Abstract
Background and aim
Chrysin is a flavonoid found in plant extracts from Passiflora species, honey and propolis. It has demonstrated anti-adipogenic activity in vitro but there are no studies substantiating the anti-obesity activity of chrysin in vivo.
Experimental procedure
The pancreatic lipase (PL) inhibitory potential of chrysin was determined by preliminary in silico screening and further confirmed by in vitro PL inhibitory assay and oral fat tolerance test (OFTT). The effect of chrysin on acute feed intake and sucrose preference test was determined in normal rats. Obesity was induced by feeding of high fructose diet (HFD) to the rats. The rats were divided into six groups: normal control, HFD control, orlistat and three doses of chrysin (25, 50 and 100 mg/kg body weight). Body weight, body mass index (BMI), abdominal circumference/thoracic circumference (AC/TC) ratio, calorie intake, adiposity index, fecal cholesterol, locomotor activity and histopathology of the adipose tissue of the rats were evaluated.
Results
Chrysin showed good affinity to PL with competitive type of inhibition. It significantly reduced serum triglycerides in OFTT. Chrysin also significantly reduced acute feed intake and sucrose preference in rats. Chrysin significantly decreased the body weight, BMI, AC/TC ratio, adiposity index, calorie intake while it significantly increased the fecal cholesterol and locomotor activity of the rats. Chrysin was found to reduce the size of the adipocytes when compared to the HFD control group.
Conclusion
Thus, chrysin exerted anti-obesity effect by inhibiting PL, reducing sucrose preference, reducing calorie intake and increasing the locomotor activity of rats.
Keywords: Adipose tissue, Locomotion, Pancreatic lipase, Sucrose preference
Abbreviations: AC/TC, ratio-abdominal circumference to thoracic circumference ratio; AUC, area under the curve; BMI, body mass index; C100, chrysin 100 mg/kg p.o. body weight; C25, chrysin 25 mg/kg p.o. body weight; C50, chrysin 50 mg/kg p.o. body weight; GLP, 1-glucagon like peptide 1; HFD, high fructose diet; NC, normal control; OFTT, oral fat tolerance test; Orli, orlistat; PL, pancreatic lipase; p-NPP, p-nitrophenyl palmitate; SEM, standard error of mean; TG, triglycerides; VC, vehicle control
Graphical abstract
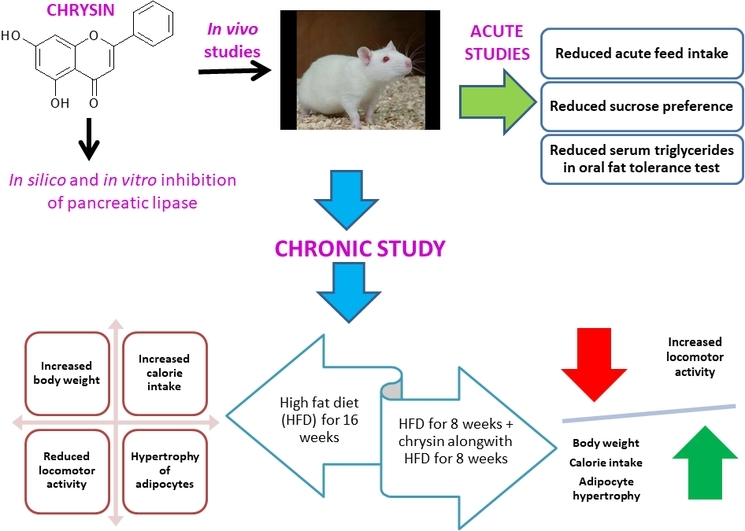
Highlights
-
•
Chrysin was found to be competitive inhibitor of pancreatic lipase.
-
•
Chrysin reduced the body weight of rats.
-
•
Chrysin reduced calorie intake of rats.
-
•
Chrysin increased the locomotor activity of obese rats.
-
•
Chrysin reduced the hypertrophy of adipocytes.
Taxonomy (classification by EVISE)
identify the disease/health condition: obesity.
the experimental approach: in silico, in vitro and in vivo studies.
the methodology: The effect of chrysin on acute feed intake, lipid absorption and sucrose preference was studied in normal rats. Thereafter, obesity was induced in the rats by feeding them high fructose diet for 16 weeks and the effect of 3 doses of chrysin was studied. The body weight, body mass index, feed intake and fructose intake was determined and the calorie intake as well as feed efficiency was calculated. The locomotor activity and fecal cholesterol of the rats was determined. The histopathological evaluation of the adipose tissue of the rats was also carried out.
1. Introduction
Overweight and obesity are defined as an abnormal or excessive fat accumulation that may impair health. Obesity occurs due to an imbalance between energy intake and expenditure. The consumption of energy dense foods coupled with a sedentary lifestyle predisposes individuals to the development of obesity.1 Drug discovery efforts towards anti-obesity drugs have not been very rewarding as many of the approved drugs cause serious adverse drug reactions (ADRs).2 Therefore, it is of paramount importance to increase the focus on screening of natural compounds for the treatment of obesity which are perceived to show fewer and/or less severe adverse effects. Higher intake of dietary flavonoids has been associated with reduced body weight in a cohort of adults living in the Mediterranean area.3
Pancreatic lipase (PL) is responsible for the absorption of dietary fats. Therefore, inhibition of PL is one of the promising strategies for the treatment of obesity. Orlistat is an approved anti-obesity drug and it inhibits PL. However, it is associated with ADRs such as steatorrhea and flatulence. Many phytoconstituents including polyphenols have shown potent inhibitory activity against PL with lesser unpleasant effects than orlistat.4 An extract of Oroxylum indicum which contains chrysin as one of the active constituents has shown PL inhibitory activity.5 Obesity is also considered as a brain disorder with reports suggesting that prolonged consumption of sweetened beverages leads to their addiction with subsequent weight gain. This is due to the release of dopamine and is analogous to some addictive states (hedonic obesity).6 Drugs that are able to target the hedonic obesity pathway can significantly curb the current epidemic of obesity. Increasing the energy expenditure is another target for anti-obesity drugs. Sibutramine, an anti-obesity drug has been reported to increase the locomotor activity and thus decrease the body weight of rats.7
Chrysin is a flavone found in propolis and many plants, predominantly in the Passiflora species. It possesses several pharmacological activities such as anti-inflammatory, neuroprotective, antidiabetic, antiatherogenic, hepatoprotective, anticancer, nephroprotective and cardioprotective.8 Chrysin has also been shown to inhibit the differentiation of adipocytes and induce the browning of white adipocytes in vitro.9 However, these effects have not been substantiated in animal models of obesity.
