Abstract
Aim and Objectives
To describe the prevalence and characteristics of olfactory dysfunction (OD) in patients with laboratory-confirmed COVID-19 infection.
Materials and Methods
This monocentric study was performed at Chest Diseases Hospital during the COVID-19 pandemic and all patients testing positive for COVID-19 over a 5-month period (April to August 2020) were recruited. Detailed history was elicited from subjects and all patients were inquired about olfactory dysfunction (OD). Patients with olfactory dysfunction were asked to complete olfactory questionnaires based on the short version of the Questionnaire of Olfactory Disorders-Negative Statements (sQOD-NS).
Results
655 patients with mild to moderate COVID-19 infection were included in the study. The prevalence rate of olfactory dysfunction was 18.47% (n = 121) with contribution of 11.60% (n = 76) and 6.87% (n = 45) from anosmia and hyposmia respectively, thereby suggesting olfactory dysfunction to be a significant clinical feature in COVID-19 patients. Males were significantly more affected by olfactory dysfunctions than females. Anosmic patients had significantly reduced sQOD-NS results as compared to hyposmic patients (significant at P < 0.05). The mean duration of OD was 7.7 days (± 4.3) and >90% patients in our study showed resolution within 14 days.
Conclusion
The early recognition of olfactory dysfunction should help to screen, identify and thereby quickly isolate mildly symptomatic COVID-19 patients from the general population and the existence of these dysfunctions may well be a prognostic factor in the course of the disease.
Keywords: Coronavirus, COVID-19, SARS-CoV-2, Olfactory dysfunction, Anosmia, Hyposmia, sQOD-NS, Kashmir
Introduction
Coronavirus disease 2019 (COVID-19) is caused by a newly discovered coronavirus. The virus is now known as the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). This current viral pandemic started from Wuhan, China, in December 2019 and has spread exponentially to the rest of the world [1]. This is the third novel coronavirus in last 17 years and phylogenetically it is closely related to bat-derived SARS like coronaviruses [2].
SARS-CoV-2 virus utilizes spiny protein S1, which is responsible for making the virion adhere to cell membrane and the host ACE2 receptor [3]. ACE2 is a functional receptor for SARS-CoV-2, and its nervous system expression and distribution indicates that SARSCoV-2 can induce neurological manifestations via direct or indirect pathways [4]. Because of the unusual morphology of the olfactory system, including the olfactory bulb and the olfactory nerve, viruses may also contribute through the cribriform plate to central nervous system infections [5, 6].
As of August 16, 2020, there were 21,294,845 confirmed cases of COVID-19 and 761,779 deaths globally [7]. COVID-19 has been reported to spread among humans across multiple sources, such as droplets, aerosols, feces and oral mucous membranes [8].
In light of the increasing number of true positives and the paucity of test kits and facilities, particularly in developing countries, it is of paramount importance to gauge the number of diagnostic symptoms of this disease in order to make a decision on self-isolation and to prevent infection transmission.
Many studies have documented common clinical symptoms of COVID-19 including fever, cough, diarrhea and fatigue [1]. In comparison, some patients may experience upper respiratory symptoms such as pharyngodynia, sore throat, nasal obstruction, rhinorrhea and alterations in olfaction [9, 10]. Viral pneumonia leading to acute respiratory distress syndrome (ARDS) and even death has been observed in more severe cases [10].
There is steadily growing observational evidence that olfactory disorders are prevalent symptoms in COVID-19 patients, in absence of rhinorrhea and nasal obstruction and can be presenting symptoms before the appearance of other symptoms such as cough and fever. Related studies come from various countries worldwide [11]. Although post viral olfactory dysfunction is common observation in ENT practice and viruses like rhinovirus, parainfluenza, Epstein-Barr virus and some coronavirus have been implicated to cause this via an inflammatory reaction of the nasal mucosa and the development of rhinorrhea, but the olfactory dysfunction that is observed in COVID-19 is usually not associated with rhinorrhea [12, 13].
The role of an otolaryngologist in COVID-19 pandemic is very crucial and, in many, otolaryngologist will be the first to evaluate COVID-19 positive patients. Since SARS-CoV-2 is most likely to be transmitted by contact and respiratory droplets (aerosols) [14], ENT examination is a high risk procedure to the healthcare provider over short distances (1.5 m).
