Abstract
The rhizome of Curculigo pilosa (CP) prepared with Corn steep liquor (CSL), is traditionally used in the treatment of diabetes mellitus. In this study, antidiabetic activity of the CSL extract and its fractions (butanol and methanol) were evaluated in streptozotocin-induced diabetic rats. Diabetes mellitus was induced by single intraperitoneal administration of streptozotocin (50 mg/kg) and diabetic rats were treated with 300 mg/kg bodyweight of the extract(s) for 28 days. Antidiabetic effect was monitored by plasma blood glucose, oral glucose tolerances test (OGGT), body weight and heamatological indices. Also serum urea, creatinine, cholesterol, high density lipoprotein, bilirubin, alkaline phosphatase, aspartate and alanine transaminases were evaluated. The levels of hepatic glutathione, catalase, superoxide dismutase, lipid peroxidation, nitric oxide and hydrogen peroxide were assessed; also histopathology of the hepatic tissues was examined. Oral administration of the extract resulted in significant (p < 0.05) reduction of plasma blood glucose (29.32% crude extract and 22.96% butanol fraction) and also increased body weight (20.61% crude extract, 13.44% butanol fraction and 6.23% methanol fraction) of diabetic rats. The heamotogical indices, plasma parameters and hepatic oxidative stress in diabetic rats were returned to near normalcy following treatment with the extract(s). The GC-MS analysis of the extract revealed the presence of stilbene, a proven antidiabetic agent, which might be responsible for the antidiabetic activity. The results obtained suggest that the CSL extract of CP could be used in management of diabetes mellitus thus providing scientific validation of its use as an antidiabetic agent.
Keywords: Corn steep liquor, Curculigo pilosa, antidiabetic, Streptozotocin
Graphical abstract
1. Introduction
Diabetes mellitus (DM) is a metabolic disease of carbohydrates and fat due to deficiency of insulin secretion or varying degree of insulin resistance.1 It is a major public health problem and has become a menace globally. According to International diabetic federation (IDF), 425 million people are currently suffering from diabetes mellitus and the number is estimated to rise to 642 million by the year 2040 and it was also estimated to cause the mortality of a person every 6 s in year 2015.2 The increase in blood glucose level of diabetic patients usually leads to excessive generation of free radicals and aberration in lipid metabolism which have been associated with virtually all complications of diabetes mellitus. Diabetic complication has reported to develop in patients with diabetes and has become one of leading causes of mortality worldwide.3 Presently, nearly half a billion people live with diabetes. Low and middle income countries carry almost 80% of the diabetes burden because they do not have adequate resources to provide preventive or medical care for their populations therefore, rely on traditional medicines.4
Traditional medical practices have been in existence since times immemorial and have been used across all tribes as a source of treatment. About 90% of the population in rural areas of developing countries relies solely on traditional medicines for their primary health care.5 In diabetes, herbal alternatives have proven to provide symptomatic relief and assist in the prevention of the secondary complications of the disease.6 Some herbs have also been proven to help in the regeneration of β-cells and overcoming insulin resistance, while some herbs are also reported to possess antioxidant activity and cholesterol-lowering action in addition to their anti-hyperglycemic effect.6
Curculigo pilosa Schum and Thonn (CP) belongs to the family of Hypoxidaceae. CP rhizome of the Curculigo genus was the first to be described of African species.7 It is known as ‘Epakun’ in Yoruba tribe of south-west Nigeria and it is commonly used as a purgative as well as for the management and treatment of hernia, infertility, diabetes, genital infections and sexually transmitted infections.8 It is also used in the manufacture of infant food and sorghum beer.9 Curculigo pilosa rhizome has been reported to be rich in essential mineral and dietary fibre.9 Apart from this, it contains high polyphenols and possesses scavenging abilities.10,11 Its antimicrobial activities have also been reported.12,13 The phytochemical screening of its extract reported the presence of phenol, phlobatannin, coumarin, saponin, steroid and terpenoid.12,14
Corn steep liquor (CSL) is a product of wet corn milling, usually a filtrate in the production of palp (a cereal-based traditional lactic acid fermented food). It is an important solvent in traditional medicine of south-west Nigeria, where it is used to form decoction and infusion for treatment of diabetes.15
Despite considerable progress in the treatment of diabetes by oral hypoglycemic agents most of the synthetic drugs used in the management of diabetes are expensive, toxic and non available especially to low and middle income earner which constitute the larger percentage of the populace. Also, with high rate of diabetes mellitus throughout the world, there is need for development of indigenous, inexpensive botanical sources for management of diabetes mellitus. Previously the acute toxicity of Corn Steep Liquor extract of Curculigo pilosa has been studied and the LD50 was found to be as high as 2828 mg/kg and therefore, it is considered to be relatively safe.14 Therefore, this present study is designed to evaluate the antidiabetic effect of Corn Steep Liquor extract of Curculigo pilosa and its solvent fractions in streptozotocin induced diabetic rats. This is in view to scientifically substantiate the indigenous use of this plant as antidiabetic herb.
2. Materials and methods
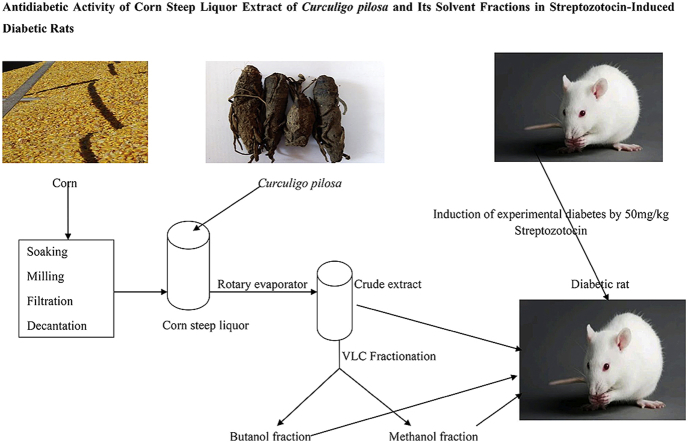
Plant Materials: The dried corn and Curculigo pilosa rhizomes used in the work were gotten from local market (Bodija), Ibadan. They were identified and authenticated in the Department of Botany, University of Ibadan. The sample vouchers (UIH-22759 and UIH-22773) were deposited in their herbarium. They were without infection. The dried corn was used for preparation of corn steep liquor. The Curculigo pilosa was washed with distilled water, sliced and shade dried in the laboratory. The shade dried samples were grinded into powdery form.
2.1. Preparation of corn steep liquor (CSL)
This was prepared by steeping corn using the method.14 Well-washed healthy, dried corn grains (1000 g) were soaked in 4 L hot water (100 °C) for 72 h. It was milled using a well-washed blender and filtered using muslin cloth. The filtrate was then allowed to settle for 24 h. The resultant supernatant (Corn steep liquor) was decanted and used for the extraction.