The present study tested the hypothesis that chrysin can exert anti-obesity effect by inhibiting PL. Thus, the aim of this study was to determine the PL inhibitory potential of chrysin preliminarily by in silico screening followed by in vitro confirmation and further corroboration of these results in vivo. Another aim of the present study was to determine the effect of chrysin on the bingeing of sweetened beverages using a sucrose preference test. The effects of chrysin on the anthropometric parameters, feed and water intake, fecal cholesterol, locomotor activity and histopathology of the adipose tissue of rats were also determined.
2. Materials and methods
2.1. Molecular docking of chrysin to pancreatic lipase
The preliminary studies on predicting the binding affinity of chrysin against human PL was carried out using molecular docking approach employing the GLIDE module in the Schrödinger suite.10 The X-ray crystal structure for human PL was imported from the protein data bank (PDB id: 1LPB).11 Chrysin structure was prepared for docking using the ligprep module in the Schrödinger suite. All the docking solutions were scored using the GLIDE standard precision scoring function.10,12
2.2. Chemicals and kits for biochemical estimations
Chrysin, porcine PL and p-nitrophenyl palmitate (p-NPP) were procured from Sigma Aldrich®, India. The rat normal chow was procured from Krishna Valley AgroTech, Maharashtra, India and fructose was obtained from Tate & Lyle, United Kingdom. Orlistat was provided as a gift sample by Macleods Pharmaceuticals Ltd., Mumbai, India. Intralipid® 20% was procured from a local pharmacy. The kits for the estimation of triglycerides (TG) and cholesterol were procured from Erba Mannheim®, Germany. All other chemicals used in this study were of analytical grade.
2.3. In vitro pancreatic lipase inhibition assay
In vitro PL inhibition assay was carried out using p-NPP as the substrate. The percentage inhibition of the test solutions was calculated using equation (1) given below
| 1 |
The IC50 of chrysin for PL was determined.12
2.4. Kinetics of PL inhibition by chrysin
To determine the kinetics of inhibition of PL by chrysin, the enzyme concentration was held constant (1 mg/ml) while the substrate (p-NPP) concentration was varied (0, 250 μM, 500 μM, 1250 μM, 2500 μM, 5000 μM and 10000 μM). Three concentrations of chrysin (0.02, 0.04 and 0.08 mM) were incubated with the different concentrations of p-NPP as described for the determination of IC50. A Lineweaver-Burk plot was used to determine the type of inhibition.13
2.5. Animals
All the animals used in this study were treated humanely as per the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Environment, Forests, and Climate Change, Government of India. The protocol was approved by the Institutional Animal Ethics Committee of T. N. Medical College and BYL Nair Charitable Hospital, Mumbai (Protocol No. IAEC/8A dated 29th February 2016). Male Wistar rats (body weight 200 ± 20 g) were procured from Bombay Veterinary College, Mumbai, India. They were housed in polypropylene cages with husk bedding in an environment maintained at 25±2 °C and relative humidity of 40–70% in an animal house with 12/12 h light/dark cycle. They had access to food and water ad libitum. The animals were allowed to acclimatize to the animal house conditions for one week prior to the start of the experiments.
2.6. Evaluation of chrysin in vivo in normal rats and high fructose diet (HFD) induced obese rats
In order to mitigate obesity, it is desirable that a compound reduces the absorption of fats, feed intake and the intake of palatable foods such as sugars.2 To determine the PL inhibitory potential of chrysin in vivo, an oral fat tolerance test (OFTT) was carried out. After a wash-out period of one week, the same animals were used for assessing the effect of chrysin on acute feed intake. The rats were given a one–week wash out period and then the sucrose preference test was carried out. Thereafter, the rats were again given a wash-out period and then the anti-obesity potential of chrysin was determined in rats fed a HFD for 16 weeks (Fig. 1).
Fig. 1.
Schematic representation of the study design.
2.7. Evaluation of chrysin in normal rats
2.7.1. OFTT
The rats were randomly divided into five groups with 8 rats per group as follows:
-
i.
Vehicle Control - fed 0.5% methylcellulose p.o.
-
ii.
Standard drug-Orlistat-45 mg/kg p.o.
-
iii.
Chrysin-25 mg/kg p.o.
-
iv.
Chrysin-50 mg/kg p.o.
-
v.
Chrysin-100 mg/kg p.o.
All the doses were administered as per kg of body weight. 0.5% methylcellulose was chosen as the vehicle as it has demonstrated good safety in long term studies.14
The rats were fasted overnight. The next day, blood was withdrawn from the retroorbital plexus for the estimation of baseline serum TG (0 h). The rats were administered vehicle, orlistat or different doses of chrysin. The doses of orlistat and chrysin used in this study were selected on the basis of available literature.15,16 Subsequently, the rats were fed Intralipid® 20% orally (10 ml/kg body weight) that is an intravenous fat emulsion containing 20% soybean oil, 1.2% egg yolk phospholipids, 2.25% glycerin, and water for injection.17 The blood of the rats was withdrawn at 1, 2, 3, 4 and 6 h after the administration of Intralipid® 20%. The serum TG levels were estimated using a commercial kit (Erba Mannheim®, GmbH) and the area under the curve (AUC) of serum TG was determined.12
2.7.2. Acute feed intake
The rats were randomly divided into five groups with 8 rats per group as follows:
-
i.
Vehicle Control - fed 0.5% methylcellulose p.o.
-
ii.
Standard drug-naltrexone 1 mg/kg p.o.
-
iii.
Chrysin-25 mg/kg p.o.
-
iv.
Chrysin-50 mg/kg p.o.
-
vi.
Chrysin-100 mg/kg p.o.
All the doses were administered as per kg of body weight.
The rats were housed individually in each cage. The feed intake was monitored for three days to determine the average daily intake of each rat.18 Thereafter, vehicle, naltrexone or chrysin was administered to the rats and the feed intake was monitored for the next 24 h.
Naltrexone was used as a standard for acute feed intake and sucrose preference test as it is known to suppress appetite and the intake of palatable foods while orlistat does not have these effects. The dose of naltrexone was selected on the basis of available literature.19,20
2.7.3. Sucrose preference test
The grouping of the animals was the same as described in section 2.7.2. The rats were housed individually and accustomed to drinking water from two bottles attached to the lid of the cage, ensuring that they could freely access both the bottles. The positions of the bottles were swapped each day to habituate them to drink from both the bottles for four days.21 Thereafter, the rats were exposed to the following treatment:
Day 1-Day 3- Exposure to one bottle of sucrose and one bottle of water with swapping of the position of the bottles every second day to ensure that there is no position bias for the rats to consume the liquid from one particular bottle.
