Abstract
Purpose
The purpose of this study was to determine quantitative testicular tissue stiffness values in normal and infertile men using shear wave elastography (SWE), and to evaluate the relationship between infertility and testicular stiffness value.
Methods
In total, 100 testes of 50 infertile patients with abnormal semen parameters were classified as group A, and 100 testes of 50 control subjects were classified as group B. These two groups were compared in terms of age, testicular volume, and SWE values. The group B testes were randomly chosen from patients who had applied for ultrasonography for any reason, and who had no testis disease and no history of infertility.
Results
The mean age of the patients was 27.83 years, and no significant difference in age was found between the groups (P = 0.133). No significant difference in testicular volume was found between the groups (P = 0.672). The SWE values were significantly higher in group A than in group B (P = 0.000 for both m/s and kPa values). SWE values had a negative correlation with mean testicular volume in group A (for m/s values: P = 0.043; for kPa values: P = 0.024).
Conclusion
SWE can be a useful technique for assessing testicular stiffness in infertile patients to predict parenchymal damage in testicular tissue that leads to an abnormality in sperm quantity. In addition, decreased testicular volume, together with increased SWE values, can reflect the degree of parenchymal damage.
Keywords: Shear wave elastography, Testis, Male infertility, Ultrasonography
Introduction
Infertility is defined as the inability to achieve a clinical pregnancy after at least 1 year of regular, unprotected sex. Infertility affects 15–20% of couples. The male factor is estimated to be present in approximately 50% of cases. About 40–50% of all male infertility cases have a sperm defect as the main cause [1]. Semen analysis can reflect testicular function and parenchymal damage [2]. Ultrasonography (US) is a clinically acceptable, non-invasive tool used in the assessment of male infertility [3]. Shear wave elastography (SWE) is a new ultrasound modality that provides quantitative information on tissues according to their stiffness. Thus, it gives information on histological changes in tissues. The basic SWE process includes the application of an US transducer that produces acoustic push pulses to generate shear waves. The propagation velocity of shear waves depends on the tissue consistency. Propagation is slower through softer tissues and faster through harder tissues. The quantitative shear wave elastography values are measured in meters per second (m/s) and kilopascals (kPa) [4, 5]. The efficacy of SWE in assessing tissue or lesion stiffness has been shown in various organs such as the thyroid, breast, and liver [5–7]. However, a limited number of studies performed with SWE have focused on testicular elasticity. Most of these studies were limited to tumors, torsion, or infarctions [8–10], although some evaluated normal testicular stiffness [11, 12]. Different elasticity values, depending on testicular volume and function, have been demonstrated using SWE [8–10, 13]. To the best of our knowledge, there are only a few studies investigating the relationship between testicular SWE evaluation and male infertility [14, 15].
The purpose of this study was to determine quantitative testicular tissue stiffness values in normal and infertile men, and to evaluate the relationship between infertility and testicular stiffness value.
Materials and methods
This controlled prospective study was conducted at the University of Health Sciences Konya Training and Research Hospital in Turkey. The study was approved by the Ethics Committee of the Selçuk University Medical Faculty in Konya, Turkey. Written, informed consent was obtained from all individuals included in the study.
The World Health Organization (WHO)’s defines infertility as “a disease of the reproductive system defined by the failure to achieve a clinical pregnancy after 12 months or more of regular unprotected sexual intercourse” [16]. All individuals included in the study met these criteria and had abnormal semen parameters. Some studies have shown that tumors, torsion, varicocele, orchitis, hydrocele, undescended testis, or operations increase testicular stiffness and SWE values [8–10, 17–19]. We thought that these conditions could modify stiffness. Therefore, we excluded affected patients from the study to evaluate only the relationship between testicular stiffness and infertility, regardless of other effects. In other words, we selected infertile patients with low sperm counts and without concomitant testicular diseases. No age restriction was applied when selecting patients for the study.
