatment in ICU. | [57] | ||
| 19 | Severe conditions lead to blood coagulation and ischemia. | [54] | ||
| 20 | Requires oxygen therapy and ventilation | [54] | ||
| 21 | Survivors recover Completely but are still contagious and prone. | Critical Cases Recovery | [54] |
3. Diagnosis
Tracking and diagnosing the suspected individual is critical in understanding the epidemiological link, suppressing the chance of transmission, and informing the management of the case. A suspected case of health problems with respiratory problems, fever, sore throat and any chance of contact with the person from the areas of persistent local transmission or patient contact with a similar travel history or those with confirmed COVID-19 infection is likely to be the carrier of the disease [58]. However, some individuals with the condition may also spread the virus without any genuine symptoms (asymptomatic) [25]. Clinical specimens from sputum, nasal secretions, blood, and broncho-alveolar lavage (BAL), collected from suspected patients used for the laboratory diagnosis [59]. There are different ways of diagnosing the disease based on clinical diagnosis, molecular diagnosis, immunodiagnostics, microbial examination, etc. [60]. The following procedures used for the determination of patients with suspected infection: real-time fluorescence (RT-PCR) to detect the positive nucleic acid of SARS-CoV-2 in sputum, throat swabs, and lower respiratory tract sample secretions [61, 62].
Nevertheless, the test's reliability is uncut, as the PCR can only identify the virus if it is present in the suspect's sample. Consequently, output about the infection becomes uncertain, leading to sometimes false-negative results depending upon the concentration and sensitivity [55]. Chest X-Ray may be other diagnostic criteria. C.T. imaging shows ground-glass opacities, bilateral infiltrates, and sub segmental consolidation, even in cases with no clinical evidence of respiratory tract infection (asymptomatic), considering being sensitive and specific diagnostic approach [63]. In cases with the negative molecular test, C.T. imaging has been used to diagnose suspects with COVID-19 infection [64]. Immunodiagnostics is also playing a crucial role in the diagnosis, where the epidemiological link of the suspected individual is found to be useful. Still, then again, the molecular correlation is negative [65]. This tests the presence of antigens (viral proteins) within the COVID-19 patients with the immunoglobulins (IgG and IgM) protein detection reagents and SARS-CoV-2 antigen detection reagents victimization the assay (ELISA) technique, diagnosed by rapid diagnostic test (RDT) from the tract of a respiratory tract of the infected individual [66]. Other lab diagnoses are generally non-specific. The count of white cells is typically normal or low and may lead to lymphocytopenia; serious illness associated with a lymphocyte count <1000 [58].
4. Treatment Strategies
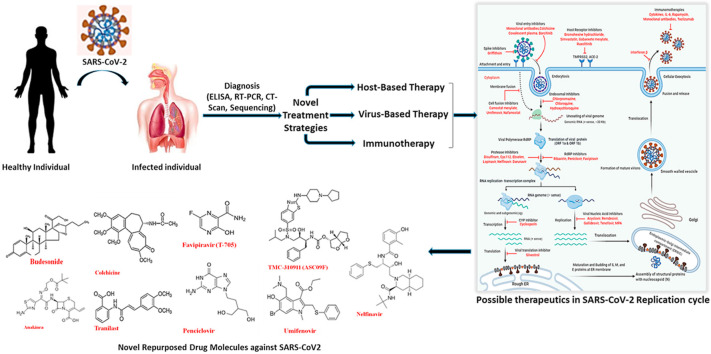
More cases of COVID-2019 are coming up with the passing of each day, and the fear of the novel coronavirus unfortunately has true becoming a pandemic disease. Treatments provide doctors with resources to support the patients to produce a vaccine. Therefore, the development of vaccines at the earliest is of the utmost to curb the disease. Further, to understand the host, genomics, epidemiological links, and transmission modes of nCoV, constant efforts are being made at international, national, and individual levels [67]. Globally, drug manufacturers, research labs, and other organizations are producing several specific kinds of pharmaceutical drugs for COVID-19 therapy. Potential drugs include medications already employed or tested for the diagnosis of certain diseases and recently discovered or specially developed medicines (Fig. 4 ). Still, scientists worldwide are working unceasingly, intending to create an optimal antiviral agent and SARS-CoV-2 vaccine [69]. Yet the screening of new small molecule drugs in combinations and other agents with potent anti-SARS-CoV-2 effects can successfully derive new and better chief compounds and agents, which may be useful in COVID-19 therapy [26]. Several methods of general aspects in discovering antiviral therapy [68], like testing of the broad-spectrum existing antiviral drugs using standard assays [69], screening of existing molecules or libraries including transcription properties in cell lines [70], new drug redevelopment of the genome specificity and biophysical understanding of the human coronavirus pathogen [71] can assess the potential therapy for the human pathogen coronavirus [70]. Further progress of clinical trials worthy of antiviral agents includes lopinavir and ritonavir, ribavirin, interferon α2b, chloroquine phosphate, protease inhibitors, interferon β, and Arbidol. SARS-CoV-2 virions can be inactivated to utilize the cause of neutralizing antibodies; inactivated vaccines could be an approach for the conventional vaccine that can relate through this strategy [26]. Doctors in different medical centers worldwide to manage their cases of COVID-19 are presently using such antiviral drugs either individually or in combinations, but the precise effectiveness remains uncertain. To date, this is still not obvious that a single medication or a mixture of several antiviral agents is sufficient for the care of COVID-19 patients [67]. Studies suggested that the corresponding enzymes drug-binding sites in SARS-CoV-2, SAR-CoV, and MERS-CoV might be similar. Therefore, the drugs used against SARS and MERS at the emergency stage may guide the quick development of specific medications to treat COVID-19 [72].
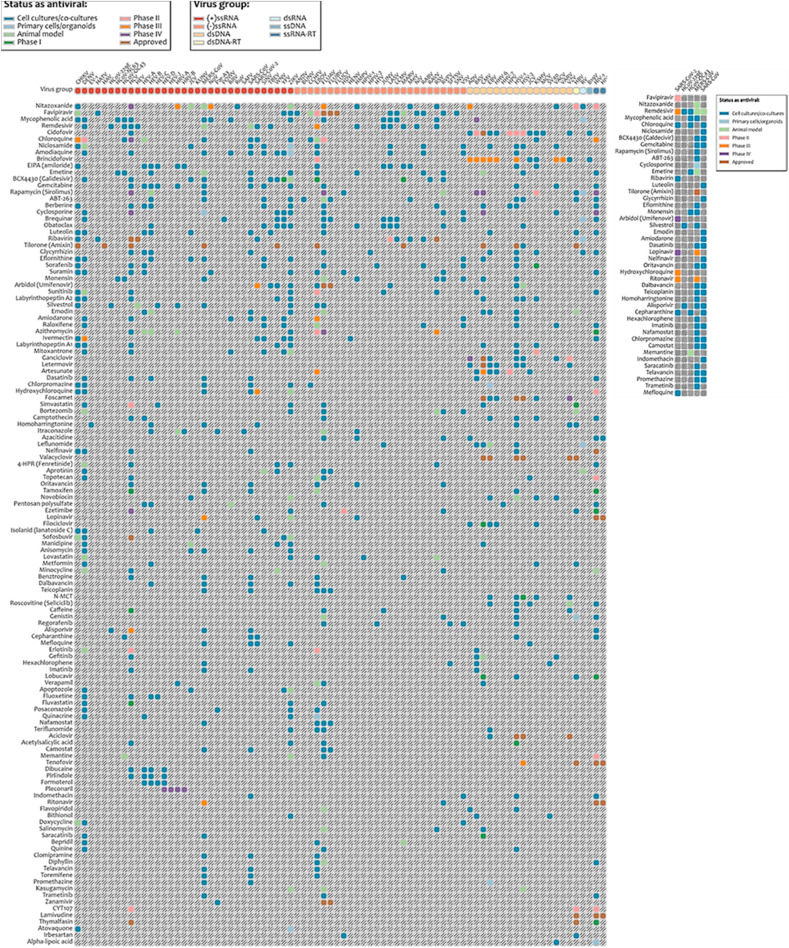
Fig. 4.
A heat-map showing the database of antiviral agents against different categories of viruses clustered in groups from highest to lowest number of targeted viruses (left) (https://drugvirus.info/). Antiviral agents in current trials against different strains of coronavirus (right). Shadings in different colours indicate different statuses of antiviral-agents; ray scale indicates no reported results. Abbreviations: ds-double-stranded; RT-reverse transcriptase; ss-single-stranded.
4.1. Drug repurposing as well as novel drugs treatment strategies
Drug repurposing also known as drug reprofiling or repositioning that assures to recognize antiviral agents for the novel coronavirus disease in a transient fashion. We also propose a perspective that antiviral combinations with a ‘double hit effect’ may present the best possibility of positive result and clinical translatability. Drug repurposing is a budding approach where existing medicines, already tested on safety parameters in humans, are rationalized to fight difficult-to-treat diseases. Such repurposed drugs may conclusively not yield a substantial clinical benefit when used individually but scrupulously combined cocktails could potentially be very effective, as demonstrated for HIV in the 1990s; the pressing challenge now being which combination. Based on the early phase clinical trials testing, broad-spectrum antiviral agents (BSAAs) that have been presumed ‘safe-in-man’ have been vaunted as good drug repurposing candidates [73]. In a highly accessible database of 120 experimental, investigational and approved agents, 31 potential candidates have been summarized for COVID-19 trials [74] (Fig. 5 ). Ideally, taking merit of the promiscuity of viral replicative mechanisms and host interactions, BSAAs target two or more viral families [75]. In the wake of COVID-19 outbreak in December 2019, fistful existing BSAAs have been expeditiously put forward into clinical trials, traversing Phases II though IV. Umifenovir (a membrane fusion inhibitor targeting viral entry) and lopinavir/ritonavir (a drug combination targeting viral protease), both are accepted for the indications of Influenza and HIV. They are presently being contemplated in various combinations in a Phase IV clinical trial for pneumonia related with COVID-19 [NCT04255017]. A novel nucleotide analog prodrug, Remdesivir, is being investigated at Phase III level for mild and moderate SARS-CoV-2 [NCT04252664]. In preclinical studies, remdesivir demonstrated activity against coronaviridae species involved in SARS-CoV and Middle East respiratory syndrome (MERS-CoV) [76]. Prominently, in a randomized, controlled trial for Ebola virus disease, remdesivir displayed an antiviral effect [77]. In accordance with promising in vitro data (NCT04261517), antimalarial hydroxychloroquine, is also being evaluated in combination therapy as Phase III agents for viral pneumonia [NCT04261517]. Chloroquine, demonstrated to have antiviral activity at entry and post-entry stages of the SARS-CoV-2 infection. It can boost the antiviral activity of remdesivir and significantly act as a synergizer of BSAAs [78]. At more initial phases, favipiravir (broad-spectrum inhibitor of viral RNA polymerase) in combination is also on a Phase II clinical trial for novel coronavirus-associated pneumonia [ChiCTR2000029544]. Ultimately, preclinical studies of ribavirin (ribonucleic analog) have demonstrated in vitro activity against SARS-CoV-2 [79].
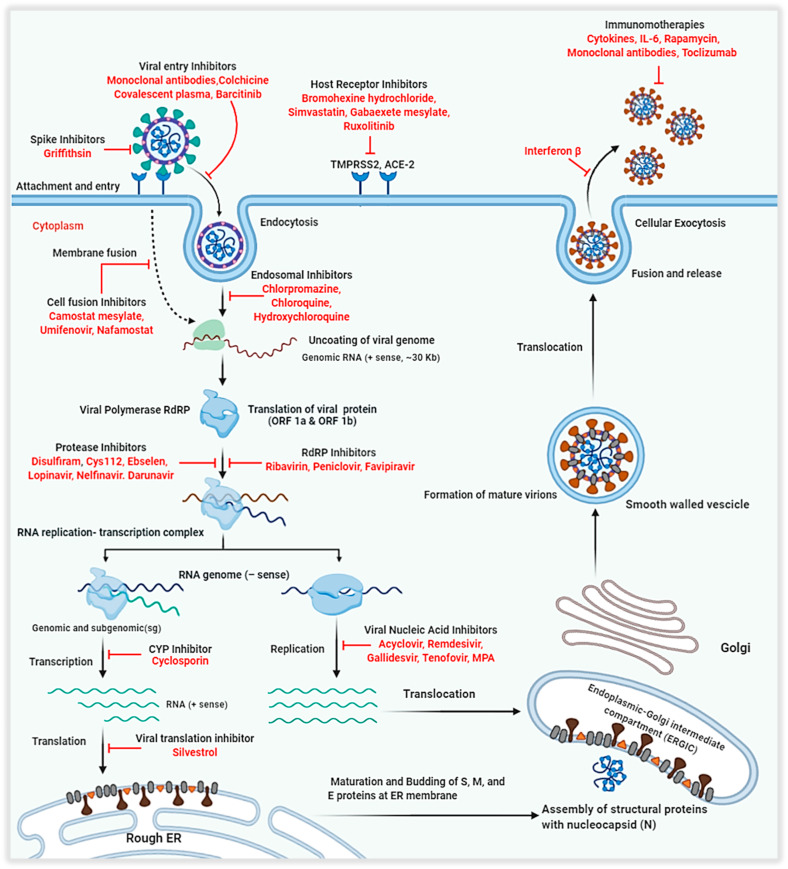
Fig. 5.
Schematic illustration of the coronavirus replication process demonstrating possible therapeutics against multiple virus-based, host-based and immunotherapy goals for repurposing coronavirus drugs used against SARS and MERS. TMPRSS2- Transmembrane protease, serine 2; ACE-2- Angiotensin-converting enzyme 2; RNA-Ribonucleic Acid; IL-6- Interleukin 6; CYP- Cytochrome P450; ORF- Open Reading Frame; RdRP- RNA dependent RNA Polymerase; MPA- Mycophenolic acid.
