Abstract
Diabetic patients are frequently afflicted with impaired wound healing where linear progression of molecular and cellular events compromised. Despite of meaningful progress in diabetic treatment, management of diabetic chronic wounds is still challenging. Jamun (Syzygium cumini) honey may be a promising candidate for diabetic wound healing and need to explore in detail.
So present study was designed to evaluate the efficacy of Jamun honey (JH) for diabetic wound healing in in vitro wound (primary fibroblasts) model and in in vivo of diabetic mice (Streptozotocin induced) model. The fibroblast cell model was studied for migratory behaviour and myofibrolasts infiltration under honey interventions via scratch/migration assay, immuno-cytochemistry and western blot. We applied FDA approved Manuka honey (MH) as positive control and JH as test honey to evaluate wound re-epithelialization, sub-epithelial connective tissue modification and angiogenesis via histo-pathological and immuno-histochemical analysis. JH (0.1% v/v) dilution has notably improved wound closure, migration with concomitant α-SMA expressions in vitro. Topical application of JH in diabetic mice model showed significant (*p ≤ 0.05) wound closure, reepithelialization, collagen deposition (I/III) and balanced the myofibroblasts formation. It also modulated vital angiogenic markers (viz HIF-1α, VEGF, VEGF R–II) significantly (*p ≤ 0.05). All these observations depicted that JH promotes sequential stages of wound healing in diabetic mice model. The results of the present study established Jamun honey as good as Manuka honey considering wound closure, re-epithelialization, collagen deposition and pro-angiogenic potential.
Keywords: Diabetic wound, Jamun honey, Wound closure, Angiogenesis, Reepithelialization
Abbreviations: DAB, 3,3′-Diaminobenzidine; DBM, Diabetic mice; DMEM, Dulbecco’s Modified Eagle Medium; ECM, Extracellular matrix; EGF, Epidermal growth factor; EMT, Epithelial–mesenchymal transition; H&E, Hematoxylin and Eosin; HIF 1 α, Hypoxia-inducible factor 1 α; IHC, Immuno-histochemistry; JH, Jamun honey; MH, Manuka honey; PI, Povidine Iodine; STZ, Streptozotocin; VEGF, Vascular endothelial growth factor; VG, van Gieson’s
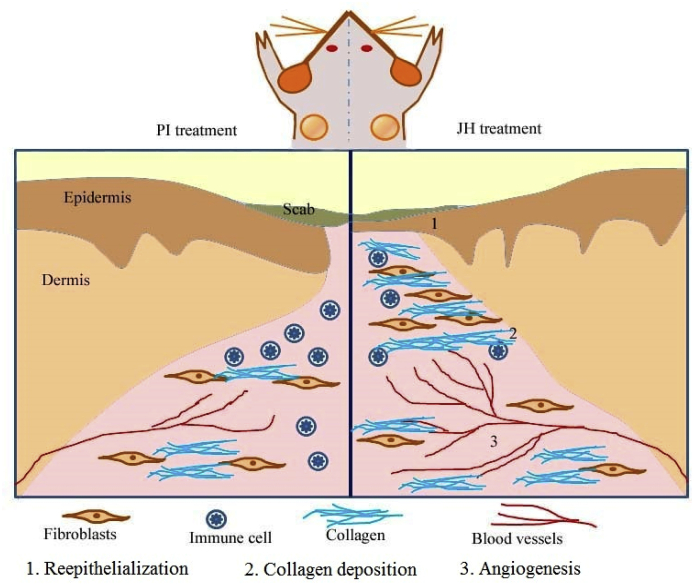
Graphical abstract
Highlights
-
•
Wound healing under Jamun honey and it’s effect on angiogenesis needs to explore.
-
•
Jamun honey promotes sequential stages of wound healing with pro-angiogenic efficacy.
-
•
Healing under Jamun honey is comparable to that of Manuka (Medical grade) honey.
1. Introduction
Cutaneous wound repair is a well orchestrated process of tissue repair in which the integrity of wounded skin continues to retain while initial skin tissue difficult to achieve.1 Diabetic patients are frequently afflicted with impaired wound healing where linear progression of molecular and cellular events compromised.2, 3, 4 Several intrinsic pathological abnormalities (neuropathy and vascular problems) and extrinsic factors (wound infection, callus formation and excessive pressure to the wound site) are mostly responsible for the complex microenvironment of diabetic wound.5 Hyperglycemia lowers the proliferation of collagen producing cells and obstructs the mechanical strength providing activity into the wound bed.6 In this regard, suitable collagen depositions is the foremost criteria of remodeling phase as it increases the tensile strength and tenders important signaling molecules towards wound area.7 The increment in collagen type I: III ratio is vital aspect of tensile strength of healing wounds.8 Decrease of wound size and extracellular matrix (ECM) deposition during healing process are the results of fibroblasts to myofibroblasts conversion.9 Myofibroblasts are present in proliferative phase of wound healing and express α-SMA (α-smooth muscle actin)10 along with vimentin,11 fibronectin12 and N-cadherin.13 The epithelial restoration process is associated with sub-epithelial myofibroblasts infiltration where c-Myc (cell proliferation marker) expression is imperative.14 Stimulation of angiogenesis is an important aspect for normal wound healing but hyperglycemic condition in diabetic wound disrupts angiogenesis and hypoxia inducible factor (HIF) -α trans-activation15,16. The vascular endothelial growth factor (VEGF) plays an important role in signaling and balancing of neo-vascularization17,18. The VEGF signaling cascade initiated by binding with transmembrane tyrosine kinase receptors – VEGFR-I and VEGFR-II. VEGF interacts with VEGFR-II to persuade the formation of new blood vessels for angiogenesis.19
Furthermore, any successful healing agent needs evaluation of composition and functional dynamism where natural honey is a promising candidate (used since long time) for its healing potentiality against tropical infections and wounds20,21. Honey with various organic and inorganic substances creates a moist healing environment, induces proliferation of cells, accumulates granulation tissue and growth factors at wound margin.2,5 It also reduces bacterial burden, inflammation, malodor, edema and exudates.22 There are several studies on wound healing potential of honey on diabetic wound but healing under Jamun honey (JH) and it’s effect on angiogenesis needs to explore further.
In this study, the healing progression of diabetic mice (DBM) and non-diabetic mice (NDM) wound model under JH (physicochemically characterized) and it’s comparison to Povidone Iodine3 (Kolluru et al., 2012)/Manuka honey5 (Maharlooei et al., 2011) were examined. Primary fibroblasts isolated from both DBM & NDM skin tissue were observed for in vitro migratory behaviour under JH and MH dilutions via phase contrast microscopy, scratch/migration assay, immuno-cytochemistry (ICC) of α-SMA and western blot analysis for α-SMA, collagen I/III. Furthermore, histo-pathological (Hematoxylin & Eosin/van Gieson’s) and immuno-histochemical (for collagen I/III, α-Smooth muscle actin, VEGF, VEGFR II and HIF1 α) staining were performed to study the diabetic wound repair in in vivo wound model.
2. Materials and methods
2.1. Materials
All chemicals and reagents used were of analytical grade obtained from Sigma-Aldrich and Merck chemicals. DMEM low glucose (Cat. No. AT007) and high glucose media (Cat. No. AT006), antimycotic antibiotic, fetal bovine serum (FBS), l-glutamine, 0.05% trypsin−EDTA solution, bovine serum albumin (BSA) were purchased from Himedia (India); streptozotocin (Sisco Research Laboratories Pvt. Ltd. Andheri, Mumbai); Primary antibodies, Anti-HIF1 alpha antibody [EP1215Y]-(ab51608); Anti-VEGF antibody [14–124]-(ab16883); Anti-VEGF Receptor 2 antibody [SP123]-(ab115805); Anti-Collagen I antibody [COL-1] ab6308, Anti-Collagen III antibody [FH-7A] ab6310 were purchased from Abcam (Cambridge, UK). α-Actin (1A4): sc-32251 were purchased from Santa Cruz biotechnology. For chromogenic detection HRP-conjugated secondary antibody and 3, 3′-diaminobenzidine (DAB) {Super Sensitive Polymer-HRP IHC Detection System kit (Cat. No. QD400-60K, BioGenex)} used, sections counter stained by Hematoxylin. Western ECL Substrate (Catalogue No- #170–5061, Bio-Rad, USA).
Physicochemically characterized Jamun honey (JH) (from Societé Naturelle, S-18 E Shakarpur, Delhi 110092, India) and Medihoney (Derma Sciences, U.S.) were used for wound healing experiments. The physical and biochemical properties of the selected honeys were given in supplementary table (ST)1 and ST2.
2.2. Study design and diabetic induction
In this study, 60 male swiss albino mice (Mus musculus) age 8–12 weeks and weight 25–35g were used. The animals were housed individually in a pathogen free environment and this study protocol was approved by Institutional Animal Care and Use Committee, IIT Kharagpur Reference number: IE-1/JC-SMST/1.14.
