Abstract
The current study was conducted to investigate the protective properties of Eucalyptus globulus leaves methanolic extract (EGLME) against diclofenac sodium (DS) induced hepatorenal and testicular toxicity in male rats. A total of 40 rats were equally divided into 4 groups, Control, Diclofenac sodium (DS), EGLME and DS + EGLME groups, respectively. DS and EGLME were administered orally at dose rate 2.5 and 100 mg/kg BW, 4 times/week for 8 weeks, respectively. Administration of DS distorted hepatorenal functions manifested by alteration of serum levels of ALT, AST, total protein and albumin, creatinine and urea with changes of histological architectures. DS caused reproductive toxicity represented by minimized sperm count, individual sperm motility and viability; depleted concentration of reduced glutathione (GSH) in testicular tissue; and decreased testosterone level with alteration in testicular histological features. In contrast, co-treatment of DS intoxicated rats with EGLME protected rats against the adverse effects of DS revealing enhancing properties of EGLME on rats’ liver, kidney and testes. In conclusion, we demonstrated that EGLME had a potent protecting property against DS induced hepatic, renal and testicular toxicity in male rats, with special concern to testicular tissue via modulation of GSH as an oxidant marker.
Taxonomy
(classification by EVISE): Diclofenac sodium toxicity (hepatorenal and testicular toxicity), co-treatment with natural herbal extract, blood biochemical assays, tissue anti-oxidants assay, histopathology and reproductive indices analyses.
Keywords: Eucalyptus globulus, Liver, Kidney, Reproductive toxicity, Reduced glutathione
Graphical abstract
Highlights of the findings and novelties
-
•
Diclofenac sodium toxicity affects hepatorenal and reproductive efficiency.
-
•
Eucalyptus globulus enhanced the altered functions and histopathology.
-
•
Eucalyptus globulus enhanced anti-oxidative molecule GSH in testes tissue.
1. Introduction
Diclofenac sodium is considered the most commonly used anti-inflammatory drug of non-steroidal nature (NSAID) and analgesic substance.1,2 It is used for medical complications like rheumatoid arthritis, inflammation, trauma, degenerative joint disease, dysmenorrhea and surgical pains.3 Diclofenac acts on decreasing the prostaglandin synthesis through inhibition of cyclooxygenase enzymes and diminishing the apoptosis.4,5 Diclofenac induces hepatotoxicity and renal toxicity as a side effect.2,6 It is known that anti-inflammatory drugs badly affect reproduction.2,3 Administration of diclofenac caused liver and kidney toxicity, besides degenerative alteration in testis, epididymis and accessory sex glands.7 Moreover, diclofenac metabolism involves the production of reactive oxygen species leading to oxidative stress and genomic DNA fragmentation.8 Furthermore, previous work has suggested that diclofenac is cytotoxic to liver via cytochrome P-450 (CYP)-mediated metabolism.9 There is clear evidence to implicate drug-induced oxidative stress as a mechanism of toxicity in numerous tissues. ROS have effects on key cellular targets, namely, DNA, lipid, and protein macromolecules. ROS may damage these critical cellular components at the molecular level, with consequent effects of ROS on cell survival mediated by kinase cascades. These factors may have a key role in initiating cell death in response to oxidative insult.10
Eucalyptus, which includes more than 700 species is the native plant of Australia. Eucalyptus globulus (E. globulus), were transferred to Europe and North Africa where they are well cultivated to the Mediterranean shores.11 Eucalyptus is mostly acclimated for cosmetics and paper industrial purposes and for traditional medicine. Recent trends in medicine depends on certain species of Eucalyptus as well.12,13 Recently, Eucalyptus is extensively cultivated for many purposes including antiseptic, chemotherapeutic, antioxidant, antimicrobial, respiratory and gastrointestinal disorders, healing, acaricidal, repellent, herbicidal and nematicidal.14, 15, 16 In this study we aimed to ameliorate and protect against the side effects of treatment with diclofenac sodium (DS) using novel extracts from natural herbal plants. To our knowledge, the protective effect of E. globulus against diclofenac induced reproductive toxicity in rats has not yet been studied. Therefore, the present study was undertaken to investigate the possible mechanism through which, E. globulus leaves methanolic extract (EGLME) deserve its protective potentials against DS induced hepatic, renal and reproductive toxicity in rats.
2. Materials and methods
2.1. Animals
A total of 40 male albino rats (Rattus norvegicus), weighing 100–120 g were used in this study. The animals were obtained from Abu Rawash, Giza Governorate, Egypt. Rats were kept in plastic cages with humidity (65%), temperature (20 ± 2 °C) and 12 h dark/light cycles and fed normal chow diet. They were acclimatized 10 days before starting the experiment. The experiments were conducted in accordance with ethical guidelines of the Animal Care and Use Committee of University of Sadat City, which followed the Guide for the Care and Use of Laboratory Animals 8th edition. Washington (DC): National Academies Press (US); 2011.
2.2. Chemicals and reagents
Diclofenac sodium (Declophen®, tablets) was purchased from Pharco Pharmaceuticals, Egypt. Diagnostic kits for assaying serum activities of alanine aminotransferase (ALT), aspartate aminotransferase (AST), urea, creatinine and reduced glutathione (GSH) in testis tissue were purchased from Bio-diagnostic company, Egypt. Diagnostic kits for assaying serum levels of total protein (Diamond diagnostics, Egypt), albumin (SPECTRUM, Egypt).
2.3. Plant extract preparation
Fresh green leaves of E. globulus, 2 kg, were obtained from road trees at Sadat City, Menoufia, Egypt (location: 30.462629, 30.782271). E. globulus leaves were identified and authenticated in Biochemistry department, Faculty of Veterinary Medicine, University of Sadat City. Methanolic extract of E. globulus leaves were prepared as follows, in brief, fresh leaves of E. globulus were rinsed thoroughly with distilled water to remove dust and debris and shade dried at room temperature then ground into powder. The powder was immersed in 80% methanol for 48 h at room temperature (22 °C) with gentle shaking. The contents were filtered through filter paper (Whatman size No. 1), then the filtrate was dried at room temperature by air current to obtain a semisolid crude extract weighing (320 g) with 16% as a crude percent. This extract was stored in airtight container at 4 °C until used.
