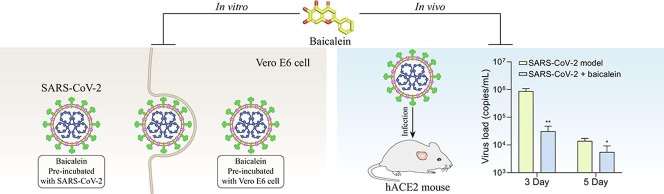
Graphical abstract
Abbreviations: ANOVA, Analysis of variance; BALF, bronchoalveolar lavage fluid; Cavg, average steady-state plasma concentration; CC50, half-cytotoxic concentration; Cmax, peak plasma concentration; COVID-19, Coronavirus Disease 2019; CPE, cytopathic effect; DENV, dengue virus; EC50, half-maximal effective concentrations; EEP, end-expiratory pause; hACE2, human angiotensin-converting enzyme 2; HIV, human immunodeficiency virus; H & E, Hematoxylin and eosin; JEV, Japanese encephalitis virus; LLOQ, lower limit of quantitation; MOI, multiplicity of infection; Mpro, main protease; PEF, peak expiratory flow; Penh, enhanced pause; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SI, selection index; ZIKV, Zika virus
Keywords: Baicalein, SARS-CoV-2, COVID-19
Abstract
Baicalein is the main active compound of Scutellaria baicalensis Georgi, a medicinal herb with multiple pharmacological activities, including the broad anti-virus effects. In this paper, the preclinical study of baicalein on the treatment of COVID-19 was performed. Results showed that baicalein inhibited cell damage induced by SARS-CoV-2 and improved the morphology of Vero E6 cells at a concentration of 0.1 μM and above. The effective concentration could be reached after oral administration of 200 mg/kg crystal form β of baicalein in rats. Furthermore, baicalein significantly inhibited the body weight loss, the replication of the virus, and relieved the lesions of lung tissue in hACE2 transgenic mice infected with SARS-CoV-2. In LPS-induced acute lung injury of mice, baicalein improved the respiratory function, inhibited inflammatory cell infiltration in the lung, and decreased the levels of IL-1β and TNF-α in serum. In conclusion, oral administration of crystal form β of baicalein could reach its effective concentration against SARS-CoV-2. Baicalein could inhibit SARS-CoV-2-induced injury both in vitro and in vivo. Therefore, baicalein might be a promising therapeutic drug for the treatment of COVID-19.
1. Introduction
The Coronavirus Disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has led to a global public health crisis. Some patients infected with SARS-CoV-2 showed respiratory distress or even respiratory failure. A massive number of non-invasive or invasive ventilators are needed for patients to achieve mechanical ventilation. Critical illness patients with respiratory failure or multiorgan failure require ICU monitoring treatment urgently [1], [2], [3], [4]. The pandemic brings a huge challenge to the public health-care system. Therefore, the drug discovery for the treatment of COVID-19 is urgent.
Several drugs have been tested for the efficacy and safety against COVID-19, such as remdesivir, chloroquine, hydroxychloroquine, and favipiravir, some of which have displayed antiviral effects against SARS-CoV-2 in vitro [5], [6]. It was reported that remdesivir, chloroquine, favipiravir could effectively protect Vero E6 cells by inhibiting the infection of SARS-CoV-2 with the half-maximal effective concentrations (EC50) values of 0.77, 1.13, and 61.88 μM, respectively 5. Unfortunately, there is still no ideal drug for the treatment of COVID-19 clinically. Although a multicenter prospective observational study demonstrated that chloroquine treatment could shorten the median time to achieve an undetectable viral RNA and the duration of fever compared to non-chloroquine control [7], the benefit-risk ratio of chloroquine treatment needs to be considered due to its large volume of distribution, the long half-life, easy accumulation, and a lethal dose of 5 g in adults [8]. Several clinical trials have evaluated the efficacy and safety of hydroxychloroquine, but whether it is beneficial or not is not consistent [9], [10]. The clinical treatment effect of chloroquine and hydroxychloroquine still needs to be verified by large-scale randomized, double-blind, controlled trials. Besides, a randomized controlled trial enrolling 240 patients, half receive favipiravir, and half receive arbidol, reported that the clinical recovery rate of favipiravir on Day 7 was not significantly different from that of arbidol [11]. In addition, an open-label RCT of lopinavir-ritonavir revealed no significant difference in the clinical improvement between lopinavir-ritonavir treatment and standard therapy [12]. The convalescent plasma therapy showed beneficial effects on severe COVID-19 patients, which could significantly improve the clinical symptoms of severe patients and decrease the viral load [13], [14]. However, this treatment also has disadvantages, including insufficient plasma supply and the risk of disease transmission. The drug discovery for COVID-19 could be a time-consuming process. And it is also a big challenge to elucidate the safety and toxicity of a new drug in a short time.
Baicalein is an active compound isolated from Scutellaria baicalensis Georgi (Huangqin), a traditional medicine with the pharmacological effects of anti-inflammation and anti-virus. Baicalein or the extract of Huangqin exhibited broad-spectrum antiviral effects, including influenza A (H1N1) virus [15], [16], Zika virus (ZIKV) [17], and dengue virus (DENV) [18], et al. Although Huangqin has been used for a long time as a traditional medicine, the plasma concentration of its active component (baicalein) after oral administration is relatively low, which weakens its efficacy. Thus, improving the absorption, tissue distribution, and bioavailability of baicalein is critical for its therapeutic effect. During the past decade, we have obtained a new crystal form of baicalein (crystal form β of baicalein) and found that this crystal form showed a higher bioavailability than the other crystal forms of baicalein. In the study, the therapeutic effects of baicalein against SARS-CoV-2 were investigated both in vivo and in vitro. An LPS-induced acute lung injury model was also carried out in mice to reveal the effects of baicalein on the related symptoms of COVID-19.
2. Materials and methods
2.1. Chemicals and reagents
The crystal forms α and β of baicalein were prepared by the Institute of Materia Medica, Chinese Academy of Medical Sciences (CAMS) and Peking Union Medical College (PUMC, Beijing, China). Crystal form β of baicalein was used in the subsequent in vivo studies and has been developed as a pharmaceutical form of Baicalein Chewable Tablets used for the clinical trial [19], [20]. Other chemicals and reagents used are as follows: remdesivir (Lalpharm Co., Ltd., Beijing, China); fetal bovine serum, RPMI 1640 medium, and DMEM medium (Gibco, Thermo Fisher Scientific, USA); LPS, hematoxylin and eosin (Sigma-Aldrich, Shanghai, China); CMC-Na and diethyl ether (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China); paraformaldehyde and xylene (Shanghai Macklin Biochemical Co., Ltd., Shanghai, China); hydrochloric acid, ammonia solution (Beijing Tong Guang Fine Chemicals Company, Beijing, China); heparin sodium (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China); alcohol (Shanghai Aladdin Biochemical Technology Co. Ltd., Shanghai China).
