Abstract
Adeno-associated virus (AAV)-based gene therapy is undergoing major expansion into clinical practice, with two treatments currently being granted Food and Drug Administration (FDA) approval. However, the presence of pre-existing neutralizing antibodies (NAB) is one of the significant hurdles for the clinical application of AAV vectors that significantly limits the patient population, which benefits from the treatment. A reliable diagnostic to evaluate the patient's seropositivity is required to ensure the effectiveness of the AAV-mediated therapeutic. Here, we describe a simple method for the determination of AAV NAB activity based on our finding that Compound C makes HEK293 cell highly permissive for infection by 10 commonly used AAV serotypes.
Keywords: adeno-associated virus, neutralizing antibodies, selective inhibitor of AMPK, assay development
Introduction
Adeno-associated virus (AAV) has been widely recognized as a safe and effective clinical-stage vector for gene therapy in a broad spectrum of inherited diseases such as Leber's congenital amaurosis, hemophilia A and B, and muscular dystrophy.1–3 Most recently, AAV-based treatments had a major ground break as two gene therapies for rare eye disease and spinal muscular atrophy were approved by the US Food and Drug Administration (FDA) and became available for patients.4,5
However, AAV seropositivity of the general patient population, due to natural virus infection and the presence of neutralizing antibodies (NAB) to commonly used AAV serotypes, limits the cohort of successful recipients of novel therapies.6–13 For that reason, numerous attempts were made to identify or develop novel AAV serotypes that can avoid neutralization.14–21 Nevertheless, regardless of the successful use of novel AAV capsid variants in animal models, they required additional preclinical evaluation before use for human application.
Thus, a reliable method for patients screening to evaluate the presence and activity of NAB for commonly used AAV serotypes is necessary. Several methods based on different principles to determine NAB titer are published, including enzyme-linked immunosorbent assay (ELISA),22 a quantitative polymerase chain reaction-based method evaluating AAV binding to cells,23 and in vivo inhibition of AAV activity by injection of a tested sample in C57BL/6 mice.24 Nevertheless, some of the methods, such as measurements of NAB activity using mice, are lengthy and hard to standardize.
An assay based on in vitro infection of cells with the AAV encoded reporter gene is easy to establish and reproduce,25–27 and it is recommended by the FDA as the assay of choice.28 In this assay, cells are transfected with reporter AAV in the presence of serial dilution of the tested sample. The titer of the NAB is determined as a sample dilution at which the transduction efficiency of the reporter gene is half of the maximum value.
Nevertheless, the challenge of identifying an appropriate cell line for all commonly used AAV serotypes26,29 and the reporter gene with sufficient sensitivity to detect reliable differences between evaluated serum samples30 remains unresolved. For example, AAV8 is a highly efficient serotype for transgene delivery to the liver in vivo; at the same time, it is hardly infectious and requires a very high multiplicity of infections (MOI) to transduce cells in vitro.
To overcome this limitation, a number of cell lines were used (HEK293, HeLa, Huh7, C2C12, etc.) for different AAV serotypes, a wide range (103–105 viral genome (vg)/cell) of AAV MOI, and a large selection of pharmacological drugs previously identified to significantly enhance the transduction of some AAV serotypes in vitro.24,26,31 In addition, a helper virus, such as Adenovirus, was previously applied to increase expression in AAV-transduced cells.24 Also, a recently published detailed protocol of NAB assay suggested using the HEK293-derived cell line 2V6.11 (not commercially available) for AAV8, which expresses the adenovirus E4 ORF gene under the control of the ecdysone-inducible promoter and ponasterone A to make these cells permissive for AAV infection.27
This study was designed to develop a protocol for reliable estimation of NAB titer against AAV8, as well as other AAV serotypes, which allow the use of a commonly available cell line such as HEK293. For that purpose, we performed limited screening by infecting HEK293 cells with AAV8-luciferase (Luc) in the presence of several pharmacologically active drugs. We identified a selective inhibitor of AMP-activated protein kinase (AMPK) Dorsomorphin, also known as Compound C (CC), as an enhancer of the infection of HEK293 cells by AAV8, and other AAV serotypes, without cytotoxic effects.
As a result of this significant observation, we developed a protocol to determine NAB titers that works efficiently for all AAV serotypes we tested.
