Abstract
Purpose
We aimed to investigate the use and effectiveness of Shear-Wave Elastography (SWE) in Hashimoto’s Thyroiditis (HT) diagnosis and compare the SWE values in HT patients with asymptomatic volunteers.
Methods
The thyroid gland parenchyma of 74 patients whose clinical and laboratory findings and ultrasonography (US) features were indicative of HT and 75 healthy, asymptomatic participants with normal laboratory values were examined using SWE. Their thyroid parenchymal echoes and thyroid gland volume were measured using B-mode US examination. Elastographic measurements were made by plotting the boundaries of thyroid gland by hand, using Free Region of Interest (ROI). The quantitative SWE values [meters/second (m/s) and kilopascal (kPa)] were compared betweent the patients and the controls. The correlation analyses between the SWE measurements and the autoantibodies [Anti-thyroid peroxidase antibody (TPOAbs) and anti-thyroglobulin antibodies (TgAbs)], thyroid-stimulating hormone (TSH), freetriiodothyronine (fT3), free-thyroxine (fT4), and thyroglobulin levels were performed.
Results
The mean thyroid SWE measurement values of HT group were significantly higher than the asymptomatic group (p < 0.001). This study proposes 29.45 kPa or 2.77 m/s as a sensitive-spesific cut-off value for HT. We revealed significant positive association between SWE values and TgAb levels, gland volume, TgAb, TPOAb levels, and a significant negative association between SWE and echogenicity (p < 0.001).
Conclusion
In the assessment of HT, SWE is a highly sensitive imaging method to estimate the degree of fibrosis and to provide objective numerical values.
Keywords: Free ROI, Hashimoto’s thyroiditis, Shear wave elastography, Ultrasonography
Introduction
Thyroiditis is a generic word that includes a range of clinical entities that influence the thyroid gland. Hashimoto’s thyroiditis (HT) is the most common autoimmune thyroid disorder. Typically, it influences females 5–8-fold more than males [1, 2]. Pathological HT characteristics are lymphocytic infiltration and varying levels of fibrosis in the interstitium [3, 4]. Fibrosis gives rise to the thyroid parenchyma getting stiffer than normal thyroid tissue [5, 6].
The major imaging technique in HT diagnosis and follow-up is ultrasonography (US). Heterogeneous hypoechoic echogenicity and lobulated contour are specific gray-scale findings; however, sensitivity of these findings are limited [7, 8]. Shear Wave Elastography (SWE) is a real-time, non-invasive, and reproducible new imaging technology which allows the quantitative assessments of tissues according to their stiffness The quantitative elasticity value is calculated in both elasticity-kilopascal (kPa) and velocity-meters/second (m/s) [9–15]. The detection of an increase in thyroid tissue stiffness with SWE may increase the sensitivity of B-mode US for the diagnosis and show the disease degree and the activity of HT. In all studies in the literature, the SWE measurements were obtained with a limited ROI without any possibility to change their dimensions [1, 7–9, 16–19]. Unlike these previous studies, we used the average SWE values by drawing thyroid gland with free Region Of Interest (ROI) manually. This is the first and only study that evaluates thyroid parenchyma by two-dimensional SWE using this measurement technique in the literature. The objective of this study was quantitatively evaluated elasticity of thyroid parenchyma by SWE using the free ROI in HT patients, correlated elasticity with serum thyroid hormones and autoantibodies, and compared them with healthy controls.
