Abstract
Coronavirus disease (COVID-19) and tuberculosis (TB) developed in 4 foreign workers living in dormitories in Singapore during April–May 2020. Clinical manifestations and atypical radiographic features of COVID-19 led to the diagnosis of TB through positive interferon-gamma release assay and culture results. During the COVID-19 pandemic, TB should not be overlooked.
Keywords: 2019 novel coronavirus disease, coronavirus disease, COVID-19, severe acute respiratory syndrome coronavirus 2, SARS-CoV-2, viruses, respiratory infections, zoonoses, tuberculosis, tuberculosis and other mycobacteria, co-infection, socioeconomic, radiography, Singapore
As the world focuses on the coronavirus disease (COVID-19) pandemic, caution must be taken to not overlook tuberculosis (TB). COVID-19 was first diagnosed in Singapore in January 2020, after cases were imported from Wuhan, China. Subsequent sustained community transmission of the virus followed a wave of imported cases from local residents returning from abroad (1). The outbreak in Singapore is being driven by spread within migrant worker dormitories. As of June 28, 2020, Singapore reported 43,459 confirmed cases of COVID-19, of which 41,010 were in dormitory residents (1). TB is endemic to Singapore; annual incidence rate is ≈40 cases/100,000 population (2), and a large proportion of cases are in nonpermanent residents.
We describe migrant workers in Singapore dormitories who were co-infected with severe acute respiratory syndrome virus 2 (SARS-CoV-2) and Mycobacterium tuberculosis. For all 4 patients, TB was diagnosed by positive interferon-gamma release assay (IGRA) (QIAGEN, https://www.qiagen.com); M. tuberculosis was isolated from pleural fluid culture from patient 4 only (Table).
Table. Epidemiologic and clinical features for 4 patients with coronavirus disease and tuberculosis, Singapore*.
| Pt no. | Age, y/sex, nationality | Initial signs/symptoms | Radiologic findings | Pleural fluid analysis | Sputum analysis | Microbiological findings | IGRA for TB | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1† |
32/M, India |
Fever, productive cough |
CXR: right upper zone and left lower zone cavitary lesions;
chest CT: irregular opacifications with central cavitation |
NA |
AFB smear negative; molecular TB analysis negative |
Sputum AFB culture negative |
+ |
Symptoms resolved; repeat CXR after starting ATT demonstrated resolution of cavitary lesions at 2 mo of treatment |
| 2 |
33/M, India |
Fever, nonproductive cough; 3-kg weight loss over 1 mo |
CXR: right-sided pleural effusion;
chest CT: loculated right-sided effusion with adjacent collapse/consolidation |
Lymphocytic exudative effusion; ADA 130 U/L;
SARS-CoV-2 PCR negative |
AFB smear negative;
molecular TB analysis negative |
Sputum and pleural fluid AFB cultures pending |
+ |
Symptoms resolved with interval improvement of CXR |
| 3† |
22/M, India |
Fever, nonproductive cough; exertional dyspnea, pleuritic chest pain |
CXR: right-sided pleural effusion with adjacent compressive atelectasis |
Lymphocytic exudative effusion; ADA 112 U/L;
SARS-CoV-2 PCR negative |
AFB smear negative;
molecular TB analysis negative |
Sputum and pleural fluid AFB cultures pending |
+ |
Symptoms resolved with interval improvement of CXR |
| 4 | 40/M, Bangladesh | Fever, productive cough; reduced effort tolerance | CXR: large left-sided pleural effusion; Chest CT: left-sided pleural effusion, bilateral patchy consolidative changes with ground-glass opacities and interlobular septal thickening | Lymphocytic exudative effusion; ADA 69 U/L; SARS-CoV-2 PCR negative | AFB smear negative; molecular TB analysis negative | Sputum AFB culture negative; pleural fluid AFB culture positive for Mycobacterium tuberculosis complex | + | Symptoms resolved with interval improvement of CXR |
*ADA, adenosine deaminase; AFB, acid-fast bacilli; ATT, anti-TB therapy; CT, computed tomography image; CXR, plain chest radiograph; IGRA, interferon gamma release assay; NA, not applicable; Pt, patient; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TB, tuberculosis; +, positive. †These patients reside in the same dormitory.
Patient 1 was a 32-year-old man from India with a 2-day history of fever and cough. He was positive for SARS-CoV-2 by reverse transcription PCR (RT-PCR) of a nasopharyngeal swab sample. Radiographs showed bilateral cavitary lung lesions (Figure 1, panel A). Sputum samples were smear negative and culture negative for acid-fast bacilli (AFB) and negative by molecular testing for M. tuberculosis nucleic acids (Cepheid Xpert MTB/RIF, https://www.cepheid.com). The IGRA for TB result was positive. In consideration of the clinical manifestations and risk factors, anti-TB therapy (ATT) was started, and interval radiographic imaging showed resolution.
Patient 2 was a 33-year-old man from India with an 8-day history of fever and cough and a 1-month history of weight loss (3 kg). He was positive for SARS-CoV-2 by RT-PCR of a nasopharyngeal swab sample. Radiographs showed a right-sided pleural effusion (Figure 1, panel B). Pleural fluid analysis revealed a lymphocytic exudative effusion with an adenosine deaminase (ADA) level of 130 U/L (reference range <40 U/L), but the fluid was negative for SARS-CoV-2 by RT-PCR. Sputum and pleural fluid were smear negative for AFB and M. tuberculosis nucleic acid negative by molecular testing; culture results are pending. IGRA was positive for TB, and ATT was started with subsequent clinical improvement.
