Abstract
Background
Since the beginning of 21st century, several major public health emergencies (PHEs) have threatened the health of people globally. Posttraumatic stress symptoms (PTSS) was one of the most concerned mental health problems. The objective of this study is to systematically estimate the prevalence of PTSS under the influence of PHEs.
Method
We searched both English and Chinese databases. This meta-analysis used a random-effects model to estimate the prevalence of PTSS. Subgroup analyses were conducted to analyze the source of heterogeneity. Meta-regression model was used to calculate the proportion of the variance explained by subgroup moderators.
Results
Forty eligible studies (n = 15,538) were identified. The results revealed a pooled prevalence of PTSS of 17.0% (95%CI: 13.5%–21.2%), higher than that of previous epidemiological survey, with high between-studies heterogeneity (Q = 1199, I2 = 96.75%, p < .001). There was variance of prevalence in different countries (4.0%–36.5%) and epidemics (12.1%–36.5%). The prevalence of PTSS showed the feature of fluctuation in the change of time (Q = 6.173, p = .290). Patients had higher prevalence (26.2%) compared to healthcare workers (HCWs) (18.5%) and community samples (12.4%) and frontline HCWs had marginally significantly higher estimated rate than general HCWs (22.2%, 95%CI:16.0%–30.1% vs. 10.4%, 95%CI: 6.4%–16.6%). The variance of prevalence screened by interview and self-reported was significant (Q = 3.393, p = .05) and studies with higher quality possessed lower prevalence (high:12.4%; moderate: 17.3%; low: 18.0%). The total variance explained by subgroup moderators was estimated 64% by meta regression model.
Limitations
Limitations include high level of heterogeneity between studies and within subgroups as well as the lack of studies with high quality and using probability sampling.
Conclusions
This study suggested that the PTSS was common under the influence of PHEs. It was crucial to further explore the psychological mechanism and effective strategies for prevention and intervention in future research with more high-quality studies.
Keywords: Posttraumatic stress symptoms, Public health emergency, Systematic review, Meta-analysis
Highlights
-
•
Experience of PTSS was relatively common (17.0%) under the influence of PHEs.
-
•
Subgroup moderators could explain 64% of the variance across studies.
-
•
The prevalence of PTSS was higher in patients and frontline healthcare workers.
-
•
More attentions should be paid to post-PHE trauma-related psychiatric symptoms.
1. Introduction
Public health emergency (PHE) refers to the sudden occurrence of major infectious diseases such as congregative disease of unknown reasons, serious food and occupational poisoning, and other events that severely threaten public health (State Council of the people’s Republic of China, 2011). Since the beginning of the 21st century, several major PHEs related to infectious disease have challenged public health globally (Holshue et al., 2020; Lai, Shih, Ko, Tang, & Hsueh, 2020; Remuzzi & Remuzzi, 2020). The ongoing PHE is coronavirus disease-2019 (COVID-19) with the first confirmed case reported in December 2019. The COVID-19 was declared a global pandemic on March 11(Director-General, 2020). Up to April 15, the confirmed COVID-19 global cases were 1,914,916 with 123,010 deaths (WHO, 2020a). Several pandemics emerged over the past two decades including SARS, Middle East respiratory syndrome (MERS), (Drosten et al., 2003; Zaki, van Boheemen, Bestebroer, Osterhaus, & Fouchier, 2012) and Ebola virus disease (EVD), formerly known as Ebola hemorrhagic fever, which started in Guinea 2014, then spread to Sierra Leone and Liberia (Holmes, Dudas, Rambaut, & Andersen, 2016). SARS has caused 8422 global cumulative cases and 916 deaths by August 7 in 2003 (WHO, 2003). At the end of September 2019, a total of 2468 laboratory-confirmed cases of MERS, including 851 associated deaths (case–fatality rate: 34.4%) were reported globally (WHO, 2019). The Ebola epidemic in Democratic Republic of the Congo has been around since 2018 (Ebola Outbreak Epidemiology Team, 2018), with 3456 confirmed cases and 2266 deaths reported up to April 12 (WHO, 2020). These major PHEs have challenged the development of human society in the new era.
Mental health consequences associated with PHEs need to be addressed as much as physical health consequences(Chen et al., 2020; Shultz, Baingana, & Neria, 2015; Yang et al., 2020). PHEs exposed individuals to multiple stressors including physical health consequences associated with disease (Zhou et al., 2020), anxiety over getting infected (Asmundson & Taylor, 2020), social isolation(Brooks et al., 2020), depression due to quarantine(Hawryluck et al., 2004), and other stressful events arising during epidemic (e.g., unemployment and loss of loved ones)(Chaves, Castellanos, Abrams, & Vazquez, 2018). One common psychological distress resulted from PHEs is posttraumatic stress disorder (PTSD), a psychological disorder that occurs after traumatic events (Lötsch, Schnyder, Goorhuis, & Grobusch, 2017; Rogers et al., 2020). Chan and Huak reported that two months after SARS outbreak, around 20% of 661 healthcare workers (HCWs) of a medium–size regional general hospital in Singapore, had a probable PTSD diagnosis (Chan & Huak, 2004). Hawryluck et al. (2004) examined the psychological effects of quarantine during SARS epidemic among individuals in Toronto, Canada, and found that 28.9% of 129 participants showed symptoms of PTSD.
Diagnosis of PTSD could be conducted by psychiatrists using uniform diagnostic criteria for symptoms (e.g. Chinese Classification and Diagnostic Criteria of Mental Disorders 3rd edition (CCMDIII), DSM-IV). Having individuals complete a self-rated psychometric scale and seeing if they met the cut-off score of PTSD could lead to a probable PTSD diagnosis (Hawryluck et al., 2004; Su et al., 2007). Individuals being diagnosed with PTSD or met cut-off score were considered to have significant posttraumatic stress symptoms (PTSS). Due to more cautious considerations on clinical diagnosis, the heterogeneity of PTSS prevalence rate caused by screening method is high (Lancee et al., 2008; Maunder et al., 2006).
In addition, the prevalence rate of PTSS under PHEs varied across time course of the PHE and population group. The number of follow-up studies on PTSS prevalence was limited and shows inconsistent results. For example, Lee et al. found that the prevalence rate of PTSS decreased over time after MERS (12–18 months: 42%–27%)(Lee et al., 2019), but another study tracked the prevalence of PTSS in SARS convalescent patients and found the rate to be stable at different time points: 40% (2.5 months), 41% (7 months), 39% (10 months), 40% (24 months), and 39% (46 months)(Hong et al., 2009). The prevalence rate of PTSS tends to be high among patients (Zhang et al., 2005). Shi et al. (2005) found that the prevalence rate in patients, first-line HCWs, second-line HCWs, general HCWs, and colleges students after SARS epidemic were 50%, 5%, 5%, 0%, and 7%, respectively. Liu et al. (2020) investigated the prevalence of PTSS in residents during COVID-19 epidemic in Wuhan, China, and found the rate was 7%. Moreover, it was still unexplored whether the prevalence under PHEs were affected by different country and epidemic.
A systematic review evaluated the psychological impact of deploying in support of the U.S. Response to Ebola by analyzing articles related to infectious disease and HCWs. It was estimated that effect size for PTSD was relatively small and non-significant (SMD = 0.12, 95%CI = −0.23 to 0.47, HCWs vs. low-risk control group)(Vyas, Delaney, Webb-Murphy, & Johnston, 2016). Another study systematically reviewed the long-term neuropsychological effects of the Ebola outbreak on survivors and found that PTSD was one of the five most common sequelaes (Lötsch et al., 2017). To our knowledge, there is still a lack of the overall estimation of the prevalence rate of PTSS under the influence of major PHEs. To fill this gap, we conducted a meta-analytic review to improve our understanding of trauma-related mental health related to PHEs and further explored variation in prevalence by country, epidemic, time period, population group, screening method, and study quality.
2. Method
We followed Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Moher, Liberati, Tetzlaff, Altman, & Prisma Group, 2009) and Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines (Stroup et al., 2000).
2.1. Search strategy
We conducted a systematic review using Pubmed, PsychoINFO, EMBASE, and SCOPUS for publications in English. Publications in Chinese were searched through China National Knowledge Infrastructure Database (CNKI), the Wanfang database, and China Science and Technology Journal Database. No restrictions on publication type that conference proceedings and dissertations were also included. The searches were concluded by March 21, 2020. Two unpublished manuscripts were included with the approval of their authors (Sun et al., 2020a, Sun et al., 2020b). The search terms for PHE were: (Severe Acute Respiratory Syndrome virus) OR (SARS) OR (SARS Coronavirus) OR (Hemorrhagic Fever) OR (Ebola) OR (Ebolavirus) OR (Avian Influenza) OR (Influenza in Bird) OR (Avian Flu) OR (Fowl Plague) OR (Bird flu) OR (MERS) OR(Middle East respiratory syndrome) OR (2019 novel coronavirus infection) OR (COVID) OR (COVID-2019) OR (coronavirus disease 2019) OR (coronavirus disease-19) OR (2019-nCoV) OR (2019 novel coronavirus disease); search terms for PTSS were: (PTSD) OR (Posttraumatic stress disorder) OR (Post-traumatic stress disorder) OR (Posttraumatic). See Supplementary Materials for details of search terms used for each database.
