Abstract
Study aims
We sought to evaluate the diagnostic performance of quantitative elastography (shear wave elastography) and to establish the optimal cutoff value to differentiate malignant and benign breast lesions using QelaXtoTM software.
Methods
We conducted a retrospective observational study of adult women with suspicious breast lesions (BIRADS 3, 4 or 5) who underwent programmed ultrasound-guided core biopsies. Breast lesions were assessed using quantitative elastography combined with B-mode ultrasound. Histopathology was used as reference standard. Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were estimated, and a ROC curve analysis was conducted. Three elastography cutoff values were considered: 36, 50 and 80 kPa.
Results
We included 143 women (mean age of 56 years) with a total of 145 breast lesions: 68 benign tumors (47.26%) and 77 malignancies (52.74%). Mean elasticity measurements of benign and malignant lesions were significantly different (24.6 kPa, SD 28.47, vs. 101.49 kPa, SD 47.38, ). Using the 50 kPa cutoff, elastography showed a global sensitivity of 87% to discriminate malignant lesions (AUC = 0.897). Moreover, sensitivity was 90.7% when lesions were located 5–40 mm below the skin surface (optimal elastographic field of view). Our false positive rate was 17.65%, comprised mainly of fibroepithelial neoplasms, fibroadenomas and fibrosis.
Conclusions
Quantitative elastography can differentiate malignant and benign breast lesions with acceptable to excellent performance. In our sample, the QelaXtoTM software showed a lower optimal cutoff than other ultrasound systems.
Keywords: Elastography, Breast cancer, Mammary ultrasonography, Elasticity imaging techniques
Introduction
Elastography, an imaging modality that evaluates the elastic properties or hardness of tissues, has shown potential in the assessment and characterization of breast lesions [1, 2]. Elastography measures small tissue displacements as external pressure is applied. Since displacement is inversely related to tissue stiffness, malignant lesions—often harder in consistency—tend to show less displacement than benign lesions or normal tissue [3, 4]. There are two main methods to evaluate tissue elasticity: strain elastography (qualitative) and shear wave elastography (quantitative) [2, 5].
Qualitative elastography offers a color-coded strain map, which depicts different tissue displacement patterns (elastographic patterns). This map is overlaid on gray-scale B-mode ultrasound images, facilitating the spatial interpretation of elastographic findings. Qualitative elastography’s main disadvantages are its operator dependency (subjective interpretation of the color map), considerable inter- and intraobserver variability and a prolonged learning curve (a 3–6 month training period is usually required to obtain reproducible results) [2, 5–8].
To overcome these limitations, shear wave elastography (SWE) was introduced in the market. This method uses acoustic waves to induce mechanical vibrations and quantifies the stiffness of a lesion by capturing and analyzing propagated shear waves. The speed of shear wave propagation is directly related to the hardness of the evaluated tissue. Tissue elasticity is measured in kilopascals (kPa) or meters per second (m/s) [7, 9], with values ranging from 0 to 180 kPa (higher values of quantitative elastography were associated with higher risk of malignancy) [7–16]. Quantitative elastography has shown promising results in several fields [17–20], and it could play a role in the early characterization of breast lesions, particularly BIRADS 3 and 4-A [21].
Even though three systematic reviews have evaluated the operational characteristics of quantitative elastography [22–24], most of them have used Toshiba and Siemens Ultrasound systems, and to this date we have not found publications establishing optimal elastography cutoff values for the QelaXtoTM software in patients with breast lesions.
Study aims We sought to evaluate the diagnostic performance of quantitative elastography and to establish the optimal cutoff value to differentiate between malignant and benign breast lesions using an Esaote MyLab Eight Ultrasound system.
Methods
Study design
We conducted a retrospective observational study of diagnostic tests performed on a consecutive sample of women with breast lesions who attended the Breast Imaging and Interventional Radiology Department in a 3rd level academic hospital in Buenos Aires, between May and September 2018.
Setting
This study was conducted at a third level academic hospital with centralized electronic health records. The Breast Imaging and Interventional Radiology Department is comprised of 10 specialists and 2 fellows, all of whom are exclusively dedicated to breast imaging. On average, the department reports 30,000 mammograms and conducts 25,000 breast ultrasounds annually. It possesses an integrated RIS/PACS system since 2010.