Methods
The study was carried out in our hospital between October 2017 and March 2019 and allowed by local research ethics committee. HT patients were diagnosed in the pediatric or adult endocrinology outpatient clinic of our hospital according to clinical evidence, thyroid hormone results, thyroid autoantibodies (TPOAbs and/or TgAbs), and thyroiditis pattern (hypoechogenicity and heterogeneous echotexture) on US examination. The patients who had thyroid nodules, Graves’ disease, thyroid gland operation, and radioactive iodine treatment history were not included in the study. All participants were examined with gray-scale US imaging and SWE via high-frequency (4–14 MHz) linear array transducer Toshiba Aplio 500 (Toshiba Medical System Corporation, Tokyo, Japan). The dimensions [length (L) × width (W) × height (H)] of both thyroid lobe were measured and thyroid volume was calculated automatically by computer (Fig. 1). In this study, the echogenicity of thyroid parenchyma was compared with submandibular gland, which was classified into four groups as hyperechoic, isoechoic, hypoechoic, or very hypoechoic. Lymph node close to thyroid gland and echogenic band in parenchyma were noted. The SWE measurements were performed in the right lobe and left lobe on the transverse and longitudinal planes after the B-mode US examination. In the SWE application, one-shot scan or continuous scan can be used. We selected the one-shot scan to be able to get higher image quality. The quality control standards for images in our study were the propagation of the contour lines must be parallel within the thyroid gland and color map pattern in the elasticity mode (kPa) and velocity mode (m/s) imaging must include the nearly all of thyroid parenchyma (colored of nearly all of thyroid parenchyma). Three images were obtained for both the right and left thyroid lobes in transverse and longitudinal planes. Tissue elasticity was defined using a color range from dark blue (lowest) to red (highest) (by default 0–120 kPa) (Fig. 2). Elastic value E (kPa) is calculated using the equation E = 3ρ (m/s)2. In the formula, m/s refers to the shear wave propagation velocity, and ρ refers to the tissue density (whose approximated value in the human body is 1) [20]. Elasticity values were measured by drawing the contours of the thyroid right and left lobe manually using the free ROI (Fig. 3). The average SWE values (m/s and kPa) of longitudinal and transverse planes were collected and divided to two; in this way, average lobe (right or left) SWE value was obtained. Then, average total thyroid SWE value was obtained by calculating the average of right and left lobes (right average SWE value + left average SWE value/2).
Fig. 1.
Measurement of the three dimensions and the volume of the right and left thyroid gland lobe on the longest transverse and longutidinal sections of a 9-year-old girl. The total thyroid volume [right lobe volume (6.8 cm3) + left lobe volume (7.2 cm3)] was measured as 14 cm3
Fig. 2.

Shear wave elastography of 15-year-old symptomatic woman with HT. In the color elastography map, there are yellow–red (stiffer tissue) and blue (softer tissue) areas
Fig. 3.
12-year-old symptomatic woman with HT. Quantitative elasticity values were measured by manually drawing the contours of the thyroid gland structure, on the propagation mode, in the longest transverse plane and longitudinal plane, using the free ROI. The quantitative elasticity values were measured as 2.70 m/s and 23.8 kPa in the transverse plane and 4.29 m/s and 59.5 kPa in the longutidinal plane. The mean quantitative elasticity values for the left thyroid lobe were measured as 3.495 m/s (2.70 + 4.29/2) and 41.65 kPa (23.8 + 59.5/2)
Statistical analysis
Statistical analysis was performed using the Statistical Package for Social Sciences (SPSS) version 24 (IBM, Armonk, NY) software to evaluate the data. The descriptive statistics were expressed as mean, standard deviation, minimum–maximum values, frequency, and percentile. The Kolmogorov–Smirnov test was used to determine the normal distribution of continuous variables. First, the definitive statistics related to the variables were assessed. Chi-square and One-way ANOVA tests were used to evaluate the differences between the two groups. Mann–Whitney U test was used to compare SWE values between patients with HT and control group. The Pearson correlation analysis was used to evaluate the relationship between SWE values and serum autoantibody levels, thyroid hormone level, and age. A P value of less than 0.05 was considered significant at a statistical level having 95% confidence level. The receiver-operating characteristic (ROC) curve analysis was used to determine the best cut-off value for the SWE values of the patients with HT and the asymptomatic group. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and diagnostic accuracy were calculated.
Results
The participants were 8–60 years old (mean 25.65 ± 13.83 years). A total of 149 participants, 74 patients and 75 controls, participated in the study. The symptomatic group included 56 females (75.7%) and 18 males (24.3%) with an age range between 8 and 60 years (mean age 28.10 ± 16.84 years). The control group consisted of 54 women (72.0%) and 21 men (28.0%), and the age range ranged from 8 to 45 years (mean age 23.24 ± 9.55 years). BMI of patient group was 28.44 ± 5.76; BMI of the control group was 27.85 ± 5.76. There were no statistically significant differences between the patients and controls in the parameters of age (p = 0.409), gender (p = 0.710), and BMI (p = 0.634).