Recent studies have shown that olfaction also affects the quality of life (QOL) [15–18] and olfactory impairment can lead to problems in diverse elements of QOL such as safety, hygiene, and nutrition. Since there have been a rapidly growing number of reports of a significant increase in the number of patients presenting with olfactory dysfunction in the absence of other symptoms, a comprehensive epidemiological review is the need of the hour to characterize olfactory disorders in infected patients.
The aim of this study is to describe the prevalence and characteristics of olfactory dysfunction (OD) in patients with laboratory-confirmed COVID-19 infection and this study is conducted to help the international community in better understanding this novel infectious disease.
Materials and Methods
This monocentric cross-sectional observational study was conducted at Chest Diseases hospital, an associated hospital of GMC Srinagar, J&K for duration of five month from April 2020 to august 2020. This is a designated hospital assigned by the Government of India to treat patients with COVID-19. This study is focused on admitted hospital patients that have been identified as having laboratory-confirmed 2019-nCoV infection. The study was reviewed and approved by the Ethical committee of our institution.
All COVID-19 positive patients were isolated with airborne and contact precautions in the designated hospital and attending staff wore personal protective equipment in accordance with the US Centers for Disease Control and Prevention guidelines [19]. Our study focused primarily on patients with mild to moderate COVID-19, described as patients without the need for intensive care. A written informed consent was obtained from all the patients enrolled in this study.
Inclusion Criteria
Individuals confirmed to have COVID-19 by SARS-CoV-2 real-time reverse transcriptase–polymerase chain reaction (RT-PCR).
Adult (>18 years).
Patients clinically stable and having mild to moderate symptoms and able to complete the questionnaire.
Exclusion Criteria
Suspected COVID-19 cases but not laboratory-confirmed.
Patients with history of olfactory dysfunctions before the study.
Patients with previous history of head trauma, acute or chronic rhinosinusitis, allergic rhinitis, dementia, malignancy or abnormal nasal anatomy.
Patients who are sick and admitted in the intensive care unit at the time of the study.
During our study period, 963 COVID-19 patients were tested positive, out of which 655 were enrolled in this study. The study was performed in accordance with the principles of the Declaration of Helsinki.
Diagnosis was confirmed by real-time PCR (RT-PCR) on nasopharyngeal and oropharyngeal swabs which were placed into a collection tube containing preservation solution for the virus.
Radiologic assessments included chest and head CT and all laboratory testing (a complete blood cell count, blood chemical analysis, coagulation testing, assessment of liver and renal function testing, C-reactive protein, creatine kinase, and lactate dehydrogenase) were performed according to the clinical needs of the patient.
Clinical and Olfactory Assessment
Clinical data was prospectively collected during the ear, nose, and throat (ENT) consultation in the isolation ward of the designated hospital. The demographic and clinical variables studied included: age, sex, smoking history, comorbidities, olfaction, general and otolaryngological symptoms. All patients were asked to complete olfactory questionnaires based on the short version of the Questionnaire of Olfactory Disorders-Negative Statements (sQOD-NS). The impact of olfactory dysfunction, if present, on the quality of life (QoL) of patients was assessed through this validated sQOD-NS. This is a 7-item patient reported outcome questionnaire and each item is rated on a scale of 0–3, with higher scores reflecting better olfactory-specific QoL. The total score ranges from 0 (severe impact on QoL) to 21 (no impact on QoL) [20]. The study wasn’t carried out with the intention to check out the association between olfactory and gustatory dysfunctions in COVID-19 positive subjects.
Statistical Methods
Data was entered in a Microsoft Excel spreadsheet and IBM-SPSS statistical software version 22 was used for analyzing the data. Continuous variables were summarized as mean and standard deviation. Chi-Square test was used to compare between categorially variables. Independent-Samples T-test was used for the comparison of the means. Two-tailed p-values were reported and a p-value < 0.05 was considered statistically significant.