Study participants
Our study included women over 18 years of age who underwent a programmed ultrasound-guided breast core biopsy due to a suspicious breast mass. We included lesions categorized as low- (BIRADS 3), medium- (BIRADS 4A, B or C) or highly suspicious of malignancy (BIRADS 5) by a previous ultrasound, according to the 5th BIRADS edition [25]. We excluded women with incomplete histopathology reports.
Index diagnostic test: quantitative elastography
The index diagnostic test was quantitative elastography (SWE) performed in combination with B-mode ultrasound, using an Esaote MyLab Eight Ultrasound System (Esaote, Genova, Italy). The Breast Imaging and Interventional Radiology Department at our institution uses a linear array transducer with a frequency range of 7.5–15 MHz and the Qelaxto software for quantitative evaluation of tissue stiffness. Elastographic evaluations of breast lesions are performed by four medical imaging specialists with exclusive dedication to breast imaging.
Using B-mode ultrasound, radiologists record lesion features such as lesion size (maximum diameter), length of breast tissue containing the suspicious lesion (vertical distance from the skin to the pectoralis muscle) and lesion depth (vertical distance from the skin to the upper margin of the nodule). Nodule stiffness is evaluated following the elastography protocol summarised in Table 1. According to published evidence, the harder the lesion measured with quantitative elastography (in kPa), the higher the probability of malignancy.
Table 1.
Summarized protocol for quantitative elastography combined with B-mode breast ultrasound for the evaluation of BIRADS 3, BIRADS 4 (A, B and C) and BIRADS 5 lesions at the Breast Imaging and Interventional Radiology Department
| Field of view (FoV) delimitation: FoV must include part of the lesion and at least 3 mm of normal adjacent breast tissue |
| The transducer is immobilized over the breast for 5–10 s to stabilize the image. Immediately after asking the patient to hold their breath, elastographic measurements are taken |
| Every nodule is measured in at least ten different areas. Mean elasticity, expressed in kiloPascals, is recorded |
| When the distance between the nodule and the skin surface is less than 5 mm, a thick coat of gel is applied between the transducer and the patient’s skin, to increase the distance between the transducer and the lesion. This allows a better inclusion of the nodule in the field of view (the Software has a fixed field of view that does not include lesions closer than 5 mm or below 50 mm) |
Given that SWE was performed before biopsy specimens were obtained, physicians performing SWE were blind to histopathologic results (reference standard).
Reference standard test: histopathology
Histopathology reports of breast core biopsies were retrieved from our institution’s centralized electronic health records and used as reference standard.
Ultrasound-guided core needle biopsies were performed using a free-hand technique and local anesthesia, with patients in the supine position. Our team uses a reusable biopsy gun (Bard-Magnum Biopsy Instrument, Covington, GA, USA) and 14-gauge cutting needles. At our center, biopsy specimens are examined by the Pathology Department following standard histopathology procedures; samples are fixed, sectioned, stained with Haematoxylin-Eosin and evaluated by optical microscopy. At least one complete section of each core is included. When a malignant lesion is diagnosed, histologic type is assessed and nuclear grade (I, II or III) is scored. Immunohistochemistry is performed to evaluate the presence of hormone receptors (estrogen and/or progesterone receptors), Her 2 Neu receptor overexpression and Ki-67 proliferation index. Pathologists working on biopsy specimens are uninformed of elastography results.
Statistical analysis
Breast lesions were classified into malignant and benign tumors according to their histopathology (gold standard). Every nodule with a pathology report of ductal breast carcinoma (in situ or invasive), invasive lobular carcinoma and/or sarcoma was considered a malignant lesion.
Once the classification was established, the different elastography cutoff values were explored by ROC curve analysis. Based on systematic reviews [22–24] and other publications [8–16, 21] that reported optimal elastography cutoff values between 36 and 80 kPa, our team explored three different cutoff values: 36, 50 and 80 kPa1.
For each pre-specified cutoff value, we calculated sensitivity, specificity, positive predictive value and negative predictive value with their respective 95% confidence intervals. False negative results (benign breast lesions with stiffness above the elastography cutoff) were identified and their histopathology reports recorded.
