Abstract
Background and aim
Renal ischemia-reperfusion is associated with inflammation and oxidative stress. As a major compound in black pepper, piperine has anti-inflammatory and anti-oxidative properties. In present study, the protective effects of oral administration of piperine in renal ischemia-reperfusion (IR) induced acute kidney injuries (AKI) were investigated.
Experimental procedure
Male Wistar rats received piperine (10 or 20 mg/kg.bw) or vehicle for 10 days. The artery and vein of both kidneys were then clamped for 30 min, followed by a 24-h reperfusion period. Concentrations of creatinine and urea-nitrogen in descending aorta blood were measured, and malondialdehyde (MDA) and ferric reducing/antioxidant power (FRAP) levels were measured in kidney tissue to evaluate the oxidative stress. Inflammation was evaluated by measuring the TNF-α and ICAM-1 mRNA expression levels in renal cortical tissue using Real Time PCR method and counting leukocytes infiltration to interstitium. Further measured were tissue damages in H & E stained sections.
Results
Renal IR reduced FRAP, while increasing the plasma concentrations of creatinine and urea-nitrogen, tissue MDA level, TNF-α and ICAM-1 mRNA expressions, leukocyte infiltration and histopathologic injuries. Piperine administration significantly reduced the plasma concentrations of creatinine and urea-nitrogen, expression of pro-inflammatory factors, oxidative stress and renal histopathologic injuries. It is to be noted that 20 mg/kg dose was more effective.
Conclusion
Our results suggest piperine protects the kidney against ischemia-reperfusion induced acute kidney injuries by its anti-inflammatory and anti-oxidative properties.
Keywords: Piperine, Ischemia-reperfusion, Acute kidney injury, Inflammation, Oxidative stress
Abbreviations: AKI, Acute kidney injury; IR, Ischemia-reperfusion; MDA, Malondialdehyde; FRAP, Ferric reducing antioxidant power; ROS, Reactive oxygen species; TNF-α, Tumor necrosis factor-α; ICAM-1, Intercellular adhesion molecule-1; NO, Nitric oxide; iNOS, Inducible nitric oxide synthase; eNOS, Endothelial nitric oxide synthase; NF-κB, Nuclear factor-κB; PBS, Phosphate buffer saline; TPTZ, Tripyridyl-s-triazine; qRT-PCR, quantitative real-time PCR; GFR, Glomerular filtration rate; IL-1, Interleukin-1; IL-6, Interleukin-6
Graphical abstract
Highlights
-
•
Renal ischemia-reperfusion increased the inflammation and oxidative stress parameters.
-
•
Ischemia-reperfusion increased histopathological damages and functional parameters.
-
•
Piperine pretreatment significantly reduced the inflammation and oxidative stress.
-
•
Piperine administration ameliorated renal function and histopathologic damages.
1. Introduction
Acute kidney injury (AKI) is a serious complication that, currently, has no specific treatment and may be observed in up to 5% of the hospitalized patients.1 A major cause of AKI is renal ischemia-reperfusion (IR). Renal injuries caused by IR include inflammation, oxidative stress, and injuries of vascular endothelium and tubular epithelium.2
Following IR, inflammation begins as a result of cell injury, resulting molecular products which activate kidney parenchymal cells and dendritic cells (DCs), entailing the secretion of chemokines.3, 4, 5 Renal ischemia leads to leukocyte infiltration and tissue injury development through up-regulating the adhesive molecules (including ICAM-1) in vascular endothelium, and synthesizing pro-inflammatory cytokines such as IL-1, IL-6 and TNF-α in kidney.5,6 Moreover, renal IR increases the synthesis of inducible nitric oxide synthase (iNOS), augmenting the production of NO which reacts with reactive oxygen species (ROS) to form peroxynitrite (ONOO−), inhibits endothelial nitric oxide synthase (eNOS), reduces NO production in endothelium, and results in vasoconstriction.7, 8, 9
Piperine (1-peperoylpiperidine) is an alkaloid found in the fruit of plants belonging to piperaceae family such as black pepper (piper nigrum) and long pepper (piper longum).10 Plants from this family have been used in traditional medicine and as a spice around the world. It has been shown that piperine has many pharmacological properties such as anti-oxidative,11,12 anti-inflammatory13,14 and vasodilator15,16 effects, and can protect the brain against ischemia-reperfusion17 and kidney against lead acetate induced nephrotoxicity.18 Several studies have been performed on the mechanism of piperine, reporting that it acts by reducing the expression of ICAM-1, TNF-α, iNOS, and NF-κB,17,19,20 inhibiting cytochrome P450 enzymes21 and p-glycoprotein activities,22,23 and preventing endoplasmic reticulum stress.24 However, the protective effect of piperine against functional disturbances, inflammation, oxidative stress and tissue damages induced by renal IR is yet to be fathomed. Therefore, the objective of the present study was to assess the protective effects of piperine against renal damages induced by 30-min bilateral renal ischemia followed by 24-h reperfusion in rat.