4.2. BSAA (Broad Spectrum Antiviral Agents) combination therapy
The poor potency of hit compounds as single agents is one of the constraints of phenotypic screens since their maximal tolerated dose is many times sub therapeutic for the new indications being focused [80]. One way to elude this concern is to assess two or more drugs acting on different cellular signalling pathways involving viral replication with limited prolixity. One more approach, which may enable researchers to curb the spectrum of individual antimicrobials for emerging and re-emerging infectious diseases, is high-throughput screening of compound libraries for synergistic combinations at the host–virus interactome level [75,81,82]. These approaches promise to resolve the often-weak activity of BSAAs by enhancing efficacy while strengthening dose mitigation, reducing duration, cost of the drug development pipeline, decreasing toxicity and reducing appearance of secondary resistance. Currently, therapies for patients with SARS-CoV-2 infection primarily repurpose the medicinal medications available are focused on symptomatic symptoms. The medication regimens include ARDS, accompanied by secondary infections, antibiotics, antiviral therapy, systemic corticosteroids, and anti-inflammatory medications [83]. Remdesivir, an anti-Ebola drug, may hold promise is found to be useful as a nucleotide analog in preventing replication of MERS-CoV in monkeys, providing a basis for a rapid test in beneficial aspects of COVID-19 treatment [84]. The treatment therapies applied in the ongoing scenario using antiviral drugs can be categorized into Virus based, Host–based, Immunotherapy and Cellular therapy.
4.3. Virus-based therapy
It utilizes monoclonal antibodies or antiviral peptides that attack specific targets at different stages of viral devices such as enzyme inhibitors, spike glycoprotein, and nucleoside analogues inhibitors [85].
4.3.1. Nucleic acid analog inhibitors
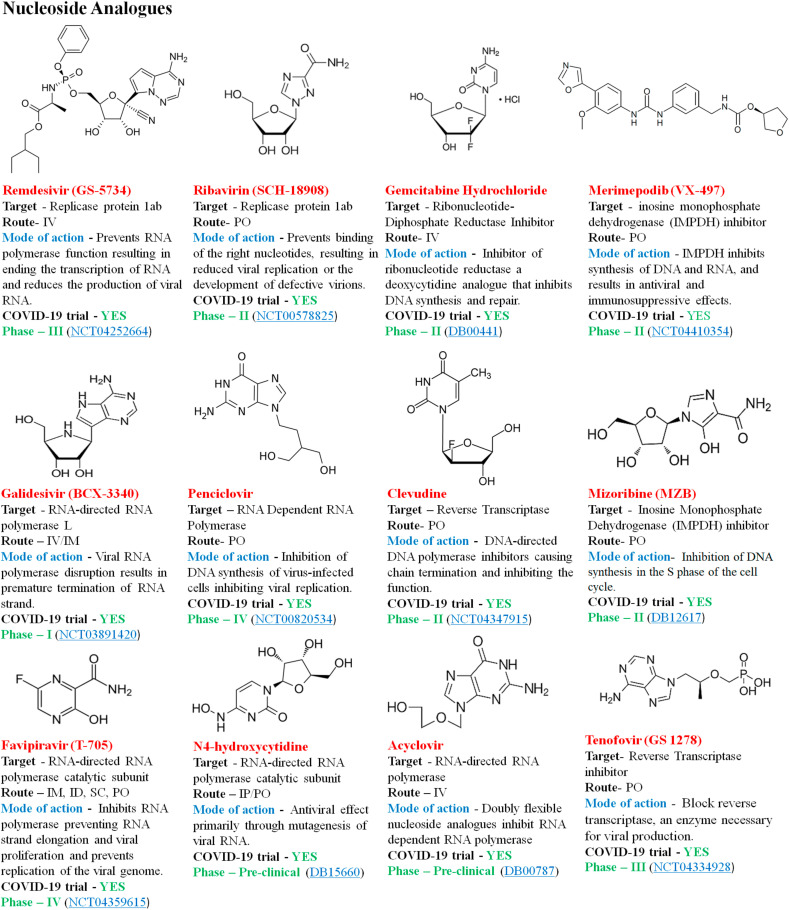
The nucleoside analogues are significant class of antiviral agents that are now widely used in the treatment of numerous types of human viruses. The nucleoside analogues mimic naturally existing nucleosides, which function by terminating the nascent DNA chain [79]. Subsequent incorporation of dinucleotides in the replication and transcription machinery of viral genomes, nucleic acid analogues via such processes change the genetic structure of the virus, resulting in decreased viral fitness with increasing successive replication period may lead to the chain termination [80]. Premature chain termination with the binding of the chain terminator reduces replication fidelity due to the incorporation of mutagens and depletion of clusters of naturally occurring nucleotides. This presents a strong resistance barrier since the structural stability of the viral binding site in the polymerase targets is large among virus families and resistance mutations [86]. The viral RNA dependent RNA polymerase (RdRP) is conserved across the genera, which ranges from the 70–100%, suggesting nucleic acid analogues could be a potent inhibitor of SARS-CoV disease [87]. Several antiviral nucleic acid analogues as evidence to support their effectiveness against SARS-CoV-2 are described (Fig. 6 ).
Fig. 6.
Re-purposed nucleoside analogues drug molecules.
4.3.2. Protease inhibitors
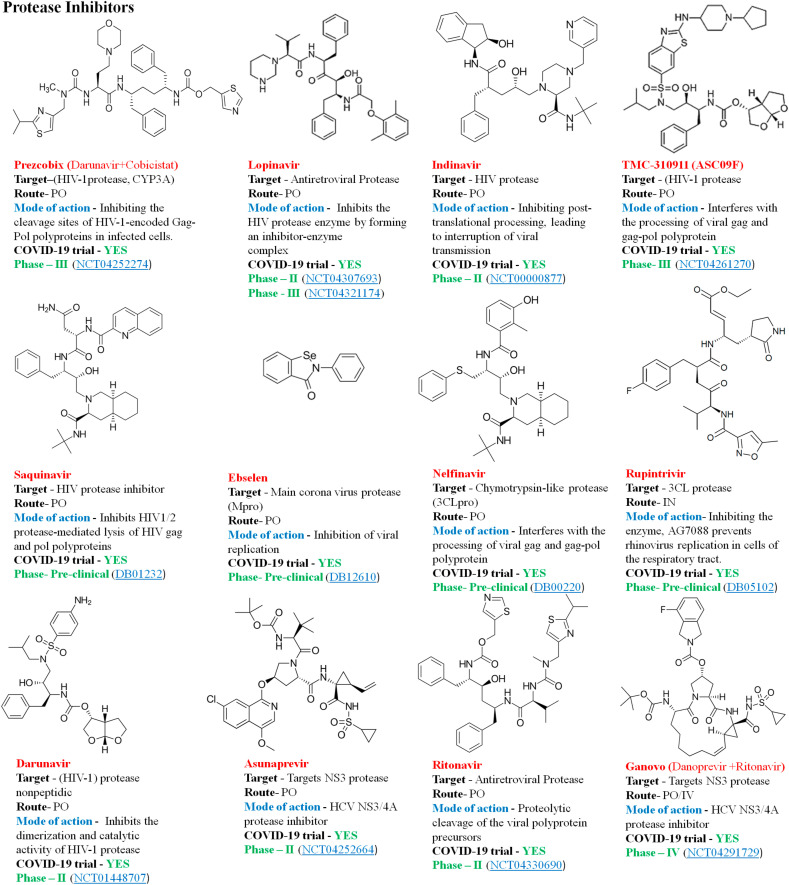
Protease inhibitors are synthetic drugs that block the activity of HIV-1 protease, an enzyme that cleaves precursor proteins into smaller fragments required for viral development, infectivity and replication [71]. Protease inhibitors mediate by binding to the active site of the protease enzyme and block the maturation of the freshly formed virions. They usually target the papain-like proteases (PLpro) and chymotrypsin-like proteases (3CLpro) in the family of coronaviridae cleaving the polyprotein (pp1a and pp1ab) [88,89]. Since both the proteins are essential for virus reproduction and regulation of host cell response, thereby are main targets in the production of antiviral drugs. Coronaviruses PLpro are multifunctional enzymes with protease activity to cycle the viral replicase polyprotein and deubiquitinating activity, which presumed to change the innate immune response to infection [90]. 3CLpro are another protease enzyme that cleaves replicase polyproteins during replication. Studies suggest that the 3CLpro has a homology of 96% between SARS-CoV and SARS-CoV2, with negligible variations between the two proteins. Therefore, the drug inhibitors of the SARS-CoV 3CLpro are likely to inhibit the SARS-CoV2 3CLpro becoming a potential target for the disease [91] Fig. 7 demonstrates some of the common protease inhibitors with the potential activity against SARS-CoV2.
Fig. 7.
Some of the common protease inhibitors.
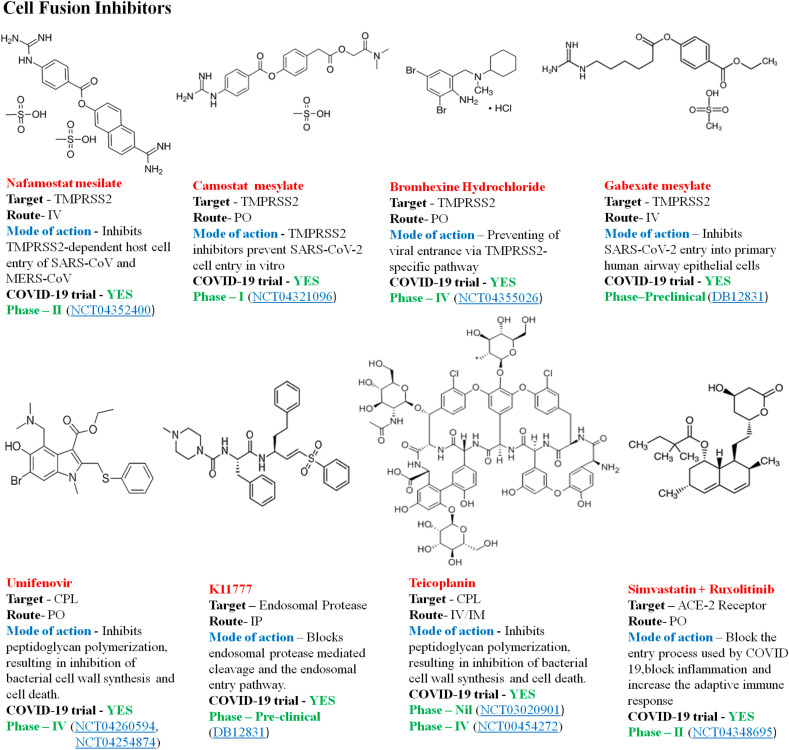
4.3.3. Virus-cell fusion inhibitors
The Spike (S) protein of the SARS-CoV2 exposed to the surface, mediates the entry of the pathogen into the host cells, which is the crucial target of antibodies neutralization upon infection [92]. ACE-2 and TMPRSS2 are the receptors, which mediates the fusion of the S protein of the SARS-CoV2 to the host cell. The fusion of the S protein to the ACE-2 receptor is by cleavage through TMPRSS2, which is essential for viral entry and spread of infection [93]. The majority of coronaviruses enter their target cells via plasma membrane fusion, the endosomal endocytosis through the protease enzyme cathepsin L (CPL) that cleaves the S glycoprotein into two subunits S1 and S2, S2 fusion with the cell membrane is another entry mechanism [94]. Inhibitors of the TMPRSS2 and CPL activity may become a potential antiviral target thereby inhibiting the mechanism of cell-fusion and entry of the SARS-CoV2 into the cells. Fig. 8 illustrates some of the cell fusion antiviral drug molecules against SARS-CoV2.
Fig. 8.
Representative viral cell fusion inhibitors against COVID-19.
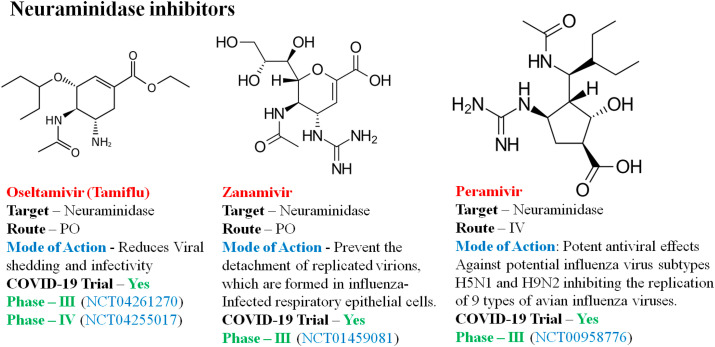
4.3.4. Neuraminidase inhibitors
Neuraminidase inhibitors (NIs) are drug molecules targeting the category of influenza A and B viruses, which are currently in the therapeutic trial of COVID-19. NIs interact with the release of progeny influenza virus from contaminated host cells, a mechanism that protects new host cells from infection and thus avoids the spread of respiratory tract infection [95]. Fig. 9 illustrates the common neuraminidase inhibitors in trial against COVID-19.
Fig. 9.
Representative neuraminidase inhibitors against COVID-19.
4.4. Host-based Therapies
It include the host immune responses and host based drug targets. Immune response improves the effect of interferon reaction in the host factors used by SARS-CoV-2 for replication influencing host-signalling pathways [85]. Drug targets emphasizes on the receptors through which the virus binds to the cellular receptors (ACE-2 and TMPRSS2) on the surface and initiates the infection [91]. This includes the antimalarial drugs and the drugs of Janus-Kinase inhibitors.
