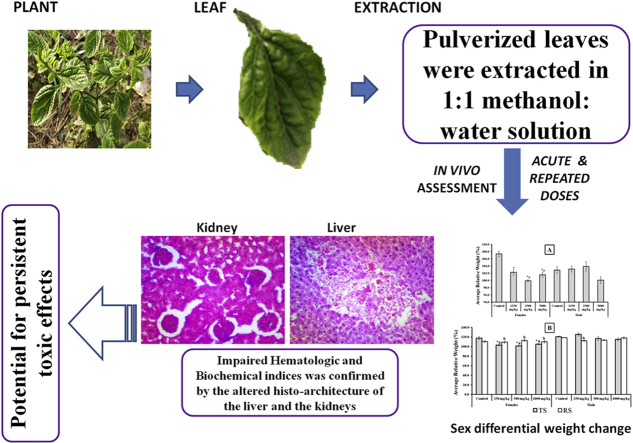
Abstract
Background and Aim
The many pharmacological potentials of Stachytarpheta cayennensis (L.C. Rich) Vahl, especially in managing central nervous system disorders, hypertension, diabetes and infections, have made it a subject of abuse, necessitating the need to ascertain its safety. This study therefore investigated the toxic effects of the leaf extract of S. cayennensis in rats following acute and 28-day repeated doses in male and female rats.
Experimental procedure
Acute and repeated dose studies were conducted in male and female groups of rats (135–150 g), using OECD 423 and 407 Tests guidelines respectively. Functional observational battery, and body weights were monitored. Blood samples were analysed for haematological and plasma biochemical indices. Organs (brain, kidneys and liver) specimen were collected and weighed. Kidney and liver specimen were subjected to histopathological analysis.
Results and conclusion
The LD50 of the extract was greater than 5000 mg/kg, p.o. (24 h) suggesting that the extract may be non-toxic. However, following single and repeated doses, the results revealed varying degree of significant (p < 0.05) changes in biochemical and heamatological indices, as well as in relative body weight and organ-body and organ-brain weight ratios. Also, histological assessment revealed evidence of liver and kidney toxicities and recovery was incomplete, as signs of toxicities were still evident after 21 days of recovery. Therefore, the extract is potentially harmful to vital organs with evidence of sex differential adverse effects and non-reversible forms of toxicity, especially with repeated usage, necessitating the need to avoid indiscriminate use.
Keywords: Stachytarpheta cayennesis, Safety assessment, Acute toxicity, Repeated dose toxicity, Organotoxicity
Graphical abstract
Highlights
-
•
The leaf extract of Stachytarpheta cayennensis induced significant changes in rats weights.
-
•
The extract caused varying significant changes in biochemical and hematological indices.
-
•
Significant alteration in histoarchitecture of liver and kidney were observed.
-
•
The observed toxic effects were persistent following period of recovery.
-
•
The toxic effects are mostly sex dependent.
List of abbreviations
- OECD
Organization for Economic Co-operation and Development
- TG
Test Guidelines
- ME and BF
Methanol extract and Butanol fraction of C. albidum seed cotyledons respectively
- FOB
Functional observatory batteries
- FWR
Female Wistar rats
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- WBC
White blood cell
- RBC
Red blood cell
- Hb
Hemoglobin concentration
- HCT
Hematocrit
- MCV
Mean corpuscular volume
- MCH
mean corpuscular hemoglobin
- MCHC
Mean corpuscular hemoglobin concentration
1. Introduction
Plants, with their numerous diversities, are essential sources of basic human needs (food, clothing and housing), as well as natural medicines for human well-being.1 Many conventional drugs originated from plant sources, such as aspirin (Salix alba), digoxin (Digitalis purpurea), quinine (Cinchona officinalis), and morphine (Papaver somniferum).1 There are documented evidence of incidences of organs toxicity from prolong ingestion of medicinal herbs.2,3 Despite these reported toxicity, the patronage of medicinal plants and related products is on the increase, due to supposed safety, availability and affordability.4,5 The increasing patronage also come with increasing tendencies for abuse, arising from indiscriminate uses, thereby necessitating the need to establish the safety of medicinal plants. In addition, the resounding calls for the integration of traditional medicine practice into the conventional modern medicine,6,7 make the determination and documentation of the safety/toxic risk potentials of medicinal plants imperatives.
Among the medicinal plants that have found usefulness traditionally and attracted scientific interests is Stachytarpheta cayennensis (L.C. Rich) Vahl8. S. cayennensis is a seed producing weedy herbaceous plant, erect, shrubby perennial, which belongs to the Verbenaceae family and can grow up to 1.5 m high.8,9 It is commonly called Brazilian tea, Blue rats tail (English), and Iruamure and Opapara in South western Nigeria.9 The seed is a two-seeded kernel or nutlet enclosed by a persistent calyx that is embedded in a shallow groove in the inflorescence axis.9,10The plant is found in several regions of the world, including Brazil, Ghana, India, Malaysia, Mexico, West Indies and Nigeria as weeds.8, 9, 10
Ethnomedicinally, S. cayennensis is used to treat various ailments such as inflammation, pain, fever, hepatic and renal disorders, helminthiasis, constipation, hypertension, stress, insomnia and diabetes.1,8,10 Essentially, the antimalarial, antifungal, antibacterial, immunomodulatory, antidiabetic and central nervous system effects of S. cayennensis have been documented.8,11, 12, 13, 14, 15 However, despite the pharmacological potentials associated with S. cayennensis, the toxicity profile of the plant remains largely unexplored. The traditional use of the leaf of S. cayennensis in the management of insomnia, evidence by the reported central nervous system effects,8 made this plant a subject of abuse, necessitating the need to establish its safety profile.
In this report, we presented the evaluation of the toxicity potential of the leaf extract of S. cayennensis using acute and 28-day repeated dose toxicity approaches. Evidence of potential sex differential adverse effects of the extract of S. cayennensis on haematological and plasma biochemical indices, as well as kidney and liver histology of male and female rats are presented.
2. Materials and methods
2.1. Collection and preparation of plant material
The plant was identified, authenticated and herbarium specimen with voucher number IFE 17620 was deposited in the Herbarium unit of the Department of Pharmacognosy, Faculty of Pharmacy, Obafemi Awolowo University (OAU) Ile-Ife, Nigeria. The plant name was also checked against http://www.theplantlist.org, an extensive source of medicinal plants, for confirmation. The leaves of S. cayennensis were collected from the wild on the campus of OAU, Ile-Ife, Nigeria, and air-dried in the laboratory at room temperature. The dried leaves were pulverized and 1 kg was soaked for 48 h in 6 L of 1:1 methanol: water solution and the marc was re-extracted twice as earlier described.8 The pooled extract was concentrated to dryness in vacuo using rotary evaporator and activated desiccators to obtain 176.5 g (17.65%) of sticky, dark crude extract.
2.2. Animals use and care
Healthy Wistar rats of both sexes (135–150 g), bred locally in the animal holdings of the Department of Pharmacology, Faculty of Pharmacy, OAU, Ile-Ife, were used in both the single and repeated dose toxicity tests. The animals were housed in standard plastic cages for at least 7 days prior to the start of the study to allow for acclimatization under natural atmospheric conditions. The animals were also fed with standard laboratory chow (Vital Feed) ® and water ad libitum. The procedure for the animal care was based on the “Guide for the Care and Use of Laboratory Animals – Eighth Edition”,16 as adopted by the Committee on care and use of laboratory animals, Obafemi Awolowo University and was given approval number PHP14/15/H/0211.
2.3. Experimental procedures
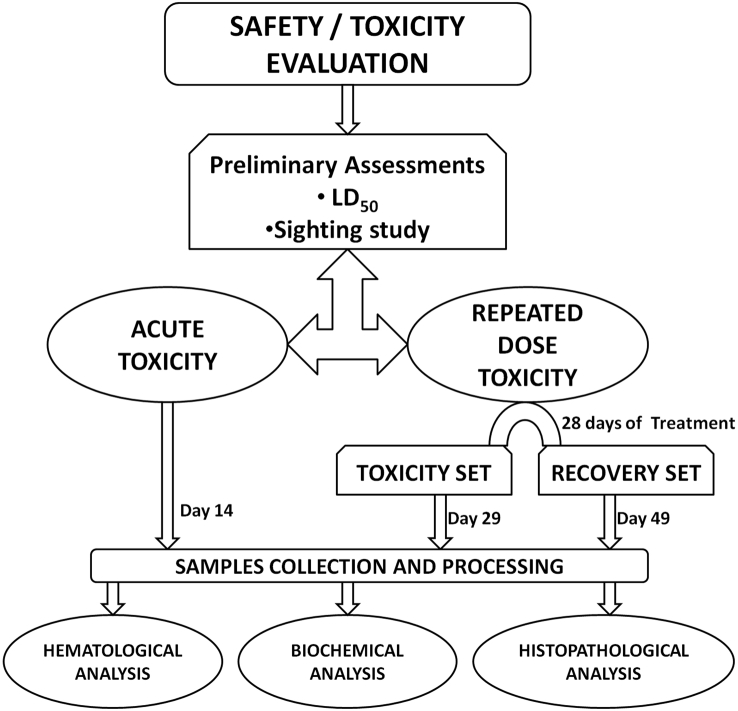
The schematic representation of the experimental procedures is given in Fig. 1.
Fig. 1.
Schematic representation of the experimental procedures. Experiments were performed separately in male and female rats.