2.2. Pharmacokinetic study of baicalein
In the oral pharmacokinetic study of rats, 10 male SD rats (240–270 g) were divided into 2 groups (5 rats in each) and orally given 200 mg/kg crystal form α or β of baicalein, respectively. The blood sample was obtained from the posterior venous plexus of rat into a heparinized test tube before administration and at 0.33, 0.66, 1, 1.5, 2, 3, 5, 7, 9, 12, 24 h after administration. Then the sample was centrifuged, and plasma was collected. For intravenous injection, 10 rats (200–240 g, half male and half female) were injected with 10 mg/kg of baicalein intravenously. At 0.03, 0.08, 0.17, 0.33, 0.5, 0.75, 1, 1.5, 2, 3, 4, 6, and 8 h after injection, blood sample was collected into heparinized test tube, and then centrifuged. 100 μL of plasma was collected. Previously, we have reported a method for simultaneous quantification of baicalein and its metabolite baicalin in monkey plasma [21]. With minor modification, the method was validated in rat plasma. And the concentration of baicalein and baicalin were simultaneously determined. The lower limit of quantitation (LLOQ) was 0.185 μM for baicalein and 0.112 μM for baicalin, respectively. All the protocols were approved by the Experimental Animal Care and Use Committee of the Institute of Materia Medica, CAMS and PUMC.
2.3. Vero E6 cell culture, SARS-CoV-2 exposure, and baicalein treatment
Two cytopathic effect (CPE) assays were carried out in this study. For one of the CPE assays, Vero E6 cells and SARS-CoV-2, with a titer of 105 TCID50/mL, were stored at −80 °C by the Pathogen Center of Institute of Laboratory Animal Science, CAMS and PUMC. Vero E6 cells were maintained in RPMI 1640 medium with 10% fetal bovine serum and cultured at 37 °C in a humidified incubator containing 5% CO2. 100 μL Vero E6 cells (5 × 104 cells/mL) were placed in the wells of 96 cell plates and cultured for 24 h. 100 µL baicalein solution was mixed with an equal volume of 100 µL TCID50 SARS-CoV-2 to the final concentrations (0.0003, 0.001, 0.003, 0.01, 0.03, 0.1 and 0.3 μM) with 4 duplicate wells at each concentration and incubated for 1 h. Then, the culture medium was discarded, and the above mixture was added into each well. Subsequently, the cells were cultured for 5 days. Normal cell control, solvent control, positive control (remdesivir) and virus control (negative control) were used at the same time. The CPE was observed under a light microscope. An observable CPE was recorded as “+”, no CPE was recorded as “−”. All these experiments were carried out in biosafety level 3 laboratory in the Institute of Laboratory Animal Science, CAMS and PUMC.
For another CPE assay, SARS-CoV-2 (108) was provided by the Guangdong Provincial Center for Disease Control and Prevention with a virus titer of 106 pfu/mL. During the experiment, DMEM medium was used to prepare baicalein into solutions of different concentrations. Then, Vero E6 cells were pretreated with baicalein for 1 h. After that, SARS-CoV-2 was added at a multiplicity of infection (MOI) of 0.05 and incubated for 1 h. After discarding the baicalein-virus mixture, the normal culture medium containing baicalein was added, and the CPE was observed under a light microscope after 48 h. Remdesivir was used as a positive control.
2.4. Establishment of hACE2 transgenic mice SARS-CoV-2 infection model and baicalein treatment
The human angiotensin-converting enzyme 2 (hACE2) transgenic mice infected with SARS-CoV-2 was used to evaluate the drug efficacy, which cannot be achieved by the wild-type mice [22]. 7-week-old male and female SPF-grade hACE2 transgenic mice (16–24 g) were provided and housed at the Institute of Laboratory Animal Science, CAMS and PUMC. Experiments were carried out in the animal biosafety level 3 facility. All procedures were reviewed and approved by the Institutional Animal Care and Use Committee of the Institute of Laboratory Animal Science, CAMS and PUMC, and were complied with all relevant ethical regulations. The mice were randomly divided into a model group and a baicalein treatment group, with 6 mice in each group. During the experiment, each mouse was infected with 50 μL of 105 TCID50 SARS-CoV-2 by intranasal infection.
Before the mice were administered by gavage, baicalein was suspended with 0.5% CMC-Na solution to form a suspension solution with a concentration of 10 mg/mL. The dose for each mouse was 200 mg/kg/day. Baicalein was administrated at 1 h after the infection of SARS-CoV-2 in mice, and once a day for 5 consecutive days, whereas the model group was given 0.5% CMC-Na solution in equal volume.
After mice were infected with SARS-CoV-2, their general symptoms were observed continuously for 5 days, and changes in body weight were recorded. On the 3rd and 5th days after infection, 3 mice were euthanized in each group to detect the viral load of lung tissue. On the 5th day, the pathology of lung tissues was examined by hematoxylin and eosin (H & E) staining. Histopathology was reviewed and scored using the reported Lung Injury Scoring System from the Official American Thoracic Society Workshop Report in a blinded manner by three qualified investigators [23].
2.5. Establishment of LPS-induced mice acute lung injury model and baicalein treatment
50 BALB/c male mice (SPF grade, 18–22 g) were acquired from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China) with the animal license number SCXK (Beijing) 2012-0001. 5 mice were kept in each cage under a specific pathogen-free condition at 24 ± 1 °C, 60–65% humidity, and 12 h dark/light cycle. Mice were supplied with standard laboratory chow and given water ad libitum. Before the experiment, mice were fed for 3 days for environment adaption. The protocol of the experiment was approved by the Experimental Animal Care and Use Committee of the Institute of Materia Medica, CAMS and PUMC. Mice were randomly divided into 5 groups with 10 mice in each group, including the normal control group, the LPS model group, and LPS + baicalein treatment groups (50, 100, and 200 mg/kg).
The aim of the experiment is to assess the beneficial effect of baicalein on LPS-induced acute lung injury in mice. For the model establishment, mice were firstly anesthetized with a small amount of ether and inhaled a total volume of 50 μL LPS (8 mg/mL). The normal control mice nasally inhaled the same volume of saline. Baicalein was dissolved with 1% CMC-Na aqueous solution. Mice in treatment groups were given baicalein (50, 100, and 200 mg/kg) by gavage at 0.5 and 12 h after inhaling LPS. The normal control group and the LPS model group were provided with an equal volume of 1% CMC-Na aqueous solution. The lung function of mice in each group was monitored at 24 h after the model was made. Blood samples and bronchoalveolar lavage fluid (BALF) were collected. Lung tissues were used for subsequent experiments.
2.5.1. Lung function monitoring
24 h after the establishment of the LPS-induced acute lung injury model, the EMKA non-invasive pulmonary function monitoring system (EMKA Technologies, Paris, France) was applied to record the lung function of mice in the conscious and unrestrained state. Respiratory function-related indicators, including peak expiratory flow (PEF), respiratory frequency, end-expiratory pause (EEP), and enhanced pause (Penh) were monitored and analyzed. Each mouse was recorded for 5 min.