4.4.1. Interferons
In clinical studies, synthetic recombinant interferon α has been shown to be successful in treating SARS patients linked with higher oxygen supply and greater detection of radiographic lung opacities than just systemic corticosteroids [71,96]. Relative to the glucocorticoid-treated community alone, the pulmonary X-ray irregular recovery period has shortened by 50% across the interferon-treated population, indicating to be an important MERS-CoV replication inhibitor [97]. Throughout the diagnosis of patients with SARS or MERS, different formulations of interferon alfa or interferon beta and other antivirals such as ribavirin and/or lopinavir – ritonavir is used [98]. Such results indicated interferon could be included in COVID-19 therapy. Table 3 illustrates some of the common interferon therapies in current clinical trials against COVID-19.
Table 3.
Some common interferons with the efficacy in the treatment in COVID-19. IFN- Interferon; IM-Intramuscular; IV-Intravenous; SC-Sub-cutaneous; PO-Oral; PFOR-Pyruvate: ferredoxin oxidoreductase; INH- Inhalation; PAR- Parenteral; IP- Intraperitoneal; IFN-α-Interferon alpha; IFN-β- Interferon beta; poly I:C- Polyinosinic-polycytidylic acid; peg–IFN–λ1a-Peginterferon lambda-1a.
| Compound | Chemical Formula | Target | Route | Mechanism of action | COVID-19 Trial Status |
|---|---|---|---|---|---|
| IFN-β-1a | C908H1408N246O252S7 | IFN-β receptor 2 | SC | Blocking of the key SARS-CoV-1 protease, which results in viral replication inhibition | Phase- 4 (NCT04350671, NCT02735707) |
| IFN-α-2b | C16H17Cl3I2N3NaO5S | IFN-α/β receptor 2 | IM, SC, IV | Inhibition of protein synthesis, inactivation of viral RNA, and enhancement of phagocytic and cytotoxic mechanisms | Phase 1 (NCT04379518) |
| IFN-α-con-1 | C860H1353N227O255S9 | IFN-α/β receptor 1/2 | SC | Induces innate antiviral immune response. | Phase-1 (NCT01227798) |
| peg–IFN–λ1a | – | IFN-α/β receptor | SC, IV | Preventing virus infection by maintaining an antiviral condition | Phase-2 (NCT04331899) |
| Nitazoxanide | C12H9N3O5S | PFOR | PO | Antiprotozoal behaviour by interfering with the electron transfer reaction based on PFOR | Phase-2 (NCT04360356) |
| Novation (Recombinant) | – | IFN-α | INH | Interferon stimulants | Phase-4 (ChiCTR2000029496) |
| IFN-β-1b | C908H1408N246O253S6 | Leukocytes | SC | Modulates immune response by reducing antigen presence and increasing T-cells suppressors | Phase-2 (NCT04350281) |
| Calderon (IFN-α-n3) | – | IFN-α/β receptor 1 | PO, PAR | Immunomodulating cytokine | Phase-2 (NCT00215826) |
| poly I:C | C19H27N7O16P2 | IFN-α | IM, IN, IV, IP | Produces antiviral effects by IFN induction and stimulation of macrophage phagocytosis | Phase- 4 (ChiCTR2000029776) |
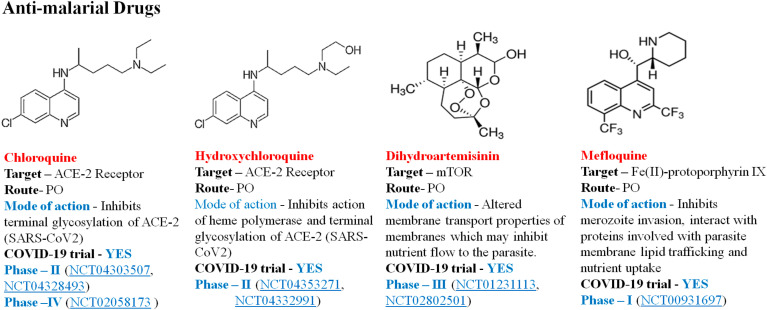
4.4.2. Antimalarial drugs
Chloroquine (CQ) and hydroxychloroquine (HCQ) are aminoquinolines and implemented for over 50 years for the prevention of malaria and autoimmune diseases [99]. It is reported to be successful in COVID-19-associated pneumonia, which prevents pneumonia exacerbation, faster conversion to negative viruses and shortens the period of the disease [100]. The SARS-CoV-2's primary target cells are enterocytes and pneumocytes when it enters into the body, which involves fusion of viral and cell membranes, and it binds to these cells with the aid of spike protein-host protein interaction [101]. Chloroquine prevents contact with the virus by elevating the endosomal pH necessary for virus/cell fusion as well as interacting with the glycosylation of virus cell receptors. Its anti-inflammatory and immunomodulative action may add to COVID-19's effectiveness [102]. Fig. 10 illustrates some antimalarial drugs in clinical trial against COVID-19.
Fig. 10.
Representative antimalarial drugs against COVID-19.
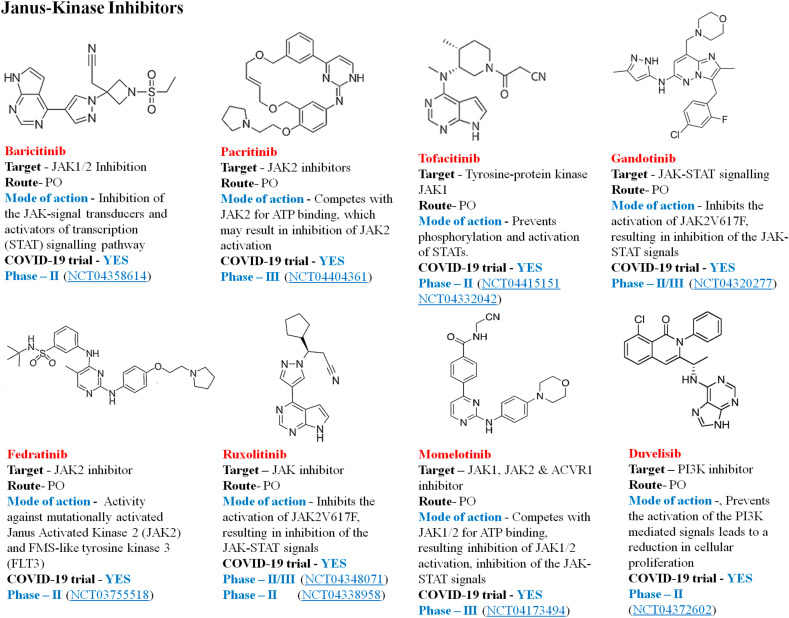
4.4.3. Janus kinase inhibitors
Numb-associated kinase (NAK) target drugs in patients with COVID-19 pneumonia reduce systemic and alveolar inflammation by inhibiting important cytokine signals involved in immune-mediated inflammatory response [103]. Studies define COVID-19's cytokine profile as close to the one of hemophagocytic lymphohistiocytosis (sHLH). IL-2, IL-7, GCSF, INF-gamma, monocyte chemoattractant protein 1 (MCP1), macrophage inflammatory protein-1 (MIP-1) alpha, and TNF-alpha are distinguished by decreased sHLH. Inhibition of JAK may be a therapeutic choice [104]. Baricitinib is a small molecule inhibitor of Janus kinase subtype 1 and 2 (JAK1, JAK2) licensed for the treatment of TNFα-antagonist-resistant rheumatoid arthritis in the EU and USA. Regardless of its dual purpose, Baricitinib was identified as a potentially beneficial agent in COVID-19 patient in vitro anti-inflammatory and antiviral function [105]. Fig. 11 describes some of the JAK inhibitors currently in clinical trial against COVID-19.
Fig. 11.
JAKs/STAT signal inhibitors in current therapeutic trial against COVID-19.
4.5. Immunotherapy
It acts through the mechanism of DNA synthesis interference, pulse depletion of immune cells, sequestration of leukocytes, immunomodulation, cytokine-targeted agents, complement inhibition, and blockage of intracellular signalling pathways, etc. [106].
4.5.1. Immunosuppressive therapies
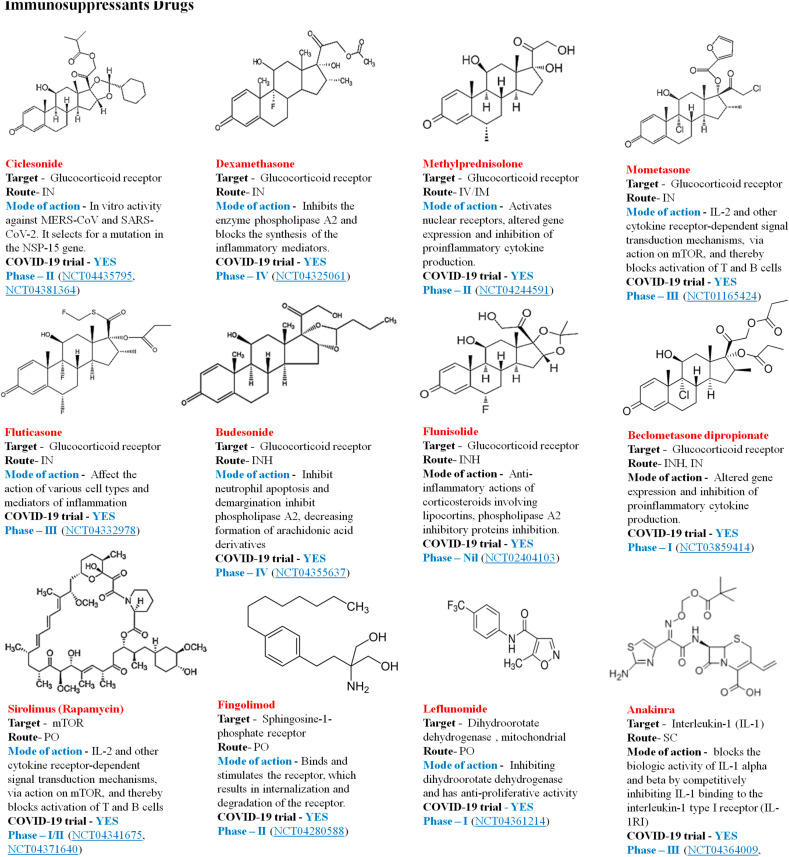
Glucocorticoids possess strong anti-inflammatory and immunosuppressive effects; methylprednisolone is the major medicinal hormone which has a strong impact on the cytokine storm created by badly affected patients COVID-20 [95]. A cytokine profile close to that seen in the macrophage activation syndrome (MAS) identified in a subgroup of COVID-19 patients [71]. Anti-cytokine therapies may be helpful for managing this community of patients with COVID-19 who undergo such a cytokine-storm syndrome. However, selective inhibition of different cytokines during acute respiratory distress syndrome or sepsis can entail risks such as reactivation of viral infections and decreased bacterial infection susceptibility [107]. Host-directed therapies in patients with extreme COVID-19 and recurrent cytokine storms could be successful against SARS-CoV-2 infection [108] Therefore, treatments of immunosuppressant effectively employed against other viruses may be used with COVID-19. Fig. 12 describes the immunosuppressant drugs in clinical trial against COVID -19.
Fig. 12.
Immunosuppressant's in current therapeutic trial against COVID-19.
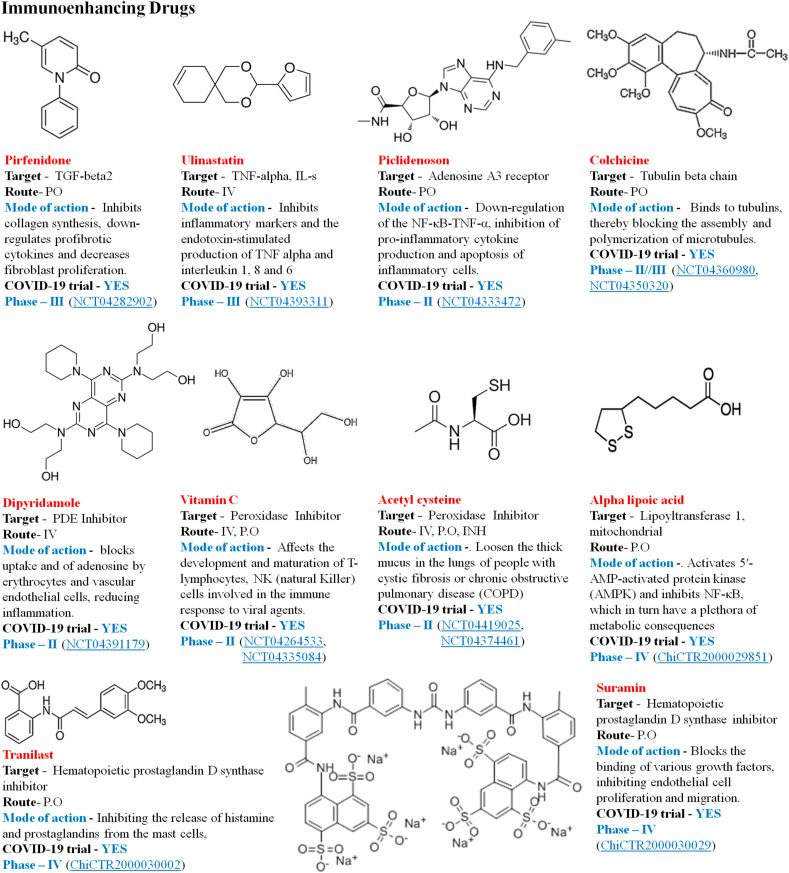
4.5.2. Immunoenhancing therapies
It includes the anti-inflammatory, antioxidant and antibody therapies. The disease's clinical characteristics include overproduction of reactive oxygen species that cause oxidative stress responses and lead to acute damage to the lung, which provides a new approach for the involvement of antioxidant therapy (NCT04466657). Vitamin C helps resist oxidative damage and increases immunity, improve antiviral ability and also avoid and manage acute lung infection and acute respiratory distress induced by certain respiratory viruses [95]. Fig. 13 illustrates some of the antioxidants and anti-inflammatory drugs in current scenario of COVID-19 clinical trials.
Fig. 13.
Antioxidant and anti-inflammatory drugs.