Results
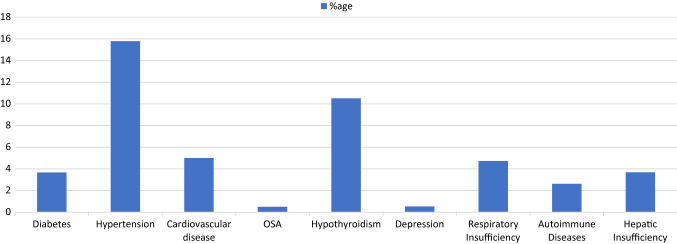
The study was completed by a total of 655 patients. The mean age was 32.7 ± 10.1 years with a range of 19–85 years. In our study, 63.20% patients (n = 414) were males and 36.80% (n = 241) were females (Table 1). Hypertension (15.79%) and hypothyroidism (10.52%) were the most frequent comorbidities in patients. Other comorbidities were less frequent (Fig. 1).
Table 1.
Gender wise distribution of cases
| Gender | Number | Percentage (%) |
|---|---|---|
| Male | 414 | 63.20 |
| Female | 241 | 36.80 |
| Total | 655 | 100 |
Fig. 1.
Comorbidities associated with COVID-19 positive cases
The majority of the study subjects had no history of smoking (74.05%). The prevalence rate of olfactory dysfunction was 18.47% with contribution of 11.60% and 6.87% from anosmia and hyposmia respectively (Table 2). There was a statistically significant difference between the 2 patient groups (with and without olfactory dysfunction) with respect to the following variables: sex and smoking history (Tables 3 and 4). The relative effect of olfactory dysfunction on men and smokers was greater as compared to women and non-smokers (significant at P < 0.05).
Table 2.
Distribution of cases with COVID-19 disease
| Covid-19 cases | Number | Percentage (%) | |
|---|---|---|---|
| Positive cases with olfactory dysfunction (OD) | Anosmia | 76 | 11.60 |
| Hyposmia | 45 | 6.87 | |
| Total | 121 | 18.47 | |
| Positive cases without olfactory dysfunction (OD) | 534 | 81.53 | |
| Total | 655 | 100 | |
Table 3.
Relationship between gender and olfactory dysfunction in COVID-19 cases
| Gender | With olfactory dysfunction | Without olfactory dysfunction | Total | p-value | |||
|---|---|---|---|---|---|---|---|
| No. | Age (%) | No. | Age (%) | No. | Age (%) | ||
| Males | 86 | 20.77 | 328 | 79.22 | 414 | 63.20 | <0.05 |
| Females | 35 | 14.52 | 206 | 85.48 | 241 | 36.80 | |
| Total | 121 | 18.47 | 534 | 81.53 | 655 | 100 | |
Table 4.
Relationship between smoking and olfactory dysfunction in COVID-19 cases
| Smoker | With olfactory dysfunction | Without olfactory dysfunction | Total | p-value | |||
|---|---|---|---|---|---|---|---|
| No. | Age (%) | No. | Age (%) | No. | Age (%) | ||
| Yes | 97 | 57.05 | 73 | 42.95 | 170 | 25.95 | <0.05 |
| No | 24 | 4.95 | 461 | 95.05 | 485 | 74.05 | |
| Total | 121 | 18.47 | 534 | 81.53 | 655 | 100 | |
Clinical Outcomes
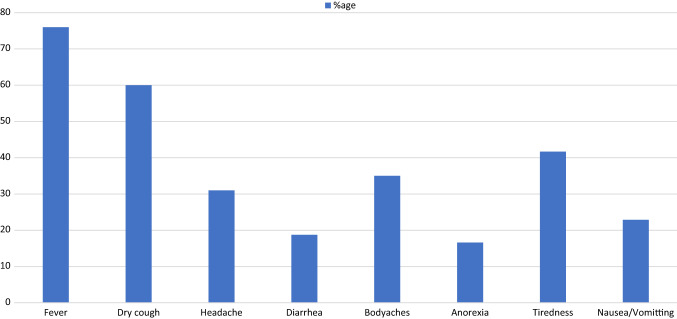
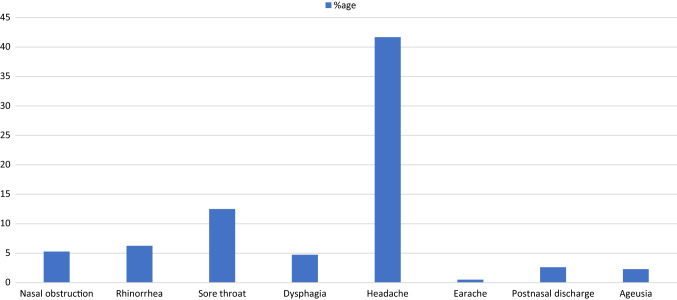
The general symptoms reported by the patients during the course of infection are illustrated in Fig. 2. The most common symptoms were fever (76%), dry cough (60%), tiredness (41.7%), bodyaches (35%), headache (31%), nausea/vomiting (22.9%), diarrhea (18.76%) and anorexia (16.6%). The otolaryngological symptoms most associated with the infection appear in Fig. 3.