Day 4 – Exposure to one bottle of sucrose and one bottle of water alongwith treatment with the vehicle or drugs.
Sucrose preference was calculated as given in equation (2) below:
| 2 |
2.8. Evaluation of the anti-obesity potential of chrysin in HFD induced obesity in rats
Fructose feeding was used to induce obesity in rats.22 The rats were divided into six groups each containing eight rats and given the following treatments:
-
1.
Normal control-The rats received the normal chow and drinking water ad libitum for 16 weeks
-
2.
HFD control- The rats received the normal chow and 30% fructose in drinking water ad libitum for 16 weeks. They were administered 1 ml of 0.5% methylcellulose (vehicle) daily by oral gavage from 9th-16th week.
-
3.
Orlistat- The rats received the normal chow and 30% fructose in drinking water ad libitum for 16 weeks. They were administered orlistat (45 mg/kg) daily by oral gavage from 9th-16th week. Orlistat was selected as a standard drug as it is the only anti-obesity drug which is approved for the long term management of obesity.
-
4.
Chrysin-25-The rats received the normal chow and 30% fructose in drinking water ad libitum for 16 weeks. They were administered chrysin (25 mg/kg body weight) daily by oral gavage from 9th-16th week
-
5.
Chrysin-50-The rats received the normal chow and 30% fructose in drinking water ad libitum for 16 weeks. They were administered chrysin (50 mg/kg body weight) daily by oral gavage from 9th-16th week
-
6.
Chrysin-100-The rats received the normal chow and 30% fructose in drinking water ad libitum for 16 weeks. They were administered chrysin (100 mg/kg body weight) daily by oral gavage from 9th-16th week Chrysin-100-The rats received the normal chow and 30% fructose in drinking water ad libitum for 16 weeks.
2.8.1. Estimation of anthropometric parameters
The body weight, body mass index (BMI), thoracic circumference (TC) and abdominal circumference (AC), AC/TC ratio of the rats were determined. The feed and water/fructose intake of the rats was monitored daily throughout the study and the average daily total calorie intake per day per rat and feed efficiency was calculated.23
2.8.2. Determination of locomotor activity using actophotometer
The locomotor activity of the rats was measured at baseline, 8th week and 16th week of the study. The rats were kept individually in the actophotometer and allowed to explore the surroundings for 30 min. Then a baseline activity count was determined for each rat. The rats received their respective treatments and then the activity count was determined again after 1 h.24
2.8.3. Estimation of fecal cholesterol
At 16 weeks, the feces of the rats were collected and air dried for the estimation of fecal cholesterol.25 The dried feces were powdered and the lipids were extracted using chloroform: methanol (2:1).26 The samples were centrifuged to remove the debris. After partitioning, the lipid film was dissolved in isopropyl alcohol. The cholesterol content was determined in the lipid film using a commercial kit (Erba Mannheim®, Germany).
2.8.4. Euthanasia and isolation of organs
Sixteen weeks after the commencement of the study, the rats were euthanized by carbon dioxide asphyxiation. The intraabdominal fat (mesenteric, retroperitoneal, omental) and epididymal fat were dissected and weighed.23 The adiposity index was calculated as shown in equation (3):
| 3 |
2.8.5. Histopathological evaluation of the adipose tissue
The pathological changes in the adipose tissue were examined by a qualified veterinary pathologist who was blinded to the identity of the samples. Briefly, the tissue was stored in buffered formalin to allow its fixation. 5 μm sections of the tissue were taken using a microtome which was then stained using hematoxylin and eosin. The stained sections were observed under a microscope at 400× magnification. The numbers of adipocytes per high power field were determined.27
2.9. Statistical analysis
Data are expressed as mean ± standard error of mean (SEM). Statistical analysis was carried out using GraphPad Prism, version 5, CA, USA. Two way ANOVA followed by Bonferroni's test was used for the statistical analysis of OFTT, acute feed intake, sucrose preference test, body weight and locomotor activity with time and treatment as the two variables. One way ANOVA followed by Tukey's multiple comparison test was used for the remaining parameters. P < 0.05 was considered to be statistically significant.
3. Results
3.1. In silico binding of chrysin to PL and in vitro inhibition of PL by chrysin
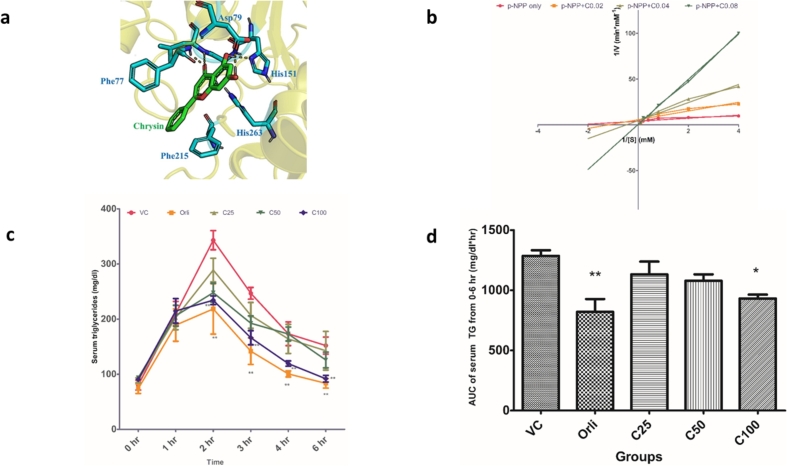
Chrysin's interactions with human PL are primarily dominated by hydrogen bonds to Gly76, Phe77, Asp79, His151, Ser152 and Leu153. Ser152 is part of the catalytic triad and covalently binds to the co-crystallized ligand, C11 alkyl phosphonate (MUP) (Fig. 2a). MUP shows two hydrogen bonds with the backbone NH group of Leu153 and Phe77, which are also shown by chrysin. This indicates that chrysin possesses important chemical features necessary to bind to human PL. Moreover, large number of hydrogen bonds, in this case, shows that it has a great potential to bind strongly to human PL. The IC50 of chrysin for PL was found to be 0.018 ± 0.006 mM. The Lineweaver-Burk plot for three graded concentrations of chrysin showed an increase in km without much change in Vmax, thereby indicating that chrysin inhibited PL competitively (Fig. 2b). Thus, PL inhibitory activity is one of the mechanisms responsible for the anti-obesity effects of chrysin.
Fig. 2.
