Abstract
The association of thyroid disease and Ménière’s disease would suggest that both are autoimmune diseases. This study aimed to investigate the relation of goiter, hypothyroidism, thyroiditis, hyperthyroidism, and autoimmune thyroiditis with Ménière’s disease. The Korean National Health Insurance Service-Health Screening Cohort data from 2002 through 2015 were used. The 8183 adult patients with Ménière’s disease were 1:4 matched with the 32,732 individuals of the control group for age, sex, income, and region of residence. The previous histories of thyroid disorders including goiter, hypothyroidism, thyroiditis, and hyperthyroidism were investigated using conditional logistic regression analyses. Subgroup analyses were conducted, including for age and sex. Smoking, alcohol consumption, obesity, Charlson Comorbidity Index, histories of benign paroxysmal vertigo, vestibular neuronitis, other peripheral vertigo, thyroid cancer, and levothyroxine medication were adjusted in the models. The histories of goiter (5.7% vs. 4.2%), hypothyroidism (4.7% vs. 3.6%), thyroiditis (2.1% vs. 1.6%), hyperthyroidism (3.6% vs. 2.5%), and autoimmune thyroiditis (0.99% vs. 0.67%) were higher in the Meniere’s disease group than in the control group (all P < 0.05). The histories of goiter, hypothyroidism, and hyperthyroidism were associated with Ménière’s disease (adjusted odds ratio (OR) = 1.19 [95% confidence interval (CI) = 1.04–1.36] for goiter, 1.21 [95% CI 1.02–1.44] for hypothyroidism, and 1.27 [95% CI 1.09–1.49] for hyperthyroidism, each of P < 0.05). In subgroup analyses, hypothyroidism was associated with Ménière’s disease in < 65-year-old women. Hyperthyroidism was related with Ménière’s disease in women overall. Thyroid diseases of goiter, hypothyroidism, and hyperthyroidism were associated with Ménière’s disease.
Subject terms: Endocrinology, Neurology
Introduction
The thyroid gland has crucial functions to regulate the endocrine systems through the hypothalamic-pituitary-thyroid axis and affect organ-specific or non-organ-specific metabolisms1,2. Therefore, metabolic syndrome, obesity, and type 2 diabetes have shown an association with thyroid diseases3. Autoimmune thyroid disease is thought to be associated with other organ-specific autoimmune diseases, such as Addison’s disease, type 1 diabetes mellitus, adrenocorticotropic hormone deficiency, and chronic active hepatitis, and non-organ-specific, such as rheumatoid arthritis, systemic sclerosis, and systemic lupus erythematosus4. Shared genetic predispositions and pathophysiology are thought to influence the association between autoimmune thyroid disease and many other autoimmune diseases4,5. The prevalence of autoimmune thyroid diseases, including Graves’ disease and chronic autoimmune thyroiditis or Hashimoto’s thyroiditis has been estimated about 1–5% in general population6. Hashimoto’s thyroiditis is the most common etiology of hypothyroidism (47%) and about 9.6% of hypothyroidism occurs due to the medication for a previous hyperthyroidism7. Although the abnormal thyroid function is primarily manifested as hyperthyroidism in Graves’ disease and hypothyroidism in Hashimoto's thyroiditis, both diseases shared common pathophysiology of genetic and epigenetic causes resulting in thyroid autoimmunity8. Thus, it could be theorized that both hyper- and hypo- thyroid function affect autoimmune diseases.
Ménière’s disease is clinically diagnosed by recurrent vertigo attacks combined with cochlear symptoms of primarily low- or mid-frequency sensorineural hearing loss, tinnitus, or ear fullness9,10. The incidence of Ménière’s disease varies according to the ethnic population, which is estimated to be about 13–200 person-years11,12. The peak age of onset of Ménière’s disease has been reported to be about 40–60 years11,12. The sudden surge of endolymphatic flow, which shifts from the pars inferior (cochlea) to the pars superior (utricle and semicircular canals), stimulates the vestibular hair cells in the cristae of the semicircular canals and may induce vertigo attack in patients with Ménière’s disease13. However, the pathophysiologic causes for the increase of endolymphatic flow are not understood and are thought to be multifactorial, including abnormal immune response and metabolic endocrine dysfunctions, such as hypothyroidism13–15. Previous studies have suggested the association of hypothyroidism with Ménière’s disease14,16,17. To our knowledge, there has been little research on thyroid diseases for a relationship with Ménière’s disease.
The hypothesis of the present study was that other thyroid diseases, besides hypothyroidism, might have an impact on the occurrence of Ménière’s disease, because of the autoimmunity and metabolic changes according to the abnormal thyroid function. To delineate the association of various thyroid diseases with Ménière’s disease, the histories of goiter, hypothyroidism, thyroiditis, and hyperthyroidism were compared between Ménière’s disease and control groups.
Materials and methods
Study population
The Ethics Committee of Hallym University (2019-10-023) approved this study. Requirement for written informed consent was waived by the Institutional Review Board of the Ethics Committee of Hallym University18. All analyses adhered to the guidelines and regulations of the ethics committee of Hallym University18. The detailed description of The Korean National Health Insurance Service-Health Screening Cohort data was described elsewhere18.
Definition of Ménière’s disease (dependent variable)
If the participants were diagnosed with ICD-10 codes H810, we classified them as Ménière's disease19. From that group, we selected participants who were treated for Ménière's disease ≥ 2 times and had an audiometric examination (claim code: E6931–E6937, F6341–F6348) as a previous study19.
Levothyroxine medications users (independent variable)
Levothyroxine medication users were selected if participants took levothyroxine medications for ≥ 3 months.