STATA 13.0 software was used for data analysis.
Results
Study participants and breast lesions
We identified 143 women who underwent ultrasound-guided breast biopsies due to suspicious breast lesions (BIRADS 3, 4 or 5) between May and September 2018. Mean age was 56 years. Two women had two breast lesions that met the inclusion criteria, and were included in the study sample, resulting in a total of 145 lesions evaluated with elastography (index test) and histopathology (reference standard test). 68 lesions were benign tumors (47.26%) and 77 were malignancies (52.74%). The patients’ baseline characteristics and their main results are described in Table 2. Women with benign lesions were significantly younger than those with malignant lesions: 47.4 (SD 15.4) vs. 64.5 (SD 15.3) years of age, respectively.
Table 2.
Baseline characteristics and main lesion measurements of patients with benign and malignant histopathology results
| Benign | Malignant | p | |||
|---|---|---|---|---|---|
| Age (years), mean (SD) | 47.4 (15.4) | 64.5 (15.3) | |||
| BIRADS ultrasound classification, counts (percentage) | 3 | 21/68 (30.88 %) | 0/77 (0 %) | ||
| 4 | A | 47/68 (69.12 %) | 6/77 (7.79 %) | ||
| B | 0/68 (0 %) | 0/68 (0 %) | |||
| C | 0/68 (0 %) | 58/77 (75.32 %) | |||
| 5 | 0/68 (0 %) | 13/ 77 (16.88 %) | |||
| Size of the lesion (mm), mean (SD) | 15.27 (11.11) | 19.6 (13.06) | 0.035 | ||
| Depth of the lesion (mm), mean (SD) | 8.44 (5.34) | 8.85 (5.10) | 0.64 | ||
| Elastography result (Kpa), mean (SD) | 24.6 (28.47) | 101.49 (47.38) |
Ductal breast carcinoma (in situ or invasive), invasive lobular carcinoma and/or sarcoma
t test for independent samples
Nonparametric trend test
Out of all 68 benign lesions, 54 (79%) were Fibroadenomas. The rest of them were fibroids (), biphasic lesions (), fat necrosis (), pseudoangiomatous stromal hyperplasia (), and foreign body granulomas (). Infiltrating ductal carcinomas () were the main type of malignant lesion (81%), followed by infiltrating lobular carcinoma (), ductal carcinoma in situ (), mucinous carcinoma (), and breast sarcoma ().
Diagnostic performance of shear wave elastography
Overall, shear wave elastography showed acceptable to excellent discriminative ability, with an AUC of 0.898 when all lesions in our dataset were included in the analysis. Selected results of the ROC curve analysis are displayed in Figs. 1 and 2, and Tables 3 and 4.
Fig. 1.
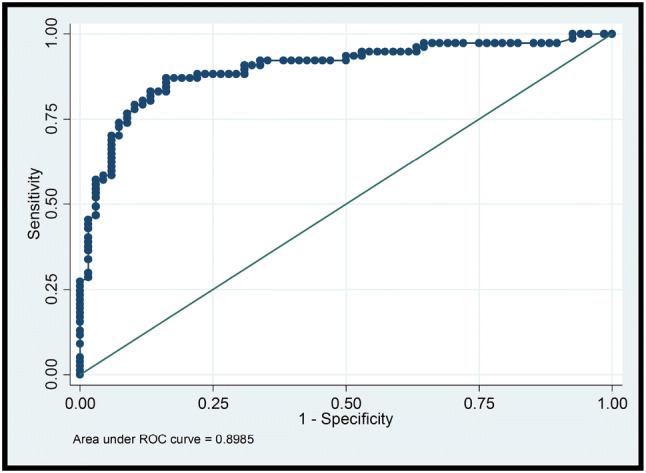
ROC curve that represents, at different cutoff values, the sensitivity and specificity of elastography for discriminating between benign and malignant tumors, considering all lesions in the study sample (). AUC = 0.8985
Fig. 2.