Protocol Development
Briefly, AAV vectors used in this study (serotypes 1, 2, 3, 5, 6, 7, 8, 9, 10, and recently identified Anc80L6532) were packaged in HEK293 cells by triple transfection with polyethyleneimine (PEI) and isolated by an iodixanol four-step gradient followed by ion-exchange column purification as described.33,34 This method provides high-purity vector preps with <10% empty capsids.35,36 The vectors contained a single-strain expression cassette with a chicken-β-actin promoter (CB)-driven fusion of firefly luciferase (Luc), and yellow fluorescent protein (YFP) genes.37,38
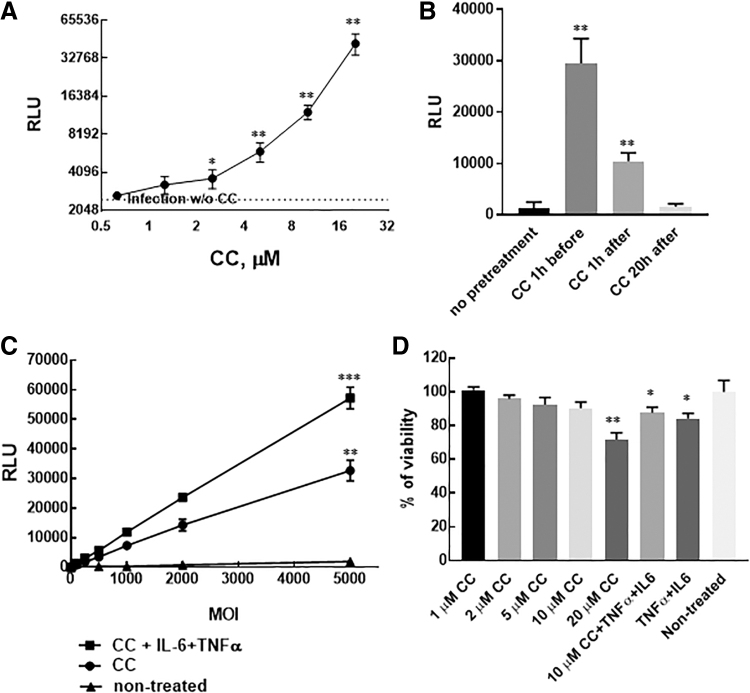
First, escalation doses of CC were used to identify the optimal drug concentration for AAV serotype 8 (Fig. 1A). Pretreatment of HEK293 cells with CC dose dependently increased luciferase activity 24 h after infection. The dose 10 μM CC was chosen for the next experiments as the minimal concentration that induces high AAV transduction; luciferase activity was five times higher compared with non-treated cells, and values were two orders higher than the background.
Figure 1.
Pretreatment of HEK293 cells with CC significantly increases the infectivity of AAV8-Luc. (A) HEK293 cells were treated with different concentrations of CC and 1 h later infected with AAV8-Luc at MOI = 2,000 vg/cell. *p < 0.05 and **p < 0.01 compared with luciferase activity in untreated cells. (B) 10 μM CC was added to HEK293 at different time points during infection with AAV8-Luc. **p < 0.01 compared with luciferase activity in untreated cells. (C) HEK293 cells were infected with different MOI (100–5,000 vg/cell) of AAV8-Luc in the presence of 10 μM CC or 10 μM CC +20 ng/mL IL-6 + 20 ng/mL TNF-α. Luciferase activity was measured 24 h later. **p < 0.01 for all MOI in the presence of CC compared with infections without pretreatment of HEK293 cells, ***p < 0.01 for MOI in the range 500–5,000 vg/cell in the presence of CC+IL-6+TNF-α compared with the infections in the presence of CC. For MOI = 250 vg/cell, *p < 0.05. (D) HEK293 cells were incubated with different concentrations of CC or with 20 ng/mL IL-6 + 20 ng/mL TNF-α for 48 h. At the end of incubation, cell counting reagent CCK-8 (APExBio, Boston, MA) was added to wells for an additional 1 h. The absorbance was measured at 450 nm. The number of cells in treated wells was compared with the number of cells in non-treated control wells, which was considered as 100% viability. *p < 0.05 and **p < 0.01 compared with non-treated controls. AAV, adeno-associated virus; CC, compound C; CCK-8, cell counting kit-8; IL-6, interleukin-6; MOI, multiplicity of infections; TNF-α, tumor necrosis factor alpha.
In the next step of protocol optimization, 10 μM CC was added to cells at different time points before or after AAV8 infection. The maximum increase in AAV8-mediated luciferase expression was observed if cells were pretreated 1 h before infection (Fig. 1B). Hence, in all subsequent experiments, HEK293 cells were pretreated with 10 μM CC 1 h before infection. Next, we used escalating doses of AAV8-Luc in the presence of CC and demonstrated the linear dose dependence of luciferase expression at a range of MOI from 100 to 5,000 vg/cell.
In addition, we also showed that pretreatment of cells with CC together with pro-inflammatory cytokines interleukin (IL)-6 (20 ng/mL) and tumor necrosis factor alpha (TNF-α) (20 ng/mL) further increased luciferase expression. It should be noted that without CC treatment with IL-6 and TNF-α only marginally increases luciferase expression (data not shown). The treatment of HEK293 cells with CC for 48 h does not induce cytotoxicity at doses up to 10 μM, at 20 μM CC and a combination of 10 μM CC with TNF-α and IL-6 mild cytotoxic effect was observed (Fig. 1D). The dramatic improvement of AAV8 transduction in vitro in the presence of CC prompted to test whether a similar effect can be achieved for other AAV serotypes.