For diabetes induction, the protocol mentioned by Brosius, 2011 was followed. In brief streptozotocin (50 mg per kg body weight) were injected intraperitonially for 5 days regularly to induce diabetes and blood glucose level was determined in order to identify diabetic mice model.23 Protocol for streptozotocin (STZ) preparation has been given in supplementary document. The identification of diabetic induced mice were based on high blood glucose level i. e ≥ 300 mg/dL, frequent urination and mean weight loss which was 10% of total body weight after five days STZ injection. 30 animals without diabetic induction named non-diabetic mice i. e NDM and another 30 diabetic induced mice named DBM. The mice treatments and code assignments were given in ST3.
2.3. Wound formation
All the surgical procedure conducted under anesthesia {ketamine (100 mg/kg) and xylazine (5 mg/kg) intraperitoneal}. The dorsum was shaved and two wounds per mice between the 6th and 8th thoracic vertebrae symmetrically spaced 5–10 mm from the vertebral column formed by surgical excision. The left side wound (left of dorsum) treated with PI (10% w/w)/MH (active leptospermum honey from Derma Sciences) as control and right side wound (right of dorsum) treated with JH soaked sterile cotton gauge. The redressing was done after 24 h for 30 days.
2.3.1. Cell culture
The protocol for primary fibroblast isolation was adopted from Seluanov et al., 2010 with some modifications.24 Details given in Supplementary figure (SF)1.
2.3.2. Scratch wound healing and migration assay
We adopted the protocol for scratch wound healing and migration assay from our previous work.25
2.3.3. Immuno-cytochemistry for α-SMA
The in vitro scratch wounds were fixed by 4% paraformaldehyde in PBS for 10 min at 25 °C. Serum blocked (10% goat serum in PBST) to reduce non-specific binding of the antibodies. Further, cells were incubated with diluted primary antibody of α-SMA. HRP conjugated secondary antibody was used for chromogenic (counter stained by Meyers Hematoxylin) staining.
2.4. Wound closure analysis
Macroscopic camera images and biopsies of mice were taken for 5th, 10th and 15th days. The biopsies were collected after giving anesthesia under guidance of expert pharmacologist. To examine the wound closure rate under JH, images were taken of wound at different temporal point macroscopically by camera (with sterile scale) and microscopically by stereozoom (1x), further the wound fraction was calculated by measuring the area of wound by using DP2 BSW software. The wound fraction formula as follows
| Wf = Wt/W0 |
Where Wf = wound fraction; Wt = wound area at time t; W0 = wound area at initial time.
2.5. Histological and immuno-histochemical staining
Wound incisional biopsies of 5 × 2 mm2 surface size and thickness ≈0.5 mm were fixed in 4% formaldehyde solution in phosphate-buffered saline and 4-μm thick microtome sections were obtained on albumin and poly l-lysine coated glass slides for histopathology and immuno-histochemistry respectively.
2.6. Hematoxylin & eosin staining
The tissue sections were de-paraffinized in xylene and re-hydration performed by incubating 100%, 90%, 80%, 70% alcohol followed by running tap water. Thereafter, these tissue sections were stained with hematoxylin for 3–5 min followed by washing and 1% Eosin applied for 3–5 min. After washing the excess stain of the section were washed and dehydrated by alcohols gradients to mount in DPX mounting medium.
2.7. van Gieson’s staining
De-paraffinized and rehydrated tissue sections were stained with hematoxylin and then dipped in 5% sodium thio-sulphate solution for 2 min. Then the sections were washed with tap water followed by 9:1 saturated Picric acid: acid fuschin 2 min treatment. Further, tissue sections were washed with water followed by alcohol gradients before mounting into DPX.
2.8. Immuno-histochemical staining
In brief, sections were deparaffinized (baking at 60 °C) and subject to antigen retrieval in a using the EZ-Retriever System V.2 (BioGenex, S R, California). Samples were blocked to reduce non-specific binding of the antibodies and incubated with specific primary antibody. Primary antibody binding was visualized using fluorophore-conjugated secondary antibody. For chromogenic staining poly-HRP secondary antibody with DAB chromogen were used and tissue sections were counterstained by Hematoxylin. Appropriate controls were put up to validate the experiments. All the antibody reactions were performed in dark at room temperature (25 °C).
2.9. Western blot
Western blot protocol was adopted from Chaudhary et al. (2017).26
2.10. Semi-quantitative evaluation of histochemical and immunohistochemical images
For reepithelialization study, the migratory behaviour of epithelial cells i.e. epithelial tongue area after 5 days was measured by AxioVision Rel. 4.7 software. The Van Gieson’s (VG) stain intensity was calculated by AxioVision Rel. 4.7 software across three equidistant points (P1 = 25 μm, P1 = 50 μm, P1 = 100 μm) underneath the basement membrane of dermis (perpendicular to the basement membrane). 30 random points were selected in each intervention and intensity values were normalized in 0–10 scale. Further, the notch box plot was created using MATLAB R2015a.
The level of immuno-histochemical staining of ECM proteins was observed by separating the counter stain by imageJ software. Further, images were converted into grey scale in order to measure the grey scale intensity of particular protein expression. The grey scale intensity was calculated and notch box were generated by MATLAB R2015a.
2.11. Statistical analysis
Statistical significance was calculated by Student’s paired t-Test with one tail distribution in Microsoft Office Excel 2007 (Microsoft Redmond, W.A.) and p value ≤ 0.05 was considered to indicate statistical significance. Result of VG intensity score were analyzed by variance between the groups i.e. ANOVA. For this one way parametric ANOVA calculated using http://www.physics.csbsju.edu/stats/anova.html (online ANOVA calculator).
3. Results
3.1. Diabetic mice identification
Diabetic mice wound model was created by giving low dose streptozotocin to swiss albino mice for five consecutive days and then excisional wound was formed on dorsal skin surface. All the experiments were completed within one month (until the animals were hyperglycemic) from wound formation in order to minimize the diabetic recovery in animals. Although streptozotocin induced diabetes in mice model have some limitation of long term metabolic and pathological attributes which are similar to human diabetes, streptozotocin induced diabetes in mice represents a comparable model to examine acute wound healing events27,28. In this study, the diabetic mice developed characteristic physiological features of diabetic humans like hyperglycemia, weight loss and compromised wound healing. Hyperglycemic condition reduces cell proliferation and alters collagen synthesis. In addition, weight loss is linked with poor nutrient intake and reported to contribute wound healing defects in diabetic animals25,29.
3.2. Efficacy of JH dilution on in vitro fibroblast wound healing
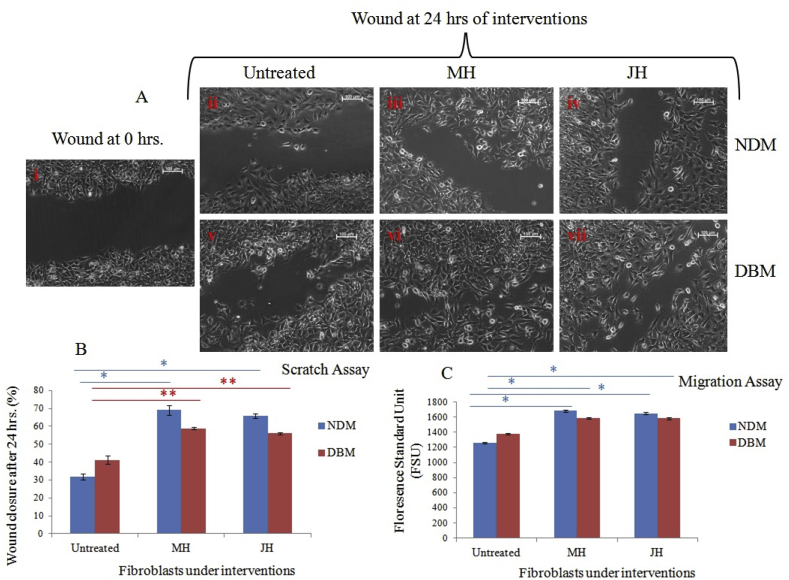
The pro-inflammatory cytokines stimulate the dermal fibroblasts at wound margins. ECM has various growth factors and cytokines to stimulate fibroblasts migration. In this regard, honey dilutions have been reported to provide a matrix at the wound interface for cell migration and proliferation. Ranzato et al. (2013) have shown that honey dilution induces tissue repair activity of fibroblasts in in vitro dermal fibroblasts monolayer.30 Considering this reference dermal fibroblast isolated from NDM and DBM mice at early passages (up to 10) were grown in vitro and treated with 0.1% v/v honey (both MH &JH) dilution in DMEM media after scratch wounding. NDM derived fibroblasts showed significant (*p ≤ 0.05) increase in wound closure rate under MH and JH dilutions in comparison to untreated one. In contrast, the DBM derived fibroblasts have non-significant (**p > 0.05) alteration in wound closure rate under MH and JH dilutions (Fig. 1 A & B). Furthermore, in migration assay of fibroblasts, the fluorescence standard unit value calculated for MH and JH dilutions. The cell migration for both DBM & NDM showed significant (*p ≤ 0.001) increase in comparison to untreated cells (Fig. 1 C).
Fig. 1.