Pancreatic lipase inhibitory potential of chrysin (a) Molecular interactions of chrysin with human PL. Chrysin is shown as cyan molecule and green represents atoms of human PL, (b) Kinetics of inhibition of pancreatic lipase by chrysin (n = 3), (c) Effect of chrysin on oral fat tolerance test (OFTT) in rats (n = 8). Data were analyzed by two-way ANOVA followed by Bonferroni's test. Values are expressed as mean ± SEM (*p < 0.05 vs VC), (d) Effect of chrysin on area under the curve (AUC) of serum triglycerides from 0 to 6 h (n = 8). Data were analyzed by one-way ANOVA followed by Tukey's multiple comparison test. Values are expressed as mean ± SEM (*p < 0.05 vs VC, **p < 0.01 vs VC).
Abbreviations: p-NPP-p-nitrophenyl palmitate, C0.02- chrysin 0.02 mM, C0.04-chrysin 0.04 mM, C0.08-chrysin 0.08 mM, VC-vehicle control, Orli-orlistat (45 mg/kg p.o.), C25-chrysin 25 mg/kg p.o., C50-chrysin 50 mg/kg p.o., C100-chrysin 100 mg/kg p.o., AUC-area under the curve.
3.2. Effect of chrysin on serum TG and AUC of serum TG in OFTT in rats
The PL inhibitory potential of chrysin was further corroborated in the OFFT in rats. There was an increase in the serum TG of rats in all the groups after administration of Intralipid® 20%. However, the rats that were pretreated with chrysin (100 mg/kg) and orlistat showed a significant decrease in serum TG when compared to vehicle control from 2 to 6 h (Fig. 2c). There was a significant decrease in the AUC of serum TG from 0 to 6 h in orlistat and chrysin (100 mg/kg) groups when compared to the vehicle control group (Fig. 2d).
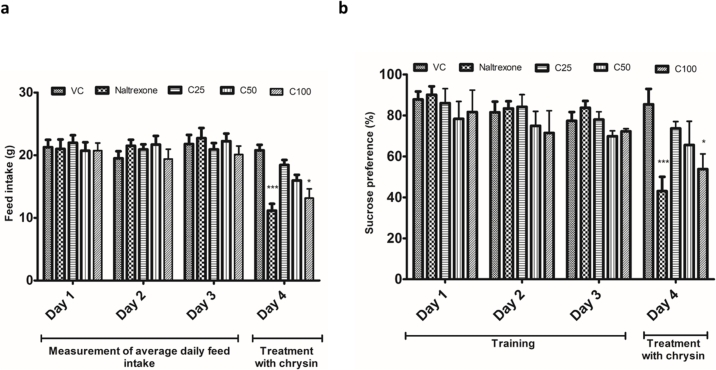
3.3. Effect of chrysin on acute feed intake and sucrose preference test in rats
There was no significant difference in the average daily feed intake of the rats in the different groups during the first three days of the study. Administration of naltrexone and chrysin (100 mg/kg) caused a significant decrease in the acute feed intake of the rats when compared to the vehicle control group (Fig. 3a). During the days 1–3, the rats in all the groups did not show any statistically significant difference in the sucrose preference. Rats treated with naltrexone and chrysin (100 mg/kg) showed a significant decrease in the sucrose preference when compared to the vehicle control group (Fig. 3b).
Fig. 3.
Effect of chrysin on (a) Acute feed intake of rats (*p < 0.05 vs VC), (b) Sucrose preference in rats. Data were analyzed by two-way ANOVA followed by Bonferroni post hoc test and expressed as mean ± SEM (n = 8 per group) (*p < 0.05 vs VC); Abbreviations: VC-vehicle control, Orli-orlistat (45 mg/kg po), C25-chrysin-25 mg/kg p.o., C50-chrysin 50 mg/kg p.o., C100-chrysin 100 mg/kg p.o.
3.4. Effect of chrysin on the anthropometric parameters, calorie intake, feed efficiency and adiposity index of rats
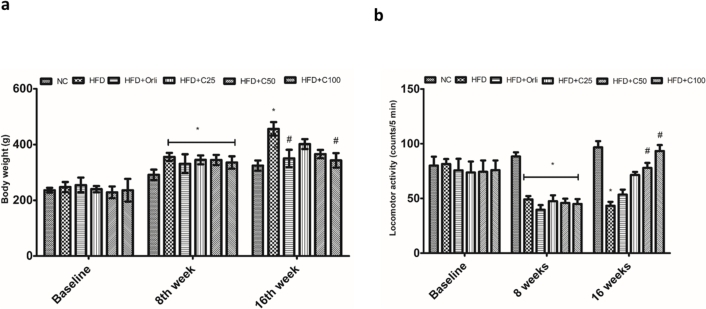
Rats fed HFD showed a significant increase in the body weight when compared to the normal control group at 8th and 16th week (Fig. 4a). Rats in the HFD group also showed a significant increase in the BMI and AC/TC ratio. There was no significant difference in the average daily feed intake between the groups. The increase in the body weight, BMI and AC/TC ratio can be attributed to an increased average daily intake of fructose and average daily calorie intake (Table 1). Chrysin (100 mg/kg) caused a significant decrease in the body weight of the rats at 16th week when compared to the HFD group (Fig. 4a). There was a significant decrease in the average daily fructose intake and average daily calorie intake in chrysin (50 mg/kg and 100 mg/kg) groups when compared to the HFD group. The feed efficiency of chrysin (100 mg/kg) and orlistat groups were significantly decreased when compared to the HFD group (Table 1). There was a significant increase in the adiposity index of rats in the HFD group when compared to the normal control group. Chrysin (100 mg/kg) significantly decreased the adiposity index of the rats (Table 1).
Fig. 4.
Effect of chrysin on (a) Body weight of rats, (b) Locomotor activity of rats.
Data were analyzed by two-way ANOVA followed by Bonferroni post hoc test and expressed as mean ± SEM (n = 8 per group). (*p < 0.05 vs NC; #p < 0.05 vs HFD).
Abbreviations: C25-chrysin-25 mg/kg p.o., C50-chrysin 50 mg/kg p.o., C100-chrysin 100 mg/kg p.o., NC-normal control, HFD-high fructose diet.
Table 1.