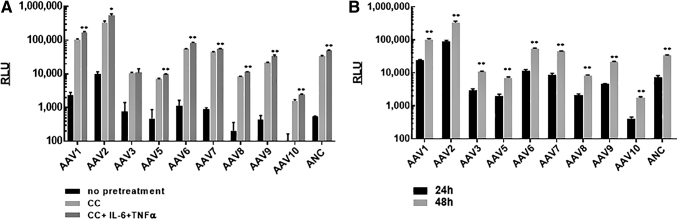
We analyzed the transduction efficiency of 10 common AAV serotypes at MOI 2,000 vg/cell. Without pretreatment, only cells infected with AAV1 and AAV2 had luciferase activity that was significantly higher than the background at 24 h (data not shown) and 48 h after infection (Fig. 2A). At the same time, pretreatment with CC allowed the measurement of sufficient luciferase activity for all tested serotypes (Fig. 2A). In addition, an extension of incubation time after infection from 24 to 48 h significantly increased luciferase activity for all serotypes (Fig. 2B).
Figure 2.
CC enhanced the infectivity of all tested AAV serotypes. (A) HEK293 cells were infected with different AAV-Luc serotypes at MOI 2,000 vg/cell in the presence of 10 μM CC, or CC+IL-6+TNF-α. Luciferase activity was measured 48 h later. For all serotypes, luciferase activity was higher in the presence of CC compared with non-treated cells (p < 0.01). *p < 0.05 and **p < 0.01 for cells infected in the presence of CC+IL6+TNF-α compared with CC only. (B) Comparison of luciferase activity at 24 and 48 h after infection. Cells were infected in the presence of CC as described in (A). *p < 0.05 and **p < 0.01 for 48 h compared with 24 h.
Such an extension of the time up to 48 h is beneficial for NAB measurements for hard-to-infect serotypes such as AAV3, 5, 10, and 8. In fact, without CC the luciferase activity for AAV10 was at a background level, and for AAV3, 5, 8, and 9 it was in only a marginally higher background. Thus, it makes it impossible to measure NAB titers for these serotypes in non-treated HEK293 cell. After pretreatment with CC, all these serotypes demonstrated a high level of luciferase expression, which allows to establish NAB titer protocol. The highest luciferase activity was observed for AAV2 (more than 1,000 times higher background in the presence of CC at 48 h) and the lowest was observed for AAV10 (8 times higher background).
We also showed that the addition of IL-6 and TNF-α to CC treatment improved infection efficiency for all serotypes, but not for AAV3 (Fig. 2A). That additional treatment can be used for some serotypes such as AAV10 in case the treatment with CC and increase of MOI still do not provide reporter gene activity enough to set up the NAB assay.
Next, we ensure that the addition of CC to media does not affect readout in NAB titer. Thus, we compared the values for AAV2 NAB titer determined in experiments with non-treated and CC pretreated HEK293 cell. AAV2 was chosen as the most infectious serotype for HEK293 cells, and therefore, NAB titers can be measured without additional stimulation of cells. Mouse serum from animals injected intramuscularly (i.m) with 1010 vg/mouse of AAV2-Luc was analyzed by the protocol described next. The results shown in Fig. 3 strongly suggest that CC does not change NAB titer and inhibition appears at the same dilution of the serum.
Figure 3.
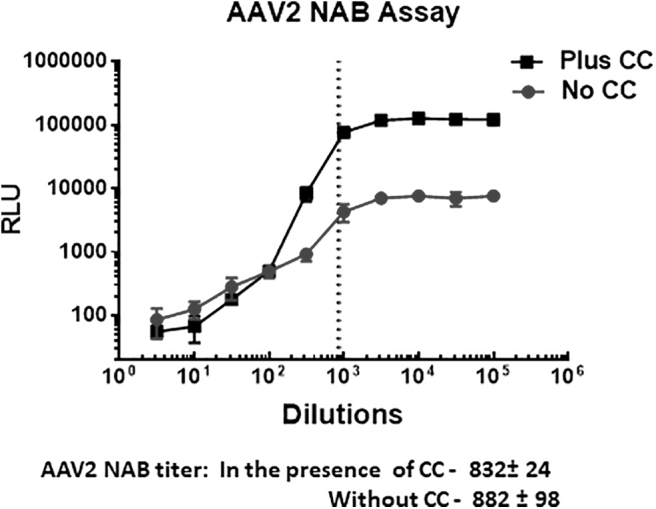
The performance of NAB assay in the presence of CC does not change NAB titer. The mouse plasma was collected 1 month after injection of AAV2 and analyzed in the presence of NAB by utilizing HEK293 cells either pretreated or non-treated with CC. Although CC significantly increased the values for luciferase activity, it did not affect NAB titer (as demonstrated overlaid vertical dotted lines corresponded to NAB titers for AAV2 measured in the presence and absence of CC). The observed difference is not statistically significant. NAB, neutralizing antibodies.