2. Materials and methods
2.1. Animals
This experimental study was performed on 28 male Wistar rats (200–250 g) obtained from laboratory animal breeding at Kermanshah University of Medical Sciences. Throughout the experiments, animals were kept under constant conditions of 23°±2 °C temperature and 12-h light/darkness cycles in polypropylene cages, with free access to standard food and water. We attempted to assign the least possible number of rats to each test group. Rats experiencing unexpected suffering (disability, reduced motility and abnormal status) were excluded from experiments and euthanized by deep anesthesia. All experiments and procedures were carried out according to the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978), and were approved by ethics committee of Kermanshah University of Medical Sciences (Approval number: IR.KUMS.REC.1396.452).
2.2. Experimental protocols
Studied animals were randomly divided into 4 groups (n = 7). The first group (sham) received vehicle (20 μl Tween 80 + 980 μl normal saline) via gavage for 10 days and on the 10th day, one hour following gavage; they underwent sham operation without clamping the arteries and veins. As in the sham group, the second one received vehicle and on day 10 they underwent surgery and clamping of the artery and vein of both kidneys for 30 min (IR group). The third and fourth groups received piperine (Sigma, USA) for 10 days, 10 or 20 mg/kg.bw, respectively and underwent 30-min ischemia and 24-h reperfusion on the 10th day.
In the present research, utilized doses of piperine were selected based on our pilot studies and previous similar studies.11,17 It should be mentioned that 50 units of heparin were injected to animals just 30-min prior to ischemia induction, ip. Sodium pentobarbital (55 mg/kg, ip) was used for anesthesia. During surgery, body temperature was controlled by a rectal thermometer and maintained constant at 37 ± 1 °C using a lamp and a heat plate. At the end of the surgery, all rats were transferred into the cages to spend a 24-h reperfusion period, during which they had free access to food and water. On the 11th day and following 24-h reperfusion period, animals were reanesthetized and a blood sample was taken from descending aorta in order to measure the plasma creatinine and urea-nitrogen concentrations. After that, right kidney was removed and immediately frozen in liquid nitrogen for oxidative stress assessment through measuring malondialdehyde (MDA) and ferric reducing antioxidant power (FRAP). For the histopathologic study, one half of the left kidney was removed, fixed in 10% formaldehyde, and stained by haematoxylin and eosin (H & E). TNF-α and ICAM-1 mRNA expression levels were further assessed in the cortex tissue of other half of the kidney by quantitative Real Time PCR method (qRT-PCR). At the end of the experiment, all rats were euthanized by deep anesthesia.25
2.3. Assessment of renal function
In order to evaluate the kidney function, plasma creatinine and urea-nitrogen concentrations were measured by use of an autoanalyzer.
2.4. Assessment of oxidative stress
To assess the oxidative stress, MDA and FRAP levels were measured in the renal tissue by colorimetric assay method. MDA is the final product of the peroxidation of membrane phospholipids by oxidative factors, and FRAP indicates total tissue antioxidant power.26,27
2.5. Assessment of inflammation
To assess inflammation, mRNA expression levels of proinflammatory factors TNF-α and ICAM-1 were measured using qRT-PCR, and beta-actin, as housekeeping gene, in renal cortex tissue. For this purpose, first total RNA was extracted using TRIzol (Bioneer-Korea) and cDNA was synthesized (Takara-China). Gene expression levels were then measured using SYBR Green and Ampliqon Kit in accordance with the manufacturer's protocol. Finally, the 2-ΔΔCT method was employed to analyze the relative expression of genes.
For TNF-α, forward primer sequence was 5′-TCTTCTCATTCCTGCTCGTG-3′ and reverse primer sequence was 5′-TTTGGGAACTTCTCCTCCTTG-3'. For ICAM-1, forward and reverse primer sequences were 5′-GGGATGGTGAAGTCTGTCAA-3′ and 5′-GGCGGTAATAGGTGTAAATGG-3′, respectively. Forward and reverse primer sequences for beta-actin were 5′-TGCTATGTTGCCCTAGACTTC-3′ and 5′-GTTGGCATAGAGGTCTTTACGG-3′, respectively.28
To further evaluate the degree of inflammation, infiltrated leukocytes were counted in 20 optical microscopic fields (each field area: 0.14 mm2) and the mean number per mm2 was calculated.26
2.6. Assessment of renal tissue injuries
Injuries to renal tissue were analyzed through studying tissue sections stained with H & E. In this regard, Bowman's space enlargement, tubular cell necrosis, vascular congestion, intratubular proteinaceous cast and perivascular edema were studied and graded in 10 microscopic fields. For this purpose, the level of each pathophysiological feature was graded according to the changes involved, scoring 0 with no changes, 1 with<20%, 2 with 20–40%, 3 with 40–60%, 4 with 60–80% and 5 with >80%. Also calculated was the total histopathologic score for statistical analysis, which was equal to the sum of all grades associated with different injuries in each group.27
2.7. Statistical analysis
SPSS-23 software was used for data analysis, presented as mean ± SEM. To compare the data on kidney function and oxidative stress in different groups, one-way analysis of variance (ANOVA) and Tukey post hoc test were used; precise p-values were calculated by LSD test, and non-parametric Krushal-Wallis and Mann-Whitney tests were used to analyze the total histopathologic score. P < 0.05 was considered as data significance level.