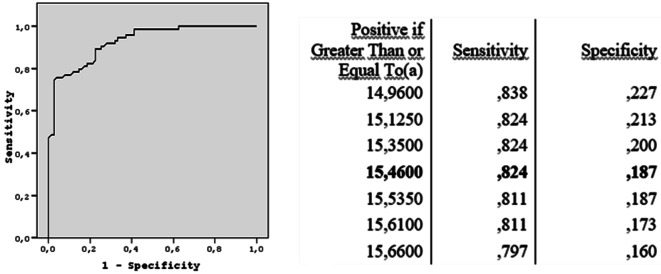
There were no echogenic band in thyroid gland and no reactive adjacent lymph node in the asymptomatic group. In the symptomatic group, 55 (74.3%) patients had lymph nodes and 47 (63.5%) had echogenic bands in gland parenchyma. Mean thyroid volume and SWE measurements of the patient and asymptomatic groups are shown in Table 1. No significant difference was found between SWE values of right and left lobes of thyroid gland in both HT and asymptomatic patients (p = 0.194). Mann–Whitney U test was performed to compare gland volume and SWE measurements, since the data did not follow the normal distribution. HT patients had significantly higher SWE values of (p < 0.001) and higher gland volume (p < 0.001) than control group. In addition, the mean quantitative SWE values of thyroid gland [(mean SWE value of the right thyroid lobe + mean SWE value of the left thyroid lobe)/2] were significantly higher in the HT group than in the control group (p < 0.001) (Table 1). The cut-off values of SWE for HT and specificity, sensitivity, NPV, PPV, and the diagnostic accuracy of these cut-off values are summarized in Table 2. The ROC curve analyses of the SWE values of HT were calculated; kPa is given in Fig. 4 and m/s is given in Fig. 5. The maximum area under curves (AUC) for mean kPa elasticity value was 0.927 and for mean m/s elasticity value was 0.926 (95% confidence interval). The mean thyroid parameters of HT and asymptomatic group are given in Table 3. Serum TSH level was significantly higher in the patient group compared to control group (p = 0.002), but sT4 hormone levels were not significantly different between the two groups (p = 0.857).
Table 1.
Volume and SWE values of the thyroid gland in patients with HT and asymptomatic group
| Symptomatic group (74) | Asymptomatic group (75) | p value | |
|---|---|---|---|
| Right lobe volume | 7.97 ± 4.75 cm3 | 7.66 ± 3.43 cm3 | < 0.001 |
| Left lobe volume | 7.06 ± 4.00 cm3 | 6.93 ± 2.96 cm3 | < 0.001 |
| Total volume | 14.98 ± 8.59 cm3 | 14.59 ± 6.32 cm3 | < 0.001 |
| Right thyroid lobe mean kPA in transverse plane | 21.90 ± 12.48 | 10.69 ± 4.38 | < 0.001 |
| Right thyroid lobe mean m/s in transverse plane | 2.50 ± 0.66 | 1.79 ± 0.33 | < 0.001 |
| Right thyroid lobe mean kPA in longutidinal plane | 26.85 ± 10.86 | 14.58 ± 5.38 | < 0.001 |
| Right thyroid lobe mean m/s in longutidinal plane | 2.81 ± 0.55 | 2.13 ± 0.33 | < 0.001 |
| Right thyroid lobe mean kPA | 24.38 ± 10.99 | 12.70 ± 4.24 | < 0.001 |
| Right thyroid lobe mean m/s | 2.66 ± 0.57 | 1.96 ± 0.28 | < 0.001 |
| Left thyroid lobe mean kPA in transverse plane | 24.07 ± 11.65 | 10.19 ± 3.93 | < 0.001 |
| Left thyroid lobe mean m/sn in transverse plane | 2.62 ± 0.61 | 1.75 ± 0.32 | < 0.001 |
| Left thyroid lobe mean kPA in longutidinal plane | 27.20 ± 12.75 | 14.37 ± 3.61 | < 0.001 |
| Left thyroid lobe mean m/s in longutidinal plane | 2.86 ± 0.60 | 2.13 ± 0.25 | < 0.001 |
| Left thyroid lobe mean kPA | 25.64 ± 11.14 | 12.28 ± 3.08 | < 0.001 |
| Left thyroid lobe mean m/s | 2.74 ± 0.56 | 1.94 ± 0.24 | < 0.001 |
| The mean kPA of thyroid gland | 25.01 ± 10.53 (10.72–68.27) | 12.49 ± 3.23 (7.72–23.67) | < 0.001 |
| The mean m/sn of thyroid gland | 2.70 ± 0.53 (1.83–4.61) | 1.94 ± 0.23 (1.55–2.61) | < 0.001 |
Table 2.