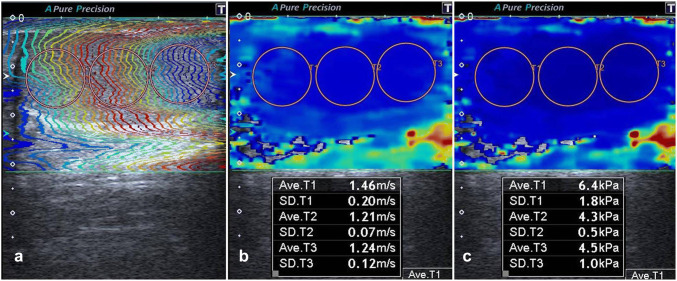
In total, 50 infertile patients with abnormal semen parameters (100 testes) and 50 control subjects (100 testes) were included in the study. The testes of the patients with infertility were classified as group A, and the testes of the normal patients who were the control group were classified as group B. The group B testes were randomly chosen from patients who had applied for US for any reason, had no testis disease, had no history of infertility, had fathered at least one child, and had agreed to participate in the study. All SWE examinations were performed by a single radiologist with 4 years of experience in SWE. All patients underwent US, including SWE examinations of the testes performed using a high-frequency (4–14 MHz) linear array transducer (Toshiba Aplio 500; Toshiba Medical System Corporation, Tokyo, Japan). All testes were measured in the largest three dimensions (length (L) × height (H) × width (W)), and the testicular volume was then automatically calculated by US device according to the formula L × W × H × 0.523. We used two-dimensional SWE, which is the newest SWI method and one that uses acoustic radiation force. We obtained the elastography images of the testes by placing the US probe very lightly on the scrotum, without applying any pressure. To prevent artifacts, we advised patients not to breathe or move, while examinations were performed. The values obtained from acquisition images with motion artifacts were not considered. We repeated measurements in 11 patients who did not cooperate or who moved during examination, and the repeated measurements provided ideal images. SWE values were measured in the longest longitudinal plane using a circular region of interest (ROI). After freezing, SWE images were viewed using three display modes: speed mode (m/s), elasticity mode (kPa), and propagation (arrival time contour) mode (Fig. 1). Tissue elasticity was indicated with a range of color between dark blue (lowest stiffness) and red (highest stiffness). Three SWE measurements were obtained from each 1/3 (upper, central and lower) region of the testis, the average of which was considered the SWE value of the corresponding testis. We hypothesized that this increased the accuracy of the numerical values obtained.
Fig. 1.
The testis of a 29-year-old patient with infertility. After freezing, SWE images were viewed using three display modes: propagation mode (a), speed mode (m/s) (b), and elasticity mode (kPa) (c). Three measurements were obtained from each testis in the longest longitudinal plane using a circular region of interest, the average of which was considered the SWE value of the corresponding testis
Statistical analysis
All statistical analyses of the data were performed using the Statistical Package for Social Sciences version 24 (IBM, Armonk, NY) software. Descriptive statistics were expressed as mean, standard deviation, minimum–maximum values, frequency, and percentile. The age, testicular volume, and SWE values (m/s and kPa) of the two groups were compared. The Kolmogorov–Smirnov test was used to verify the normal distribution of the data. The non-parametric Mann–Whitney U test was performed to assess the age, volume, m/s, and kPa values, since these values were not normally distributed. A P value of < 0.05 was considered statistically significant with a 95% confidence level.
Results
A total of 100 patients were enrolled in the study, and 200 testes were examined. Data are presented as mean ± standard deviation (SD). The mean age of patients included in the study was 27.83 (± 7.63) years, and the mean testicular volume was 15.39 (± 4.61) ml. The mean age was 28.33 (± 6.67) years in group A, and 27.34 (± 8.49) years in group B, indicating no statistically significant difference in age between the two groups (P = 0.133).
The mean testicular volumes in the two groups were as follows: 15.34 (± 5.24) ml in group A and 15.44 (± 3.92) ml in group B. The Mann–Whitney U test revealed no significant difference in the testicular volume between the two groups (P = 0.672).