Fig. 2.
General symptoms associated with COVID-19 infection
Fig. 3.
Otolaryngological symptoms associated with COVID-19 infection
The most common otolaryngological symptom observed in COVID-19 positive subjects was headache (41.7%) and sore throat (12.5%). Rest of ENT symptoms were less frequent (Fig. 3).
Olfactory Analysis
A total of 121 study participants (18.47%) had olfactory dysfunction consistent with the COVID-19 infection. Of which, 76 patients (11.60%) were anosmic, and 45 (6.87%) were hyposmic. In 14.29% patient’s olfactory dysfunction evolved prior to appearance of general/otolaryngologic symptoms whereas in 54.29% and 31.42% cases OD was noted at the same time or after the advent of general/otolaryngologic symptoms respectively. The mean duration of OD was 7.7 days (± 4.3), and >90% patients in our study showed resolution within 14 days. The result of olfactory dysfunction on patient QOL is reported in Table 5. Anosmic patients had significantly reduced sQOD-NS results as compared to hyposmic patients (significant at P < 0.05).
Table 5.
Short version of Questionnaire of Olfactory Disorders-Negative Statements of patient (sQOD-NS)
| Short version QOD-NS items | Anosmia | Hyposmia |
|---|---|---|
| The changes in my sense of smell make me feel isolated. | 1.81 ± 0.64 | 2.76 ± 0.42 |
| Because of the changes in my sense of smell, I have problems with taking part in activities of daily life | 1.40 ± 0.65 | 2.38 ± 0.62 |
| The changes in my sense of smell make me feel angry | 1.63 ± 0.64 | 2.38 ± 0.48 |
| Because of the changes in my sense of smell, I go to restaurants less often than I used to. | 1.90 ± 0.84 | 2.23 ± 0.69 |
| Because of the problems with my sense of smell, I eat less than before (loss of appetite) | 2.27 ± 0.44 | 2.46 ± 0.49 |
| Because of the changes in my sense of smell, I try harder to relax. | 1.59 ± 0.57 | 2.23 ± 0.79 |
| I am worried that I will never get used to the changes in my sense of smell. | 2.31 ± 0.46 | 2.38 ± 0.48 |
| Short version QOD-NS total score | 12.95 ± 2.01 | 16.84 ± 2.31 |
Discussion
Olfactory dysfunction (OD) as an indicator of COVID-19 infection has been reported by many studies since the onset of this pandemic. In recent months, a significant number of otolaryngologists have documented the concurrent symptoms of a sudden anosmia or hyposmia in COVID-19 positive cases. When working with OPD patients, otolaryngologists need to be diligent so that diagnosis of COVID 19 is not delayed and serious attention should be paid to inquiring about the loss of sense of smell in any suspected COVID-19 patient as coronaviruses have historically been associated with encephalitis and chronic demyelination [21]. Moreover, patients presenting with anosmia during COVID-19 pandemic should be ruled out for this viral disease.
The mean age of our population was 32.7 (± 10.1) years, and 63.20% were males. Patients with olfactory dysfunction (n = 121) seemed to be younger with a predominance of males and had fewer comorbidities. This finding was in contrast to the studies conducted earlier [22–28]. Males have greater predisposition to olfactory dysfunction because they have the higher likelihood of exposure to harmful agents. In addition, estrogen and progesterone might have favorable impacts in peripheral or central olfactory region stem cells, that could delay olfactory decline in women [29, 30]. Moreover, neural function has propensity to diminish more rapidly in men as compared to women [31, 32]. There is a need for future research to analyze the possible gender disparities in the development of olfactory dysfunction in COVID-19 positive patients.