Definition of Goiter (independent variable)
Goiter was defined if the participants were diagnosed with ICD-10 codes E04 (Other nontoxic goiter). Among them, we selected participants who were treated for goiter ≥ 2 times.
Definition of hypothyroidism (independent variable)
Hypothyroidism was defined if the participants were diagnosed with ICD-10 codes E02 (Subclinical iodine-deficiency hypothyroidism) and E03 (Other hypothyroidism). Among them, we selected participants who underwent treatment ≥ 2 times.
Definition of thyroiditis (independent variable)
Thyroiditis was defined if the participants were diagnosed with ICD-10 codes E06 (Thyroiditis). Among them, we selected the participants who treated for it ≥ 2 times.
Definition of hyperthyroidism (independent variable)
Hyperthyroidism was defined if the participants were diagnosed with ICD-10 codes E05 (hyperthyroidism). Among them, we selected the participants who treated it ≥ 2 times.
Definition of autoimmune thyroiditis (independent variable)
Autoimmune thyroiditis was defined if the participants were diagnosed with ICD-10 codes E063 (autoimmune thyroiditis). Among them, we selected the participants who were treated ≥ 2 times.
Participant selection
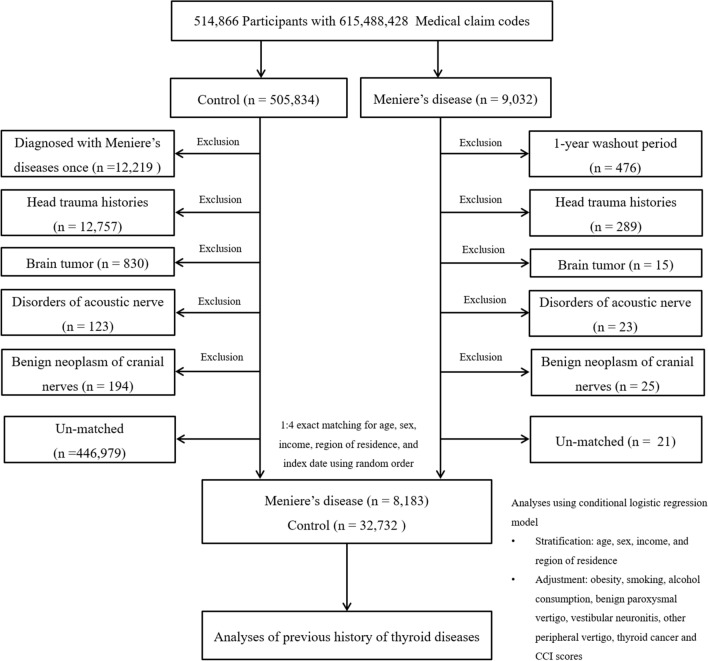
Patients with Ménière’s disease were selected from 514,866 participants with 615,488,428 medical claim codes from 2002 through 2015 (n = 9032)19. The control group was included if participants were not diagnosed with Ménière’s disease from 2002 through 2015 (n = 505,834)19. To select a patient diagnosed with Ménière’s disease for the first time, patients diagnosed with Ménière’s disease in 2002 were excluded (washout periods, n = 476)19. Control participants were excluded if the participants were diagnosed with Ménière’s disease once (n = 12,219)19. Participants who were treated for head trauma (ICD-10 codes: S00 to S09, diagnosed by neurologists, neurosurgeons, or emergency medicine doctors) ≥ 2 times with head and neck CT evaluations (Claim codes: HA401–HA416, HA441–HA443, HA451–HA453, HA461–HA463, or HA471–HA473) were excluded (n = 289 patients with Ménière’s disease, n = 12,757 control participants)19. Participants who were treated for brain tumor (ICD-10 codes: C70 to C72) ≥ 2 times (n = 15 for Ménière’s disease, n = 830 for control), disorders of the acoustic nerve (ICD-10 codes: H933) ≥ 2 times (n = 23 for Ménière’s disease, n = 123 for control) and benign neoplasm of the cranial nerves (ICD-10 codes: D333) ≥ 2 times (n = 25 for Ménière’s disease, n = 194 for control) were excluded19. Patients with Ménière’s disease were 1:4 matched with control participants for age, sex, income, and region of residence19. To minimize the selection bias, the control participants were selected in a randomized order18. The index date of each patient with Ménière’s disease was set as the time of treatment of Ménière’s disease19. The index date of control participants was set as the index date of their matched patient with Ménière’s disease19. Therefore, each patient with Ménière’s disease matched with control participants had the same index date19. During the matching procedure, 21 patients with Ménière’s disease and 446,979 control participants were excluded19. Ultimately, 8,183 patients with Ménière’s disease were 1:4 matched with 32,732 control participants (Fig. 1)19.
Figure 1.
A schematic illustration of the participant selection process used in the present study. Of a total of 514,866 participants, 8,183 of Meniere’s disease patients were 1:4 matched with 32,732 control participants for age, sex, income, and region of residence.
Covariates
Age groups were divided into 5-year intervals: 40–44, 45–49, 50–54, and 85+ years old (Total of 10 age groups)20. Income groups were classified as 5 classes (class 1 [lowest income]–5 [highest income]). The region of residence was grouped into urban and rural areas following our previous study20.
Tobacco smoking was categorized based on the participant’s current smoking status (nonsmoker, past smoker, and current smoker)18. Alcohol consumption was categorized based on the frequency of alcohol consumption (< 1 time a week and ≥ 1 time a week). Obesity was measured using body mass index (BMI, kg/m2)18. BMI was categorized as < 18.5 (underweight), ≥ 18.5 to < 23 (normal), ≥ 23 to < 25 (overweight), ≥ 25 to < 30 (obese I), and ≥ 30 (obese II) based on the Asia–Pacific criteria following the Western Pacific Regional Office (WPRO) 200021. Missing BMI (23/43,290 [0.053%]) was replaced by mean values of variable from final selected participants.