4.5.3. Antibody therapies
Other strategy of the Immunoenhancing therapy includes the plasma therapy (convalescent plasma) and the monoclonal antibody therapy. Studies suggest intervention of plasma therapy and immunoglobulin on patients infected with 2019-nCoV could improve clinical outcome [109]. Plasma therapy is a form of passive immunotherapy, with multivalent treatment wherein, different antibodies collected from recovered individual's
Serum to create convalescent blood products (CBPs), and administered into critically ill patients with SARS-CoV-2 infections [110]. Monoclonal antibody treatment applies to removing B-cells from patients in healing period, which then develop unique antiviral therapy antibodies administered to unrecovered patients. Monoclonal antibodies limit the amount of memory B cells that have neutralizing, and unique consequences produced by cloning the antibody genes [111]. Such antibodies may offer an effective solution for emergency prophylaxis and SARS-CoV-2 treatment, whereas vaccinations and experimental medications are undergoing alternate and more time intensive development [112]. Intravenous immunoglobulin can be the strongest long-term immunomodulator of all ages and can aid inhibit the release of proinflammatory cytokines and improve the production of anti-inflammatory mediators [113]. In fact, thymosin alpha-1 (Ta1) may be an immune aid for patients with SARS, successfully controlling epidemic transmission [114]. Therefore, intravenous immunoglobulin and Ta1 could be employed as COVID-19 therapies (Table 4 ).
Table 4.
Antibody therapies with effectiveness in current trial activity against COVID-19. TNF- α - Tumor Necrosis Factor alpha; VEGF - Vascular Endothelial Growth Factor; IL-6- Interleukin 6; IL-17 –Interleukin 17; PD-L1/L2- Programmed death-ligand 1/ligand 2; CCR5- Chemokine Receptor type 5; GM–CSF–R -Granulocyte-Macrophage Colony-Stimulating Factor receptor; FCGR1A- High Affinity Immunoglobulin Gamma Fc receptor I; IV-Intravenous; SC-Sub-cutaneous; IVIG- Intravenous immunoglobulin.
| Compound | Chemical Formula | Target | Route | Mechanism of action | COVID-19 Trial Status |
|---|---|---|---|---|---|
| Adalimumab | C6428H9912N1694O1987S46 | TNF- α | S·C | Inhibiting the interaction of TNF- α with the cell surface TNF receptors neutralizing TNF- α bioactivity. | Phase- 4 (ChiCTR2000030089) |
| Bevacizumab | C6638H10160N1720O2108S44 | VEGF | I·V | Binds and blocks VEGF | Phase 2 (NCT04275414) |
| Eculizumab | C6442H9910N1694O2034S50 | Complement C5 | I·V | Inhibits terminal complement system including the development of membrane attack complex. | Phase- 2 (NCT04346797, NCT04288713) |
| Sarilumab | C6388H9918N1718O1998S44 | IL-6 receptor | I·V | Binds to receptor variants of IL-6, preventing IL-6 pro- and trans-inflammatory signalling cascades. | Phase-3 (NCT04345289) Phase-2 (NCT04315298) |
| Siltuximab | C6450H9932N1688O2016S50 | IL-6 receptor | I·V | Inhibits attachment to soluble and membrane-bound IL-6 receptors and thereby inhibits lymphocyte proliferation. | Phase-3 (NCT04330638) Completed (NCT04322188) |
| Ixekizumab | C6492H10012N1728O2028S46 | IL-17 receptor | S·C | Inhibit IL-17 A from connecting to receptor by attenuating an interleukin-mediated inflammatory response 17 A | Phase-3 (NCT02757352) |
| Tocilizumab | C6428H9976N1720O2018S42 | IL-6 receptor | I·V | Inhibits signal transduction by binding sIL-6R and mIL-6R | Phase- 2 (NCT04331808) Phase- 3 (NCT04320615) |
| Nivolumab | C6362H9862N1712O1995S42 | PD-L1/L2 receptor | I·V | Binds to PD-1, blocking PD-L1 and PD-L2 from inhibiting T-cell function, returning tumor-specific T-cell response to a patient | Phase- 2 (NCT04343144, NCT04413838) |
| Leronlimab | C6534H10036N1720O2040 S42 | CCR5 | S·C | Binds to several extracellular CCR5 receptor sites, inhibiting HIV from reaching the cell | Phase-2 (NCT04347239) |
| Lenzilumab | C6474H10024N1748O2010S42 | GM–CSF–R | I·V | Neutralizes GM-CSF binding to and blocking GM-CSF binding to its receptor, thus stopping GM–CSF–mediated signalling to myeloid progenitor cells | Phase-3 (NCT04351152) |
| Mavrilimumab | C6706H10438N1762O2104S54 | GM–CSF–R | S·C | Inhibits GM–CSF–R | Phase-2 (NCT04397497, NCT04399980) |
| Gimsilumab | C6726H10428N1764O2184S38 | GM–CSF–R | I·V | Inhibition by targeting GM-CSF itself or by targeting the GM-CSF receptor complex. | Phase-2 (NCT04351243) |
| IVIG | C6332H9826N1692O1980S42 | FCGR1A | IV | Reduce the inflammatory response to extreme SARS-CoV-2 infection, including the existence of autoreactive antibodies targeting cytokines or targeting to certain antibodies' vector domains | Phase-2/3 (NCT04261426) |
4.6. Cellular therapy
It implicates bone marrow mesenchymal stem cell engineering to release therapeutic factors that effectively reduce pulmonary inflammation and edema in ARDS [95]. Cell-based methods, mainly of mesenchymal stem (stromal) cells (MSCs), demonstrated protection and possible efficacy in patients with acute respiratory distress syndrome (ARDS), while not yet thoroughly established with ARDS caused by respiratory virus [115]. Table 5 explains the cellular therapy (stem cells) with the prolonged mechanism of action in different clinical trial against COVID-19 treatment.
Table 5.
Stem Cells Therapy in the effective clinical trial against COVID-19 treatment. TNF- α - Tumor Necrosis Factor alpha; VEGF - Vascular Endothelial Growth Factor; IL-6- Interleukin 6; IL-17 –Interleukin 17; PD-L1/L2- Programmed death-ligand 1/ligand 2; CCR5- Chemokine Receptor type 5; GM–CSF–R -Granulocyte-Macrophage Colony-Stimulating Factor receptor; FCGR1A- High Affinity Immunoglobulin Gamma Fc receptor I; IV-Intravenous; SC-Sub-cutaneous; IVIG- Intravenous immunoglobulin.
| Stem Cells | Source | Route | Mechanism of action | COVID-19 Trial |
|---|---|---|---|---|
| UC-MSCs | Adipose Tissue, Umbilical cord, Placenta | IV | Preventing the stormy release of cytokines by the immune system and promoting endogenous repair by stem cell reparations. | Phase-1 (NCT04333368) |
| NestaCell® | Mesenchymal stem cells | IV | The release of anti-inflammatory, immunomodulatory, anti-fibrogenic and trophic functional biomolecules. | Phase-2 (NCT04315987) |
| Cord Blood Stem cells | Umbilical cord & Placenta | IV, IM | Initiate progenitor cell proliferation and tissue repair characterized by extracellular vesicle (EV) secretions and soluble factors | (NCT04393415) |
| MenSCs | Menstrual blood | IA, IV | Boost myocardial infarction owing to stimulation of lung-embolized cells to secrete anti-inflammatory protein | Phase-Pre-clinical (ChiCTR2000029606) |
| BM-Allo MSC | Bone Marrow | IV, IA, IM, I-OCUL |
Found to have immunomodulatory effects, to reduce ARDS-related lung inflammation. | Phase-1 (NCT04397796) |
| WJ-MSCs | Umbilical cord | IV, IT, IM, IN | Pro-angiogenic activity mediator through the secretion of angiogenin, interleukin-8, protein-1 monocyte chemoattractant, and endothelial growth factor | Phase-1 (NCT04313322, NCT04390152) |
| UC-MSCs | Umbilical cord | IV | Preventing the stormy release of cytokines by the immune system and promoting endogenous repair by stem cell reparations | Phase-2 (NCT04288102) |
| PLX-PAD | Placenta | IM | Modulate the cytokine surge for immune system integrity and reduce tissue harm associated with SARS-CoV-2 ARDS | Phase-2 (NCT04389450) |
| CYNK-001 | Placenta | IV, ITUMOR |
Production of cytolytic perforin and granzyme molecules that may contribute to the killing of viral pathogens contaminated cells | Phase-1/2 (NCT04365101) |
| HB-adMSCs | Adipose Tissue | IV | Cell replacements | Phase-2 (NCT04348435; NCT04362189) |
| CAStem | Embryo | IV | Cellular replacement and organ regeneration | Phase-2 (NCT04331613) |
4.7. Proteasome activator PA28γ-dependent degradation of COVID-19 (nucleocapsid protein)
The SARS-CoV-2 nucleocapsid protein (from now on, alluded to as nCoV N) represents the biggest extent of viral structure proteins and is the most plentiful protein in infection contaminated cells. Its essential capacity is to bundle the viral RNA genome into a ribonucleoprotein complex, the capsid [116]. The nucleocapsid protein encoded by SARS-CoV-2 can go about as a viral inhibitory factor of RNA impedance in cells [117]. Besides, it has been demonstrated that the N protein of SARS-CoV can tweak the host cell hardware and it might serve in a regulatory job during the viral life cycle [118]. Subsequently, the nucleocapsid protein is a pivotal multifunctional protein, engaged with the procedure of infection disease, replication, and bundling [119].
An expanding number of studies have indicated that the proteasome is related with viral disease, and there is proof that HCV core proteins can be corrupted through the PA28γ-20S framework. That is, the nuclear retention and stability of HCV core proteins are controlled by PA28γ-subordinate pathways with the end goal that the pathogenicity of HCV might be accomplished through this pathway [120]. Additionally, the human immunodeficiency infection 1 (HIV-1) Tat protein can repress the peptidase action of the 20S proteasome by contending with the 11 S/PA28 controller (REG) for connecting at the REG/Tat-proteasome-binding (RTP) site and meddling with antigen processing [121]. It has been indicated that the hepatitis B virus X protein-derived polypeptide, cultivating the α4 proteasome subunit restricting motif, debilitates the actuation of 20S proteasomes by PA28 [122]. The coxsackievirus infection can be improved by proteasome activator PA28γ advancing p53 corruption [123], like the system of debasement seen in the HBx infection [124]. Moreover, protein p30 connections with PA28γ may likewise influence ATM capacities and increment cell endurance [125].
On the contrary, PA28γ functions as a co-repressor of HTLV-1 p30 to stifle viral replication and is imperative for the upkeep of control viral latency [126]. Hence, past investigations have indicated that PA28γ is firmly associated with the HTLV-1 virus and performs a permanent role in the development and dissemination of the virus. Furthermore, the capacity of PA28γ to advance viral protein debasement proposes its contribution in viral pathogenesis.
Studies additionally have exhibited that the novel coronavirus nucleocapsid protein (N) shares about 90% amino acid sequence affinity with SARS coronavirus. SARS coronavirus N protein antibodies can cross-respond with novel coronavirus, however, cannot give cross-resistance. Like SARS-CoV, nCoV N proteins can repress RNA impedance (RNAi) to beat the host protection [127]. Early examination uncovered the engagement of the ubiquitin-proteasome framework (UPS) in numerous stages of the coronavirus infection cycle and determined UPS as a prospective drug target to regulate the eminence of coronavirus infection [128].
An ongoing report displayed that a novel ubiquitination-independent pathway could administer the protein levels of nCoV N, and PA28γ could monitor the plethora of nCoV N by managing its endurance. Additionally, study results revealed that PA28γ connects with nCoV N and advances its intracellular degradation. SARS coronavirus nucleocapsid protein is a significant viral structural protein and an essential pointer in early prognosis owing to its multitude and high preservation in cells. Examining nucleocapsid protein structural function, how it takes part in transcription and translation, the molecular mechanism in virus tainted cells, and the management of gene expression will aid to fully comprehend SARS coronaviruses and find compelling strategies for curbing and management of related illnesses [116]. When SARS-CoV-2 attacks the human body, it elicits liaison with human immune cells, thereby enabling the immune cells to generate enormous IFN-γ, and IFN-γ can actuate PA28γ [127]. Therefore, invigorates proteasome action bringing about degradation of the coronavirus N protein. Therefore, virus production is obstructed, and multiplication and dissemination are enormously improved.
Conclusively, PA28γ could intercede with the degradation of nCoV N. These outcomes propose that the PA28γ interplay has a significant role in controlling 20 S proteasome activity and promotes our comprehension of the pathogenesis of 2019-nCoV. Continued work is important to find the precise zone of nCoV N synergy with PA28γ.
5. Progress in the development of vaccine of COVID-19
The emerging COVID-19 pandemic has inspired various medical agencies and companies to design candidates for vaccinations against this new disease. The coming months would undoubtedly see an ever-changing world as multiple applicants for the vaccine pass across the production process. As of June 22, 2020, there are 195 target vaccine candidates in the clinical trial processes across the world, as per the data obtained from Vaccine Center at the London School of Hygiene & Tropical Medicine. These target vaccine candidates categorized into different types, i.e., RNA, DNA, non-replicating viral vectors, replicating viral vectors, inactivated, live attenuated, protein subunit, other/unknown vaccines (Table 6 ).
Table 6.