The correlation between smoking and olfaction may be the consequence of the pro-inflammatory effect on the olfactory epithelium of the chemicals found in cigarette smoke [33]. Olfactory dysfunction has also been attributed to the effects of smoking on vascular system and brain [34]. Our research found low smoker prevalence (25.95%) in the study population. This finding was similar to study done by Speth et al. [24], who found a prevalence of 8.8%. However, in our study a statistically significant association was found between smoking and anosmia/hyposmia (significant at P < 0.05). The adverse effects of smoking on clinical outcome of COVID-19 patients has also been demonstrated by Kaye et al. [35].
In this study, 655 COVID-19 patients were examined for olfactory dysfunction, out of which 76 patients (11.60%) had anosmic symptoms and 45 subjects (6.87%) had hyposmia. In the present study, the prevalence of olfactory dysfunction was 18.47% in COVID-19 patients. Compared to Klopfenstein’s study the prevalence is lower as 47% of their patients reported anosmia [36]. Anosmia was also reported by Lechien et al. [22] in up to 86% of their patients and Paderno et al. [25] documented OD in 83% of their patients. Tong et al. in a meta-analysis review reported that the prevalence rate of the 10 investigations studying olfactory dysfunction in COVID-19 patients was 52.73% [37]. These impairments have been more frequently reported in European studies [24]. Nonetheless, the lower prevalence of olfactory dysfunction in our study population is consistent with findings from other Asian studies. Although only few studies on the prevalence of olfactory dysfunctions in Asia have been published so far, one of these studies by Mao et al. documented hyposmia in 5.1% of subjects [3]. Raid et al. reported that the prevalence rate of olfactory dysfunction was 24.82% [38] and Prasun et al. showed that the olfactory dysfunctions were seen in 14.8% of Indian patients [39].
The possible mechanism of difference in prevalence of olfactory dysfunction between European and Asian populations remains to be investigated at the moment, and many potential mechanisms have been hypothesized. First, the virus surface protein mutation, spike S protein, and nucleocapsid N protein, providing the virus with stability to prevent its entrance into the cell [40]. Second, genetic differences between ethnic groups and their role in viruses affecting particular systems. Third, SARS-CoV-2 affinity for tissues and potential human genetic characteristics [22]. Finally, difference in the degree of expression of ACE2 in various tissues which can affect the sensitivity and outcomes of COVID-19 infection [41]. Hence, even though prevalence of OD in our study sample is lower than that of European subjects, OD in COVID-19 cases does form a significant clinical feature.
Our patients had similar general symptoms as those documented in other studies [1, 8, 42–44], with fever and dry cough being the most common symptoms in our study population. Furthermore, we found that diarrhea was documented in less than 20% of patients, as was reported in study by Li X et al. [45].
In our study population, otolaryngological symptoms other than OD were particularly less prevalent compared to European cohorts. These findings are similar to study done by Chen et al. who reported rhinorrhea in 4% patients [44] and Guan et al. who reported nasal obstruction in 5% of its study subjects [1]. It is critical to discuss such differences between Asians and Europeans cohorts in future studies.
Post viral olfactory loss (POL) can be caused by various kinds of viruses like coronaviruses such as HCoV-229E [11]. In our study the mean duration of OD was 7.7 days (± 4.3), and >90% patients showed resolution within 14 days. These findings showing a fast recovery time are consistent with the studies by Kaye et al. [35] and Raid et al. [38]. In our study, the median onset of olfactory dysfunction was 3 days after infection onset.
Our study design included a questionnaire for evaluating olfaction-specific QOL in COVID-19 patients with olfactory dysfunction, and we used the short version of sQOD-NS for this purpose [20]. Anosmic patients had a substantially lower sQOD-NS score relative to hyposmic subjects (significant at P < 0.05) at the time of assessment. These findings correlated with the study of Lechien et al. [22].