Cut-off values of SWE (kPa and m/s) for HT and sensitivity, specificity PPV, NPV, and diagnostic accuracy of these cut-off values
| Cut-off value | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Diagnostic accuracy (%) |
|---|---|---|---|---|---|
| 15.4600 kPa | 82.4 | 81.3 | 81.3 | 82.4 | 81.8 |
| 2.1500 m/s | 85.1 | 78.7 | 79.7 | 84.2 | 81.8 |
Fig. 4.
ROC curve analysis of the SWE values of HT and the best cut-off value for elasticity (kPa)
Fig. 5.
ROC curve analysis of the SWE values of HT and the best cut-off value for velocity (m/s)
Table 3.
Mean thyroid function parameters of the symptomatic group and asymptomatic group
| Symptomatic group (74) (mean ± SD) | Asymptomatic group (75) (mean ± SD) | |
|---|---|---|
| TSH | 8.86 ± 20.78 | 2.73 ± 1.28 |
| fT4 | 1.43 ± 0.66 | 1.31 ± 0.15 |
| fT3 | 4.36 ± 3.79 | – |
| Thyroglobulin | 25.00 ± 99.64 | – |
| TPOAbs | 547.11 ± 375.39 | – |
| TgAbs | 549.78 ± 833.55 | – |
There was a significant strong positive correlation between age and SWE values (both m/s and kPa) (for kPa p = 0.002, Pearson coefficient 0.249, for m/s p = 0.002, Pearson coefficient 0.256). The correlation analysis between the lab tests (TSH, fT4, fT3, thyroglobulin, TPOAbs, TgAbs) and SWE values is shown in Table 4. There was a significant positive correlation between TgAbs levels and SWE values (both m/s and kPa) (p = 0.001); however, there was no significant correlation between the SWE values and TPOAbs, Tiroglobulin, TSH, fT3, and fT4 levels (p > 0.05). There was a significant positive association between total thyroid volume and TgAbs, TPOAbs levels (p = 0.001), but there was no significant association between total gland volume and thyroglobulin levels (p > 0.05) (Table 4). In the entire control group, the laboratory values were within normal limits and euthyroid. In the symptomatic patient group, 51 (68.9%) of the patients were euthyroid. Hypothyroidism was detected in 13 (17.6%) of the symptomatic patients and hyperthyroidism in 10 (13.5%) (Table 5). In the symptomatic group, no difference existed between these subgroups (in patients with euthyroid, hyperthyroid, and hypothyroid) at a significant level in terms of mean SWE numerical values, according to the one-way ANOVA test (for m/s p = 0.640, f = 0.449; for kPa p = 0.688, and f = 0.376). Grouping according to thyroid echogenecity and average SWE values is shown in Table 6. While thyroid parenchyma echogenicity decreased, m/s and kPa values increased. There was a significant association between parenchyma echogenicity and kPa and m/s in HT patients; however, there was no significant association between parenchyma echogenicity and m/s in the control group. There was a strong negative correlation between thyroid parenchyma echogenicity and kPa and m/s in both HT and asymptomatic groups (Table 7).
Table 4.