2.4. Gas chromatograph technique for characterization of bioactive constituents of Eucalyptus globulus
The potential bioactive constituents of Eucalyptus globulus methanolic leaf extract was characterized by using a Hewlett- Packard HP 5890-Series II gas chromatograph equipped with a Hewlett-Packard HP 5971 series mass detector (MS). Briefly, a representative sample (0.2 g) of Eucalyptus Globulus methanol extract was extracted with 10 mL of methylene chloride. A 1 μl of the sample was injected onto VF-Xms Agilent capillary column of (30 m × 0.25 mm ID × 0.25 μm film thickness). The initial oven temperature of the GC was 40 °C for 4 min and the temperature then programmed at a rate of 5 °C/min to 270 °C. The injector temperature and detector temperature were 270 and 250 °C, respectively, and the carrier gas was He of 99.99% purity. The m/z (ratio of mass to charge) values, which represent the fragment ions of the compounds were recorded for each compound.
2.5. Experimental design
To evaluate protective potentials of E. globulus leaves methanolic extract (EGLME) against diclofenac sodium (DS) induced toxicity. Animals were assigned into 4 groups (n = 10/group) as the following, control group, were received distilled water, DS group, only diclofenac sodium intoxicated, rats were given orally diclofenac sodium, 2.5 mg/kg BW, 4 times per week for 8 weeks dissolved in distilled water. E. globulus leaves methanolic extract (EGLME) group, rats were given orally EGLME, 100 mg/kg BW, 4 times per week for 8 weeks. DS + EGLME group, rats were diclofenac sodium intoxicated as DS group and co-treated with EGLME as in the EGLME group.
2.6. Blood and tissues sampling
Blood samples were collected at the end of the 8th week of the experiment. Rats were anaesthetized with Diethyl ether. Blood samples were collected from orbital venous plexus. Sera samples were separated and stored at −20 °C until used for investigation of biochemical parameters. Furthermore, rats were sacrificed, and livers, kidneys and testes were removed, washed with normal physiological saline solution and divided into 2 parts. The first part was stored at −80 °C and used for assaying reduced glutathione (GSH) (antioxidant defense system biomarker) in testicular tissue. The second part was kept in 10% neutral formalin for histo-pathological examination.
2.7. Preparation of testicular tissue homogenate for measuring of reduced glutathione (GSH)
Before dissection, tissues were perfused with phosphate buffer saline solution (PBS) pH 7.4 containing 0.16 mg/ml heparin to remove red blood cells and clots. Testicular tissues (222 mg) were homogenized by using 2 ml cold buffer (50 mM potassium phosphate, pH 7.5 and 1 mM EDTA for GSH). Tissue homogenate was centrifuged at 4000 rpm for 15 min at 4 °C. The supernatant was aspirated and stored at −80 °C till analysis.
2.8. Biochemical and hormonal assay
We have measured serum activities of ALT and AST,17 serum total protein concentration,18 serum albumin concentration,19 serum urea concentration20 and serum creatinine level.21 Serum total testosterone level was measured using a competitive immunoassay using direct chemiluminescent technology (The ADVIA Centaur® CP Immunoassay System, Siemens Healthcare Diagnostics, USA). GSH content of testicular tissue was determined according to the procedure described by.22
2.9. Epididymal sperm preparation
The rats’ dissected cauda epididymis was kept in 1 ml of pre-warmed phosphate buffer saline. Gentle agitation along with tearing of the tissue was applied to make spermatozoa leak into the PBS.23 Each sample was briefly incubated at 37 °C for 20 min for further sperm analysis.
2.10. Evaluation of sperm count and motility
The motile sperm percents were estimated under (40 × ) using phase-contrast microscope with hot stage. Sperm motility (%) was categorized as proportion of progressive (rapid and slow) and non-progressive spermatozoa. Total number of motile sperm was calculated as; motility (%) = number of motile sperm/total number of sperms x100. Whereas total sperm count in a drop of the resulting sperm suspension was determined using a Neubauer chamber as described by.24,25
2.11. Evaluation of sperm viability
The viability was evaluated using eosin stain. Briefly, mixing 10 μl of sperm sample with 10 μl of dye (0.5% eosin stain) on a microscope slide and covered with a coverslip. A total of 200 sperm cells were counted shortly after addition of dye.23 Evaluation of live (unstained) and dead (red stained) spermatozoa eas carried out using light microscopy under X40.
2.12. Histopathological examination
Following necropsy, tissue specimens from liver, kidneys and testes were collected and rapidly fixed in 10% neutral buffered formalin solution. The fixed specimens were trimmed, washed, dehydrated in ascending grades of ethyl alcohol, cleared in methyl benzoate and processed through the conventional paraffin embedding technique. A size of 3–5 μm sections were gained from paraffin blocks using microtome (LEICA RM 2135) then routinely stained by haematoxylin and eosin (H & E) stain according to Ref. 26. Prepared slides were checked using light microscopy and photographed using a digital Leica photomicroscope (LEICA, DMLB, Germany).
2.13. Semiquantitative histopathological evaluation
The histopathological lesions in liver, kidney and testes tissues were examined in three rats from each group and five randomly selected sections were examined from each rat. Semiquantitative evaluation was performed in examined fields (n = 15) according to the percentage, degree and extent of tissue damage and were scored according to Michael, 2008 as follows: (−): normal appearance (absence of pathological lesion 0%), (+): mild (<25% of sections), (++): moderate (25–50% of sections), (+++): severe (51–75% of sections), and (++++): very severe (>75% of sections).
2.14. Statistical analysis
The significant differences between groups were analyzed using (GraphPad Prism 5, GraphPad Software, Inc., San Diego, CA, USA) with a one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test for pairwise comparison of data from the multiple groups. Results were statistically significant when P < 0.05.
3. Results
3.1. Characterization of bioactive constituents of Eucalyptus globulus
The potential bioactive constituents of Eucalyptus globulus methanolic leaf extract was characterized by using a Hewlett- Packard HP 5890-Series II gas chromatograph and revealed 4 compounds o-Isopropylanisole, Spathulenol, 2-Butanone, 4-(2,6,6-trimethyl-2-cyclohexen-1-yl)- and β-Gurjurene (Table 1).
Table 1.