2.3.1. Median lethal dose (LD50) determination and sighting study
The median lethal dose (LD50) was determined using Organisation for Economic, Co-operation and Development (OECD) Test Guideline (TG) 425 17, with earlier reported LD50 value in mice13 used as the starting point. With no mortality observed at 5000 mg/kg, a confirmatory test was conducted to validate the observation. Using LD50 as guide, and monitoring functional observational battery (FOB),18, 19, 20 the sighting study21,22 was conducted to determine the optimal doses for the acute and repeated dose toxicity studies, and humane endpoint criteria.
2.3.2. Single dose (acute) toxicity study
Twenty each of males and females (nulliparous non-pregnant) rats were used in this study. Each category (male or female rats) were randomly allotted into four groups (control and three test groups, n = 5) and the study procedure was adapted from OECD TG 423 22. The graded doses of the leaf extract of S. cayennensis (1250, 2500, 5000 mg/kg body weight) and distilled water (control) were orally administered to rats following an overnight fast. The volume of administered doses was not more than 1 ml/100 g body weight. Cage side observation using FOB was monitored continuously for the first 30 min, then at regular 30 min interval for the next 4 h, then regularly thereafter till 24 h and daily till day 14. The body weight of each animal was determined prior to administration of the test extract, day 1 and every 48 h thereafter. On day 14, rats were sacrificed for samples collection.
2.3.3. Repeated dose toxicity and recovery studies
Forty each of male and female nulliparous non-pregnant rats were randomly allotted into four groups of 10 rats (male or female) each. The procedure for the repeated dose toxicity study was adapted from OECD TG 407 23. Groups 1–3 (male or female category) were daily orally administered 1000, 500, and 250 mg/kg body weight of the extract, while the control was given distilled water. The volume of administered doses was not more than 1 ml/100 g body weight. Following the 28 days of repeated daily dosing for both male and female categories, the animals in each test group were randomly separated into two sub-groups of 5 rats each. A set of sub-groups was regarded as Toxicity set (TS), while the other was regarded as Recovery set (RS). The RS (male and female) were further allowed a 21 days of non-dosing recovery period. Detailed physical examinations were done daily for 28 days. Cage side observation of animals immediately after administration of the extract was carried out as in single dose toxicity tests. The body weight for each animal was taken daily prior to dosing. The rats in the TS were sacrificed on day 29, while the rats in the RS were sacrificed on day 49.
2.3.4. Samples collection
For both acute and repeated dose toxicity study, rats were sacrificed by cervical dislocation, and blood samples were collected into EDTA K3 tubes for haematological and biochemical analysis, while organs (brain, kidney and liver) samples were collected, weighed, and kidney and liver were preserved in 10%v/v formalin in normal saline for histopathological assessment.
2.4. Biochemical assays
Blood plasma for biochemical assays were obtained from the blood samples following centrifugation at 3000 rpm for 5 min. Aspartate transaminase (AST) and Alanine transaminase (ALT), creatinine level, bilirubin, and Urea were estimated using standard laboratory kits (Randox Laboratories Limited, Crumlin, County Antrim, BT294QY, United Kingdom), as per manufacturer's instructions.
2.5. Haematological assays
Haematological analysis of the blood samples was performed using an automated hematology analyzer (2800 Hematology Auto-Analyzer).24 Parameters which were evaluated included white blood cell (WBC), red blood cell (RBC), hemoglobin concentration (Hg), hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC).
2.6. Histopathological examination
The kidneys and liver specimens from each rat were immediately stored in 10%v/v formalin in normal saline after gross histological examination and dehydrated using increasing concentrations of isopropyl alcohol (80–100%). Paraffin sections at 5 μm thickness were made from the paraffin embedded organs using a Leica rotary microtome (Bright B5143 Huntington, England). This was followed by routine staining with hematoxylin and eosin which involved the process of deparaffinization, hydration, staining, rinsing and clearing in xylene.25,26 Slides were viewed under light microscope with photomicrographs taken with a Leica DM750 Camera Microscope (X 400). Histopathological lesions were scored using semi-quantitative approach as follows: 0 for normal, 1 (1%–30%) for mild, 2 (31%–70%) for moderate, and 3 (>70%) for severe.
2.7. Data presentation and statistical analysis
Data were expressed as mean ± standard error of mean (SEM), and significant differences were determined using Student's t-test and/or one–way analysis of variance (ANOVA) followed by Dunnett's post hoc test using Graph Pad Prism version 5.01 (Graph Pad software, San Diego, California, U.S.A). Differences were considered significant at p < 0.05.
3. Results
3.1. Cage-side observations with FOBs
The preliminary assessment during sighting study revealed that the extract did not show any critical effects that could lead to death. In fact, the LD50, which was greater than 5000 mg/kg, suggests that the extract could be practically non-toxic. As such, none of the FOB was selected as endpoint criterion for the purpose of monitoring adverse effects that could lead to death and the highest acute dose was therefore selected to be 5000 mg/kg. However, taken into consideration the potential cumulative effects of the extract following repeated administration, the doses used for repeated dose toxicity study were taken as one-fifth of the acute toxicity study. Expectedly, continuous monitoring during single and repeated dose administration confirmed that at the tested doses, none of the observed adverse effects led to death. Specifically, following single and repeated dose administration, the results (Table S1) show no overt sign of intoxication in both sexes, across the dose levels (5000, 2500 and 1250 mg/kg for acute, and 1000, 500 and 250 mg/kg for repeated doses) and within the first 24 h. Only normal behavioural changes (lethargy, repeated head flicking, mouth scratching and transient hypokinesia) which appeared within the first 4 h and gradually wore off, were observed. No mortality was recorded throughout the periods of the experiments, in both sexes and across all the dose levels.
3.2. Effects of the extract on relative body weights
Following acute dose administration, control and 1250 mg/kg showed significant increases in weights in most of the days when compared to day 0 (Table S2). However, with increase in dose, reduction in relative weights were observed, though, these changes were not significantly different from the control. Interestingly, following repeated doses, weekly assessment of the weights revealed significant increases in all tested doses when compared with day 0 (Table S3). However, the increases in weights were significantly lower than observed in controls, and lower in female compared to male rats. The recovery maintained the increases in weights, and showed that while it may take longer time to catch up with the control, the observed effects of the extract on weight may be reversible.
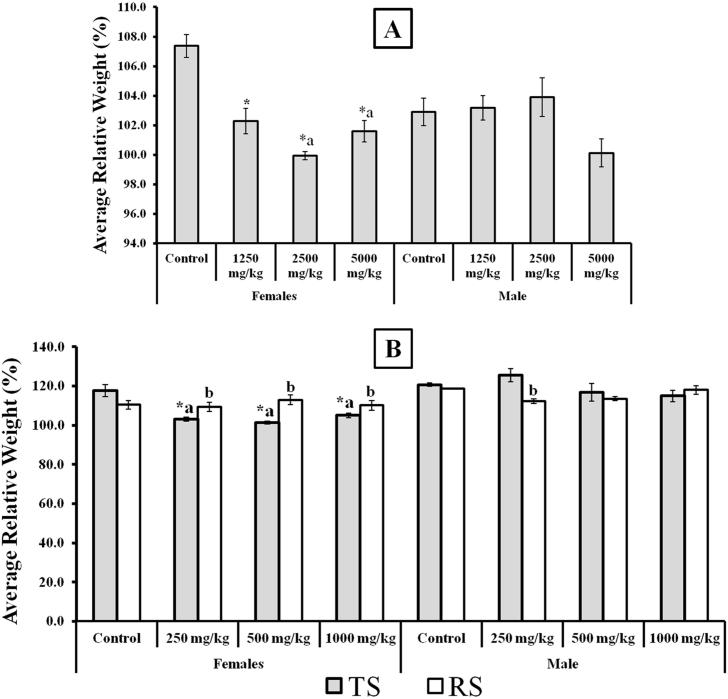
Furthermore, to better appreciate the effects of the extract on relative body weights, we analysed the average weight change over the experimental periods. The results is presented in Fig. 2. The results revealed a sex differential effects of the extract on relative body weights of male and female rats. For instance, in both acute and repeated dose studies, the results showed significant decreases in weights of female rats in all tested doses when compared to control. Also at higher doses of 2500 and 5000 mg/kg in acute, and in all doses of repeated oral administration, the female also showed significant weight reduction when compared to male counterpart. However, the recovery showed potential for regaining the lost weights.
Fig. 2.
Average relative change in body weights following single and repeated dose administration of the extract of Stachytarpheta cayennensis. Data were expressed as mean ± SEM, n = 5, p < 0.05. *Compare with respective controls (male or female). a Compare effect on female with male for each dose level. b Compare with respective Toxicity set. All statistical analysis were done using Student's T Test for pairwise comparison.
3.3. Effects of the extract on organ-body weights and organ-brain weights ratios
Apart from the result for the highest dose of 5000 mg/kg group for male liver-body weight ratio and female kidney-body weight ratio, as well as all tested doses for female liver-body weight ratio in repeated dose toxicity, no other significant changes in organ-body weight and organ-brain weight ratios were observed. (Table 1). While this may suggest lack of potential toxic effects on the organs, the significant changes in female liver-body weight ratio, further support the sex differential toxic effect of the extract seen with relative weight change. However, it should be noted that the observed higher increase in liver-body weight ratio at 1000 mg/kg repeated dose administration, was not significantly different when compared with lower doses.