Cell migration assay under honey dilutions in DMEMF-12 media (v/v %); A. Representative phase contrast (10x) image of three independent wound healing experiments of primary fibroblasts. Control at T = 0 h. (i); (ii-iv) untreated, MH dilution and JH dilution at T = 24 h of NDM fibroblasts respectively; (v-vi) untreated, MH dilution and JH dilution at T = 24 h of DBM fibroblasts respectively. B. Graph represents the % wound closure [(pre wound area-post wound area)/pre wound area x 100] of cell population within 24 h. For each sample 3 field were counted. (*p ≤ 0.05 and **p > 0.05), C. Graph represents the cell migration of cell population within 24 h (*p ≤ 0.05 and **p > 0.05).
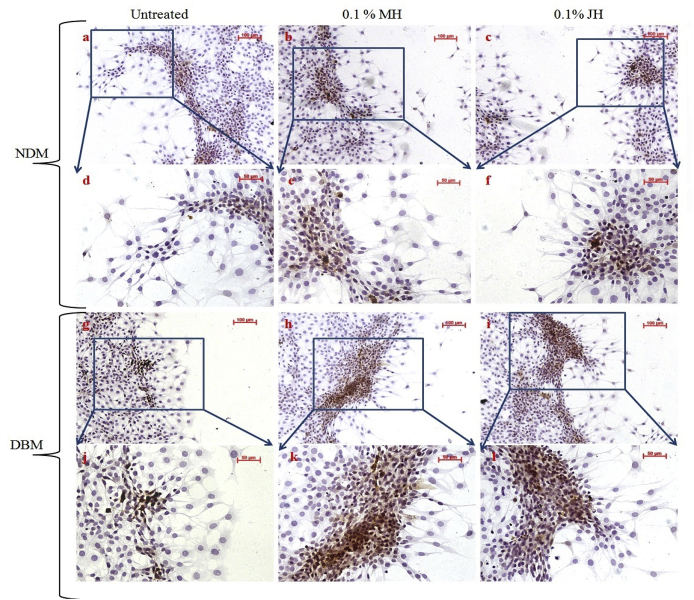
The prime myofibroblast marker i.e. α- SMA expressions in NDM/DBM fibroblasts were examined. Fig. 2 depicted that α-SMA was differentially expressed under honey dilutions where marginal/migratory cells showed more α-SMA expression.
Fig. 2.
Microphotographs for chromogenic expression of α-SMA in fibroblast cell population; α-SMA expression (10x objective magnification) of NDM isolated fibroblasts in untreated, MH dilution and JH dilution respectively (a–c); enlarged view (20× objective magnification) of NDM isolated fibroblasts in untreated, MH dilution and JH dilution (d–f); α-SMA expression (10x objective magnification) of DBM isolated fibroblasts in untreated, MH dilution and JH dilution respectively (g–i); enlarged view (20× objective magnification) of NDM isolated fibroblasts in untreated, MH dilution and JH dilution (j–l).
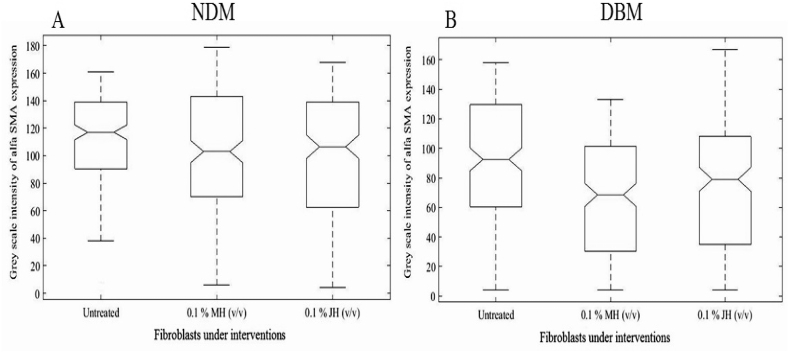
To find the efficacy of JH and MH in inducing α-SMA expression the grey scale intensity of DAB (separated by color deconvolution in image J software) expression were calculated and notch box plot was created. A significant (p ≤ 0.05) up-regulation of α-SMA expression was observed for honey dilution treated group in comparison to control both for NDM and DBM. However, non-significant (p ≥ 0.05) alterations of α-SMA expression were observed between MH and JH treated group for NDM and DBM (Fig. 3). In addition, the western blot analysis for α-SMA expression also supported the above notion (ICC analysis). The western blot analysis of collagen I and III showed significant (p ≤ 0.05) alteration in expression for MH/JH dilution treated groups (NDM & DBM) in comparison to untreated (Fig. 4). A non significant (p ≤ 0.05) alteration of α-SMA expressions after 18 h honey (MH and JH) dilution incubation validated similar behaviour of both honey (Manuka as well as Jamun) in inducing fibroblast to myofibroblasts transformation.
Fig. 3.
Notch box plot for grey scale intensity of α-SMA expression for NDM isolated fibroblasts (A) and for DBM isolated fibroblasts (B) under selected study groups where significant alteration (p ≤ 0.05) between untreated and honey dilution (MH &JH) treated groups and non-significant (p ≥ 0.05) change between MH and JH dilutions (both for NDM and DBM) was found.
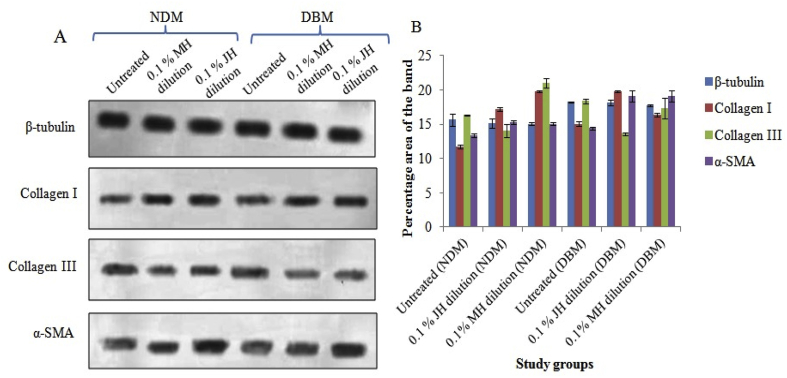
Fig. 4.
(A) Western blot study of collagen I/III and α-SMA expression of in vitro fibroblast wound under interventions. B. Graph representing semi-quantitative analysis of target proteins using percentage area of band where significant alteration (p ≤ 0.05) between untreated and honey dilution (MH &JH) treated groups and non-significant (p ≥ 0.05) change for MH vs JH dilutions (both for NDM and DBM) was found.
3.3. Non-invasive assessment of wound closure
Wound pictures were taken by camera at day 0, day 5, day 10 and day 15 showed in SF 2. Fig. 5A showed the representative images grabbed by stereozoom microscope. Wound fraction was measured from these images where MH and JH treated wounds were found to have significant (*p < 0.05) wound closure in comparison to PI treated wound both in NDM and DBM mice (Fig. 5B and C).
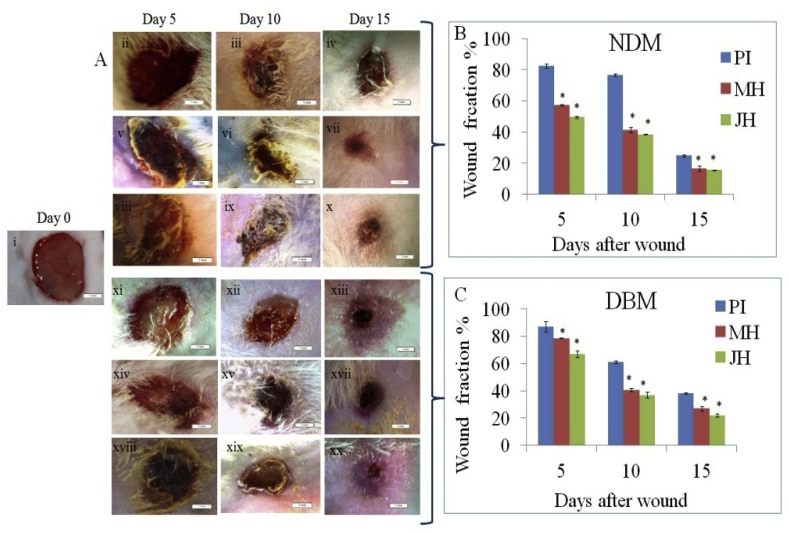
Fig. 5.
(A) Stereozoom images (1x) of wound bed: (i) day 0, (ii-iv) PI treated, (v-vii) MH treated and (viii-x) JH treated at day 5, 10 and 15 in NDM; (xii-xiv) PI treated, (xv-xvii) MH treated and (xviii-xx) JH treated at day 5, 10 and 15 in DBM. Wound fraction % of NDM, B. & DBM C (*p ≤ 0.05).
3.4. Impact of JH on reepithelialization, collagen and α-SMA expression
Effect of JH dressings on healing wound bed was examined by Hematoxylin and eosin (H & E) stained microtome sections (Fig. 6). H & E stained tissue sections at day 5 [Fig. 6A (1–6)], represented epithelial tongue formation from the margins of wound in order to reconstitute the epidermal barrier. Finally, the regenerated epithelial cells covered the dermal defect at 15th day [SF3]. The re-epithilialization area of wound margin under each intervention at 5th day was measured by using Axiovision Rel. 4.7 software and the re-epithilialization graph plotted (Fig. 6 B). H & E observations showed that compared to PI treated wound, the re-epithelialization area was significantly (*p ≤ 0.05) increased under honey interventions both in NDM and DBM (Fig. 6). After 15 days of interventions basement membrane was clearly visible and wound gap was healed with several inflammatory cells. However, in PI treated wound (NDM and DBM) some defects (loose connection) were observed in dermal epidermal junctional region (SF3 iii and vi) which was a fully compact structure in honey treated groups [SF3 (iv-viii)].