The Charlson Comorbidity Index (CCI) has been used widely to measure disease burden using 17 comorbidities22,23. In our study, we excluded cancer and metastatic cancer from CCI score. CCI was measured as the continuous variable (0 [no comorbidities] through 29 [multiple comorbidities])22,23.
Regarding Ménière’s disease, benign paroxysmal vertigo (ICD-10 codes: H811), vestibular neuronitis (ICD-10 codes: H812), other peripheral vertigo (ICD-10 codes: H813), and thyroid cancer (ICD-10 codes: C73) were additionally assigned if participants were treated ≥ 2 times19.
Statistical analyses
The general characteristics between the Ménière’s disease and control groups were compared using the Chi-square test19.
To analyze the odds ratios (ORs) with 95% confidence intervals (CIs), the conditional logistic regression model for Ménière’s disease in thyroid diseases was used19. The crude model 1 (adjusted for obesity, smoking, alcohol consumption, benign paroxysmal vertigo, vestibular neuronitis, other peripheral vertigo, thyroid cancer and CCI scores), and model 2 (additionally adjusted for Synthroid, goiter, hypothyroidism, thyroiditis, and hyperthyroidism in model 1) were used19. Because of levothyroxine medication, goiter, hypothyroidism, thyroiditis, and hyperthyroidism histories were closely related (S1 Table), we adjusted for them in model 219. The analyses were stratified by age, sex, income, and region of residence19.
For the subgroup analyses, we divided participants by age and sex (< 65 years old and ≥ 65 years old; men and women) and by income and region of residence (low and high; urban and rural) using crude, model 1, and model 219.
Two-tailed analyses were performed, and significance was defined as P values < 0.05. The SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) were used for statistical analyses19.
Results
The rates of goiter, hypothyroidism, thyroiditis, hyperthyroidism, and autoimmune thyroiditis were higher in the group with Ménière’s disease than in the control group (5.7% vs. 4.2% for goiter, 4.7% vs. 3.6% for hypothyroidism, 2.1% vs. 1.6% for thyroiditis, 3.6% vs. 2.5% for hyperthyroidism, and 0.99% vs. 0.67% for autoimmune thyroiditis, Table 1). The rate of levothyroxine medication was 4.8% in the Ménière’s disease group and 3.6% in the control group. Age, sex, income, and region of residence were exactly matched between the Ménière’s disease and control groups (P = 1.000). The distributions of obesity, smoking status, alcohol consumption, CCI score, benign paroxysmal vertigo, vestibular neuronitis, and other peripheral vertigo were significantly different between the Ménière’s disease and control groups (all P < 0.001).
Table 1.
General characteristics of participants.
| Characteristics | Total participants | ||
|---|---|---|---|
| Meniere’s disease (n, %) | Control (n, %) | P value | |
| Age (years old) | 1.000 | ||
| 40–44 | 123 (1.5) | 492 (1.5) | |
| 45–49 | 537 (6.6) | 2148 (6.6) | |
| 50–54 | 1199 (14.7) | 4796 (14.7) | |
| 55–59 | 1407 (17.2) | 5628 (17.2) | |
| 60–64 | 1349 (16.5) | 5396 (16.5) | |
| 65–69 | 1285 (15.7) | 5140 (15.7) | |
| 70–74 | 1151 (14.1) | 4604 (14.1) | |
| 75–79 | 736 (9.0) | 2944 (9.0) | |
| 80–84 | 316 (3.9) | 1264 (3.9) | |
| 85 + | 80 (1.0) | 320 (1.0) | |
| Sex | 1.000 | ||
| Male | 2885 (35.3) | 11,540 (35.3) | |
| Female | 5298 (64.7) | 21,192 (64.7) | |
| Income | 1.000 | ||
| 1 (lowest) | 1397 (17.1) | 5588 (17.1) | |
| 2 | 1038 (12.7) | 4152 (12.7) | |
| 3 | 1259 (15.4) | 5036 (15.4) | |
| 4 | 1707 (20.9) | 6828 (20.9) | |
| 5 (highest) | 2782 (34.0) | 11,128 (34.0) | |
| Region of residence | 1.000 | ||
| Urban | 3445 (42.1) | 13,780 (42.1) | |
| Rural | 4738 (57.9) | 18,952 (57.9) | |
| Obesitya | < 0.001* | ||
| Underweight | 167 (2.0) | 832 (2.5) | |
| Normal | 2782 (34.0) | 11,587 (35.4) | |
| Overweight | 2290 (28.0) | 8752 (26.7) | |
| Obese I | 2694 (32.9) | 10,442 (31.9) | |
| Obese II | 250 (3.1) | 1119 (3.4) | |
| Smoking status | |||
| Nonsmoker | 6640 (81.1) | 25,851 (79.0) | < 0.001* |
| Past smoker | 829 (10.1) | 3017 (9.2) | |
| Current smoker | 714 (8.7) | 3864 (11.8) | |
| Alcohol consumption | |||
| < 1 time a week | 6209 (75.9) | 23,867 (72.9) | < 0.001* |
| ≥ 1 time a week | 1974 (24.1) | 8865 (27.1) | |
| CCI score | |||
| 0 | 5128 (62.7) | 22,183 (67.8) | < 0.001* |
| 1 | 1690 (20.7) | 5447 (16.6) | |
| 2 | 834 (10.2) | 3071 (9.4) | |
| 3 | 246 (3.0) | 903 (2.8) | |
| ≥ 4 | 285 (3.5) | 1128 (3.5) | |
| Benign paroxysmal vertigo | 2800 (34.2) | 2170 (6.6) | < 0.001* |
| Vestibular neuronitis | 900 (11.0) | 467 (1.4) | < 0.001* |
| Other peripheral vertigo | 1913 (23.4) | 1517 (4.6) | < 0.001* |
| Thyroid cancer | 81 (0.9) | 296 (1.0) | 0.469 |
| Period of taking levothyroxine | |||
| < 3 month | 7793 (95.2) | 31,557 (96.4) | < 0.001* |
| ≥ 3 month | 390 (4.8) | 1175 (3.6) | |
| Goiter | 462 (5.7) | 1377 (4.2) | < 0.001* |
| Hypothyroidism | 385 (4.7) | 1168 (3.6) | < 0.001* |
| Thyroiditis | 174 (2.1) | 518 (1.6) | < 0.001* |
| Hyperthyroidism | 292 (3.6) | 805 (2.5) | < 0.001* |
| Autoimmune thyroiditis | 81 (0.99) | 219 (0.67) | 0.002* |
CCI Charlson comorbidity index.