Effect of chrysin on the body composition, dietary intakes and fecal cholesterol of rats.
| NC | HFD | HFD + Orlistat | HFD + C25 | HFD + C50 | HFD + C100 | |
|---|---|---|---|---|---|---|
| BMI (g/cm2) | 0.66 ± 0.03 | 0.89 ± 0.03* | 0.67 ± 0.02# | 0.79 ± 0.01 | 0.72 ± 0.01# | 0.69 ± 0.02# |
| AC/TC ratio | 1.11 ± 0.02 | 1.36 ± 0.04* | 1.17 ± 0.02# | 1.32 ± 0.05 | 1.23 ± 0.03# | 1.19 ± 0.43# |
| Avg. daily feed intake per day per rat (g/day) | 16.86 ± 1.09 | 14.97 ± 1.32 | 10.69 ± 1.3 | 11.5 ± 0.75 | 12.53 ± 1.37 | 13.53 ± 1.59 |
| Avg. daily water or fructose intake per day per rat (ml/day) | 27 ± 1.66 | 37.73 ± 1.82* | 31.25 ± 1.98* | 27.32 ± 1.1# | 24.81 ± 1.23# | 22.83 ± 1.22# |
| Avg. daily calorie intake per day per rat (kcal/day) | 40.91 ± 3.38 | 81.37 ± 4.91* | 70.0 ± 3.95* | 58.33 ± 3.56 | 56.19 ± 2.09# | 51.91 ± 1.51# |
| Feed efficiency (g/kcal) | 1.92 ± 0.15 | 3.32 ± 0.39* | 1.99 ± 0.33# | 2.61 ± 0.15 | 2.37 ± 0.17 | 1.48 ± 0.19# |
| Adiposity index (%) | 1.39 ± 0.21 | 4.17 ± 0.28* | 2.3 ± 0.26# | 3.67 ± 0.24 | 3.23 ± 0.31 | 2.83 ± 0.30# |
| Fecal cholesterol (mg/g) | 0.2 ± 0.03 | 0.69 ± 0.08* | 1.37 ± 0.09# | 0.72 ± 0.12 | 0.83 ± 0.09 | 1.19 ± 0.2# |
Data are expressed as mean ± SEM of 8 rats per group. Data were analyzed by one-way ANOVA followed by Tukey's multiple comparison test (*p < 0.05 vs NC; #p < 0.05 vs HFD).
Abbreviations: NC-Normal Control, HFD-High fructose diet, C25-Chrysin-25 mg/kg, C50-Chrysin-50 mg/kg, C100-Chrysin-100 mg/kg, BMI-body mass index, AC/TC ratio-abdominal circumference/thoracic circumference ratio.
3.5. Effect of chrysin on the locomotor activity of rats
HFD resulted in a significant decrease in the locomotor activity of the rats when compared to the rats that received the normal chow. Chrysin (50 mg/kg and 100 mg/kg) increased the locomotor activity of rats when compared to the HFD group (Fig. 4b). Thus, increase in the energy expenditure of rats is another mechanism contributing to the anti-obesity activity of chrysin.
3.6. Effect of chrysin on the fecal cholesterol content of rats
HFD caused a significant increase in the fecal cholesterol content of the rats when compared to the normal control group. There was a significant increase in the fecal cholesterol content of the orlistat and chrysin (100 mg/kg) treated rats when compared to the HFD group (Table 1).
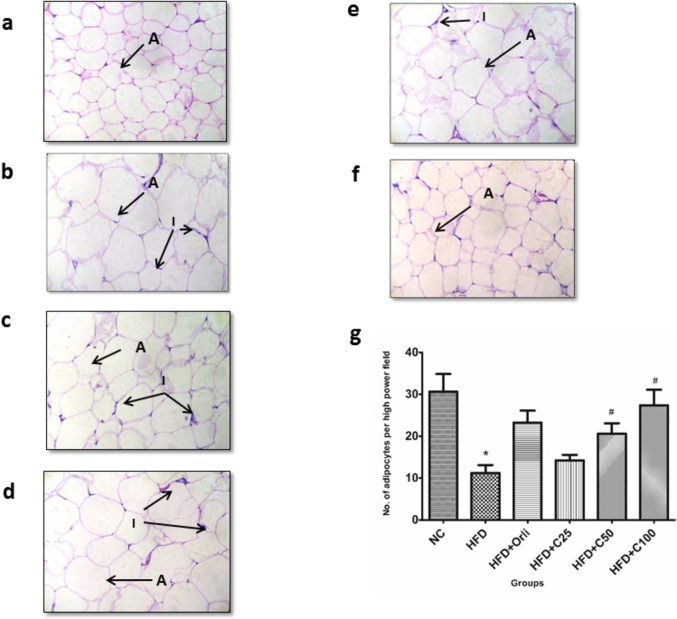
3.7. Effect of chrysin on the histopathology of adipose tissue of rats
Rats in the normal control group showed normal adipocytes (Fig. 5a) while the rats in the HFD group showed hypertrophy of adipocytes and mild inflammation when compared to the normal control group (Fig. 5b). The size of the adipocytes in the orlistat group was smaller than the HFD group but there was mild inflammation (Fig. 5c). The adipose tissue of chrysin (25 mg/kg) treated rats showed mild inflammation and hypertrophy of adipocytes when compared to the normal control group (Fig. 5d). Chrysin (50 mg/kg and 100 mg/kg) groups showed smaller adipocytes and less inflammation when compared to the HFD group (Fig. 5e and f). Rats treated with chrysin (100 mg/kg) showed a significant increase in the number of adipocytes per high power field when compared to the HFD group (Fig. 5g). Thus, chrysin was able to reduce the hypertrophy of adipocytes and ameliorate the inflammation in the adipose tissue of rats.
Fig. 5.
Effect of chrysin on the histopathology of adipose tissue of rats fed a high fructose diet. The tissue samples were stained with hematoxylin and eosin and observed at a magnification of 400× (a) Adipose tissue from rats provided with normal chow and water showing normal adipocytes, (b) Adipose tissue from rats provided with high fructose diet (HFD) alongwith the normal chow showing enlarged adipocytes and mild inflammation compared to normal control, (c) Adipose tissue from rats on HFD treated with orlistat for 8 weeks, (d) Adipose tissue from rats on HFD treated with chrysin 25 mg/kg for 8 weeks, (e) Adipose tissue from rats on HFD treated with chrysin 50 mg/kg for 8 weeks, (f) Adipose tissue from rats on HFD treated with chrysin 100 mg/kg for 8 weeks, (g) Number of adipocytes per high power field in the adipose tissue of rats in the different groups. Data was analyzed by one-way ANOVA followed by Tukey's multiple comparison test. Values are expressed as mean ± SEM (n = 8 per group) (*p < 0.05 vs NC; #p < 0.05 vs HFD). Abbreviations: A-adipocytes, I-mild inflammation.