2.2. Inclusion/exclusion criteria
The following inclusion criteria were applied:1) participants under the influence of PHE; 2) prevalence of PTSS in study samples being surveyed and reported; 3) use of measures that have good psychometrics to assess PTSS or retrieve from reliable records.
The following studies were excluded from this meta-analysis:1) commentary, editorial, case report, or review; 2) not reporting prevalence data; 3) studies explicitly referring to different disorders, such as depressive symptoms. 4) articles that could not be retrieved in full-text form through online databases, library requests or email correspondence with the authors of the studies.
2.3. Data extraction and coding
Two authors screened the title and/or abstracts independently, and then the full text of the studies was retrieved and independently assessed based on the inclusion and exclusion criteria. The two independent authors also applied a uniform data extraction form to record data such as authors, publication year, countries, epidemic, time after epidemic, study participants, screen tools, sample size, the prevalence of PTSS and other subgroup data, etc. For studies (k = 3) with multiple assessment points, a mean value of combined results was calculated when estimating the overall prevalence rate; all time points were used as a separate study when estimating the prevalence rate across different time points. The ‘time after epidemic’ was estimated based on information provided by articles and records on the World Health Organization website (e.g. summary table of SARS cases by country)(WHO, 2003). When the same samples were assessed using multiple assessments, a mean value of combined prevalence rate was calculated. When multiple studies were based on the same dataset, the study with a larger sample size was included. Interrater reliability was calculated for continuous variables (e.g. event rate, sample size) using intraclass correlation coefficients (ICC) and for categorical moderators (e.g. study quality, country, epidemic). Interrater agreement was high (ICC = 0.99 and Kappa = 0.77 for continuous and categorical variables, respectively) across studies, and discrepancies were resolved through discussion.
We divided the participants in the studies into three categories: patients, healthcare workers (HCWs), and community samples. Patients referred to individuals with diseases related to PHEs. HCWs referred to individuals who provided medical services. Frontline HCWs referred to medical workers who had direct contact with patients. Community samples included individuals who were in quarantine, college students, residents, persons visiting the community primary health care, and patients in psychiatric department (mixed sample).
2.4. Quality assessment
The quality of each study was assessed by a Risk of Bias Tool for prevalence studies developed by Hoy et al. (2012). The tool consists of 10 items, divided into external validity and internal validity subscales. The external validity subscale has four items, including representation of the national population, sampling frame, random sampling, and nonresponse bias. The internal validity subscale has six items: data collected directly from the subjects or a proxy, case definition, quality of instruments, consistency of data collection mode, the length of the prevalence period, and the calculation of prevalence. Each item was assigned a score of 1 (yes, high quality) or 0 (no, low quality), thus the total score ranged from 1 to 10. Consistent with previous studies (Tang, Tang, Ren, & Wong, 2019), the quality of each study was classified as high (> 8), moderate (6–8), or low (≤5). Two investigators (Yaoguang Zhou and Luna Sun) independently assigned the scores, and inconsistencies were resolved by discussion. The detail of quality assessment is shown in Supplementary Materials.
2.5. Meta-analytic procedures
Due to the between-studies heterogeneity, a random effects model was applied to estimate the mean value of prevalence rate, and it gave an overall estimate across studies weighted by sampling error and true variance (Borenstein, Hedges, & Rothstein, 2007). The Q-value was chosen as an indicator of heterogeneity, with p less than 0.05 suggesting a significant heterogeneity across studies. In addition, we made a raw comparison between the estimated mean prevalence rate with the PTSD prevalence rate in National Epidemiologic Survey(NES)(Pietrzak, Goldstein, Southwick, & Grant, 2011). NES refers to an up-to-date assessment of the lifetime prevalence and Axis I comorbidity of DSM-IV PTSD and partial PTSD, with a representative sample of the civilian, noninstitutionalized U.S. population (n = 34,653).
We performed subgroup analysis stratified by countries, epidemic, time periods, population groups, screening method, and study quality. Since there were more homogeneous groups in included studies (i.e. studies from China, studies in SARS, studies within 6 months after epidemic, studies in sample of community samples, HCWs, and patients), sensitivity analyses were conducted with subgroup moderators mentioned above in those groups. Sensitivity analyses were also performed by leave one out analysis. Meta-regression model was built by using maximum likelihood calculation to find moderators that explained the majority of the variance across studies. Publication bias was assessed using funnel plots and Egger's tests. Comprehensive Meta-Analysis (V3, Biostat, Englewood, NJ, USA) was used for the meta-analyses.
3. Results
3.1. Study selection and study characteristics
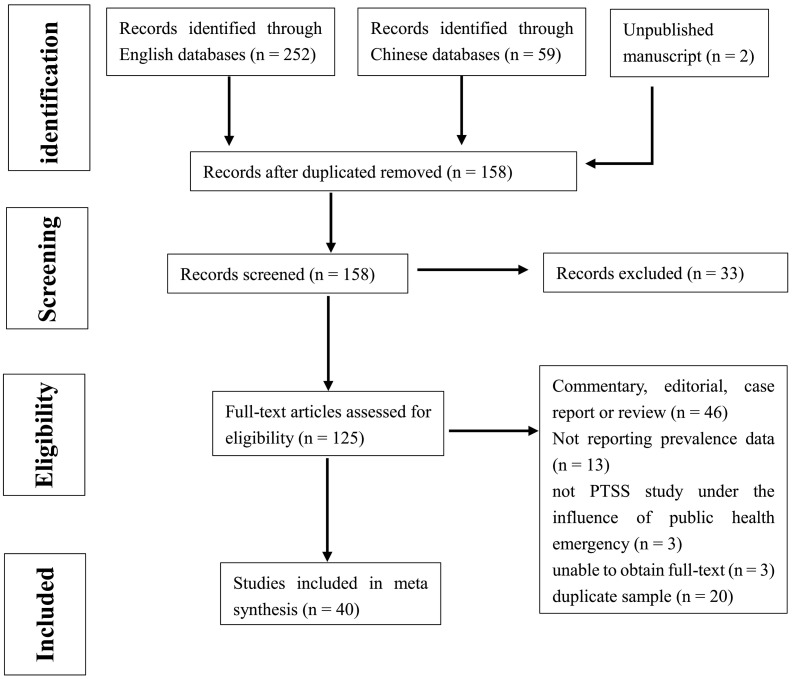
The screening process is shown in Fig. 1 . Based on the search terms, we found a total of 158 non-duplicate articles. Of these, 33 were excluded by screening titles and abstracts, leaving a total of 125 full texts to be assessed for eligibility. After the full text screening, 40 articles met the inclusion criteria. Of the 85 articles that were excluded, 46 were commentary, editorial, case report, or review, 13 did not report prevalence data, 3 were not PTSS study conducted under the influence of public health emergency, 20 had duplicate samples and 3 were not accessible in full-text-form.
Fig. 1.
PRISMA Flowchart of Study Selection.
Table 1 shows 40 included studies with a total of 15,538 participants. Sample sizes ranged from 29 to 3564. These studies were from 8 countries and 5 PHEs (SARS, Ebola, Crimean-Congo hemorrhagic fever, MERS, COVID-19), with three major population groups participating in these studies: patients, HCWs, and community samples. Fourteen studies obtained their samples from patients, 17 from HCWs and 13 from community samples. The investigation was conducted on 0–46 months from the time of epidemic. Dates of publication ranged from 2004 to 2020. Ten self-report scales were used to identify PTSS: The Impact of Event Scale—Revised (IES-R), the Impact of Event Scale (IES), the PTSD checklist (PCL), the PTSD self-rating scale (PTSD-SS), Davidson Trauma Scale-Chinese version(DTS-C), the posttraumatic diagnostic scale for DSM-5 (PDS-5), the Impact of Event Scale-6 (IES-6, a shortened version of the full IES-R), the PTSD Checklist-Specific (PCL-S), PTSD checklist Cuvukuab version (PCL—C), and PTSD checklist for DSM-5(PCL-5). Three interview scales were used to identify PTSD diagnose: Composite International Diagnostic Interview-PTSD, Version 2.1 (CIDI 2.1), Clinician-Administered PTSD Scale (CAPS), and PTSD symptom scale–Interview (PSS-I). Regarding study quality, 3 out of 40 studies are of high quality, 34 are of moderate quality, and 3 are of low quality.