The major antioxidant compounds identified in Eucalyptus globulus leaf extract.
| Retention time (min) | Area % | Compound name | Structure |
|---|---|---|---|
| 25.95 | 3.05 | o-Isopropylanisole | 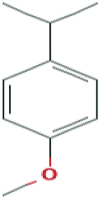 |
| 33.27 | 48.43 | Spathulenol | 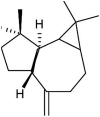 |
| 36.73 | 16.21 | 2-Butanone, 4-(2,6,6-trimethyl-2-cyclohexen-1-yl)- | 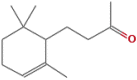 |
| 36.91 | 3.06 | β-Gurjurene | 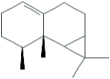 |
3.2. Eucalyptus globulus ameliorated diclofenac sodium induced alteration in liver function biomarkers
Rats of the DS group showed significantly elevated (p < 0.05) serum activities of ALT, AST and significantly reduced serum levels of total protein and albumin compared to those of rats in control group. However, co-treatment of rats with EGLME and diclofenac sodium in group DS + EGLME, significantly reduced (p < 0.05) serum activity of ALT, AST and significantly increased serum levels of total protein and albumin compared to those of rats in DS group. Administration of rats in EGLME group had no significant effects on the mentioned parameters compared to control group (Table 2).
Table 2.
Effect of Diclofenac sodium and/or Eucalyptus globulus leaves methanolic extract on serum liver and kidney functions biomarkers, testicular tissue Reduced Glutathione (GSH) and serum total testosterone.
| Control | DS | EGLME | DS + EGLME | |
|---|---|---|---|---|
| ALT (U/ml) | 16.22b ± 0.6915 | 23.54a±1.246 | 14.73b ± 0.4963 | 17.70b ± 0.2793 |
| AST (U/ml) | 90.93c±0.5586 | 104.0a±1.436 | 89.81c±0.7327 | 96.56b ± 1.203 |
| Albumin (g/dl) | 3.140a±0.07385 | 2.129b ± 0.03621 | 2.964a,c±0.02467 | 2.875c±0.03846 |
| Total protein (g/dl) | 8.268a±0.04716 | 6.108b ± 0.2360 | 8.228a±0.1588 | 7.806a±0.2040 |
| Creatinine (mg/dl) | 0.3095b ± 0.02231 | 0.7937a±0.1208 | 0.4683b ± 0.01068 | 0.4048b ± 0.02005 |
| Urea (mg/dl) | 46.74b ± 0.8980 | 58.11a±0.4042 | 46.14b ± 0.5095 | 47.51b ± 0.9046 |
| Reduced Glutathione mmol/g testicular tissue | 11.84a±0.06594 | 10.77b ± 0.01453 | 11.74a±0.1564 | 11.48a±0.08881 |
| Testosterone (ng/dl) | 2.460a±0.2552 | 1.177b ± 0.1969 | 2.960a±0.09512 | 2.623a±0.2229 |
- Mean value ± SEM (standard error of mean). The mean difference is significant at P < 0.05. The values carrying different superscript letters (a, b, c) in the same row were statistically different.
3.3. Eucalyptus globulus ameliorated diclofenac sodium induced alteration in kidney function biomarkers
Rats of the DS group showed significantly elevated (p < 0.05) serum activities of creatinine and urea compared to those of rats in control group. However, co-treatment of rats with EGLME and diclofenac sodium in group DS + EGLME, significantly reduced (p < 0.05) serum activity of creatinine and urea compared to those of rats in DS group. Administration of rats in EGLME group had no significant effects on the mentioned parameters compared to control group (Table 2).
3.4. Eucalyptus globulus ameliorated diclofenac sodium induced alteration in reproductive parameters
3.4.1. Regulation of reduced glutathione (GSH) concentration in testicular tissue
Rats of the DS group showed significantly decreased (p < 0.05) GSH concentration in testicular tissue compared to those of rats in control group. However, co-treatment of rats with EGLME and diclofenac sodium in group DS + EGLME, significantly enhanced (p < 0.05) GSH concentration in testicular tissue compared to those of rats in DS group. Administration of rats in EGLME group had no significant effects on GSH concentration compared to control group (Table 2).
3.4.2. Regulation of serum total testosterone level
Rats of the DS group showed significantly decreased (p < 0.05) serum total testosterone level compared to those of rats in control group. However, co-treatment of rats with EGLME and diclofenac sodium in group DS + EGLME, significantly enhanced (p < 0.05) serum total testosterone level compared to those of rats in DS group. Administration of rats in EGLME group had no significant effects on serum total testosterone level compared to control group (Table 2).
3.4.3. Eucalyptus regulated sperm parameters
3.4.3.1. Total sperm count
Rats of the DS group showed significantly decreased (p < 0.05) total sperm count compared to those of rats in control group. However, co-treatment of rats with EGLME and diclofenac sodium in group DS + EGLME, significantly increased (p < 0.05) total sperm count compared to those of rats in DS group. Administration of rats in EGLME group significantly increased (p < 0.05) total sperm count compared to those of rats in control and DS groups (Table 3).
Table 3.
Effect of Diclofenac sodium and/or Eucalyptus globulus leaves methanolic extract on sperm parameters (sperm count, motility and viability).
| Variables | Control | DS | EGLME | DS + EGLME |
|---|---|---|---|---|
| Count ( × 106) | 33.16a ± 2.45 | 19.33b ± 1.11 | 45.8c ±0.91 | 39ac ± 2.52 |
| Rapid motility (%) (Grade a) | 25a ± 1.66 | 10.91b ± 0.62 | 38.57c ± 2.62 | 21.25a ± 1.85 |
| Slow motility (%) (Grade b) | 35a ± 0.96 | 18.5b ± 2.07 | 37a ± 1.83 | 33.33a ± 1.66 |
| Non progressive motility (%) (Grade c) | 22 ± 0.79 | 22.85 ± 1.13 | 17.5 ± 1.72 | 25.83 ± 1.13 |
| Immotile sperm (%) (Grade d) | 18a ± 0.7 | 47.74b ± 2.98 | 6.93c ± 1.09 | 19.59a ± 0.7 |
| Total motility (%) (Grade a, b, c) | 82a ± 4.27 | 52.26b ± 7.69 | 93.07c ± 1.13 | 80.41a ± 1.13 |
| Viability (%) | 80a ± 0.96 | 58.33b ± 1.88 | 86.42c ± 1.73 | 78.5a± 1.36 |
- Mean value ± SEM (standard error of mean). The mean difference is significant at P < 0.05. The values carrying different superscript letters (a, b, c) in the same row were statistically different.