2.2. Extraction of sample
One thousand grams (1000 g) of the powered Curculigo pilosa was extracted with 10 L of corn steep liquor, After 72 h, the extract was filtered using muslin cloth and the filtrate was concentrated using rotary evaporator. The extracts were kept in refrigerator until use.
2.3. Fractionation of sample
One hundred gram (100 g) Crude Corn steep liquor extract was adsorbed with silica gel (ratio 1:2); the adsorbed mixture was loaded on Buchner funnel already packed with silica gel. The funnel was eluted with butanol and methanol successively under pressure. The eluted samples were concentrated using rotary evaporator to give butanol and methanol fractions respectively.
2.4. Experimental animals
Healthy Wistar albino rats weighing 80–100 g were obtained from the animal house of the Department of Physiology, College of Medicine, University of Ibadan. They were fed with standard pellet diet (Ladokun feeds) and water was given ad libitum. They were kept under a constant 12-hr light and dark cycle. The animals were acclimatized for 2 weeks before the induction of experimental diabetes. The experimental protocols were conducted in accordance with ethical guidelines as approved by Institutional animal care use and research ethics committee for care and use of laboratory animals (UI-ACUREC/App/03/2017/006).
2.5. Induction of diabetes mellitus
Experimental Diabetes was induced in overnight fasted experimental rats by a single intraperitoneal injection of streptozotocin (50 mg/kg body weight) dissolved in 0.1 M freshly prepared cold citrate buffer pH 4.5.16 After 72 h for development of diabetes, blood glucose was measured and rats with fasting blood glucose greater than 15.2 mmol/l were considered diabetic and used in the present study.
2.6. Experimental design
After the successful induction of experimental diabetes, the rats were divided into six groups each containing a minimum of 6 rats.
Control: Control rats treated with distilled water
Dcontrol: Diabetic control rats treated with distilled water
CRD: Diabetic rats treated with CSL extract (300 mg/kg)
BUTH: Diabetic rats treated with Butanol fraction of CSL extract (300 mg/kg)
METH: Diabetic rats treated with Methanol fraction of CSL extract (300 mg/kg)
GLBC: Diabetic rats treated with Glibenclamide (1 mg/kg).
Body weight and plasma glucose level measurements were conducted weekly during the experiment. Plasma glucose level was measured using one-touch glucometer. The dosage of the extracts was adjusted every week to accommodate changes in body weight to maintain the same dosage throughout the experiment. They were administered orally for 28 days using intragastric tube. After 28 days, the rats were fasted overnight and euthanized under anesthesia (Sodium pentobarbitone). The blood was collected with or without anticoagulant sample bottles for plasma and serum respectively.
2.7. Oral glucose tolerance test (OGTT)
On the 28th day, the rats were fasted overnight and 1 g/kg glucose was given orally to all the groups of rats. Blood samples were collected from the tail vein of all the animals at 0, 30, 60, 90, and 120 min after the glucose administration.17 The blood glucose levels were determined using one-touch glucometer.
2.8. Haematological indices determination
After four weeks of treatment with the extracts, blood samples collected with anticoagulant were used for the determination of the haematological indices using 3-part differential haematology auto analyzer, Surgifield, England following manufacturer's instruction. These include packed cell volume (PCV), heamoglobin (hg), red blood cell (RBC) and white blood cell (WBC).
2.9. Biochemical assays
Blood collected into sample bottles without anticoagulant were centrifuged at 3000 g for 15 min to obtained serum. Serum urea was assayed by the method of,18 serum creatinine by the method of,19 serum cholesterol by the method of,20 serum high density lipoprotein by the method of,20 serum total bilirubin by the method of,21 serum aspartate transaminase (AST) by the method of,22 serum alanine transaminase (ALT) by the method of,22 and serum alkaline phosphatase (ALP) by the method of.23 All determinations were carried out using diagnostic kits (Randox diagnostic kit, UK). Atherogenic index (AI) and Cardiac risk ratio (CRR) were calculated following the method of24 and,25 respectively.
2.10. Determination of hepatic hyperglycemia-induced oxidative damage
Hepatic tissues from control and experimental groups of rats were excised, rinsed with ice-cold saline and homogenized in 0.1 M phosphate buffer (pH 7.4) using Teflon homogenizer and centrifuged at 10,000×g for 10 min at 4 °C. The supernatant obtained was used for the biochemical estimations. The protein content in the liver homogenate was estimated by the method of.26 Lipid peroxidation was quantified by the method of.27 Nitric oxide (NO) level was assessed by the method of.28 Reduced glutathione was determined using the method of.29 Hydrogen peroxide (H2O2) generation was determined according to the method of.30 Catalase (CAT) activity was determined by the method of Clairborne.31 Superoxide dismutase (SOD) activity was determined by the method of.32
2.11. Histological observations hepatic tissues
Tissue histology was done by the method of.33 Hepatic tissues were fixed in 10% formalin for 24 h. The tissue was processed using automatic tissue processor. The tissues were blocked in paraffin wax and the blocks were trimmed to expose the tissue surface using a rotary microtome. The surfaces were allowed to cool on ice before sectioning. The tissues were sectioned at 4 μm. The sections were floated on water bath at 55 °C and dried at 60 °C for 1 h. Sections were stained with hematoxylin-eosin (for structural derangement). Histological changes in the stained sections were viewed and analyzed under the optical microscope by a pathologist without prior knowledge of the groups.
2.12. Gas chromatography - mass spectrum analysis (GC-MS)
An aliquot of 1 μl of sample solution of the sample was injected into the column with the injector temperature at 350 °C. GC oven temperature started at 60 °C and holding for 2 min until it was raised to 260 °C at the rate of 5 °C/min, without holding. Holding was allowed at 280 °C for 9 min with program rate of 5 °C/min. The injector and detector temperatures were set at 250 °C and 280 °C respectively. Ion source temperature was maintained at 200 °C. The mass spectrum of Compounds in samples was obtained by electron ionization at 70 eV and the detector was operated in scan mode from 45 to 450 amu (atomic mass units). A scan interval of 0.5 s and fragments from 45 to 450 Da was maintained. The total running time was 54 min. Interpretation on mass spectrum GC-MS was conducted using the database of National Institute Standard and Technology (NIST) having more than 62,000 patterns. The spectrum of the unknown component was compared with the spectrum of the known components stored in the NIST library. The name, molecular weight and structure of the components of the test materials were ascertained.
2.13. Statistical analysis
Data are expressed as the mean ± SD of at least three measurements. The significance of the differences between the means of the samples were established by the analysis of variance (ANOVA) P < 0.05 using SPSS statistics 20 and charts were drawn with graph pad prism 5.