Table 1.
Organ - body weights and organ-brain weight ratio following single and repeated doses of the extract.
| Treatment Group | Organ - Body weights ratio |
Organ - Brain weights ratio |
|||||
|---|---|---|---|---|---|---|---|
| Liver | Kidney | Brain | Liver | Kidney | |||
| Acute Toxicity | Control | Male | 3.20 ± 0.15 | 0.73 ± 0.06 | 1.37 ± 0.06 | 2.36 ± 0.12 | 0.53 ± 0.03 |
| Female | 3.78 ± 0.13a | 0.77 ± 0.04 | 1.25 ± 0.13 | 3.04 ± 0.12a | 0.62 ± 0.02a | ||
| 1250 mg/kg | Male | 3.56 ± 0.09 | 0.72 ± 0.03 | 1.30 ± 0.06 | 2.76 ± 0.14 | 0.56 ± 0.01 | |
| Female | 4.03 ± 0.09a | 0.82 ± 0.04 | 1.25 ± 0.07 | 3.29 ± 0.20 | 0.67 ± 0.02a | ||
| 2500 mg/kg | Male | 3.20 ± 0.08 | 0.62 ± 0.02 | 1.21 ± 0.04 | 2.65 ± 0.07 | 0.51 ± 0.02 | |
| Female | 3.88 ± 0.10a | 0.74 ± 0.04a | 1.28 ± 0.08 | 3.09 ± 0.23 | 0.58 ± 0.03 | ||
| 5000 mg/kg | Male | 3.60 ± 0.07* | 0.77 ± 0.03 | 1.37 ± 0.04 | 2.65 ± 0.11 | 0.57 ± 0.03 | |
| Female | 3.72 ± 0.17 | 0.63 ± 0.04*a | 1.20 ± 0.03a | 3.12 ± 0.19 | 0.60 ± 0.00 | ||
| Repeated Dose TS | Control | Male | 4.07 ± 0.30 | 0.65 ± 0.04 | 0.96 ± 0.03 | 4.33 ± 0.24 | 0.70 ± 0.06 |
| Female | 4.95 ± 0.15a | 0.85 ± 0.33 | 1.05 ± 0.02 | 4.35 ± 0.41 | 0.75 ± 0.02 | ||
| 250 mg/kg | Male | 4.17 ± 0.15 | 0.74 ± 0.04 | 0.96 ± 0.02 | 4.34 ± 0.18 | 1.77 ± 0.74 | |
| Female | 3.79 ± 0.25* | 0.77 ± 0.04 | 1.12 ± 0.06 | 3.48 ± 0.21a | 0.70 ± 0.03 | ||
| 500 mg/kg | Male | 3.71 ± 0.06 | 0.59 ± 0.07 | 0.88 ± 0.04 | 4.26 ± 0.24 | 0.79 ± 0.05 | |
| Female | 3.76 ± 0.23* | 0.72 ± 0.04 | 1.03 ± 0.05a | 3.71 ± 0.20 | 0.71 ± 0.04 | ||
| 1000 mg/kg | Male | 4.09 ± 0.18 | 0.80 ± 0.07 | 1.08 ± 0.04 | 3.80 ± 0.28 | 0.75 ± 0.06 | |
| Female | 4.21 ± 0.25* | 0.69 ± 0.04 | 0.98 ± 0.06 | 4.44 ± 0.32 | 0.72 ± 0.04 | ||
| Repeated Dose RS | Control | Male | 4.22 ± 0.13 | 0.72 ± 0.03 | 1.09 ± 0.07 | 3.93 ± 0.19 | 0.69 ± 0.08 |
| Female | 4.48 ± 0.30 | 0.74 ± 0.03 | 1.02 ± 0.03 | 4.38 ± 0.06 | 0.72 ± 0.02 | ||
| 250 mg/kg | Male | 4.40 ± 0.19 | 0.73 ± 0.04 | 0.95 ± 0.03 | 4.70 ± 0.33 | 0.78.±0.05 | |
| Female | 3.89 ± 0.14 | 0.69 ± 0.03 | 0.94 ± 0.05b | 4.16 ± 0.21b | 0.74 ± 0.03 | ||
| 500 mg/kg | Male | 4.26 ± 0.12b | 0.66 ± 0.03 | 0.95 ± 0.03 | 4.56 ± 0.23 | 0.70 ± 0.01 | |
| Female | 3.97 ± 0.22 | 0.71 ± 0.02 | 0.97 ± 0.07 | 4.16 ± 0.20 | 0.74 ± 0.04 | ||
| 1000 mg/kg | Male | 3.93 ± 0.12 | 0.63 ± 0.33 | 0.95 ± 0.03b | 4.14 ± 0.05 | 0.66 ± 0.03 | |
| Female | 4.16 ± 0.30 | 0.70 ± 0.04 | 0.92 ± 0.02* | 4.55 ± 0.36 | 0.76 ± 0.06 | ||
Data were expressed as mean ± SEM, n = 5, and p < 0.05, TS = Toxicity Set, RS = Recovery set.
*Compare with respective controls (male or female) was done using ANOVA followed by Dunnett's post hoc test; while.
Compare effect on female with male for each dose level and.
Compare with respective Toxicity set were done using Student's T-test. All statistics were done using Graph Pad Prism version 5.01 (Graph Pad software, San Diego, California, U.S.A).
3.4. Effects of the extract on hematological indices
The significant changes following single and repeated dose administration when compared with control, were mostly observed in platelets, HCT and MCV (Table 2a, Table 2bb). However, these changes were more pronounced in repeated doses, suggesting potential for cumulative toxic effects of the extracts. In contrast to what was observed with weight changes, the significant changes in hematological indices can be seen mostly in male rats, with significant difference in values when compared to females (Platelets, HCT, MCV),. Apart from the observed changes in platelets, HCT and MCV, in general, other hematological indices did not show any significant changes in all tested doses when compared to control, except for female WBC at 250 mg/kg TS, RBC at 1250 mg/kg and haemoglobin (Hg) at 2500 mg/kg of acute doses, as well as 500 mg/kg TS, and male MCHC at 1000 mg/kg RS. Though results also showed potential for recovery, the observed significant differences between TS and RS in platelets, HCT and MCV, may be an indication of delayed recovery from the toxic effects of the extract.
Table 2a.
Hematological parameters following single and 28 days repeated dose oral administration of the extract of S. Cayennensis.
| Treatment Groups | WBC (x109/L) |
Platelet (x109/L) |
RBC (x1012/L) |
Haematocrit (%) |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | Male | Female | ||
| Acute Toxicity | Control | 5.00 ± 0.39 | 5.04 ± 0.60 | 502.60 ± 50.37 | 686.40 ± 23.61a | 7.38 ± 0.31 | 8.30 ± 0.14a | 56.96 ± 1.41 | 57.30 ± 0.60 |
| 1250 mg/kg | 5.00 ± 0.59 | 4.50 ± 0.23 | 430.00 ± 38.17 | 644.80 ± 38.64a | 7.22 ± 0.19 | 7.17 ± 0.44* | 56.32 ± 1.73 | 48.40 ± 1.33*a | |
| 2500 mg/kg | 5.00 ± 0.88 | 6.40 ± 1.08 | 490.60 ± 42.00 | 641.20 ± 45.93a | 6.86 ± 0.70 | 7.70 ± 8.20 | 46.80 ± 2.72* | 54.50 ± 1.22a | |
| 5000 mg/kg | 4.60 ± 0.80 | 5.52 ± 0.36 | 672.40 ± 35.83* | 642.80 ± 44.56 | 7.86 ± 0.06 | 7.19 ± 0.84 | 48.10 ± 2.43* | 57.50 ± 0.64a | |
| Repeated Dose TS | Control | 4.90 ± 0.47 | 5.60 ± 0.71 | 408.80 ± 43.00 | 479.20 ± 44.73 | 6.90 ± 0.85 | 6.99 ± 0.12 | 40.50 ± 1.53 | 44.72 ± 1.92 |
| 250 mg/kg | 4.90 ± 0.69 | 3.56 ± 0.52* | 609.40 ± 48.92* | 652.40 ± 25.00* | 7.10 ± 0.46 | 7.60 ± 0.25 | 52.50 ± 1.11* | 51.12 ± 1.58* | |
| 500 mg/kg | 5.10 ± 0.53 | 3.68 ± 0.74 | 657.60 ± 47.73* | 677.20 ± 15.01* | 6.70 ± 0.80 | 7.45 ± 0.21 | 45.30 ± 2.47 | 53.68 ± 2.17*a | |
| 1000 mg/kg | 4.30 ± 0.78 | 6.40 ± 1.01 | 655.40 ± 41.99* | 706.40 ± 35.56* | 7.68 ± 0.41 | 7.11 ± 0.26 | 49.60 ± 2.01* | 48.80 ± 1.89 | |
| Repeated Dose RS | Control | 5.60 ± 3.73 | 5.20 ± 0.59 | 569.60 ± 41.77b | 711.20 ± 50.45b | 6.80 ± 0.84 | 6.45 ± 0.70 | 49.80 ± 1.25b | 44.60 ± 2.40 |
| 250 mg/kg | 3.60 ± 0.65 | 4.40 ± 0.57 | 323.20 ± 43.00*b | 611.80 ± 40.53a | 7.90 ± 0.12 | 5.93 ± 0.81a | 56.70 ± 1.19* | 44.50 ± 2.69a | |
| 500 mg/kg | 3.40 ± 0.77 | 5.10 ± 0.69 | 314.80 ± 49.93* | 587.80 ± 46.75a | 6.22 ± 0.86 | 6.45 ± 0.74 | 44.30 ± 1.11* | 46.00 ± 2.75 | |
| 1000 mg/kg | 3.82 ± 0.79 | 5.30 ± 0.68 | 269.00 ± 27.4*b | 613.60 ± 43.00a | 5.35 ± 0.43b | 5.60 ± 0.63 | 41.80 ± 1.13*b | 42.40 ± 1.99b | |
Data were expressed as mean ± SEM, n = 5, and p < 0.05, TS = Toxicity Set, RS = Recovery set.