*Chi-square test. Significance at P < 0.05.
aObesity (BMI, body mass index, kg/m2) was categorized as < 18.5 (underweight), ≥ 18.5 to < 23 (normal), ≥ 23 to < 25 (overweight), ≥ 25 to < 30 (obese I), and ≥ 30 (obese II).
The histories of goiter, hypothyroidism, and hyperthyroidism were related with the increased OR for Ménière’s disease in model 2. The odds for Ménière’s disease were highest in hyperthyroidism, followed by hypothyroidism and goiter (adjusted OR 1.19, 95% CI 1.04–1.36 for goiter; adjusted OR 1.21, 95% CI 1.02–1.44 for hypothyroidism; adjusted OR 1.27, 95% CI 1.09–1.49 for hyperthyroidism, Table 2).
Table 2.
Crude and adjusted odd ratios (95% confidence interval) for Meniere’s disease in levothyroxine, goiter, hypothyroidism, thyroiditis, hyperthyroidism, and autoimmune thyroiditis.
| Characteristics | Odd ratios for Meniere’s disease | |||||
|---|---|---|---|---|---|---|
| Crudea | P value | Model 1a,b | P value | Model 2a,c | P value | |
| Total participants (n = 40,915) | ||||||
| Levothyroxine | 1.27 (1.12–1.44) | < 0.001* | 1.28 (1.09–1.50) | 0.003* | 0.94 (0.76–1.16) | 0.583 |
| Goiter | 1.37 (1.23–1.53) | < 0.001* | 1.28 (1.13–1.45) | < 0.001* | 1.19 (1.04–1.36) | 0.011* |
| Hypothyroidism | 1.34 (1.19–1.51) | < 0.001* | 1.34 (1.17–1.53) | < 0.001* | 1.21 (1.02–1.44) | 0.030* |
| Thyroiditis | 1.35 (1.14–1.61) | < 0.001* | 1.23 (1.01–1.49) | 0.041* | 1.05 (0.85–1.29) | 0.667 |
| Hyperthyroidism | 1.47 (1.28–1.69) | < 0.001* | 1.37 (1.18–1.60) | < 0.001* | 1.27 (1.09–1.49) | 0.003* |
| Autoimmune thyroiditis | 1.49 (1.15–1.92) | 0.002* | 1.26 (0.94–1.69) | 0.122 | ||
CCI Charlson Comorbidity Index.
*Conditional logistic regression model, significance at P < 0.05.
aModels stratified by age, sex, income, and region of residence.
bModel 1 was adjusted for obesity, smoking, alcohol consumption, benign paroxysmal vertigo, vestibular neuronitis, other peripheral vertigo, thyroid cancer, and CCI scores.
cModel 2 was adjusted for model 1 plus levothyroxine, goiter, hypothyroidism, thyroiditis, hyperthyroidism.