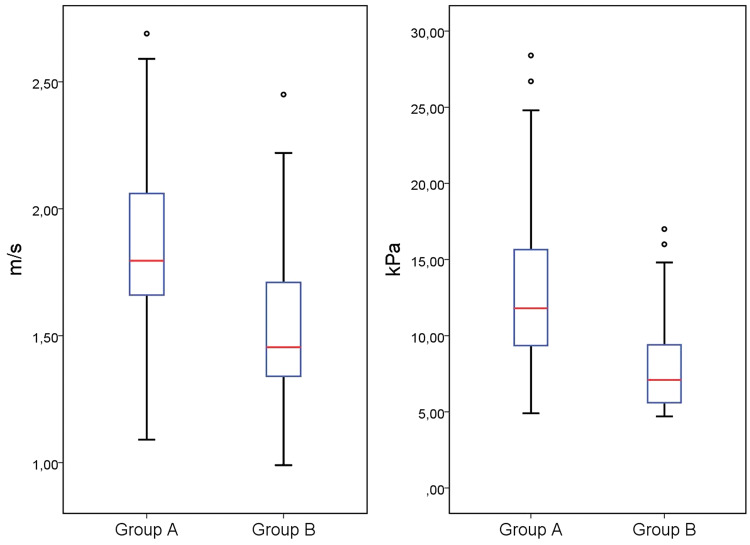
The mean SWE values were 1.85 (± 0.31) m/s and 12.82 (± 5.19) kPa in group A, and 1.53 (± 0.25) m/s and 8.01 (± 3.02) kPa in group B. The mean SWE values were higher in group A than in group B. The Mann–Whitney U test revealed a significant difference in the m/s and kPa values between the two groups (for m/s and kPa values: P = 0.000). The total number of testes, mean age, and testicular volume, as well as the SWE values of the groups, are listed in Table 1. Median values with 25th and 75th percentiles of m/s and kPa values for each group are illustrated in the boxplot in Fig. 2.
Table 1.
Total number of testes, mean age, testicular volume, and SWE values of the testes according to the groups
| Group | Total number (%) | Mean ± SD | |||
|---|---|---|---|---|---|
| Age (years) | Volume (ml) | m/s | kPa | ||
| A | 100 (50%) | 28.33 ± 6.67 | 15.34 ± 5.24 | 1.85 ± 0.31 | 12.82 ± 5.19 |
| B | 100 (50%) | 27.34 ± 8.49 | 15.44 ± 3.92 | 1.53 ± 0.25 | 8.01 ± 3.02 |
| A + B | 200 (100%) | 27.83 ± 7.63 | 15.39 ± 4.61 | 1.69 ± 0.33 | 10.42 ± 4.87 |
Fig. 2.
Boxplot of median values with 25th and 75th percentiles of m/s and kPa values for each group
Furthermore, a significant negative correlation was found between testicular volume and SWE values in group A (for m/s values: P = 0.043; for kPa values: P = 0.024), but no significant correlation was found between testicular volume and SWE values in group B (for m/s values: P = 0.704; for kPa values: P = 0.736).
Discussion
Infertility is the inability of a sexually active, non-contracepting couple to achieve pregnancy in 1 year.[16]. Although the estimates vary, approximately 15% of couples trying to get pregnancy fail at the end of a year. Approximately 7% of men worldwide are affected by infertility. Male factors constitute 50% of all infertility cases [1]. There are many reasons for infertility in men. These include anatomical or genetic abnormalities, infections, varicocele, gonadotoxins, anti-sperm antibodies, smoking, anabolic steroid use, and a history of testicular surgery, trauma or chemoradiotherapy, etc. [1]. Semen analysis is used to evaluate the male partners in couples with infertility [20]. Approximately 45–50% of all infertility cases have a sperm defect as the main cause. The most common abnormality of semen analysis is oligospermia [1].
US provides a non-invasive and real-time assessment in cases where an intratesticular pathology is suspected. According to the American Institute of Ultrasound in Medicine (AIUM), US is an admissible modality used in the evaluation of male infertility [3]. It can be used to assess testicular size, as this commonly relates to spermatogenesis [3]. Tissue palpation is used by urologists to assess scrotal stiffness, but is a subjective method and requires experience. A spermiogram can indirectly reflect parenchymal damage [2]. Testicular biopsy can be performed to assess the histological features of parenchymal damage, but is no longer routinely recommended, as it is an invasive procedure. SWE is a new ultrasound modality that gives information about tissue stiffness. With SWE, stiffness can be assessed by marking the relevant area both for focal lesions and diffuse diseases. Testis tumor, torsion, infarction, varicocele, hydrocele, and undescended testis were evaluated using SWE in some studies [8–10, 17–19]. Researchers reported significantly higher SWE values in these patients.