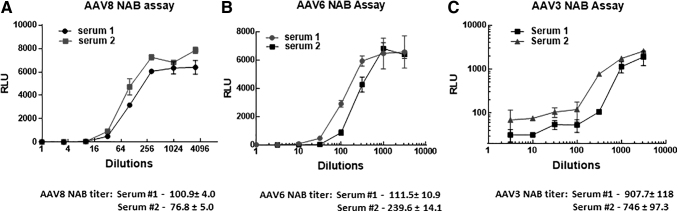
Finally, we demonstrate the successful measurements of NAB titers by using the protocol described next for three different AAV serotypes 8, 6, and 3 with mouse serum collected from animals injected i.m. with corresponding AAVs (Fig. 4).
Figure 4.
Examples of NAB assay for different serotypes performed with HEK293 cell pretreated with CC. For each AAV, serotype mice were injected with 1010 vg/animal and serum was collected 3 weeks later for NAB assay. (A) Assay for AAV8. (B) Assay for AAV6. (C) Assay for AAV3.
The interaction of AAV vectors with host cells occurs through multiple steps: virus attachment to target cells by binding to receptor and co-receptors, entry by endocytosis, intracellular trafficking, nuclear translocation, capsid uncoating, and vector-mediated gene expression.39–45 Investigation into which steps in this complex process CC affect HEK293 cell infection with AAV is out of the scope of this study. Since CC, which at first was identified as an AMPK inhibitor, also inhibits other kinases,46 we can speculate that its effect can be partially explained by preventing AAV capsid phosphorylation with these kinases and subsequent AAV degradation by proteasome machinery.47,48
In summary, our findings resulted in the development of easily setting up a universal protocol for the analysis of AAV-specific NAB for commonly used serotypes. However, such an enhancement in the transduction efficiency of AAV vectors by CC can be used for the development of other infection-based in vitro assays.
Protocol
The following protocol provides a detailed example of utilizing HEK293 cells pretreated with CC alone or together with TNF-α and IL-6 to estimate the titer of AAV-specific NAB in tested serum samples. In general, AAV vectors expressing luciferase are incubated with serial dilutions of tested serum sample, and they are then added to HEK293 cells pretreated with CC. The expression of luciferase is analyzed 24 or 48 h later in cells by measuring enzyme activity using Bright-Glo luciferase substrate. NAB titer corresponds to the dilution of the test serum sample at which 50% of the luciferase signal is inhibited compared with the “virus only” control.
Of note, the protocol does not include the description of AAV preparation, titration, and quality control. However, these are important steps for the reproducibility of data across the labs and should be taken into consideration before setting up the NAB assay.
Materials
The essential materials required to complete protocol are listed in Table 1.
Table 1.
Essential materials required to complete protocol
| Reagents | Supplier | Specific Handling | Storage Conditions |
|---|---|---|---|
| Compound C in solution | EMD Biosciences, 171261 | Hazardous in case of skin contact (irritant), of eye contact (irritant), use standard procedures to avoid contact with skin and eyes | −20°C |
| 293T cell line | ATCC | Use low passages | In liquid nitrogen |
| FBS | Thermofisher (Gibco), 16000044 | Heat inactivate at 56°C for 30 min before use | −80°C |
| Dulbecco's modified Eagle's medium | Thermofisher (Gibco), 11965084 | +4°C | |
| Reporter AAV/luciferase stocks | Produced according to lab protocol | Store in aliquots to reduce thaw/freeze cycles. | −20°C |
| Bright Glo luciferase assay system | Promega, E2610, E2620, E2650 | The lyophilized Bright-Glo™ Substrate contains DTT and is, therefore, classified as hazardous. The reconstituted reagent is not known to present any hazards, as the concentration of DTT is <1%. | −20°C |
| Trypsin-EDTA (0.05%), phenol red | Thermofisher (Gibco), 25300120 | −20°C or up to 1 week at +4°C | |
| PBS | Thermofisher (Gibco), 20012027 | Room temperature |
AAV, adeno-associated virus; DTT, dithiothreitol; EDTA, ethylenediaminetetraacetic acid; FBS, fetal bovine serum; PBS, phosphate-buffered saline.
Supplies
Plates, 96-well with black or white walls and flat, clear-bottom, tissue culture-treated.
Polypropylene or other low-absorption plates, 96-well, U- or V-bottomed, sterile.
Equipment
CO2 incubator.
Automotive cell counter (Countess II; ThermoFisher).
BioTek Synergy Neo 2 or any other spectrophotometer with the capability to read luminescence in multi-well plates.
Multichannel pipette.
Reagents' preparation
Complete media: Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and antibiotics. To 1 L of DMEM add 100 mL of heat-inactivated FBS and 10 mL of 10 × penicillin/streptomycin. Store at 4°C for 1 month.
Test samples: Serum, plasma, or any other biological liquid. Heat inactivate at 56°C for 30 min. Samples can be stored at −80°C in aliquots.
Diluent: FBS should be heat inactivated (HI) at 56°C for 30 min before use as a diluent. Aliquots of HI-FBS can be stored in aliquots at −80°C.
CC is purchased as 10 mM stock solution in DMSO. It is stored in aliquots at −20°C.