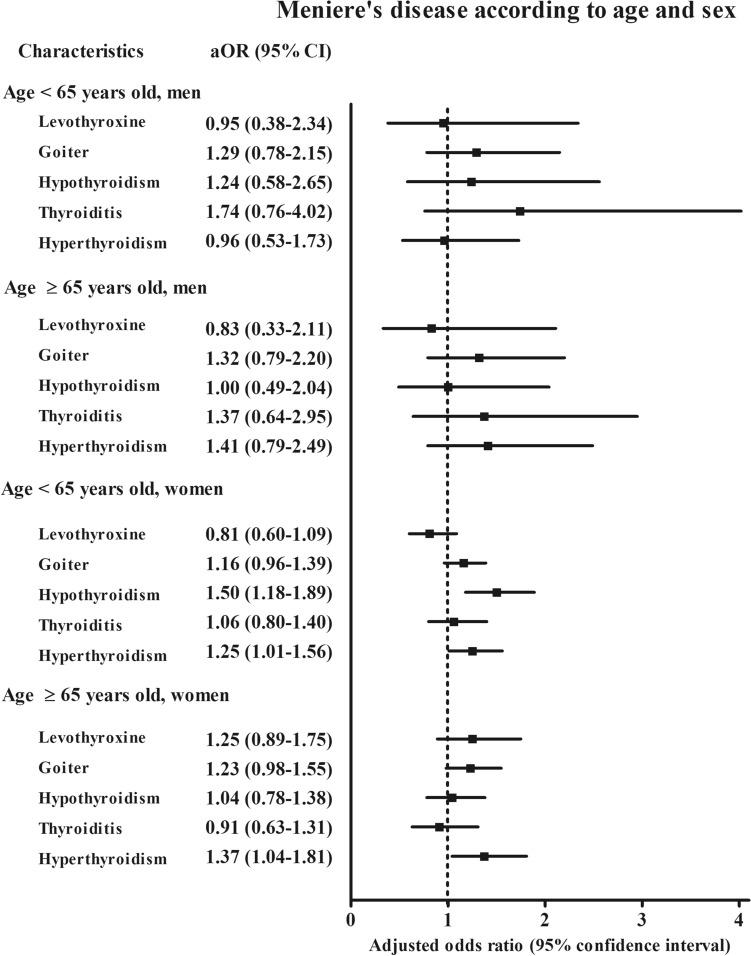
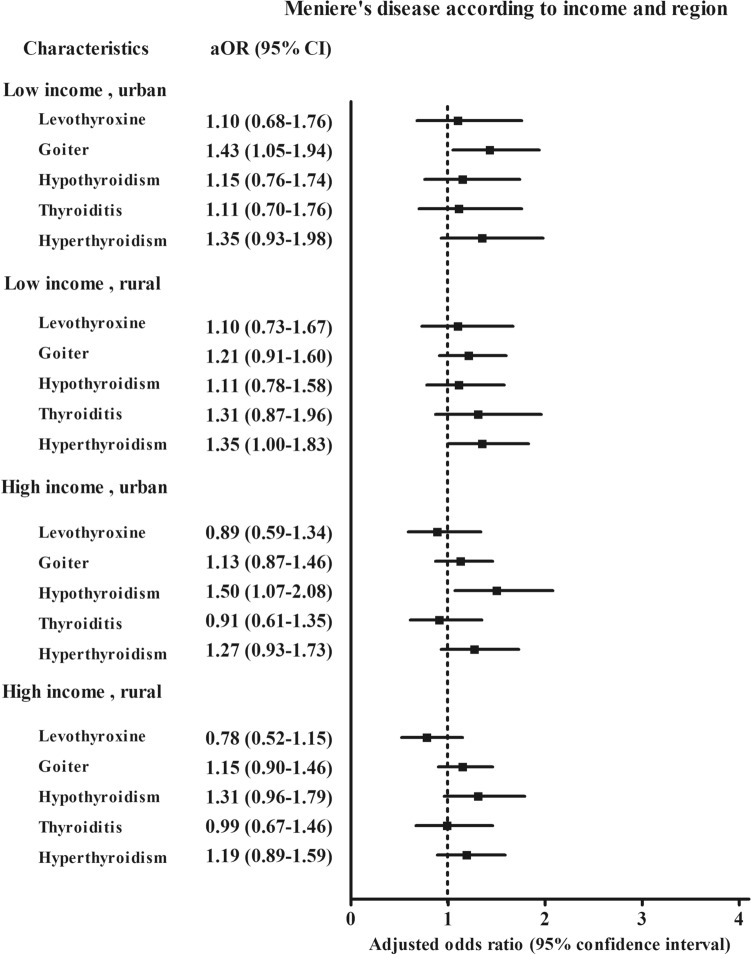
According to age and sex, hyperthyroidism was associated with the higher odds for Ménière’s disease in the women’s subgroups (adjusted OR 1.25, 95% CI 1.01–1.56 for the < 65-year-old women group and adjusted OR 1.37, 95% CI 1.04–1.81 for the ≥ 65-year-old women group) (Fig. 2 and S2 Table). Hypothyroidism was associated with a 1.5-fold higher odds for Ménière’s disease in the < 65-year-old women subgroup (95% CI 1.18–1.89). According to income and region of residence, goiter was 1.43 times more likely to be associated with Ménière’s disease in the low income, urban subgroup (95% CI 1.05–1.94, Fig. 3 and S3 Table). Hyperthyroidism was 1.35 times more likely associated with Ménière’s disease in the low income, rural subgroup (95% CI 1.00–1.98). Hypothyroidism was 1.5 times more likely to be associated with Ménière’s disease in the high income, urban subgroup (95% CI 1.07–2.08).
Figure 2.
The odds ratios (95% confidence interval) of levothyroxine medication, goiter, hypothyroidism, thyroiditis, and hyperthyroidism for Meniere’s disease according to age and sex.
Figure 3.
The odds ratios (95% confidence interval) of levothyroxine medication, goiter, hypothyroidism, thyroiditis, and hyperthyroidism for Meniere’s disease according to region of residence.
Discussion
Ménière’s disease was positively related with the previous histories of goiter, hypothyroidism, and hyperthyroidism in the present study. Women showed a consistent association of Ménière’s disease with hypothyroidism (< 65 years old) and hyperthyroidism. This study adjusted for levothyroxine medication and other thyroid diseases, thereby separately analyzing the relation of each thyroid disease with Ménière’s disease.
Previous studies described the relation of hypothyroidism with Ménière’s disease14,17. However, most prior studies were limited with a small sample size17,24. In a case–control study, the rate of thyroid hormone medication was higher in patients with Ménière’s disease than in the age- and sex-matched control group (32% [16/50] vs. 4% [2/50], P < 0.001)17. In another small case series, the thyroxin medication improved the symptoms of Ménière’s disease patients with hypothyroidism (100%, 12/12)24. A cohort study conducted among 27,050 individuals in Taiwan demonstrated a 1.31-fold higher odds for hypothyroidism in patients with Ménière’s disease (95% CI 1.14–1.51)16. However, they did not consider other thyroid diseases.