3.4.3.2. Sperm individual motility
Rats of the DS group showed significantly decreased (p < 0.05) total sperm individual motility compared to those of rats in control group. However, co-treatment of rats with EGLME and diclofenac sodium in group DS + EGLME, significantly increased (p < 0.05) total sperm individual motility compared to those of rats in DS group. Administration of rats in EGLME group significantly increased (p < 0.05) total sperm individual motility compared to those of all groups (Table 3).
3.4.3.3. Sperm viability
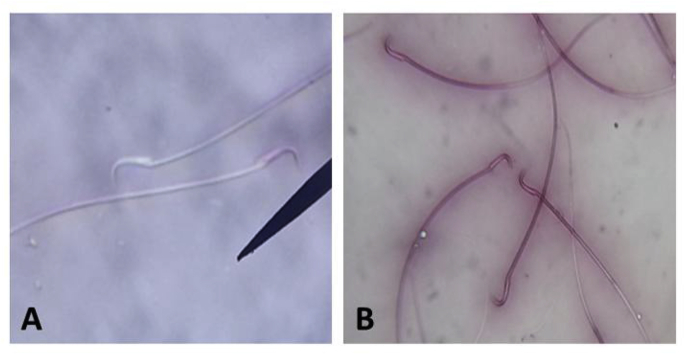
Rats of the DS group showed significantly decreased (p < 0.05) sperm viability compared to those of rats in control group. However, co-treatment of rats with EGLME and diclofenac sodium in group DS + EGLME, significantly increased (p < 0.05) sperm viability compared to those of rats in DS group. Administration of rats in EGLME group significantly increased (p < 0.05) sperm viability compared to those of all groups (Table 3) (Fig. 1).
Fig. 1.
Epididymal rat sperm stained with Eosin stain. (A) colorless live sperms (B) red stained dead sperms.
3.5. Eucalyptus globulus attenuated diclofenac sodium induced pathological alterations in rats’ hepatic tissues
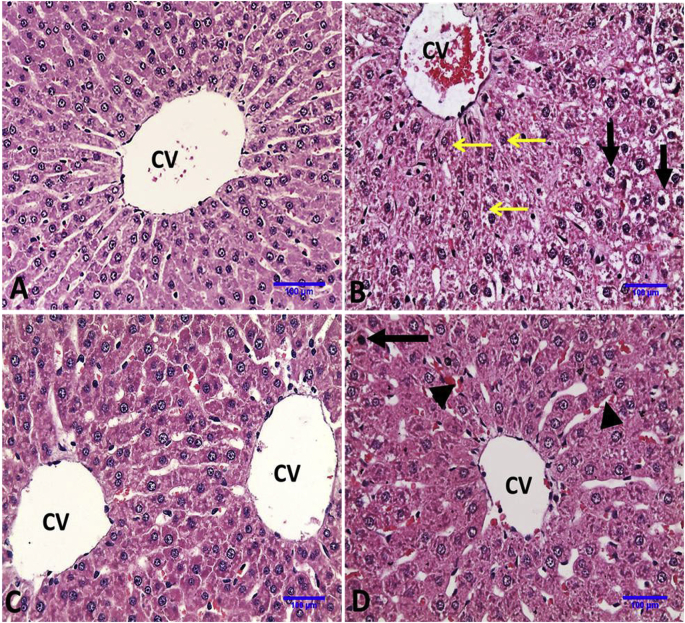
Normal hepatocytes arranged in trabecular manner around normal central vein were observed in liver of control and E. globulus administrated rats. Administration of DS induced histo-pathological alterations in the liver including congestion of central and portal veins, granular degeneration of hepatocytes with granular cytoplasm, diffuse hydropic degeneration and vacuolation of hepatocytes in liver parenchyma. On the other hand, administration of EGLME with DS protected the liver from diclofenac toxicity and only single cell necrosis with pyknotic nucleus and mild congestion in hepatic sinusoids were observed (Fig. 2 and Table 4).
Fig. 2.
Representative photomicrographs of liver sections of rats in different groups (Haematoxylin and Eosin stain X20). A, Control group: showing normal histologic architecture of liver. B, DS group: showing congestion of central vein, granular degeneration of hepatocytes with granular cytoplasm around central vein (thin arrows) and hepatocytes with diffuse hydropic degeneration and vacuolation (thick arrows). C, EGLME: showing normal histology as control group. D, DS + EGLME group: showing more normal hepatocytes arranged in trabecular manner around central vein, single cell necrosis with pyknotic nucleus (arrow) and mild congestion in hepatic sinusoids (head arrows).
Table 4.
Lesions in liver, kidney and testes from all groups and its histopathological grades.
| Control | DS | EGLME | DS + EGLME | |
|---|---|---|---|---|
| Liver lesions | ||||
| Congestion of central &portal vein | – | +++ | – | – |
| Congestion in hepatic sinusoids | – | +++ | + | + |
| Coagulative necrosis in hepatocytes | – | +++ | – | + |
| Granular degeneration of hepatocytes | – | +++ | – | + |
| Hydropic degeneration | – | +++ | – | + |
| kidney lesions | ||||
| Shrinkage of glomerular tuft with increase of Bowman’s space | – | +++ | – | + |
| Necrosis with pyknotic nuclei of glomerular cells | – | +++ | – | + |
| Focal coagulative necrosis of renal tubules | – | +++ | – | – |
| Vacuolation of epithelial lining renal tubules. | – | +++ | – | – |
| Hyaline and cellular casts | – | ++++ | – | + |
| Interstitial edema | – | ++ | – | - |
| Testes lesions | ||||
| Degenerated atrophied seminiferous tubules | – | +++ | – | + |
| Desquamation of spermatogenic epithelium & debris in the lumen | – | +++ | – | + |
| Loss of primary and secondary spermatocytes | – | ++++ | – | + |
| Testicular degeneration with appearance of spermatid giant cell | – | +++ | – | – |
| Congestion of interstitial blood vessels | – | ++++ | – | + |
| Edematous fluid and vacuolation in the interstial tissue | – | ++++ | – | – |
3.6. Eucalyptus globulus reduced diclofenac sodium induced pathological alterations in rats’ renal tissues
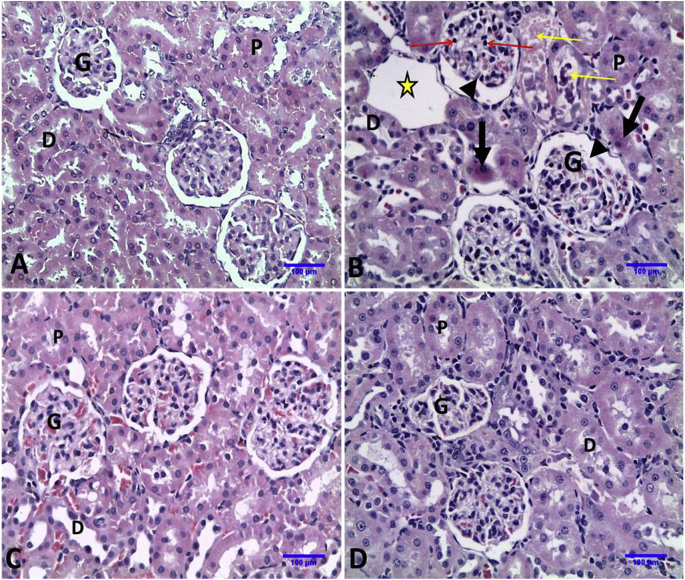
Normal histologic architecture of glomeruli, proximal and distal convoluted tubules were observed in kidneys of control and E. globulus administrated rats. Administration of DS resulted in shrinkage of glomerular tuft with increase of Bowman’s space, necrosis with pyknotic nuclei of glomerular cells, coagulative necrosis of some proximal convoluted tubules, damaged distal convoluted tubules with hyaline and cellular casts and interstitial edema. More normal glomeruli and renal tubules were observed in DS + EGLME group (Fig. 3 and Table 4).