Bright Glo Luciferase assay system: Before first use, transfer the contents of one bottle of Bright-Glo Buffer to one bottle of Bright-Glo Substrate. Mix by inversion until the substrate is thoroughly dissolved. Store in aliquots at −20°C. According to the manufacturer's instructions, each aliquot can be subjected to freeze/thaw up to seven times without loss of activity if it is thawed at temperatures below 25°C.
Experimental Procedure
Day 0
-
(1)
Remove serum-containing medium from HEK293 cell culture; then, gently wash cells twice with 100 μL of phosphate-buffered saline (PBS). Cells should be at a low passage and ∼80% confluent without being overgrown. Harvest cells by trypsinization and perform a cell count.
-
(2)
Resuspend cells in complete DMEM culture medium at 200,000 cells/mL. Seed cells in 96-well plates with black or white walls and a clear bottom: 20,000 cells/well in 100 μL of complete media. Incubate cells overnight in a CO2 incubator at 37°C.
Day 1
-
(1)
Observe cells in the plate under a microscope. Cells should be 50–80% confluent.
Warm up serum-free DMEM. Thaw the stock solutions of CC (10 mM in DMSO), and analyze reporter AAV-Luc for serotype.
-
(2)
Do not change media in the plate with cells. Activate cells by adding 50 μL/well of serum-free DMEM containing 30 μM CC (note that the final concentration is 10 μM). For example, for the whole 96-well plate, 6 mL of DMEM supplemented with 18 μL CC will be needed.
Put the plate back in a CO2 incubator for 1 h.
-
(3)
Prepare serial dilutions of test samples by using HI-FBS as a diluent in 96-well plates with a U or V bottom.
An example of the dilution strategy is given in Table 2.
Table 2.
Preparation of the dilution cascade for the test samples (enough for three repeats × 10 μL)
| Dilution Factor | Volume of Test Sample | Volume of Diluent (μL) | |
|---|---|---|---|
| Dilution 1 | 1:1 | 40 μL of undiluted material | 0 |
| Dilution 2 | 1:4 | 10 μL of dilution 1 | 30 |
| Dilution 3 | 1:16 | 10 μL of dilution 2 | 30 |
| Dilution 4 | 1:64 | 10 μL of dilution 3 | 30 |
| Dilution 5 | 1:256 | 10 μL of dilution 4 | 30 |
| Dilution 6 | 1:1,024 | 10 μL of dilution 5 | 30 |
| Dilution 7 | 1:4,096 | 10 μL of dilution 6 | 30 |
Positive control: HI-FBS alone.
HI-FBS, heat-inactivated FBS.
-
(4)
Prepare the working solution of AAV. First, calculate the working concentration of AAV, which is based on the number of cells in wells and multiplicity of infection (MOI) will be used.
Example: If at the day of infection cells are 50% confluent (assuming that 100% confluence consists of 50,000 cells/well) and in each well AAV-Luc will be added at MOI = 2,000 vg/well in 5 μL, the working virus concentration is calculated as follows:
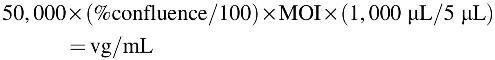 |
50,000 × (50/100) × 2,000 × 200 = 1 × 1010 vg/mL-working concentration of AAV-Luc
For the whole 96-well plate, 800 μL of AAV-Luc plus extra is needed (if to mix 25 μL of AAV for each sample in triplicate, then 96 wells/3 × 25 μL = 800 μL).
If the stock of AAV8-Luc is 1 × 1012 vg/mL, it needs to be diluted with PBS to working concentration 1 × 1010 vg/mL (dilution factor 100); then for 1 mL of working concentration of AAV, 10 μL of stock AAV-Luc should be diluted with 990 μL PBS.
-
(5)
Mix 25 μL of each dilution of the sample with 25 μL AAV-Luc (ratio 1:1) in new 96-well plates with a U or V bottom and incubate for 1 h at 37°C. For positive control, mix 25 μL AAV-Luc AAV with 25 μL diluent. For negative controls (background), mix 25 μL diluent (HI-FBS) with 25 μL PBS.
-
(6)
Add AAV/sample mix to the plate with CC pretreated HEK293 cells at 10 μL of mix per well. Each sample dilution should be analyzed at least in triplicate. Also include positive control with maximum (MAX) infection level (AAV mixed with diluent HI-FBS), and background wells (no AAV): HI-FBS mixed with PBS (MIN). An example of a plate layout is shown in Table 3.
-
(7)
Wrap the plate in aluminum foil and incubate in a CO2 incubator for 24 h.
Table 3.