*compare with respective controls (male or female) was done using ANOVA followed by Dunnett's post hoc test; while.
a compare effect on female with male for each dose level and b compare with respective Toxicity set were done using Student's T-test. All statistics were done using Graph Pad Prism version 5.01 (Graph Pad software, San Diego, California, U.S.A).
Table 2b.
Hematological parameters following single and 28 days repeated dose oral administration of the extract of S. Cayennensis.
| Treatment Groups | Haemoglobin (g/dl) |
MCV (fl) |
MCH (pg) |
MCHC (g/dl) |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | Male | Female | ||
| Acute Toxicity | Control | 13.70 ± 0.42 | 14.80 ± 0.25 | 77.18 ± 1.72 | 69.04 ± 0.74a | 18.56 ± 0.73 | 17.83 ± 0.39 | 24.05 ± 1.83 | 25.83 ± 0.85 |
| 1250 mg/kg | 13.50 ± 0.58 | 12.94 ± 0.87 | 78.01 ± 1.92 | 67.5 ± 1.77a | 18.7 ± 0.77 | 18.05 ± 1.31 | 23.97 ± 2.31 | 26.74 ± 2.2 | |
| 2500 mg/kg | 12.60 ± 1.02 | 13.70 ± 0.37* | 68.22 ± 3.42* | 70.78 ± 1.42 | 18.37 ± 1.72 | 17.79 ± 8.57 | 26.92 ± 3.74 | 25.14 ± 1.59 | |
| 5000 mg/kg | 13.70 ± 0.19 | 14.60 ± 0.21a | 61.20 ± 2.49* | 79.97 ± 1.48a | 17.43 ± 0.25 | 20.31 ± 1.05a | 28.48 ± 2.62 | 25.39 ± 0.85 | |
| Toxicity Set | Control | 13.10 ± 0.68 | 13.44 ± 0.20 | 58.70 ± 2.38 | 63.98 ± 2.04 | 18.99 ± 1.53 | 19.23 ± 0.32 | 32.35 ± 2.21 | 30.05 ± 2.12 |
| 250 mg/kg | 14.20 ± 0.08 | 19.70 ± 4.55 | 73.94 ± 1.57* | 67.26 ± 1.83a | 20.00 ± 0.54 | 25.92 ± 4.8 | 27.05 ± 1.19 | 38.54 ± 6.13 | |
| 500 mg/kg | 13.20 ± 0.47 | 14.48 ± 0.39* | 67.61 ± 3.27 | 72.05 ± 2.38* | 19.70 ± 1.27 | 19.44 ± 0.6 | 29.14 ± 2.94 | 26.97 ± 2.56 | |
| 1000 mg/kg | 14.30 ± 0.55 | 14.20 ± 0.46 | 64.58 ± 2.42 | 78.64 ± 2.15* a | 18.62 ± 0.96 | 19.97 ± 0.72 | 28.83 ± 2.56 | 29.1 ± 2.35 | |
| Recovery Set | Control | 11.48 ± 1.10 | 13.30 ± 0.45 | 73.24 ± 2.09b | 69.15 ± 3.1a | 16.88 ± 1.94 | 20.62 ± 1.15 | 38.52 ± 2.35 | 29.82 ± 2.85 |
| 250 mg/kg | 11.90 ± 1.69 | 13.80 ± 0.60 | 71.77 ± 1.31 | 75.04 ± 3.5 | 15.06 ± 1.81b | 23.27 ± 1.41a | 32.43 ± 2.88 | 31.01 ± 3.29 | |
| 500 mg/kg | 10.40 ± 1.49 | 13.30 ± 0.41 | 71.22 ± 1.97 | 71.32 ± 3.49 | 16.72 ± 2.35 | 20.62 ± 1.15 | 42.8 ± 2.6b | 28.91 ± 3.16a | |
| 1000 mg/kg | 10.88 ± 1.54 | 13.40 ± 0.29 | 78.13 ± 1.56b | 75.71 ± 2.62a,b | 20.34 ± 1.97 | 23.93 ± 0.92b | 49.91 ± 2.67*,b | 31.6 ± 2.28a | |
Data were expressed as mean ± SEM, n = 5, and p < 0.05, TS = Toxicity Set, RS = Recovery set.
*Compare with respective controls (male or female) was done using ANOVA followed by Dunnett's post hoc test; while.
Compare effect on female with male for each dose level and.
Compare with respective Toxicity set were done using Student's T-test. All statistics were done using Graph Pad Prism version 5.01 (Graph Pad software, San Diego, California, U.S.A).
3.5. Effects of the extract on biochemical indices
Following acute and repeated dose administration, the results revealed a consistent, yet mostly dose dependent significant changes in the values of biochemical indices, in both male and female rats (Table 3). It should be noted however, that AST was significantly higher than the control in all tested doses in acute and RS, and significantly lower in TS. Also, AST is generally higher than ALT, except at 1000 mg/kg for female TS. However, the use of AST/ALT ratio can provide a better view of the effects of the test agent.27, 28, 29, 30 Our results showed a consistently greater than 1 AST/ALT ratio in all tested doses of acute study, and dose dependent reduction in the ratio following repeated doses. Also, there are significant increases in creatinine and urea at higher doses, especially in male rats. In general, female rats appeared to show more sensitivity to the effects of the extract on biochemical indices than male rats. At recovery, though the results showed a reversal of observed effects with TS, the significantly higher AST and ALT when compared to control pointed to possible irreversibility of toxic effects in a manner similar to acute test. However, there were reduction in the values of urea and creatinine, signalling potential for recovery from accumulative toxic effects on the kidney.
Table 3.
Change in Plasma Biochemical indices following single and 28 days repeated dose administration of the extract of S. Cayennensis.
| Treatment Groups | AST (Ul) |
ALT (Ul) |
Urea (mmol/l) |
Creatinine (mg/dl) |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | Male | Female | ||
| Acute Toxicity | Control | 257.71 ± 3.82 | 233.58 ± 3.60 | 72.28 ± 4.19 | 86.27 ± 1.84 | 7.99 ± 0.46 | 8.77 ± 0.32 | 3.780 ± 0.17 | 3.98 ± 0.17 |
| 1250 mg/kg | 284.14 ± 0.79* | 393.33 ± 1.30*a | 75.98 ± 4.42 | 156.25 ± 4.38*a | 7.21 ± 0.74 | 8.60 ± 0.55 | 4.37 ± 0.09 | 5.84 ± 0.34*a | |
| 2500 mg/kg | 281.79 ± 3.75* | 304.70 ± 2.28*a | 55.36 ± 1.21* | 88.60 ± 3.20a | 7.80 ± 0.29 | 7.94 ± 0.70 | 5.97 ± 0.13* | 5.07 ± 0.21*a | |
| 5000 mg/kg | 334.60 ± 2.09* | 316.95 ± 2.62*a | 84.54 ± 1.76* | 95.91 ± 1.54*a | 9.25 ± 0.69* | 9.92 ± 0.31 | 6.83 ± 0.12* | 4.54 ± 0.53a | |
| Repeated Dose TS | Control | 231.19 ± 3.09 | 225.65 ± 3.24 | 79.47 ± 2.10 | 74.74 ± 2.86 | 7.72 ± 0.25 | 7.08 ± 0.38 | 3.31 ± 0.26 | 3.42 ± 0.29 |
| 250 mg/kg | 209.86 ± 2.52* | 168.91 ± 1.05*a | 67.13 ± 0.80* | 81.54 ± 3.30a | 8.24 ± 1.26 | 8.07 ± 0.24 | 7.34 ± 2.90* | 3.30 ± 0.36a | |
| 500 mg/kg | 176.42 ± 3.27* | 158.35 ± 0.49*a | 77.14 ± 0.76 | 113.91 ± 2.29*a | 9.79 ± 0.40* | 8.11 ± 0.21a | 5.57 ± 0.12* | 4.62 ± 0.25a | |
| 1000 mg/kg | 166.11 ± 2.58* | 129.23 ± 0.64*a | 90.48 ± 1.52* | 150.04 ± 2.85*a | 13.26 ± 1.89* | 8.75 ± 0.20*a | 5.66 ± 0.31* | 4.22 ± 0.17a | |
| Repeated Dose RS | Control | 245.75 ± 4.17 | 236.4 ± 3.48 | 75.96 ± 4.56 | 78.09 ± 3.58 | 7.92 ± 0.50 | 7.14 ± 0.40 | 3.08 ± 0.14 | 3.10 ± 0.20 |
| 250 mg/kg | 331.16 ± 7.47*b | 250.32 ± 4.45ab | 68.50 ± 1.32 | 101.19 ± 4.61*ab | 5.76 ± 0.30*b | 5.62 ± 0.25*b | 5.43 ± 0.14*b | 3.49 ± 0.44a | |
| 500 mg/kg | 307.09 ± 3.63*b | 280.98 ± 2.80*ab | 110.86 ± 2.40*b | 108.80 ± 2.70* | 6.42 ± 0.26b | 5.35 ± 0.10*ab | 3.94 ± 0.29b | 2.99 ± 0.38b | |
| 1000 mg/kg | 348.74 ± 2.85*b | 270.77 ± 2.17*ab | 115.9 ± 2.83*b | 123.23 ± 0.96*ab | 6.83 ± 0.24b | 5.13 ± 0.37*ab | 3.88 ± 0.27b | 3.14 ± 0.16b | |
Data were expressed as mean ± SEM, n = 5, and p < 0.05, TS = Toxicity Set, RS = Recovery set.