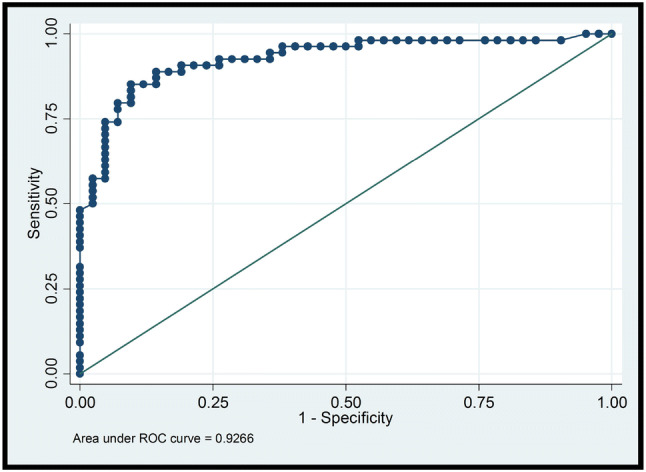
ROC curve that depicts shear wave elastography’s diagnostic performance for the subset of lesions located within the optimal field of view (). AUC = 0.9266
Table 3.
Sensitivity, specificity, positive predictive value and negative predictive value of pre-specified elastography cutoffs for all lesions in the dataset (; BI-RADS 3, 4 and 5)
| Elastography cutoff value | Sensitivity (95% CI) | Specificity (95% CI) | Predictive value | |
|---|---|---|---|---|
| Positive | Negative | |||
| 36 kPa | 68/77 | 52/68 | 68/84 | 52/61 |
| 88.3% | 76.5% | 80.9% | 85.% | |
| (79–94.5) | (64.6–85.9) | (70.9–88.7) | (73.8–93) | |
| 50 kPa | 67/77 | 56/68 | 67/79 | 56/66 |
| 87% | 82.35% | 85% | 84.8% | |
| (77–94) | (71–90) | (75–91.9) | (73.9–92.5) | |
| 80 kPa | 54/77 | 64/68 | 54/58 | 64/87 |
| 70.12% | 94% | 93.1% | 73.6% | |
| (58.6–80) | (85.8–98.4) | (83.3–98.1) | (63–82.4) | |
The test was considered positive when the cutoff value was exceeded
Table 4.
Sensitivity, specificity, positive predictive value and negative predictive value of pre-specified elastography cutoffs for lesions located in the optimal field of view to 40 mm ()
| Elastography cutoff value | Sensitivity (95% CI) | Specificity (95% CI) | Predictive value | |
|---|---|---|---|---|
| Positive | Negative | |||
| 36 kPa | 50/54 | 30/42 | 50/62 | 30/34 |
| 92.6% | 71.4% | 80.6% | 88.2% | |
| (82.1–97.9) | (55.4–84.3) | (68.6–89.6) | (72.5–96.7) | |
| 50 kPa | 49/54 | 33/42 | 49/58 | 33/38 |
| 90.7% | 78.6% | 84.5% | 86.8% | |
| (79.7–96.9) | (63.2–89.7) | (72.6–92.6) | (71.9–95.6) | |
| 80 kPa | 40/54 | 40/42 | 40/42 | 40/54 |
| 74.1% | 95.2% | 95.2% | 74.1% | |
| (60.3–85) | (83.8–99.4) | (83.8–99.4) | (60.3–85) | |
The test was considered positive when the cutoff value was exceeded
Figure 1 depicts sensitivity and specificity estimates when all lesions are considered (, including 49 [33.8%] outside the optimal field of view). The corresponding diagnostic performance metrics are reported in Table 3.
When we considered the subset of lesions located within the optimal field of view (96 lesions; 66.2%), AUC was 0.927. Figure 2 shows a ROC curve after this second analysis and Table 4 demonstrates the sensitivity, specificity, positive and negative predictive values of elastography for the considered cutoff values, considering this subset of lesions ().