Table 1.
Study characteristics of the included studies.
| Author(s) year | Country | epidemic | Time after epidemic (month) |
participants | Screening tool | Cutoffs | Valid sample size | Cases above cutoffs | prevalence | Quality rating |
|---|---|---|---|---|---|---|---|---|---|---|
| Chan & Huak, 2004 | Singapore | SARS | 2 | HCWs | IES | 30 | 661 | 127 | 0.19 | 7 |
| Hawryluck et al., 2004 | Canada | SARS | 0 | persons in quarantine | IES-R | 20 | 129 | 35 | 0.27 | 5 |
| Sim et al., 2004 | Singapore | SARS | 0 | medical stuff | IES-R | DMS-IV criteria | 277 | 26 | 0.09 | 7 |
| Tham et al., 2004 | Singapore | SARS | 6 | HCWs | IES | 26 | 96 | 17 | 0.18 | 7 |
| Cai et al., 2004 | China | SARS | 10 | patients | NR | NR | 29 | 1 | 0.03 | 6 |
| Lau et al., 2005 | China | SARS | 6 | residents | IES | NR | 818 | 128 | 0.16 | 5 |
| Wu et al., 2005 | China | SARS | 1 | patients | IES-R | 2(mean) | 195 | 11 | 0.06 | 8 |
| Liu et al., 2005 | China | SARS | 3 | patients | IES-R | 20 | 117 | 65 | 0.56 | 7 |
| Shi et al., 2005 | China | SARS | 6 | patients, HCWs, college students | PCL | NR | 162 | 9 | 0.06 | 7 |
| Yan et al., 2005 | China | SARS | 3 | patients | CIDI2.1 | NR | 286 | 28 | 0.10 | 7 |
| Zhang et al., 2005 | China | SARS | 3 | patients, HCWs, community sample | IES-R | 20 | 296 | 115 | 0.39 | 7 |
| Kwek et al., 2006 | Singapore | SARS | 3 | patients | IES | 26 | 63 | 26 | 0.41 | 7 |
| Lee et al., 2006 | China | SARS | 2 | residents | IES-R | 26 | 146 | 13 | 0.09 | 7 |
| Maunder et al. 2006a | Canada | SARS | 19 | HCWs | IES | 26 | 769 | 96 | 0.12 | 7 |
| Gao et al., 2006 | China | SARS | 3 | patients | PTSD-SS + CCMDIII criteria | 48 | 67 | 31 | 0.46 | 8 |
| 12 | 67 | 26 | 0.39 | |||||||
| ≥12 | 67 | 37 | 0.55 | |||||||
| Lin et al., 2007 | China | SARS | 1.5 | HCWs | DTS-C | 40 | 83 | 16 | 0.19 | 7 |
| Su et al., 2007 | China | SARS | 0 | nurses | DTS-C | 23 | 102 | 29 | 0.28 | 7 |
| Yang et al., 2007 | China | SARS | 12 | HCWs | CCMDIII criteria | – | 112 | 5 | 0.04 | 7 |
| Lancee et al. 2008a | Canada | SARS | 19 | HCWs | CAPS | – | 139 | 2 | 0.01 | 7 |
| Reynolds et al., 2008 | Canada | SARS | 1 | persons in quarantine | IES-R | 20 | 1057 | 148 | 0.14 | 8 |
| Hong et al., 2009 | China | SARS | 2.5 | patients | IES + CCMDIII criteria | NR | 70 | 28 | 0.40 | 8 |
| 7 | 61 | 25 | 0.41 | |||||||
| 10 | 57 | 22 | 0.39 | |||||||
| 24 | 58 | 23 | 0.40 | |||||||
| 46 | 57 | 24 | 0.39 | |||||||
| Mak et al., 2009 | China | SARS | 30 | patients | IES-R | 2(mean) | 90 | 23 | 0.26 | 8 |
| Wu et al., 2009 | China | SARS | 36 | hospital employees | IES-R | 20 | 549 | 55 | 0.10 | 9 |
| Sim et al., 2010 | Singapore | SARS | 2 | persons visiting the community primary health care | IES-R | DMS-IV criteria | 415 | 107 | 0.26 | 7 |
| Gul et al., 2012 | Turkey | Crimean-Congo hemorrhagic fever | 12 | patients | DSM-IV-TR criteria | – | 54 | 10 | 0.19 | 7 |
| Betancourt et al., 2016 | Sierra Leone | Ebola | 0 | general public | PSS-I | NR | 1008 | 114 | 0.11 | 10 |
| Keita, Taverne, et al., 2017 | Guinea | Ebola | 0 | patients in psychiatric department | NR | NR | 68 | 4 | 0.04 | 7 |
| Keita et al.(2)2017 | Guinea | Ebola | 24 | patients with depressed symptom | NR | NR | 33 | 3 | 0.09 | 5 |
| Colorado, 2018 | Sierra Leone | Ebola | 0 | aid workers | PDS-5 | 30 | 403 | 163 | 0.40 | 7 |
| Jalloh et al., 2018 | Sierra Leone | Ebola | 0 | general public | IES-6 | 9 | 3564 | 570 | 0.16 | 9 |
| Lee et al., 2018 | Korea | MERS | 0 | hospital workers | IES-R | 26 | 359 | 183 | 0.51 | 7 |
| Sipos et al., 2018 | Liberia | Ebola | 0 | active duty soldiers,medical mission | PCL-S | 50 and DSM-IV criteria | 173 | 7 | 0.04 | 8 |
| Lee et al., 2019 | Korea | MERS | 12 | survivors | IES-R | 25 | 52 | 22 | 0.42 | 6 |
| 18 | 52 | 14 | 0.27 | |||||||
| Huang et al., 2020 | China | COVID-19 | 0 | HCWs | PTSD-SS | 50 | 230 | 63 | 0.27 | 8 |
| Jung et al., 2020 | Korea | MERS | 2 | nurses | IER-R | 25 | 147 | 37 | 0.25 | 7 |
| Liu et al., 2020 | China | COVID-19 | 0 | residents(hardest-hit) | PCL-5 | DSM-5 criteria | 285 | 20 | 0.07 | 8 |
| Sun, Yi, et al., 2020b | China | COVID-19 | 0 | residents | PCL-5 | 33 | 2091 | 96 | 0.05 | 8 |
| Li et al., 2020 | China | COVID-19 | 0 | nurses | PCL-C | 38 | 205 | 104 | 0.51 | 8 |
| Tian et al., 2020 | China | COVID-19 | 0 | villager | PTSD-SS | 50 | 87 | 2 | 0.02 | 7 |
| Sun, Sun, et al., 2020a | China | COVID-19 | 0 | patients | PCL-5 | DSM-5 criteria | 190 | 43 | 0.23 | 8 |
a. These two studies used different screening tools for the same participants. The mean value of the results was obtained when analyzing overall estimated pooled prevalence.
3.2. Prevalence of PTSS under the influence of PHEs
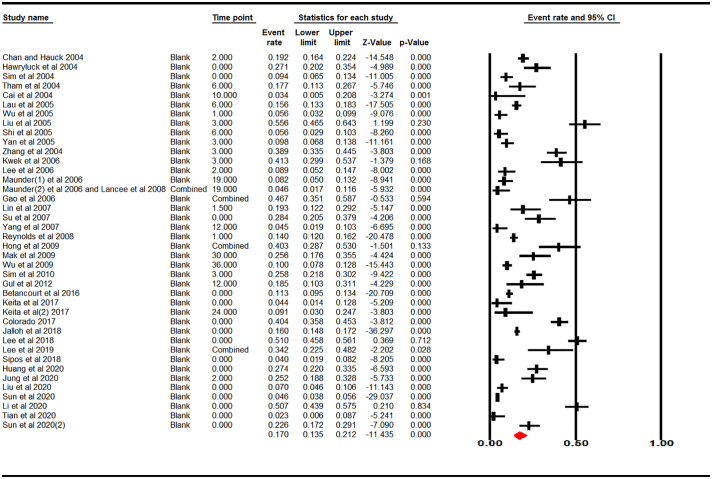
The overall pooled estimates of PTSS reported by 40 studies yielded a crude summary of prevalence of 17.0% (95% CI: 13.5%–21.2%) with significant heterogeneity present (Q = 1199, I2 = 96.75%, p < .001). See Fig. 2 .
Fig. 2.
Estimated prevalence of PTSS during or after PHE.
Note. Estimated prevalence = 0.170 (95% CI: 0.135–0.212).