3. Results
3.1. Effects of piperine on IR induced renal functional disturbances
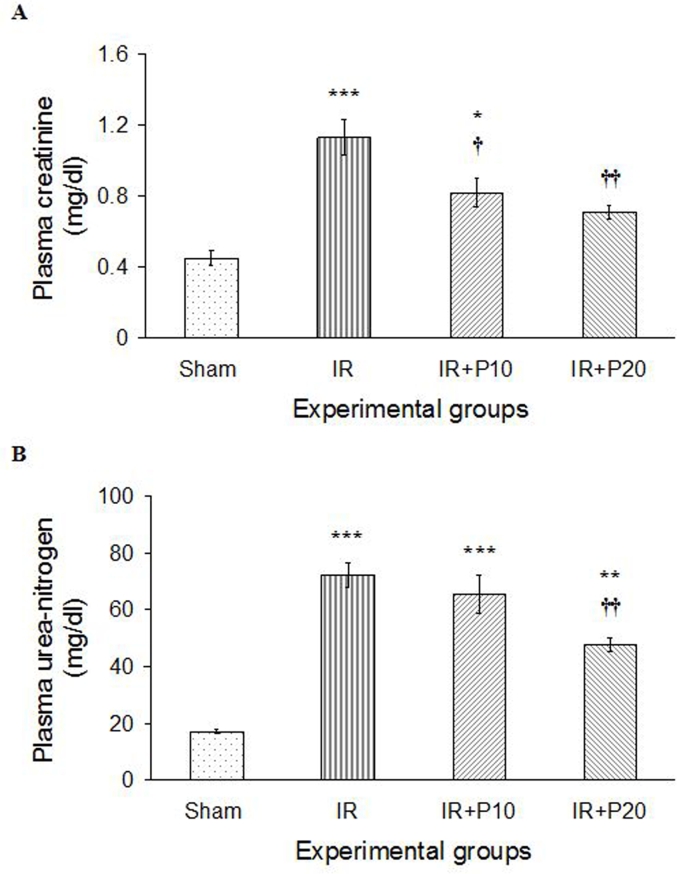
Renal IR resulted in increased concentrations of plasma creatinine and urea-nitrogen, compared with the sham group (P < 0.001). Piperine gavage reduced creatinine concentration in IR + P10 and IR + P20 groups (P < 0.05 and P < 0.01, respectively), in comparison with IR group, a reduction greater in IR + P20 group, whose value reached the level observed in the sham group (Fig. 1A). Moreover, piperine at 20 mg/kg was able to significantly reduce plasma urea-nitrogen concentration compared to IR group (P < 0.01), yet it was still higher than that of the sham group (P < 0.01). However, piperine with 10 mg/kg could not significantly reduce plasma urea-nitrogen concentration in comparison with IR group (Fig. 1B).
Fig. 1.
Plasma creatinine (A) and urea-nitrogen (B) levels in sham, ischemia/reperfusion (IR), IR + P10 or IR + P20 groups. Data are presented as mean ± SE (n = 7).
*P < 0.05, **P < 0.01, ***P < 0.001 in comparison with the sham group.
†P < 0.05, ††P < 0.01 in comparison with IR group.
3.2. Effects of piperine on IR induced oxidative stress
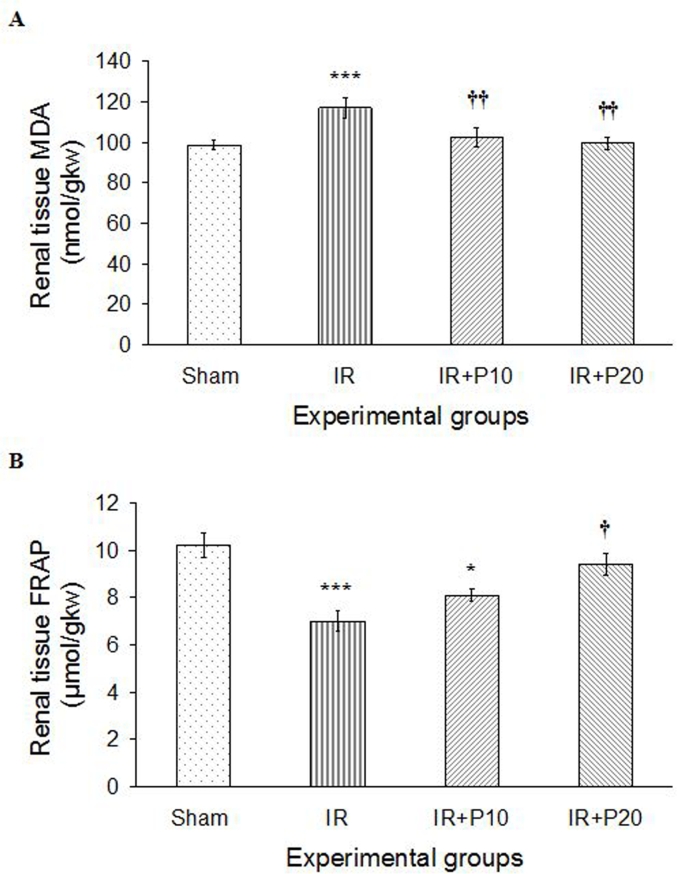
Thirty-minute renal ischemia followed by 24 h of reperfusion increased phospholipids peroxidation (MDA) in IR group, compared to the sham one (P < 0.001). Piperine gavage with both doses of 10 and 20 mg/kg.bw was able to reduce MDA level in comparison to IR group (P < 0.01 for both). Therefore, MDA level in both groups reached its level in sham group (Fig. 2A). On the other hand, renal IR reduced the total antioxidant capacity (FRAP) in kidney tissue (P < 0.001). Although both studied doses of piperine could augment FRAP level in comparison to IR group, only the increase in IR + P20 group was significant (Fig. 2B).
