Abstract
A 20-year-old male without any symptoms was referred for heart murmur on a medical examination. A thrill was palpable at the upper left sternal border. His cardiac murmur showed respiratory variation. The systolic murmur was louder (Levine grade IV/VI) during expiration and diminished during inspiration (Levine grade I/VI). He was thin and had a narrow thoracic cage in the anteroposterior direction due to straight back syndrome (SBS). An echocardiogram and a right ventriculogram showed changes in the diameter of the right ventricular outflow tract (RVOT) on respiration. During expiration, the RVOT was compressed and narrow, while it was expanded during inspiration. Cardiac catheterization demonstrated a 10-mmHg of pressure gradient across the RVOT during expiration but no pressure gradient during inspiration. Thus, respiratory compression to the RVOT by a narrow thoracic cage due to SBS was the cause of the cardiac murmur with respiratory alterations. Our case highlights the importance of physical examination, including an inspection of the patient’s physique.
<Learning objective: When examining a patient with a cardiac murmur, respiratory alterations of cardiac murmurs should be auscultated. In these cases, straight back syndrome would be one of the differential diagnoses and should be considered. During a physical examination, inspection of the patient’s physique is also important.>
Keywords: Straight back syndrome, Cardiac murmur, Respiratory variation, Right ventricular outflow tract
Introduction
Straight back syndrome (SBS) is a congenital disease characterized by a deformed thoracic spine. Specifically, normal kyphosis of a thoracic spine is absent, and a thoracic spine is more straight than normal. SBS usually occurs in young and slim patients. Rawlings first reported SBS as a pseudo-heart syndrome in 1960 [1]. In patients with SBS, a cardiac murmur is sometimes auscultated; however, detailed examinations typically do not detect any cardiac abnormalities. Therefore, SBS is referred to as pseudo-heart syndrome and is often overlooked [2]. It has also been reported that SBS mimics some cardiac diseases that produce abnormal cardiac murmurs such as atrial septal defect, pulmonary stenosis, or mitral regurgitation [3]. The mechanisms by which SBS produces a cardiac murmur have not been well investigated.
Case report
The subject was a 20-year-old male who was completely asymptomatic and had an unremarkable medical history. A cardiac murmur was identified during a medical examination at his university. A cardiac anomaly was suspected at a neighboring clinic; therefore, he was referred to our hospital for further evaluation. He was thin, with a height of 175 cm and a body weight of 57 kg. His body mass index was 18.6 kg/m2 (Fig. 1A). A thrill was palpable at the upper left sternal border. A cardiac systolic murmur was clearly auscultated at the upper left sternal border.
Fig. 1.
(A) The patient is very thin, and physiological kyphosis of the thoracic spine is not observed. (B) Chest radiography revealed a cardiothoracic ratio of 40%. In the lateral image, the loss of normal kyphosis of the thoracic spine is observed, suggesting straight back syndrome. (C) A diagnostic criterion of straight back syndrome.
In straight back syndrome, the measured distance from the mid-anterior surface of the eighth thoracic (T8) spine to the vertical line that connects between the top of anterior border of the T4 spine to the anterior surface of the inferior part of the T12 is less than 1.2 cm. (D) A three-dimensional computed tomography clearly reveals the loss of normal kyphosis of the thoracic spine, referred to as straight back syndrome. This loss of kyphosis compresses the whole heart, especially the outflow tract of the right ventricle.
Interestingly, the murmur showed respiratory variations (Online Soundtrack 1). The systolic murmur was louder (Levine grade IV/VI) during expiration and diminished during inspiration (Levine grade I/VI). His electrocardiogram was normal, and the chest radiograph revealed a cardiothoracic ratio of 40% (Fig. 1B). In the lateral image, we observed loss of physiological kyphosis of the thoracic spine. The measured distance from the mid-anterior surface of the eighth thoracic spine (T8) to the vertical line that connects between the top of anterior border of the T4 spine to the anterior surface of the inferior part of the T12 was less than 1.2 cm (Fig. 1C), suggesting SBS [4]. An echocardiogram did not show any cardiac anomalies such as atrial septal defect, pulmonary stenosis, or mitral regurgitation. A three-dimensional computed tomography also revealed a straight-shaped thoracic spine (Fig. 1D). The straight back caused narrowing of the thoracic cage in the anteroposterior direction through the loss of normal kyphosis of the thoracic spine and compressed the whole heart, especially the right ventricular outflow tract (RVOT).
We hypothesized that his thin thoracic cage and straight back caused the systolic murmur and its alterations during respiration. When he expired, the length of the thoracic cage shortened in the anteroposterior direction. This physically narrowed the RVOT and caused the pressure gradient across the RVOT to increase, which made the murmur louder than that detected during inspiration. To prove this hypothesis, we performed a phonocardiogram, a more precise echocardiogram, and cardiac catheterization.