There are various limitations to the present study. First, subjective olfaction tests such as the UPSIT and Sniffin Sticks tests were not employed in this study because of the contagious potential of this infection and to preserve the safety protocols established in our institution and we recommend a further research to use an objective evaluation of these olfactory variations. Second, our subset of patients consisted of mild to moderate COVID-19 patients with little comorbidity and they may not be indicative of the infected communities.
Having said that, our study is the main monocentric cohort of confirmed COVID-19 cases in Jammu and Kashmir and this study is the first to evaluate the prevalence of olfactory dysfunction in COVID-19 positive subjects in this region and the above mentioned limitations need to be considered in future studies for evaluating and characterizing olfactory functions in COVID-19 patients, as any addition to our insight will help us clinicians in early diagnosis and treatment of this disorder.
Conclusion
Olfactory Impairment’s significantly influence daily activities. The early recognition of olfactory dysfunction should help to screen, identify and thereby quickly isolate mildly symptomatic COVID-19 patients from the general population and the existence of these dysfunctions may well be a prognostic factor in the course of the disease. Current epidemiological evidence shows a relatively high prevalence of olfactory dysfunction in a wide range of populations. Therefore, we recommend that the international scientific community must consider new onset anosmia/hyposmia as significant symptoms of the COVID-19 infection and that primary physicians and otolaryngologists need to be vigilant of this ostensible presentation. This research provides evidence for using olfactory dysfunction as a symptom for increased screening of COVID-19 infections in an attempt to minimize the risk of disease transmission.
Acknowledgments
Conflict of interest
None declared.
Ethical Approval
The study was approved by the Institutional Ethics Committee.
Funding
No funding sources.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Naveed Nazir Shah, Email: naveedazirshah@yahoo.com.
Raj Tajamul Hussain, Email: raj.tajamul@gmail.com.
Bibliography
- 1.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11(7):995–998. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- 5.Koyuncu OO, Hogue IB, Enquist LW. Virus infections in the nervous system. Cell Host Microbe. 2013;13(4):379–393. doi: 10.1016/j.chom.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baig AM. Neurological manifestations in COVID-19 caused by SARS-CoV-2. CNS Neurosci Ther. 2020;26(5):499–501. doi: 10.1111/cns.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200816-covid-19-sitrep-209.pdf?sfvrsn=5dde1ca2_2
- 8.Huang C, Wang Y, Li X et al (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China [published correction appears in Lancet. 2020 Jan 30]. Lancet 395(10223):497–506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed]
- 9.Lovato A, de Filippis C, Marioni G. Upper airway symptoms in coronavirus disease 2019 (COVID-19) Am J Otolaryngol. 2020;41(3):102474. doi: 10.1016/j.amjoto.2020.102474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lovato A, de Filippis C (2020) Clinical presentation of COVID-19: a systematic review focusing on upper airway symptoms [published online ahead of print, 2020 Apr 13]. Ear Nose Throat J:145561320920762. 10.1177/0145561320920762 [DOI] [PubMed]
- 11.Suzuki M, Saito K, Min WP, et al. Identification of viruses in patients with postviral olfactory dysfunction. Laryngoscope. 2007;117(2):272–277. doi: 10.1097/01.mlg.0000249922.37381.1e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Riel D, Verdijk R, Kuiken T. The olfactory nerve: a shortcut for influenza and other viral diseases into the central nervous system. J Pathol. 2015;235(2):277–287. doi: 10.1002/path.4461. [DOI] [PubMed] [Google Scholar]