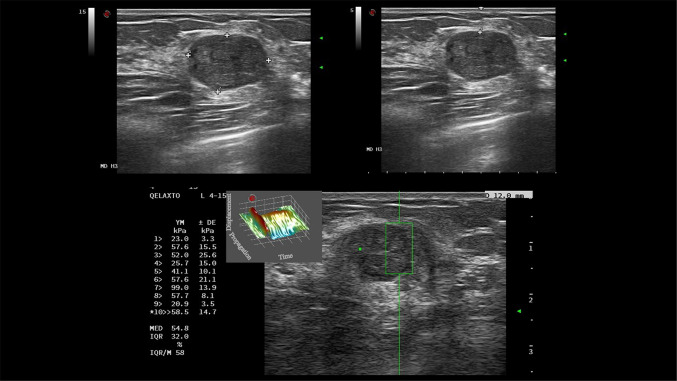
Figure 3 shows an example of a malignant lesion correctly identified by elastography; Fig. 4, an example of a correctly identified benign lesion.
Fig. 3.
67-year-old patient. Ultrasound (a) revealed a solid, hypoechoic, irregular mass with spiculated margins and antiparallel orientation, at 10 o’clock position of the right breast. Length of breast tissue containing the suspicious lesion (vertical distance from the skin to the pectoralis muscle) and lesion depth (vertical distance from the skin to the upper margin of the nodule) were recorded. Quantitative elastography (b) showed an elasticity of 92 kPa. Histopathology report: invasive ductal carcinoma (ER+, PGR+, Her 2 Neu−, Ki 67: 10%)
Fig. 4.
42-year-old patient with a hypoechoic solid mass, with microlobulated margins. Tissue elasticity measured by quantitative elastography was 38 kPa. Core biopsy report: involutive fibroadenoma
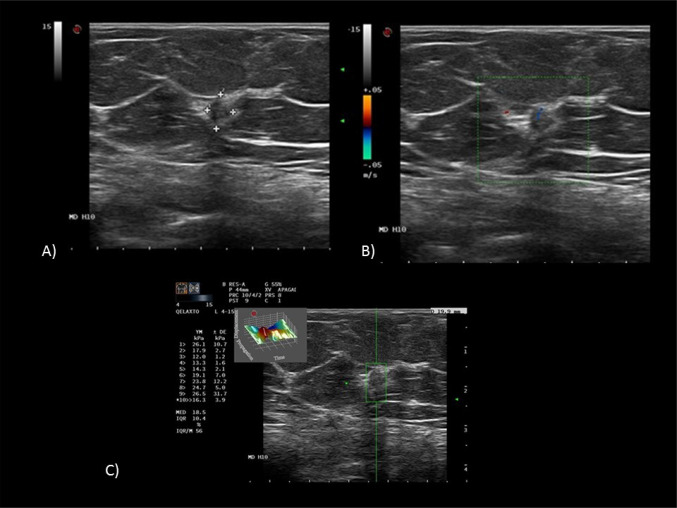
Using the 50 kPa cutoff, 10 out of 77 malignant lesions (13%) were erroneously classified as benign (false negative results). This group included 7 infiltrating ductal carcinomas: 6 were located outside the optimal field of view—including 2 with necrotic areas, which might yield lower elasticity measurements. The remaining false negative results were one ductal carcinoma in situ (DCIS) and 2 mucinous carcinomas, which are typically softer than other malignant breast lesions. Figures 5 and 6 display lesions that yielded a false positive and false negative result, respectively.
Fig. 5.
45 year-old patient with a false positive result. B-mode ultrasound (a) showed an hypoechoic solid mass with microlobulated margins categorized as BIRADS 4A. The elastographic measurement is 54.8 kPa. Histopathology by core biopsy: biphasic tumor
Fig. 6.
72-year-old patient with a false negative result. Ultrasound (a) showed a solid, hypoechoic, irregular mass with spiculated margins, with an antiparallel orientation at 10 o’clock position of the right breast. Doppler evaluation (b) shows peripheral vascularization. Quantitative elastography (c) measurement was 18.5 kPa. Histopathology report: ductal carcinoma in situ
Discussion
In the evaluation of breast masses with quantitative elastography, we found a statistically significant difference between elastographic measurements of benign and malignant lesions: mean tissue stiffness was 24.6 kPa (SD 28.47) vs 101.49 kPa (SD 47.38) respectively (). These outcomes are consistent with previous work by other authors [8–16, 21].