In our study, a significant difference in SWE values was observed between the testes of infertile men (group A) and the testes of healthy individuals (group B). The SWE values (m/s and kPa) of group A were significantly higher than those of group B. These findings suggested that the pathological changes in testicular tissue that led to an abnormality in sperm quantity were associated with an increase in testicular stiffness. Therefore, SWE seems to be a reliable technique for quantitatively measuring changes in testicular tissue stiffness in patients with infertility. The potential role of SWE in evaluation of testicular tissue damage has also been demonstrated by histological examination of rabbits in a previous study [9]. Those researchers found increased stiffness values and decreased spermatogenesis following different artificial torsion protocols. Although our findings were not confirmed by histopathological examination, we have found SWE to be a useful technique to evaluate testicular damage in infertile patients with abnormal semen parameters. To the best of our knowledge, there are only a few studies investigating the relationship between testicular SWE evaluation and male infertility [14, 15]. Yavuz et al. [14] found a negative correlation between testicular SWE values and sperm count, and much like we do, they argued that the histological damage in testicular tissue that led to a decrease in sperm count was mostly correlated with an increase in testicular stiffness. In their study, they classified infertile patients according to sperm count, but did not use a control group consisting of non-infertile subjects. Unlike them, we preferred to compare infertile patients with normal subjects rather than to compare them within themselves. When choosing infertile patients, Yavuz et al. also excluded patients with obstructive causes, but did not exclude patients with other testis diseases (varicocele, history of tumor, torsion operation, etc.) that may affect testicular stiffness. Unlike them, we have excluded these kinds of patients from our study to evaluate only the relationship between testicular stiffness and infertility, regardless of other effects. Rocher et al. [15] excluded patients with testis diseases other than varicocele from their study. Their sample consisted of normal and infertile patients. They classified infertile patients as having oligoasthenoteratospermia, obstructive azoospermia (OA), Klinefelter syndrome non-obstructive azoospermia (KS-NOA), non-Klinefelter syndrome non-obstructive azoospermia, and varicocele. In our opinion, although varicocele may be a cause of infertility, it may have affected the SWE results, since it is itself a cause of testicular stiffness [17]. Rocher et al. observed that stiffness was higher in patients with KS-NOA than in those with other causes of infertility. Furthermore, there was no significant difference in testis stiffness between normal population and OA patients, unlike with other causes. As can be seen, SWE values were generally found to be high in infertile patients, although with different classifications. Nevertheless, further studies using larger sample sizes are needed to confirm the efficacy of the SWE technique in routine infertility evaluation.
In addition, no significant difference in testicular volume was observed between the two groups. A significant negative correlation was found between testicular volume and SWE values (m/s and kPa) in group A; however, no significant correlation was found between testicular volume and SWE values in group B. Unlike in normal subjects, testicular volume in infertile patients showed a negative correlation with SWE values. We think that testicular volume can also be a reliable parameter for reflecting the degree of parenchymal damage in patients with infertility and abnormal semen parameters. Like us, Li et al. [21] detected a significantly negative correlation in strain ratios and testicular volume in infertile patients. However, Rocher et al. [15] did not find a correlation between SWE values and testicular volume in infertile patients. This difference may be explained by the differences in strain and shear wave elastography technique. Similarly, the results of studies regarding whether testicular volume is a useful parameter for predicting the degree of interstitial fibrosis are contradictory [22–25]. However, high SWE values may be a more reliable parameter than testicular volume for reflecting interstitial fibrosis, and thus the severity of damage, since there is more similarity between the SWE findings of these studies on this subject.
Several studies have evaluated testes using SWE [8–10, 17–19]. Similar to the studies conducted on other organs, those conducted on testes also performed SWE measurements in limited areas with a constant small-circle ROI. However, a standard area for evaluation was not established in these studies. SWE values may differ in different parts of the same organ. Therefore, in this study, we used three different ROIs on the different parts of each testis and obtained three measurements, the average of which was considered the SWE value of the corresponding testis. This was thought to increase the accuracy of the numerical values obtained. Nevertheless, studies assessing the effect of different types of ROI on SWE measurements are needed.