2.5.2. H & E staining
H & E staining was used for the pathological analysis of lung tissues. Firstly, part of the left lung tissue of the mouse was immersed in 4% paraformaldehyde and fixed for 48 h. Then, the cut surface of the fixed lung tissue is trimmed, dehydrated, cleared, replaced, and embedded. The trimmed wax block was placed on the microtome, cut into 4 μm/piece, and placed in a 55 °C oven for 1 h. The sections were then dewaxed, replaced, washed with triply distilled water for 5 min, stained by hematoxylin for 8 min, and washed with water. Tissue sections were treated with 1% hydrochloric acid alcohol solution for 30 sec. After rinsed with water, the tissue sections were treated with 1% ammonia solution for 30 sec and followed by rinse. Finally, tissue sections were stained with eosin for 3 min, and the water in the sections was replaced with a gradient concentration of alcohol. The sections were put into xylene I for 5 min and then xylene II for 5 min. After covered with neutral gum, the sections were observed under a microscope (Nikon, Japan). Lung injury was scored in a blinded manner by three qualified investigators using the Lung Injury Scoring System from the Official American Thoracic Society Workshop Report [23].
2.5.3. Determination of IL-1β, TNF-α, and IL-6 in mice serum
Cytokines play vital roles in inflammation response. To find out whether baicalein inhibited the release of cytokines in LPS-induced acute lung injury model, the cytokines in mice serum were measured at 24 h after LPS stimulation. The concentrations of IL-1β, TNF-α, and IL-6 were determined using the commercial ELISA kits (4A Biotech Co., Ltd, Beijing, China). All the experiments were carried out according to the manufactures' instructions.
2.5.4. Counting of total, neutrophil and macrophage cells in BALF
For cell counting, the trachea near the neck of the mouse was exposed, and tracheal intubation was performed. The trachea was lavaged with pre-cold sterilized 0.3 mL PBS for three times to obtain BALF. Total cells were counted by a hemocytometer. The Wright-Giemsa stain assay was applied for the counting of neutrophil and macrophage cells.
2.6. Statistical analysis
The data were expressed as mean with standard deviation. Statistical analysis was carried out using GraphPad Prism 7.0 software (GraphPad Software Inc., CA, USA). Unpaired t-test, one-way analysis of variance (ANOVA), or two-way ANOVA followed by Bonferroni's multiple comparisons were carried out. Results were considered statistically significant at P < 0.05.
3. Results
3.1. The pharmacokinetic study of different crystal forms of baicalein
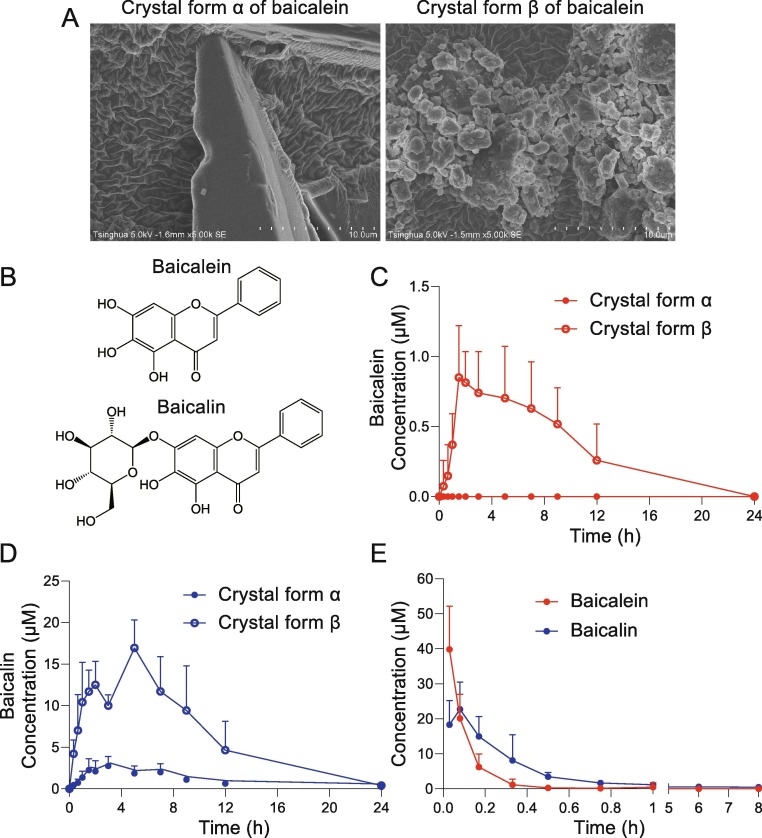
The absorption of different crystal states of baicalein (α and β form, Fig. 1 A) were studied. Baicalin is the main Phase II metabolite of baicalein (Fig. 1B). The plasma concentration–time curves of baicalein and baicalin were shown (Fig. 1C and D). After oral administration of α crystal form of baicalein (200 mg/kg), the plasma concentration of the parent drug baicalein was lower than its LLOQ (0.185 μM), while that of the metabolite baicalin was higher than its LLOQ (0.112 μM). Therefore, only the metabolite baicalin could be detected, but not the parent drug baicalein. The peak plasma concentration (Cmax) of the metabolite was 2.85 ± 1.21 μM, and the AUC0-24h of the metabolite was 23.66 ± 8.80 μM·h. After oral administration of crystal form β of baicalein, the Cmax of baicalein was 1.04 ± 0.22 μM, and the AUC0-24h of baicalein was 6.44 ± 3.00 μM·h. The Cmax of baicalin was 17.27 ± 2.98 μM, and the AUC0-24h was 146.94 ± 51.60 μM·h. Taking the crystal form α as a reference, the relative bioavailability of the crystal form β is 648.33% of crystal form α, indicating that the absorption of baicalein after the change of the crystal form from α to β is obviously improved. The plasma concentration–time curves after the intravenous injection of 10 mg/kg baicalein in rats were shown (Fig. 1E). The AUC0-8h of baicalein was 5.18 ± 0.96 μM·h, and the AUC0-8h of baicalin was 11.00 ± 3.34 μM·h. Based on the AUC values of baicalein and baicalin after 200 mg/kg oral and 10 mg/kg intravenous administration in rats, the absolute bioavailability of crystal forms α and β of baicalein was calculated to be 7.31% and 47.40%, respectively. The crystal form β of baicalein was prepared and used in the major experiments because of its higher bioavailability than crystal form α.
Fig. 1.
The pharmacokinetic study of baicalein and the comparison between crystal form α and β. (A) Scanning electron micrographs (SEM) of crystal forms α and β of baicalein. (B) Chemical structure of baicalein and baicalin. (C) Plasma concentration–time curve of baicalein after oral administration of crystal forms α and β of baicalein (200 mg/kg) in rats (n = 10). (D) Plasma concentration–time curve of baicalin after oral administration of crystal forms α and β of baicalein (200 mg/kg) in rats (n = 10). (E) Plasma concentration–time curve of baicalein and baicalin after intravenous injection of baicalein (10 mg/kg) in rats (n = 10). Results were shown as mean + SD.