Using a 50 kPa cutoff value, elastography showed an overall sensitivity of 87% (95% CI 77–94) to detect malignant lesions (Table 2); these results are consistent with previously reported meta-analyses [22–24]. Moreover, sensitivity exceeded 90% (90.7%, 95% CI 79.7–96.9) in the subgroup of lesions located between 5 and 40 mm below the skin surface (Table 3).
As for specificity, even though our overall estimate (82.35%; 95% CI 71–90) was slightly inferior than the ones reported in the previously mentioned meta-analyses [22–24], there is a clear overlapping of their confidence intervals: Liu and col. (86.6%; 95% CI 83.3–89.4); Xue and col. (89%; 95% CI 84–92, in caucasian population) and Luo and col. (84.9%; 95% CI 82.6–86.9).
Our false positive rate was 17.65% (95% CI 10–29), comparable to those published by Kim [26] and Yoon [27], which were 11% (13–18) and 36.6% (30–44) respectively. Our false positive results were comprised of biphasic tumors, fibroadenomas and fibrosis, coinciding with other international reports [8–16, 21]. Our global false negative rate was 13% (95% CI 6–23), which lies within the bounds reported by Suvannarerg [8]: (12.5–17.7%) and is also comparable to those communicated by Kim [26] and Yoon [27], which were 22% (12–37) and 20.6% (15–27) respectively. Most of our false negative results were located outside the optimal field of view. The remaining false negative results consisted of a DCIS or lesions with mucinous and/or necrotic components, which are typically softer than other malignant lesions; these findings are consistent with previous reports by the mentioned authors.
Our data suggest that the use of a gel coat between the transducer and the patient’s skin (to increase the distance between the transducer and the lesion and thus include the lesion within the optimal field of view) does not improve diagnostic performance sufficiently, since discriminative ability (AUC) was higher for the subset of lesions located within the optimal field of view.
We obtained an optimal cutoff value that is lower than the ones reported by previous studies. This finding can probably be explained by different measurement equipments and/or techniques (for example, different software and equipments, different field of view sizes, different number of measurements).
Our study’s limitations include its reduced sample size and its retrospective design. Furthermore, women in our study sample have not undergone qualitative elastography, which might have improved our diagnostic performance if areas of the lesions whose color suggested a higher level of stiffness were selected for a subsequent evaluation with quantitative elastography.
Our quantitative evaluation of SWE’s diagnostic performance does not take B-mode ultrasound findings into account. Although this approach provides valid performance estimates for SWE measurements, findings should be generalized with caution, since tissue stiffness is rarely used as a standalone measure in clinical practice. Specialists usually consider both SWE and B-mode findings when evaluating suspicious lesions, as B-mode can help characterize breast lesions in various settings [28, 29]. Furthermore, our study focused on a dichotomous classification of breast lesions (benign vs. malignant), and it did not assess the correlation of elastographic measurements with tumor histotype, grade and/or hormone receptor status.
As previously mentioned, a disadvantage of shear wave elastography with the equipment we used is that the field of view is fixed and cannot be adjusted to measure lesions at less than 5 mm below the skin surface or deeper than 40 mm. This setback appears to be only partially solved by the use of a gel coat between the transducer and the skin.
Based on our results, which are concordant with the previously mentioned meta-analyses, quantitative elastography has shown an adequate diagnostic performance and can be an important supporting tool for the differential diagnosis of benign and malignant breast lesions. It is a reliable, reproducible and non-invasive technique, relatively easy to perform and of low cost, given that it only requires the addition of a software package to the traditional ultrasound equipments, and it usually lasts only 5 min longer than a conventional B-mode ultrasound.
Conclusions
Quantitative elastography showed acceptable to excellent discriminative ability to differentiate benign and malignant breast lesions. Most false negative results were ductal carcinomas located outside the optimal field of view or lesions with a necrotic or mucinous component. In our study, the QelaXtoTM software showed a lower optimal cutoff value (50 kPa) than the ones reported for other ultrasound systems.
Funding
This Study was founded by the Radiology Department of the Hospital Italiano de Buenos Aires.
Compliance with ethical standards
Conflict of interest
The authors declare no conflicts of interest.
Ethical standards
This study was conducted in accordance with the Helsinki Declaration of 1975 and its late amendments. The study protocol was approved by the institutional Ethics Committee, with IRB approval number 3797.