Under a raw comparison (17.0% vs 6.6% and 6.4%), the 95%CI lower limit (13.5%) of estimated prevalence in current study is higher than the upper limit of prevalence of PTSD (9.9%) and partial PTSD (10.1%) in a National Epidemiologic Survey(NES), suggesting a significant difference on the level of α = 0.05. See Table 2 .
Table 2.
Raw comparison between NES of prevalence of PTSD and the PTSS prevalence in the current study.
| National Epidemiologic Surveya |
Current study |
||
|---|---|---|---|
| Partial PTSD | Full PTSD | PTSS | |
| n | 34,653 | 15,538 | |
| Prevalence rate | 0.066 | 0.064 | 0.170 |
| Lower limit(95%CI) | 0.031 | 0.029 | 0.135 |
| Upper limit(95%CI) | 0.101 | 0.099 | 0.212 |
a. NES refers to an up-to-date assessment of the lifetime prevalence and Axis I comorbidity of DSM-IV PTSD and partial PTSD, with a representative sample of the civilian, noninstitutionalized U.S. population.
3.3. Subgroup analysis
To further determine the source of heterogeneity, meta-analyses stratified by multiple moderators were conducted (See Table 3 ). When stratified by countries, there was significant heterogeneity in the estimates of PTSS prevalence (p < .001). Specifically, the prevalence of each country were as follows: Canada, 12.4% (95CI%: 7.0%–21.2%); China, 16.2% (95CI%: 10.7%–23.8%); Guinea, 6.3% (95CI%: 2.9%–13.4%); Korea, 36.5% (95CI%: 20.6%–56.0%); Liberia, 4.0% (95CI%: 1.9%–8.2%); Sierra Leone, 20.3% (95CI%: 10.0%–33.6%); Singapore, 20.8% (95CI%: 14.1%–29.5%); Turkey, 18.5% (95CI%: 10.3%–31.1%). When stratified by China vs other countries (16.2%, k = 21 vs 17.8%, k = 19), there was no significant heterogeneity between two groups (p = .700). Between-group Q value was reduced to 0.418 (Q value was 31.931 when grouping by different countries). Subsequent analysis of prevalence of PTSS on different variables among studies from China was presented at Table S1.
Table 3.
Estimated PTSS prevalence on different variables.
| k | n | Estimated rate | Lower limit (95% CI) |
upper limit (95% CI) |
Q-value | p for heterogeneity test |
|
|---|---|---|---|---|---|---|---|
| Country | |||||||
| Canada | 4 | 1955 | 0.124 | 0.070 | 0.212 | 27.574 | <0.001 |
| China | 21 | 6210 | 0.162 | 0.107 | 0.238 | 700.040 | <0.001 |
| Guinea | 2 | 101 | 0.063 | 0.029 | 0.134 | 0.836 | 0.361 |
| Korea | 3 | 558 | 0.365 | 0.206 | 0.560 | 28.333 | <0.001 |
| Liberia | 1 | 173 | 0.040 | 0.019 | 0.082 | 0.000 | 1.000 |
| Sierra Leone | 3 | 4975 | 0.203 | 0.100 | 0.336 | 163.101 | <0.001 |
| Singapore | 5 | 1512 | 0.208 | 0.141 | 0.295 | 42.938 | <0.001 |
| Turkey | 1 | 54 | 0.185 | 0.103 | 0.311 | 0.000 | 1.000 |
| Between group | 31.931 | <0.001 | |||||
| China vs other countries | |||||||
| China | 21 | 6210 | 0.162 | 0.107 | 0.232 | 496.261 | <0.001 |
| Other countries | 19 | 9328 | 0.178 | 0.134 | 0.238 | 700.040 | <0.001 |
| Between group | 0.148 | 0.070 | |||||
| Epidemic | |||||||
| COVID-19 | 6 | 3088 | 0.135 | 0.046 | 0.331 | 370.286 | <0.001 |
| Crimean-Congo hemorrhagic fever | 1 | 54 | 0.185 | 0.103 | 0.311 | 0.000 | 1.000 |
| Ebola | 6 | 5294 | 0.121 | 0.067 | 0.210 | 188.901 | <0.001 |
| MERS | 3 | 558 | 0.365 | 0.206 | 0.560 | 28.833 | <0.001 |
| SARS | 24 | 6589 | 0.174 | 0.134 | 0.223 | 403.181 | <0.001 |
| Between group | 7.921 | 0.095 | |||||
| SARS vs other PHEs | |||||||
| SARS | 24 | 6589 | 0.174 | 0.134 | 0.223 | 795.223 | <0.001 |
| Other PHEs | 16 | 8949 | 0.165 | 0.106 | 0.246 | 403.181 | <0.001 |
| Between group | 0.049 | 0.826 | |||||
| Time period (month) | |||||||
| 0 | 15 | 9171 | 0.161 | 0.103 | 0.242 | 809.485 | <0.001 |
| 1–3 | 13 | 3603 | 0.237 | 0.171 | 0.320 | 262.458 | <0.001 |
| 4–6 | 3 | 1076 | 0.124 | 0.071 | 0.207 | 11.068 | 0.004 |
| 7–12 | 6 | 432 | 0.256 | 0.141 | 0.419 | 38.503 | <0.001 |
| 13–24 | 6 | 1118 | 0.192 | 0.079 | 0.397 | 76.584 | <0.001 |
| >24 | 3 | 696 | 0.230 | 0.086 | 0.484 | 45.095 | <0.001 |
| Between group | 6.173 | 0.290 | |||||
| Population group | |||||||
| Community sample | 13 | 9791 | 0.124 | 0.091 | 0.166 | 266.269 | <0.001 |
| Healthcare workers | 16 | 4383 | 0.185 | 0.127 | 0.262 | 468.199 | <0.001 |
| frontline HCWs | 13 | 1695 | 0.222 | 0.160 | 0.301 | 134.500 | <0.001 |
| general HCWs | 9 | 1193 | 0.104 | 0.064 | 0.166 | 33.132 | <0.001 |
| Between group | 6.788 | 0.009 | |||||
| patients | 14 | 1364 | 0.262 | 0.171 | 0.379 | 189.347 | <0.001 |
| Between group | 8.529 | 0.014 | |||||
| Screening method | |||||||
| Based on interview | 10 | 1886 | 0.108 | 0.063 | 0.181 | 92.905 | <0.001 |
| Based on self-report scale | 30 | 13,791 | 0.191 | 0.148 | 0.243 | 1085.232 | <0.001 |
| Between group | 3.693 | 0.051 | |||||
| Study quality | |||||||
| High | 3 | 5121 | 0.124 | 0.090 | 0.168 | 23.336 | <0.001 |
| Moderate | 34 | 9437 | 0.173 | 0.129 | 0.228 | 1045.855 | <0.001 |
| Low | 3 | 980 | 0.180 | 0.107 | 0.287 | 11.522 | 0.003 |
| Between group | 2.875 | 0.238 | |||||
When stratified by different PHEs, there was no significant heterogeneity in the estimates of PTSS prevalence (p = .095). Specifically, prevalence in each epidemic were as follows: COVID-19, 13.5% (95CI%: 4.6%–33.1%); Crimean-Congo hemorrhagic fever, 18.5% (95CI%: 10.3%–31.1%); Ebola, 12.1% (95CI%: 6.7%–21.0%); MERS, 36.5% (95CI%: 20.6%–56.0%); SARS, 17.4% (13.4%–22.3%). When stratified by SARS vs other PHEs (17.4%, k = 24 vs 16.5%, k = 16), heterogeneity was not significant (p = .826). Between-group Q value was reduced to 0.049 (Q value was 7.21 when grouping by different PHEs). Subsequent analysis of prevalence of PTSS on different variables among SARS-related studies was presented in Table S2.
The prevalence of PTSS fluctuated along with the time course. And there was no significant heterogeneity on different time period (p = .290). During the epidemic, the estimated PTSS prevalence rate was 16.1% (95CI%: 10.3%–24.2%). The estimated rate after 1–3 moths, 4–6 months, 7–12 months, 13–24 months, >24 months were respectively 23.7% (95CI%: 17.1%–32.0%), 12.4% (95CI%: 7.1%–20.7%), 25.6% (95CI%: 14.1%–41.9%), 19.2% (95CI%: 7.9%–39.7%), 23.0% (95CI%:8.6%–48.4%). Subsequent analysis of prevalence of PTSS on different variables among studies within 6 months was presented in Table S3.