3.2. Baicalein inhibited SARS-CoV-2-induced Vero E6 cell injury
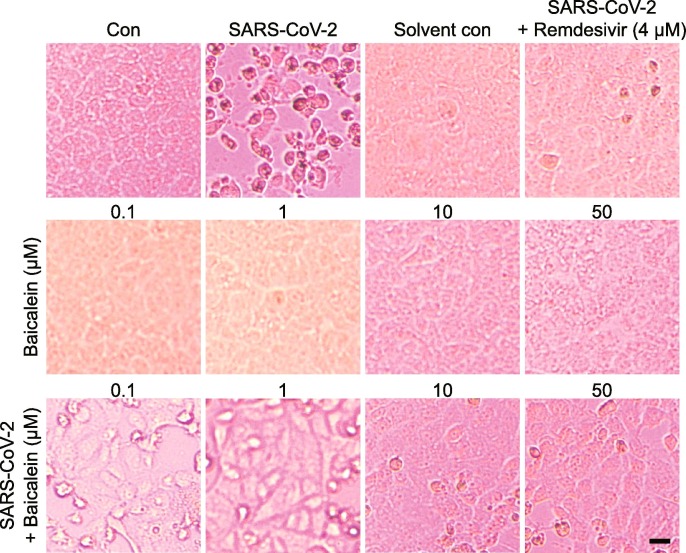
To investigate whether baicalein has the anti-SARS-CoV-2 effect, two CPE assays were carried out. For one of the CPE assays (Table 1 ), different concentrations of baicalein solution were pre-incubated with SARS-CoV-2 for 1 h, then the incubated solutions were added to the cells for the anti-virus study. The results showed that baicalein at the concentration range of 0.1–0.3 μM could completely protect cells against the SARS-CoV-2 induced injury. The positive control remdesivir also completely protected cells from damage at the concentration of 0.1 μM. This result indicated that baicalein had a direct killing effect on the virus. For another CPE assay, cells were pretreated with baicalein (0.1–50 μM) for 1 h before SARS-CoV-2 was added. Results indicated that baicalein could slightly alleviate cells damage caused by SARS-CoV-2 at the concentration of 0.1 and 1 μM. And only a few cells showed lesion when treated with 10 and 50 μM of baicalein (Fig. 2 ). Taken together, these results suggested that baicalein had a significant anti-SARS-CoV-2 effect at the concentration of 0.1 μM and above.
Table 1.
Baicalein inhibited SARS-CoV-2-induced Vero E6 cell injury.
| Compound | Concentration (μM) | CPE result (n = 4) | |||
|---|---|---|---|---|---|
| Baicalein | 0.3 | – | – | – | – |
| 0.1 | – | – | – | – | |
| 0.03 | – | + | + | + | |
| 0.01 | + | + | + | + | |
| 0.003 | + | + | + | + | |
| 0.001 | + | + | + | + | |
| 0.0003 | + | + | + | + | |
| Remdesivir | 0.1 | – | – | – | – |
| 0.03 | – | + | + | + | |
| Normal control | – | – | – | – | |
| Solvent control | – | – | – | – | |
| SARS-CoV-2 control | + | + | + | + | |
An observable CPE was recorded as “+”, no CPE was recorded as “−”.
Different concentrations of baicalein or remdesivir solution were pre-incubated with SARS-CoV-2 for 1 h, then the incubated solutions were added to the cells for the anti-virus study. The results showed that baicalein at the concentration range of 0.1–0.3 μM could completely protect cells against the SARS-CoV-2 induced injury, indicating that If an effective concentration of baicalein and virus are directly mixed in advance, the virus might be completely killed, thus protecting the cells from the subsequent damage caused by SARS-CoV-2.
Fig. 2.
Baicalein inhibited SARS-CoV-2-induced Vero E6 cell injury. The CPE assay was carried out in Vero E6 cells infected with SARS-CoV-2. Cells were pretreated with drugs for 1 h. Then SARS-CoV-2 virus (MOI = 0.05) was added and incubated for 1 h. After discarding the drug-virus mixture, the normal culture medium containing the drug was added, and the CPE was observed under a light microscope after 48 h. Baicalein showed cell-protective effects against SARS-CoV-2 in the concentration range of 0.1 to 50 μM. Scale bar = 25 μM.
3.3. Baicalein inhibited the loss of body weight, reduced virus load and relieved lung injury in mice infected with SARS-CoV-2
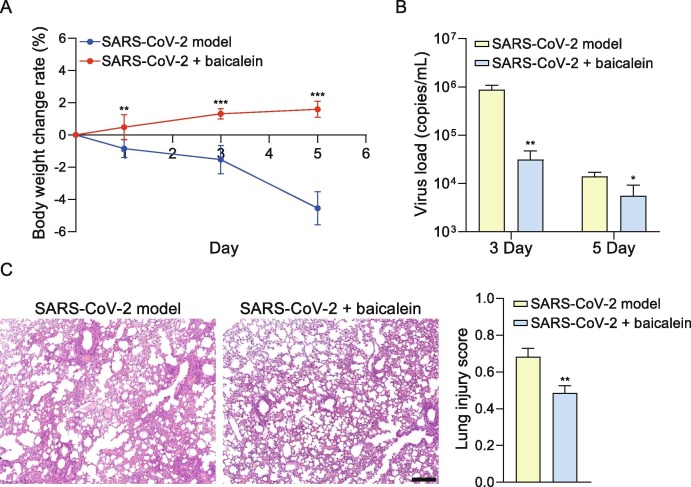
The general condition of mice was observed during the experiment. The hairs and body weight were lost in some mice of the model group. The loss rate of average body weight is up to 4.55% on the 5th day in the model group, while the baicalein treatment group showed an increasing rate of 1.59% on the 5th day. The average body weight of mice in the baicalein group was significantly higher than that in the model group on the 1st, 3rd, and 5th day (P < 0.01, P < 0.001 and P < 0.001, Fig. 3 A). In the model group, the virus load of the lung tissue on the 3rd and 5th days after infection was 105.94 and 104.14 copies/mL, while the viral load in baicalein group on the 3rd and 5th days after the infection was 104.45 and 103.36 copies/mL, which were significantly lower than that in the model group, respectively (P < 0.01 and P < 0.05, Fig. 3B). The widening and cell infiltration of the alveolar septum, and a small amount of cell infiltration around the blood vessels were observed in the lung tissue of the hACE2 transgenic mice infected with SARS-CoV-2 on the 5th day. These pathological injuries have also been observed in the baicalein treatment group, compared with the model group, the treatment of baicalein relieved the inflammatory cell infiltration of lung tissue (Fig. 3C).
Fig. 3.
Baicalein inhibited the loss of the body weight, reduced virus load and relieved lung injury in mice infected with SARS-CoV-2. (A) The average body weight change rate of mice in model and baicalein groups. Compared with the model group, the average body weight of mice in the baicalein group was significantly increased on the 1st (n = 6), 3rd (n = 6), and 5th (n = 3) day (P < 0.01, P < 0.001 and P < 0.001). Statistical significance was assessed using two-way ANOVA with Bonferroni's multiple comparisons test. (B) Baicalein significantly inhibited the virus load of the lung tissue on the 3rd and 5th days after infection (**P < 0.01 and *P < 0.05). Statistical significance was assessed using unpaired t test. (C) The baicalein relieved inflammatory cell infiltration of lung tissue caused by SARS-CoV-2, according to the results of H & E staining. Statistical significance of lung injury score was assessed using unpaired t test. The values are presented as mean ± SD. *P < 0.05, **P < 0.01 and ***P < 0.01 vs. SARS-CoV-2 infection model group (scale bar = 250 μm).