Fig. 2.
A, Renal tissue lipid peroxidation level (MDA) and B, total antioxidant capacity (FRAP) in sham, ischemia/reperfusion (IR), IR + P10 or IR + P20 groups. Data are presented as mean ± SE (n = 7).
*P < 0.05, ***P < 0.001 in comparison with the sham group.
†P < 0.05, ††P < 0.01 in comparison with IR group.
3.3. Effects of piperine on IR induced renal inflammation
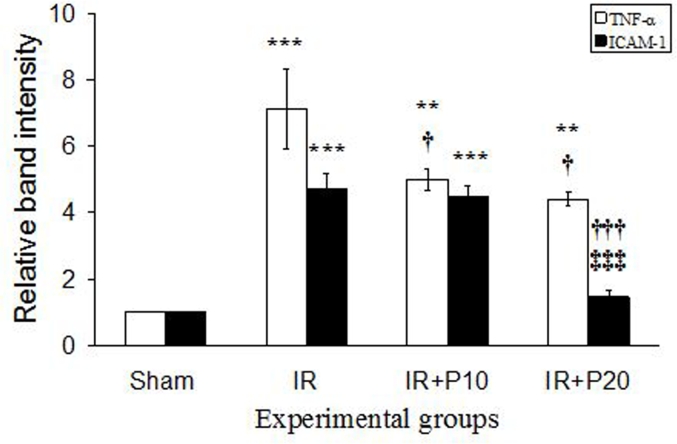
As seen in Fig. 3, IR increased TNF-α and ICAM-1 mRNA expression levels in renal cortical tissue compared to the sham group (P < 0.001 for both factors). Following piperine gavage, TNF-α mRNA expression was reduced in both IR + P10 and IR + P20 groups relative to IR group (P < 0.05), but their levels were still significantly higher than that in the sham group. In addition, piperine reduced ICAM-1 mRNA expression level in comparison to IR group, which was significant only at 20 mg/kg (P < 0.001). So that ICAM-1 expression level in this group reached its value in the sham group.
Fig. 3.
Representative mRNA fold change expression for tumor necrotic factor-α (TNF- α) and intercellular adhesion molecule-1 (ICAM-1) in the renal cortical tissue of the sham, ischemia/reperfusion (IR), IR + P10 or IR + P20 groups.
**P < 0.01, ***P < 0.001 in comparison with the sham group.
†P < 0.05, †††P < 0.001 in comparison with IR group.
‡‡‡P < 0.001 in comparison with IR + P10 group.
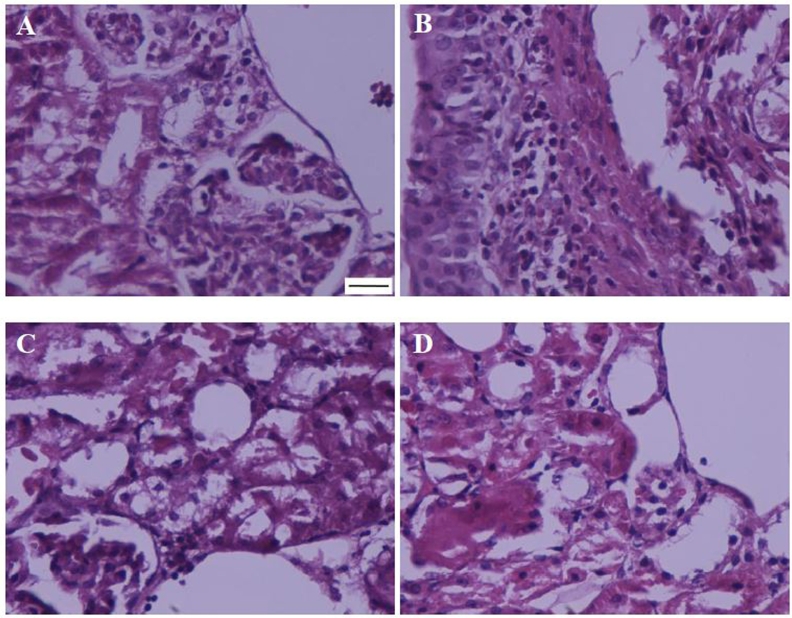
Leukocyte infiltration degree to interstitium increased following renal ischemia-reperfusion, which was reduced by both studied doses of piperine, a reduction more severe in IR + P20 group (Fig. 4 & Table 1).
Fig. 4.
Representing leukocytes infiltration in the kidney of sham (A), ischemia/reperfusion (B), IR + P10 (C), or IR + P20 (D) groups. (Haematoxylin-Eosin, 400×; scale bar: 200 μm).
Table 1.