Example of assay plate layout
| Sample 1 |
Sample 2 |
Sample 3 |
Sample 4 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| A | 1:1 | 1:1 | 1:1 | 1:1 | 1:1 | 1:1 | 1:1 | 1:1 | 1:1 | 1:1 | 1:1 | 1:1 |
| B | 1:4 | 1:4 | 1:4 | 1:4 | 1:4 | 1:4 | 1:4 | 1:4 | 1:4 | 1:4 | 1:4 | 1:4 |
| C | 1:16 | 1:16 | 1:16 | 1:16 | 1:16 | 1:16 | 1:16 | 1:16 | 1:16 | 1:16 | 1:16 | 1:16 |
| D | 1:64 | 1:64 | 1:64 | 1:64 | 1:64 | 1:64 | 1:64 | 1:64 | 1:64 | 1:64 | 1:64 | 1:64 |
| E | 1:256 | 1:256 | 1:256 | 1:256 | 1:256 | 1:256 | 1:256 | 1:256 | 1:256 | 1:256 | 1:256 | 1:256 |
| F | 1:1,024 | 1:1,024 | 1:1,024 | 1:1,024 | 1:1,024 | 1:1,024 | 1:1,024 | 1:1,024 | 1:1,024 | 1:1,024 | 1:1,024 | 1:1,024 |
| G | 1:4,096 | 1:4,096 | 1:4,096 | 1:4,096 | 1:4,096 | 1:4,096 | 1:4,096 | 1:4,096 | 1:4,096 | 1:4,096 | 1:4,096 | 1:4,096 |
| H | MAX | MAX | MAX | MAX | MAX | MAX | MIN | MIN | MIN | MIN | MIN | MIN |
MAX, maximum AAV infection level: mixed with diluent (HI-FBS); MIN, background levels (no AAV): HI-FBS mixed with PBS.
Day 2
-
(1)
Thaw an aliquot of Bright Glo Luciferase assay reagent at room temperature in the dark. For one 96-well plate 6 mL of reagent is sufficient.
-
(2)
Remove the plate from a CO2 incubator. Dump media from the plate by turning it upside down quickly. Tap the rest of the media on paper towels. Fill the plate with 50 μL PBS/well.
-
(3)
Set up a spectrophotometer to read chemiluminescence. If the spectrophotometer is BioTek, the optimal read conditions are: 500 ms, gain 125–135. Add 50 μL of Bright Glo substrate to each well, incubate the plate for 3 min in the dark, and finally read. For maximal light intensity, samples should be measured within 15 min of reagent addition.
Calculation of anti-AAV NAB titer
First method
NAB titer is defined as the neutralizing titer of the sample and is the first dilution at which 50% or greater inhibition of the luciferase expression is measured.
It can be quantified manually by subtracting average background values from all measurements and then by calculating the percent of the total luciferase expression:
 |
The first dilution of the sample with 50% or greater inhibition of the luciferase activity is used to determine the neutralizing titer. For example, if 50% or greater inhibition is observed at a 1:10 dilution of the sample, the titer is reported as 10.
Alternative method
Another way to determine NAB titer is by using GraphPad Prizm or any other suitable software. Place data as an XY file, where X is testing sample dilutions and Y is testing luminescence measurements in triplicate. Then, go to the results folder to analyze data: Use XY analysis→Nonlinear regression (curve fit)→[Agonist] versus response→Variable slope (four parameters). It will calculate EC50, which corresponds to NAB titer. Note that the use of this analysis depends on the appropriate inhibition curve with well-defined plateaus at the minimum and maximum dilutions. In the case when the values at the highest dilution of a tested sample are much lower than the maximum value, the assay should be repeated with more diluted samples.
Troubleshooting
A summary of critical steps of the protocol with possible troubleshooting is explained in Table 4.
Table 4.
Summary of critical steps of the protocol with possible troubleshooting
| Problem | Solution |
|---|---|
| High variability in readout across triplicate wells | The major source of such variability is unequal number of cells in the different wells. The HEK293 cell is readily detached from the plate during the trypsinization step, but it does not dissociate easily from each other. Ensure that cells form a single-cell suspension at the step of plating. In addition, wrapping the plate in aluminum foil during incubation time in a CO2 incubator will help to maintain even temperature across the plate and as result more even growing. |
| Since HEK293 cells detach easily, to prevent the loss of cells during the assay, avoid aspiration of media or plate washing. | |
| Low level of luciferase readout | The aliquot of reporter AAV lost activity or the titer was miscalculated. Take another aliquot or re-titer virus. |
| The quality of the HEK293 cell is also very important. Cells should be of low passage and be 50–70% confluent at the beginning of the experiment. | |
| To increase the signal for serotypes with low infectivity, several approaches can be utilized. (1) Time of incubation can be extended from 24 to 48 h. (2) Multiplicity of infections can be increased. However, make sure that luciferase signal is dose dependent and is not saturated. (3) For many serotypes, pretreatment of HEK293 cell with Compound C together with interleukin-6 and tumor necrosis factor alpha will additionally increase the luciferase readout. | |
| The RLU of MAX luciferase signal is significantly lower than some of the dilutions of the test sample | FBS is used as a diluent and it inhibits AAV infection by itself. Different providers and a lot of FBS should be tested on the ability to affect the AAV infectivity. |
Authors' Contributions
K.K. and G.A. developed the concept of the project and designed experiments. K.K. performed experiments. K.K. and G.A. wrote the article.