3. Results
The effect of Corn steep liquor extract of Curculigo pilosa and its solvent fractions on blood glucose level was shown in Table 1. There was significant (P < 0.05) decrease in the level of blood glucose of CSL extract and its butanol fraction treated group when compared with diabetic untreated group but was significantly (P < 0.05) elevated when compared with undiabetic group. The effect on body weight is showed in Table 2. A significant decrease in the body weight of the diabetic rats is observed but upon administration of the extracts and glibenclamide there was significant (P < 0.05) restoration of the body weight to near normalcy. Also Table 3 showed the effect of the extracts and glibenclamide on the some heamatological (PCV, Hb, RBC and WBC) indices in the diabetic rats. A significant (P < 0.05) decrease was found in the diabetic rats which were returned to near normalcy with the treatment.
Table 1.
Effect of CSL Extract of Curculigo pilosa and its solvent fractions on blood glucose (mmol/l) in streptozotocin-induced diabetic rats.
| Initial | 7th day | 14th day | 21st day | 28th day | |
|---|---|---|---|---|---|
| Control | 5.00 ± 0.82 | 5.50 ± 0.47 | 5.47 ± 0.38 | 5.57 ± 0.82 | 5.43 ± 0.75 |
| Dcontrol | 19.20 ± 1.92a | 20.40 ± 1.88a | 25.72 ± 2.63a | 28.72 ± 2.80a | 30.08 ± 3.52a |
| CRD | 21.25 ± 1.70a | 18.46 ± 3.20a | 16.72 ± 3.41ab | 15.58 ± 2.28ab | 15.02 ± 3.04ab |
| BUTH | 21.36 ± 1.99a | 17.46 ± 2.90ab | 17.06 ± 3.23ab | 16.60 ± 3.08ab | 16.40 ± 2.64ab |
| METH | 21.06 ± 1.41a | 22.72 ± 1.52a | 23.05 ± 2.21a | 24.18 ± 3.68ab | 24.05 ± 2.06ab |
| GLBC | 23.60 ± 1.03a | 20.20 ± 0.72a | 18.32 ± 1.98ab | 16.60 ± 1.32ab | 14.10 ± 1.90ab |
Data are expressed as mean ± SEM, n = 6, Initial means 72hr of streptozotocin injection.
a p < 0.05 when experimental groups were compared with control group, b p < 0.05 when experimental groups were compared with diabetic control group.
Table 2.
Effect of CSL Extract of Curculigo pilosa and its solvent fractions on body weight (g) in streptozotocin-induced diabetic rats.
| Initial | 7th day | 14th day | 21st day | 28th day | |
|---|---|---|---|---|---|
| Control | 123.7 ± 2.33 | 142.5 ± 4.40 | 171.5 ± 3.15 | 178.5 ± 3.70 | 190.0 ± 3.80 |
| Dcontrol | 126.3 ± 2.89 | 100.5 ± 3.50a | 105.8 ± 3.22a | 99.7 ± 2.50a | 94.5 ± 2.90a |
| CRD | 121.3 ± 1.91 | 118.8 ± 2.67a | 131.3 ± 3.10ab | 140.0 ± 3.70ab | 152.8 ± 3.30ab |
| BUTH | 125.5 ± 2.80 | 113.5 ± 3.12a | 133.5 ± 1.91ab | 141.8 ± 4.11ab | 145.0 ± 3.12ab |
| METH | 123.3 ± 2.11 | 110.5 ± 3.04a | 122.8 ± 3.11ab | 128.5 ± 2.55ab | 131.5 ± 3.11ab |
| GLBC | 125.0 ± 2.30 | 117.5 ± 2.85a | 125.8 ± 2.71ab | 139.0 ± 2.81ab | 143.1 ± 3.70ab |
Data are expressed as mean ± SEM, n = 6, Initial means 72hr of streptozotocin injection.
a p < 0.05 when experimental groups were compared with control group, b p < 0.05 when experimental groups were compared with diabetic control group.
Table 3.
Effect of CSL Extract of Curculigo pilosa and its solvent fractions on heamatological indices in streptozotocin-induced diabetic rats.
| PCV (%) | Hb(mg/dl) | RBC(10ˆ6) | WBC(10ˆ3) | |
|---|---|---|---|---|
| Control | 41.37 ± 1.34 | 157.33 ± 5.23 | 7.53 ± 0.14 | 8.75 ± 0.90 |
| Dcontrol | 36.19 ± 0.44a | 137.00 ± 4.50a | 6.14 ± 0.19a | 11.50 ± 0.70a |
| CRD | 40.47 ± 0.82b | 152.67 ± 3.67b | 6.83 ± 0.05b | 10.80 ± 0.57a |
| BUTH | 37.74 ± 0.31a | 147.67 ± 5.17ab | 6.22 ± 0.22ab | 11.20 ± 0.55a |
| METH | 36.78 ± 1.74a | 151.33 ± 4.50ab | 6.35 ± 0.11a | 9.83 ± 0.82b |
| GLBC | 39.17 ± 1.69b | 154.33 ± 5.17b | 6.91 ± 0.42b | 9.67 ± 1.21b |
Data are expressed as mean ± SEM, n = 6.
a p < 0.05 when experimental groups were compared with control group, b p < 0.05 when experimental groups were compared with diabetic control group.
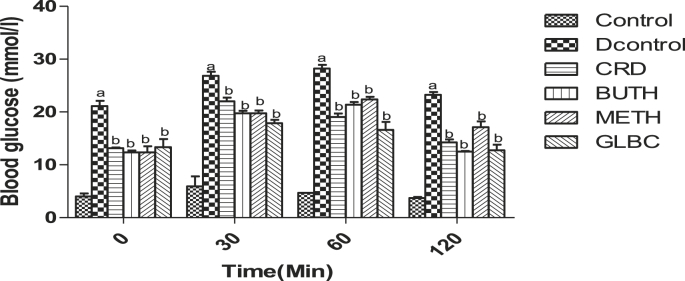
The effect of CSL extract of Curculigo pilosa and its solvent fractions on the streptozotocin induced diabetic rats after an oral glucose challenge was showed in Fig. 1. The blood glucose level in the control and diabetic rats treated with the crude extract and its butanol fraction were elevated to a maximum value at 30 min after glucose load and declined to near basal levels at 120 min, whereas, in streptozotocin-induced diabetic rats, the increase in blood glucose level was still high after 120 min.
Fig. 1.
Effect of CSL extract of Curculigo pilosa and its solvent fractions on OGTT (mmol/l) in streptozotocin-induced diabetic rats. a p < 0.05 when experimental groups were compared with control group, b p < 0.05 when experimental groups were compared with diabetic control group.