4. Discussion
The outcomes of this study indicate that the anti-obesity potential of chrysin can be attributed to its PL inhibitory potential, ability to reduce acute feed intake and sucrose preference in normal rats. In HFD induced obesity, chrysin reduced the average daily calorie intake, increased the fecal cholesterol excretion and the locomotor activity of rats. Chrysin also reduced the hypertrophy of adipocytes and inflammation in the adipose tissue of rats. Thus, chrysin demonstrated anti-obesity effects in rats by suppressing the appetite, reducing the intake of palatable foods, increasing the locomotor activity and reducing the hypertrophy of adipocytes and inflammation in the adipose tissue. However, these findings need to be assessed in different experimental settings for further validation and acceptance at the clinical level.
PL is responsible for the metabolism of TG into monoglycerides and free fatty acids which are then absorbed by the small intestine. Thus, inhibiting PL seems to be one of the relatively safe and effective strategies for the treatment of obesity.28 The molecular modeling studies suggested good affinity of chrysin to PL with interactions similar to the co-crystallized ligand (MUP). These results were confirmed in the PL inhibitory assay using p-NPP as a substrate, which demonstrated that chrysin is a competitive inhibitor of PL. The administration of chrysin to rats fed Intralipid® further corroborated the PL inhibitory potential of chrysin. Therefore, inhibition of PL is one of the mechanisms of action for the anti-obesity effects of chrysin.
A potential anti-obesity drug must be able to reduce the energy intake.29 Naltrexone is an antagonist of the μ-opioid receptors and it has been shown to reduce the feed intake in rats and hence it was deemed to be the appropriate standard to compare the effects of chrysin on the acute feed intake of rats. Rats that were given a single dose of chrysin showed a statistically significant and dose-dependent decrease in the acute feed intake when compared to the vehicle treated rats. This shows that chrysin possesses anorectic effect. Body weight gain can be attributed to two mechanisms-homeostatic and hedonic.30 Hedonic obesity is regulated by the reward system to satisfy the need of pleasure associated with the consumption of certain foods like beverages rich in sugars.31 The activation of the μ-opioid receptors in the pallidum and nucleus accumbens in the brain increases the hedonic reactions to sweet tastes. Naltrexone, by virtue of blocking the μ-opioid receptors reduced the preference of rats for sucrose.32 Rats that were treated with chrysin showed a decrease in the sucrose consumption in the sucrose preference test, indicating that chrysin may exert some effect on the reward pathway in the brain and thus reduce the bingeing on sugars like sucrose. These results indicate that chrysin may act on the hypothalamus and modulate the intake of palatable foods. This warrants further investigation of the effect of chrysin on the signaling pathways in the brain which regulate appetite. Chrysin has also shown to inhibit the activity of dipeptidyl peptidase-4 which may justify its effects on satiety and thus decrease in feed and sucrose intake.33.
Chronic feeding of fructose to rats results in an increase in the body weight and abdominal obesity.34 When the rats were provided fructose ad libitum along with the normal chow, they consumed more of the palatable fructose than the normal chow. This is because fructose stimulates the secretion of a hormone, ghrelin which increases the consumption of fructose.35 Feeding the rats with HFD resulted in an increase in the body weight of rats, BMI, AC/TC ratio and adiposity index when compared to the normal control group. Rats that were treated with chrysin showed a statistically significant and dose-dependent decrease in the body weight, BMI, AC/TC ratio and adiposity index. Feed efficiency is a measure of body weight gain in relation to the energy intake.36 The reduction in the BMI and adiposity index by chrysin may prove beneficial not only to mitigate obesity but also protect against its co-morbidities. The findings of this study corroborate the beneficial metabolic effects of chrysin in aged rats and rats fed fructose reported by other investigators.37,38
Energy expenditure can be increased by increasing the physical activity, increasing the basal metabolic rate and increasing the thermogenesis.39 Increase in the body weight is associated with lethargy and reduced physical activity that leads to further increase in the body weight.40 Rats fed HFD showed a significant decrease in their locomotor activity when compared to the rats that received the standard diet. Interventions that increase the locomotor activity have been shown to result in a decrease in body weight.7,24 Chrysin significantly increased the locomotor activity of rats. Thus, increase in the energy expenditure of rats is yet another mechanism of action of chrysin. Moreover, Choi et al. have reported that chrysin can increase the expression of genes responsible for the browning of adipocytes such as uncoupling protein-1 (UCP-1) and peroxisome proliferator activated receptor gamma co-factor 1 alpha (PGC-1α) which also supports the effect of chrysin on increasing the energy expenditure of rats.9
Hypertrophy of adipocytes occurs due to the increased storage of fats which is a characteristic feature of adipose tissue of obese individuals.41 Moreover, obesity also results in the influx of macrophages in the adipose tissue that secrete certain adipokines like leptin which further aggravate the disease.42 The number of adipocytes per high power field was used as a measure for comparing the size of the adipocytes between the different groups.23 Chrysin reduced the size of the adipocytes as well as inflammation in the adipose tissue of rats. Chrysin has demonstrated anti-inflammatory activity in obese mice by inducing the anti-inflammatory M2 macrophages and decreasing the pro-inflammatory M1 macrophages.43.
The strengths of this study include the systematic screening of chrysin for anti-obesity potential through in silico, in vitro and in vivo models. The free access to fructose in the 16 week study is analogous to the increased fructose consumption in the real-world setting and simulates hedonic obesity in humans. Chrysin was administered by oral gavage that ensured a fixed daily intake when compared to mixing it in the rat feed. Thus, the results of this study have greater translational value. Thus, this study provides a proof-of-concept that chrysin possesses anti-obesity activity and could be a promising molecule to combat obesity owing to its pleiotropic effects. However, the limitations of the study include the inability to evaluate the effect of chrysin on the appetite regulating hormones such as leptin, ghrelin and adiponectin which will be addressed in the future studies. Another limitation of the study is the inability to measure the effect of chrysin on the basal metabolic rate of the rats. Future experiments will evaluate the effects of chrysin on energy intake and expenditure using indirect calorimetry.
5. Conclusion
The present study demonstrated that chrysin showed good affinity to PL and inhibited it in vitro and in vivo. Chrysin supplementation for eight weeks alongwith the HFD reduced the body weight and abdominal obesity in the rats when compared to the HFD group. Chrysin treated rats showed an increase in the fecal cholesterol content which reinforces its PL inhibitory potential. Chrysin also reduced the hypertrophy of adipocytes and inflammation in the adipose tissue of rats. Thus, chrysin is a promising phytoconstituent for the management of obesity.
Conflicts of interest
The authors have no conflicts to declare.