Pipeline of vaccines in clinical trials for the treatment of COVID-19 across the globe.
| Country | Candidate | Type | Organization | Phase of Development |
|---|---|---|---|---|
| RNA vaccines | ||||
| United States | LUNAR-COV19 | RNA | Arcturus Therapeutics & Duke- NUS | Pre-clinical development |
| Germany, China | BNT162 | RNA | Biotech, Fosun Pharma Pfizer |
Pre-clinical development Phase I/II (NCT04368728) |
| Germany | CVnCoV | RNA | CureVac | Pre-clinical development; Phase I |
| Belgium | mRNA TriMix vaccine | RNA | eTheRNA Immunotherapies | Pre-clinical development |
| United States | mRNA-1273 | RNA | Moderna NIAID |
Phase I (NCT04283461) Phase I/II (800057258) Phase III (700320962) |
| Japan | LNP-encapsulated mRNA vaccine | RNA | University of Tokyo Daiichi-Sankyo |
Pre-clinical development |
| Belgium | ZIP-1642 | RNA | Ziphius Therapeutics Ghent University |
Pre-clinical development |
| United Kingdom | Self-amplifying RNA vaccine | RNA | Imperial College London | Pre-clinical development Phase I |
| Spain | RNA vaccine | RNA | Centro Nacional Biotecnología (CNB–CSIC) | Pre-clinical development |
| China | RNA vaccine (VLP) | RNA | Fudan University/Shanghai Jiao Tong University/RNACure Biopharma (VLP) | Pre-clinical development |
| France | LNP-mRNA vaccine | RNA | Sanofi Pasteur, Translate Bio | Pre-clinical development |
| Russia | RNA vaccine | RNA | BIOCAD | Pre-clinical development |
| China | RNA vaccine | RNA | China CDC, Tongji University Stermina Therapeutics |
Pre-clinical development |
| Thailand, United States |
RNA vaccine | RNA | Chula VRC University of Pennsylvania |
Pre-clinical development |
| United Kingdom, United States | RNA vaccine | RNA | Emergex Vaccines George Mason University |
Pre-clinical development |
| Russia | RNA vaccine | RNA | FSRI SRC VB VECTOR, Rospotrebnadzor, Koltsovo |
Pre-clinical development |
| China | RNA vaccine (RBD) | RNA | Fudan University/Shanghai Jiao Tong University RNACure Biopharma(RBD) | Pre-clinical development |
| Korea | RNA vaccine | RNA | GeneOne Life Science Houston Methodist |
Pre-clinical development |
| United States | RNA vaccine | RNA | Greenlight Biosciences | Pre-clinical development |
| United States | RNA vaccine | RNA | HDT BioCorp | Pre-clinical development |
| Spain | mRNA vaccine | RNA | IDIBAPS-Hospital Clinic (RNA) | Pre-clinical development |
| Germany | RNA vaccine | RNA | Max Planck Institute of Colloids and Interfaces (RNA) | Pre-clinical development |
| United States | RNA vaccine | RNA | RNAimmune Inc. | Pre-clinical development |
| United States | RNA vaccine | RNA | Rochester Clinical Research | Pre-clinical development |
| DNA vaccines | ||||
| United States | DNA vaccine | DNA | Immunomic Therapeutics Epivax, PharmaJet |
Pre-clinical development |
| United States | Fusogenix DNA vaccine | DNA | Entos Pharmaceuticals | Pre-clinical development |
| Korea | GX-19 | DNA | Genexine Consortium | Pre-clinical development |
| United States | INO-4800 | DNA | Inovio Pharmaceuticals | Pre-clinical development; Phase I (NCT04336410) |
| India | DNA-plasmid vaccine | DNA | Zydus Cadila | Pre-clinical development |
| Canada | bac-TRL-Spike | DNA | Symvivo Corporation | Phase I (NCT04334980) |
| Sweden, United Kingdom | DNA vaccine | DNA | Karolinska Institute Cobra Biologics |
Pre-clinical development |
| Japan | DNA vaccine | DNA | Osaka University, AnGes Takara Bio |
Pre-clinical development |
| United Kingdom United States | DNA vaccine | DNA | Scancell University of Nottingham |
Pre-clinical development |
| Italy | DNA vaccine | DNA | Takis Biotech, Evvivax Applied DNA Sciences |
Pre-clinical development |
| United Kingdom | DNA vaccine | DNA | University of Cambridge DIOSynVax |
Pre-clinical development |
| Canada | DNA vaccine | DNA | University of Waterloo | Pre-clinical development |
| Non-replicating viral vector vaccines | ||||
| United States | T-COVID | Non-replicating viral vector | Altimmune | Pre-clinical development |
| United States | AdCOVID | Non-replicating viral vector | Altimmune; The University of Alabama at Birmingham |
Pre-clinical development |
| China | Ad5-nCoV | Non-replicating viral vector | CanSino Biological Inc. Beijing Institute of Biotechnology |
Pre-clinical development Phase I (NCT04313127) Phase II (NCT04341389) |
| Spain | Non-replicating viral vector vaccine | Non-replicating viral vector | CNB–CSIC (viral vector) | Pre-clinical development |
| Germany | MVA-S encoded vaccine | Non-replicating viral vector | DZIF - German Center for Infection Research | Pre-clinical development |
| Russia | Gam-COVID Vaccine | Non-replicating viral vector | Gamaleya Research Institute of Epidemiology and Microbiology | Pre-clinical development Phase I (NCT04436471) Phase II (NCT04437875) |
| China | MVA-VLP vaccine | Non-replicating viral vector | GeoVax & BravoVax | Pre-clinical development |
| United States | Ad5 S (GREVAX) | Non-replicating viral vector | Greffex | Pre-clinical development |
| Spain | MVA-S vaccine | Non-replicating viral vector | IDIBAPS-Hospital Clinic (viral vector) | Pre-clinical development |
| United States | hAd5COVID19-Spike/Nucleocapsid | Non-replicating viral vector | ImmunityBio NantKwest |
Pre-clinical development |
| United States | Ad26 vaccine | Non-replicating viral vector | Johnson & Johnson BARDA |
Pre-clinical development |
| United States | AAVCOVID | Non-replicating viral vector | Massachusetts Eye and Ear General Hospital | Pre-clinical development |
| United Kingdom | OraPro-COVID-19 | Non-replicating viral vector | Stabilitech Biopharma Ltd | Pre-clinical development |
| United States | CORAVAX | Non-replicating viral vector | Thomas Jefferson University | Pre-clinical development |
| China | Adenovirus-vectored vaccine | Non-replicating viral vector | Tsinghua University | Pre-clinical development |
| Canada | Dendritic cell-based vaccine | Non-replicating viral vector | University of Manitoba | Pre-clinical development |
| United Kingdom | AZD1222/ChAdOx1-S | Non-replicating viral vector | University of Oxford AstraZeneca |
Pre-clinical development Phase I (DB15656) Phase II (NCT04324606) |
| Finland | Pan-Corona | Non-replicating viral vector | Valo Therapeutics Ltd | Pre-clinical development |
| United States | Oral recombinant vaccine | Non-replicating viral vector | Vaxart Emergent Biosolutions |
Pre-clinical development |
| Georgia | (PIV5)-based vaccine | Non-replicating viral vector | University of Georgia University of Iowa | Pre-clinical development |
| Replicating viral vector vaccines | ||||
| Russia, United States |
Replicating viral vector vaccine | Replicating viral vector | BIOCAD IEM |
Pre-clinical development |
| India, United States |
CoroFlu (M2SR) | Replicating viral vector | FluGen Bharat Biotech UW-Madison |
Pre-clinical development |
| Russia | Replicating viral vector vaccine | Replicating viral vector | FSRI SRC VB VECTOR, Rospotrebnadzor, Koltsovo |
Pre-clinical development |
| United States, Germany | VSV vector vaccine | Replicating viral vector | IAVI Merck |
Pre-clinical development |
| United States | TNX-1800 | Replicating viral vector | Tonix Pharma/Southern Research | Pre-clinical development |
| Netherlands | NDV-SARS-CoV-2/Spike | Replicating viral vector | Intravacc Wageningen Bioveterinary Research Utrecht University |
Pre-clinical development |
| Belgium | YF17D vector vaccine | Replicating viral vector | KU Leuven | Pre-clinical development |
| United Kingdom | APM vector vaccine | Replicating viral vector | Lancaster University | Pre-clinical development |
| Canada | VSV-S | Replicating viral vector | University of Western Ontario | Pre-clinical development |
| India | Replicating viral vector vaccine | Replicating viral vector | Zydus Cadila | Pre-clinical development |
| Inactivated vaccines | ||||
| China | CpG 1018 | Inactivated | Sinovac/Dynavax | Phase I (700321277) |
| China | SCB-2019 | Inactivated | Clover Biopharmaceuticals AUS Pty Ltd | Pre-clinical development Phase I (NCT04405908) |
| China | Inactivated vaccine | Inactivated | Beijing Minhai Biotechnology Co | Pre-clinical development |
| China | Inactivated vaccine | Inactivated | Institute of Medical Biology, Chinese Academy of Medical Sciences |
Pre-clinical development Phase I/II |
| Japan | Inactivated vaccine | Inactivated | Osaka University BIKEN NIBIOHN |
Pre-clinical development |
| Kazakhstan | Inactivated vaccine | Inactivated | Research Institute for Biological Safety Problems | Pre-clinical development |
| China | Inactivated + CpG 1018 | Inactivated | Valneva/Dynavax | Pre-clinical development |
| Live-attenuated vaccines | ||||
| United States India | Deoptimized live attenuated vaccines |
Live-attenuated | Codagenix Serum Institute of India |
Pre-clinical development |
| Germany | Live attenuated, measles virus | Live-attenuated | DZIF - German Center for Infection Research | Pre-clinical development |
| India, Australia | Deoptimized live attenuated vaccines |
Live-attenuated | Indian Immunologicals Ltd Griffith University |
Pre-clinical development |
| Protein subunit vaccines | ||||
| Japan | Capsid like particle vaccine | Protein subunit | AdaptVac | Pre-clinical development |
| United States | D-Crypt™ | Protein subunit | Akers Biosciences Premas Biotech |
Pre-clinical development |
| China | RBD-Dimer vaccine | Protein subunit | Anhui Zhifei Longcom Biopharmaceutical Institute of Microbiology, Chinese Academy of Sciences |
Pre-clinical development |
| Slovakia | Peptide vaccine | Protein subunit | Axon Neuroscience | Pre-clinical development |
| Thailand | Plant-based (RBD-Fc + Adjuvant) | Protein subunit | Baiya Phytopharm/Chula Vaccine Research Center | Pre-clinical development |
| Italy | OMV-based vaccine | Protein subunit | BiOMVis Srl University of Trento |
Pre-clinical development |
| United States | EPV-CoV-19 | Protein subunit | EpiVax | Pre-clinical development |
| United States | NVX-CoV2373 | Protein subunit | Novavax | Pre-clinical development Phase I (NCT04368988) |
| United States | PittCoVacc | Protein subunit | University of Pittsburgh | Pre-clinical development |
| United States | Linebacker and Equivir | Protein subunit | Impact BioMedical | Pre-clinical development |
| Israel | (IBV) Vaccine | Protein subunit | Migal Galilee Research Institute | Pre-clinical development |
| United States | Ii-Key peptide vaccine | Protein subunit | Generex & EpiVax | Pre-clinical development |
| United States | FlowVax™ | Protein subunit | Flow Pharma | Pre-clinical development |
| Canada | VXL-301 VXL-302 VXL-303 |
Protein subunit | Vaxil BioTherapeutics | Pre-clinical development |
| United States | gp-96 vaccine | Protein subunit | Heat Biologics University Of Miami |
Pre-clinical development |
| Canada | DPX COVID-19 | Protein subunit | IMV Inc. | Pre-clinical development |
| Switzerland | TaliCoVax19 | Protein subunit | InnoMedica | Pre-clinical development |
| China, United Kingdom |
COVID-19 XWG-03 | Protein subunit | Innovax Biotech; Xiamen University; GSK | Pre-clinical development |
| India | Protein subunit (RBD) | Protein subunit | Biological E Ltd* | Pre-clinical development |
| United States | Protein subunit, nanoparticle vaccine | Protein subunit | LakePharma Inc. | Pre-clinical development |
| Netherlands, United States | OMV-peptide vaccine | Protein subunit | Intravacc Epivax |
Pre-clinical development |
| United States | PDS0203 PDS0204 |
Protein subunit | PDS Biotechnology | Pre-clinical development |
| Israel | RBD-based vaccine | Protein subunit | Neovii Tel Aviv University |
Pre-clinical development |
| United States | S–2P protein + CpG 1018 | Protein subunit | Medigen Vaccine Biologics Corporation/NIAID/Dynavax | Pre-clinical development |
| United States | IC-BEVS | Protein subunit | Vabiotech | Pre-clinical development |
| Canada | Adjuvanted microsphere peptide | Protein subunit | VIDO-InterVac University of Saskatchewan |
Pre-clinical development |
| Australia, United States |
Molecular clamp vaccine | Protein subunit | University of Queensland GSK; Dynavax |
Pre-clinical development |
| Japan | VLP recombinant protein vaccine | Protein subunit | Osaka University BIKEN NIBIOHN (subunit) |
Pre-clinical development |
| Other vaccines | ||||
| United States | AVI-205 | Other | AbVision, Inc. | Pre-clinical development |
| United States | AV-COVID-19 | Other | Aivita Biomedical Inc | Pre-clinical development Phase I (NCT04386252) |
| Germany | VLP vaccine | Other | ARTES Biotechnology | Pre-clinical development |
| Australia | ERC SARS-Cov-2 vaccine | Other | ERC Worldwide | Pre-clinical development |
| Canada | Self-assembling vaccine | Other | HaloVax Hoth Therapeutics |
Pre-clinical development |
| United States | V-SARS plasma inactivated | Other | Immunitor Inc | Pre-clinical development Phase I (NCT04380532) |
| United Kingdom | AD Domer VLP vaccine | Other | Imophoron Ltd Bristol University |
Pre-clinical development |
| Sweden | ISR-50 | Other | ISR Immune System Regulation | Pre-clinical development |
| United States | TerraCoV2 | Other | Oragenics | Pre-clinical development |
| China | aAPC vaccine | Other | Shenzhen Geno-Immune Medical Institute (APC) | Pre-clinical development Phase I (NCT04299724) |
| China | LV-SMENP-DC | Other | Shenzhen Geno-Immune Medical Institute (D.C.) | Pre-clinical development Phase I (NCT04276896) |
| United States | 1c-SApNP VLP vaccine | Other | Ufovax | Pre-clinical development |
| United States | rOMV vaccine | Other | Versatope Umass (OMV) |
Pre-clinical development |
| Canada | Nanoparticle vaccine | Other | University of Laval | Pre-clinical development |
6. Future directions
In the present scenario, prompt transmission of SARS-CoV-2 across various nations has linked to fierce illness that make it very serious public health risk. In various controversies of strong researches, officially substantiated effective therapy does not exist at the current level, thereby an urgent need for proven therapy for treatment as well as prophylaxis of COVID-19 patients. Recognizing the global public health threat of zoonotic diseases and to decrease the viral spread, wild animal trafficking must be prohibited with closing of live animal markets trading in wildlife. Further, decontaminating reagents and facilities for routine cleaning of hands should be arranged at the public services. Agents that can possibly serve as an alternative route of transmission like faecal and urine samples should be dealt with physical contact. Travel screenings and other prominent control measures to restrain the further attack of the virus should be implemented at global scale. A considerable number of significant unanswered questions must be addressed. Statistics on the frequency of testing, percentage of cases that turned positive should be checked to aware if the rate remains constant or variable. A small number of pediatric cases have been reported so far; either it accounts for scarcity of testing rates or a typical insufficiency of infection and/or susceptibility in them. How many populations have developed severe disease that have been tested so far, and how many confirmed cases have been found with asymptomatic condition? These central questions would provide a frame of reference to which a precise and rational public health measures would be conducted.