The prevalence of PTSS among patients (26.2%, 95%CI: 13.1%–37.9%) was significantly higher than community samples (12.4%, 95%CI: 9.1%–16.6%), and HCWs had a moderate prevalence rate (18.5%, 95%CI: 12.7%–26.2%), with significant heterogeneity among these three groups (p = .014). In group of HCWs, frontline HCWS had marginally significantly higher PTSS prevalence rate than general HCWs (22.2%, 95%CI:16.0%–30.1% vs. 10.4%, 95%CI: 6.4%–16.6%). The heterogeneity was significant (p = .009). Subsequent analysis of prevalence of PTSS on different variables among different population groups was presented in Table S4-S6.
When stratified by screening method, the estimated pooled prevalence rate screened by interview was lower than that screened by self-report scale (10.8%, 95%CI: 6.3%–18.1% vs. 19.1%, 95%CI: 14.8%–24.3%), with marginally significant heterogeneity between these two groups (p = .051). In terms of study quality, there was no significant heterogeneity (p = .238) among studies with high (12.4%, 95%CI: 0.09%–16.8%), moderate (17.3%, 95%CI: 12.9%–22.8%) and low (18.0%, 95%CI: 10.7%–28.7%) quality, with higher quality possessing lower estimated prevalence rate.
3.4. Meta regression model
Subgroup moderators were all included as covariates of a meta regression model, except epidemic which caused high collinearity with other variables. Thirty-five studies were available from regression analysis based on datasets. The test of the model showed that Q = 50.93, df = 17, p < .001, suggesting the model was significantly efficient on explaining the variance across studies. The proportion of total between-study variance explained by the model was 64% (R2 = 0.64, See Table 4 ).
Table 4.
Summary of meta-regression model using country, time, population group, screening method and study quality to explain variance across studies.
| Covariates included sequentially a | k | ΔTau2 | ΔR2 | Test of a single covariate within model |
||
|---|---|---|---|---|---|---|
| Q value | df | p | ||||
| Country | 35 | −0.2317 | 0.25 | 10.51 | 7 | 0.1614 |
| Time | −0.0781 | 0.09 | 4.93 | 5 | 0.4246 | |
| Population group | −0.1715 | 0.18 | 11.59 | 2 | 0.0035 | |
| Screening method | −0.0740 | 0.08 | 6.90 | 1 | 0.0086 | |
| Study quality | −0.0338 | 0.04 | 3.43 | 2 | 0.1802 | |
| Tau2 | R2 | Test of the model | ||||
| The entire model | 0.3336 | 0.64 | 50.93 | 17 | <0.001 | |
a. The covariates were all included as dummy variables in the meta-regression model. Each had a reference group.
3.5. Sensitivity analysis
Sensitivity analysis, carried out leave one out analysis by omitting each study, demonstrated that no individual study affected the prevalence estimate of PTSS under the influence of PHE more than 4.7%. Details were presented in Fig. S1.
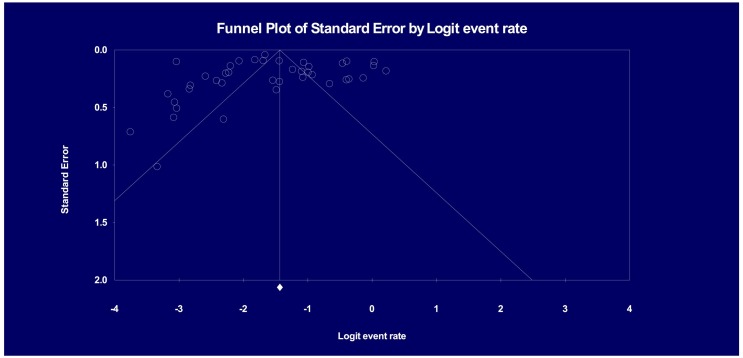
3.6. Publication bias
Visual inspection of the funnel plot of studies revealed significant asymmetry (see Fig. 3 ). Evidence of substantial publication bias was not identified using Egger's test (p = .41).
Fig. 3.
Funnel plot.
Note. Egger's Test of the Intercept shows intercept (B0) as-0.35502, with 95% confidence interval (−3.54659, 2.83655), t = 0.22519, df = 38. p = .41152.
4. Discussion
This systematic meta-analysis of 40 studies involving 15,538 participants under the influence of PHEs yielded an estimated prevalence of PTSS of 17.0%.
As reported in results, the Wave 2 NES on Alcohol and Related Conditions reported the lifetime full PTSD and partial PTSD were 6.4% and 6.6% in the United States, respectively (Pietrzak et al., 2011). Although the true deviation was obscure due to the bias confounded in meta-analytic result (e.g., mixed samples, time period), the prevalence of PTSS following PHEs was higher than the lifetime prevalence of general population. Furthermore, another study investigated the 12 month PTSD prevalence in the worldwide scale found that the prevalence rate was 1.1% in the total sample (Karam et al., 2014). Those evidences preliminarily demonstrated that more attention should be paid to the trauma-related psychological impacts caused by PHEs.
Subgroup analysis showed that the prevalence rate varied across countries and epidemics, but this variance became minimal when stratified by China and other countries (from 4.0%–36.5% to 16.2%–17.8%), and SARS and other PHEs (from 13.5–36.5% to 16.5%–17.4%). With increased studies included in each subgroup, the pooled prevalence was closer to the overall estimate of 17.0%, suggesting the representativeness of our results. However, the results should still be taken with caution due to the heterogeneity in each subgroup. Among the included studies, there were relatively high correlations between country and epidemic. For instance, the prevalence of PTSS under COVID-19 and SARS was mainly reported in China, while EVD was mainly reported in African countries. After controlling one variable (country or epidemic, Table S1-S2) or setting a multivariate regression model, our results preliminarily showed country or epidemic might not have a significant effect on prevalence rate; However, there was a considerable amount of within-group heterogeneity. Nevertheless, the cross-cultural variations in PTSS prevalence have been previously reported in multiple large-scale investigations. In 2005, Kessler and colleagues have reported lifetime PTSD prevalence in the USA of 7.8% and 12-month prevalence of 3.9%(Kessler et al., 2005). Creamer, Burgess, and McFarlane (2001) have reported a much lower 12-month PTSD prevalence (1.3%) in Australia, despite comparable levels of exposure to trauma. Relative to the USA, the prevalence of PTSD is lower in Canada (2.7% for woman and 1.2% for men) (Stein, Walker, Hazen, & Forde, 1997), China (0.2%) (Karam et al., 2014), and 12 European countries (1.1%) (Darves-Bornoz et al., 2008). Therefore, there was complexity in the cross-cultural differences on the morbidity of PTSD and it could be still implicated that trauma-related mental health emergency strategies towards PHEs ought to be rigorously planned on the reference to those differences. In addition, to truly understand the impact of different PHEs on PTSS, a comparative study of more homogenous population groups in different epidemics was needed but relevant research is rare.
The PTSS prevalence fluctuated over time, which was mainly related to mixed characteristics in general population samples, healthcare workers, and patients. The PTSS prevalence in the general population was relatively low, with the highest prevalence emerging on 1–3 months after epidemic, and only available for three time windows (0, 1–3, 4–6). There was a trend of declining in prevalence of HCWs and constant high level in patients. But the studies for each time window in each population group were highly heterogeneous or limited in numbers (Table S3-S6). Notably, there is a lack of obvious decline in prevalence rate, with peak observed among 7–12 months after epidemic. The presence of PTSS could be delayed relative to the occurrence of traumatic event(Carty, O'Donnell, & Creamer, 2006), along with the possibility of suffering from subsequent traumatic event (Pietrzak et al., 2011). Meanwhile, PTSS could exist in a long time after index trauma. For instance, according to an investigation in the US army, approximately 9000 veterans endured PTSS 40 years after the Vietnam War (Marmar et al., 2015). To more accurately describe the longitudinal trajectory of PTSS under the PHEs, future studies should conduct longer follow-up studies among different populations, as well as examine the protective and risk factor towards PTSS. In conclusion, these results preliminarily suggested that the prevalence was relatively high in a long time period. The wide range time effect of PTSS implicated the crucial need for consistent intervention and care towards posttraumatic psychiatry after PHEs.
Patients who had a higher risk of confronted with stressful experiences (e.g. fear of deterioration) and physical lesion, had the highest prevalence among participant groups. The estimated prevalence of patients in the current study was comparable to another systematic review that reported the prevalence of PTSD symptoms in adult critical care survivors was 19.8% (Righy et al., 2019). HCWs also had a relatively high prevalence of PTSS, with frontline HCWs yielding a higher estimated rate than general HCWs. As a systematic review concluded, work-related critical incidents are positively related to PTSS(de Boer et al., 2011).The front-line personnel are in direct contact with patients, facing greater challenges in work intensity and complexity, as well as safety protection. The PTSS of the community samples is relatively low, while the estimated rate was still higher than the majority of the other trauma-related conditions reported in a international survey(Kessler et al., 2017). Some effective control measures towards the epidemic, such as quarantine, may also be positively correlated with PTSS(Brooks et al., 2020). Hence it is necessary to develop targeted psychological protection in PHE based on the posttraumatic characteristics of different populations.