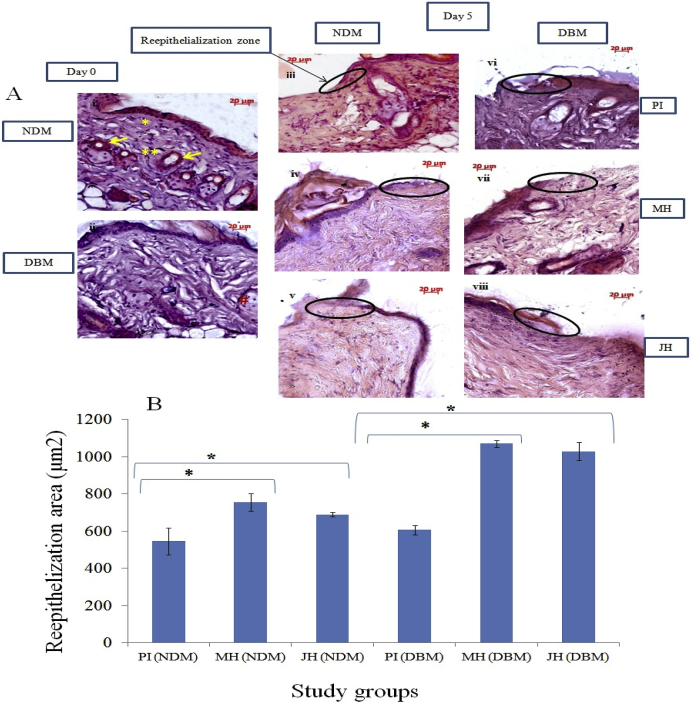
Fig. 6.
Histo-pathological (H&E) examination of wound bed of NDM & DBM under interventions (10x objective magnification): A. normal skin (a0 = NDM) and (b0 = DBM) at day 0; [(1–3) NDM group] & [(4–6) DBM group] reepithelization area under PI, MH, JH at day 5; B. Graph representing the reepithelialization area (μm2) at day 15 under selected study groups (*p ≤ 0.05), * indicated reticular dermis, ** indicated papillary dermis and # indicated sebaceous gland. Hair follicles indicated by yellow arrows.
The VG studies also showed improvement in collagen population under honey treated group. The collagen intensity [at three equidistance points (P1, P2 and P3) from basal layer] under honey treatment followed the intensity pattern of normal skin (Fig. 7 and Table 1) for both NDM and DBM. However, PI treated group showed major differences at P1, P2 and P3 grey scale intensity in comparison to normal for NDM and DBM.
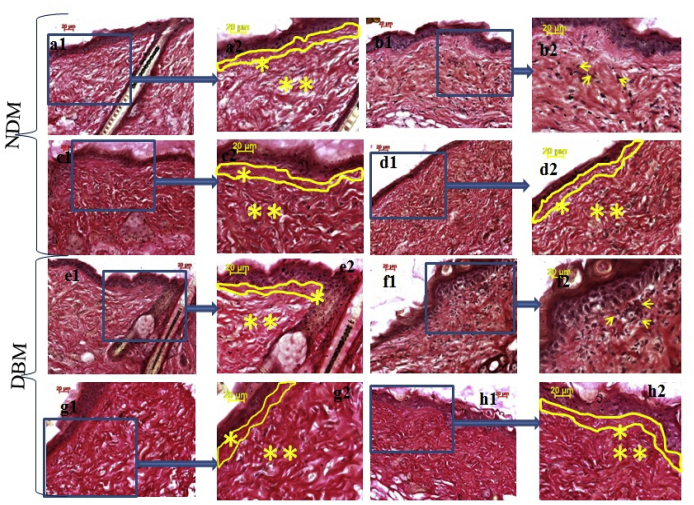
Fig. 7.
Histo-pathological (VG) examination of wound bed of NDM & DBM under interventions (10x objective magnification): a1. normal skin NDM, a2. enlarged view of a1, b1. PI treated wound of NDM at day 15, b2. enlarged view of b1, c1. MH treated wound of NDM at day 15, c2. enlarged view of c1, d1. JH treated wound of NDM at day 15, d2. enlarged view of d1; e1. normal skin DBM, e2. enlarged view of e1, f1. PI treated wound of DBM at day 15, f2. enlarged view of f1, g1. MH treated wound of DBM at day 15, g2. enlarged view of g1, h1. JH treated wound of DBM at day 15, h2. enlarged view of h1. Demarcation of basement membrane and papillary dermis indicated by yellow lines, ** showed reticular dermis and * showed papillary dermis.
Table 1.
ANOVA for evaluating VG intensity differences.
| NDM | ||||||
|---|---|---|---|---|---|---|
| Length lying below the basement membrane | Normal | PI | MH | JH | F-value | P-value |
| P1 = 25 μm | 102.9 ± 3.2 | 120.4.0 ± 6.02 | 95.78 ± 8 | 92.2 ± 6.2 | 39.52 | <.0001 |
| P2 = 50 μm | 121.4 ± 6.2 | 121.8 ± 5.02 | 106 ± 6.1 | 95 ± 3.1 | 34.42 | <.0001 |
| P3 = 100 μm |
128.4 ± 6 |
98.8 ± 5.8 |
122.3 ± 6 |
96.2 ± 7.8 |
64.13 |
<.0001 |
| DBM |
||||||
| P1 = 25 μm | 120 ± 4.6 | 120.1 ± 7 | 104.7 ± 5.8 | 115.5 ± 7 | 12.99 | <.0001 |
| P2 = 50 μm | 122.6 ± 6.5 | 109 ± 6 | 118.1 ± 5.8 | 128.8 ± 7.5 | 15.53 | <.0001 |
| P3 = 100 μm | 128.9 ± 7.4 | 101 ± 4.1 | 114.5 ± 5 | 131 ± 5.8 | 57.5 | <.0001 |
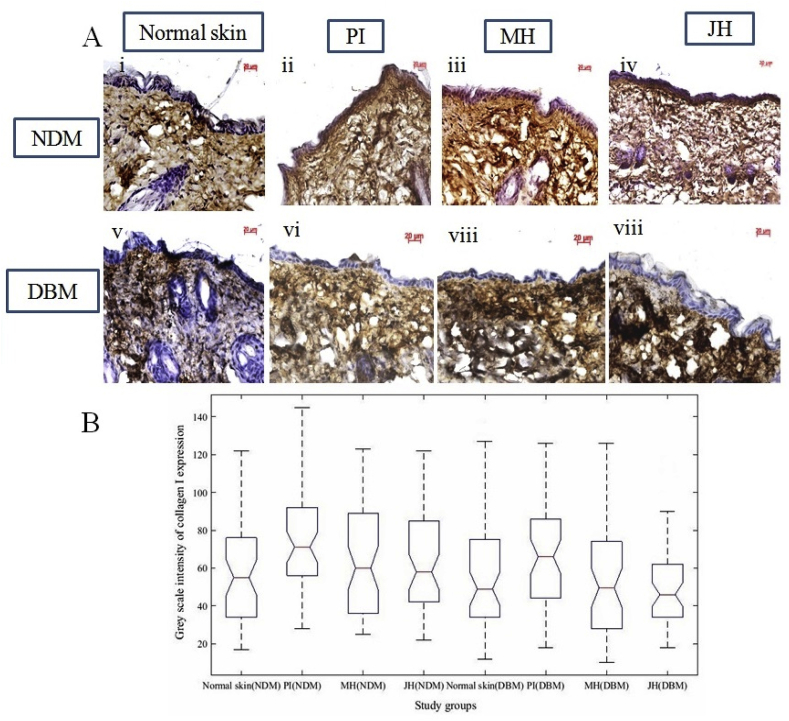
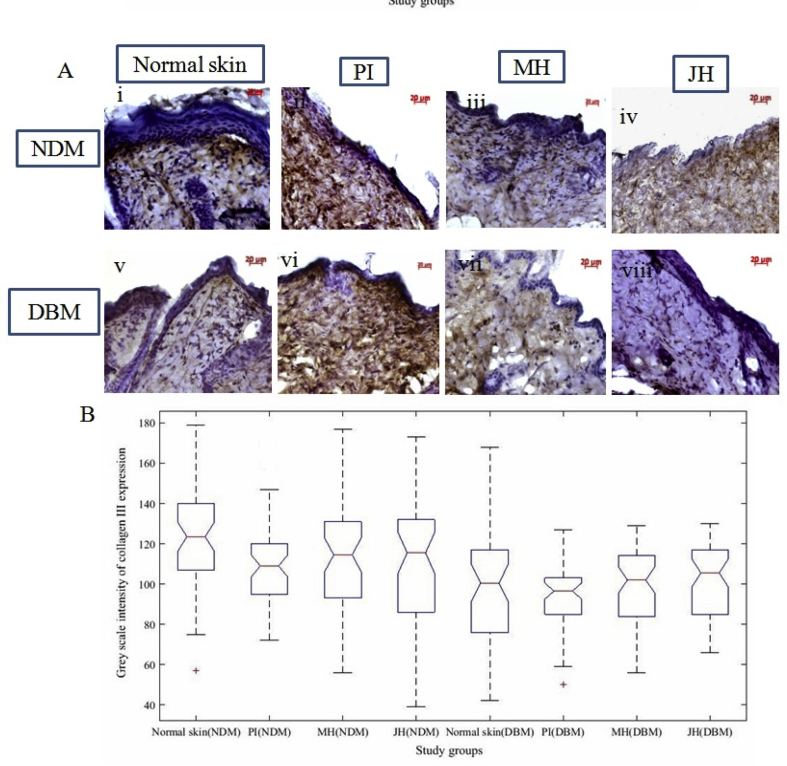
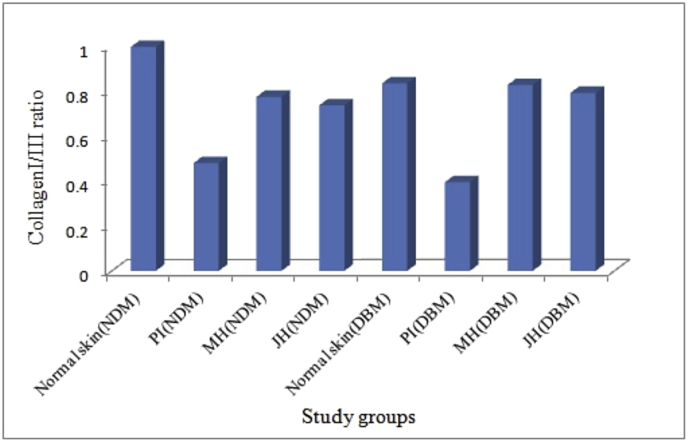
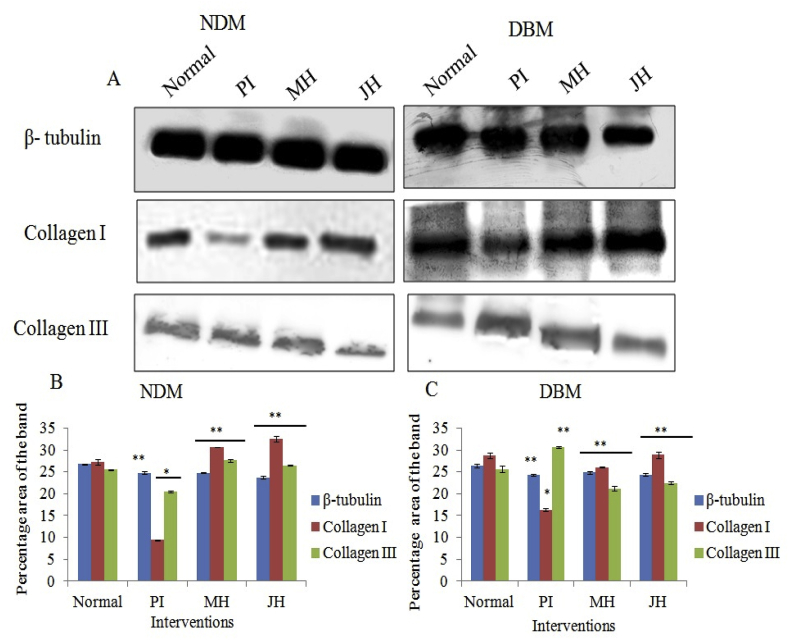
Further, dermal repair of major collagens i.e. collagen I and III were determined via IHC analysis. Fig. 8 A & 10A are the representative image of collagen I and III expression of healed wound at day 15th day under interventions. The box plot observation of grey scale intensity for NDM and DBM illustrated non-significant (p ≥ 0.05) alteration in collagen I expression for honey treated group and a significant (p ≤ 0.05) decrease in collagen I expression for PI treated group in comparison to normal counterpart (Fig. 8B). These