The effect of piperine on renal ischemia/reperfusion induced histopathologic damages.
| Histopathologic damages | Experimental groups |
|||
|---|---|---|---|---|
| Sham | IR | IR + P10 | IR + P20 | |
| Bowman's space enlargement | 0.1 | 5 | 3.4 | 1 |
| Tubular cell necrosis | 0 | 5 | 4.2 | 0.7 |
| Vascular congestion | 0.2 | 4.3 | 3.8 | 1.1 |
| Intra-tubular proteinaceous casts | 0 | 4.6 | 2.9 | 0.8 |
| Perivascular edema | 0 | 3.8 | 3.1 | 1.2 |
| Leukocyte infiltration | 0 | 5 | 3.3 | 0.6 |
| Total histopathologic score | 0.3 | 27.7 *** | 20.5 *** † | 5.4 * ††† |
Histopathological scores in rats underwent sham operation and received normal saline (Sham), ischemia/reperfusion and received normal saline (IR), or piperine at 10 or 20 mg/kg (IR + P10 and IR + P20).
*P < 0.05, ***P < 0.001, in comparison with control group.
†P < 0.05, †††P < 0.001, in comparison with IR group.
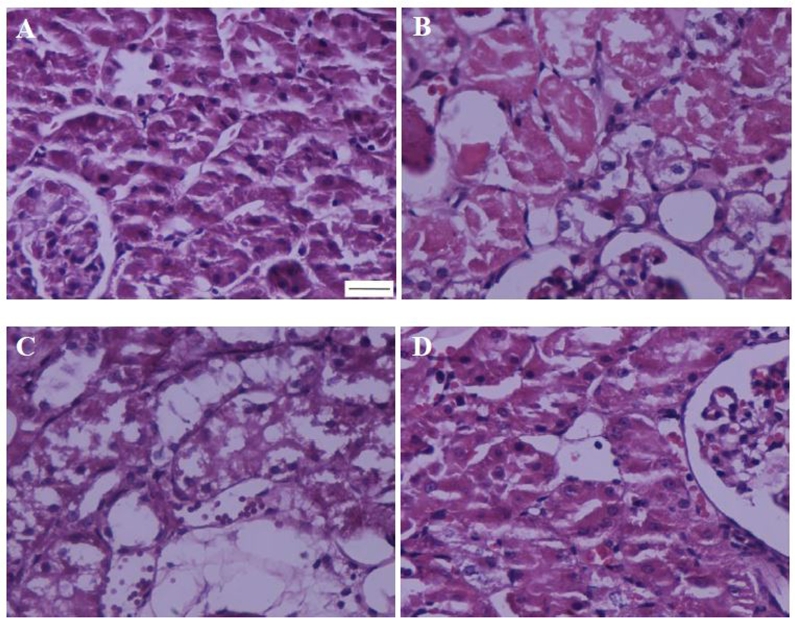
3.4. Effects of piperine on IR induced renal tissue injuries
Renal IR resulted in Bowman's space enlargement, tubular cell necrosis, vascular congestion, intratubular proteinaceous cast formation and perivascular edema (Fig. 5 & Table 1). In this regard, the total histopathologic score was 27.7 in IR group, significantly higher than that of the sham group (P < 0.001). Compared with IR group, piperine reduced all such injuries in IR + P10 and IR + P20 groups, although its efficacy was much higher with 20 mg/kg.
Fig. 5.
Representing histopathologic alterations in the kidney of sham (A), ischemia/reperfusion (B), IR + P10 (C), or IR + P20 (D) groups. (Haematoxylin-Eosin, 400×; scale bar: 200 μm).
4. Discussion
The present study demonstrated for the first time that piperine gavage protects kidney against 30-min ischemia and 24-h reperfusion induced injuries in rat. Piperine was administered by gavage. Previous studies have shown that in oral administration; about 97% of administered piperine is absorbed without any alteration.29
In the present research, renal IR increased plasma creatinine and urea-nitrogen concentrations. Given the reverse relationship between plasma creatinine concentration and glomerular filtration rate (GFR), it is likely that the increase in the plasma creatinine and urea-nitrogen concentrations is due to the decrease in GFR. It has been shown that different factors are at play in reducing GFR following IR, among which, mention can be made of the back-leak of filtered substances (due to damaged tubular epithelial cells), activation of tubuloglomerular feedback because of increased NaCl delivery to macula densa, and increased vascular resistance.1,2
Following renal IR, the expression of the inducible nitric oxide synthase (iNOS) increases in tubules, augmenting NO production. Such NO produces peroxynitrite (ONOO−) after reacting with reactive oxygen species (ROS), inhibits eNOS, and reduces NO production from endothelium and, as a result, vasoconstriction.7, 8, 9 It has been shown that endotoxin-induced renal injury is more pronounced in eNOS knockout mice.30
The results of the present study showed that piperine gavage reduced plasma creatinine and urea-nitrogen concentrations dose-dependently. Different studies have shown that piperine has vasodilator properties and protects the vessels.15,16 Furthermore, piperine results in reduced iNOS expression and production, and a reduction in kidney tissue damage in lead acetate induced nephrotoxicity.13,17, 18, 19 It can therefore be concluded that piperine increases GFR and consequently reduces plasma creatinine and urea-nitrogen concentrations through reducing vascular resistance and tubular epithelial cell damages. The results of the present study also indicated that piperine reduced cellular injuries following ischemia-reperfusion.