The prevalence screened by interview which was constantly regarded as a more objective way to evaluate the PTSS was estimated lower than by self-reported assessment. It could be inferred that positive PTSD confirmed by interview tended to be discreet in diagnostic process, for more comprehensive evaluation of psychopathological symptoms, traumatic events, and considerations of following consequences of a psychiatric verdict being needed (Bonfils et al., 2018), while the PTSS points rated by self-reported scales were constantly seen as a reference to the symptom severity. It was also demonstrated that studies with higher quality were estimated at a higher rate than lower ones, which is similar to another systematic review studying the prevalence of the depress symptoms in Chinese young adolescents (Tang et al., 2019). The above results suggested that the difference in psychometric measurements and the study quality may lead to bias in the estimated PTSS prevalence related to PHEs.
The strength of this study is thoroughly reviewed studies that examined PTSS under the influence of PHEs and applied a multivariate meta-regression model to investigate variances explained by subgroup moderators. Nevertheless, several limitations should be noted. First, there was a relatively high risk of bias in included studies, with only a few studies used probability sampling and had a high study quality. Second, there is a high heterogeneity (36%) among studies remaining largely unexplained by the variables investigated. Third, the main results remained to be updated for the COVID-19 epidemic since there are ongoing surveys concerning the trauma-related mental health. Fourth, the study was not pre-registered and might lead to potential bias by post hoc decisions in review methods (Liberati et al., 2009). Lastly, samples included in this study could not represent the general population, leading to an imbalance of sample composition and biased estimation of prevalence.
5. Conclusion
In summary, our study suggests that PTSS are frequently experienced by various populations under PHEs and the impact can be long-lasting. Several strategies can be taken to improve the accuracy of the estimated prevalence rate. For example, future studies may include larger-scale samples by probability sampling, use statistical methods to eliminate the variance between screening by interview and self-report, utilize measurements with good psychometric properties and valid cutoffs for the targeted population, and conduct a longer follow-up. Moreover, since the correlated factors of PTSS under the PHEs had been studied in previous articles, more high-quality investigations and systematic reviews are needed to explore the characteristics and mechanisms of PTSS under PHEs. Finally, more randomized controlled trials are needed to identify effective prevention, intervention, or treatment pointing at PTSS under the PHEs.
(Betancourt et al., 2016; Cai, Liu, Zhou, Liu, & Qin, 2004; Chan & Huak, 2004; Colorado, 2018; Gao et al., 2006; Gul et al., 2012; Hawryluck et al., 2004; Hong et al., 2009; Huang, Han, Luo, Ren, & Zhou, 2020; Jalloh et al., 2018; Jung, Jung, Lee, & Kim, 2020; Keita et al., 2017; Keita et al., 2017; Kwek et al., 2006; Lancee, Maunder, & Goldbloom, 2008; Lau et al., 2005; Lee et al., 2019; Lee, Kang, Cho, Kim, & Park, 2018; Lee, Chi, Chung, & Chou, 2006; Li et al., 2020; Lin et al., 2007; Liu et al., 2020; Liu, Lu, Xu, & Zhang, 2005; Mak, Chu, Pan, Yiu, & Chan, 2009; Maunder et al., 2006; Reynolds et al., 2008; Shi et al., 2005; Sim, Chan, Chong, Chua, & Soon, 2010; Sim, Phui, Yiong, & Soon, 2004; Sipos, Kim, Thomas, & Adler, 2018; Su et al., 2007; Sun et al., 2020a; Sun et al., 2020b; Tham et al., 2004; Tian, Zhang, & Qian, 2020; Wu, Chan, & Ma, 2005; Wu et al., 2009; Yan, Guo, Li, Yan, & Liu, 2005; Yang et al., 2007; Zhang et al., 2005)
Funding
This work was supported by the National Natural Science Foundation of China [grant numbers: 32071086] and Military Medical Research Foundation [grant numbers: CWS20J007].
Authors' contributions
YZ, ZS, YW, CX contributed to the writing, and the meta analysis of this article, who are co-first authors, WL leaded the whole study, including putting forward this study, carrying out the study, and was the corresponding author. LS, ZS contributed to search process, study inclusion, and data extraction/coding and quality assessment.
Declaration of Competing Interest
The authors declare that they have no conflicts of interest.
Acknowledgements
The authors thank Professor Lynda Song and Sabina Lingxiao Xu Ph.D. from University of Leeds, UK, and Xiaoqian Yu Ph.D. from University of South Florida, USA, for language editing.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cpr.2020.101938.
Appendix A. Supplementary data
Supplementary material
References
- Asmundson G.J.G., Taylor S. Coronaphobia: Fear and the 2019-nCoV outbreak. Journal of Anxiety Disorders. 2020;70 doi: 10.1016/j.janxdis.2020.102196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betancourt T.S., Brennan R.T., Vinck P., VanderWeele T.J., Spencer-Walters D., Jeong J.…Pham P. Associations between mental health and Ebola-related health Behaviors: A regionally representative cross-sectional survey in post-conflict Sierra Leone. PLoS Medicine. 2016;13(8) doi: 10.1371/journal.pmed.1002073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer J., Lok A., Van’t Verlaat E., Duivenvoorden H.J., Bakker A.B., Smit B.J. Work-related critical incidents in hospital-based health care providers and the risk of post-traumatic stress symptoms, anxiety, and depression: A meta-analysis. Social Science & Medicine. 2011;73(2):316–326. doi: 10.1016/j.socscimed.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfils K.A., Lysaker P.H., Yanos P.T., Siegel A., Leonhardt B.L., James A.V.…Davis L.W. Self-stigma in PTSD: Prevalence and correlates. Psychiatry Research. 2018;265:7–12. doi: 10.1016/j.psychres.2018.04.004. [DOI] [PubMed] [Google Scholar]
- Borenstein M., Hedges L.V., Rothstein H. Wiley; Chichester, England: 2007. Introduction to meta-analysis. [Google Scholar]
- Brooks S.K., Webster R.K., Smith L.E., Woodland L., Wessely S., Greenberg N., Rubin G.J. The psychological impact of quarantine and how to reduce it: Rapid review of the evidence. Lancet. 2020;395(10227):912–920. doi: 10.1016/s0140-6736(20)30460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai L., Liu Z., Zhou D., Liu Y., Qin P. Results of tracing of 29 SARS patients with compllcatiom at rehabilitation stage. China Tropical Medcine. 2004;4(4):538–539,542. doi: 10.3969/j.issn.1009-9727.2004.04.018. [DOI] [Google Scholar]
- Carty J., O’Donnell M.L., Creamer M. Delayed-onset PTSD: A prospective study of injury survivors. Journal of Affective Disorders. 2006;90(2–3):257–261. doi: 10.1016/j.jad.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Chan A.O.M., Huak C.Y. Psychological impact of the 2003 severe acute respiratory syndrome outbreak on health care workers in a medium size regional general hospital in Singapore. Occupational Medicine. 2004;54(3):190–196. doi: 10.1093/occmed/kqh027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaves C., Castellanos T., Abrams M., Vazquez C. The impact of economic recessions on depression and individual and social well-being: The case of Spain (2006-2013) Social Psychiatry and Psychiatric Epidemiology. 2018;53(9):977–986. doi: 10.1007/s00127-018-1558-2. [DOI] [PubMed] [Google Scholar]
- Chen S., Yang J., Yang W., Wang C., Bärnighausen T. COVID-19 control in China during mass population movements at New Year. Lancet. 2020;395(10226):764–766. doi: 10.1016/s0140-6736(20)30421-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colorado E.E. A mixed-method study of aid workers in Sierra Leone during the 2014–2015 ebola epidemic: Exploring psychological distress, trauma, resilience, and coping. Dissertation Abstracts International Section A: Humanities and Social Sciences. 2018;79 7-A(E), No-Specified. [Google Scholar]
- Creamer M., Burgess P., McFarlane A.C. Post-traumatic stress disorder: Findings from the Australian National Survey of Mental Health and Well-being. Psychological Medicine. 2001;31(7):1237–1247. doi: 10.1017/s0033291701004287. [DOI] [PubMed] [Google Scholar]
- Darves-Bornoz J.M., Alonso J., de Girolamo G., de Graaf R., Haro J.M., Kovess-Masfety V.…Gasquet I. Main traumatic events in Europe: PTSD in the European study of the epidemiology of mental disorders survey. Journal of Traumatic Stress. 2008;21(5):455–462. doi: 10.1002/jts.20357. [DOI] [PubMed] [Google Scholar]