The inflammatory or metabolic changes in the patients with thyroid diseases could have an impact on the inner ear inflammation and homeostasis of endolymphatic flow. The association of inflammation with thyroid dysfunction has been acknowledged25. For instance, inflammatory cytokines including tumor necrosis factor α and interleukin 1 and 6 reduced the expressions of sodium/iodine symporters, in that impeded the iodide uptake in the thyroid gland25,26. Moreover, the association of thyroid diseases with the metabolic disease of obesity has been reported based on the inflammatory and metabolic etiology27,28. The inflammatory or degenerative changes of the inner ear epithelia could increase the risk of Ménière’s disease. Although the contribution of autoimmune response to the pathogenesis of Ménière’s disease has been suggested, only the immune complexes were observed only in about 7% of patients with Ménière’s disease and the biomarker for autoimmunity in Ménière’s disease is still elusive29. A few endotypes of Ménière’s diseases were suggested with different etiologies inducing hypoplasia or inflammation or degeneration of the endolymphatic sac30,31. The maintenance of ionic and non-ionic compositions of endolymph is crucial for transduction of acceleration in the vestibular labyrinth, which consists of a unique composition of low calcium levels of endolymph (280 μM) compared to perilymph (1 Mm)32,33. Thus, the perturbation of this composition due to altered metabolism could impact the vestibular function. Indeed, it was suggested that the abnormal metabolisms of patients with thyroid disease could induce the endolymphatic hydrops and Ménière’s disease14,15. Hypothyroidism supposedly changes the composition of endolymphatic fluid through the diffusion of thyroid autoantibody complexes in the endolymph34. In addition, the common anion exchanger such as pendrin, which is encoded by Solute Carrier 26A4 (SLC26A4), could be defected in the patients with goiter and/or hypothyroidism. Therefore, altering the endolymph composition and endocochlear potential, as in the case of Pendred syndrome35.
Although autoimmune thyroiditis did not show a statistically significant association with Ménière’s disease in this study, the abnormal autoimmune responses in the patients with autoimmune thyroid disease could influence the inner ear autoimmune dysfunctions related with Ménière’s disease36–38. Patients with Ménière’s disease had a higher rate of thyroid autoantibodies of anti-thyroperoxidase antibody, anti-TSH receptor antibody, anti-thyroperoxidase antibody, anti-thyroglobulin antibody compared to control and acute unilateral peripheral vestibulopathy groups38. Ménière’s disease, especially in bilateral cases, has been reported to be related with immune dysfunctions including allergy36,37,39,40. A prospective case–control study suggested the higher rate of positivity to cellular and humoral autoimmune tests in 10 bilateral patients with Ménière’s disease36. In addition, the prevalence of systemic autoimmune diseases, such as rheumatoid arthritis, systemic lupus erythematosus, and ankylosing spondylitis was higher in 690 patients with Ménière’s disease than in the general population41. In particular, familial cases of Ménière’s disease have been reported to harbor some genetic factors, such as certain human leucocyte antigens29,42. Therefore, it was presumed that Ménière’s disease could be included in the spectrum of autoimmune inner ear disease41. Autoimmune mechanism may have an influence on hypothyroidism, hyperthyroidism, and thyroiditis8. Therefore, the possible mediating role of autoimmunity with respect to thyroid diseases in Ménière’s disease cannot be excluded in this study.
Both hypo- and hyperthyroidism were related to Ménière’s disease in this study. These associations could have originated from the common pathophysiology among the thyroid diseases, such as autoimmune responses and inflammation4,5. In addition, the effects of treatment of abnormal thyroid function could influence the overlapping of thyroid diseases. For instance, the treatment of hypothyroidism could induce a status of hyperthyroidism. Thus, it was reported that the treatment of hypothyroidism accounted for approximately 9.6% of causes of hypothyroidism7. Moreover, it was reported that about 14% of patients with Ménière’s disease showed a state of hyperthyroidism due to l-thyroxine therapy38.
The present study was based on a large, representative national cohort. The large study population guaranteed a sufficient number of control population matched for age, sex, income, and region of residence. In addition, potential confounders were comprehensively reviewed and adjusted for obesity: smoking; alcohol consumption; comorbidities using CCI score; other vestibular diseases of benign paroxysmal positional vertigo, vestibular neuronitis, and other peripheral vertigo; levothyroxine medication; and other thyroid diseases of goiter, hypothyroidism, thyroiditis, and hyperthyroidism. The use of the health claim data meant that the thyroid status could not be measured by thyroid function tests because there was a possibility that the thyroid status was heterogenous among participants. In addition, subclinical or untreated thyroid diseases could be misclassified. For Ménière’s disease, the vestibular function tests could not be assessed; thus the severity and management of Ménière’s disease might vary among participants. However, the correlation of vestibular function tests with the types and severity of Ménière’s disease could also be variable, and the clinical otovestibular symptoms assessed by the otologist might be more reliable for the diagnosis of Ménière’s disease. The endotypes of Ménière’s disease, including a degenerating distal endolymphatic sac and hypoplastic endolymphatic sac, could not be differentiated in the present study31. Although we could not access low- to-medium frequency sensorineural hearing loss, which is accompanied in the definite Ménière’s disease, the participants who underwent pure tone audiometry were enrolled, thus, probable Ménière’s disease might be included in this study43.
Conclusion
Thyroid diseases of goiter, hypothyroidism, and hyperthyroidism were related with the increased risk of Ménière’s disease. The associations of hypo- and hyperthyroidism with Ménière’s disease were consistent in women.
Supplementary information
Acknowledgements
We would like to thank Editage (www.editage.co.kr) for English language editing.