*compare with respective controls (male or female) was done using ANOVA followed by Dunnett's post hoc test; while.
a compare effect on female with male for each dose level and b compare with respective Toxicity set were done using Student's T-test. All statistics were done using Graph Pad Prism version 5.01 (Graph Pad software, San Diego, California, U.S.A).
3.6. Effects of the extract on organs histology
3.6.1. Effects of the extract on kidney
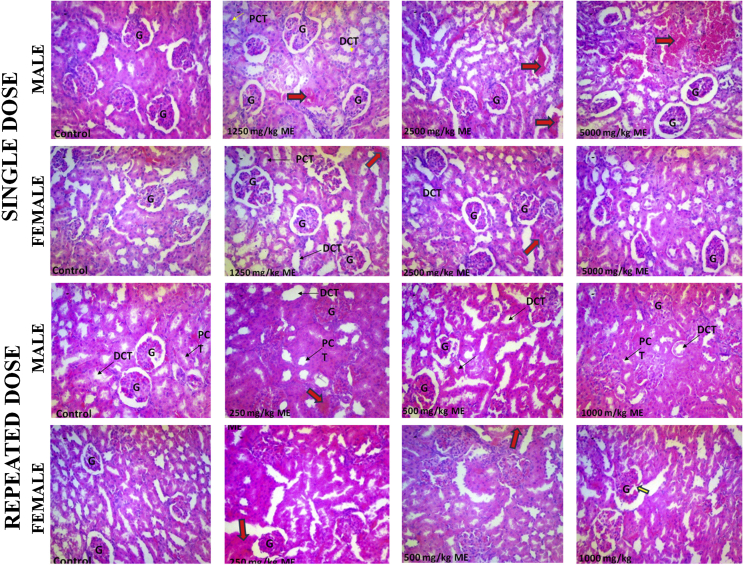
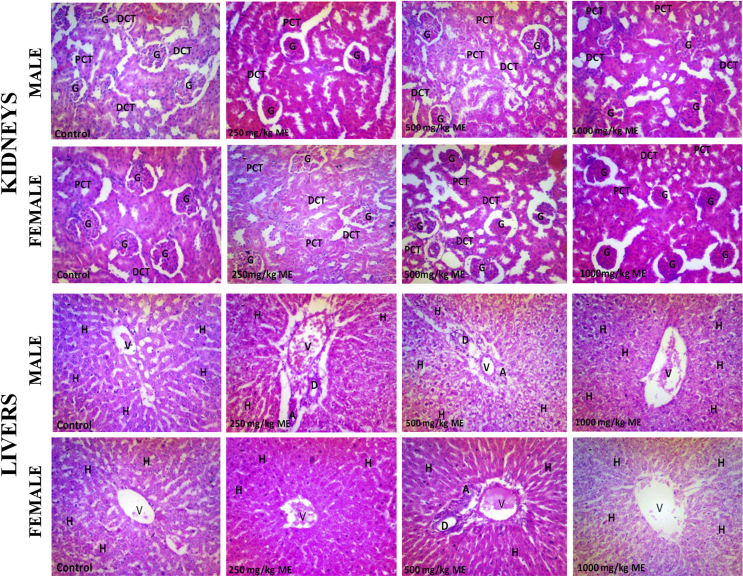
Assessment of the effect of the test substances on the cellular architecture of the kidney revealed that the control shows good histoarchitecture, clear distinct proximal and distal tubules; and distinct vascular and urinary poles, with few additional mitotic cells seen in female control (Fig. 3). Following single and 28 days of repeated administration of the extract of S. cayennensis, the renal histology of both male and female rats revealed pathological lesions which appears to increase in severity with increasing doses. Common to all dose levels in both single and repeated doses are tissue haemorrhage, abortive glomeruli, abundant intersititium, and prominent nucleoli (Fig. 3). For acute toxicity, other features include spindle cell stroma (2500 mg/kg, male), scanty mitotic body (2500 mg/kg, female) and mild glomerular atrophy (1250 mg/kg, female). For repeated doses, other features found in all tested doses in male and female rats are disrupted histoarchitecture, and atrophic glomerulus and fibrotic changes in the vascular path (Fig. 3). Differences from one test group to the other are mostly negligible and females seem to be less affected than the males.
Fig. 3.
Histopathology of male and female rat kidney following single and repeated dose administration of the extract of Stachytarpheta cayennensis. DCT, distal convoluted tubule; PCT, proximal convoluted tubule; G, glomerulus. Red arrows were used to identify pathological changes including presence of renal lesions/tissue haemorrhages. Staining was done using H&E and magnification was ×400.
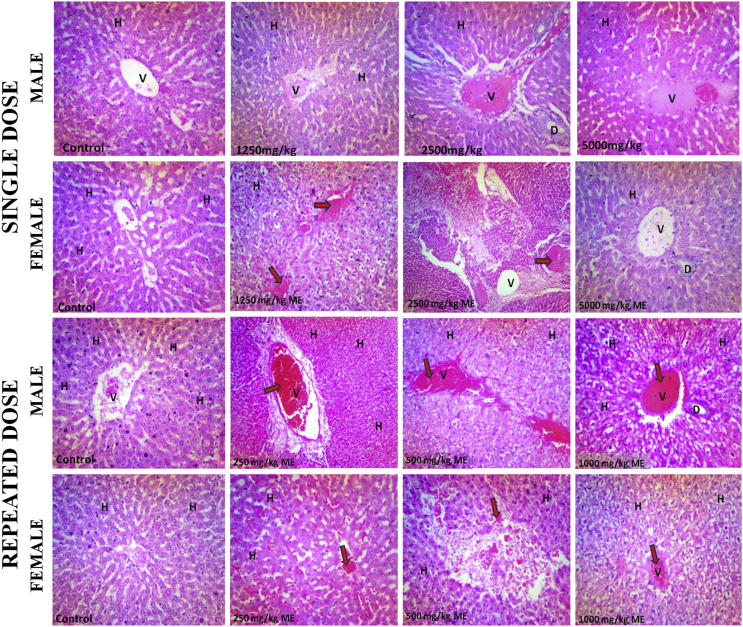
The recovery group maintained most of the histopathological features seen in toxicity set, including evidence of mild to severe tissue haemorrhage, and abortive glomeruli (Fig. 5). In addition, male rats showed tortuous tubular cells which may be due to inflammation or oedema and vacuolated interstitium (1000 mg/kg), distinct poles (500 mg/kg), and distorted tubules and wide distance between glomerulus and bowman capsule (250 mg/kg). However, in female rats recovery group, most glomeruli appeared normal and interstitium was adequate (1000 mg/kg), with adequate but vacuolated interstitium and tubular system (500 mg/kg) and tortuous tubules and indistinct bowman capsule (250 mg/kg) (Fig. 5).
Fig. 5.
Histopathology of male and female rat Kidneys and livers following 21 days of recovery after repeated dose administration of methanol extract of Stachytarpheta cayennensis. DCT, distal convoluted tubule; PCT, proximal convoluted tubule; G, glomerulus; H, hepatocyte; V, central vein; D, bile duct. Staining was done using H&E and magnification was ×400.
3.6.2. Effects of the extract on liver
The histopathological assessment of the liver shows mitotic bodies, clear sinusoids, cords of hepatocytes, well outlined central vein and portal triad; and conspicuous nucleoli in the controls (male and female) (Fig. 4). However, following single and 28 days repeated doses of the extract of S. cayennensis, the liver histology of male and female rats revealed pathological lesions characterized with mostly distorted histoarchitecture. Histological features common to all groups of rats include mild to severe haemorrhage, prominent nucleoli, few mitotic bodies, pyknotic nuclei, and vascular congestion (Fig. 4). In addition, repeated dose toxicity set revealed enlarged central vein due to vascular congestion, with prominent nucleoli (1000 mg/kg, male), clear signs of tissue necrosis, and loss or severe disruption of hepatocytes arrangement (500 mg/kg, male and 1000 mg/kg, female) (Fig. 4). However, the severity of the pathology did not appear to be dose dependent, differences from one test group to other are mostly negligible and females seem to be more tolerant than the males.
Fig. 4.
Histopathology of male and female rat livers following single and repeated dose administration of methanol extract of Stachytarpheta cayennensis. H, hepatocyte; V, central vein; D, bile duct. Red arrows were used to identify pathological changes including presence of hepatic lesions. Staining was done using H&E and magnification was ×400.