- Director-General W. 2020. WHO Director-General’s opening remarks at the Mission briefing on COVID-19 - 2 April 2020. (WHO Director-General Speech) [Google Scholar]
- Drosten C., Günther S., Preiser W., van der Werf S., Brodt H.R., Becker S.…Doerr H.W. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. The New England Journal of Medicine. 2003;348(20):1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- Ebola Outbreak Epidemiology Team Outbreak of Ebola virus disease in the Democratic Republic of the Congo, April-May, 2018: An epidemiological study. Lancet. 2018;392(10143):213–221. doi: 10.1016/s0140-6736(18)31387-4. [DOI] [PubMed] [Google Scholar]
- Gao H., Hui W., Lan X., Wei H., Hu Y., Li R., Jiao Z. A follow-up study posttraumatic stress disorder of SARS patients after discharge. Chinese Journal of Rehabilitation Medcine. 2006;21(11):1003–1004,1026. doi: 10.3969/j.issn.1001-1242.2006.11.018. [DOI] [Google Scholar]
- Gul S., Gul E.U., Yesilyurt M., Ozturk B., Kuscu F., Ergonul O. Health-related quality of life and the prevalence of post-traumatic stress disorder among Crimean-Congo hemorrhagic fever survivors. Japanese Journal of Infectious Diseases. 2012;65(5):392–395. doi: 10.7883/yoken.65.392. [DOI] [PubMed] [Google Scholar]
- Hawryluck L., Gold W.L., Robinson S., Pogorski S., Galea S., Styra R. SARS control and psychological effects of quarantine, Toronto, Canada. Emerging Infectious Diseases. 2004;10(7):1206–1212. doi: 10.3201/eid1007.030703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes E.C., Dudas G., Rambaut A., Andersen K.G. The evolution of Ebola virus: Insights from the 2013-2016 epidemic. Nature. 2016;538(7624):193–200. doi: 10.1038/nature19790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H.…Pillai S.K. First Case of 2019 Novel Coronavirus in the United States. The New England Journal of Medicine. 2020;382(10):929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong X., Currier G.W., Zhao X., Jiang Y., Zhou W., Wei J. Posttraumatic stress disorder in convalescent severe acute respiratory syndrome patients: A 4-year follow-up study. General Hospital Psychiatry. 2009;31(6):546–554. doi: 10.1016/j.genhosppsych.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoy D., Brooks P., Woolf A., Blyth F., March L., Bain C., Buchbinder R. Assessing risk of bias in prevalence studies: Modification of an existing tool and evidence of interrater agreement. Journal of Clinical Epidemiology. 2012;65(9):934–939. doi: 10.1016/j.jclinepi.2011.11.014. [DOI] [PubMed] [Google Scholar]
- Huang J.Z., Han M.F., Luo T.D., Ren A.K., Zhou X.P. Mental health survey of 230 medical staff in a tertiary infectious disease hospital for COVID-19. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2020;38(03):192–195. doi: 10.3760/cma.j.cn121094-20200219-00063. on Chinese data base-CNKI. [DOI] [PubMed] [Google Scholar]
- Jalloh M.F., Li W., Bunnell R.E., Ethier K.A., O’Leary A., Hageman K.M.…Redd J.T. Impact of Ebola experiences and risk perceptions on mental health in Sierra Leone, July 2015. BMJ Global Health. 2018;3(2) doi: 10.1136/bmjgh-2017-000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung H., Jung S.Y., Lee M.H., Kim M.S. Assessing the presence of post-traumatic stress and turnover intention among nurses post-Middle East respiratory syndrome outbreak: The importance of supervisor support. Workplace Health & Safety. 2020 doi: 10.1177/2165079919897693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karam E.G., Friedman M.J., Hill E.D., Kessler R.C., McLaughlin K.A., Petukhova M.…Koenen K.C. Cumulative traumas and risk thresholds: 12-month PTSD in the world mental health (WMH) surveys. Depression and Anxiety. 2014;31(2):130–142. doi: 10.1002/da.22169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keita M.M., Doukoure M., Chantereau I., Sako F.B., Traore F.A., Soumaoro K.…Diallo M. Survivors of epidemic recent disease Ebola virus in psychiatric hospital service national Donka in Guinea: Psychopathological and psychotherapeutic study. L’Evolution Psychiatrique. 2017;82(1):127–142. doi: 10.1016/j.evopsy.2016.07.004. [DOI] [Google Scholar]
- Keita M.M., Taverne B., Sy Savané S., March L., Doukoure M., Sow M.S.…PostEboGui Study G. Depressive symptoms among survivors of Ebola virus disease in Conakry (Guinea): Preliminary results of the PostEboGui cohort. BMC Psychiatry. 2017;17(1):127. doi: 10.1186/s12888-017-1280-8. Apr 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R.C., Aguilar-Gaxiola S., Alonso J., Benjet C., Bromet E.J., Cardoso G.…Koenen K.C. Trauma and PTSD in the WHO World Mental Health Surveys. European Journal of Psychotraumatology. 2017;8(sup5) doi: 10.1080/20008198.2017.1353383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R.C., Berglund P., Demler O., Jin R., Merikangas K.R., Walters E.E. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kwek S.-K., Chew W.-M., Ong K.-C., Ng A.W.-K., Lee L.S.-U., Kaw G., Leow M.K.-S. Quality of life and psychological status in survivors of severe acute respiratory syndrome at 3 months postdischarge. Journal of Psychosomatic Research. 2006;60(5):513–519. doi: 10.1016/j.jpsychores.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C.C., Shih T.P., Ko W.C., Tang H.J., Hsueh P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. International Journal of Antimicrobial Agents. 2020;55(3) doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancee W.J., Maunder R.G., Goldbloom D.S., Coauthors for the Impact of, S. S Prevalence of psychiatric disorders among Toronto hospital workers one to two years after the SARS outbreak. Psychiatr Serv. 2008;59(1):91–95. doi: 10.1176/ps.2008.59.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau J.T.F., Yang X., Pang E., Tsui H.Y., Wong E., Wing Y.K. SARS-related perceptions in Hong Kong. Emerging Infectious Diseases. 2005;11(3):417–424. doi: 10.3201/eid1103.040675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T.M.C., Chi I., Chung L.W.M., Chou K.L. Ageing and psychological response during the post-SARS period. Aging & Mental Health. 2006;10(3):303–311. doi: 10.1080/13607860600638545. [DOI] [PubMed] [Google Scholar]
- Lee S.M., Kang W.S., Cho A.-R., Kim T., Park J.K. Psychological impact of the 2015 MERS outbreak on hospital workers and quarantined hemodialysis patients. Comprehensive Psychiatry. 2018;87:123–127. doi: 10.1016/j.comppsych.2018.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.H., Shin H.S., Park H.Y., Kim J.L., Lee J.J., Lee H.…Han W. Depression as a mediator of chronic fatigue and post-traumatic stress symptoms in middle east respiratory syndrome survivors. Psychiatry Investigation. 2019;16(1):59–64. doi: 10.30773/pi.2018.10.22.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Mi Y., Chu J., Zhu L., Zhang z., Lian L., Liu L. Investigation and analysis of novel coronavirus first-line nurses' posttraumatc emergency disorder. Journal of Nurse Training. 2020:1–5. [Google Scholar]
- Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gotzsche P.C., Ioannidis J.P., Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Medicine. 2009;6(7) doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C.Y., Peng Y.C., Wu Y.H., Chang J., Chan C.H., Yang D.Y. The psychological effect of severe acute respiratory syndrome on emergency department staff. Emergency Medicine Journal. 2007;24(1):12–17. doi: 10.1136/emj.2006.035089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Lu Z., Xu Y., Zhang K. Analysis on posttraumatic stress disorder of subjects recoverd from SARS. Chinese Journal of Public Health. 2005;21(12):1457–1458. doi: 10.3321/j.issn:1001-0580.2005.12.040. [DOI] [Google Scholar]
- Liu N., Zhang F., Wei C., Jia Y., Shang Z., Sun L.…Liu W. Prevalence and predictors of PTSS during COVID-19 Outbreak in China Hardest-hit Areas: Gender differences matter. Psychiatry Research. 2020 doi: 10.1016/j.psychres.2020.112921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lötsch F., Schnyder J., Goorhuis A., Grobusch M.P. Neuropsychological long-term sequelae of Ebola virus disease survivors - a systematic review. Travel Medicine and Infectious Disease. 2017;18:18–23. doi: 10.1016/j.tmaid.2017.05.001. [DOI] [PubMed] [Google Scholar]