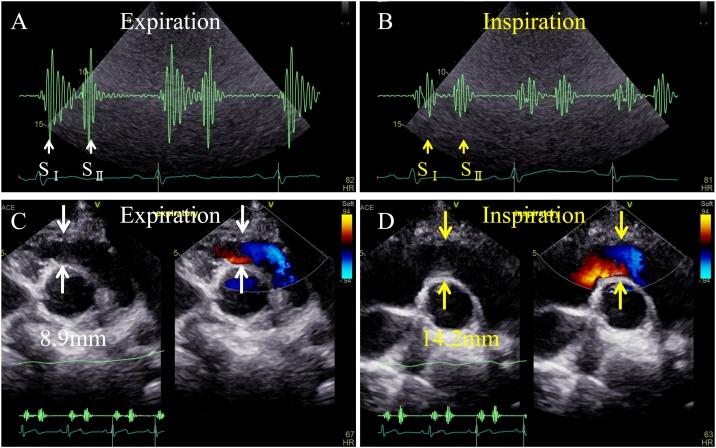
The phonocardiogram showed that the heart sounds and the systolic murmur were louder during expiration and quieter during inspiration (Fig. 2A, B). On echocardiogram, the diameter of the RVOT was 8.9 mm during expiration and enlarged to 14.2 mm during inspiration at the end-systolic phase (Fig. 2C, D, Online Movies 1, 2). Simultaneous pressure waveforms of the main pulmonary artery and the RV revealed a 10-mmHg of pressure gradient across the RVOT during expiration; however, no pressure gradient was observed during inspiration (Fig. 3A). The RVOT area measured by Gorlin’s formula during catheterization was 4.75 cm2 in the expiratory phase, which enlarged to 9.67 cm2 in inspiration. In a lateral image of the right ventriculogram at end-systole, the narrowest portion of the RVOT was 7.9 mm in diameter during expiration and enlarged to 9.5 mm in diameter during inspiration (Fig. 3B, C, Online Movies 3, 4).
Fig. 2.
(A) A phonocardiogram during expiration shows that the heart sounds and the systolic murmur are louder. (B) A phonocardiogram during inspiration shows that the heart sounds and the systolic murmur are diminished. (C) On a transthoracic echocardiogram, the right ventricle just beneath the outflow tract is 8.9 mm in diameter in the anteroposterior direction during expiration. The white arrows suggest the narrowest portion of the right ventricle, which is the proximal end of the outflow tract. (D) On a transthoracic echocardiogram, the right ventricle just beneath the outflow tract is widened to 14.2 mm in diameter in the anteroposterior direction during inspiration. The yellow arrows suggest the narrowest portion of the right ventricle, which is the proximal end of the outflow tract.
Fig. 3.
(A) Simultaneous pressure waveforms of the main pulmonary artery and the right ventricle reveal a 10-mmHg of pressure gradient across the right ventricular outflow tract during expiration, and no pressure gradient during inspiration. (B) A lateral image of the right ventriculogram at end-systole during expiration. The narrowest portion of the right ventricular outflow tract is 7.9 mm in diameter. (C) A lateral image of the right ventriculogram at end-systole during inspiration. The narrowest portion of the right ventricular outflow tract is enlarged to 9.5 mm in diameter.
Thus, respiratory-related changes of the thoracic cage in the anteroposterior direction through SBS affected the dimension of the RVOT and altered the pressure gradient across the RVOT and the intensity of the systolic murmur.
Discussion
SBS was first introduced as a pseudo-heart syndrome without cardiac abnormalities [1]. However, patients with SBS sometimes present with cardiac disease [5], [6]. Mitral valve prolapse was the most common associated cardiac condition, having been reported in about 64% of SBS cases [7]. The bicuspid aortic valve was also observed in patients with SBS. Still, it was rare [8]. SBS also can affect the respiratory system by causing compression to the trachea [7].
Davies et al. reported the diagnostic criteria of SBS in 1980 [4]. When drawing a line connecting T4 and T12, the height of this vertical line to the anterior border to T8 is longer than 2.0 cm in normal subjects. However, in patients with SBS, the height is less than 1.2 cm (Fig. 1C).
A case with SBS and alterations of the intensity of the systolic ejection murmur during respiration was reported [9]. In this case, an echocardiogram showed respiratory variations of the RVOT flow velocity (i.e. the peak velocity was increased during expiration and decreased during inspiration). Therefore, respiratory-altered compression of the RVOT between the thoracic spine and the sternum was considered as a cause of respiratory alterations of the cardiac murmur. However, morphological changes of the RVOT, along with respiratory status, have not been investigated in patients with SBS.
In our case, the RVOT was compressed between the sternum anteriorly and the thoracic spine posteriorly (Online Fig. 1). The stenosis in the RV was the proximal end of the outflow tract; therefore, the hemodynamics was also similar to a double-chambered RV. In a case of fixed RVOT stenosis (e.g. valvular stenosis), it is considered that cardiac murmur increases during inspiration when RV stroke volume increases. However, in the current case, the stenosis of RVOT changed according to respiratory phase and became narrower during expiration, therefore it is reasonable that the heart murmur during expiration increased even if RV stroke volume decreased during expiration (Online Figs. 2, 3).