Renal IR resulted in an increase in MAD and a decrease in FRAP levels in kidney tissue. Malondialdehyde is the end product of phospholipids peroxidation by ROS, while FRAP represents the total antioxidant capacity of the tissue. Therefore, increased levels of MDA along with decreased levels of FRAP reflect the oxidative stress induction in renal tissue following IR. Depletion of ATP due to ischemia activates deleterious proteases and phospholipases, causing oxidative stress during reperfusion.2 Moreover, renal blood flow circulation change is not uniform following IR, and hypoxic regions may exist alongside those with normal circulation, interactions of which can increase ROS production.31,32 NO produced by iNOS reacts with these ROSs to produce peroxynitrite, which is much more potent than ROS.7 In addition to directly affecting the cells, ROS enhances vascular responsiveness to vasoconstrictors, thereby increasing vascular resistance.33
In this study, piperine gavage reduced MDA and increased FRAP, reflecting its anti-oxidative properties. It has been shown that piperine supplementation considerably reduces puromycin induced H2O2 production,34 increases antioxidative capacity of tissues, decreases lipid peroxidation,11 reduces endoplasmic reticulum oxidative stress24 and augments superoxide dismutase and glutathione peroxidase from renal tissue following lead acetate induced nephrotoxicity.18 Via its antioxidant effect, piperine is able to reduce the oxidative stress in renal tissue following IR, appearing as a decrease in MDA and an increase in FRAP.
Our results indicate that renal IR enhanced TNF-α and ICAM-1 mRNA expression level, and augmented leukocytes infiltration to interstitium, which indicates the induction of inflammation in renal tissue. Following IR, leukocytes attach to activated endothelial cells, resulting in their infiltration and exacerbation of injuries through the formation of congestion in peritubular capillaries, stimulation of ROS production, and cytokines release.35,36 It has further been shown that renal IR leads to the up-regulation of adhesive molecules such as ICAM-1, P-selection and E-selection in vascular endothelium,37,38 and synthesis of proinflammatory cytokines including IL-1, IL-6 and TNF-α in kidneys6; ICAM-1 inhibition results in renal protection against IR induced injuries.38 In the present study, administration of piperine attenuated TNF-α and ICAM-1 mRNA expression and the leukocytes infiltration induced by IR, a finding in line with the results of other studies where piperine was able to reduce the expression of NF-κB, TNF-α and ICAM-1 in different models of inflammation and brain IR.13,17,19 Accordingly, it can be concluded that one approach to renal protection by piperine against IR induced injuries is to inhibit inflammation by reducing TNF-α and ICAM-1 mRNA expressions, hence reducing leukocytes infiltration, as observed in the present study.
We found that renal IR resulted in histopathologic injuries including Bowman's space enlargement, tubular cell necrosis, vascular congestion, intratubular proteinaceous casts and perivascular edema in kidneys. In case of ischemia-reperfusion, the mechanism of cellular injuries begins with ATP depletion which leads to cytoskeleton disruption, brush border loss, and translocation of Na+-K+ pump from basolateral to apical membrane.39,40 In addition, damaged epithelial cells and reduced tubular reabsorption increase tubular sodium concentration. Increased sodium polymerizes Tamm-Horsfall protein, conducing to the cast formation.41 In this study, administration of piperine reduced all tissue injuries induced by IR, which can be attributed to the reduction in inflammation, oxidative stress and/or the increase in renal blood flow circulation.
It has further been demonstrated that renal IR increases the amounts of p-glycoprotein, and lack of p-glycoprotein protect kidneys against IR.23 Han et al. reported that piperine inhibited p-glycoprotein,22 which might be another way of protecting the kidney against IR, a subject to be further studied in the future.
5. Conclusions
It can be concluded that piperine gavage for 10 days improves renal function and protects it against 30-min ischemia followed by 24-h reperfusion through the mitigation of inflammation, oxidative stress and histopathologic injuries, where 20 mg/kg dose of piperine is more efficient than 10 mg/kg.
Conflict of interest statement
The authors declare no competing of interest.
Funding
This work was supported by the research deputy of Kermanshah University of Medical Sciences, Kermanshah, Iran (grant no. 96496 to HN).