3.4. Baicalein alleviated LPS-induced acute lung injury in mice
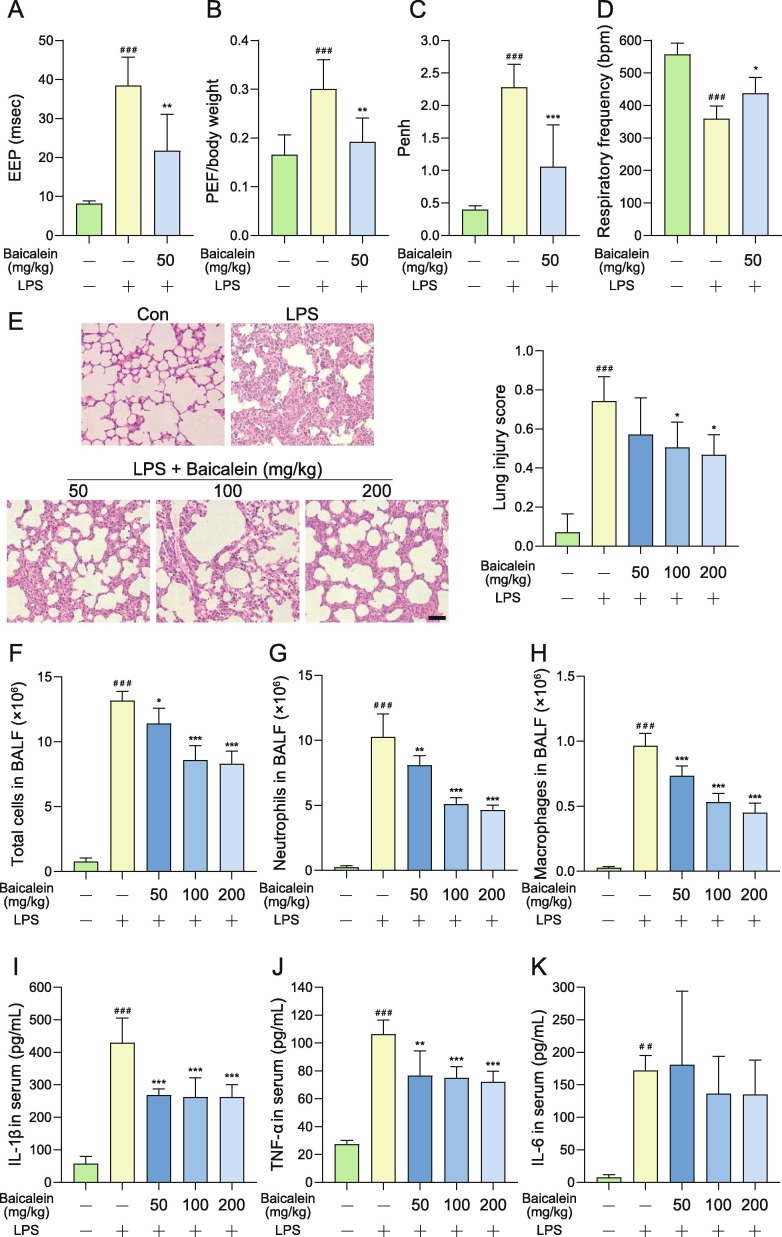
To check if baicalein could alleviate the acute lung injury induced by LPS, baicalein was orally administrated to mice. Compared with the normal control group, the EEP of the LPS model group was significantly longer (P < 0.001), the PEF/body weight and the Penh were significantly increased (P < 0.001), and the respiratory frequency was significantly decreased (P < 0.001). Whereas baicalein at a dose of 50 mg/kg had a significant effect on shortening the EEP in mice with lung injury compared with the LPS model group (P < 0.01, Fig. 4 A). Baicalein also significantly reduced the PEF/body weight of mice with lung injury (P < 0.01, Fig. 4B), and remarkably reduced the Penh in mice with lung injury (P < 0.001, Fig. 4C). In addition, baicalein also increased the respiratory frequency of mice injured by LPS (P < 0.05, Fig. 4D).
Fig. 4.
Baicalein alleviated LPS-induced acute lung function injury in mice. Baicalein was orally administrated at a dose of 50 mg/kg in mice. Lung function was monitored at 24 h after LPS stimulation. (A) Baicalein significantly shortened the EEP in mice with lung injury. (B) Baicalein significantly reduced the PEF/body weight of mice with lung injury. (C) Baicalein significantly reduced the enhanced pause (Penh) in mice with lung injury. (D) Baicalein increased the respiratory frequency of mice injured by LPS. (E) The treatment of baicalein reduced the lung cell infiltration compared with the LPS model group according to the results of H & E staining (scale bar = 50 μm). (F-H) Baicalein significantly reduced the total cell (F), neutrophils (G), and macrophages (H) in bronchial perfusion fluid. (I-K) Baicalein significantly reduced the concentrations of IL-1β (I) and TNF-α (J) in mice serum compared with the LPS model group. But no significant different was found on the concentration of IL-6 (K). The values are presented as mean + SD. Statistical significance was assessed using one-way ANOVA with Bonferroni’s multiple comparisons test. ##P < 0.01 and ###P < 0.001 vs. control group, *P < 0.05, **P < 0.01 and ***P < 0.001 vs. LPS model group.
As shown in Fig. 4E, in the lung tissue of BALB/c mice inhaled by LPS, the alveolar space was replaced by a large number of infiltrated cells, and the structure of alveoli was changed. However, compared with the LPS model group, the treatment of baicalein reduced lung cell infiltration. Compared with the normal control group, the total cells, neutrophils, and macrophages were significantly increased in BALF of the LPS model group (P < 0.001). Baicalein could significantly reduce the total cell in BALF at the doses of 50, 100, and 200 mg/kg (Fig. 4F). In addition, baicalein could also dose-dependently reduce the neutrophils and macrophages in BALF of mice with lung injury (Fig. 4G and H). The concentrations of inflammatory cytokines in serum of mice, including IL-1β, TNF-α, and IL-6, were significantly increased in the LPS model group (Fig. 4I - K). Baicalein significantly reduced the concentration of IL-1β and TNF-α compared with the LPS model group at 50, 100, and 200 mg/kg.
4. Discussion
Baicalein is the main active compound in Huangqin, a traditional medicinal herb with multiple pharmacological activities. Our previous results showed that baicalein protected against MPTP-induced neurotoxicity in mice [24], [25], inhibited 6-hydroxydopamine-induced neurotoxicity in vitro and in vivo [26], [27], and alleviated dementia caused by chronic cerebral hypoperfusion [28]. In this study, baicalein was found to have anti-SARS-CoV-2 activity and reduce lung injury caused by SARS-CoV-2 in mice. In addition, baicalein could alleviate LPS-induced acute lung injury, regulate the respiratory function, and reduce the inflammatory cells in BALF in mice with acute lung injury.