Acknowledgements
This work was supported by a grant awarded to the first author, Ms Sarayu Pai from University Grants Commission, New Delhi, India (Letter no.F.25-1/2014-15 (BSR)/No. F.5-63/2007 (BSR) dated 16 Feb 2015). The authors wish to thank Dr Ashish Mungantiwar, Senior Vice President, Macleods Pharmaceuticals Ltd. for providing us with a gift sample of orlistat. The authors are also grateful to Ms Deepali Ganachari and Ms Jaya Verma, Research Fellows at Dept. of Clinical Pharmacology, T. N. Medical College and BYL Nair Hospital for their help during animal experimentation and Dr Sanjay Pawar, veterinary pathologist for carrying out the histopathological evaluation of the adipose tissue of rats.
Footnotes
The present study involves the evaluation of chrysin as an anti-obesity drug. Chrysin was found to inhibit pancreatic lipase in silico, in vitro and in vivo. In high fructose diet fed rats, chrysin reduced the body weight of the rats, increased the excretion of cholesterol in the feces and increased the locomotor activity of rats. Chrysin reduced the hypertrophy of adipocytes and reduced the inflammation in the adipose tissue of rats. Thus, chrysin was found to be a potential anti-obesity candidate in rats.
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Heymsfield S.B., Wadden T.A. Mechanisms, pathophysiology and management of obesity. N Engl J Med. 2017;376(3):254–266. doi: 10.1056/NEJMra1514009. [DOI] [PubMed] [Google Scholar]
- 2.Narayanaswami V., Dwoskin L.P. Obesity: current and potential pharmacotherapeutics and targets. Pharmacol Ther. 2017;170:116–147. doi: 10.1016/j.pharmthera.2016.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marranzano M., Ray S., Godos J., Galvano F. Association between dietary flavonoids intake and obesity in a cohort of adults living in the Mediterranean area. Int J Food Sci Nutr. 2018;69(8):1020–1029. doi: 10.1080/09637486.2018.1452900. [DOI] [PubMed] [Google Scholar]
- 4.Buchholz T., Melzig M.F. Polyphenolic compounds as pancreatic lipase inhibitors. Planta Med. 2015;81(10):771–783. doi: 10.1055/s-0035-1546173. [DOI] [PubMed] [Google Scholar]
- 5.Mangal P., Khare P., Jagtap S., Bishnoi M., Kondepudi K.K., Bhutani K.K. Screening of six Ayurvedic medicinal plants for anti-obesity potential: an investigation on bioactive constituents from Oroxylum indicum (L.) Kurz bark. J Ethnopharmacol. 2017;197:138–146. doi: 10.1016/j.jep.2016.07.0706. [DOI] [PubMed] [Google Scholar]
- 6.Stice E., Figlewicz D.P., Gosnell B.A., Levine A.S., Pratt W.E. The contribution of brain reward circuits to the obesity epidemic. Neurosci Biobehav Rev. 2013;37(9 Pt A):2047–2058. doi: 10.1016/j.neubiorev.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Golozoubova V., Strauss F., Malmlof K. Locomotion is the major determinant of sibutramine induced increase in energy expenditure. Pharmacol Biochem Behav. 2006;83(4):517–527. doi: 10.1016/j.pbb.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 8.Mani R., Natesan V. Chrysin: sources, beneficial pharmacological activities, and molecular mechanism of action. Phytochemistry. 2018;145:187–196. doi: 10.1016/j.phytochem.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 9.Choi J.H., Yun J.W. Chrysin induces brown fat-like phenotype and enhances lipid metabolism in 3T3-L1 adipocytes. Nutrition. 2016;32(9):1002–1010. doi: 10.1016/j.nut.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 10.Friesner R.A., Banks J.L., Murphy R.B. Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J Med Chem. 2004;47(7):1739–1749. doi: 10.1021/jm0306430. [DOI] [PubMed] [Google Scholar]
- 11.Egloff M.P., Marguet F., Buono G., Verger R., Cambillau C., van Tilbeurgh H. The 2.46 A resolution structure of the pancreatic lipase-colipase complex inhibited by a C11 alkyl phosphonate. Biochemistry. 1995;34(9):2751–2762. doi: 10.1021/bi00009a003. [DOI] [PubMed] [Google Scholar]
- 12.Pai S.A., Martis E.A.F., Joshi S.G., Munshi R.P., Juvekar A.R. Plumbagin exerts antiobesity effects through inhibition of pancreatic lipase and adipocyte differentiation. Phytother Res. 2018;32(8):1631–1635. doi: 10.1002/ptr.6085. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y., Wang J., Yanagita R.C. Effects of two sulfated triterpene saponins echinoside A and holothurin A on the inhibition of dietary fat absorption and obesity reduction. Biosci Biotechnol Biochem. 2014;78(1):139–146. doi: 10.1080/09168451.2014.877830. [DOI] [PubMed] [Google Scholar]
- 14.Gad S.C., Cassidy C.D., Aubert N., Spainhour B., Robbe H. Nonclinical vehicle use in studies by multiple routes in multiple species. Int J Toxicol. 2006;25(6):499–521. doi: 10.1080/10915810600961531. [DOI] [PubMed] [Google Scholar]
- 15.Kazmi I., Afzal M., Rahman S., Iqbal M., Imam F., Anwar F. Antiobesity potential of ursolic acid stearoyl glucoside by inhibiting pancreatic lipase. Eur J Pharmacol. 2013;709(1-3):28–36. doi: 10.1016/j.ejphar.2013.02.032. [DOI] [PubMed] [Google Scholar]
- 16.Pushpavalli G., Kalaiarasi P., Veeramani C., Pugalendi K.V. Effect of chrysin on hepatoprotective and antioxidant status in D-galactosamine-induced hepatitis in rats. Eur J Pharmacol. 2010;631(1-3):36–41. doi: 10.1016/j.ejphar.2009.12.031. [DOI] [PubMed] [Google Scholar]
- 17.Intralipid® 20% https://www.accessdata.fda.gov/drugsatfda_docs/label/2007/017643s072,018449s039lbl.pdf Available at. Accessed 21st March 2019.