Author Disclosure
K.K. and G.A. hold provisional patents related to the protocol described in the current article. G.A. has several issued patents related to AAV vectors that have been licensed to various gene therapy companies.
Funding Information
This project was supported by NIH/NIGM 1R01HL131586 and startup funds from the Hormel Institute.
References
- 1. High KA, Aubourg P. rAAV human trial experience. Methods Mol Biol 2011;807:429–457 [DOI] [PubMed] [Google Scholar]
- 2. Mendell JR, Rodino-Klapac L, Sahenk Z, et al. Gene therapy for muscular dystrophy: lessons learned and path forward. Neurosci Lett 2012;527:90–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jacobson SG, Cideciyan AV, Roman AJ, et al. Improvement and decline in vision with gene therapy in childhood blindness. N Engl J Med 2015;372:1920–1926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Smalley E. First AAV gene therapy poised for landmark approval. Nat Biotechnol 2017;35:998–999 [DOI] [PubMed] [Google Scholar]
- 5. Keeler AM, Flotte TR. Recombinant adeno-associated virus gene therapy in light of luxturna (and zolgensma and glybera): where are we, and how did we get here? Annu Rev Virol 2019;6:601–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mingozzi F. AAV immunogenicity: a matter of sensitivity. Mol Ther 2018;26:2335–2336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mingozzi F, High KA. Therapeutic in vivo gene transfer for genetic disease using AAV: progress and challenges. Nat Rev Genet 2011;12:341–355 [DOI] [PubMed] [Google Scholar]
- 8. Calcedo R, Wilson JM. Humoral immune response to AAV. Front Immunol 2013;4:341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Halbert CL, Miller AD, McNamara S, et al. Prevalence of neutralizing antibodies against adeno-associated virus (AAV) types 2, 5, and 6 in cystic fibrosis and normal populations: implications for gene therapy using AAV vectors. Hum Gene Ther 2006;17:440–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li C, Narkbunnam N, Samulski RJ, et al. Neutralizing antibodies against adeno-associated virus examined prospectively in pediatric patients with hemophilia. Gene Ther 2012;19:288–294 [DOI] [PubMed] [Google Scholar]
- 11. Boutin S, Monteilhet V, Veron P, et al. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum Gene Ther 2010;21:704–712 [DOI] [PubMed] [Google Scholar]
- 12. Nathwani AC, Tuddenham EG, Rangarajan S, et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med 2011;365:2357–2365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Verdera HC, Kuranda K, Mingozzi F. AAV vector immunogenicity in humans: a long journey to successful gene transfer. Mol Ther 2020;28:723–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li C, Wu S, Albright B, et al. Development of patient-specific AAV vectors after neutralizing antibody selection for enhanced muscle gene transfer. Mol Ther 2016;24:53–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tse LV, Klinc KA, Madigan VJ, et al. Structure-guided evolution of antigenically distinct adeno-associated virus variants for immune evasion. Proc Natl Acad Sci U S A 2017;114:E4812–E4821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kotterman MA, Schaffer DV. Engineering adeno-associated viruses for clinical gene therapy. Nat Rev Genet 2014;15:445–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pekrun K, De Alencastro G, Luo QJ, et al. Using a barcoded AAV capsid library to select for clinically relevant gene therapy vectors. JCI Insight 2019;4:e131610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Paulk NK, Pekrun K, Zhu E, et al. Bioengineered AAV capsids with combined high human liver transduction in vivo and unique humoral seroreactivity. Mol Ther 2018;26:289–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ogden PJ, Kelsic ED, Sinai S, et al. Comprehensive AAV capsid fitness landscape reveals a viral gene and enables machine-guided design. Science 2019;366:1139–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li C, Diprimio N, Bowles DE, et al. Single amino acid modification of adeno-associated virus capsid changes transduction and humoral immune profiles. J Virol 2012;86:7752–7759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huttner NA, Girod A, Perabo L, et al. Genetic modifications of the adeno-associated virus type 2 capsid reduce the affinity and the neutralizing effects of human serum antibodies. Gene Ther 2003;10:2139–2147 [DOI] [PubMed] [Google Scholar]
- 22. Nathwani AC, Davidoff AM, Hanawa H, et al. Sustained high-level expression of human factor IX (hFIX) after liver-targeted delivery of recombinant adeno-associated virus encoding the hFIX gene in rhesus macaques. Blood 2002;100:1662–1669 [DOI] [PubMed] [Google Scholar]