findings were supported by western blot analysis where, MH and JH treated wound showed non-significant (**p ≥ 0.05) change collagen I in comparison to normal skin (day 0). A significant (*p ≤ 0.05) decrease of collagen I expression was observed for PI treated wound (Fig. 8). Box plot (grey scale intensity) of collagen III expression showed non-significant (p ≥ 0.05) alteration for MH and JH treated wound and a significant (p ≤ 0.05) increase was found for PI treated wound in comparison to normal counterpart both for NDM and DBM (Fig. 9B). The western blot analysis was also corroborative with this observation (Fig. 10). The collagen I and III ratio analysis depicted that under honey intervention, the healed skin approached toward the normal skin collagen I/III ratio (Fig. 11).
Fig. 8.
Immuno-histochemical examination of collagen I expression under interventions (10x objective magnification): A. normal skin (i = NDM) and (v = DBM) at day 0; (ii-iv) PI, MH, JH treated wound under NDM group and (vi-viii) PI, MH, JH treated wound under DBM group for collagen I respectively. B. Notch box plot for grey scale intensity of collagen I expression, where normal skin vs PI treated wound showed significant (p ≤ 0.05) decrease (both for NDM and DBM) and JH vs MH treated wound of NDM and DBM showed non-significant (p ≥ 0.05) change.
Fig. 9.
Immuno-histochemical examination of collagen III expression under interventions (10x objective magnification): A. normal skin (i = NDM) and (v = DBM) at day 0; (ii-iv) PI, MH, JH treated wound under NDM group and (vi-viii) PI, MH, JH treated wound under DBM group for collagen III respectively. B. Notch box plot for grey scale intensity of collagen III expression, where PI treated wound showed significant (p ≤ 0.05) increase in comparison to normal skin (both for NDM and DBM) and JH vs MH treated wound of NDM and DBM showed non-significant (p ≥ 0.05) change.
Fig. 10.
Collagen I and III ratio of grey scale intensity under each intervention.
Fig. 11.
Western blot study of collagen I & III of healed wound under (NDM & DBM) interventions: A. Representative western blot of β-tubulin, collagen I & III expression, & C. Graph representing semi-quantitative analysis of target proteins using percentage area of band for NDM (B) and DBM (C) (**p > 0.05) (*p ≤ 0.05).
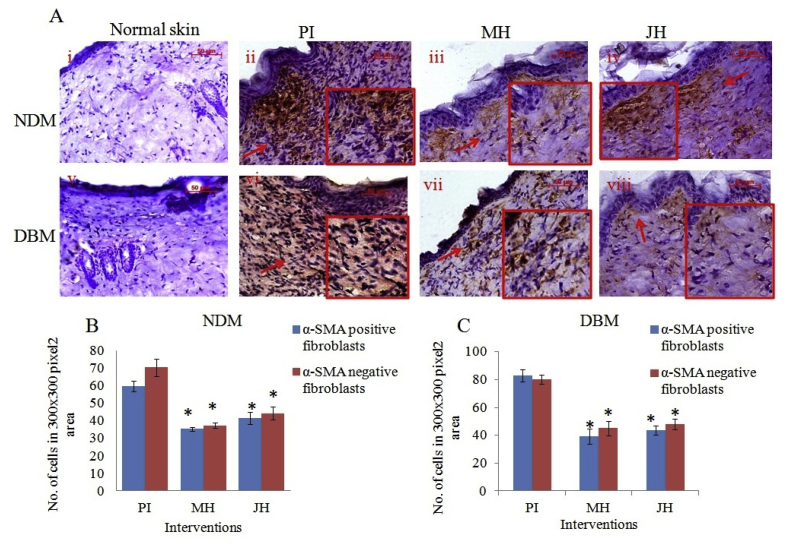
From Fig. 12 A three different areas (300 × 300 pixel2) were selected to count α-SMA (a marker of myofibroblast) positive and negative fibroblasts cells. The α-SMA positive fibroblast cells were decreased significantly (*p ≤ 0.05) under JH and MH treated wound in comparison to PI treated both for NDM and DBM which indicated gradual disappearance myofibroblasts (Fig. 12 B and C).
Fig. 12.
Immuno-histochemical examination of α-SMA expression under interventions (10x objective magnification): A. normal skin (i = NDM) and (v = DBM) at day 0; (ii-iv) PI, MH, JH treated wound under NDM group and (vi-viii) PI, MH, JH treated wound under DBM group respectively, Graph representing α-SMA positive and negative cell count in 300 × 300 pixel2 area of NDM (B) and DBM (C) (*p ≤ 0.05).