In our case, peak pressure gradient during expiration was 10 mmHg, which was equivalent to 1.6 m/s of peak flow velocity. In general, a 1.6 m/s of flow velocity is inadequate to cause a louder systolic murmur with thrill. However, the fact was that the cause of systolic murmur was the stenosis of RVOT, and the murmur was loud enough to accompany with thrill. We cannot provide an exact explanation of the mechanism, however, we have a consideration. A 1.6 m/s of peak flow velocity caused the vibration of the tricuspid chordae tendineae, especially, that of medially papillary muscles, which was attached to the border between the sinus portion and infundibulum of the RV. A 1.6 m/s of peak flow velocity also caused the vibration of the leaflets of the pulmonary valve. These vibrations could contribute to such a loud murmur with thrill. Thus, only a 1.6 m/s of velocity could cause a Levine Ⅳ/Ⅵ systolic murmur and the mechanism was similar to that observed in musical murmur, one of innocent murmurs.
To the best of our knowledge, our case is the first report that describes a mechanism of cardiac murmur during respiration in patients with SBS.
A funnel chest, another type of a thoracic cage deformity, which is characterized by a sternal depression and a narrowing of a thoracic cage in the anteroposterior direction, can also cause a compression of RV and alterations of the intensity of the systolic ejection murmur during respiration [10].
Conclusion
In patients with SBS, the systolic cardiac murmur exacerbates during expiration because the narrowed thorax compresses the RVOT and induces a pressure gradient across the RVOT. SBS should be considered as a differential diagnosis when a patient presents with an abnormal cardiac systolic murmur, yet without definite findings of cardiac disease. Our case highlights the importance of the physical examination, including an inspection of the patient’s physique.
Financial support
This study received no specific grant from any funding agency, commercial, or not-for-profit entity.
Ethical standards
The patient provided informed consent for publication.
Conflict of interest
The authors declare that there is no conflict of interest.
Acknowledgments
None.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.jccase.2020.07.008.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Rawlings M.S. The “straight back” syndrome, a new cause of pseudoheart disease. Am J Cardiol. 1960;5:333–338. doi: 10.1016/0002-9149(60)90080-1. [DOI] [PubMed] [Google Scholar]
- 2.Chaothaweel L. Straight back syndrome: a misleading condition in cardiology, demonstrated with magnetic resonance imaging. Bangkok Med J. 2014;8:52–58. [Google Scholar]
- 3.Deleon A.C., Jr, Perloff J.K., Twigg H., Majd M. The straight back syndrome: clinical cardiovascular manifestations. Circulation. 1965;32:193–203. doi: 10.1161/01.cir.32.2.193. [DOI] [PubMed] [Google Scholar]
- 4.Davies M.K., Mackintosh P., Cayton R.M., Page A.J., Shiu M.F., Littler W.A. The straight back syndrome. Q J Med. 1980;49:443–460. [PubMed] [Google Scholar]
- 5.Bon Tempo C.P., Ronan J.A., Jr, de Leon A.C., Jr, Twigg H.L. Radiographic appearance of the thorax in systolic click-late systolic murmur syndrome. Am J Cardiol. 1975;36:27–31. doi: 10.1016/0002-9149(75)90863-2. [DOI] [PubMed] [Google Scholar]
- 6.Salomon J., Shah P.M., Heinle R.A. Thoracic skeletal abnormalities in idiopathic mitral valve prolapse. Am J Cardiol. 1975;36:32–36. doi: 10.1016/0002-9149(75)90864-4. [DOI] [PubMed] [Google Scholar]
- 7.Grillo H.C., Wright C.D., Dartevelle P.G., Wain J.C., Murakami S. Tracheal compression caused by straight back syndrome, chest wall deformity, and anterior spinal displacement: techniques for relief. Ann Thorac Surg. 2005;80:2057–2062. doi: 10.1016/j.athoracsur.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 8.Ansari A. The "straight back" syndrome: current perspective more often associated with valvular heart disease than pseudoheart disease: a prospective clinical, electrocardiographic, roentgenographic, and echocardiographic study of 50 patients. Clin Cardiol. 1985;8:290–305. doi: 10.1002/clc.4960080509. [DOI] [PubMed] [Google Scholar]
- 9.Esser S.M., Monroe M.H., Littmann L. Straight back syndrome. Eur Heart J. 2009;30:1752. doi: 10.1093/eurheartj/ehp197. [DOI] [PubMed] [Google Scholar]
- 10.Deviggiano A., Carrascosa P., Vallejos J., Bellia-Munzon G., Vina N., Rodriguez-Granillo G.A. Relationship between cardiac MR compression classification and CT chest wall indexes in patients with pectus excavatum. J Pediatr Surg. 2018;53:2294–2298. doi: 10.1016/j.jpedsurg.2018.05.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.