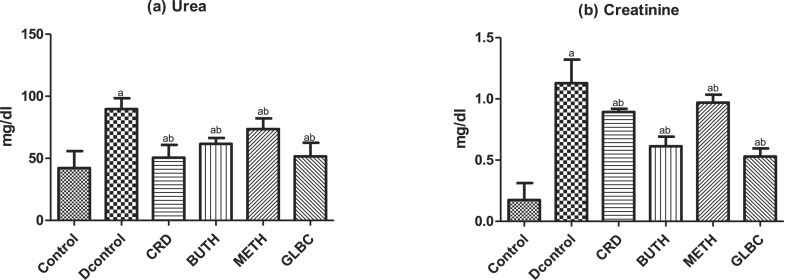
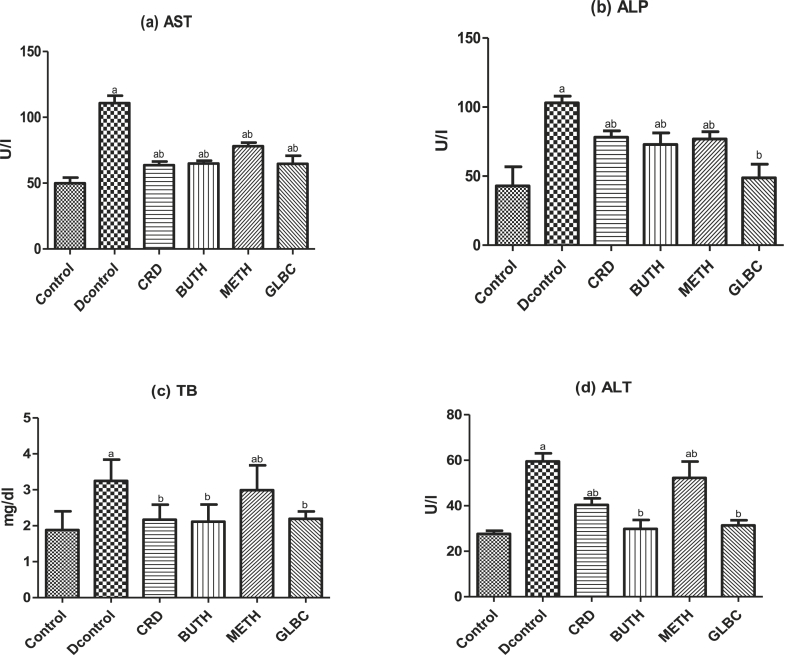
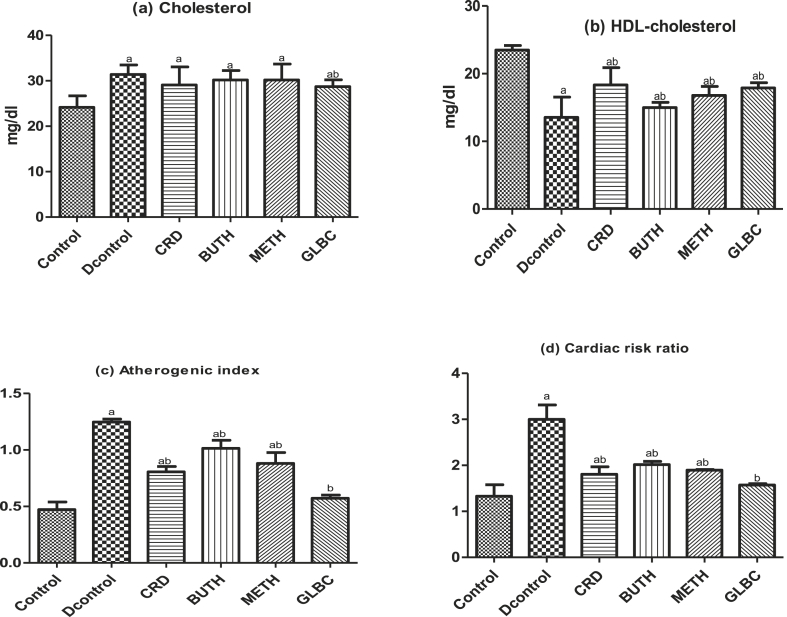
The effect of CSL extract of Curculigo pilosa on kidney function tests (Urea and Creatinine), liver function test (TB, ALT, AST, ALP) and lipid profiles (Cholesterol, HDL-cholesterol, atherogenic index and cardiac risk ratio) in streptozotocin-induced rats were showed in Fig. 2, Fig. 3, Fig. 4 respectively. Induction of experimental diabetes by streptozotocin led to significant (P < 0.05) alteration in the level of these biochemical parameters. However, oral administration of CSL extract of Curculigo pilosa for 28 days was able to significantly bring the levels to near normalcy.
Fig. 2.
Effect of CSL extract of Curculigo pilosa and its solvent fractions on kidney damage biomarkers (a) Urea (b) Creatinine in streptozotocin-induced diabetic rats. a p < 0.05 when experimental groups were compared with control group, b p < 0.05 when experimental groups were compared with diabetic control group.
Fig. 3.
Effect of CSL extracts of Curculigo pilosa and its solvent fractions on liver damage biomarkers (a) AST (b) ALP (c) TB (d) ALT in streptozotocin-induced diabetic rats. a p < 0.05 when experimental groups were compared with control group, b p < 0.05 when experimental groups were compared with diabetic control group.
Fig. 4.
Effect of CSL extracts of Curculigo pilosa and its solvent fractions on lipid profiles (a) Cholesterol (b) HDL-Cholesterol (c) Atherogenic index (d) Cardiac risk ratio in streptozotocin-induced diabetic rats. a p < 0.05 when experimental groups were compared with control group, b p < 0.05 when experimental groups were compared with diabetic control group.
The effect of CSL extract of Curculigo pilosa and its solvent fractions on hyperglycemia mediated oxidative stress in hepatic tissues of streptozotocin induced diabetic rats was showed in Fig. 5a–f. Significantly (P < 0.05) higher level of markers of oxidative stress (LPO, NO and H2O2) were found in diabetic rats when compared to the control rats. Also, GSH and activities of enzymic antioxidants (CAT and SOD) were significantly (P < 0.05) lowered in the diabetic rats when compared with the control rats. However, treatment with CSL extract of Curculigo pilosa or its butanol fraction was able to significantly (P < 0.05) lower the elevated oxidative stress makers (LPO, NO and H2O2) and raised the level of decreased GSH, CAT and SOD in the hepatic tissues of the diabetic rats.
Fig. 5.