Acknowledgment
The authors thank medical biology research center personnel for their kindly cooperation.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Basile D.P., Yoder M.C. Renal endothelial dysfunction in acute kidney ischemia reperfusion injury. Cardiovasc Haematol Disord - Drug Targets. 2014;14:3–14. doi: 10.2174/1871529x1401140724093505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abuelo J.G. Normotensive ischemic acute renal failure. N Engl J Med. 2007;357:797–805. doi: 10.1056/NEJMra064398. [DOI] [PubMed] [Google Scholar]
- 3.Chen G.Y., Nunez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rabb H., Griffin M.D., McKay D.B. Inflammation in AKI: current understanding, key questions, and knowledge gaps. J Am Soc Nephrol. 2016;27:371–379. doi: 10.1681/ASN.2015030261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kurts C., Panzer U., Anders H.J., Rees A.J. The immune system and kidney disease: basic concepts and clinical implications. Nat Rev Immunol. 2013;13:738–753. doi: 10.1038/nri3523. [DOI] [PubMed] [Google Scholar]
- 6.Thurman J.M. Triggers of inflammation after renal ischemia/reperfusion. Clin Immunol. 2007;123:7–13. doi: 10.1016/j.clim.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goligorsky M.S., Brodsky S.V., Noiri E. Nitric oxide in acute renal failure: NOS versus NOS. Kidney Int. 2002;61:855–861. doi: 10.1046/j.1523-1755.2002.00233.x. [DOI] [PubMed] [Google Scholar]
- 8.Ling H., Edelstein C., Gengaro P. Attenuation of renal ischemia-reperfusion injury in inducible nitric oxide synthase knockout mice. Am J Physiol Renal Physiol. 1999;46:F383–F390. doi: 10.1152/ajprenal.1999.277.3.F383. [DOI] [PubMed] [Google Scholar]
- 9.Chatterjee P.K., Patel N., Sivarajah A. GW274150, a potent and highly selective inhibitor of iNOS, reduces experimental renal ischemia/reperfusion injury. Kidney Int. 2003;63:853–865. doi: 10.1046/j.1523-1755.2003.00802.x. [DOI] [PubMed] [Google Scholar]
- 10.Selvendiran K., Thirunavukkarasu C., Singh J.P., Padmavathi R., Sakthisekaran D. Chemopreventive effect of piperine on mitochondrial TCA cycle and phase-I and glutathione-metabolizing enzymes in benzo(a)pyrene induced lung carcinogenesis in Swiss albino mice. Mol Cell Biochem. 2005;271:101–106. doi: 10.1007/s11010-005-5615-2. [DOI] [PubMed] [Google Scholar]
- 11.Vijayakumar R.S., Surya D., Nalini N. Antioxidant efficacy of black pepper (Piper nigrum L.) and piperine in rats with high fat diet induced oxidative stress. Redox Rep. 2004;9:105–110. doi: 10.1179/135100004225004742. [DOI] [PubMed] [Google Scholar]
- 12.Mujumdar A.M., Dhuley J.N., Deshmukh V.K., Raman P.H., Naik S.R. Anti-Inflammatory activity of piperine. Jpn J Med Sci Biol. 1990;43:95–100. doi: 10.7883/yoken1952.43.95. [DOI] [PubMed] [Google Scholar]
- 13.Ying X., Chen X., Cheng S., Shen Y., Peng L., Xu H.Z. Piperine inhibits IL-β induced expression of inflammatory mediators in human osteoarthritis chondrocyte. Int Immunopharmacol. 2013;17:293–299. doi: 10.1016/j.intimp.2013.06.025. [DOI] [PubMed] [Google Scholar]
- 14.Wang-Sheng C., Jie A., Jian-Jun L., Lan H., Zeng-Bao X., Chang-Qing L. Piperine attenuates lipopolysaccharide (LPS)-induced inflammatory responses in BV2 microglia. Int Immunopharmacol. 2017;42:44–48. doi: 10.1016/j.intimp.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Booranasubkajorn S., Huabprasert S., Wattanarangsan J., Chotitham P., Jutasompakorn P., Laohapand T. Vasculoprotective and vasodilatation effects of herbal formula (Sahatsatara) and piperine in spontaneously hypertensive rats. Phytomedicine. 2017;24:148–156. doi: 10.1016/j.phymed.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 16.Taqvi S.I., Shah A.J., Gilani A.H. Blood pressure lowering and vasomodulator effects of piperine. J Cardiovasc Pharmacol. 2008;52:452–458. doi: 10.1097/FJC.0b013e31818d07c0. [DOI] [PubMed] [Google Scholar]
- 17.Vaibhav K., Shrivastava P., Javed H. Piperine suppresses cerebral ischemia-reperfusion-induced inflammation through the repression of COX-2, NOS-2, and NF-κB in middle cerebral artery occlusion rat model. Mol Cell Biochem. 2012;367:73–84. doi: 10.1007/s11010-012-1321-z. [DOI] [PubMed] [Google Scholar]
- 18.Sudjarwo S.A., Eraiko K., Sudjarwo G.W., Koerniasari Protective effects of piperine on lead acetate induced-nephrotoxicity in rats. Iran J Basic Med Sci. 2017;20:227–231. doi: 10.22038/IJBMS.2017.9487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu D., Wang Y., Chen Z. The protective effect of piperine on dextran sulfate sodium induced inflammatory bowel disease and its relation with pregnane X receptor activation. J Ethnopharmacol. 2015;169:109–123. doi: 10.1016/j.jep.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 20.Kim H.G., Han E.H., Jang W.S. Piperine inhibits PMA-induced cyclooxygenase-2 expression through downregulating NF-κB, C/EBP and AP-1 signaling pathways in murine macrophages. Food Chem Toxicol. 2012;50:2342–2348. doi: 10.1016/j.fct.2012.04.024. [DOI] [PubMed] [Google Scholar]