The recovery group maintains some of the features of the toxicity group, including tissue haemorrhage (Fig. 5). In addition, also seen in the male recovery group are few pigmented nuclei, portal triad showing mild fibrotic extensions, disrupted cordlike arrangement of hepatocytes and scanty nucleolar prominence (1000 mg/kg, male). Also seen are abundant pigments in the nucleoli, and minimal nucleolar prominence (500 mg/kg, male), and mild fibrotic extensions at the triad (250 mg/kg, male) (Fig. 5). However, the female rats recovery set appear to show improved features when compared to male counterpart. These include abundant pigmented nuclei, well outlined cords of hepatocytes and normal central veins and portal triad (1000 mg/kg, female), normal sinusoid with well outlined cords of hepatocytes and pigmented cell (500 mg/kg, female). Having signs of vascular congestion, diffused cellular necrosis and portal triads appears normal (250 mg/kg, female) (Fig. 5).
4. Discussion
Plants are sources of many potent and efficacious drugs1,10 and herbal medicine constitute a larger proportion of the health care needs of developing countries.4,5 However, despite the inherent benefits of medicinal plants, and the perceived safety/non-toxic nature, available evidence have shown their involvement in the aetiology of various forms of toxicity,31,32 making it imperative to investigate their potential toxicity.33 By determining the effects of the extract of S. cayennensis on body and organ weights, hematological and biochemical indices, and organs histology, following single and repeated doses, we seek to establish its safety and provide recommendations on the safe use of this plant for medicinal purposes.
In this study, the LD50 of the extract, which was found to be greater than 5000 mg/kg, p.o. in both male and female rats, provided an initial evidence of the potential safety of the extract.34 Also, lack of mortality and observable adverse effects from FOB in the treated rats throughout the observation period of 14 days (single dose), 28 days (TS) and 49 days (RS), coupled with general lack of significant changes in organ-body and organ-brain weights ratio, further supports the idea of potential safety of the extract. However, it should be noted that a change in body weight is an uncomplicated and sensitive index to study the detrimental effects of drugs and chemicals.35 In general, toxic nature of the drug could lead to abnormalities in body weight36 and a decrease in body weight could indicate a substantial degree of toxicity, while a reduced body weight gain represents only a mild form of intoxication.37,38 Furthermore, organ weight, organ/body weight and organ/brain weight ratios are a more sensitive indicators of drug toxicity, making any subtle alteration of significant importance for further investigation.35, 36, 37, 38 Essentially, organ-brain weight ratio provides a more realistic assessment of the toxic effects of a test agent where variation in body weight is inevitable, as substances that alter body weight do not generally alter brain weight.35, 36, 37 Therefore, the significant reduction in relative body weight in female rats (Fig. 2), coupled with significant alteration in female liver-body weight ratio following repeated doses (Table 1), suggest that the female rats may be more sensitive to both the acute and cumulative effects of the extract than male rats. While this may also be an indication of a sex specific potential toxic effects of the extract on female rats as compared to male rats,36,37 the lack of significant differences among the tested doses in organ-brain weight ratio suggest that the observed changes may not be of toxicological significance.
Evaluation of hematological parameters is an important and sensitive index, considered to be vital in toxicity studies during extrapolations of experimental data to clinical study.39 Available evidence have shown that the consumption of toxic plants or agent can cause alterations in the hematological profile40,41 and drugs associated with toxic effect could cause organ dysfunction and significant alteration in hematological biomarkers.33 In this study, platelets and two other RBC indices (HCT and MCV) were for the most part, significantly altered, especially in male rats. Platelets are produced by the bone marrow through the stimulation of myeloid stem cells by thrombopoietin.39 Therefore, the observed dose dependent decrease (male rats) or increase (female rats) in platelets (Table 2a, Table 2b), resulting from high or cumulative toxic effects of the extracts, may suggest that the extracts have inhibitory or stimulatory effect on thrombopoietin.39,42 Circulating platelets could be increased (thrombocytosis) as a result of toxic agent mediated inflammation and/or abnormal bleeding, or platelets can be reduced (thrombocytopenia) by trapping in the spleen, reduced platelet production or increased destruction.39,42, 43, 44 Apart from thrombocytosis, increased level of free Hb and WBC may also be associated with inflammation arising from assault on vital organs. In fact, increased level of WBC (specifically leukocytes), has been shown to correlate well with C-reactive protein (CRP), an important marker of inflammation.39,53 In this study, however, general lack of significant changes in WBC correlated well with the reported anti-inflammatory property of S. Cayennensis,15 indicating that inflammation may not have played any significant role in the observed toxic effect of the extract. The significant reduction at 250 mg/kg in female rats, may further explain the sex differential effects of the extract. This differential sensitivity to the effect of the extract was further confirmed by lack of significant changes in WBC among the doses of the extract in male rats as opposed to significantly higher WBC at 1000 mg/kg in female when compared to 250 and 500 mg/kg.
Furthermore, hematocrit (HCT) is an indication of the percentage of the red cells in the total blood, and provides an indication of the oxygen carrying capacity or efficiency of the RBC. The observed decrease (male rats) or increase (female rats) in HCT could be an indication of clinical condition associated with abnormally low (anaemia) or high HCT (polycythemia) respectively.39,43,44 The variation in effects of the extracts on HCT in male and female rats, vis-a-vis a decrease in HCT with increasing doses in male rats, and increase in HCT with increase in doses in female rats, is an added evidence of potential sex differential effects of the extract. While the observed significant decrease in HCT and MCV at higher doses of 2500 and 5000 mg/kg suggests iron deficiency anaemia, lack of significant changes in RBC, Hb, MCH and MCHC when compared with control, and thus, lack of positive correlation with Hb suggested otherwise. However, a significant reduction in HCT can be caused by an insufficient production of healthy RBC with normal size and shape, an increased number of WBC, deficiencies in vitamin or mineral and overhydration.39,43,44 In this study, while the RBC count and Hb concentration appear normal with no significant changes when compared with control, the correlation between the HCT and MCV gave an indication that at higher doses, the extract may have caused an insufficient production of healthy RBC with normal size and shape. Specifically, it appears that the RBC that were produced has a higher proportion of erythrocytes with smaller sizes (as indicated by MCV), leading to decrease in HCT, and suggesting iron deficiency hypochromic microcytic anemia.39,43,44 Polycythemia could result from hyperosmotic conditions arising from high dosage of toxic agents.39,43 On the other hand, MCV measures the average volume or size of RBC,39,43 and a low MCV (microcytic) is consistent with anaemia and thalassaemia syndromes, and an elevation (macrocytic) could be a reference to deficiencies in vitamin B12 and folate.39,43,44 Furthermore, the dose related further reduction in platelets and HCT during recovery, though mostly significant in male rats, may be a pointer to the inability of rats to overcome the toxic effects of the extract within the allotted time frame or an indication of delayed recovery or persistent toxic effects of the extract.
Liver and kidney play key roles in metabolic processes and assessment of the health status of various organ including liver and kidney among others requires multiple blood biomarkers.45,46 The vital functions of the liver and kidneys make them subject of frequent attacks by toxic compounds. Though their sensitivity and specificity are limited, AST, ALT, urea and creatinine evaluation still constitutes reliable indices of liver and kidney health.45, 46, 47 For instance, in a toxic environment, blood levels of AST and ALT are known to significantly increase,29,46 potentially resulting from the destruction of liver cells.46 However, due to distinctive abundance of ALT in cytoplasm of liver cells, ALT has been commonly used as a more specific marker to quantify suspected liver cell damage.46, 47, 48 Also, while urea, a marker of acute renal dysfunction, is the first acute marker following renal injury, creatinine, a marker of chronic renal dysfunction, is the most dependable renal marker and increases only when the significant renal function is lost.49,50 Therefore, the consistently greater than 1 AST/ALT ratio in all tested doses of acute study, and the dose dependent reduction in the ratio following repeated doses, suggest a different pattern of mechanism of toxic effects. Also, significant increases in creatinine and urea at higher doses, especially in male rats provided supports for the potential extract induced acute and chronic kidney dysfunction.45,51 At recovery, the significantly higher AST and ALT, as well as consistently higher than 1 AST/ALT ratio pointed to possible irreversibility of accumulated toxic effects in a manner similar to acute test. On the other hand, the reduction in the values of urea and creatinine, signalled potential for recovery from accumulative toxic effects on the kidney. However, the effects of the extract on the enzymes must be correlated with histological findings.52
Histopathological findings following single and repeated doses of the extract (Fig. 3, Fig. 4, Fig. 5) provided clear evidence of organ toxicity, similar to those associated with known hepatotoxins and nephrotoxins.52,54, 55, 56 It also provided the much needed correlation with observed changes in biochemical indices (Table 3). However, differences in liver and kidney histology do not seem to follow a specific pattern for both single and 28 days of repeated doses. The severity appears to be dose dependent but appears to be less in females than male, suggesting sex differential organ toxicity. Sex differential is now a critical factor in toxicological assessment due largely to observed inter- and intra-species variability in responses to different toxic agents.57, 58, 59 Contrary to the biochemical evidence presented in this study (Table 3), that shows that female rats may be more sensitive to the effects of the extract of S. cayennensis than male rats, the reverse was the case going by the histopathological evidence (Fig. 3, Fig. 4, Fig. 5). Several factors could be responsible for the observed variability; including the rate of absorption and elimination of the toxic agent, interaction of the test agent with lipids, the nature of the test agent and its metabolites, the interaction of the test agent with organ specific target enzymes, and variability in organ specific gene expression.57,58,60 In fact, several reports have documented several-folds increased susceptibility of male animals to certain liver and kidney toxicants than females.61, 62, 63 With higher level of cytochromes P450 gene expression in male rats and the reported reduced susceptibility of female rats to substances metabolized by P450 enzymes,58,60,64 it is possible that metabolism and the potential effects of the extract on the metabolic enzymes may have played critical role in the observed histopathology in this study. For instance, it is possible that the extract may have ability to suppress or inhibit metabolic enzymes activities, as evidenced by its reported inhibitory activities,8,12 thereby increasing the duration of exposure to the toxic agent. On the other hand, the extract may induce P450 enzymes activity, which may lead to an increase in toxic metabolites. In both instances, male rats will be on the receiving end. Meanwhile, the RS suggest incomplete recovery or persistence of deleterious effect of the extract on the organs.