Presently, there is no approved treatment therapy for COVID-19 is available. However, virologists and frontline clinicians have been experimenting with virus based and host-based therapeutics since the outbreaks in the China. After a plethora of research search, we herein mention few pre-existing and novel therapeutic strategies for emerging scientists to discover drugs and/or vaccines to inhibit this viral outbreak that could assist in developing novel therapeutics for COVID-19 treatment.
-
1.
For optimal outcomes, antiviral therapies like remdesivir, lopinavir/ritonavir and umifenovir could be initiated before the viral replication attains its peak level. Ribavarin is generally ineffective as a monotherapy and may be beneficial an add-on therapy.
-
2.
The use of corticosteroids should be limited to indicating comorbidities. Owing to lack of data in COVID-19, IVIG is usually not recommended.
-
3.
Due to the conflicting outcomes in coronavirus studies, the efficacy of interferon is still unclear.
-
4.
Chloroquine and hydroxychloroquine demonstrated in vitro inhibition of SARS-CoV-2, and whether the benefits outweigh the risk of dysrhythmias remain inconclusive.
-
5.
One of the great signs of COVID-19 is the so-called ‘cytokine storm’ due to attack of SARS-Cov-2 in the lungs. A cytokine family member Interleukin-6 (IL-6) inhibitors might be valuable for patients who developed cytokine discharge syndrome. Considering, mesenchymal stem cells (MSCs) therapy could contribute against SARS-CoV-2 viruses attack because of their immune modulatory, anti-inflammatory, and restorative ability linked to their stemness to the arsenal of treatments for COVID-19. For potential clinical recovery, remdesivir might be regarded early in the course of illness expeditiously before disease progression. Before concluding on efficacy, more well-designed RCTs are warranted in COVID-19 therapies.
-
6.
Apart from mediating the virus entry, ACE2 also displays protective role in the pathophysiological process of virus-induced ALI but the sequential role of ACE2 still remains unclear in the whole disease process and high-quality clinical trials and real-world data are potentially required to answer the question.
-
7.
Although, in view of its key role in disease pathogenesis and pathophysiology, ACE2 has inspired comprehensive interests and plan of action targeting ACE2 and its ligand-COVID-19 spike protein; this might render novel method in the prevention and management of COVID-19.
-
8.
Vaccine development in progress by Oxford University and AstraZeneca's lead candidate AZD1222 is the most advanced one among all the samples currently in development is likely to enter the third phase of the trial in the upcoming week. On the other hand, Moderna/NIAID candidate, mRNA-1273 enters the final phase of the trial study in evaluating immunogenicity, dose levels and adverse effects towards the safer efficacy, as previous trials reported safer immune response in all the volunteers tested.
-
9.
Researchers are attempting cellular therapies like monoclonal antibodies (Tocilizumab, Adalimumab, Siltuximab etc.) to create antibody dependent therapies to suppress and/or neutralize SARS-CoV-2. The virus structural and genetic similarity to SARS-CoV may assist in the creation of new approaches for COVID-19 therapy.
-
10.
Numerous epidemiologic associations evidently advocate a rational hint that the MMR vaccine may grant protection to the COVID-19 virus as well. An immediate exploration of utilizing the already available MMR vaccine in controlled studies is urgently required to demonstrate a protective benefit. Epidemiologic research indicates its efficacy equivalent as a COVID-19 vaccine, and this could be set in motion within months, probably saving thousands of lives with an earlier deployment as compared to other vaccines under development.
-
11.
As discussed above, study results have envisaged that the ubiquitin-proteasome framework performs a crucial role during several stages of the coronavirus disease. N protein of SARS-CoV-2 could be debased by PA28γ in vitro. This may show that PA28γ is a controller for SARS-CoV-2 N protein debasement. This stipulates some insight for understanding the earlier debatable physiological function of the proteasome-dependent debasement of the SARS-CoV-2 N protein during pathogenesis of COVID-19. Understanding the meticulous role of PA28γ may give us new shrewdness into virus-cell interplay and lead to a more noteworthy comprehension of the pathogenicity of 2019-nCoV infection.
-
12.
Scientist should also work on investigating molecules that may target autophagy. Blocking of autophagy inhibits virus entry into cells, however, antigen presentation by macrophages blocks the activation of adaptive immunity on T cells as well as B cells. Consequently, the autophagy inhibitors would be best administered after achieving the adaptive immune response against the COVID-19, which often takes almost 5–6 days after the onset of disease.
-
13.
Scientist should target human proteases involved in activation “Viral Spike protein”. They may be transmembrane proteases, secreted proteases and intracellular endoplasmic reticulum (ER) proteases. Although the researches are conducting on exploration of human proteases that might activate and cleave SARS-CoV-2 Spike protein, the intracellular ER proteases can continue to activate Spike protein and induce viral membrane fusion after initial endocytosis activated by extracellular proteases. Few drugs having ability of blocking extracellular proteases (For instance; camostat, nafamostat, and cobicistat) are now in clinical trials.
7. Conclusion
The COVID-19 pandemic has emerged as the biggest public health threat with a potential to end up rivalling the 1918 influenza flu. This crisis has brought unprecedented challenges in the management of those who are afflicted, by overwhelming healthcare systems and causing great stress to the healthcare workforce. During such crises, generation of timely evidence for treatment options is crucial. Taking into consideration that in absence of any effective action and lack of effective therapeutic approach along with inadequate wide implementation of social distancing, the situation will remain same until 2022 with 90% of the global population affected with COVID-19 and evident mortality in over 40 million people. Consequently, it is rational to maintain methods and public health measures until potent and effective drugs/vaccines are discovered. An appropriate immunomodulatory diet, proper mental support, adherence to standards and combinatorial therapies will be effective in the long run against COVID-19. Moreover, future viral outbreaks resulting from pathogens of zoonotic origin are likely to continue, hence, comprehensive measures must be devised apart from curbing this outbreak. Special emphasis and endeavor to shield and decrease transmission should be employed in sensitive populations including children, health care providers, and elderly people. Owing to the weak immune system which permits rapid progression of viral infection, early mortality cases of COVID-19 outbreak were prominently seen in elderly people, Taking into account the rising incidence of CoV emergence in livestock animal populations and the identification of novel CoVs in reservoir species proves the high vulnerability of CoVs beyond public-health intervention strategies. Consequently, the design and development of vaccines for SARS-CoV-2 is equally crucial in addition to developing new drugs and clinical trials of old drugs. Experience from SARS-CoV and MERS-CoV indicates for significant emphasis on establishing animal models which can summarize various aspects of human disease and determinants of vaccine safety and efficacy.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cbi.2020.109298.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Guan W., Ni Z., Hu Y., Liang W., Ou C., He J., Zhong N. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. https://www.nejm.org/doi/10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adhikari S.P., Meng S., Wu Y., Mao Y., Ye R., Wang Q.…Zhou H. Epidemiology, causes, clinical manifestation and diagnosis, prevention, and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infect. Dis. Pover. 2020;9(1):2–12. doi: 10.1186/s40249-020-00646-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li H., Liu S.M., Yu X.H., Tang S.L., Tang C.K. Coronavirus disease 2019 (COVID-19): current status and future perspectives. Int. J. Antimicrob. Agents. 2020;55(5):105951. doi: 10.1016/j.ijantimicag.2020.105951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahn D.G., Shin H.J., Kim M.H., Lee S., Kim H.S., Myoung J., Kim B.T., Kim S.J. Current status of epidemiology, diagnosis, therapeutics, and vaccines for novel coronavirus disease 2019 (COVID-19) J. Microbiol. Biotechnol. 2020;30(3):313–324. doi: 10.4014/jmb.2003.03011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bosch B.J., Zee R.V., Haan C.A., Rottier P.J. The coronavirus spike protein is a class I virus fusion protein: structural and functional characterization of the fusion core complex. J. Virol. 2003;77(16):8801–8811. doi: 10.1128/jvi.77.16.8801-8811.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cui J., Li F., Shi Z. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019;17(3):181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiss S.R., Leibowitz J.L. Coronavirus pathogenesis. Adv. Virus Res. 2011;81:85–164. doi: 10.1016/b978-0-12-385885-6.00009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geller C., Varbanov M., Duval R. Human coronaviruses: insights into environmental resistance and its influence on the development of new antiseptic strategies. Viruses. 2012;4(11):3044–3068. doi: 10.3390/v4113044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W., China Novel Coronavirus Investigating. Research Team A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D., Fouchier R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012;367(19):1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 11.De Groot Raoul, Baker Susan, Baric Ralph, Enjuanes Luis, Gorbalenya Alexander, Holmes Kathryn, Perlman Stanley, Poon Leo, Rottier Peter, Talbot Pierre, Woo Patrick, Ziebuhr John. Elsevier; Oxford: 2012. Coronaviridae. Virus Taxonomy: Classification and Nomenclature of Viruses; pp. 806–828. [Google Scholar]
- 12.Shereen M.A., Khan S., Kazmi A., Bashir N., Siddique R. COVID-19 infection: origin, transmission, and characteristics of human coronaviruses. J. Adv. Res. 2020;16(24):91–98. doi: 10.1016/j.jare.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng V.C., Lau S.K., Woo P.C., Yuen K.Y. Severe acute respiratory syndrome coronavirus as an agent of emerging and reemerging infection. Clin. Microbiol. Rev. 2007;20(4):660–694. doi: 10.1128/CMR.00023-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhong N.S., Zheng B.J., Li Y.M., Poon Xie Z.H., Chan K.H., Li P.H., Tan S.Y., Chang Q., Xie J.P., Liu X.Q., Xu J., Li D.X., Yuen K.Y., Peiris, Guan Y. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People's Republic of China, in February 2003. Lancet. 2003;362(9393):1353–1358. doi: 10.1016/s0140-6736(03)14630-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu D., Wu T., Liu Q., Yang Z. vol. 94. 2020. The SARS-CoV-2 Outbreak: what We Know. International Journal of Infectious Diseases. 44-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo Y.R., Cao Q.D., Hong Z.S., Tan Y.Y., Chen S.D., Jin H.J., Tan K.S., Wang D.Y., Yan Y. The origin, transmission, and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Milit. Med. Res. 2020;7(1):11. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ji W., Wang W., Zhao X., Zai J., Li X. Cross‐species transmission of the newly identified coronavirus 2019‐nCoV. J.Med. Virol. 2020;92(4):433–440. doi: 10.1002/jmv.25682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng Z.J., Shan J. 2019 Novel coronavirus: where we are and what we know. Infection. 2020;48(2):155–163. doi: 10.1007/s15010-020-01401-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lam T.T., Jia N., Zhang Y.W., Shum M.H., Jiang J.F., Zhu H.C., Tong Y.G., Shi Y.X., Ni X.B., Liao Y.S., Li W.J., Jiang B.G., Wei W., Yuan T.T., Zheng K., Cui X.M., Li J., Pei G.Q., Qiang X., Cheung W.Y., Cao W.C. Identifying SARS-CoV-2-related coronaviruses in Malayan pangolins. Nature. 2020 doi: 10.1038/s41586-020-2169-0. [DOI] [PubMed] [Google Scholar]