- 23. Guo P, Zhang J, Chrzanowski M, et al. Rapid AAV-neutralizing antibody determination with a cell-binding assay. Mol Ther Methods Clin Dev 2019;13:40–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang M, Crosby A, Hastie E, et al. Prediction of adeno-associated virus neutralizing antibody activity for clinical application. Gene Ther 2015;22:984–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Markusic DM, Nichols TC, Merricks EP, et al. Evaluation of engineered AAV capsids for hepatic factor IX gene transfer in murine and canine models. J Transl Med 2017;15:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Falese L, Sandza K, Yates B, et al. Strategy to detect pre-existing immunity to AAV gene therapy. Gene Ther 2017;24:768–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Meliani A, Leborgne C, Triffault S, et al. Determination of anti-adeno-associated virus vector neutralizing antibody titer with an in vitro reporter system. Hum Gene Ther Methods 2015;26:45–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Immunogenicity Testing of Therapeutic Protein Products—Developing and Validating Assays for Anti-Drug Antibody Detection; Guidance for Industry. Food and Drug Administration (FDA). 2019. https://www.regulations.gov/docket?D=FDA-2009-D-0539 (last accessed July6, 2020)
- 29. Gorovits B, Fiscella M, Havert M, et al. Recommendations for the development of cell-based anti-viral vector neutralizing antibody assays. AAPS J 2020;22:24. [DOI] [PubMed] [Google Scholar]
- 30. Jiang T, Xing B, Rao J. Recent developments of biological reporter technology for detecting gene expression. Biotechnol Genet Eng Rev 2008;25:41–75 [DOI] [PubMed] [Google Scholar]
- 31. Calcedo R, Chichester JA, Wilson JM. Assessment of humoral, innate, and T-cell immune responses to adeno-associated virus vectors. Hum Gene Ther Methods 2018;29:86–95 [DOI] [PubMed] [Google Scholar]
- 32. Landegger LD, Pan B, Askew C, et al. A synthetic AAV vector enables safe and efficient gene transfer to the mammalian inner ear. Nat Biotechnol 2017;35:280–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pandya J, Ortiz L, Ling C, et al. Rationally designed capsid and transgene cassette of AAV6 vectors for dendritic cell-based cancer immunotherapy. Immunol Cell Biol 2014;92:116–123 [DOI] [PubMed] [Google Scholar]
- 34. Krotova K, Day A, Aslanidi G. An engineered AAV6-based vaccine induces high cytolytic anti-tumor activity by directly targeting DCs and improves ag presentation. Mol Ther Oncolytics 2019;15:166–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Grieger JC, Soltys SM, Samulski RJ. Production of recombinant adeno-associated virus vectors using suspension HEK293 cells and continuous harvest of vector from the culture media for GMP FIX and FLT1 clinical vector. Mol Ther 2016;24:287–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zolotukhin S, Byrne BJ, Mason E, et al. Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Ther 1999;6:973–985 [DOI] [PubMed] [Google Scholar]
- 37. Pandya M, Britt K, Hoffman B, et al. Reprogramming immune response with capsid-optimized AAV6 vectors for immunotherapy of cancer. J Immunother 2015;38:292–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sayroo R, Nolasco D, Yin Z, et al. Development of novel AAV serotype 6 based vectors with selective tropism for human cancer cells. Gene Ther 2016;23:18–25 [DOI] [PubMed] [Google Scholar]
- 39. Ding W, Zhang L, Yan Z, et al. Intracellular trafficking of adeno-associated viral vectors. Gene Ther 2005;12:873–880 [DOI] [PubMed] [Google Scholar]
- 40. Harbison CE, Chiorini JA, Parrish CR. The parvovirus capsid odyssey: from the cell surface to the nucleus. Trends Microbiol 2008;16:208–214 [DOI] [PubMed] [Google Scholar]
- 41. Nonnenmacher M, Weber T. Intracellular transport of recombinant adeno-associated virus vectors. Gene Ther 2012;19:649–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Madigan VJ, Yuziuk JA, Chiarella AM, et al. Ring finger protein 121 is a potent regulator of adeno-associated viral genome transcription. PLoS Pathog 2019;15:e1007988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Xiao PJ, Mitchell AM, Huang L, et al. Disruption of microtubules post-virus entry enhances adeno-associated virus vector transduction. Hum Gene Ther 2016;27:309–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dudek AM, Zabaleta N, Zinn E, et al. GPR108 is a highly conserved AAV entry factor. Mol Ther 2020;28:367–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dudek AM, Pillay S, Puschnik AS, et al. An alternate route for adeno-associated virus (AAV) entry independent of AAV receptor. J Virol 2018;92:e02213-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dasgupta B, Seibel W. Compound C/dorsomorphin: its use and misuse as an AMPK inhibitor. In: Neumann D and Viollet B, eds. AMPK: Methods and Protocols. New York, NY: Springer New York, 2018:195–202 [DOI] [PubMed] [Google Scholar]
- 47. Yan Z, Zak R, Luxton GW, et al. Ubiquitination of both adeno-associated virus type 2 and 5 capsid proteins affects the transduction efficiency of recombinant vectors. J Virol 2002;76:2043–2053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Aslanidi GV, Rivers AE, Ortiz L, et al. Optimization of the capsid of recombinant adeno-associated virus 2 (AAV2) vectors: the final threshold? PLoS One 2013;8:e59142. [DOI] [PMC free article] [PubMed] [Google Scholar]