- 21.Reen R.K., Wiebel F.J., Singh J. Piperine inhibits aflatoxin B1-induced cytotoxicity and genotoxicity in V79 Chinese hamster cells genetically engineered to express rat cytochrome P4502B1. J Ethnopharmacol. 1997;58:165–173. doi: 10.1016/s0378-8741(97)00104-9. [DOI] [PubMed] [Google Scholar]
- 22.Han Y., Chin Tan T.M., Lim L.Y. In vitro and in vivo evaluation of the effects of piperine on P-gp function and expression. Toxicol Appl Pharmacol. 2008;230:283–289. doi: 10.1016/j.taap.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 23.Huls M., Schoeber J.P., Verfaillie C.M. Deficiency of either P-glycoprotein or breast cancer resistance protein protect against acute kidney injury. Cell Transplant. 2010;19:1195–1208. doi: 10.3727/096368910X504478. [DOI] [PubMed] [Google Scholar]
- 24.Hammad A.S., Ravindran S., Khalil A., Munusamy S. Structure-activity relationship of piperine and its synthetic amide analogs for therapeutic potential to prevent experimentally induced ER stress in vitro. Cell Stress Chaperones. 2017;22:417–428. doi: 10.1007/s12192-017-0786-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahmoudzadeh L., Najafi H., Changizi-Ashtiyani S., Mohamadi yarijani Z. Anti-inflammatory and protective effects of Saffron extract in ischemia-reperfusion induced acute kidney injury. Nephrology (Carlton). 2017;22:748–754. doi: 10.1111/nep.12849. [DOI] [PubMed] [Google Scholar]
- 26.Najafi H., Mohamadi Yarijani Z., Changizi-Ashtiyani S. Protective effect of Malva sylvestris L. extract in ischemia-reperfusion induced acute kidney and remote liver injury. PLoS One. 2017;12 doi: 10.1371/journal.pone.0188270. e0188270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohamadi Yarijani Z., Godini A., Madani S.H., Najafi H. Reduction of cisplatin-induced renal and hepatic side effects in rat through antioxidative and anti-inflammatory properties of Malva sylvestris L. extract. Biomed Pharmacother. 2018;106:1767–1774. doi: 10.1016/j.biopha.2018.07.115. [DOI] [PubMed] [Google Scholar]
- 28.Mohamadi Yarijani Z., Najafi H., Shackebaeia D., Madani S.H., Modarresi M., Jassemid S.V. Amelioration of renal and hepatic function, oxidative stress, inflammation and histopathologic damages by Malva sylvestris extract in gentamicin induced renal toxicity. Biomed Pharmacother. 2019;112:108635. doi: 10.1016/j.biopha.2019.108635. [DOI] [PubMed] [Google Scholar]
- 29.Bhat B.G., Chandrasekhara N. Studies on the metabolism of piperine: absorption, tissue distribution and excretion of urinary conjugates in rats. Toxicology. 1986;40:83–92. doi: 10.1016/0300-483x(86)90048-x. [DOI] [PubMed] [Google Scholar]
- 30.Wang W., Mitra A., Poole B. Endothelial nitric oxide synthase-deficient mice exhibit increased susceptibility to endotoxin-induced acute renal failure. Am J Physiol Renal Physiol. 2004;287:F1044–F1048. doi: 10.1152/ajprenal.00136.2004. [DOI] [PubMed] [Google Scholar]
- 31.Lee C.G., Kim J.G., Kim H.J. Discovery of an integrative network of microRNAs and transcriptomics changes for acute kidney injury. Kidney Int. 2014;86:943–953. doi: 10.1038/ki.2014.117. [DOI] [PubMed] [Google Scholar]
- 32.Becker G.J., Hewitson T.D. Animal models of chronic kidney disease: useful but not perfect. Nephrol Dial Transplant. 2013;28:2432–2438. doi: 10.1093/ndt/gft071. [DOI] [PubMed] [Google Scholar]
- 33.Zou A.P., Li N., Cowley A.W., Jr. Production and actions of superoxide in the renal medulla. Hypertension. 2001;37:547–553. doi: 10.1161/01.hyp.37.2.547. [DOI] [PubMed] [Google Scholar]
- 34.Liu H., Bigler S.A., Henegar J.R., Baliga R. Cytochrome P450 2B1 mediates oxidant injury in puromycin-induced nephrotic syndrome. Kidney Int. 2002;62:868–876. doi: 10.1046/j.1523-1755.2002.00515.x. [DOI] [PubMed] [Google Scholar]
- 35.Basile D.P., Anderson M., Sutton T.A. Pathophysiology of acute kidney injury. Comp Physiol. 2012;2:1303–1353. doi: 10.1002/cphy.c110041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kinsey G.R., Li L., Okusa M.D. Inflammation in acute kidney injury. Nephron Exp Nephrol. 2008;109 doi: 10.1159/000142934. e102–e107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akcay A., Nguyen Q., Edelstein C.L. Mediators of inflammation in acute kidney injury. Mediat Inflamm. 2009;2009:137072. doi: 10.1155/2009/137072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kelly K.J., Williams W., Colvin R.B. Intercellular adhesion molecule-1-deficient mice are protected against ischemic renal injury. J Clin Investig. 1996;97:1056–1063. doi: 10.1172/JCI118498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lameire N., Van Biesen W., Vanholder R. Acute renal failure. Lancet. 2005;365:417–430. doi: 10.1016/S0140-6736(05)17831-3. [DOI] [PubMed] [Google Scholar]
- 40.Devarajan P. Update on mechanisms of ischemic acute kidney injury. J Am Soc Nephrol. 2006;17:1503–1520. doi: 10.1681/ASN.2006010017. [DOI] [PubMed] [Google Scholar]
- 41.Wangsiripaisan A., Gengaro P.E., Edelstein C.L., Schrier R.W. Role of polymeric Tamm-Horsfall protein in cast formation: oligosaccharide and tubular fluid ions. Kidney Int. 2001;59:932–940. doi: 10.1046/j.1523-1755.2001.059003932.x. [DOI] [PubMed] [Google Scholar]