Studies have reported the broad-spectrum anti-virus properties of baicalein or the extract of Huangqin against influenza A (H1N1) virus [16], [29], [30], [31], influenza A (H5N1) virus [32], human immunodeficiency virus (HIV) [33], [34], DENV [35], [36], Sendai virus [37], ZIKV [17], and Japanese encephalitis virus (JEV) [38], et al. Baicalein (240, 480, and 960 mg/kg/day) prevented the death of mice, increased the mean time to death, alleviated lung consolidation, and decreased the lung virus titer of BALB/c mice infected with the influenza A/FM1/1/47 (H1N1) virus [16]. Baicalein showed synergistic activity with ribavirin against influenza A (H1N1) virus infection in mice, especially at doses of baicalein (400 mg/kg/day) and ribavirin (50 mg/kg/day) [29]. Baicalein could significantly inhibit the in vitro replication of Pandemic 2009 H1N1 virus (IC50 = 0.018 μM), as well as the Seasonal 2007 influenza A virus [30]. Baicalein concentration-dependently inhibited the A/FM1/1/47 (H1N1) and A/Beijing/32/92 (H3N2) influenza viruses via interference with the mid-late mRNA synthesis [31]. It reduced H5N1 infectious titers, and inhibited H5N1-induced the release of IL-6 and IL-8, indicating an additional anti-inflammation effect [32]. Baicalein also showed activity against HIV-l integrase [33], [34]. The replication of DENV-2 was suppressed by baicalein with the IC50 value of 6.46 μg/mL and selection index (SI) value of 17.8 when treated after the adsorption of virus to the Vero E6 cells. When cells were treated 5 h before infection followed by up to 4 days post infection, the IC50 value was 5.39 μg/mL and SI value was 21.3. Baicalein exhibited an anti-adsorption effect with IC50 value of 7.14 μg/mL, and a direct virucide effect against DENV-2 with the IC50 value of 1.55 μg/mL [35]. Another antiviral study revealed that baicalin, the metabolite of baicalein, could inhibit the replication of DENV-2 (IC50 = 13.5 μg/mL), exhibit virucide effect (IC50 = 8.74 μg/mL), and suppress virus adsorption (IC50 = 18.07 μg/mL) [36]. After oral administration, baicalein significantly reduced the lung virus titers of mice infected with Sendai virus, and protected mice from death at the doses of 200, 400, and 800 mg/kg/day [37]. Baicalein and baicalin could reduce the replication of ZIKV up to 10 h post virus infection [17]. In addition, baicalein showed anti-JEV activity with the IC50 value of 14.28 μg/mL when added to Vero cells after virus adsorption, exhibited anti-adsorption effect with the IC50 value of 7.27 μg/mL, and displayed direct extracellular virucide activity with the IC50 value of 3.44 μg/mL [38].
Baicalein had strong anti-SARS-CoV-2 activity in Vero E6 cells. It was reported that the half-cytotoxic concentration (CC50) of baicalein and baicalin was over 0.2 mM in Vero E6 cells and that baicalein and baicalin dose-dependently inhibited the replication of SARS-CoV-2 with the EC50 values of 1.69 and 10.27 μM, and the SI values greater than 118 and 19, respectively [39]. Another study indicated that baicalein inhibited the replication of SARS-CoV-2 with the EC50 value of about 17.6 μM. Two CPE assays were carried out in this study. For one of the CPE assays, different concentrations of baicalein solution were pre-incubated with SARS-CoV-2 for 1 h, then the incubated solutions were added to the cells for the anti-virus study. The results of this assay indicated that baicalein at the concentration range of 0.1–0.3 μM could completely protect cells against the SARS-CoV-2-induced injury, revealing a direct killing effect on SARS-CoV-2. If an effective concentration of baicalein and virus are directly mixed in advance, the virus might be completely killed, thus protecting the cells from the subsequent damage caused by SARS-CoV-2. For another CPE assay, cells were pretreated with baicalein (0.1–50 μM) for 1 h before SARS-CoV-2 was added to the cells. This assay showed that baicalein could slightly alleviate cell damage caused by SARS-CoV-2 at the concentration of 0.1 and 1 μM, and obviously protect the cells at the concentration of 10 and 50 μM. Both results indicated that baicalein could alleviate cell damage caused by SARS-CoV-2 at the concentration range of 0.1 μM and above, indicating a promising anti-SARS-CoV-2 activity.
To improve the bioavailability of baicalein, its different crystal forms were prepared. Polymorphism is a common phenomenon in small molecule drugs. The characteristics of different crystal forms not only affect the physical and chemical properties of the drugs, but also change their pharmacokinetic properties. Good pharmacokinetic property includes a reasonable absorption rate and an adequate blood concentration, which could be achieved after oral administration. The absolute bioavailability of baicalein was previously reported to be 7.46% [40]. In this study, the absolute bioavailability of different crystal forms of baicalein was calculated based on the AUC values of 200 mg/kg oral and 10 mg/kg intravenous administration in rats. Crystal form β of baicalein showed a higher absolute bioavailability than that of crystal form α of baicalein (47.40% vs. 7.31%), which indicated that the absorption of baicalein after the change of the crystal form from α to β was obviously improved. It is worth mentioning that due to the different species, the dose of 200 mg/kg for rats in the pharmacokinetic study cannot be equivalently converted with the dose of 200 mg/kg/day for hACE2 transgenic mice in the anti-SARS-CoV-2 experiment. The pharmacokinetic studies of baicalein were also performed in dogs and monkeys in our lab to support the clinical trial [21], [41]. Based on the findings of the new advantage crystal form of baicalein, the Baicalein Chewable Tablets were prepared for the patients. The Cmax values after administration of single-dose Baicalein Chewable Tablets (200, 400 and 800 mg) in the healthy volunteers were 0.0427, 0.0757, and 0.1309 μM for baicalein, and 0.4077, 0.5261, and 0.9602 for baicalin, respectively [19]. And the average steady-state plasma concentration (Cavg) values after 10 days administration at multiple-dose of 200, 400 and 800 mg were 0.0312, 0.0565 and 0.1130 μM for baicalein, and 0.2545, 0.6774, and 0.8477 μM for baicalin, respectively [20]. The Cmax at 800 mg single-dose and the Cavg at 800 mg multiple-dose of baicalein were above the in vitro effective concentration (0.1 μM) against SARS-CoV-2. In addition, our previous results of the phase I clinical trial indicated that the single-dose (100–2800 mg) of baicalein showed no signs of obvious liver or kidney toxicity, and no obvious accumulation of baicalein was detected in the multiple-dose (200–800 mg) study. The single-dose (100–2800 mg) and multiple-dose (200–800 mg) of baicalein were safe and well-tolerated [19], [20].
The data of animal experiments are of great significance for supporting clinical trials. Baicalein (200 mg/kg/day) significantly increased body weight and inhibited virus replication in hACE2 transgenic mice infected with SARS-CoV-2. The average body weight loss rate is up to 4.55% in the model group, whereas the treatment of baicalein increased the average weight with the highest increasing rate of 1.59%. In addition, baicalein could lower the viral load of lung tissue in infected mice. The major symptom of COVID-19 caused by SARS-CoV-2 is acute lung injury. Our results showed that the lung tissue of mice infected with SARS-CoV-2 for 5 days showed inflammatory cell infiltration. Compared with the model group, the baicalein treatment group relieved the lesions of lung tissue and reduced inflammatory cell infiltration. Baicalein had strong anti-SARS-CoV-2 activity and significantly reduced lung injury by SARS-CoV-2 in mice.