Patient 3 was a 22-year old man from India with a 10-day history of fever and cough (associated with exertional dyspnea) and pleuritic chest pain. He was positive for SARS-CoV-2 by RT-PCR of a nasopharyngeal swab sample. Radiographs showed a right-sided pleural effusion (Figure 1, panel C). Pleural fluid analysis revealed a lymphocytic exudative effusion with an ADA level of 112 U/L and interleukin-6 (IL-6) level of >1,000 pg/mL, but the fluid was negative for SARS-CoV-2 by RT-PCR. Sputum and pleural fluid were smear negative for AFB and negative for M. tuberculosis nucleic acids by molecular testing; culture results are pending. IGRA was positive for TB and ATT was started; symptoms subsequently resolved.
Patient 4 was a 40-year old man from Bangladesh with a 3-day history of fever and cough. He was positive for SARS-CoV-2 by RT-PCR from a nasopharyngeal swab sample. Radiographs showed a left-sided pleural effusion with bilateral consolidation (Figure 1, panel D). Pleural fluid analysis revealed a lymphocytic exudative effusion with an ADA level of 62 U/L and an IL-6 level of >1,000 pg/mL, but the fluid was negative for SARS-CoV-2 by RT-PCR. Sputum and pleural fluid were smear negative for AFB and negative for M. tuberculosis nucleic acids by molecular testing, but the IGRA for TB was positive. ATT was started, and pleural fluid cultures were subsequently positive for M. tuberculosis.
All 4 patients were workers who resided in dormitories and had COVID-19 but atypical radiographic features; typical radiographic features for COVID-19 patients include ground-glass opacities, multifocal patchy consolidation, and peripheral interstitial changes (3). Despite confirmed diagnoses of COVID-19, the 4 patients’ pulmonary radiologic findings were more consistent with those for TB, highlighting the value of considering other pulmonary pathologic conditions for patients with COVID-19.
Risk factors for TB include low socioeconomic status and overcrowded living conditions (4). Of note, patients 1 and 3 resided in the same dormitory. Migrant worker dormitories are often inadequately ventilated and crowded, resulting in residents being more susceptible to infectious diseases, including dengue, Zika, and varicella (5,6). The same working and living conditions have served as a catalyst for the rapid transmission of SARS-CoV-2, and potentially TB, in this population. Improving screening processes and living conditions and implementing routine vaccination strategies for this population may prevent future infectious disease outbreaks.
As the COVID-19 pandemic continues, care for patients with TB may be compromised as additional strains are placed on essential services. The 4 cases we report highlight a serious public health issue. Precautionary measures must be undertaken to be vigilant of an epidemic within the ongoing pandemic—TB. To ensure that care is not compromised, clinicians treating these at-risk populations should be aware of possible co-infection with M. tuberculosis and SARS-CoV-2 in patients with atypical radiographic features of COVID-19.
Figure.
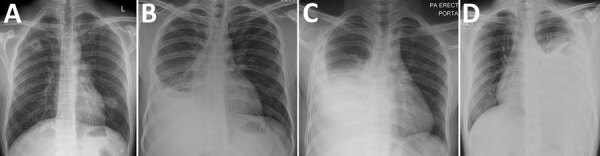
Plain chest radiographs of 4 patients with severe acute respiratory syndrome coronavirus 2 and Mycobacterium tuberculosis co-infection, Singapore. A) Patient 1, showing bilateral cavitary lesions; B) patient 2, showing a large right-sided loculated pleural effusion and adjacent consolidation; C) patient 3, showing a large right-sided pleural effusion with adjacent compressive atelectasis; D) patient 4, showing a large left-sided pleural effusion with adjacent consolidation.
Biography
Dr. Tham is an infectious diseases senior resident in the Department of Medicine at the National University Hospital of Singapore. His research interests include virology and public health.
Footnotes
Suggested citation for this article: Tham SM, Lim WY, Lee CK, Loh J, Premkumar A, Yan B, et al. Four patients with COVID-19 and tuberculosis, Singapore, April–May 2020. Emerg Infect Dis. 2020 Nov [date cited]. https://doi.org/10.3201/eid2611.202752
References
- 1.Ministry of Health Singapore. COVID-19 situation report [cited 2020 Jun 29]. https://covidsitrep.moh.gov.sg
- 2.Ministry of Health Singapore. Communicable diseases surveillance in Singapore [cited 2020 May 15]. https://www.moh.gov.sg/docs/librariesprovider5/diseases-updates/communicable-diseases-surveillance-in-singapore-2018210c9a3beaa94db49299c2da53322dce.pdf
- 3.Shi H, Han X, Jiang N, Cao Y, Alwalid O, Gu J, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425–34. 10.1016/S1473-3099(20)30086-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Narasimhan P, Wood J, Macintyre CR, Mathai D. Risk factors for tuberculosis. Pulm Med. 2013;2013:828939. 10.1155/2013/828939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sadarangani SP, Lim PL, Vasoo S. Infectious diseases and migrant worker health in Singapore: a receiving country’s perspective. J Travel Med. 2017;24:1–9. 10.1093/jtm/tax014 [DOI] [PubMed] [Google Scholar]
- 6.Ho ZJM, Hapuarachchi HC, Barkham T, Chow A, Ng LC, Lee JMV, et al. ; Singapore Zika Study Group. Outbreak of Zika virus infection in Singapore: an epidemiological, entomological, virological, and clinical analysis. Lancet Infect Dis. 2017;17:813–21. 10.1016/S1473-3099(17)30249-9 [DOI] [PubMed] [Google Scholar]