Informed consent
As we worked with de-identified retrospective data, informed consent was waived for this retrospective study.
Consent for publication
All authors expressed explicit consent for the publication of this manuscript.
Code availability
Data-analysis code is available upon request.
Footnotes
For example, Lee et al. informed that the elastographic values for malignant masses were on average 119.0 () kPa and the benign lesions were 41.4 () kPa, whereas the elastographic values published by Chang et al. were slightly superior. The malignant masses had a medium elasticity of 153.3 () kPa and the benign, 46.1 ( kPa).
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Garra BS, Cespedes EI, Ophir J, et al. Elastography of breast lesions: initial clinical results. Radiology. 1997;202(1):79–86. doi: 10.1148/radiology.202.1.8988195. [DOI] [PubMed] [Google Scholar]
- 2.Itoh A, Ueno E, Tohno E, et al. Breast disease: clinical application of US elastography for diagnosis. Radiology. 2006;239(2):341–350. doi: 10.1148/radiol.2391041676. [DOI] [PubMed] [Google Scholar]
- 3.Sigrist RMS, Liau J, Kaffas AE, Chammas MC, Willmann JK. Ultrasound elastography: review of techniques and clinical applications. Theranostics. 2017;7(5):1303–1329. doi: 10.7150/thno.18650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bamber J, Cosgrove D, Dietrich CF, et al. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 1: basic principles and technology. Ultraschall Med. 2013;34(2):169–184. doi: 10.1055/s-0033-1335205. [DOI] [PubMed] [Google Scholar]
- 5.Ricci P, Maggini E, Mancuso E, et al. Clinical application of breast elastography: state of the art. Eur J Radiol. 2014;83(3):429–437. doi: 10.1016/j.ejrad.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 6.Barr RG, Managuli RA. A clinical study comparing the diagnostic performance of assist strain ratio against manual strain ratio in ultrasound breast elastography. Ultrasound Q. 2019;35(1):82–87. doi: 10.1097/RUQ.0000000000000398. [DOI] [PubMed] [Google Scholar]
- 7.Barr RG, Zhang Z. Shear-wave elastography of the breast: value of a quality measure and comparison with strain elastography. Radiology. 2015;275(1):45–53. doi: 10.1148/radiol.14132404. [DOI] [PubMed] [Google Scholar]
- 8.Suvannarerg V, Chitchumnong P, Apiwat W, et al. Diagnostic performance of qualitative and quantitative shear wave elastography in differentiating malignant from benign breast masses, and association with the histological prognostic factors. Quant Imaging Med Surg. 2019;9(3):386–398. doi: 10.21037/qims.2019.03.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Athanasiou A, Tardivon A, Tanter M, et al. Breast lesions: quantitative elastography with supersonic shear imaging-preliminary results. Radiology. 2010;256(1):297–303. doi: 10.1148/radiol.10090385. [DOI] [PubMed] [Google Scholar]
- 10.Tozaki M, Fukuma E. Pattern classification of ShearWave elastography images for differential diagnosis between benign and malignant solid breast masses. Acta radiol. 2011;52(10):1069–1075. doi: 10.1258/ar.2011.110276. [DOI] [PubMed] [Google Scholar]
- 11.Chang JM, Moon WK, Cho N, et al. Clinical application of shear wave elastography (SWE) in the diagnosis of benign and malignant breast diseases. Breast Cancer Res Treat. 2011;129(1):89–97. doi: 10.1007/s10549-011-1627-7. [DOI] [PubMed] [Google Scholar]
- 12.Evans A, Whelehan P, Thomson K, et al. Quantitative shear wave ultrasound elastography: initial experience in solid breast masses. Breast Cancer Res. 2010;12(6):R104. doi: 10.1186/bcr2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berg WA, Cosgrove DO, Doré CJ, et al. Shear-wave elastography improves the specificity of breast US: the BE1 multinational study of 939 masses. Radiology. 2012;262(2):435–449. doi: 10.1148/radiol.11110640. [DOI] [PubMed] [Google Scholar]
- 14.Lee SH, Chang JM, Kim WH, et al. Added value of shear-wave elastography for evaluation of breast masses detected with screening US imaging. Radiology. 2014;273(1):61–69. doi: 10.1148/radiol.14132443. [DOI] [PubMed] [Google Scholar]