3.5. Angiogenesis under topical application of JH on diabetic wound model
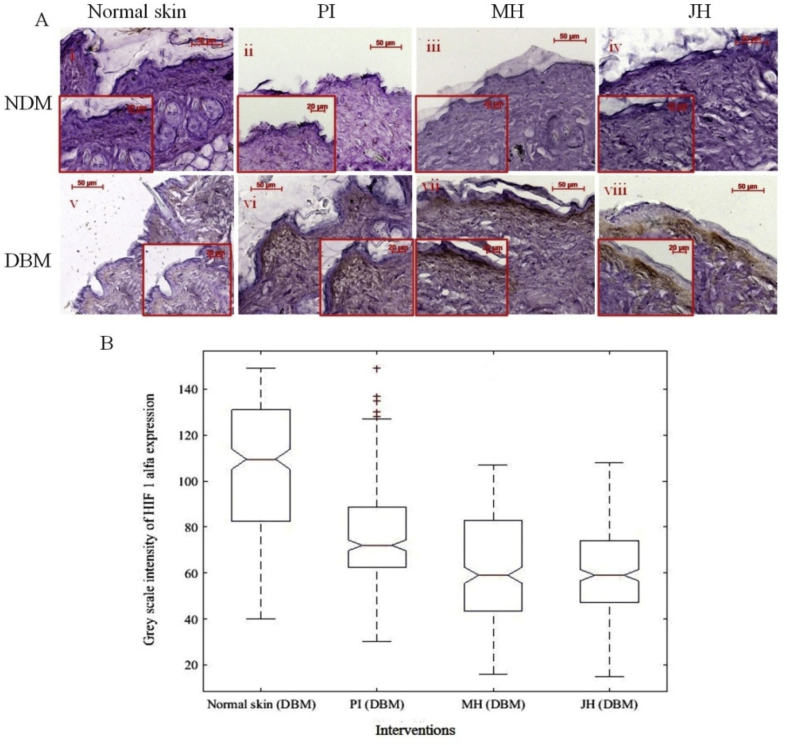
In our study we found that HIF-1α expression intensity and distribution were substantially increased in basement membrane of the post intervention samples. Whereas, in normal diabetic skin tissue expression level was comparably lower than post interventions, indicated the absence of chronic ambience (Fig. 13A). The IHC analysis (via notch boxplot) for HIF-1α was found to be significantly (p < 0.05) low in diabetic skin (before wound) in comparison to honey (MH and JH) interventions (Fig. 13 B). HIF-1α also associated with VEGF which is an essential mediator of neovascularization during healing process.15,16
Fig. 13.
Immuno-histochemical examination of HIF 1α expression under interventions (10x objective magnification): A. normal skin (i = NDM) and (v = DBM) at day 0; (ii-iv) PI, MH, JH treated wound under NDM group and (vi-viii) PI, MH, JH treated wound under DBM group respectively, Notch box plot for grey scale intensity of HIF 1α expression for DBM (B) p ≤ 0.05 was considered to be significant increase for normal skin vs treatments (PI,MH,JH).
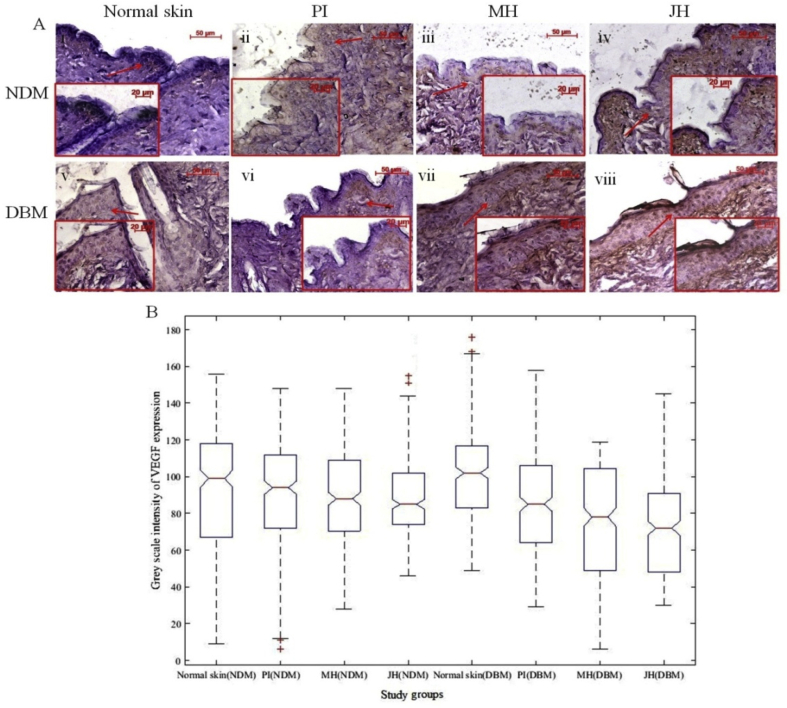
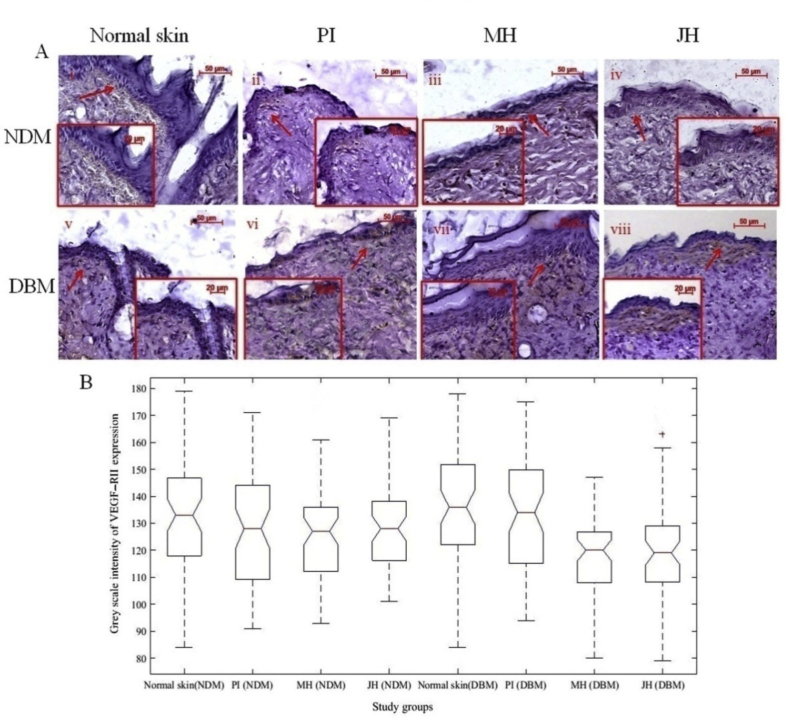
The transactivation of HIF-1α reduces under hyperglycemic condition which was indicated by down regulation of HIF-1α in diabetic skin. The surplus reactive oxygen species (ROS) generation and oxidative stress contributed by high glucose ambience which causes impairment of HIF-1α regulation in diabetes.31 Several studies were reported about the relevance of various hydroxylase inhibitors viz. DMOG, DFX, etc. which have antioxidant activity to attenuate and to stimulate HIF-1 α in diabetic wounds.15 In the present study, antioxidant components of JH and their radical scavenging activity facilitated the structural and functional regulation of HIF-1α in diabetic wound ambience. Further, the reduced transactivation of HIF-1α under hyperglycemic condition resulted into inhibition of prime angiogenic factors like VEGF and VEGFR-II. In normal skin of DBM, expression of VEGF was considerably low and mostly present in the basal region whereas after honey treatment, expression level noticeably increased (Fig. 14 A and B). Expression of VEGFR-II was also augmented in honey treated wound which was less in normal diabetic skin (Fig. 15A and B).
Fig. 14.
Immuno-histochemical examination of VEGF expression under intervention s (10x objective magnification): A. normal skin (i = NDM) and (v = DBM) at day 0; (ii-iv) PI, MH, JH treated wound under NDM group and (vi-viii) PI, MH, JH treated wound under DBM group respectively, Notch box plot for grey scale intensity of VEGF expression (B) where treated wound (PI, MH & JH) showed significant (p ≤ 0.05) increase (both for NDM & DBM) in comparison to normal counterpart and JH vs MH treated wound of NDM and DBM showed non-significant (p ≥ 0.05) change.
Fig. 15.
Immuno-histochemical examination of VEGF R–II expression under interventions (10x objective magnification): A. normal skin (i = NDM) and (v = DBM) at day 0; (ii-iv) PI, MH, JH treated wound under NDM group and (vi-viii) PI, MH, JH treated wound under DBM group respectively, Notch box plot for grey scale intensity of VEGF RII expression (B) where treated wound (PI, MH & JH) showed significant (p ≤ 0.05) increase in comparison to normal counterpart (both for NDM & DBM) and JH vs MH treated wound of NDM and DBM showed non-significant (p ≥ 0.05) change.
4. Discussion
The normal wound healing process involves sequential, synchronized and self-resolving steps whereas these processes are prolong and become ineffective in diabetes.32 In normal wound healing process collagen remodeling, degradation and scar maturation occur in organized way but in diabetic condition due to hyperglycemia glycation of collagen, loss of conformity, and resistance to enzymatic degradation lead to alteration of healing homeostasis33,34. Despite of meaningful progress in diabetic treatment, management of diabetic chronic wounds is still challenging. Nowadays researchers are developing polyphenolics based novel polyherbal formulations to overcome the hyperglycemic condition in diabetes35,36. Polyphenolics are naturally present in honey which reduces the ROS and assist wound healing especially for the diabetic one37,38. The Ayurvedic texts cited the goodness of Honey (Madhu) because of its sweetness, astringent, healing and anti-inflammatory potentials39,40. But very few studies have been tried to decipher the molecular mechanism behind the significance of this ancient therapeutic agent in wound healing specially for collagen modification and angiogenesis38,41.