Effect of CSL extracts of Curculigo pilosa and its solvent fractions on hepatic (a) GSH (b) LPO (c) NO (d) H2O2 (e) Catalase (f) SOD in streptozotocin-induced diabetic rats. a p < 0.05 when experimental groups were compared with control group, b p < 0.05 when experimental groups were compared with diabetic control group.
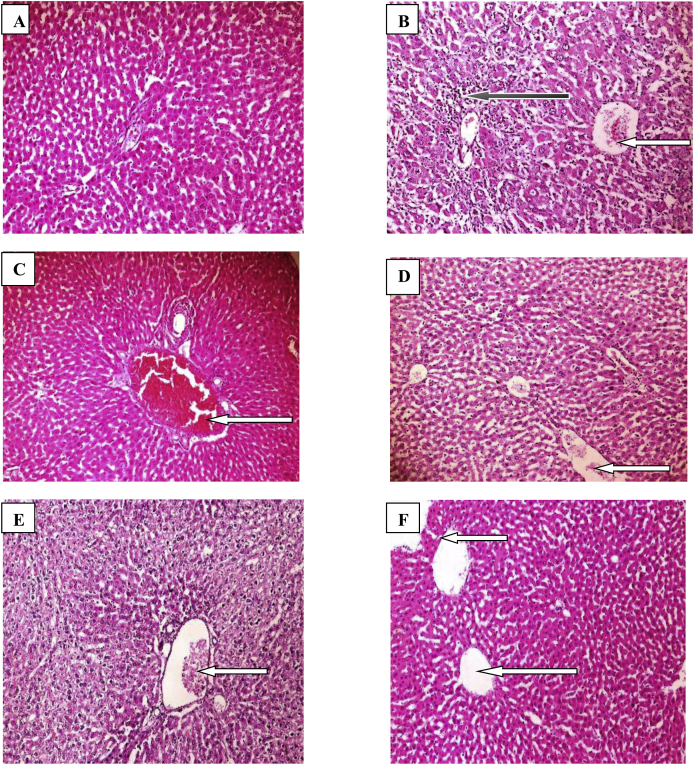
Photomicrographs of hepatic sections stained with hematoxylin and eosin was showed in Fig. 6a–f. Control rats showed normal central venules without congestion, the morphology of the hepatocytes appear normal, no pathological lesion seen. Untreated diabetic rats (6 b) showed very poor morphology, the liver parenchyma is moderately fibrotic, there is severe to chronic infiltration of inflammatory cells involving the sinusoids, central venules are normal without congestion but few perivascular infiltration and few apoptotic hepatocytes seen. Diabetic rats treated with CRD (6c) showed normal central venules without congestion (white arrow), however, there is mild perivascular infiltration seen, the morphology of the hepatocytes show cytoplasmic infiltration by fat and vacuolation, the sinusoids appear normal and not infiltrated. Diabetic rats treated with BUTH (6 d) showed mildly congested central venules, the morphology of the hepatocytes show cytoplasmic vacuolation and foamy cytoplasms, the sinusoids appear mildly infiltrated. Diabetic rats treated with METH (6e) showed normal central venules without congestion, some of the hepatocytes appear normal while others exhibit vesicular nuclei and foamy cytoplasms, the sinusoids appear mildly infiltrated by inflammatory cells. Diabetic rats treated with GLBC (6f) showed normal central venules without congestion, the morphology of the hepatocytes appear normal, the sinusoids appear normal and not infiltrated, no pathological lesion seen.
Fig. 6.
Photomicrographs ( × 100) of hepatic tissue of control and experimental groups of rat (stained with H&E). A: Control, B: Dcontrol, C: CRD, D: BUTH, E: METH, F: GLBC.
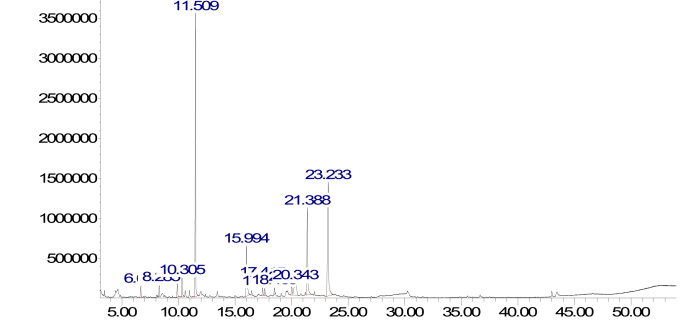
The GC-MS analysis of crude extract of curculigo pilosa revealed fourteen peaks (Fig. 7). The phytochemicals present are showed in Table 4. The prominent phytochemicals include thymine, stilbene, 4-Methoxybenzene-1,2-diol and salicyl alcohol.
Fig. 7.
Chromatogram of Corn steep liquor extract of Curculigo pilosa by GC-MS.
Table 4.
Compounds found in the CSL extract of Curculigo pilosa using GC-MS.
| RT | Compound | Nature of Compound | Peak area | Biological activity |
|---|---|---|---|---|
| 6.669 | Thymine | Pyrimidine | 1.35 | Antimicrobial |
| 8.282 | 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- | Flavonoid fraction | 1.19 | Antioxidant |
| 9.902 | Stilbene | Polyphenol | 2.67 | Antidiabetic, anticancer, antioxidants |
| 10.305 | Thiazole | Aromatic | 2.81 | Antibacterial, antifungal, antioxidant hypertension |
| 11.509 | Salicyl alcohol | Phenol | 32.78 | Antioxidant, |
| 15.990 | 4-Methoxybenzene-1,2-diol | Phenol | 11.20 | Anticancer, antioxidant |
| 17.415 | 3,4-Dimethoxythiophenol | Aromatic | 1.96 | # |
| 17.615 | Benzene, 1-methyl-3-[(4-methylphenyl)methyl]- | Aromatic | 1.08 | # |
| 18.468 | 4,5-Dihydro-3-furoic acid | Organic acid | 1.25 | # |
| 19.520 | Bicyclo [2.2.1]heptan-2-ol | Alcohol | 1.13 | Antiasthmatics |
| 20.001 | 2,5-Dihydroxypropiophenone | 1.48 | Antipsychotics | |
| 21.386 | Benzoic acid | Aromatic | 14.67 | Preservatives, antioxidant, antimicrobial |
| 25.31 | 3,7-Benzofurandiol | Aromatic | 25.31 | # |
| 43.455 | 2-Propyl-1,3-benzodioxane | Aromatic | 1.14 | # |
Source: Dr.Duke's phytochemical and ethnobotanical databases
4. Discussion
Streptozotocin (STZ, 2-deoxy-2-(3-(methyl-3-nitrosoureido)-d-glucopyranose) is usually used to induced experimental diabetes in animal models. STZ is an alkylating agent which causes the breakage of DNA that induces the activation of poly-ADP-ribose synthetase leading to nicotinamide adenine dinucleotide (NAD) depletion and subsequent inhibition of insulin synthesis and secretion.34
Medicinal plants are widely used especially in developing countries for the management of diabetes mellitus. In this present study, the antidiabetic effect of CSL extract of Curculigo pilosa and it solvent fraction was evaluated in streptozotocin-induced diabetic rats.