Cytokine storm is an excessive response produced by the body to external stimuli due to the dysregulation of the immune response. The clinical studies of COVID-19 have shown that cytokine storms are common in severely infected patients, which could cause damage to pulmonary capillary endothelial cells, lung and alveolar epithelial cells, resulting in acute respiratory distress syndrome or multiple organ dysfunction syndromes. The cytokine storm is closely related to the severity of the patient's condition, and the concentration of IL-1β and other inflammatory cytokines in the patient's blood is significantly higher than that of healthy adults [42]. The infiltration of inflammatory cells is one of the signs of lung injury [22]. Our previous research showed that baicalein had anti-inflammatory effects in some disease models [43], [44], [45]. In the LPS-induced acute lung injury model, baicalein significantly inhibited the inflammatory cell infiltration, and decreased the levels of IL-1β and TNF-α in serum, suggesting promising inhibitory effects on the release of cytokines. And baicalein also improved respiratory function of lung injury in mice by significantly shortening EEP, reducing PEF, and Penh, thus alleviating the acute lung injury induced by LPS. Baicalein might have multiple effects in the treatment of COVID-19, including direct antiviral effects and inflammation inhibition effects.
Since the pandemic of COVID-19, crystal structures of multiple therapeutic targets have been reported, which is meaningful for studying the action mechanism of drugs and designing new drugs based on these targets. The highly conserved Mpro of SARS-CoV-2 is important in producing viral protein. Baicalein has been confirmed to bind to Mpro with high affinity by X-ray protein crystallography method [39]. The IC50 for baicalin to inhibit the Mpro activity of SARS-CoV-2 was 0.39 μM [46].
In the study, baicalein was found to protect the Vero cells against SARS-CoV-2 at a concentration of 0.1 μM and above. Baicalein significantly inhibited the body weight loss, the replication of the virus in lung tissue, and relieved the lung lesions caused by SARS-CoV-2 in hACE2 transgenic mice. Furthermore, baicalein also improved the lung respiratory function, inhibited inflammatory cell infiltration, and decreased the levels of IL-1β and TNF-α in serum in LPS-induced acute lung injury of mice. Taken together, baicalein might be a potential therapeutic drug for the treatment of COVID-19.
CRediT authorship contribution statement
Junke Song: Methodology, Data curation, Writing - original draft, Writing - review & editing. Li Zhang: Methodology, Validation, Investigation. Yanfeng Xu: Methodology. Dezhi Yang: Methodology, Validation, Investigation. Li Zhang: Methodology. Shiying Yang: Methodology. Wen Zhang: Methodology, Data curation. Jinhua Wang: Methodology. Shuo Tian: Methodology. Shengqian Yang: Methodology. Tianyi Yuan: Methodology. Ailin Liu: Methodology. Qi Lu: Methodology. Fengdi Li: Methodology. Hongqi Liu: Methodology. Biyu Hou: Methodology. Xiaozhong Peng: Conceptualization, Methodology. Yang Lu: Conceptualization, Project administration. Guanhua Du: Conceptualization, Project administration, Funding acquisition, Writing - original draft, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by grants from the Chinese Academy of Medical Sciences Emergency Project of COVID-19 (2020HY320001), CAMS Innovation Fund for Medical Sciences (2017-I2M-1-010, and 2016-I2M-3-007, China), and Technology Major Projects for “Major New Drugs Innovation and Development” (2018ZX09711001-012).
References
- 1.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 2.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhatraju P.K., Ghassemieh B.J., Nichols M., Kim R., Jerome K.R., Nalla A.K. Covid-19 in critically ill patients in the Seattle region - case series. N. Engl. J. Med. 2020;382(21):2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu J., Cao R., Xu M., Wang X., Zhang H., Hu H. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:16. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang M., Li M., Xiao F., Pang P., Liang J., Tang T. Preliminary evidence from a multicenter prospective observational study of the safety and efficacy of chloroquine for the treatment of COVID-19. Nat. Sci. Rev. 2020;7(9):1428–1436. doi: 10.1093/nsr/nwaa113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong Y.K., Yang J., He Y. Caution and clarity required in the use of chloroquine for COVID-19. Lancet Rheumatol. 2020;2(5) doi: 10.1016/s2665-9913(20)30093-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang W., Cao Z., Han M., Wang Z., Chen J., Sun W. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ. 2020;369 doi: 10.1136/bmj.m1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun J., Chen Y., Fan X., Wang X., Han Q., Liu Z. Advances in the use of chloroquine and hydroxychloroquine for the treatment of COVID-19. Postgrad. Med. 2020:1–10. doi: 10.1080/00325481.2020.1778982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.C. Chen, Y. Zhang, J. Huang, P. Yin, Z. Cheng, J. Wu, et al., Favipiravir versus arbidol for COVID-19: A randomized clinical trial, medRxiv (2020), https://doi.org/10.1101/2020.03.17.20037432.
- 12.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G. A trial of lopinavir-ritonavir in adults hospitalized with severe covid-19. N. Engl. J. Med. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shen C., Wang Z., Zhao F., Yang Y., Li J., Yuan J. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323(16):1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duan K., Liu B., Li C., Zhang H., Yu T., Qu J. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc. Natl. Acad. Sci. U. S. A. 2020;117(17):9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ji S., Li R., Wang Q., Miao W.J., Li Z.W., Si L.L. Anti-H1N1 virus, cytotoxic and Nrf2 activation activities of chemical constituents from Scutellaria baicalensis. J. Ethnopharmacol. 2015;176:475–484. doi: 10.1016/j.jep.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 16.Xu G., Dou J., Zhang L., Guo Q., Zhou C. Inhibitory effects of baicalein on the influenza virus in vivo is determined by baicalin in the serum. Biol. Pharm. Bull. 2010;33(2):238–243. doi: 10.1248/bpb.33.238. [DOI] [PubMed] [Google Scholar]
- 17.Oo A., Teoh B.T., Sam S.S., Bakar S.A., Zandi K. Baicalein and baicalin as Zika virus inhibitors. Arch. Virol. 2019;164(2):585–593. doi: 10.1007/s00705-018-4083-4. [DOI] [PubMed] [Google Scholar]
- 18.Zandi K., Lim T.-H., Rahim N.-A., Shu M.-H., Teoh B.-T., Sam S.-S. Extract of Scutellaria baicalensis inhibits dengue virus replication. BMC Complement. Altern. Med. 2013;13(1):91. doi: 10.1186/1472-6882-13-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li M., Shi A., Pang H., Xue W., Li Y., Cao G. Safety, tolerability, and pharmacokinetics of a single ascending dose of baicalein chewable tablets in healthy subjects. J. Ethnopharmacol. 2014;156:210–215. doi: 10.1016/j.jep.2014.08.031. [DOI] [PubMed] [Google Scholar]