Thus, present study elucidates the efficacy of JH for diabetic healing and its molecular mechanism particularly how JH modulates the inflammation, hypoxia, re-epithelialization and sub-epithelial connective tissue arrangement like collagen formation and angiogenesis. The reason for using JH was based on effective anti-oxidative properties and traditionally Jamun (Syzygium cumini) was also proven to have important therapeutic effects for diabetic patients24,36,42. Further, the rationales of using MH as standard was based on various scientific evidences which proved its antioxidant, anti-inflammatory, diabetic wound healing as well as broad spectrum antibacterial property.43
Though in vitro wound models are insufficient to represent in vivo situations, but in the context of restitution of wound gap which involve migration, proliferation and conversion of dermal fibroblasts to myofibroblasts it may become contributory. Myofibroblasts are characterized by expression of α-smooth muscle actin (α-SMA) within cytoplasmic stress fibers. In our previous study we have investigated the mechanism of action of JH for cell proliferation and migration in normoxic and hypoxic conditions though expression analysis of E-cadherin, cytoskeletal protein F-actin, p63 and hypoxia marker HIF 1α 25. Further, it was also reported that Manuka honey activates murine fibroblasts via p38 signaling pathway.30 To investigate the mechanism underlying healing effect through cellular migration, we performed scratch wound assay, migration assay and ICC for α-SMA expressions. Interestingly, impact of JH dilution (0.1% v/v) on in vitro wound healing of primary fibroblast from NDM and DBM skin, were comparable to same dilution of MH in terms of wound closure, migration and α-SMA expressions (Fig. 1, Fig. 2, Fig. 3, Fig. 4). These observations established the chemotactic activity of honey dilutions to stimulate fibroblasts (NDM/DBM) cells under mechanical wounding.
Based on in vitro findings we evaluated wound healing potential of JH in in vivo diabetic model where MH and JH treated wound fractions were comparable at day 5, 10 and 15 both in DBM and NDM. Topical application of JH and MH both were successful in treating wounds for NDM and DBM, the success of honey (JH and MH) treated wounds were higher (*p < 0.05) than PI as per wound contraction analysis (SF2 & Fig. 5) and re-epithelialization area measurement (Fig. 6). The semi-quantitative analysis of VG stain showed comparable intensity trend (P1, P2 and P3 from basal layer) for honey treated wounds and normal skin both for NDM and DBM (Fig. 7 and Table 1). Further, a non-significant (**p ≥ 0.05) alteration in collagen I and III expression under honey (JH and MH) treated wounds in comparison normal skin (day 0) validated the reformation of collagen. As discussed above that altered biomechanical properties of collagen fibers in diabetes are due to uncontrolled cross-linking, glycation and refusal of enzymatic digestion. In this context, Topham, J. (2002) mentioned the importance of honey preparations on wound bed to persuade wound-healing proteoglycans along with modest quantities of collagen production.44 Honey also contains ascorbic acid to influence the hydroxylation of proline and lysine in order to produce nascent collagen.45 During healing of an open wound fibroblastic cells convert into myofibroblasts under guidance of ECM and synthesize collagen types I and III. The modulation of fibroblasts to myofibroblasts has an important role during wound healing such as collagen synthesis however, myofibroblasts count recede by apoptosis after re-epithelilization.46 In our in vivo study we found that α-SMA positive fibroblast significantly (*p ≤ 0.05) reduced under honey intervention (Fig. 12 B and C) indicated the apoptotic reduction of extra myofibroblasts. The controlled formation, existence and apoptotic reduction of extra myofibroblasts are imperative for normal wound healing process.47
The skin injury induces inflammatory cells, platelets, fibroblasts and injured endothelial cells secrete angiogenic mediators with growth factors to form new blood vessels in granulation tissue.15,16 Further, wound generated hypoxia also induces angiogenic growth factors via hypoxia inducible factor (HIF 1 α). However, angiogenesis is decreased in diabetic wound with reduction in inflammatory cells and growth factors.48 In our study we found modulation of important angiogenic markers (viz HIF-1α, VEGF, VEGF R–II) which actually inhibited in hyperglycemic ambience in diabetic wounds, demonstrated the pro-angiogenic potential of JH (Fig. 13, Fig. 14, Fig. 15). The sugar components present in honey (viz. fructose and oligosaccharides) along with antioxidants have synergistic effect to mitigate hyperglycemic effect. In this regard, Erejuwa O.O. (2012) also mentioned the hypo-glycemic effect of sugar components particularly fructose and oligosaccharides.49 We can say that honey is a complex system where every component plays a specific role in each step of wound healing. Therefore, it was concluded that topical application of JH on hyperglycemic diabetic wounds has a positive impact on wound contraction, re-epithelialization, collagen modification and angiogenesis events (Fig. 16).
Fig. 16.
Proposed hypothesis for mechanism of action of Jamun honey (JH) under diabetic wound.
5. Conclusions
Considering the efficacy of honey as an alternative medicine for diabetic wounds, this study depicted the healing potential of Jamun honey (physicochemically characterized) in in vitro fibroblast wound and in vivo diabetic mice (streptozotocin induced) model. The wound healing potential of Jamun honey was as good as Manuka honey (medical grade honey) considering wound closure, reepithelialization, collagen I/III and α-SMA expressions. The chemotactic activities and pro-angiogenic potential of Jamun honey established it’s efficacy as a therapeutic natural agent for diabetic wound healing. This study will also inspire future researchers to decipher its molecular system level mechanism of the traditionally acclaimed healing potential of honey.
Declaration of competing interest
The authors declare that there are no conflicts of interests associated with this publication.
Acknowledgement
This work was financially supported by the Ministry of Human Resource Development, Government of India, New Delhi, India (IIT/SRIC/SMST/NIC/2013-14/228, dated 16-04-2014) and the University Grant Commission, New Delhi, India (ref: F. No.19-6/2011(i) EU-IV, dated November 30, 2011).
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jtcme.2019.10.002.
Contributor Information
Amrita Chaudhary, Email: chaudharybt8@gmail.com.
Jyotirmoy Chatterjee, Email: jchatterjee@smst.iitkgp.ac.in.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Leoni G., Neumann P., Sumagin R., Denning T.L., Nusrat A. Wound repair: role of immune–epithelial interactions. Mucosal Immunol. 2015;8:959–968. doi: 10.1038/mi.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Falanga V. Wound healing and its impairment in the diabetic foot. The Lancet. 2005;366:1736–1743. doi: 10.1016/S0140-6736(05)67700-8. [DOI] [PubMed] [Google Scholar]
- 3.Kolluru G.K., Bir S.C., Kevil C.G. Endothelial dysfunction and diabetes: effects on angiogenesis, vascular remodeling, and wound healing. Int J Vasc Med. 2012;2012:918267. doi: 10.1155/2012/918267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ning J., Zhao H., Chen B., Mi E.Z., Yang Z., Qing W. Argon mitigates impaired wound healing process and enhances wound healing in vitro and in vivo. Theranos. 2019;9:477. doi: 10.7150/thno.29361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maharlooei M.K., Bagheri M., Solhjou Z., Jahromi B.M., Akrami M., Rohani L. Adipose tissue derived mesenchymal stem cell (AD-MSC) promotes skin wound healing in diabetic rats. Diabetes Res Clin Pract. 2011;93:228–234. doi: 10.1016/j.diabres.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 6.Witte M.B., Thornton F.J., Tantry U., Barbul A. L-Arginine supplementation enhances diabetic wound healing: involvement of the nitric oxide synthase and arginase pathways. Metabol. 2002;51:1269–1273. doi: 10.1053/meta.2002.35185. [DOI] [PubMed] [Google Scholar]
- 7.Diegelmann R.F., Evans M.C. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci. 2004;9:283–289. doi: 10.2741/1184. [DOI] [PubMed] [Google Scholar]
- 8.Xue M., Jackson C.J. Extracellular matrix reorganization during wound healing and its impact on abnormal scarring. Adv Wound Care. 2015;4:119–136. doi: 10.1089/wound.2013.0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Darby I.A., Laverdet B., Bonté F., Desmoulière A. Fibroblasts and myofibroblasts in wound healing. Clin Cosmet Investig Dermatol. 2014;7:301. doi: 10.2147/CCID.S50046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hinz B., Celetta G., Tomasek J.J., Gabbiani G., Chaponnier C. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol Biol Cell. 2001;12:2730–2741. doi: 10.1091/mbc.12.9.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Germain L., Jean A., Auger F.A., Garrel D.R. Human wound healing fibroblasts have greater contractile properties than dermal fibroblasts. J Surg Res. 1994;57:268–273. doi: 10.1006/jsre.1994.1143. [DOI] [PubMed] [Google Scholar]