Author contributions
H.G.C. designed the study; J.H.W., C.M., and H.G.C. analyzed the data; S.Y.K. and H.G.C. drafted and revised the paper; J.H.W. draw figures; all authors approved the final version of the manuscript.
Funding
This work was supported in part by a research Grants (NRF-2018-R1D1A1A02085328 and 2020R1A2C4002594) from the National Research Foundation (NRF) of Korea.
Data availability
Releasing of the data by the researcher is not allowed legally. All data are available from the database of the National health Insurance Sharing Service (NHISS) https://nhiss.nhis.or.kr/. NHISS allows data access, at a particular cost, for any researcher who promises to follow the research ethics. Data of this article can be downloaded from the website after promising to follow the research ethics.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-75404-y.
References
- 1.Mullur R, Liu YY, Brent GA. Thyroid hormone regulation of metabolism. Physiol. Rev. 2014;94:355–382. doi: 10.1152/physrev.00030.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoermann R, Midgley JE, Larisch R, Dietrich JW. Homeostatic control of the thyroid-pituitary axis: perspectives for diagnosis and treatment. Front. Endocrinol. (Lausanne) 2015;6:177. doi: 10.3389/fendo.2015.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehran L, Amouzegar A, Azizi F. Thyroid disease and the metabolic syndrome. Curr. Opin. Endocrinol. Diabetes Obes. 2019;26:256–265. doi: 10.1097/MED.0000000000000500. [DOI] [PubMed] [Google Scholar]
- 4.Jenkins RC, Weetman AP. Disease associations with autoimmune thyroid disease. Thyroid. 2002;12:977–988. doi: 10.1089/105072502320908312. [DOI] [PubMed] [Google Scholar]
- 5.Inoue N, et al. Associations between autoimmune thyroid disease prognosis and functional polymorphisms of susceptibility genes, CTLA4, PTPN22, CD40, FCRL3, and ZFAT, previously revealed in genome-wide association studies. J. Clin. Immunol. 2012;32:1243–1252. doi: 10.1007/s10875-012-9721-0. [DOI] [PubMed] [Google Scholar]
- 6.McLeod DS, Cooper DS. The incidence and prevalence of thyroid autoimmunity. Endocrine. 2012;42:252–265. doi: 10.1007/s12020-012-9703-2. [DOI] [PubMed] [Google Scholar]
- 7.Diez JJ. Hypothyroidism in patients older than 55 years: an analysis of the etiology and assessment of the effectiveness of therapy. J. Gerontol. A Biol. Sci. Med. Sci. 2002;57:M315–320. doi: 10.1093/gerona/57.5.m315. [DOI] [PubMed] [Google Scholar]
- 8.Tomer Y. Mechanisms of autoimmune thyroid diseases: from genetics to epigenetics. Annu. Rev. Pathol. 2014;9:147–156. doi: 10.1146/annurev-pathol-012513-104713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goebel JA. 2015 Equilibrium committee amendment to the 1995 AAO-HNS guidelines for the definition of Meniere's disease. Otolaryngol. Head Neck Surg. 2016;154:403–404. doi: 10.1177/0194599816628524. [DOI] [PubMed] [Google Scholar]
- 10.Kim CH, Shin JE, Yoo MH, Park HJ. Direction-changing and direction-fixed positional nystagmus in patients with vestibular neuritis and meniere disease. Clin. Exp. Otorhinolaryngol. 2019;12:255–260. doi: 10.21053/ceo.2018.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruderer SG, Bodmer D, Stohler NA, Jick SS, Meier CR. Population-based study on the epidemiology of Meniere's disease. Audiol. Neurootol. 2017;22:74–82. doi: 10.1159/000475875. [DOI] [PubMed] [Google Scholar]
- 12.Alexander TH, Harris JP. Current epidemiology of Meniere's syndrome. Otolaryngol. Clin. North Am. 2010;43:965–970. doi: 10.1016/j.otc.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Gibson WPR. Meniere's disease. Adv. Otorhinolaryngol. 2019;82:77–86. doi: 10.1159/000490274. [DOI] [PubMed] [Google Scholar]
- 14.Shambaugh GE., Jr Endocrine aspects of Meniere's disease. Laryngoscope. 1959;69:1027–1032. doi: 10.1288/00005537-195908000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Powers WH. Metabolic aspects of Meniere's disease. Laryngoscope. 1972;82:1716–1725. doi: 10.1288/00005537-197209000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Lin WL, et al. Hypothyroidism is an independent risk factor for Meniere's disease: a population-based cohort study. Medicine Baltimore. 2019;98:e15166. doi: 10.1097/MD.0000000000015166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brenner M, Hoistad DL, Hain TC. Prevalence of thyroid dysfunction in patients with Meniere's disease. Arch. Otolaryngol. Head. Neck Surg. 2004;130:226–228. doi: 10.1001/archotol.130.2.226. [DOI] [PubMed] [Google Scholar]
- 18.Kim SY, Min C, Oh DJ, Choi HG. Tobacco smoking and alcohol consumption are related to benign parotid tumor: a nested case-control study using a national health screening cohort. Clin. Exp. Otorhinolaryngol. 2019;12:412–419. doi: 10.21053/ceo.2018.01774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim SY, Lee CH, Min C, Park IS, Choi HG. Bidirectional analysis of the association between Meniere's disease and depression: two longitudinal follow-up studies using a national sample cohort. Clin. Otolaryngol. 2020 doi: 10.1111/coa.13558. [DOI] [PubMed] [Google Scholar]
- 20.Kim SY, Min C, Oh DJ, Choi HG. Bidirectional association between GERD and asthma: two longitudinal follow-up studies using a national sample cohort. J. Allergy Clin. Immunol. Pract. 2020;8:1005–1013. doi: 10.1016/j.jaip.2019.10.043. [DOI] [PubMed] [Google Scholar]
- 21.WHO/IASO/IOTR. The Asia-Pacific Perespective: Redefining Obesity and its Treatment. Health Communications Australia Pty Ltd (2000).