In this study, the enhanced elevation of blood glucose level due to administration of STZ was lowered by oral administration of CSL extract and butanol fraction. This reduction suggested the hypoglycemic activity of the extracts and might be due to its insulin stimulatory effect on the remnant β-cells or the ability to promote the regeneration of aberrant β-cells and overcoming insulin resistance. The destruction of β-cells by STZ causes some physico-metabolic abnormalities such as body weight loss, and increase in food and water intake.35 In this study, a pronounce weight loss was noted in the diabetic rats but oral administration of extracts was able to improve the body weight of the diabetic rats. The improvement in body weight of diabetic rats suggested the enhanced control of glucose homeostasis after treatment with the extracts. An oral glucose tolerance test (OGTT) is a more reliable marker of early abnormalities in glucose homeostasis than fasting blood glucose and it reflects hepatic gluconeogenesis and uptake of glucose from blood into skeletal muscle and adipose tissue after a meal.36 An excessive elevation of blood glucose level in diabetic rats following oral load of glucose was exhibited which was an indication of impaired glucose tolerance. Diabetic rats treated with the extracts were able to mitigate the impaired glucose tolerance thereby further confirm insulin stimulatory effect of the extracts on the remnant β cells.
Anaemia is a common occurrence in diabetes, particularly in patients with overt nephropathy or renal impairment.37 Anaemia occurrence in diabetes mellitus is due to the increased non-enzymatic glycosylation of red blood cell membrane proteins, which correlates with hyperglycaemia.38 Other findings in diabetic patients have revealed several metabolic and functional abnormalities of their red blood cells, including reduced erythrocyte survival,39 systemic inflammation, occult blood loss and haematinic deficiencies.40 The observed decrease in heamoglobin, packed cell volume and red blood cells in STZ-induced diabetic rats implies haematological disorders. Treatment with our extracts for 28 days was able to restore the values to near normalcy. This might not be unconnected with improved glucose homeostasis of the treated diabetic rats.
The elevation of biomarker enzymes (alanine aminotransferase, aspartate aminotransferase and alkaline phosphatase) and bilirubin in the bloodstream is an indication of hepatocellular damage that showed these enzymes that have leaked into the bloodstream.41 It has also been hypothesized that elevation in bilirubin, ALT, AST and ALP are possible predictors of type 2 diabetes.42 In this study, induction of diabetes by STZ is accompanied by increased in the level of these enzymes in the bloodstream, however the reversal of these enzymes activity in extract-treated diabetic rats towards baseline is an evidence of the prevention of cellular and tissue damage by our extracts. This capacity may further strengthen the optimized lipid metabolism in the liver of diabetic rats.43 Also In STZ-induced diabetes, the elevation in level of blood glucose is usually accompanied by an increase in plasma cholesterol, triglycerides, LDL and VLDL and decreases in HDL.44 During diabetes, activation of hormone-sensitive lipase during insulin deficiency is characterized by increased release of free fatty acids from adipose tissue. Therefore, the marked hyperlipidaemia that is usually seen in diabetes may be regarded as a consequence of the uninhibited actions of lipolytic hormones on fat depots.43 In this study, there was a marked alteration in the total cholesterol and high density lipoprotein concentrations in the STZ-induced diabetic rats. Administration of our extracts was able to normalize the observed abnormal lipid status in the treatment group. This might be due to better insulin control in the treated group.
STZ-induced diabetes is usually characterized with elevation of the serum level of urea and creatinine which was considered as significant markers of renal dysfunction.45 Creatinine and urea are byproduct of body metabolism, which are usually excreted in kidney. In the present study, the oral treatment of our extracts for 28 days significantly decreased the serum creatinine and urea levels in diabetic rats. Therefore, it may be concluded that the STZ-induced renal changes in the diabetic rats were averted in the treated group.
STZ-induced diabetes is also characterized by increased production of reactive oxygen species (ROS) which are involved in the etiology of several diabetic complications including diabetic nephropathy and hepatic damage.46 Several studies have shown that supraphysiological glucose enhances reactive oxygen species production in mesangial cells of the kidney glomerular and provokes necrosis, inflammation and oxidative stress in hepatic tissues.47,48 The elevated reactive ROS deplete the antioxidant defenses of the liver thus making it more vulnerable to hyperglycemia-mediated oxidative damage. In this study, markers of oxidative stress were elevated in diabetic rats; Lipid peroxides, hydroperoxides and protein carbonyls which are the secondary products of oxidative stress are unleashed during diabetes.49 The oral treatment of our extracts was able to avert the oxidative stress by decreasing the level of MDA, hydroperoxides and nitrites in the liver of the diabetic rats. Diabetes is often associated with a notable decline in the level of intracellular antioxidants due to their detoxifying effects on enormous free radicals produced by supraphysiological glucose. Also, the decrease in the enzymic antioxidant in the STZ-induced diabetic rats was almost normalized by the oral admistration of our extract(s). The protective nature of the extract on the hyperglycemia induced oxidative stress in hepatic tissues of the diabetic rats was further established by histopathological examination. The obvious structural aberrations include fibrotic parenchyma, chronic infiltration of inflammatory cells involving the sinusoids, few perivascular infiltration and few apoptotic hepatocytes. The administration of the extract was able to minimize these structural aberrations in the diabetic rats.
The GC-MS analysis of the extract revealed the presence of many phytochemicals including stilbene, a phytochemical with proven antidiabetic and antioxidant properties.50, 51, 52, 53 The antidiabetic activity of the extract might not be unconnected the stilbene present in it.
5. Conclusion
Conclusively, the results of the present study clearly justified the traditional use of CSL extract of Curculigo pilosa as an antidiabetic drug. The extract and its butanol fraction possess hypoglycemic ability, normalized aberrant lipid profile and some enzyme markers of organ's damage in the serum. Also hyperglycemia mediated oxidative stress in hepatic tissue was averted.