- 20.Pang H., Xue W., Shi A., Li M., Li Y., Cao G. Multiple-ascending-dose pharmacokinetics and safety evaluation of baicalein chewable tablets in healthy chinese volunteers. Clin. Drug Invest. 2016;36(9):713–724. doi: 10.1007/s40261-016-0418-7. [DOI] [PubMed] [Google Scholar]
- 21.Tian S., He G., Song J., Wang S., Xin W., Zhang D. Pharmacokinetic study of baicalein after oral administration in monkeys. Fitoterapia. 2012;83(3):532–540. doi: 10.1016/j.fitote.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 22.Bao L., Deng W., Huang B., Gao H., Liu J., Ren L. The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice. Nature. 2020;583(7818):830–833. doi: 10.1038/s41586-020-2312-y. [DOI] [PubMed] [Google Scholar]
- 23.Matute-Bello G., Downey G., Moore B.B., Groshong S.D., Matthay M.A., Slutsky A.S. An official American Thoracic Society workshop report: features and measurements of experimental acute lung injury in animals. Am. J. Respir. Cell Mol. Biol. 2011;44(5):725–738. doi: 10.1165/rcmb.2009-0210ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng Y., He G., Mu X., Zhang T., Li X., Hu J. Neuroprotective effect of baicalein against MPTP neurotoxicity: behavioral, biochemical and immunohistochemical profile. Neurosci. Lett. 2008;441(1):16–20. doi: 10.1016/j.neulet.2008.05.116. [DOI] [PubMed] [Google Scholar]
- 25.Mu X., He G.R., Yuan X., Li X.X., Du G.H. Baicalein protects the brain against neuron impairments induced by MPTP in C57BL/6 mice. Pharmacol. Biochem. Behav. 2011;98(2):286–291. doi: 10.1016/j.pbb.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 26.Mu X., He G., Cheng Y., Li X., Xu B., Du G. Baicalein exerts neuroprotective effects in 6-hydroxydopamine-induced experimental Parkinsonism in vivo and in vitro. Pharmacol. Biochem. Behav. 2009;92(4):642–648. doi: 10.1016/j.pbb.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y.H., Yu H.T., Pu X.P., Du G.H. Baicalein prevents 6-hydroxydopamine-induced mitochondrial dysfunction in SH-SY5Y cells via inhibition of mitochondrial oxidation and up-regulation of DJ-1 protein expression. Molecules. 2013;18(12):14726–14738. doi: 10.3390/molecules181214726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He X.L., Wang Y.H., Gao M., Li X.X., Zhang T.T., Du G.H. Baicalein protects rat brain mitochondria against chronic cerebral hypoperfusion-induced oxidative damage. Brain Res. 2009;1249:212–221. doi: 10.1016/j.brainres.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 29.Chen L., Dou J., Su Z., Zhou H., Wang H., Zhou W. Synergistic activity of baicalein with ribavirin against influenza A (H1N1) virus infections in cell culture and in mice. Antiviral Res. 2011;91(3):314–320. doi: 10.1016/j.antiviral.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 30.Hour M.J., Huang S.H., Chang C.Y., Lin Y.K., Wang C.Y., Chang Y.S. Baicalein, ethyl acetate, and chloroform extracts of Scutellaria baicalensis inhibit the neuraminidase activity of pandemic 2009 H1N1 and seasonal influenza a viruses. Evid. Based Complement. Alternat. Med. 2013;2013 doi: 10.1155/2013/750803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Su Z.Z., Dou J., Xu Z.P., Guo Q.L., Zhou C.L. A novel inhibitory mechanism of baicalein on influenza A/FM1/1/47 (H1N1) virus: interference with mid-late mRNA synthesis in cell culture. Chin. J. Nat. Med. 2012;10(6):415–420. doi: 10.1016/S1875-5364(12)60081-8. [DOI] [Google Scholar]
- 32.Sithisarn P., Michaelis M., Schubert-Zsilavecz M., Cinatl J., Jr. Differential antiviral and anti-inflammatory mechanisms of the flavonoids biochanin A and baicalein in H5N1 influenza A virus-infected cells. Antiviral Res. 2013;97(1):41–48. doi: 10.1016/j.antiviral.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 33.Fesen M.R., Pommier Y., Leteurtre F., Hiroguchi S., Yung J., Kohn K.W. Inhibition of HIV-1 integrase by flavones, caffeic acid phenethyl ester (CAPE) and related compounds. Biochem. Pharmacol. 1994;48(3):595–608. doi: 10.1016/0006-2952(94)90291-7. [DOI] [PubMed] [Google Scholar]
- 34.Hu J.Z., Bai L., Chen D.G., Xu Q.T., Southerland W.M. Computational investigation of the anti-HIV activity of Chinese medicinal formula Three-Huang Powder. Interdiscip Sci. 2010;2(2):151–156. doi: 10.1007/s12539-010-0074-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zandi K., Teoh B.T., Sam S.S., Wong P.F., Mustafa M.R., Abubakar S. Novel antiviral activity of baicalein against dengue virus. BMC Complement. Altern. Med. 2012;12:214. doi: 10.1186/1472-6882-12-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moghaddam E., Teoh B.T., Sam S.S., Lani R., Hassandarvish P., Chik Z. Baicalin, a metabolite of baicalein with antiviral activity against dengue virus. Sci. Rep. 2014;4:5452. doi: 10.1038/srep05452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dou J., Chen L., Xu G., Zhang L., Zhou H., Wang H. Effects of baicalein on Sendai virus in vivo are linked to serum baicalin and its inhibition of hemagglutinin-neuraminidase. Arch. Virol. 2011;156(5):793–801. doi: 10.1007/s00705-011-0917-z. [DOI] [PubMed] [Google Scholar]
- 38.Johari J., Kianmehr A., Mustafa M.R., Abubakar S., Zandi K. Antiviral activity of baicalein and quercetin against the Japanese encephalitis virus. Int. J. Mol. Sci. 2012;13(12):16785–16795. doi: 10.3390/ijms131216785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.H. Su, S. Yao, W. Zhao, M. Li, J. Liu, W. Shang, et al., Discovery of baicalin and baicalein as novel, natural product inhibitors of SARS-CoV-2 3CL protease in vitro, bioRxiv (2020), https://doi.org/10.1101/2020.04.13.038687.
- 40.Xing J., Chen X.-Y., Zhong D.-F. Absorption, excretion and enterohepatic circulation of baicalein in rats. Asian J. Pharmacodyn. Pharmacokinet. 2006;6:29–37. doi: 10.1016/j.lfs.2005.04.072. [DOI] [Google Scholar]
- 41.Tian S., Du L.-D., Wang S.-B., He G.-R., Yang T., Li X.-X. Pharmacokinetic study of baicalein and its major metabolites after iv administration in dogs. Chin. Herb. Med. 2011;3(3):196–201. doi: 10.3969/j.issn.1674-6384.2011.03.005. [DOI] [Google Scholar]
- 42.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Duan D.D., Wang K.X., Zhou Y.Z., Qin X.M., Gao L., Du G.H. Baicalein exerts beneficial effects in d-galactose-induced aging rats through attenuation of inflammation and metabolic dysfunction. Rejuvenation Res. 2017;20(6):506–516. doi: 10.1089/rej.2017.1919. [DOI] [PubMed] [Google Scholar]
- 44.Zhang X., Yang Y., Du L., Zhang W., Du G. Baicalein exerts anti-neuroinflammatory effects to protect against rotenone-induced brain injury in rats. Int. Immunopharmacol. 2017;50:38–47. doi: 10.1016/j.intimp.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 45.Yang S., Wang H., Yang Y., Wang R., Wang Y., Wu C. Baicalein administered in the subacute phase ameliorates ischemia-reperfusion-induced brain injury by reducing neuroinflammation and neuronal damage. Biomed. Pharmacother. 2019;117 doi: 10.1016/j.biopha.2019.109102. [DOI] [PubMed] [Google Scholar]
- 46.H. Liu, F. Ye, Q. Sun, H. Liang, C. Li, R. Lu, et al., Scutellaria baicalensis extract and baicalein inhibit replication of SARS-CoV-2 and its 3C-like protease in vitro, bioRxiv (2020), https://doi.org/10.1101/2020.04.10.035824. [DOI] [PMC free article] [PubMed]