Fig. 3.
Representative photomicrographs of Kidney sections of rats in different groups (Haematoxylin and Eosin stain X20). A, Control group: showing normal histologic architecture of kidney; glomeruli (G) and normal renal tubules {proximal convoluted tubules (P) and distal convoluted tubules (D)}. B, DS group: showing shrinkage of glomerular tuft with increase of Bowman’s space (head arrows), necrosis with pyknotic nuclei of glomerular cells (red arrows), coagulative necrosis of some proximal convoluted tubules (thick arrows), damaged distal convoluted tubules with hyaline and cellular casts (yellow arrows) and interstitial edema (star). C, EGLME group: showing normal histology as control group. D, DS + EGLME group: showing more normal glomeruli and normal renal tubules (proximal and distal convoluted tubules).
3.7. Eucalyptus globulus restored diclofenac sodium induced pathological deteriorations in rats’ testicular tissues
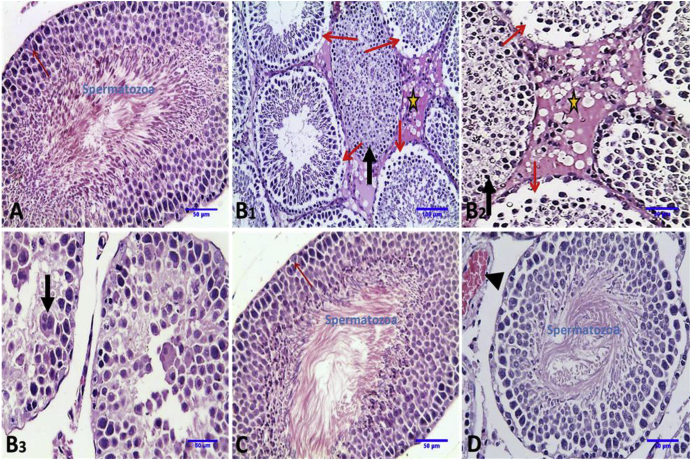
The histo-architecture of testes of control and E. globulus administrated groups showed compactly arranged seminiferous tubules with germinal layers of all stages of spermatogenesis, sertoli cells, normal lumen of seminiferous tubules which contain spermatozoa, normal interstitium and interstitial Leydig cells. Testes of DS-administrated rats showed degenerated seminiferous tubules; some with desquamation of all spermatogenic epithelium in their lumen, other seminiferous tubules with loss of primary and secondary spermatocytes and testicular degeneration with appearance of spermatid giant cell beside edematous fluid and vacuolation in the interstitial tissue. More or less normal seminiferous tubules with appearance of spermatozoa in their lumen were observed in DS + EGLME group (Fig. 4 and Table 4).
Fig. 4.
Representative photomicrographs of transverse sections of rat testicular tissue in different groups (Haematoxylin and Eosin stain). A, Control group: showing normal histologic architecture of seminiferous tubules (st), sertoli cells (red arrows) with formation of mature spermatozoa in the lumen of seminiferous tubules (X20). B, DS group: 1: showing degenerated seminiferous tubules; some with desquamation of all spermatogenic epithelium in their lumen (thick arrow), other seminiferous tubules with loss of primary and secondary spermatocytes (thin arrow) and edematous fluid and vacuolation in the interstial tissue (star) (X10). 2: showing higher magnification of 1 (X20). 3: showing seminiferous tubules with desquamated and necrosed spermatogenic epithelium and testicular degeneration with appearance of spermatid giant cell (thick arrow) (X20). C. EGLME group: showing normal histology seminiferous tubules as control group (X20). D, DS + EGLME group: showing more normal seminiferous tubules with appearance of spermatozoa in their lumen (X20).
4. Discussion
Diclofenac sodium (DS) is one of the non-steroidal anti-inflammatory drugs (NSAIDs) with reversible inhibition of cyclooxygenase and causing serious clinical sequelae in human with acute NSAID overdose and these include convulsions, metabolic acidosis, coma and acute renal failure,27 and also were known for their side effects in rats including hepatotoxicity and renal toxicity,2,6 and seriously affect reproduction.2,3 Our study aimed to ameliorate the side effects of DS using a novel extract from natural herbal plants. The present study was undertaken to investigate the possible mechanism through which, E. globulus leaves methanolic extract (EGLME) protects against DS induced hepatic, renal and reproductive toxicity in rats.