Our study had some limitations. First, we could not perform histopathological evaluation of the testes. However, it is not a preferred method to conduct biopsies on infertile patients. Second, only one operator performed all of the examinations, so we could not evaluate inter-observer variability. Third, the SWE measurements were taken in a single section in the longitudinal plane. We believe that if the measurements were taken in more than one section, the reliability of the SWE values might increase. Fourth, we could not classify the patients according to cause of infertility, because we included patients in our study who were failing to achieve a clinical pregnancy after 12 months or more of regular unprotected sexual intercourse, regardless of cause. Finally, we looked at testicular stiffness in infertile patients with abnormal semen parameters, but did not correlate SWE values with sperm count. Correlating SWE values with sperm count may provide additional information in future studies.
In conclusion, the results of our study suggest that SWE can be used as an effective technique for assessing testes in infertile patients to predict parenchymal damage that leads to a decrease in sperm count. In the infertile group, a negative correlation exists between testicular volume and SWE values. Therefore, decreased testicular volume, together with increased SWE values, can reflect the degree of parenchymal damage. Nevertheless, further studies are needed to identify the pathologic changes underlying the increased stiffness in testes of infertile patients.
Acknowledgements
We would like to thank Funda Gokgoz Durmaz for providing help in the statistical analysis of the study.
Author contributions
HE: project development, data collection, and manuscript writing. MSD: project development and data collection. BÖ: data analysis and manuscript writing. HC: data collection and data analysis. DÖ: data collection. İbrahim EG: data analysis and manuscript writing.
Funding
No financial support was received.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Katz DJ, Teloken P, Shoshany O. Male infertility—the other side of the equation. Aust Fam Physician. 2017;46(9):641–646. [PubMed] [Google Scholar]
- 2.Laven JSE, Haans LCF, Mali WP, te Velde ER, Wensing CJ, Eimers JM. Effects of varicocele treatment in adolescents: a randomised study. Fertil Steril. 1992;58(4):756–762. doi: 10.1016/S0015-0282(16)55324-2. [DOI] [PubMed] [Google Scholar]
- 3.Jurewicz M, Gilbert BR. Imaging and angiography in male factor infertility. Fertil Steril. 2016;105(6):1432–1442. doi: 10.1016/j.fertnstert.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 4.Sigrist RMS, Liau J, Kaffas AE, Chammas MC, Willmann JK. Ultrasound elastography: review of techniques and clinical applications. Theranostics. 2017;7:1303–1329. doi: 10.7150/thno.18650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song EJ, Sohn YM, Seo M. Diagnostic performances of shear-wave elastography and B-mode ultrasound to differentiate benign and malignant breast lesions: the emphasis on the cutoff value of qualitative and quantitative parameters. Clin Imaging. 2018;50:302–307. doi: 10.1016/j.clinimag.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 6.Ozturker C, Karagoz E, Incedayi M. Non-invasive evaluation of liver fibrosis: 2-D shear wave elastography, transient elastography or acoustic radiation force impulse imaging? Ultrasound Med Biol. 2016;42:3052. doi: 10.1016/j.ultrasmedbio.2016.03.033. [DOI] [PubMed] [Google Scholar]
- 7.Magri F, Chytiris S, Capelli V, et al. Shear wave elastography in the diagnosis of thyroid nodules: feasibility in the case of coexistent chronic autoimmune Hashimoto’s thyroiditis. Clin Endocrinol (Oxf) 2012;76:137–141. doi: 10.1111/j.1365-2265.2011.04170.x. [DOI] [PubMed] [Google Scholar]
- 8.Marsaud A, Durand M, Raffaelli C, et al. Elastography shows promise in testicular cancer detection. Prog Urol. 2015;25:75–82. doi: 10.1016/j.purol.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 9.Zhang X, Lv F, Tang J. Shear wave elastography (SWE) is reliable method for testicular spermatogenesis evaluation after torsion. Int J Clin Exp Med. 2015;8(5):7089–7097. [PMC free article] [PubMed] [Google Scholar]