- 20.Corman V.M., Muth D., Niemeyer D., Drosten C. Hosts and sources of endemic human coronaviruses. Adv. Virus Res. 2018:163–188. doi: 10.1016/bs.aivir.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhong J., Tang J., Ye C., Dong L. The immunology of COVID-19: is immune modulation an option for treatment? Lancet Rheumatol. 2020;2(7):28–36. doi: 10.1016/S2665-9913(20)30120-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seto W.H., Tsang D., Yung R.W., Ching T.Y., Ng T.K., Ho M., Ho L.M., Peiris J.S. Effectiveness of precautions against droplets and contact in preventing nosocomial transmission of severe acute respiratory syndrome (SARS) Lancet. 2003;361:1519–1520. doi: 10.1016/s0140-6736(03)13168-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loon S.C., Teoh S.C., Oon L.L., Se-Thoe S.Y., Ling A.E., Leo Y.S., Leong H.N. The severe acute respiratory syndrome coronavirus in tears. Br. J. Ophthalmol. 2004;88(7):861–863. doi: 10.1136/bjo.2003.035931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peiris J.S., Guan Y., Yuen K.Y. Severe acute respiratory syndrome. Nat. Med. 2004;10:588–597. doi: 10.1038/nm1143. (S12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chin A., Chu J., Perera M., Hui K., Yen H., Chan M., Peiris M., Poon L. Stability of SARS-CoV-2 in different environmental conditions. Lanc. Mic. 2020;1:10. doi: 10.1016/S2666-5247(20)30003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yuen K.S., Ye Z.W., Fung S.Y., Chan C.P., Jin D.Y. SARS-CoV-2 and COVID-19: the most important research questions. Cell Biosci. 2020;10:40. doi: 10.1186/s13578-020-00404-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han H., Men K., Wang X., Li Y., Zhang G., Hu J., Gao Y. Estimate the incubation period of coronavirus 2019 (COVID-19) Med. 2020 doi: 10.1016/j.compbiomed.2023.106794. https://doi:10.1101/2020.02.24.20027474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lei S., Jiang F., Su W., Chen C., Chen J., Mei W., Zhan L.Y., Jia Y., Zhang L., Liu D., Xia Z.Y., Xia Z. Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID-19 infection. Clin. Med. 2020;21:100331. doi: 10.1016/j.eclinm.2020.100331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walls A.C., Tortorici M.A., Snijder J., Xiong X., Bosch B., Rey F.A., Veesler D. Tectonic conformational changes of a coronavirus spike glycoprotein promote membrane fusion. Proc. Natl. Acad. Sci. Unit. States Am. 2017;114(42):11157–11162. doi: 10.1073/pnas.1708727114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahmad S., Hafeez A., Siddiqui S.A., Ahmad M., Mishra S. A review of COVID-19 (coronavirus disease- 2019) diagnosis, treatments, and prevention. EJMO. 2020;4(2):116–125. https://DOI:10.14744/ejmo.2020.90853 [Google Scholar]
- 31.Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020;109 doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by the novel coronavirus from wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J. Virol. 2020;94(7) doi: 10.1128/JVI.00127-20. 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jaimes J.A., Millet J.K., Stout A.E., André N.M., Whittaker G.R. A tale of two viruses: the distinct spike glycoproteins of feline coronaviruses. Viruses. 2020;12(1):83. doi: 10.3390/v12010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoffmann M., Kleine-Weber H., Krüger N., Müller M., Drosten C., Pöhlmann S. The novel coronavirus 2019 (2019-nCoV) uses the SARS-coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry into target cells. BioRx. 2020 doi: 10.1101/2020.01.31.929042. [DOI] [Google Scholar]
- 35.Turner A.J., Hiscox J.A., Hooper N.M. ACE2: from vasopeptidase to SARS virus receptor. Trends Pharmacol. Sci. 2004;25(6):291–294. doi: 10.1016/j.tips.2004.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shulla A., Heald-Sargent T., Subramanya G., Zhao J., Perlman S., Gallagher T. A transmembrane serine protease is linked to the severe acute respiratory syndrome coronavirus receptor and activates virus entry. J. Virol. 2010;85(2):873–882. doi: 10.1128/jvi.02062-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Donoghue M., Hsieh F., Baronas E., Godbout K., Gosselin M., Stagliano N.…Acton S. A novel angiotensin-converting enzyme–related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ. Res. 2000;87(5):1–9. doi: 10.1161/01.RES.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 38.Raizada M.K., Ferreira A.J. ACE2: a new target for cardiovascular diseases therapeutics. J. Cardiovasc. Pharmacol. 2007;50(2):112–119. doi: 10.1097/fjc.0b013e3180986219. [DOI] [PubMed] [Google Scholar]
- 39.Yan R., Zhang Y., Li Y., Xia L., Guo Y., Zhou Q. Structural basis for the recognition of SARSCoV-2 by full-length human ACE2. Science. 2020;367(6485):1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Driggin E., Madhavan M.V., Bikdeli B., Chuich T., Laracy J., Biondi-Zoccai G., Parikh S.A. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J. Am. Coll. Cardiol. 2020;75(18):2352–2371. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nguyen A.A., Habiballah S.B., Platt C.D., Geha R.S., Chou J.S., McDonald D.R. Immunoglobulins in the treatment of COVID-19 infection: proceed with caution! Clin. Immunol. 2020;216:108459. doi: 10.1016/j.clim.2020.108459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Z., Wu M., Guo J., Yao J., Liao X., Song S., Han M., Li J., Duan G., Zhou Y., Wu X., Zhou Z., Wang T., Hu M., Chen X., Fu Y., Lei C., Dong H., Zhou Y., Jia H., Chen X., Yan J. Caution on kidney dysfunctions of 2019-nCoV patients. Med. 2020 doi: 10.1101/2020.02.08.20021212. [DOI] [Google Scholar]
- 43.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L., Tai Y., Bai C., Gao T., Song J., Xia P., Dong J., Zhao J., Wang F.S. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. The Lancet. Respir. Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Argyropoulos K.V., Serrano A., Hu J., Black M., Feng X., Shen G., Call M., Kim M.J., Lytle A., Belovarac B., Vougiouklakis T., Lin L.H., Moran U., Heguy A., Troxel A., Snuderl M., Osman I., Cotzia P., Jour G. Association of initial viral load in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) patients with outcome and symptoms. Am. J. Pathol. 2020;190(9):1881–1887. doi: 10.1016/j.ajpath.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lauer S.A., Grantz K.H., Bi Q., Jones F.K., Zheng Q., Meredith H.R.…Lessler J. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann. Intern. Med. 2020;172(9):577–582. doi: 10.7326/m20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Siordia J.A., Jr. Epidemiology and clinical features of COVID-19: a review of current literature. J. Clin. Virol. 2020;127:104357. doi: 10.1016/j.jcv.2020.104357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang W., Tang J., Wei F. An updated understanding of the outbreak of 2019 novel coronavirus (2019-nCoV) in Wuhan, China. J. Med. Virol. 2020;92(4):441–447. doi: 10.1002/jmv.25689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carlos W.G., Dela Cruz C.S., Cao B., Pasnick S., Jamil S. Novel wuhan (2019-nCoV) coronavirus. Am. J. Respir. Crit. Care Med. 2020;201(4) doi: 10.1164/rccm.2014P7. P7–P8. [DOI] [PubMed] [Google Scholar]
- 49.Ren L., Wang Y., Wu Z., Xiang Z., Guo L., Xu T.…Wang J. Identification of a novel coronavirus causing severe pneumonia in humans. Chinese Med J. 2020;133(9):1015–1024. doi: 10.1097/cm9.0000000000000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Verity R., Okell L.C., Dorigatti I., Winskill P., Whittaker C., Imai N., Ferguson N.M. The Lancet Infectious Diseases. 2020. Estimates of the severity of coronavirus disease 2019: a model-based analysis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.World Health Organization . WHO; Geneva: 2020. Report of the WHO – China Joint Mission on Coronavirus Disease 2019. COVID-19. [Google Scholar]
- 52.Centers for Disease Control and Prevention . 2010. Coronavirus Disease 2019.https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html [Google Scholar]
- 53.Gu J., Han B., Wang J. COVID-19: gastrointestinal manifestations and potential fecal–oral transmission. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ñamendys-Silva S.A. Respiratory support for patients with COVID-19 infection. The Lancet. Respir. Med. 2020;8(4):e18. doi: 10.1016/S2213-2600(20)30110-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pang J., Wang M.X., Ang I., Tan S., Lewis R.F., Chen J.I., Gutierrez R.A., Gwee S., Chua P., Yang Q., Ng X.Y., Yap R.K., Tan H.Y., Teo Y.Y., Tan C.C., Cook A.R., Yap J.C., Hsu L.Y. Potential rapid diagnostics, vaccine, and therapeutics for 2019 novel coronavirus (2019-nCoV): a systematic review. J. Clin. Med. 2020;9(3):623. doi: 10.3390/jcm9030623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang N., Wang L., Deng X., Liang R., Su M., He C., Jiang S. Recent advances in the detection of respiratory virus infection in humans. J. Med. Virol. 2020;92:408–417. doi: 10.1002/jmv.25674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hartman M.E., Hernandez R.A., Patel K., Wagner T.E., Trinh T., Lipke A.B., Yim E.T., Pulido J.N., Pagel J.M., Youssef S.J., Mignone J.L. COVID-19 respiratory failure: targeting inflammation on VV-ECMO support. Am. Soc. Art. Int. Org. 2020;66(6):603–606. doi: 10.1097/MAT.0000000000001177. 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Singhal T. A review of coronavirus disease-2019 (COVID-19) Indian J. Pediatr. 2020;87(4):281–286. doi: 10.1007/s12098-020-03263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kannan S., Shaik Syed Ali P., Sheeza A., Hemalatha K. COVID-19 (Novel Coronavirus, 2019) recent trends. Eur. Rev. Med. Pharmacol. Sci. 2020;24(4):2006–2011. doi: 10.26355/eurrev_202002_20378. [DOI] [PubMed] [Google Scholar]
- 60.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., Ren R., Leung K., Lau E., Wong J.Y., Xing X., Xiang N., Wu Y., Li C., Chen Q., Li D., Liu T., Zhao J., Liu M., Tu W., Feng Z. Early transmission dynamics in wuhan, China, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Drosten C., Günther S., Preiser W., Werf S.V., Brodt H., Becker S.…Doerr H.W. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348(20):1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 62.Li T., Wei C., Li W., Hongwei F., Shi J. Peking union medical college hospital's "new coronavirus infected pneumonia" diagnosis and treatment program (V2.0) Med. J. Pek. Uni. Med. Coll. Hos. 2020 http://kns.cnki.net/kcms/detail/11.5882.r.20200130.1430.002.html [Google Scholar]
- 63.Ooi G.C., Khong P.L., Müller N.L., Yiu W.C., Zhou L.J., Ho J.C., Lam B., Nicolaou S., Tsang K.W. Severe acute respiratory syndrome: temporal lung changes at thin-section CT in 30 patients. Radiology. 2004;230(3):836–844. doi: 10.1148/radiol.2303030853. [DOI] [PubMed] [Google Scholar]
- 64.Huang P., Liu T., Huang L., Liu H., Lei M., Xu W., Liu B. Use of chest CT in combination with negative RT-PCR assay for the 2019 novel coronavirus but high clinical suspicion. Radiology. 2020;295(1):22–23. doi: 10.1148/radiol.2020200330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Meyer B., Drosten C., Müller M.A. Serological assays for emerging coronaviruses: challenges and pitfalls. Virus Res. 2014;194:175–183. doi: 10.1016/j.virusres.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang P., Wang X. COVID-19: a new challenge for human beings. Cell. Mol. Immunol. 2020;17(5):555–557. doi: 10.1038/s41423-020-0407-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sahu K.K., Mishra A.K., Lal A. COVID-2019: update on epidemiology, disease spread, and management. Monaldi Arch. Chest Dis. 2020;90(1):197–205. doi: 10.4081/monaldi.2020.1292. [DOI] [PubMed] [Google Scholar]
- 68.Lu H., Stratton C.W., Tang Y.W. Outbreak of pneumonia of unknown etiology in Wuhan, China: the mystery and the miracle. J. Med. Virol. 2020;92(4):401–402. doi: 10.1002/jmv.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Channappanavar R., Fett C., Mack M., Eyck P.P., Meyerholz D.K., Perlman S. Sex-based differences in susceptibility to severe acute respiratory syndrome coronavirus infection. J. Immunol. 2017;198(10):4046–4053. doi: 10.4049/jimmunol.1601896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim Y., Liu H., Kankanamalage A.C.G., Weerasekara S., Hua D.H., Groutas W.C. Correction: reversal of the progression of fatal coronavirus infection in cats by a broad-spectrum coronavirus protease inhibitor. PLoS Pathog. 2016;12(5) doi: 10.1371/journal.ppat.1005650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zumla A., Chan J.F., Azhar E.I., Hui D.S., Yuen K.Y. Coronaviruses - drug discovery and therapeutic options. Nature reviews. Drug. Disc. 2016;15(5):327–347. doi: 10.1038/nrd.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Morse J.S., Lalonde T., Xu S., Liu W.R. Learning from the past: possible urgent prevention and treatment options for severe acute respiratory infections caused by 2019-nCoV. Chembiochem: Europ. J. Chem. Biol. 2020;21(5):730–738. doi: 10.1002/cbic.202000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Broad-spectrum antiviral agents (BSAAs) and viruses they inhibit. Drug Virus. 2020 https://drugvirus.info/ [Google Scholar]
- 74.Andersen P.I., Ianevski A., Lysvand H., Vitkauskiene A., Oksenych V., Bjørås M., Telling K., Lutsar I., Dumpis U., Irie Y., Tenson T., Kantele A., Kainov D.E. Discovery and development of safely- man broad-spectrum antiviral agents. Int. J. Infect. Dis.: Pub. Int. Soc. Infect. Dis. 2020;93:268–276. doi: 10.1016/j.ijid.2020.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bösl K., Ianevski A., Than T.T., Andersen P.I., Kuivanen S., Teppor M., Zusinaite E., Dumpis U., Vitkauskiene A., Cox R.J., Kallio-Kokko H., Bergqvist A., Tenson T., Merits A., Oksenych V., Bjørås M., Anthonsen M.W., Shum D., Kaarbø M., Vapalahti O., Kandasamy R.K. Common nodes of virus-host interaction revealed through an integrated network analysis. Front. Immunol. 2019;10:2186. doi: 10.3389/fimmu.2019.02186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gordon C.J., Tchesnokov E.P., Feng J.Y., Porter D.P., Götte M. The antiviral compound remdesivir potently inhibits RNA-dependent RNA polymerase from Middle East respiratory syndrome coronavirus. J. Biol. Chem. 2020 doi: 10.1074/jbc.ac120.013056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mulangu S., Dodd L.E., Davey R.T., Jr., Tshiani Mbaya O., Proschan M., Mukadi D., Lusakibanza Manzo M., Nzolo D., Tshomba Oloma A., Ibanda A., Ali R., Coulibaly S., Levine A.C., Grais R., Diaz J., Lane H.C., Muyembe-Tamfum J.J., PALM Writing Group. Sivahera B., Camara M.…PALM Consortium Study Team A randomized, controlled trial of Ebola virus disease therapeutics. N. Engl. J. Med. 2019;381(24):2293–2303. doi: 10.1056/NEJMoa1910993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.LiverTox . National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda (MD): 2012. Clinical and Research Information on Drug-Induced Liver Injury.https://www.ncbi.nlm.nih.gov/books/NBK548938/ Nucleoside Analogues. [PubMed] [Google Scholar]