- 18.Yimam M., Jiao P., Hong M. Appetite suppression and antiobesity effect of a botanical composition composed of Morus alba, Yerba mate and Magnolia officinalis. J Obes. 2016;2016:4670818. doi: 10.1155/2016/4670818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Avena N.M., Bocarsly M.E., Murray S., Gold M.S. Effects of baclofen and naltrexone, alone and in combination, on the consumption of palatable food in male rats. Exp Clin Psychopharmacol. 2014;22(5):460–467. doi: 10.1037/a0037223. [DOI] [PubMed] [Google Scholar]
- 20.Taha S.A., Norsted E., Lee L.S. Endogenous opioids encode relative taste preference. Eur J Neurosci. 2006;24(4):1220–1226. doi: 10.1111/j.1460-9568.2006.04987.x. [DOI] [PubMed] [Google Scholar]
- 21.Bueter M., Miras A.D., Chichger H. Alterations of sucrose preference after Roux-en-Y gastric bypass. Physiol Behav. 2011;104(5):709–721. doi: 10.1016/j.physbeh.2011.07.025. [DOI] [PubMed] [Google Scholar]
- 22.Bellamkonda R., Karuna R., SasiBhusana Rao B. Beneficial effect of Commiphora mukul ethanolic extract against high fructose diet induced abnormalities in carbohydrate and lipid metabolism in Wistar rats. J Tradit Complement Med. 2017;8(1):203–211. doi: 10.1016/j.jtcme.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pai S.A., Munshi R.P., Panchal F.H. Plumbagin reduces obesity and nonalcoholic fatty liver disease induced by fructose in rats through regulation of lipid metabolism, inflammation and oxidative stress. Biomed Pharmacother. 2019;111:686–694. doi: 10.1016/j.biopha.2018.12.139. [DOI] [PubMed] [Google Scholar]
- 24.Razavi B.M., Lookian F., Hosseinzadeh H. Protective effects of green tea on olanzapine-induced metabolic syndrome in rats. Biomed Pharmacother. 2017;92:726–731. doi: 10.1016/j.biopha.2017.05.113. [DOI] [PubMed] [Google Scholar]
- 25.Kim H., Wang Q., Shoemaker C.F., Zhong F., Bartley G.E., Yokoyama W.H. Polysaccharide gel coating of the leaves of Brasenia schreberi lowers plasma cholesterol in hamsters. J Tradit Complement Med. 2014;5(1):56–61. doi: 10.1016/j.jtcme.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Folch J., Lees M., Sloane Stanley G.H. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226(1):497–509. [PubMed] [Google Scholar]
- 27.Pascual-Serrano A., Arola-Arnal A., Suarez-Garcia S. Grapeseed proanthocyanidin supplementation reduces adipocyte size and increases adipocyte number in obese rats. Int J Obes. 2017;41(8):1246–1255. doi: 10.1038/ijo.2017.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lunagariya N.A., Patel N.K., Jagtap S.C., Bhutani K.K. Inhibitors of pancreatic lipase: state of the art and clinical perspectives. EXCLI J. 2014;13:897–921. [PMC free article] [PubMed] [Google Scholar]
- 29.Kakkar A.K., Dahiya N. Drug treatment of obesity: current status and future prospects. Eur J Intern Med. 2015;26(2):89–94. doi: 10.1016/j.ejim.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 30.Tulloch A.J., Murray S., Vaicekonyte R., Avena N.M. Neural responses to macronutrients: hedonic and homeostatic mechanisms. Gastroenterology. 2015;148(6):1205–1218. doi: 10.1053/j.gastro.2014.12.058. [DOI] [PubMed] [Google Scholar]
- 31.Kenny P.J. Reward mechanisms in obesity: new insights and future directions. Neuron. 2011;69(4):664–679. doi: 10.1016/j.neuron.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coulter A.A., Rebello C.J., Greenway F.L. Centrally acting agents for obesity: Past, present, and future. Drugs. 2018;78(11):1113–1132. doi: 10.1007/s40265-018-0946-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalhotra P., Chittepu V.C.S.R., Osorio-Revilla G., Gallardo-Velázquez T. Structure-activity relationship and molecular docking of natural product library reveal chrysin as a novel dipeptidyl peptidase-4 inhibitor: an integrated in silico and in vitro study. Molecules. 2018;23(6):1368. doi: 10.3390/molecules23061368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tappy L. Fructose-containing caloric sweeteners as a cause of obesity and metabolic disorders. J Exp Biol. 2018;221(Pt suppl 1) doi: 10.1242/jeb.164202. pii: jeb164202. [DOI] [PubMed] [Google Scholar]
- 35.Lindqvist A., Baelemans A., Erlanson-Albertsson C. Effects of sucrose, glucose and fructose on peripheral and central appetite signals. Regul Pept. 2008;150(1-3):26–32. doi: 10.1016/j.regpep.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 36.Prunet-Marcassus B., Desbazeille M., Bros A. Melatonin reduces body weight gain in Sprague Dawley rats 484 with diet-induced obesity. Endocrinology. 2003;144(12):5347–5352. doi: 10.1210/en.2003-0693. [DOI] [PubMed] [Google Scholar]
- 37.Farkhondeh T., Abedi F., Samarghandian S. Chrysin attenuates inflammatory and metabolic disorder indices in aged male rat. Biomed Pharmacother. 2019;109:1120–1125. doi: 10.1016/j.biopha.2018.10.059. [DOI] [PubMed] [Google Scholar]
- 38.Andrade N., Andrade S., Silva C. Chronic consumption of the dietary polyphenol chrysin attenuates metabolic disease in fructose-fed rats. Eur J Nutr. 2019 doi: 10.1007/s00394-019-01895-9. [DOI] [PubMed] [Google Scholar]
- 39.Vickers S.P., Jackson H.C., Cheetham S.C. The utility of animal models to evaluate novel anti-obesity agents. Br J Pharmacol. 2011;164(4):1248–1262. doi: 10.1111/j.1476-5381.2011.01245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chin S.H., Kahathuduwa C.N., Binks M. Physical activity and obesity: what we know and what we need to know. Obes Rev. 2016;17(12):1226–1244. doi: 10.1111/obr.12460. [DOI] [PubMed] [Google Scholar]
- 41.Stenkula K.G., Erlanson-Albertsson C. Adipose cell size: importance in health and disease. Am J Physiol Regul Integr Comp Physiol. 2018;315(2):R284–R295. doi: 10.1152/ajpregu.00257.2017. [DOI] [PubMed] [Google Scholar]
- 42.Engin A. The pathogenesis of obesity-associated adipose tissue inflammation. Adv Exp Med Biol. 2017;960:221–245. doi: 10.1007/978-3-319-48382-5_9. [DOI] [PubMed] [Google Scholar]
- 43.Feng X., Qin H., Shi Q. Chrysin attenuates inflammation by regulating M1/M2 status via activating PPARγ. Biochem Pharmacol. 2014;89(4):503–514. doi: 10.1016/j.bcp.2014.03.016. [DOI] [PubMed] [Google Scholar]