Our findings reported that DS induced alteration in liver function biomarkers as it significantly elevated ALT, AST activities; significantly reduced serum total protein and albumin levels; and these findings were in accordance with the previous study reported by28,29 and30; who showed that liver injury resulted from NSAIDs including diclofenac sodium may be proposed to their acidic moiety or reactive metabolites that bind to host proteins inducing cellular injury; and agreed with31 who denoted that diclofenac form significant alterations in serum protein contents by interference in protein metabolism. Furthermore, our results were in parallel with32 who reported that increased levels of serum glutamic oxaloacetic transaminase (GOT) and serum glutamic pyruvic transaminase (GPT) indicate a marker of liver injury and damage liver cell. Moreover, diclofenac affects serum glutamic pyruvic transaminase (GPT) transaminases and change the concentration of glutamate and aspartate in the extra-cellular environment.33
Kidney is the organ for drug filtration, concentration, and excretion. The capillary filtration is the main method for excretion of soluble boundless medication, as an example, diclofenac.34 Prostaglandins are to blame for the regulation of nephritic blood flow, capillary filtration, modulation of coagulase unharness, hollow particle transport, and water metabolism.34
The present study revealed that, DS badly affected the renal function as represented by significantly elevated serum creatinine and urea when compared with control group (Table 2). As noticed from previous reports, the kidney is extremely active in the metabolism and synthesis of prostaglandins,35 diclofenac prevents the synthesis of prostaglandin by inhibiting COX pathway in the kidney36 resulting in renal dysfunction and pathophysiological alterations.35 In addition, it is well established that male fertility can be affected and impaired by several kinds of toxicants through their direct effects on testicular tissue or disrupt the endocrine activities of male reproduction.37, 38, 39, 40, 41., 42 DS significantly decreased serum total testosterone level and reproductive sperm parameters such as sperm count, sperm individual motility and viability when compared to those of rats in control group (Table 3), these findings were similar to those obtained by43 and in harmony with the current results, diclofenac sodium decreased sperm count, sperm motility and density and inhibited prostaglandins synthesis in rats.43,44 Additionally,45 reported a significant decrease in the daily sperm production in the diclofenac treated rats besides a significant decrease in sperm motility and count with a significant decrease in the live/dead sperm in the diclofenac administrated rats, and we attributed these effects to the histopathological alterations noticed in testes of rats in DS group (Fig. 4). These findings were contrary with that, administration of diclofenac affected serum cholesterol and glucose concentrations which in turn reduced androgen in treated group as cholesterol is the precursor of the androgens.46 Treatment with diclofenac sodium salts reduced testosterone levels, feed intake and body weight.45 For understanding the mechanism behind the adverse effects of DS in testes, we analyzed the antioxidant marker, reduced glutathione (GSH) and our results revealed that there was a great depletion in GSH concentration in the testicular tissue and this agreed with previous studies by,47 about the relation between diclofenac toxicity and GSH tissue concentrations.
On the other hand, we reported the enhancing properties of our novel methanol extract from Eucalyptus globulus leaves. In the concurrent study, Eucalyptus globulus ameliorated diclofenac sodium induced alteration in liver and kidney function biomarkers. Interestingly, Eucalyptus globulus ameliorated diclofenac sodium induced alteration in reproductive parameters through a mechanism depending on improving the antioxidant system inside the cell resulting in significant enhancement of GSH concentration in testicular tissue (Table 2). Administration of EGLME increased the serum total testosterone level, sperm parameters including sperm count, sperm individual motility, and sperm viability, in our opinion, that improvement was a result to the antioxidant properties of Eucalyptus. We hypothesized that Eucalyptus supplementation stimulated reduced glutathione antioxidant in testicular tissues and protected spermatozoa from loss of motility.
From the histopathological point of view, in the current study, absence of secondary spermatocytes, spermatids and spermatozoa indicated the impairment of spermatogenesis at the late spermatocyte stage, which could be due to reduced testosterone level caused by diclofenac. Multinucleate giant cells were observed in the seminiferous tubules possibly due to fusion of macrophages to engulf deteriorated spermatogenic cells. Parallel with current data, diclofenac induced degenerative changes during treatment directly or indirectly via modulation of testosterone metabolism.48,49 Moreover,7 mentioned that treatments with diclofenac 0.5 mg/kg and 1.0 mg/kg in mice impaired seminiferous tubules and form degeneration, vacuolation, apoptosis in spermatogonia, primary spermatocytes, secondary spermatocyte and spermatids as well as in Sertoli cells and Leydig cells. These changes may lead to androgen disrupting activities as well as cellular supportive nutritive cellular toxicity.50,51 Here, the desquamated spermatogenic cells reflected the degenerative changes in the seminiferous tubules. These degenerative changes in the testes were not associated with lymphocyte or neutrophil aggregation supporting the fact that apoptosis was involved in diclofenac induced testicular toxicity. Interestingly, E. globulus restored the normal testicular structure. Some researches had pointed that apoptosis is the way by which the damaged spermatogenic cells were removed (Jahuukainen et al., 2000). Sertoli cells play important role in spermatogenesis.52 Leydig cells are main source of androgen production. Both types of cells can be affected by toxicants and chemical drugs.53 Alteration in the function of these cells may lead to changes in hormonal balance, disturbance in the process of spermatozoa development and impaired male fertility.48 Sertoli cells were degenerated, dilated intercellular space among germ cells, sloughing of the germ cells and accumulation in the lumen of seminiferous tubules which disturbed testicular tissue.54 Moreover, Diclofenac having drastic effects on testicular structure especially spermatogenic cell lines, interstitial cells and sertoli cells.55 In conclusion, we demonstrated for the first time that EGLME had a potent protecting properties against DS induced hepatic, renal and reproductive testicular toxicity in male rats, with special concern to testicular tissue via modulation of cellular antioxidant markers especially the reduced glutathione, we recommend for conducting further deep studies on farm animals for validation of the EGLME as a.liver, kidney and testicular natural protector.