- 13.Nordin S, Brämerson A. Complaints of olfactory disorders: epidemiology, assessment and clinical implications. Curr Opin Allergy Clin Immunol. 2008;8(1):10–15. doi: 10.1097/ACI.0b013e3282f3f473. [DOI] [PubMed] [Google Scholar]
- 14.Trilla A. One world, one health: the novel coronavirus COVID-19 epidemic. Un mundo, una salud: la epidemia por el nuevo coronavirus COVID-19. Med Clin (Barc) 2020;154(5):175–177. doi: 10.1016/j.medcli.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keller A, Malaspina D (2013) Hidden consequences of olfactory dysfunction: a patient report series. BMC Ear Nose Throat Disord 13(1):8. Published 2013 Jul 23. 10.1186/1472-6815-13-8 [DOI] [PMC free article] [PubMed]
- 16.Landis BN, Konnerth CG, Hummel T. A study on the frequency of olfactory dysfunction. Laryngoscope. 2004;114(10):1764–1769. doi: 10.1097/00005537-200410000-00017. [DOI] [PubMed] [Google Scholar]
- 17.Miwa T, Furukawa M, Tsukatani T, Costanzo RM, DiNardo LJ, Reiter ER. Impact of olfactory impairment on quality of life and disability. Arch Otolaryngol Head Neck Surg. 2001;127(5):497–503. doi: 10.1001/archotol.127.5.497. [DOI] [PubMed] [Google Scholar]
- 18.Katotomichelakis M, Simopoulos E, Zhang N, et al. Olfactory dysfunction and asthma as risk factors for poor quality of life in upper airway diseases. Am J Rhinol Allergy. 2013;27(4):293–298. doi: 10.2500/ajra.2013.27.3903. [DOI] [PubMed] [Google Scholar]
- 19.CDC (2020) Interim infection prevention and control recommendations for patients with suspected or confirmed coronavirus disease 2019 (COVID-19) in healthcare settings. https://www.cdc.gov/coronavirus/2019-ncov/healthcare-facilities/dialysis.html
- 20.Mattos JL, Edwards C, Schlosser RJ, et al. A brief version of the questionnaire of olfactory disorders in patients with chronic rhinosinusitis. Int Forum Allergy Rhinol. 2019;9(10):1144–1150. doi: 10.1002/alr.22392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masters P, Perlman S (2013) Coronaviridae. In: Knipe D, Howley P (eds) Fields virology, 6th edn. Lippincott Williams & Wilkins, pp 825–858
- 22.Lechien JR, Chiesa-Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277(8):2251–2261. doi: 10.1007/s00405-020-059651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qiu C, Cui C, Hautefort C et al (2020) Olfactory and gustatory dysfunction as an early identifier of COVID-19 in adults and children: an international multicenter study [published online ahead of print, 2020 Jun 16]. Otolaryngol Head Neck Surg:194599820934376. 10.1177/0194599820934376 [DOI] [PMC free article] [PubMed]
- 24.Speth MM, Singer-Cornelius T, Oberle M, Gengler I, Brockmeier SJ, Sedaghat AR. Olfactory dysfunction and sinonasal symptomatology in COVID-19: prevalence, severity, timing, and associated characteristics. Otolaryngol Head Neck Surg. 2020;163(1):114–120. doi: 10.1177/0194599820929185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paderno A, Mattavelli D, Rampinelli V et al (2020) Olfactory and gustatory outcomes in COVID-19: a prospective evaluation in nonhospitalized subjects [published online ahead of print, 2020 Jun 30]. Otolaryngol Head Neck Surg:194599820939538. 10.1177/0194599820939538 [DOI] [PMC free article] [PubMed]
- 26.Chary E, Carsuzaa F, Trijolet JP, et al. Prevalence and recovery from olfactory and gustatory dysfunctions in COVID-19 infection: a prospective multicenter study. Am J Rhinol Allergy. 2020;34(5):686–693. doi: 10.1177/1945892420930954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vaira LA, Deiana G, Fois AG, et al. Objective evaluation of anosmia and ageusia in COVID-19 patients: single-center experience on 72 cases. Head Neck. 2020;42(6):1252–1258. doi: 10.1002/hed.26204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee Y, Min P, Lee S, Kim SW (2020) Prevalence and duration of acute loss of smell or taste in COVID-19 patients. J Korean Med Sci 35(18):e174. Published 2020 May 11. 10.3346/jkms.2020.35.e174 [DOI] [PMC free article] [PubMed]