- 12.Welch M.P., Odland G.F., Clark R. Temporal relationships of F-actin bundle formation, collagen and fibronectin matrix assembly, and fibronectin receptor expression to wound contraction. J Cell Biol. 1990;110:133–145. doi: 10.1083/jcb.110.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maeda M., Johnson K.R., Wheelock M.J. Cadherin switching: essential for behavioral but not morphological changes during an epithelium-to-mesenchyme transition. J Cell Sci. 2005;118:873–887. doi: 10.1242/jcs.01634. [DOI] [PubMed] [Google Scholar]
- 14.Raymond M., Marchbank T., Moyer M.P., Playford R.J., Sanderson I.R., Kruidenier L. IL-1β stimulation of CCD-18co myofibroblasts enhances repair of epithelial monolayers through Wnt-5a. Am J Physiol Gastrointest Liver Physiol. 2012;303:G1270–G1278. doi: 10.1152/ajpgi.00458.2011. [DOI] [PubMed] [Google Scholar]
- 15.Botusan I.R., Sunkari V.G., Savu O. Stabilization of HIF-1α is critical to improve wound healing in diabetic mice. Proc Natl Acad Sci. 2008;105:19426–19431. doi: 10.1073/pnas.0805230105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Covello K.L., Simon M.C. HIFs, hypoxia, and vascular development. Curr Top Dev Biol. 2004;62:37–54. doi: 10.1016/S0070-2153(04)62002-3. [DOI] [PubMed] [Google Scholar]
- 17.Baraka A.M., Guemei A., Gawad H.A. Role of modulation of vascular endothelial growth factor and tumor necrosis factor-alpha in gastric ulcer healing in diabetic rats. Biochem Pharmacol. 2010;79:1634–1639. doi: 10.1016/j.bcp.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Dehghan M.H., Mirmiranpour H., Faghihi-Kashani S. Inhibitory effect of curcumin on angiogenesis in a streptozotocin-induced diabetic rat model: an aortic ring assay. J Tradit Complement Med. 2016;6:437–441. doi: 10.1016/j.jtcme.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olsson A.K., Dimberg A., Kreuger J. VEGF receptor signalling--in control of vascular function. Nat Rev Mol Cell Biol. 2006 May;7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 20.Aziz C.B.A., Ismail C.A.N., Hussin C.M.C. The antinociceptive effects of Tualang honey in male Sprague-Dawley rats: a preliminary study. J Tradit Complement Med. 2014;4:298–302. doi: 10.4103/2225-4110.139115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pereira R.F., Bartolo P.J. Traditional therapies for skin wound healing. Adv Wound Care. 2016;5:208–229. doi: 10.1089/wound.2013.0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tandara A.A., Mustoe T.A. Oxygen in wound healing—more than a nutrient. World J Surg. 2004;28:294–300. doi: 10.1007/s00268-003-7400-2. [DOI] [PubMed] [Google Scholar]
- 23.Brosius F. 2011. Low Dose Streptozotocin Induction Protocol (Mouse) [Google Scholar]
- 24.Seluanov A., Vaidya A., Gorbunova V. Establishing primary adult fibroblast cultures from rodents. JoVE (Journal of Visualized Experiments) 2010 doi: 10.3791/2033. e2033-e2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chaudhary A., Bag S., Barui A., Barui A., Banerjee P., Chatterjee J. Honey dilution impact on in vitro wound healing: normoxic and hypoxic condition. Wound Repair Regen. 2015;23:412–422. doi: 10.1111/wrr.12297. [DOI] [PubMed] [Google Scholar]
- 26.Chaudhary A., Bag S., Banerjee P., Chatterjee J. Honey extracted polyphenolics reduce experimental hypoxia in human keratinocytes culture. J Agric Food Chem. 2017;65:3460–3473. doi: 10.1021/acs.jafc.7b00366. [DOI] [PubMed] [Google Scholar]
- 27.Romana-Souza B., Nascimento A.P., Monte-Alto-Costa A. Propranolol improves cutaneous wound healing in streptozotocin-induced diabetic rats. Eur J Pharmacol. 2009;611:77–84. doi: 10.1016/j.ejphar.2009.03.053. [DOI] [PubMed] [Google Scholar]
- 28.Shi H.P., Most D., Efron D.T., Witte M.B., Barbul A. Supplemental L-arginine enhances wound healing in diabetic rats. Wound Repair Regen. 2003;11:198–203. doi: 10.1046/j.1524-475x.2003.11308.x. [DOI] [PubMed] [Google Scholar]
- 29.Schäffer M.R., Tantry U., Efron P.A., Ahrendt G.M., Thornton F.J., Barbul A. Diabetes-impaired healing and reduced wound nitric oxide synthesis: a possible pathophysiologic correlation. Surgery. 1997;121:513–519. doi: 10.1016/s0039-6060(97)90105-7. [DOI] [PubMed] [Google Scholar]
- 30.Ranzato E., Martinotti S., Burlando B. Honey exposure stimulates wound repair of human dermal fibroblasts. Burns Trauma. 2013;1:32. doi: 10.4103/2321-3868.113333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barui A., Banerjee P., Chaudhary A., Conjeti Sailesh, Mondal Bikash, Dey Susmita. Evaluation of angiogenesis in diabetic lower limb wound healing using a natural medicine: a quantitative approach. Wound Med. 2014;6:26–33. [Google Scholar]
- 32.Hwang D.J., Lee K.M., Park M.S. Association between diabetic foot ulcer and diabetic retinopathy. PLoS One. 2017;12 doi: 10.1371/journal.pone.0175270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aronson D., Rayfield E.J. How hyperglycemia promotes atherosclerosis: molecular mechanisms. Cardiovasc Diabetol. 2002;1:1. doi: 10.1186/1475-2840-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Snedeker J.G., Gautieri A. The role of collagen crosslinks in ageing and diabetes-the good, the bad, and the ugly. Muscles Ligaments Tendons J. 2014;4:303. [PMC free article] [PubMed] [Google Scholar]
- 35.Dinakaran S.K., Chelle S., Avasarala H. Profiling and determination of phenolic compounds in poly herbal formulations and their comparative evaluation. J Tradit Complement Med. 2019;9:319–327. doi: 10.1016/j.jtcme.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghorbani A. Clinical and experimental studies on polyherbal formulations for diabetes: current status and future prospective. J Integr Med. 2014;12:336–345. doi: 10.1016/S2095-4964(14)60031-5. [DOI] [PubMed] [Google Scholar]
- 37.Alam F., Islam M.A., Gan S.H., Khalil M.I. Honey: a potential therapeutic agent for managing diabetic wounds. Evid Based Complement Alternat Med. 2014;2014:169130. doi: 10.1155/2014/169130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarkar S., Chaudhary A., Saha T.K., Amit Kumar Das, Chatterjee Jyotirmoy. Modulation of collagen population under honey assisted wound healing in diabetic rat model. Wound Med. 2018;20:7–17. [Google Scholar]
- 39.Shamshuddin N.S.S., Zohdi R.M. Gelam honey attenuates ovalbumin-induced airway inflammation in a mice model of allergic asthma. J Tradit Complement Med. 2018;8(1):39–45. doi: 10.1016/j.jtcme.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chaudhary A., Bag S., Mandal M., Krishna Karri S.P., Barui A., Rajput M. Modulating prime molecular expressions and in vitro wound healing rate in keratinocyte (HaCaT) population under characteristic honey dilutions. J Ethnopharmacol. 2015;166:211–219. doi: 10.1016/j.jep.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 41.Ediriweera E., Premarathna N. Medicinal and cosmetic uses of bee’s honey–A review. Ayu. 2012;33:178. doi: 10.4103/0974-8520.105233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Srivastava S., Chandra D. Pharmacological potentials of Syzygium cumini: a review. J Sci Food Agric. 2013;93:2084–2093. doi: 10.1002/jsfa.6111. [DOI] [PubMed] [Google Scholar]
- 43.Alam F., Islam M.A., Gan S.H., Khalil M.I. Honey: a potential therapeutic agent for managing diabetic wounds. Evid Based Complement Alternat Med. 2014;2014:169130. doi: 10.1155/2014/169130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Topham J. Why do some cavity wounds treated with honey or sugar paste heal without scarring? J Wound Care. 2002;11:53–58. doi: 10.12968/jowc.2002.11.2.26372. [DOI] [PubMed] [Google Scholar]
- 45.Sarkar S., Mukhopadhyay A., Chaudhary A. Therapeutic interfaces of honey in diabetic wound pathology. Wound Med. 2017;18:21–32. [Google Scholar]
- 46.Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol. 2003;200:500–503. doi: 10.1002/path.1427. [DOI] [PubMed] [Google Scholar]
- 47.Goldberg M.T., Han Y.P., Yan C., Michael C.S., Warren L.G. TNF-α suppresses α-smooth muscle actin expression in human dermal fibroblasts: an implication for abnormal wound healing. J Investig Dermatol. 2007;127:2645–2655. doi: 10.1038/sj.jid.5700890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baraka A.M., Guemei A., Gawad H.A. Role of modulation of vascular endothelial growth factor and tumor necrosis factor-alpha in gastric ulcer healing in diabetic rats. Biochem Pharmacol. 2010;79:1634–1639. doi: 10.1016/j.bcp.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 49.Erejuwa O.O., Sulaiman S.A., Wahab M.S.A. Fructose might contribute to the hypoglycemic effect of honey. Molecules. 2012;17:1900–1915. doi: 10.3390/molecules17021900. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.