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Malhotra S., Rana D., Patel V. Comparison of analgesic, anti-inflammatory and anti-pyretic efficacy of diclofenac, paracetamol and their combination in experimental animals. Int J Basic Clin Pharmacol. 2013;2(4):458–465. [Google Scholar]
- 2.Vohra F., Raut A. Comparative efficacy, safety, and tolerability of diclofenac and aceclofenac in musculoskeletal pain management: a systematic review. Indian J Pain. 2016;30:3–6. [Google Scholar]
- 3.Thanagari B.S. Haemato-biochemical alterations induced by diclofenac sodium toxicity in Swiss albino mice. Vet World. 2012;5:417–419. [Google Scholar]
- 4.Mayorek N., Naftali-Shani N., Grunewald M. Diclofenac inhibits tumor growth in a murine model of pancreatic cancer by modulation of VEGF levels and arginase activity. PLoS One. 2010;5:1–10. doi: 10.1371/journal.pone.0012715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aprioku J.S., Nwidu L.L., Amadi C.N. Evaluation of toxicological profile of ibuprofen in wistar albino rats. Am J Biomed Sci. 2014;6:32–40. [Google Scholar]
- 6.Gan T.J. Diclofenac: an update on its mechanism of action and safety profile. Curr Med Res Opin. 2010;26:1715–1731. doi: 10.1185/03007995.2010.486301. [DOI] [PubMed] [Google Scholar]
- 7.Vyas A, Purohit A, Ram H. Assessment of dose-dependent reproductive toxicity of diclofenac sodium in male rats. Drug Chem Toxicol. 2019;42(5):478.–-486. doi: 10.1080/01480545.2017.1421659. [DOI] [PubMed] [Google Scholar]
- 8.Inoue A., Muranaka S., Fujita H., Kanno T., Tamai H. Molecular mechanism of diclofenac-induced apoptosis of promyelocytic leukemia: dependency on reactive oxygen species, Akt, Bid, cytochrome and caspase pathway. Free Radic Biol Med. 2004;37:1290–1299. doi: 10.1016/j.freeradbiomed.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Schmitz G., Stauffert I., Sippel H., Lepper H., Estler C.J. Toxicity of diclofenac to isolated hepatocytes. J Hepatol. 1992;14:408–409. doi: 10.1016/0168-8278(92)90196-v. [DOI] [PubMed] [Google Scholar]
- 10.Deavall Damian G, Martin Elizabeth A, Horner Judith M, Ruth R. Drug-induced oxidative stress and toxicity. J Toxicol. 2012;2012 doi: 10.1155/2012/645460. Article ID 645460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hasegawa T., Takano F., Takata T., Niiyama M., Ohta T. Bioactive monoterpene glycosides conjugated with gallic acid from the leaves of Eucalyptus globulus. Phytochemistry. 2008;69:747–753. doi: 10.1016/j.phytochem.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 12.Mason A.P., Ibrahim K., Ingleby K., Munro C.R., Wilson J. Mycorrhizal development and growth of inoculated Eucalyptus globulus (Labill) seedlings in wet and dry conditions in the glasshouse. For Ecol Manag. 2000;128:269–277. [Google Scholar]
- 13.Mulyaningsih S., Sporer F., Zimmermann S., Reichling J., Wink M. Synergistic properties of the terpenoids aromadendrene and 1, 8-cineole from the essential oil of Eucalyptus globulus against antibiotic-susceptible and antibiotic-resistant pathogens. Phytomedicine. 2010;17:1061–1066. doi: 10.1016/j.phymed.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 14.Nakhaee A., Bokaeian M., Saravani M., Farhangi A., Akbarzadeh A. Attenuation of oxidative stress in streptozotocin-induced diabetic rats by Eucalyptus globulus. Indian J Clin Biochem. 2009;24(4):419–425. doi: 10.1007/s12291-009-0075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dhibi A., Mbarki S., Elfeki A., Hfaiedh N. Eucalyptus globulus extract protects upon acetaminophen- induced kidney damages in male rat. Bosn J Basic Med Sci. 2014;14(2):99–104. doi: 10.17305/bjbms.2014.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dhakad A.K., Pandey V.V., Beg S., Rawat J.M., Singh A. Biological, medicinal and toxicological significance of Eucalyptus leaf essential oil: a review. J Sci Food Agric. 2018;98:833–848. doi: 10.1002/jsfa.8600. [DOI] [PubMed] [Google Scholar]
- 17.Reitman S., Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 18.Gornall A.C., Bardawill C.J., David M.M. Determination of serum proteins by means of biuret reaction. J Biol Chem. 1949;177:751–766. [PubMed] [Google Scholar]
- 19.Doumas B.T., Watson W.A., Biggs H.G. Albumin standards and the measurement of serum albumin with bromocresol green. Clin Chim Acta. 1971;31:87–96. doi: 10.1016/0009-8981(71)90365-2. [DOI] [PubMed] [Google Scholar]
- 20.Fawcett J.K., Scott J.E. A rapid and precise method for the determination of urea. J Chim Pathol. 1960;13:156–159. doi: 10.1136/jcp.13.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murray R.L. Mosby Co.; St. Louis. Toronto. Princeton: 1984. Creatinine. Kaplan A. Clin. Chem. The C. V; pp. 1261–1266. 418. [Google Scholar]
- 22.Beutler E., Duron O., Kelly B.M. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963;61:882–888. [PubMed] [Google Scholar]
- 23.Talebi A.R., Khalili M.A., Nahangi H., Abbasi A.M., Anvari M. Evaluation of epididymal necrospermia following experimental chronic spinal cord injury in rat. Iran J Reproductive Med. 2007;5:171–176. [Google Scholar]
- 24.Belsy M.A., Moghissi K.S., Eliasson R., Paulsen C.A., Callegos A.J., Prasad M.R.,N. Press Concern; Singapore: 1980. Laboratory Manual for the Examination of Human Semen and Semen Cervical Mucus Interaction. [Google Scholar]
- 25.Yari A., Sarveazad A., Asadi E. Efficacy of Crocus sativus L. on reduction of cadmium induced toxicity on spermatogenesis in adult rats. Andrologia. 2016;48:1244–1252. doi: 10.1111/and.12568. [DOI] [PubMed] [Google Scholar]
- 26.Bancroft J.D., Gamble M. sixth ed. Churchill, Living Stone; New York, London: 2008. Theory and Practice of Histological Techniques; pp. 440–450. [Google Scholar]