- 29.Pawluski JL, Brummelte S, Barha CK, Crozier TM, Galea LA. Effects of steroid hormones on neurogenesis in the hippocampus of the adult female rodent during the estrous cycle, pregnancy, lactation and aging. Front Neuroendocrinol. 2009;30(3):343–357. doi: 10.1016/j.yfrne.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 30.Lennington JB, Yang Z, Conover JC (2003) Neural stem cells and the regulation of adult neurogenesis. Reprod Biol Endocrinol 1:99. Published 2003 Nov 13. 10.1186/1477-7827-1-99 [DOI] [PMC free article] [PubMed]
- 31.Lipnicki DM, Sachdev PS, Crawford J et al (2013) Risk factors for late-life cognitive decline and variation with age and sex in the Sydney Memory and Ageing Study. PLoS One 8(6):e65841. Published 2013 Jun 14. 10.1371/journal.pone.0065841 [DOI] [PMC free article] [PubMed]
- 32.Doty R, Shaman P, Applebaum S, Giberson R, Siksorski L, Rosenberg L (1984) Smell identification ability: changes with age. Science 226(4681):1441–1443. http://www.jstor.org/stable/1693918. Accessed 15 Sep 2020 [DOI] [PubMed]
- 33.Vent J, Robinson AM, Gentry-Nielsen MJ, et al. Pathology of the olfactory epithelium: smoking and ethanol exposure. Laryngoscope. 2004;114(8):1383–1388. doi: 10.1097/00005537-200408000-00012. [DOI] [PubMed] [Google Scholar]
- 34.Schubert CR, Cruickshanks KJ, Fischer ME, et al. Carotid intima media thickness, atherosclerosis, and 5-year decline in odor identification: the Beaver Dam Offspring Study. J Gerontol A Biol Sci Med Sci. 2015;70(7):879–884. doi: 10.1093/gerona/glu158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaye R, Chang CWD, Kazahaya K, Brereton J, Denneny JC., 3rd COVID-19 anosmia reporting tool: initial findings. Otolaryngol Head Neck Surg. 2020;163(1):132–134. doi: 10.1177/0194599820922992. [DOI] [PubMed] [Google Scholar]
- 36.Klopfenstein T, Kadiane-Oussou NJ, Toko L, et al. Features of anosmia in COVID-19. Med Mal Infect. 2020;50(5):436–439. doi: 10.1016/j.medmal.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tong JY, Wong A, Zhu D, Fastenberg JH, Tham T. The prevalence of olfactory and gustatory dysfunction in COVID-19 patients: a systematic review and meta-analysis. Otolaryngol Head Neck Surg. 2020;163(1):3–11. doi: 10.1177/0194599820926473. [DOI] [PubMed] [Google Scholar]
- 38.Al-Ani RM, Acharya D (2020) Prevalence of anosmia and ageusia in patients with COVID-19 at a Primary Health Center, Doha, Qatar [published online ahead of print, 2020 Aug 19]. Indian J Otolaryngol Head Neck Surg:1–7. 10.1007/s12070-020-02064-9 [DOI] [PMC free article] [PubMed]
- 39.Mishra P, Gowda V, Dixit S, Kaushik M (2020) Prevalence of new onset anosmia in COVID-19 patients: is the trend different between European and Indian population? [published online ahead of print, 2020 Jul 21]. Indian J Otolaryngol Head Neck Surg:1–4. 10.1007/s12070-020-019868 [DOI] [PMC free article] [PubMed]
- 40.Benvenuto D, Giovanetti M, Ciccozzi A, Spoto S, Angeletti S, Ciccozzi M. The 2019-new coronavirus epidemic: evidence for virus evolution. J Med Virol. 2020;92(4):455–459. doi: 10.1002/jmv.25688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li W, Zhang C, Sui J, et al. Receptor and viral determinants of SARS-coronavirus adaptation to human ACE2. EMBO J. 2005;24(8):1634–1643. doi: 10.1038/sj.emboj.7600640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Z, Yang B, Li Q, Wen L, Zhang R. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis. 2020;71(15):769–777. doi: 10.1093/cid/ciaa272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu J, Liu J, Zhao X, Liu C, Wang W, Wang D, Xu W, Zhang C, Yu J, Jiang B, Cao H, Li L. Clinical characteristics of imported cases of coronavirus disease 2019 (COVID-19) in Jiangsu Province: a multicenter descriptive study. Clin Infect Dis. 2020;71(15):706–712. doi: 10.1093/cid/ciaa199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li X, Geng M, Peng Y, Meng L, Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal. 2020;10(2):102–108. doi: 10.1016/j.jpha.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]