- 80.Pruijssers A.J., Denison M.R. Nucleoside analogues for the treatment of coronavirus infections. Curr. Opin. Virol. 2019;35:57–62. doi: 10.1016/j.coviro.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sun W., Sanderson P.E., Zheng W. Drug combination therapy increases successful drug repositioning. Drug Discov. Today. 2016;21(7):1189–1195. doi: 10.1016/j.drudis.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zheng W., Sun W., Simeonov A. Drug repurposing screens and synergistic drug-combinations for infectious diseases. Br. J. Pharmacol. 2018;175(2):181–191. doi: 10.1111/bph.13895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wu R., Wang L., Kuo H.-C.D., Shannar A., Peter R., Chou P.J., Kong A.-N. An update on current therapeutic drugs treating COVID-19. Curr. Phram. Rep. 2020;6:56–70. doi: 10.1007/s40495-020-00216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wit E.D., Feldmann F., Cronin J., Jordan R., Okumura A., Thomas T.…Feldmann H. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc. Natl. Acad. Sci. Unit. States Am. 2020;117(12):6771–6776. doi: 10.1073/pnas.1922083117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Holshue M.L., Debolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H., Pillai S.K. First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 2020;382(10):929–936. doi: 10.1056/nejmoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jordan P.C., Stevens S.K., Deval J. Nucleosides for the treatment of respiratory RNA virus infections. Antivir. Chem. Chemother. 2018;26:1–19. doi: 10.1177/2040206618764483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sheahan T.P., Sims A.C., Graham R.L., Menachery V.D., Gralinski L.E., Case J.B., Baric R.S. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aal3653. eaal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jean S.-S., Lee P.-I., Hsueh P.-R. Treatment options for COVID-19: the reality and challenges. J. Microbiol. Immunol. Infect. 2020;53:436–443. doi: 10.1016/j.jmii.2020.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Thiel V., Ivanov K.A., Putics Á., Hertzig T., Schelle B., Bayer S., Ziebuhr J. Mechanisms and enzymes involved in SARS coronavirus genome expression. J. Gen. Virol. 2003;84:2305–2315. doi: 10.1099/vir.0.19424-0. [DOI] [PubMed] [Google Scholar]
- 90.Mielech A.M., Kilianski A., Baez-Santos Y.M., Mesecar A.D., Baker S.C. MERS-CoV papain-like protease has demethylating and deubiquitinating activities. Virology. 2014;450–451:64–70. doi: 10.1016/j.virol.2013.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gil C., Ginex T., Maestro I., Nozal V., Barrado-Gil L., Cuesta-Geijo M.Á., Martinez COVID-19: drug targets and potential treatments. J. Med. Chem. 2020 doi: 10.1021/acs.jmedchem.0c00606. [DOI] [PubMed] [Google Scholar]
- 92.Depfenhart M., De Villiers D., Lemperle G., Meyer M., Di Somma S. Internal and Emergency Medicine. 2020. Potential new treatment strategies for COVID-19: is there a role for bromhexine as add-on therapy? [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Belouzard S., Millet J.K., Licitra B.N., Whittaker G.R. Mechanisms of coronavirus cell entry mediated by the viral spike protein. Viruses. 2012;4(6):1011–1033. doi: 10.3390/v4061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Turk V., Stoka V., Vasiljeva O., Renko M., Sun T., Turk B. Cysteine cathepsins: from structure, function, and regulation to new frontiers. Biochem. Biophys. Act. 2012;1824(1):68–88. doi: 10.1016/j.bbapap.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yang C.L., Qiu X., Zeng Y.K., Jiang M., Fan H.R., Zhang Z.M. Coronavirus disease 2019: a clinical review. Eur. Rev. Med. Pharmacol. Sci. 2020;24(8):4585–4596. doi: 10.26355/eurrev_202004_21045. [DOI] [PubMed] [Google Scholar]
- 96.Loutfy M.R., Blatt L.M., Siminovitch K.A., Ward S., Wolff B., Lho H., Pham D.H., Deif H., LaMere E.A., Chang M., Kain K.C., Farcas G.A., Ferguson P., Latchford M., Levy G., Dennis J.W., Lai E.K., Fish E.N. Interferon alfacon-1 plus corticosteroids in severe acute respiratory syndrome: a preliminary study. J. Am. Med. Assoc. 2003;290(24):3222–3228. doi: 10.1001/jama.290.24.3222. [DOI] [PubMed] [Google Scholar]
- 97.Mustafa S., Balkhy H., Gabere M.N. Current treatment options and the role of peptides as potential therapeutic components for Middle East Respiratory Syndrome (MERS): a review. J. Infect. Pub. Hea. 2018;11(1):9–17. doi: 10.1016/j.jiph.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cinatl J., Morgenstern B., Bauer G., Chandra P., Rabenau H., Doerr H.W. Treatment of SARS with human interferons. Lanc. 2003;362(9380):293–294. doi: 10.1016/s0140-6736(03)13973-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lu C.C., Chen M.Y., Lee W.S., Chang Y.L. Potential therapeutic agents against COVID- 19: what we know so far. J. Chin. Med. Assoc. 2020;83(6):534–536. doi: 10.1097/JCMA.0000000000000318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gao J., Tian Z., Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in the treatment of COVID-19 associated pneumonia in clinical studies. Biosci. Trend. 2020;14(1):72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 101.Badyal D.K., Mahajan R. Chloroquine: can it be a novel drug for COVID-19. Int. J. App. Basic Med. Res. 2020;10(2):128–130. doi: 10.4103/ijabmr.IJABMR_141_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vincent M.J., Bergeron E., Benjannet S., Erickson B.R., Rollin P.E., Ksiazek T.G., Seidah N.G., Nichol S.T. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol. J. 2005;2:69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Stebbing J., Phelan A., Griffin I., Tucker C., Oechsle O., Smith D., Richardson P. COVID- 19: combining antiviral and anti-inflammatory treatments. The Lancet. Infect. Dis. 2020;20(4):400–402. doi: 10.1016/S1473-3099(20)30132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J., HLH Across Speciality Collaboration, UK COVID-19: consider cytokine storm syndromes and immunosuppression. Lanc. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Potì F., Pozzoli C., Adami M., Poli E., Costa L.G. Treatments for COVID-19: emerging rugs against the coronavirus. Acta Biomed. 2020;91(2):118–136. doi: 10.23750/abm.v91i2.9639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pawlitzki M., Zettl U.K., Ruck T., Rolfes L., Hartung H.P., Meuth S.G. Merits and culprits of immunotherapies for neurological diseases in times of COVID-19. Biomed. 2020;56:102822. doi: 10.1016/j.ebiom.2020.102822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Delang L., Neyts J. Medical treatment options for COVID-19. European heart journal. Acute Cardio. Care. 2020;9(3):209–214. doi: 10.1177/2048872620922790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y.…Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/s0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.AminJafari A., Ghasemi S. The possible of Immunotherapy for COVID-19: a systematic review. Int. Immunopharm. 2020;83:106455. doi: 10.1016/j.intimp.2020.106455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shen C., Wang Z., Zhao F., Yang Y., Li J., Yuan J., Wang F., Li D., Yang M., Xing L., Wei J., Xiao H., Yang Y., Qu J., Qing L., Chen L., Xu Z., Peng L., Li Y., Zheng H., Liu L. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. J. Am. Med. Assoc. 2020;323(16):1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bhagavathula A.S., Aldhaleei W.A., Rovetta A., Rahmani J. Vaccines and drug therapeutics to lock down novel coronavirus disease 2019 (COVID-19): a systematic review of clinical trials. Cure. 2020;12(5) doi: 10.7759/cureus.8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kumar G.V., Jeyanthi V., Ramakrishnan S. A short review on antibody therapy for COVID- 19. New Microb. New Infect. 2020;35:100682. doi: 10.1016/j.nmni.2020.100682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gilardin L., Bayry J., Kaveri S.V. Intravenous immunoglobulin as a clinical immunomodulating therapy. CMAJ (Can. Med. Assoc. J.): Can. Med. Assoc. J. 2015;187(4):257–264. doi: 10.1503/cmaj.130375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kumar V., Jung Y.S., Liang P.H. Anti-SARS coronavirus agents: a patent review (2008 - present) Expert Opin. Ther. Pat. 2013;23(10):1337–1348. doi: 10.1517/13543776.2013.823159. [DOI] [PubMed] [Google Scholar]
- 115.Khoury M., Cuenca J., Cruz F.F., Figueroa F.E., Rocco P., Weiss D.J. Current status of cell-based therapies for respiratory virus infections: applicability to COVID-19. Eur. Respir. J. 2020;55(6):2000858. doi: 10.1183/13993003.00858-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chang C.K., Hou M.H., Chang C.F., Hsiao C.D., Huang T.H. The SARS coronavirus nucleocapsid protein--forms and functions. Antivir. Res. 2014;103:39–50. doi: 10.1016/j.antiviral.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mu J., Xu J., Zhang L., Shu T., Wu D., Huang M., Ren Y., Li X., Geng Q., Xu Y., Qiu Y., Zhou X. Life Sciences, 1–4. Advance online publication; 2020. SARS-CoV-2-encoded nucleocapsid protein acts as a viral suppressor of RNA interference in cells. Science China. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Surjit M., Liu B., Chow V.T., Lal S.K. The nucleocapsid protein of severe acute respiratory syndrome-coronavirus inhibits the activity of cyclin-cyclin-dependent kinase complex and blocks S phase progression in mammalian cells. J. Biol. Chem. 2006;281(16):10669–10681. doi: 10.1074/jbc.M509233200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fu J., Chen R., Hu J., Qu H., Zhao Y., Cao S., Wen X., Wen Y., Wu R., Zhao Q., Ma X., Huang X. Identification of a novel linear B-cell epitope on the nucleocapsid protein of porcine deltacoronavirus. Int. J. Mol. Sci. 2020;21(2):648. doi: 10.3390/ijms21020648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Moriishi K., Okabayashi T., Nakai K., Moriya K., Koike K., Murata S., Chiba T., Tanaka K., Suzuki R., Suzuki T., Miyamura T., Matsuura Y. Proteasome activator PA28gamma-dependent nuclear retention and degradation of hepatitis C virus core protein. J. Virol. 2003;77(19):10237–10249. doi: 10.1128/jvi.77.19.10237-10249.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Huang X., Seifert U., Salzmann U., Henklein P., Preissner R., Henke W., Sijts A.J., Kloetzel P.M., Dubiel W. The RTP site shared by the HIV-1 Tat protein and the 11S regulator subunit alpha is crucial for their effects on proteasome function, including antigen processing. J. Mol. Biol. 2002;323(4):771–782. doi: 10.1016/s0022-2836(02)00998-1. [DOI] [PubMed] [Google Scholar]
- 122.Stohwasser R., Holzhütter H.G., Lehmann U., Henklein P., Kloetzel P.M. Hepatitis B virus HBx peptide 116-138 and proteasome activator PA28 compete for binding to the proteasome alpha 4/MC6 subunit. Biol. Chem. 2003;384(1):39–49. doi: 10.1515/BC.2003.005. [DOI] [PubMed] [Google Scholar]
- 123.Gao G., Wong J., Zhang J., Mao I., Shravah J., Wu Y., Xiao A., Li X., Luo H. Proteasome activator REGgamma enhances coxsackieviral infection by facilitating p53 degradation. J. Virol. 2010;84(21):11056–11066. doi: 10.1128/JVI.00008-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yeom S., Jeong H., Kim S.S., Jang K.L. Hepatitis B virus X protein activates proteasomal activator 28-gamma expression via the upregulation of p53 levels to stimulate virus replication. J. Gen. Virol. 2018;99(5):655–666. doi: 10.1099/jgv.0.001054. [DOI] [PubMed] [Google Scholar]
- 125.Anupam R., Datta A., Kesic M., Green-Church K., Shkriabai N., Kvaratskhelia M., Lairmore M.D. Human T-lymphotropic virus type 1 p30 interacts with REGgamma and modulates ATM (ataxia telangiectasia mutated) to promote cell survival. J. Biol. Chem. 2011;286(9):7661–7668. doi: 10.1074/jbc.M110.176354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ko N.L., Taylor J.M., Bellon M., Bai X.T., Shevtsov S.P., Dundr M., Nicot C. PA28γ is a novel corepressor of HTLV-1 replication and controls viral latency. Blood. 2013;121(5):791–800. doi: 10.1182/blood-2012-03-420414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Schwarz K., van Den Broek M., Kostka S., Kraft R., Soza A., Schmidtke G., Kloetzel P.M., Groettrup M. Overexpression of the proteasome subunits LMP2, LMP7, and MECL-1, but not PA28 alpha/beta, enhance the presentation of an immunodominant lymphocytic choriomeningitis virus T cell epitope. J. Immun. 2000;165(2):768–778. doi: 10.4049/jimmunol.165.2.768. 1950. [DOI] [PubMed] [Google Scholar]
- 128.Raaben M., Posthuma C.C., Verheije M.H., te Lintelo E.G., Kikkert M., Drijfhout J.W., Snijder E.J., Rottier P.J., de Haan C.A. The ubiquitin-proteasome system plays an important role during various stages of the coronavirus infection cycle. J. Virol. 2010;84(15):7869–7879. doi: 10.1128/JVI.00485-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.