- 10.Kantarci F, CebiOlgun D, Mihmanli I. Shear-wave elastography of segmental infarction of the testis. Korean J Radiol. 2012;13:820–822. doi: 10.3348/kjr.2012.13.6.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trottmann M, Marcon J, D'Anastasi M, et al. Shear-wave elastography of the testis in the healthy man—determination of standard values. Clin Hemorheol Microcirc. 2016;62(3):273–281. doi: 10.3233/CH-162046. [DOI] [PubMed] [Google Scholar]
- 12.Marcon J, Trottmann M, Rübenthaler J, Stief CG, Reiser MF, Clevert DA. Shear wave elastography of the testes in a healthy study collective—differences in standard values between ARFI and VTIQ techniques. Clin Hemorheol Microcirc. 2016;64(4):721–728. doi: 10.3233/CH-168052. [DOI] [PubMed] [Google Scholar]
- 13.De Zordo T, Stronegger D, Pallwein-Prettner L, et al. Multiparametric ultrasonography of the testicles. Nat Rev Urol. 2013;10(3):135–148. doi: 10.1038/nrurol.2012.255. [DOI] [PubMed] [Google Scholar]
- 14.Yavuz A, Yokus A, Taken K, Batur A, Ozgokce M, Arslan H. Reliability of testicular stiffness quantification using shear wave elastography in predicting male fertility: a preliminary prospective study. Med Ultrason. 2018;20(2):141–147. doi: 10.11152/mu-1278. [DOI] [PubMed] [Google Scholar]
- 15.Rocher L, Criton A, Gennisson JL, et al. Testicular shear wave elastography in normal and infertile men: a prospective study on 601 patients. Ultrasound Med Biol. 2017;43(4):782–789. doi: 10.1016/j.ultrasmedbio.2016.11.016. [DOI] [PubMed] [Google Scholar]
- 16.Zegers-Hochschild F, Adamson GD, de Mouzon J, et al. International Committee for Monitoring Assisted Reproductive Technology; World Health Organization. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology. Fertil Steril. 2009;92:1520–1524. doi: 10.1016/j.fertnstert.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 17.Dede O, Teke M, Daggulli M, Utangaç M, Baş O, Penbegül N. Elastography to assess the effect of varicoceles on testes: a prospective controlled study. Andrologia. 2016;48(3):257–261. doi: 10.1111/and.12440. [DOI] [PubMed] [Google Scholar]
- 18.Kocaoglu C, Durmaz MS, Sivri M. Shear wave elastography evaluation of testes with non-communicating hydrocele in infants and toddlers: a preliminary study. J Pediatr Urol. 2018;14(5):445.e1–445.e6. doi: 10.1016/j.jpurol.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 19.Durmaz MS, Sivri M, Sekmenli T, Kocaoğlu C, Çiftçi İ. Experience of using shear wave elastography imaging in evaluation of undescended testes in children: feasibility, reproducibility, and clinical potential. Ultrasound Q. 2018;34(4):206–212. doi: 10.1097/RUQ.0000000000000344. [DOI] [PubMed] [Google Scholar]
- 20.Keel BA, Stembridge T, Pineda G, Serafy NT. Lack of standardization in performance of the semen analysis among laboratories in the United States. Fertil Steril. 2002;78:603–608. doi: 10.1016/S0015-0282(02)03296-X. [DOI] [PubMed] [Google Scholar]
- 21.Li M, Du J, Wang Z, Li F. The value of sonoelastography scores and the strain ratio in differential diagnosis of azoospermia. J Urol. 2012;188:1861–1866. doi: 10.1016/j.juro.2012.07.031. [DOI] [PubMed] [Google Scholar]
- 22.Kurtz MP, Zurakowski D, Rosoklija I, et al. Semen parameters in adolescents with varicocele: association with testis volume differential and total testis volume. J Urol. 2015;193(5):1843–1847. doi: 10.1016/j.juro.2014.10.111. [DOI] [PubMed] [Google Scholar]
- 23.Keene DJ, Sajad Y, Rakoczy G, Cervellione RM. Testicular volume and semen parameters in patients aged 12 to 17 years with idiopathic varicocele. J Pediatr Surg. 2012;47(2):383–385. doi: 10.1016/j.jpedsurg.2011.11.035. [DOI] [PubMed] [Google Scholar]
- 24.Aragona F, Ragazzi R, Pozzan GB, et al. Correlation of testicular volume, histology and LHRH test in adolescents with idiopathic varicocele. Eur Urol. 1994;26(1):61–66. doi: 10.1159/000475344. [DOI] [PubMed] [Google Scholar]
- 25.Sigman M, Jarow JP. Ipsilateral testicular hypotrophy is associated with decreased sperm counts in infertile men with varicoceles. J Urol. 1997;158(2):605–607. doi: 10.1016/S0022-5347(01)64567-1. [DOI] [PubMed] [Google Scholar]