Acknowledgement
We thank Dr John Oludele Olanlokun of Biomembrane and Biotechnology laboratories, Department of Biochemistry, University of Ibadan for his technical assistance during the fractionation of the sample and induction of experimental diabetes.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Oyedemi S.O., Adewusi E.A., Aiyegoro O.A., Akinpelu D.A. Antidiabetic and haematological effect of aqueous extract of stem bark of Afzelia Africana (Smith) on streptozotocin-induced diabetic Wistar rats. Asian Pac J Trop Biomed. 2011;1(5):353–358. doi: 10.1016/S2221-1691(11)60079-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.International Diabetes Federation the IDF Diabetes Atlas. eighth ed. 2017. [Google Scholar]
- 3.Snehal N.M., Jayesh B.D., Sangita B.K., Archana R.J. Attenuation of diabetic nephropathy in streptozotocin-induced diabetic rats by Punica granatum Linn. leaves extract. J Tradit Complemen Med. 2017;7:273–280. doi: 10.1016/j.jtcme.2016.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.International Diabetes Federation the IDF Diabetes Atlas. seventh ed. 2015. [Google Scholar]
- 5.Zurina H., Mun F.Y., Mariam A., Ahmad P.M.Y. Antidiabetic properties and mechanism of action of Gynura procumbens water extract in streptozotocin-induced diabetic rats. Molecules. 2010;15:9008–9023. doi: 10.3390/molecules15129008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edwin J., Siddaheswar B.J., Dharam C.J. Diabetes and herbal medicines Iranian. J Pharmacol Ther. 2008;7:97–106. [Google Scholar]
- 7.Palazzino G., Gale C., Federici E., Monache F., Cometa M., Palmery M. Benzylbenzoate and nor lignan glucosides from Curculigo pilosa: structural analysis and in vitro vascular activity. Phytochemistry. 2000;55:411–417. doi: 10.1016/s0031-9422(00)00256-9. [DOI] [PubMed] [Google Scholar]
- 8.Nie Y., Dong X., He Y. Medicinal plants of genus Curculigo: traditional uses and a phytochemical and ethnopharmacological review. J Ethnopharmacol. 2013;147:547–563. doi: 10.1016/j.jep.2013.03.066. [DOI] [PubMed] [Google Scholar]
- 9.Sofidiya M.O., Oduwole B., Bamgbade E., Odukoya O., Adenekan S. Nutritional composition and antioxidant activities of Curculigo pilosa (Hypoxidaceae) rhizome African. J Biotechnol. 2011;10(75):17275–17281. [Google Scholar]
- 10.Karigidi K.O., Ojebode M.E., Anjorin O.J., Omiyale B.O., Olaiya C.O. Antioxidant activities of Curculigo pilosa and Gladilous psittascinus against lipid peroxidation in rat's liver and heart J Herbs. Spices Med Plants. 2019;25(1):1–10. [Google Scholar]
- 11.Adefegha Stephen A., Oyeleye Sunday I., Oboh Ganiyu. African crocus (Curculigo pilosa) and wonderful kola (Buchholzia coriacea) seeds modulate critical enzymes relevant to erectile dysfunction and oxidative stress. J Complement Integr Med. 2018 doi: 10.1515/jcim-2016-0159. [DOI] [PubMed] [Google Scholar]
- 12.Gbadamosi I.T., Egunyomi A. Phytochemical screening and in vitro anti-candidal activity of extracts and essential oil of Curculigo pilosa (Schum and Thonn) Engl. Hypoxidaceae. Afr. J. Biotechnol. 2010;9(8):1236–1240. [Google Scholar]
- 13.Olaiya C.O., Idowu PA Karigidi K. Antioxidative and antimicrobial activities of corn steep liquor anti-diabetic herb extracts. Ann Food Sci Technol. 2016;17(2):272–279. [Google Scholar]
- 14.Olaiya C.O., Karigidi K.O. Hypoglycemic effects of corn steep liquor extracts in streptozotocin-induced diabetic rats. Int J Biochem Res Rev. 2016;13(2):1–8. [Google Scholar]
- 15.Soladoye M.O., Chukwuma E.C., Owa F.P. An ‘Avalanche’ of plant species for the traditional cure of diabetes mellitus in South-Western Nigeria. J Nat Prod Plant Resour. 2012;2(1):60–72. [Google Scholar]
- 16.Rakieten N., Radkarni M.R. Studies on the diabetogenic action of streptozotocin (NSC-37917) Cancer Chemother Rep. 1963;29:91–98. [PubMed] [Google Scholar]
- 17.Du Vigneaud V., Karr W.G. Carbohydrates utilization rate of disappearance of d-glucose from the blood. J Biol Chem. 1925;66:281–300. [Google Scholar]
- 18.Weatherburn M.W. Colorimetric methods for serum urea determination. Anal Chem. 1967;39:971. [Google Scholar]
- 19.Bartels H., Bohmer M. In vitro determination of Creatinine and Urea. Clin Chem. 1972;2:37–193. doi: 10.1016/0009-8981(72)90432-9. [DOI] [PubMed] [Google Scholar]
- 20.Allain G.C., Poon L.S., Chan C.S., Richmond W. Quantitative determination of cholesterol using enzymatic colorimetric method. Clin Chem. 1974;20:470–475. 1974. [PubMed] [Google Scholar]
- 21.Jendrassik L., Grof P. In vitro determination of total and direct bilirubin. Biochemica. 1938;297:81. [Google Scholar]
- 22.Reitman S., Frankel S. A colorimetric method for the determination of serum glutamic oxaloacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 23.Rec GSCC (DGKC) Colorimetric method for serum alkaline phosphatase determination. J Clin Chem Clin Biochem. 1972;10:182. [Google Scholar]
- 24.Takasaki Y. Serum lipid levels and factors affecting atherogenic index in Japanese children. J Physiol Anthropol Appl Hum Sci. 2005;24:511–515. doi: 10.2114/jpa.24.511. [DOI] [PubMed] [Google Scholar]
- 25.Ikewuchi C.J., Ikewuchi C.C. Alteration of plasma lipid profiles and atherogenic indices by Stachytarpheta jamaicensis L (Vahl) Boikemistri. 2009;21:71–77. [Google Scholar]
- 26.Bradford M.M. A rapid and sensitive for the quantitation of microgram quantitites of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 27.Farombi E.O., Tahnteng J.G., Agboola A.O., Nwankwo J.O., Emerole G.O. Chemoprevention of 2-acetylaminofluorene-induced hepatotoxicity and lipid peroxidation in rats by kolaviron-a Garcinia kola seed extract. Food Chem Toxicol. 2000;38:535–541. doi: 10.1016/s0278-6915(00)00039-9. [DOI] [PubMed] [Google Scholar]