5. Conclusion
In conclusion, the extract of S. cayennensis may be assumed to be non-toxic judging from the lack of serious alteration in functional and behavioural observations (FOB), lack of mortality following the adminstration of the stated doses in acute and repeated dose toxicity studies, and greater than 5000 mg/kg LD50. However, the observed significant, and at times, persistent toxic effects on some hematological and biochemical indices, as well as histopathological findings, showed the potential of the extract to effect toxic action on the body at higher doses or when given repeatedly. Also, our findings revealed sex differential toxic effects. Therefore, our study has provided evidence of altered haematological and biochemical indices, as well as histological architecture of kidneys and liver with evidence of non-reversible forms of toxicity. There is greater need to take caution and avoid abuse and indiscriminate uses of the leaves of S. cayennensis. This is more important going by the potential for cumulative toxic effects from continuous usage.
Taxonomy (classification by EVISE)
Blood Analysis, Histopathology, Behavioural Toxicity, Acute Kidney Injury, Chronic Kidney Disease, Acute-on-Chronic Liver Failure.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of interest
None.
Conflicts of interest
The Authors declare no conflict of interest.
Footnote
None.
Acknowledgments
We acknowledge the assistance we received on histology from Dr. S. O. A. Odukoya of the Department of Anatomy and Cell Biology, Faculty of Basic Medical Science, Obafemi Awolowo, Ile – Ife, Nigeria. We also thank Mr. I. I. Ogunlowo, a botanist in the Herbarium unit of the Department of Pharmacognosy, Faculty of Pharmacy, Obafemi Awolowo University (OAU) Ile-Ife, Nigeria, for his assistance towards the identification and collection of the plant material.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jtcme.2019.05.001.
Contributor Information
Oladotun A. Olayode, Email: oladotunolayode@yahoo.com.
Michael Oluwatoyin Daniyan, Email: mdaniyan@oauife.edu.ng, toyinpharm@gmail.com.
Gbola Olayiwola, Email: gbolayiw@oauife.edu.ng.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Gurib-Fakim A. Medicinal plants: traditions of yesterday and drugs of tomorrow. Mol Asp Med. 2006;27(1):1–93. doi: 10.1016/j.mam.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad I.A., Farrukh F., Owais M. Herbal medicines: prospects and constraints. In: Ahmad I.,F., Aqil, Owais M., editors. Modern Phytomedicine: Turning Medicinal Plants into Drugs. -VCH Verlag GmbH & Co.; Germany: 2006. pp. 59–78. [DOI] [Google Scholar]
- 3.Bandaranayake W.M. Quality control, screening, toxicity, and regulation of herbal drugs, In: Ahmad I.A.F., Owais M., editors. Modern Phytomedicine: Turning Medicinal Plants into Drugs. VCH Verlag GmbH & Co.; Germany: 2006. pp. 25–57. [Google Scholar]
- 4.Hosseinzadeh S., Jafarikukhdan A., Hosseini A., Armand R. The application of medicinal plants in traditional and modern medicine: a review of. Thymus vulgaris Int J Clin Med. 2015;06(09):635–642. doi: 10.4236/ijcm.2015.69084. [DOI] [Google Scholar]
- 5.Mahomoodally M.F. Traditional medicines in Africa: an appraisal of ten potent african medicinal plants. Evid Based Complement Altern Med ECAM. 2013;2013:617459. doi: 10.1155/2013/617459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agostinho A.B. vol. 2. Univ Forum; 2011. Integration of Traditional Medicine in Health Systems in Africa.http://www.universitasforum.org/index.php/ojs/article/view/68 Accessed 2. [Google Scholar]
- 7.Krah E., de Kruijf J., Ragno L. Integrating traditional healers into the health care system: challenges and opportunities in rural northern Ghana. J Community Health. 2018;43(1):157–163. doi: 10.1007/s10900-017-0398-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olayiwola G., Ukponmwan O., Olawode D. Sedative and anxiolytic effects of the extracts of the leaves of Stachytarpheta cayennensis in mice. A J Trad Compl and Alter Med. 2013;10:568–579. doi: 10.4314/ajtcam.v10i6.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akobundu I., Agyakwa W.C. second ed. International Institute of Tropical Agriculture; Ibadan: 1998. Handbook of West Africa Weeds. [Google Scholar]
- 10.Iwu M.M. second ed. CRC Press; 2014. Handbook of African Medicinal Plants.https://www.crcpress.com/Handbook-of-African-Medicinal-Plants-Second-Edition/Iwu/p/book/9781466571976 [Google Scholar]
- 11.Okokon J.E., Ettebong E., Antia B.S. In vivo antimalarial activity of ethanolic leaf extract of Stachytarpheta cayennensis. Indian J Pharmacol. 2008;40(3):111. doi: 10.4103/0253-7613.42303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okoronkwo N.E., Echeme J.O., Onwuchekwa E.C. Cholinesterase and bacterial inhibitory activities of Stachytarpheta cayennensis. Acad Res Int. 2012;2(3):209. [Google Scholar]
- 13.Okoye T.C., Akah P.A., Ezike A.C. Immunomodulatory effects of Stachytarpheta cayennensis leaf extract and its synergistic effect with artesunate. BMC Complement Altern Med. 2014;14(1):1–8. doi: 10.1186/1472-6882-14-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Onofre S.B., Kagimura F.Y., Mattiello S.P. Antifungal activity of the aqueous extract of Stachytarpheta cayennensis,(Rich.) Vahl.(Verbenaceae), on oral candida species. J Med Plants Res. 2015;9(2):42–47. doi: 10.5897/JMPR2014.5667. [DOI] [Google Scholar]
- 15.Penido C., Costa K.A., Futuro D.O. Anti-inflammatory and anti-ulcerogenic properties of Stachytarpheta cayennensis ( L . C . Rich ) Vahl. J Ethnopharmacol. 2006;104:225–233. doi: 10.1016/j.jep.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 16.National Research Council (US) eighth ed. National Academies Press (US); Washington (DC): 2011. Guide for the Care and Use of Laboratory Animals.http://www.ncbi.nlm.nih.gov/books/NBK54050/ [Google Scholar]
- 17.OECD . 2008. Test Guideline 425: Acute Oral Toxicity – up and Down Procedure. Paris. [Google Scholar]
- 18.Gauvin D.V., Yoder J.D., Holdsworth D.L. The standardized functional observational battery: its intrinsic value remains in the instrument of measure: the rat. J Pharmacol Toxicol Methods. 2016;82:90–108. doi: 10.1016/j.vascn.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 19.Mathiasen J.R., Moser V.C. The irwin test and functional observational battery (FOB) for assessing the effects of compounds on behavior, physiology, and safety Pharmacology in rodents. Curr Protoc Pharmacol. 2018:e43. doi: 10.1002/cpph.43. September. [DOI] [PubMed] [Google Scholar]
- 20.Moser V. The functional observational battery in adult and developing rats. Neurotoxicology. 2000;Dec 21(6):989–996. [PubMed] [Google Scholar]
- 21.OECD. 2008. Guidelines for the Testing of Chemicals: Repeated Dose 28-Day Oral Toxicity Study in Rodents. [DOI] [Google Scholar]
- 22.OECD. 2001. Oral Toxicity - Acute Toxic Class Method, Test Guideline No. 423. Paris. [Google Scholar]
- 23.OECD . OECD; Paris, France.: 2008. Guideline for the Testing of Chemicals 407 on Repeated Dose 28-Day Oral Toxicity Study in Rodents. [Google Scholar]
- 24.Ode S.A., Adamu M., Saror D.I. Determination of haematocrit using Mindray BC-2800Vet® automated haematology analyser and microhaematocrit method: a comparative study. Sokoto J Vet Sci. 2017;15(2):62. doi: 10.4314/sokjvs.v15i2.9. [DOI] [Google Scholar]
- 25.Feldman A.T., Wolfe D. Histopathology. Methods in Molecular Biology. Humana Press; New York, NY: 2014. Tissue processing and hematoxylin and eosin staining; pp. 31–43. [DOI] [PubMed] [Google Scholar]
- 26.Slaoui M., Fiette L. Histopathology procedures: from tissue sampling to histopathological evaluation. In: Jean-Charles Gautier., editor. vol. 691. Springer Science+Business Media, LLC; 2011. pp. 69–82. (Drug Safety Evaluation: Methods and Protocols). Methods in molecular biology (Clifton, N.J.) [DOI] [PubMed] [Google Scholar]
- 27.Bartoš V., Dastych M., M.D. In: Clinical Biochemistry. prof MUDr Jaroslav Racek D., MUDr Daniel Rajdl P., editors. Charles University Karolinum Press; 2016. [Google Scholar]