- 27.Hunter L.J., Wood D.M., Dargan P.I. The patterns of toxicity and management of acute nonsteroidal anti-inflammatory drug (NSAID) overdose. Open Access Emerg Med. 2011;3:39–48. doi: 10.2147/OAEM.S22795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bessone F. Nonsteroidal anti-inflammatory drugs: what is the actual risk of liver damage. World J Gastroenterol. 2010;16(45):5651–5661. doi: 10.3748/wjg.v16.i45.5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kishida T., Onozato T., Kanazawa T., Tanaka S., Kuroda J. Increase in covalent binding of 5-hydroxydiclofenac to hepatic tissues in rats co-treated with lipopolysaccharide and diclofenac: involvement in the onset of diclofenac-induced idiosyncratic hepatotoxicity. J Toxicol Sci. 2012;37(6):1143–1156. doi: 10.2131/jts.37.1143. [DOI] [PubMed] [Google Scholar]
- 30.Gomaa S. Adverse effects induced by diclofenac, ibuprofen, and paracetamol toxicity on immunological and biochemical parameters in Swiss albino mice. J Basic Appl Zool. 2018;79:5. [Google Scholar]
- 31.Subramanian S. Diclofenac induced toxic manifestations on adjuvant induced arthritic rats peripheral and reproductive organ of male wistar rats Rattus norvegicus. J Toxicol Environ Health Sci. 2009;1:12–21. [Google Scholar]
- 32.Kucera O. Acetaminophen toxicity in rat and mouse hepatocytes in vitro. Drug Chem Toxicol. 2016;545:1–9. doi: 10.1080/01480545.2016.1255953. [DOI] [PubMed] [Google Scholar]
- 33.Saran R.P., Purohit A., Ram H. A comparative patho-physiological study of diclofenac and meloxicam induced toxicity. Gallus Domestics. 2016;4(7):71–84. [Google Scholar]
- 34.Ahmed A.Y., Gad A.M., Abd El-Raouf O.M. Curcumin ameliorates diclofenac sodium-induced nephrotoxicity in male albino rats. J Biochem Mol Toxicol. 2017;31 doi: 10.1002/jbt.21951. [DOI] [PubMed] [Google Scholar]
- 35.Yasmeent T., Qureshi G.S., Perveen S. J Pak Med Assoc. 2007;57:349–351. [PubMed] [Google Scholar]
- 36.Laurence L., Bruce A., Björn C., Goodman & Gilman’s . eleventh ed. The McGraw-Hill; 2006. The Pharmacological Basis of Therapeutics. [Google Scholar]
- 37.Jeng H.A. Exposure to endocrine disrupting chemicals and male reproductive health. Front Public Health. 2014;2(55) doi: 10.3389/fpubh.2014.00055. 55–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sweeney M.F. Environmental endocrine disruptors: effects on the human male reproductive system. Rev Endocr Metab Disord. 2015;16(4):341–357. doi: 10.1007/s11154-016-9337-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mahmoudi R. Green tea improves rat sperm quality and reduced cadmium chloride damage effect in spermatogenesis cycle. J Med Life. 2018;11(4):371–380. doi: 10.25122/jml-2018-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chemek Involvement of testicular DAAM1 expression in zinc protection against cadmium-induced male rat reproductive toxicity. J Cell Physiol. 2018;233(1):630–640. doi: 10.1002/jcp.25923. [DOI] [PubMed] [Google Scholar]
- 41.Aly Testicular toxicity of gentamicin in adult rats: ameliorative effect of lycopene. Hum Exp Toxicol. 2019;38(11):1302–1313. doi: 10.1177/0960327119864160. [DOI] [PubMed] [Google Scholar]
- 42.Taherdehi Ghorbani. Evaluating the protective role of ascorbic acid in malathion-induced testis tissue toxicity of male rats. Int J Prev Med. 2019;17(10):45. doi: 10.4103/ijpvm.IJPVM_253_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jana K., Sen P.C. Environmental toxicants induced male reproductive disorders: identification and mechanism of action. In: Acree B., editor. Toxicity and Drug Testing. InTechOpen. Intech; Sciyo: 2012. [Google Scholar]
- 44.Brunetti L. Cafeteria diet increases prostaglandin E2 levels in rat prostate, kidney and testis. Int J Immunopathol Pharmacol. 2010;23:1073–1078. doi: 10.1177/039463201002300411. [DOI] [PubMed] [Google Scholar]
- 45.Ademuyiwa J.A., Adeolu R.J., Adeniyi S.A. Protective effects of cyclohexyl methyl dithiocarbamates sodium salts on diclofenac induced reproductive toxicity in male albino rats. Biochem Pharmacol. 2014;4:155. [Google Scholar]
- 46.English K.M. Men with coronary artery disease have lower levels of androgens than men with normal coronary angiograms. Eur Heart J. 2000;21:890–894. doi: 10.1053/euhj.1999.1873. [DOI] [PubMed] [Google Scholar]
- 47.Hickey J., Raje R., Reid V., Gross S., Ray S. Free Radic Biol Med. 2001;31(2):139–152. doi: 10.1016/s0891-5849(01)00560-3. [DOI] [PubMed] [Google Scholar]
- 48.Monsees T.K., Franz M., Gebhardt S., Winterstein U., Schill W.B., Hayatpour J. Sertoli cells as a target for reproductive hazards. Andrologia. 2000;32:239–246. doi: 10.1046/j.1439-0272.2000.00391.x. [DOI] [PubMed] [Google Scholar]
- 49.Sarkar U. Integrated assessment of diclofenac biotransformation, pharmacokinetics, and omics-based toxicity in a three-dimensional human liver immunocompetent coculture system. Drug Metab Dispos. 2017;45(7):855–866. doi: 10.1124/dmd.116.074005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Obeys A.K., Karim A., Mahood S. Histological study of the effect of piroxicam on testes of albino mice Mus musculus. J Univ Anbar Pure Sci. 2013;7(2):1–11. [Google Scholar]
- 51.Frungieri M.B. Cyclooxygenase and prostaglandins in somatic cell populations of the testis. Reproduction. 2015;149:R169–R180. doi: 10.1530/REP-14-0392. [DOI] [PubMed] [Google Scholar]
- 52.Sapori P.M.H., Chatelain P., Saez J.M. In vitro interaction between Sertoli cells and steroidogenic cells. Biochem Biophys Res Commun. 1986;134:957–962. doi: 10.1016/s0006-291x(86)80513-7. [DOI] [PubMed] [Google Scholar]
- 53.Papadakis V., Vlachopapadopoulu W., Van Syckle K. Gonadal function in young patients successfully treated for Hodgkin’s disease. Med Pediater Oncol. 1999;32:366–372. doi: 10.1002/(sici)1096-911x(199905)32:5<366::aid-mpo10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 54.Campion S. Male reprotoxicity and endocrine disruption. In: Luch A., editor. vol. 101. Springer International Publishing AG; 2012. pp. 315–360. (Molecular, Clinical and Environmental Toxicology. Experientia Supplementum). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mohan D., Sharma S. 3rd International Conference on Climate Change. Forest Resource and Environment; India: 2011. Histopathological alteration in the testes of mice exposed to Diclofenac Sodium. [Google Scholar]