- 28.Green L.C., Wagner D.A., Glogowski J., Skiper P.L., Wishnock J.S., Tannenbaum S.R. Analysis of nitrate, nitrite and [15 N] nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 29.Jollow D.J., Mitchell J.R., Zampaglione N., Gillette J.R. Bromobenzene induced liver necrosis: protective role of glutathione and evidence for 3,4 bromobenzene oxide as the hepatotoxic metabolite. Pharmacol. 1974;11:151–169. doi: 10.1159/000136485. [DOI] [PubMed] [Google Scholar]
- 30.Wolff S.P. Ferrous ion oxidation in the presence of ferric ion indicator xylenol orange for measurement of hydroperoxides. Methods Enzymol. 1994;233:182–189. [Google Scholar]
- 31.Clairborne A. Catalase activity. In: Greewald A.R., editor. Handbook of Methods for Oxygen Radical Research. CRC Press; Boca Raton, FL: 1995. pp. 237–242. [Google Scholar]
- 32.Misra H.P., Fridovich I. The role of superoxide anion in the autooxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–3175. [PubMed] [Google Scholar]
- 33.Avwioro O.G. Claverianum press; Nigeria: 2010. Histochemistry and Tissue Pathology, Principle and Techniques. [Google Scholar]
- 34.Virag L., Szabo´ C. The therapeutic potential of poly (ADP-ribose) polymerase inhibitors. Pharmacol Rev. 2002;54:375–429. doi: 10.1124/pr.54.3.375. [DOI] [PubMed] [Google Scholar]
- 35.Palsamy P., Subramanian S. Resveratrol, a natural phytoalexin, normalizes hyperglycemia in streptozotocin-nicotinamide induced experimental diabetic rats. Biomed Pharmacother. 2008;62:598–605. doi: 10.1016/j.biopha.2008.06.037. [DOI] [PubMed] [Google Scholar]
- 36.Singleton J.R., Smith A.G., Russell J.W., Feldman E.L. Microvascular complications of impaired glucose tolerance. Diabetes. 2003;52:2867–2873. doi: 10.2337/diabetes.52.12.2867. [DOI] [PubMed] [Google Scholar]
- 37.Thomas M.C., Cooper M.E., Rossing K., Parving H.H. Anaemia in diabetes: is there a rationale to TREAT? Diabetologia. 2006;49:1151–1157. doi: 10.1007/s00125-006-0215-6. [DOI] [PubMed] [Google Scholar]
- 38.Kennedy L., Baynes J.W. Non-enzymatic glycosylation and the chronic complications of diabetes: an overview. Diabetologia. 1984;26:93–98. doi: 10.1007/BF00281113. [DOI] [PubMed] [Google Scholar]
- 39.Thomas M.C., Tsalamandris C., MacIssaac R., Jerums G. Anemia in diabetes; an emerging complication of microvascular disease. Curr Diabetes Rev. 2005;1:107–126. doi: 10.2174/1573399052952587. [DOI] [PubMed] [Google Scholar]
- 40.Jones R.L., Peterson C.M. Hematologic alterations in diabetes mellitus. Am J Med. 1981;70:339–352. doi: 10.1016/0002-9343(81)90771-3. [DOI] [PubMed] [Google Scholar]
- 41.Jaeschkle H., Gores G.J., Cederbaum A.I., Hinson J.A., Pessayre D., Lemaster J.J. Mechanisms of hepatotoxicity. Toxicol Sci. 2002;65:166–176. doi: 10.1093/toxsci/65.2.166. [DOI] [PubMed] [Google Scholar]
- 42.Elizabeth H., Harris M.D. Elevated liver function tests in type 2 diabetes. Clin Diabetes. 2005;23:115–119. [Google Scholar]
- 43.Subbiah R., Kasiappan R., Karuran S., Sorimuthu S. Beneficial effects of aloe vera leaf gel extract on lipid profile status in rats with streptozotocin diabetes. Clin and Exp Pharmacol Physiol. 2006;33:232–237. doi: 10.1111/j.1440-1681.2006.04351.x. [DOI] [PubMed] [Google Scholar]
- 44.Mitra S.K., Gopumadhavan S., Muralidhar T.S., Anturlikar S.D., Sujatha M.B. Effect of D-400, a herbomineral preparation on lipid profile, glycated hemoglobin and glucose tolerance in streptozotocin induced diabetes in rats. Indian J Exp Biol. 1995;33:798–800. [PubMed] [Google Scholar]
- 45.Nain P., Saini V., Sharma S., Nain J. Antidiabetic and Antioxidant Potential of Emblica officinalis Gaertn. Leaves extract in streptozotocin-induced type-2 diabetes mellitus (T2DM) rats. J Ethnopharmacol. 2012;142:65–71. doi: 10.1016/j.jep.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 46.Cheng D., Liang B., Li Y. Antihyperglycemic effect of Ginkgo biloba extract in streptozotocin-induced diabetes in rats. BioMed Res Int. 2013:1–7. doi: 10.1155/2013/162724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brownlee M. The pathobiology of diabetic complications. Diabetes. 2005;54(6):1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 48.Palsamy P., Sivakumar S., Subramanian S. Resveratrol attenuates hyperglycemia-mediated oxidative stress, proinflammatory cytokines and protects hepatocytes ultrastructure instreptozotocin–nicotinamide-induced experimental diabetic rats. Chem Biol Interact. 2010;186:200–210. doi: 10.1016/j.cbi.2010.03.028. [DOI] [PubMed] [Google Scholar]
- 49.Evans J.L., Goldfine I.D., Maddux B.A., Grodsky G.M. Oxidative stress and stress activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev. 2002;23:599–622. doi: 10.1210/er.2001-0039. [DOI] [PubMed] [Google Scholar]
- 50.Frombauma M., Clanche S.L., Bonnefont-Rousselot D., Borderie D. Antioxidant effects of resveratrol and other stilbene derivatives on oxidative stress and NO bioavailability: potential benefits to cardiovascular diseases. Biochimie. 2012;94:269–276. doi: 10.1016/j.biochi.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 51.Heo S., Jin Y., Jung J.M., Wang M. Antidiabetic properties of 2,5-Dihydroxy-4,3’-Di(ß-D-Glucopyranosyloxy)-trans-Stilbene from mulberry (Morus bombycis koidzumi) root in streptozotocin-induced diabetic rats. J Med Food. 2010;4:602–607. doi: 10.1089/jmf.2006.0241. [DOI] [PubMed] [Google Scholar]
- 52.Kerem Z., Bilkis I., Flaishman M., Sivan L. Antioxidant activity and inhibition of r-glucosidase by trans-resveratrol, piceid, and a novel trans-stilbene from the roots of Israeli Rumex bucephalophorus L. J Agric Food Chem. 2006;54:1243–1247. doi: 10.1021/jf052436+. [DOI] [PubMed] [Google Scholar]
- 53.Lv L., Shao X., Wang L., Huang D., Ho C., Sang S. Stilbene glucoside from Polygonum multiflorum thunb.: a novel natural inhibitor of advanced glycation end product formation by trapping of methylglyoxal. J Agric Food Chem. 2010;58:2239–2245. doi: 10.1021/jf904122q. [DOI] [PubMed] [Google Scholar]