- 28.Basten G. Introduction to Clinical Biochemistry. Interpreting Blood Results; 2011. Liver function tests (LFTs) and enzymes; pp. 40–45. [Google Scholar]
- 29.Nosrati A., Alizadeh A., Hashemi-soteh M.B. Hepatotoxicity and ALT/AST enzymes activities change in therapeutic and toxic doses consumption of acetaminophen in rats. Int Biol Biomed J. 2017;3(3):119–124. [Google Scholar]
- 30.Sharma O.P. Clinical biochemistry of hepatotoxicity. J Clin Toxicol. 2011;1(3) doi: 10.4172/2161-0495.S4-001. [DOI] [Google Scholar]
- 31.Knöss W. Toxicity of herbal medicines: from past to present to future. In: Pelkonen O., Duez P., Vuorela P.M., Vuorela H., editors. Toxicology of Herbal Products. Springer International Publishing; Cham: 2017. pp. 1–9. [DOI] [Google Scholar]
- 32.Obidike I., Salawu O. vol. 4. INTECH; 2013. pp. 63–88. (Screening of Herbal Medicines for Potential Toxicities. New Insights into Toxicity and Drug Testing). [Google Scholar]
- 33.Arome D., Chinedu E. The importance of toxicity testing. J Pharm Biosci. 2014;4(2013):146–148. [Google Scholar]
- 34.Hodge H.C., Sterner J.H. Tabulation of toxicity classes. Am Ind Hyg Assoc Q. 1949;10(4):93–96. doi: 10.1080/00968204909344159. [DOI] [PubMed] [Google Scholar]
- 35.Bailey S.A., Zidell R.H., Perry R.W. Relationships between organ weight and body/brain weight in the rat: what is the best analytical endpoint? Toxicol Pathol. 2004;32(4):448–466. doi: 10.1080/01926230490465874. [DOI] [PubMed] [Google Scholar]
- 36.Nirogi R., Goyal V.K., Jana S., Pandey S.K. Gothi A. What suits best for organ weight Analysis: review of relationship between organ weight and body/brain weight for rodent toxicity studies. Int J Pharm Sci Res. 2014;5(4):1525–1532. doi: 10.13040/IJPSR.0975-8232.5(4).1525-32. doi: [DOI] [Google Scholar]
- 37.Michael B., Yano B., Sellers R.S. Evaluation of organ weights for rodent and non-rodent toxicity studies: a review of regulatory guidelines and a survey of current practices. Toxicol Pathol. 2007;35(5):742–750. doi: 10.1080/01926230701595292. [DOI] [PubMed] [Google Scholar]
- 38.Piao Y., Liu Y., Xie X. Change trends of organ weight background data in sprague dawley rats at different ages. J Toxicol Pathol. 2013:29–34. doi: 10.1293/tox.26.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arika W., Nyamai D., Musila M., Ngugi M., Njagi E. Hematological markers of in vivo toxicity. J Hematol Thromboembolic Dis. 2016;4(2):4–10. doi: 10.4172/2329-8790.1000236. [DOI] [Google Scholar]
- 40.Sani D., Sanni S., Sandabe U.K., Ngulde S.I. Effect of intake of aqueous stem extract of Anisopus mannii on haematological parameters in rats. Int J Appl Res Nat Prod. 2009;2(3):22–28. [Google Scholar]
- 41.Zahmati M., Saljooghi A.S. The evaluation of deferasirox on hematological parameters after lead administration. Asian Pac J Med Toxicol. 2016;5:124–129. [Google Scholar]
- 42.Li J., Xia Y., Kuter D. Interaction of thrombopoietin with the platelet complements receptor in plasma: binding, internalization, stability and pharmacokinetics. Br J Haematol. 1999;106:345–356. doi: 10.1046/j.1365-2141.1999.01571.x. [DOI] [PubMed] [Google Scholar]
- 43.Hall J.E. thirteenth ed. vol. 33. Saunders, United States of America; 2016. Red blood cells, anemia and polycythemia; pp. 445–454. (Guyton and Hall Textbook of Medical Physiology). [Google Scholar]
- 44.Leach M. Interpretation of the full blood count in systemic disease – a guide for the physician. J R Coll Phys Edinb. 2014;44:36–41. doi: 10.4997/JRCPE.2014.109. [DOI] [PubMed] [Google Scholar]
- 45.Fassett R.G., Venuthurupalli S.K., Gobe G.C., Coombes J.S., Cooper M.A., Hoy W.E. Biomarkers in chronic kidney disease : a review. Kidney Int. 2011;80(8):806–821. doi: 10.1038/ki.2011.198. [DOI] [PubMed] [Google Scholar]
- 46.Yang X., Schnackenberg L.K., Shi Q., Salminen W.F. Biomakers in Toxicoogy. 2014. Hepatic toxicity biomarkers; pp. 241–259. [DOI] [Google Scholar]
- 47.Campion S., Aubrecht J., Boekelheide K. The current status of biomarkers for predicting toxicity. Expert Opin Drug Metabol Toxicol. 2013;9(11):1–28. doi: 10.1517/17425255.2013.827170. The. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ferreira S.A., Guimarães A.G., Ferrari F.C., Carneiro C.M., Paiva N.C.N.D. Assessment of acute toxicity of the ethanolic extract of Lychnophora pinaster (Brazilian arnica) Rev Bras Farmacogn. 2014;24(5):553–560. [Google Scholar]
- 49.Arneson W., Brickell J. Assessment of renal function. In: (Arneson W., Brickell J., editors. Clinical Chemistry: A Laboratory Perspective. first ed. F.A. Davis Company.; Philadelphia: 2007. [Google Scholar]
- 50.Ferguson M.A. Biomarkers of nephrotoxic acute kidney injury. Toxicology. 2008;245(3):182–193. doi: 10.1016/j.tox.2007.12.024.Biomarkers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vaidya V.S., Ferguson M.A., Bonventre J.V. Biomarkers of acute kidney injury. Annu Rev Pharmacol Toxicol. 2008;48:463–493. doi: 10.1146/annurev.pharmtox.48.113006.094615. Biomarkers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Iweala E.E.J., Obichi I.C., Omotosho O.E. Biochemical and histological responses of hepatotoxic rats fed Musa paradisiaca L. supplemented diet. Int J Pharmacol. 2011;4:471–477. [Google Scholar]
- 53.Hemelrijck M.V., Holmberg L., Garmo H. Association between levels of C-reactive protein and leukocytes and cancer: three repeated measurements in the Swedish AMORIS study. Cancer Epidemiol Prev Biomark. 2011;20(3):428–437. doi: 10.1158/1055-9965.EPI-10-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Husain H., Latief U., Ahmad R. Pomegranate action in curbing the incidence of liver injury triggered by Diethylnitrosamine by declining oxidative stress via Nrf2 and NFκB regulation. Sci Rep. 2018;8(1):8606. doi: 10.1038/s41598-018-26611-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Latief U., Husain H., Ahmad R. β-Carotene supplementation ameliorates experimental liver fibrogenesis via restoring antioxidant status and hepatic stellate cells activity. J Funct Foods. 2018;49:168–180. doi: 10.1016/j.jff.2018.08.027. [DOI] [Google Scholar]
- 56.Mukherjee D., Ahmad R. Resveratrol attenuates Nitrosodiethylamine-induced liver injury in anti-inflammatory manner via regulating cyclooxygenase-2. J Food Biochem. 2018;42(5) doi: 10.1111/jfbc.12594. [DOI] [Google Scholar]
- 57.Calabrese E.J. Editorial: sex differences in susceptibility to toxic industrial chemicals. Br J Ind Med. 1986;43(9):577–579. doi: 10.1136/oem.43.9.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gochfeld M. Sex differences in human and animal toxicology: toxicokinetics. Toxicol Pathol. 2017;45(1):172–189. doi: 10.1177/0192623316677327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee S.K. Sex as an important biological variable in biomedical research. BMB Rep. 2018;51(4):167–173. doi: 10.5483/BMBRep.2018.51.4.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Itoh Y., Arnold A.P. Are females more variable than males in gene expression? Meta-analysis of microarray datasets. Biol Sex Differ. 2015;6:18. doi: 10.1186/s13293-015-0036-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pohl L.R., George J.W., Satoh H. Strain and sex differences in chloroform-induced nephrotoxicity. Different rates of metabolism of chloroform to phosgene by the mouse kidney. Drug Metab Dispos. 1984;12(3):304–308. [PubMed] [Google Scholar]
- 62.Ali B.H., Ismail T.H.B., Bashir A.A. Sex difference in the susceptibility of rats to gentamicin nephrotoxicity: influence of gonadectomy and hormonal replacement therapy. Indian J Pharmacol. 2001;33(5):369–373. [Google Scholar]
- 63.Pohjanvirta R., Miettinen H., Sankari S., Hegde N., Lindén J. Unexpected gender difference in sensitivity to the acute toxicity of dioxin in mice. Toxicol Appl Pharmacol. 2012;262(2):167–176. doi: 10.1016/j.taap.2012.04.032. [DOI] [PubMed] [Google Scholar]
- 64.Trevisan A., Chiara F., Mongillo M., Quintieri L., Cristofori P. Sex-related differences in renal toxicodynamics in rodents. Expert Opin Drug Metabol Toxicol. 2012;8(9):1173–1188. doi: 10.1517/17425255.2012.698262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.