- Mak I.W.C., Chu C.M., Pan P.C., Yiu M.G.C., Chan V.L. Long-term psychiatric morbidities among SARS survivors. General Hospital Psychiatry. 2009;31(4):318–326. doi: 10.1016/j.genhosppsych.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmar C.R., Schlenger W., Henn-Haase C., Qian M., Purchia E., Li M.…Kulka R.A. Course of posttraumatic stress disorder 40 years after the Vietnam war: Findings from the National Vietnam Veterans Longitudinal Study. JAMA Psychiatry. 2015;72(9):875–881. doi: 10.1001/jamapsychiatry.2015.0803. [DOI] [PubMed] [Google Scholar]
- Maunder R.G., Lancee W.J., Balderson K.E., Bennett J.P., Borgundvaag B., Evans S.…Wasylenki D.A. Long-term psychological and occupational effects of providing hospital healthcare during SARS outbreak. Emerging Infectious Diseases. 2006;12(12):1924–1932. doi: 10.3201/eid1212.060584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D.G., Prisma Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Medicine. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzak R.H., Goldstein R.B., Southwick S.M., Grant B.F. Prevalence and Axis I comorbidity of full and partial posttraumatic stress disorder in the United States: Results from Wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. Journal of Anxiety Disorders. 2011;25(3):456–465. doi: 10.1016/j.janxdis.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remuzzi A., Remuzzi G. COVID-19 and Italy: What next? Lancet. 2020;395(10231):1225–1228. doi: 10.1016/s0140-6736(20)30627-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds D.L., Garay J.R., Deamond S.L., Moran M.K., Gold W., Styra R. Understanding, compliance and psychological impact of the SARS quarantine experience. Epidemiology and Infection. 2008;136(7):997–1007. doi: 10.1017/S0950268807009156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Righy C., Rosa R.G., da Silva R.T.A., Kochhann R., Migliavaca C.B., Robinson C.C.…Falavigna M. Prevalence of post-traumatic stress disorder symptoms in adult critical care survivors: A systematic review and meta-analysis. Critical Care. 2019;23(1):213. doi: 10.1186/s13054-019-2489-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J.P., Chesney E., Oliver D., Pollak T.A., McGuire P., Fusar-Poli P.…David A.S. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: A systematic review and meta-analysis with comparison to the COVID-19 pandemic. The Lancet Psychiatry. 2020 doi: 10.1016/s2215-0366(20)30203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi T., Jiang C., Jia S., Liu Q., Zhang J., Qi X. Post-traumatic stress disorder and related factors following the severe acute respiratory syndrome. Zhonghua Linchuan Kangfu. 2005;44:9–13. [Google Scholar]
- Shultz J.M., Baingana F., Neria Y. The 2014 Ebola outbreak and mental health: current status and recommended response. JAMA. 2015;313(6):567–568. doi: 10.1001/jama.2014.17934. [DOI] [PubMed] [Google Scholar]
- Sim K., Chan Y.H., Chong P.N., Chua H.C., Soon S.W. Psychosocial and coping responses within the community health care setting towards a national outbreak of an infectious disease. Journal of Psychosomatic Research. 2010;68(2):195–202. doi: 10.1016/j.jpsychores.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim K., Phui N.C., Yiong H.C., Soon W.S.W. Severe acute respiratory syndrome-related psychiatric and posttraumatic morbidities and coping responses in medical staff within a primary health care setting in Singapore. Journal of Clinical Psychiatry. 2004;65(8):1120–1127. doi: 10.4088/JCP.v65n0815. [DOI] [PubMed] [Google Scholar]
- Sipos M.L., Kim P.Y., Thomas S.J., Adler A.B. U.S. service member deployment in response to the Ebola crisis: The psychological perspective. Military Medicine. 2018;183(3–4):e171–e178. doi: 10.1093/milmed/usx042. [DOI] [PubMed] [Google Scholar]
- State Council of the people’s Republic of China . State council bulletin. State council; 2011. Regulations and preparedness for and response to emergent public health hazards. [Google Scholar]
- Stein M.B., Walker J.R., Hazen A.L., Forde D.R. Full and partial posttraumatic stress disorder: Findings from a community survey. The American Journal of Psychiatry. 1997;154(8):1114–1119. doi: 10.1176/ajp.154.8.1114. [DOI] [PubMed] [Google Scholar]
- Stroup D.F., Berlin J.A., Morton S.C., Olkin I., Williamson G.D., Rennie D.…Thacker S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- Su T.-P., Lien T.-C., Yang C.-Y., Su Y.L., Wang J.-H., Tsai S.-L., Yin J.-C. Prevalence of psychiatric morbidity and psychological adaptation of the nurses in a structured SARS caring unit during outbreak: A prospective and periodic assessment study in Taiwan. Journal of Psychiatric Research. 2007;41(1–2):119–130. doi: 10.1016/j.jpsychires.2005.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L., Sun Z., Wu L., Zhu Z., Zhang F., Shang Z.…Liu W. Journal of Affective Disorders (under review); 2020. Prevalence and Risk Factors of Acute Posttraumatic Stress Symptoms during the COVID-19 Outbreak in Wuhan, China. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L., Yi B., Pan X., Shang Z., Wu L., Jia Y.…Liu W. e-Life (under review); 2020. Psychological impact on 190 cases of COVID-19 in Wuhan, China: an observational from two centers. [Google Scholar]
- Tang X., Tang S., Ren Z., Wong D.F.K. Prevalence of depressive symptoms among adolescents in secondary school in mainland China: A systematic review and meta-analysis. Journal of Affective Disorders. 2019;245:498–507. doi: 10.1016/j.jad.2018.11.043. [DOI] [PubMed] [Google Scholar]
- Tham K.Y., Tan Y.H., Loh O.H., Tan W.L., Ong M.K., Tang H.K. Psychiatric morbidity among emergency department doctors and nurses after the SARS outbreak. Annals of the Academy of Medicine, Singapore. 2004;33(5 Suppl):S78–S79. [PubMed] [Google Scholar]
- Tian Y., Zhang Y., Qian Z. Investigation on the emotional status of villagers in a rural area of Hangzhou after the implementation of closure measures during the prevention and control of 2019-nCoV infection. Health Research. 2020;40(1) 16–18+21. [Google Scholar]
- Vyas K.J., Delaney E.M., Webb-Murphy J.A., Johnston S.L. Psychological impact of deploying in support of the U.S. response to Ebola: A systematic review and Meta-analysis of past outbreaks. Military Medicine. 2016;181(11):e1515–e1531. doi: 10.7205/MILMED-D-15-00473. [DOI] [PubMed] [Google Scholar]
- WHO . WHO official website; 2003. Summary table of SARS cases by country, 1 November 2002 - 7 August 2003. [Google Scholar]
- WHO . 2019. Situation update of MERS-CoV. (WHO official website) [Google Scholar]
- WHO . World Health Organization; 2020. Coronavirus disease 2019 (COVID-19): Situation report—86. [Google Scholar]
- WHO Ebola in the Democratic Republic of the Congo——Health emergency update. WHO webisite: emegencies. 2020 [Google Scholar]
- Wu K.K., Chan S.K., Ma T.M. Posttraumatic stress, anxiety, and depression in survivors of severe acute respiratory syndrome (SARS) Journal of Traumatic Stress. 2005;18(1):39–42. doi: 10.1002/jts.20004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P., Fang Y., Guan Z., Fan B., Kong J., Yao Z., Hoven C.W. The psychological impact of the SARS epidemic on hospital employees in China: Exposure, risk perception, and altruistic acceptance of risk. The Canadian Journal of Psychiatry / La Revue canadienne de psychiatrie. 2009;54(5):302–311. doi: 10.1177/070674370905400504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan F., Guo Z., Li S., Yan J., Liu M. Surveys on mental status of subjects recoverd from SARS. Chinese Mental Health Journal. 2005;18(10):675–677. [Google Scholar]
- Yang L., Wu X., Zhang Y., Li M., Liu G., Gao Y.…He M. Mental Health Status of Medical Staffs Fighting SARS: a Long-dated Investigation. China Journal of Health Psychology. 2007;6:567–569. [Google Scholar]
- Yang Y., Li W., Zhang Q., Zhang L., Cheung T., Xiang Y.T. Mental health services for older adults in China during the COVID-19 outbreak. Lancet Psychiatry. 2020 doi: 10.1016/s2215-0366(20)30079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D., Fouchier R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. The New England Journal of Medicine. 2012;367(19):1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- Zhang K., Xu Y., Liu Z., Yang H., Song L., Xue Y.…Feng M. Controlled study on posttraumatic stress disorder among patients with severe acute respiratory syndrome and first-line hospital staffs as well as the public in prevalent areas. Zhongguo Linchuang Kangfu. 2005;12:94–96. [Google Scholar]
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z.…Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 doi: 10.1016/s0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material