- 15.Au FWF, Ghai S, Moshonov H, et al. Diagnostic performance of quantitative shear wave elastography in the evaluation of solid breast masses: determination of the most discriminatory parameter. AJR Am J Roentgenol. 2014;203(3):W328–36. doi: 10.2214/AJR.13.11693. [DOI] [PubMed] [Google Scholar]
- 16.Evans A, Whelehan P, Thomson K, et al. Differentiating benign from malignant solid breast masses: value of shear wave elastography according to lesion stiffness combined with greyscale ultrasound according to BI-RADS classification. Br J Cancer. 2012;107(2):224–229. doi: 10.1038/bjc.2012.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erdoğan H, Durmaz MS, Özbakır B, et al. Experience of using shear wave elastography in evaluation of testicular stiffness in cases of male infertility. J Ultrasound. 2020 doi: 10.1007/s40477-020-00430-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kara T, Ateş F, Durmaz MS, et al. Assessment of thyroid gland elasticity with shear-wave elastography in Hashimoto’s thyroiditis patients. J Ultrasound. 2020 doi: 10.1007/s40477-020-00437-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paternostro R, Reiberger T, Bucsics T. Elastography-based screening for esophageal varices in patients with advanced chronic liver disease. World J Gastroenterol. 2019;25(3):308–329. doi: 10.3748/wjg.v25.i3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ozgokce M, Batur M, Alpaslan M, et al. A comparative evaluation of cataract classifications based on shear-wave elastography and B-mode ultrasound findings. J Ultrasound. 2019;22(4):447–452. doi: 10.1007/s40477-019-00400-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gheonea IA, Stoica Z, Bondari S. Differential diagnosis of breast lesions using ultrasound elastography. Indian J Radiol Imaging. 2011;21(4):301–305. doi: 10.4103/0971-3026.90697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu B, Zheng Y, Huang G, et al. Breast lesions: quantitative diagnosis using ultrasound shear wave elastography—a systematic review and meta-analysis. Ultrasound Med Biol. 2016;42(4):835–847. doi: 10.1016/j.ultrasmedbio.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 23.Luo J, Cao Y, Nian W, et al. Benefit of shear-wave elastography in the differential diagnosis of breast lesion: a diagnostic meta-analysis. Med Ultrason. 2018;1(1):43–49. doi: 10.11152/mu-1209. [DOI] [PubMed] [Google Scholar]
- 24.Xue Y, Yao S, Li X, Zhang H. Value of shear wave elastography in discriminating malignant and benign breast lesions: a meta-analysis. Medicine. 2017;96(42):e7412. doi: 10.1097/MD.0000000000007412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.American College of Radiology (2013) ACR BI-RADS Atlas: Breast Imaging Reporting and Data System. Mammography, Ultrasound, Magnetic Resonance Imaging, Follow-up and Outcome Monitoring, Data Dictionary
- 26.Kim MY, Choi N, Yang JH, Yoo YB, Park KS. False positive or negative results of shear-wave elastography in differentiating benign from malignant breast masses: analysis of clinical and ultrasonographic characteristics. Acta Radiol. 2015;56(10):1155–1162. doi: 10.1177/0284185114551400. [DOI] [PubMed] [Google Scholar]
- 27.Yoon JH, Jung HK, Lee JT, Ko KH. Shear-wave elastography in the diagnosis of solid breast masses: what leads to false-negative or false-positive results? Eur Radiol. 2013;23(9):2432–2440. doi: 10.1007/s00330-013-2854-6. [DOI] [PubMed] [Google Scholar]
- 28.Alikhassi A, Azizi F, Ensani F. Imaging features of granulomatous mastitis in 36 patients with new sonographic signs. J Ultrasound. 2020;23(1):61–68. doi: 10.1007/s40477-019-00392-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carlino G, Rinaldi P, Giuliani M, et al. Ultrasound-guided preoperative localization of breast lesions: a good choice. J Ultrasound. 2019;22(1):85–94. doi: 10.1007/s40477-018-0335-0. [DOI] [PMC free article] [PubMed] [Google Scholar]