- 22.Quan H, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am. J. Epidemiol. 2011;173:676–682. doi: 10.1093/aje/kwq433. [DOI] [PubMed] [Google Scholar]
- 23.Quan H, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med. Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 24.Santosh UP, Rao MS. Incidence of hypothyroidism in Meniere's disease. J. Clin. Diagn. Res. 2016;10:MC01–MC03. doi: 10.7860/JCDR/2016/17587.7759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ajjan RA, et al. The sodium iodide symporter gene and its regulation by cytokines found in autoimmunity. J. Endocrinol. 1998;158:351–358. doi: 10.1677/joe.0.1580351. [DOI] [PubMed] [Google Scholar]
- 26.Schumm-Draeger PM. Sodium/iodide symporter (NIS) and cytokines. Exp. Clin. Endocrinol. Diabetes. 2001;109:32–34. doi: 10.1055/s-2001-11018. [DOI] [PubMed] [Google Scholar]
- 27.Longhi S, Radetti G. Thyroid function and obesity. J. Clin. Res. Pediatr. Endocrinol. 2013;5(Suppl 1):40–44. doi: 10.4274/jcrpe.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fontenelle LC, et al. Thyroid function in human obesity: underlying mechanisms. Horm. Metab. Res. 2016;48:787–794. doi: 10.1055/s-0042-121421. [DOI] [PubMed] [Google Scholar]
- 29.Gallego-Martinez A, Lopez-Escamez JA. Genetic architecture of Meniere's disease. Hear Res. 2019 doi: 10.1016/j.heares.2019.107872. [DOI] [PubMed] [Google Scholar]
- 30.Frejo L, et al. Extended phenotype and clinical subgroups in unilateral Meniere disease: a cross-sectional study with cluster analysis. Clin. Otolaryngol. 2017;42:1172–1180. doi: 10.1111/coa.12844. [DOI] [PubMed] [Google Scholar]
- 31.Bachinger D, et al. Endotype-phenotype patterns in Meniere's disease based on gadolinium-enhanced MRI of the vestibular aqueduct. Front. Neurol. 2019;10:303. doi: 10.3389/fneur.2019.00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marcus DC, Wangemann P. Cochlear and Vestibular Function and Dysfunction. Physiology and Pathology of Chloride Transporters and Channels in the Nervous System—From molecules to diseases. Amsterdam: Elsevier; 2009. pp. 421–433. [Google Scholar]
- 33.Hudspeth AJ, Gillespie PG. Pulling springs to tune transduction: adaptation by hair cells. Neuron. 1994;12:1–9. doi: 10.1016/0896-6273(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 34.Modugno GC, et al. A relationship between autoimmune thyroiditis and benign paroxysmal positional vertigo? Med. Hypotheses. 2000;54:614–615. doi: 10.1054/mehy.1999.0905. [DOI] [PubMed] [Google Scholar]
- 35.Kopp P, Bizhanova A. Clinical and molecular characteristics of Pendred syndrome. Ann. Endocrinol. (Paris) 2011;72:88–94. doi: 10.1016/j.ando.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 36.Hughes GB, Kinney SE, Barna BP, Calabrese LH. Autoimmune reactivity in Meniere's disease: a preliminary report. Laryngoscope. 1983;93:410–417. doi: 10.1002/lary.1983.93.4.410. [DOI] [PubMed] [Google Scholar]
- 37.Hughes GB, Barna BP, Kinney SE, Calabrese LH, Nalepa NJ. Clinical diagnosis of immune inner-ear disease. Laryngoscope. 1988;98:251–253. doi: 10.1288/00005537-198803000-00001. [DOI] [PubMed] [Google Scholar]
- 38.Fattori B, et al. Possible association between thyroid autoimmunity and Meniere's disease. Clin. Exp. Immunol. 2008;152:28–32. doi: 10.1111/j.1365-2249.2008.03595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suzuki M, Kitahara M. Immunologic abnormality in Meniere's disease. Otolaryngol. Head Neck Surg. 1992;107:57–62. doi: 10.1177/019459989210700109. [DOI] [PubMed] [Google Scholar]
- 40.Weinreich HM, Agrawal Y. The link between allergy and Meniere's disease. Curr. Opin. Otolaryngol. Head Neck Surg. 2014;22:227–230. doi: 10.1097/MOO.0000000000000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gazquez I, et al. High prevalence of systemic autoimmune diseases in patients with Meniere's disease. PLoS ONE. 2011;6:e26759. doi: 10.1371/journal.pone.0026759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xenellis J, Morrison AW, McClowskey D, Festenstein H. HLA antigens in the pathogenesis of Meniere's disease. J. Laryngol. Otol. 1986;100:21–24. doi: 10.1017/s0022215100098698. [DOI] [PubMed] [Google Scholar]
- 43.Lopez-Escamez JA, et al. Diagnostic criteria for Meniere's disease. J. Vestib. Res. 2015;25:1–7. doi: 10.3233/VES-150549. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Releasing of the data by the researcher is not allowed legally. All data are available from the database of the National health Insurance Sharing Service (NHISS) https://nhiss.nhis.or.kr/. NHISS allows data access, at a particular cost, for any researcher who promises to follow the research ethics. Data of this article can be downloaded from the website after promising to follow the research ethics.