Correlation analysis between the laboratory values (TSH, fT4, fT3, Tiroglobulin, TPOAbs, and TgAbs) and SWE values (kPa and m/s) in patients with HT
| Correlation analysis between | p value | Pearson coefficient |
|---|---|---|
| TSH and kPa | 0.093 | 0.113 |
| TSH and m/s | 0.169 | 0.138 |
| fT4 and kPa | 0.209 | 0.104 |
| fT4 and m/s | 0.374 | 0.073 |
| fT3 and kPa | 0.886 | 0.017 |
| fT3 and m/s | 0.837 | 0.024 |
| Tiroglobulin and m/s | 0.091 | 0.198 |
| Tiroglobulin and kPa | 0.064 | 0.217 |
| TPOAbs and m/s | 0.094 | 0.196 |
| TPOAbs and kPa | 0.065 | 0.216 |
| TgAbs and m/s | 0.001* | 0.516 (positive correlation) |
| TgAbs and kPa | 0.001* | 0.564 (positive correlation) |
| TgAbs and volume | 0.001* | 0.421(positive correlation) |
| TPOAbs and volume | 0.001* | 0.403(positive correlation) |
| Thyroglobulin and volume | 0.647 | − 0.054 |
* P value of less than 0.05 was considered significant at a statistical level having 95% confidence level
Table 5.
Mean SWE values of patients in the symptomatic group were compared to euthyroid, hypothyroid, and hyperthyroid
| Patients with Hashimato’s thyroiditis (74 patients) | Mean kPa | Mean m/s |
|---|---|---|
| Euthyroid (51 patients, 68.9%) (TSH, fT3, and fT4 in normal range) | 24.44 ± 8.86 | 2.68 ± 0.58 |
| Hypothyroid (13 patients, 17.6%) (TSH increased, and fT3 and fT4 decreased) | 27.31 ± 12.70 | 2.83 ± 0.63 |
| Hyperthyroid (10 patients, 13.5%) (TSH decreased, and fT3 and fT4 increased) | 24.85 ± 8.96 | 2.66 ± 0.46 |
Table 6.
Mean SWE values according to the thyroid gland parenchyma echogenicity in patients with HT and asymptomatic group
| Asymptomatic group | Symptomatic group | Total | Mean kPa | Mean m/sn | |
|---|---|---|---|---|---|
| Hyperechogenic | 53 (70.7%) | 6 (8.1%) | 59 | 13.4076 (± 4.4325) | 2.0088 (± 0.3027) |
| Isoechogenic | 22 (29.3%) | 22 (29.7%) | 44 | 17.2802 (± 8.2781) | 2.2448 (± 0.49104) |
| Hypoechogenic | 0 | 31 (41.9%) | 31 | 25.6306 (± 11.37851) | 2.7461 (± 0.55160) |
| Marked hypoechogenic | 0 | 15 (20.3%) | 15 | 29.4673 (± 11.23881) | 2.9207 (± 0.56110) |
Table 7.
Correlation analysis between thyroid parenchyma echogenicity and SWE values
| Correlation between | p value | Pearson correlation coefficient | |
|---|---|---|---|
| Patients with HT (74) | Ecogenity and kPa | 0.019* | 0.272 |
| Ecogenity and m/s | 0.017* | 0.277 | |
| Asymptomatic Group (75) | Ecogenity and kPa | 0.470 | 0.085 |
| Ecogenity and m/s | 0.511 | 0.077 | |
| All participants (149) | Ecogenity and kPa | 0.001* | 0.572 |
| Ecogenity and m/s | 0.001* | 0.596 |
* P value of less than 0.05 was considered significant at a statistical level having 95% confidence level
Discussion
This study revealed that the mean SWE values of the thyroid gland in HT patients were higher at a significant level than of those in control group. Increased thyroid stiffness was positively correlated with HT progression and thyroid fibrosis [16]. The pathological HT effects on thyroid parenchyma regarding the use of SWE with comparison to the control subjects have been evaluated in several studies. In all these studies, the elasticity values were reported to be significantly higher in HT group [2, 7, 16–18, 21–24].
In studies that evaluated thyroid gland parenchyma using SWE, semiquantitative SWE values were measured in nodules [12–15]; the quantitative elasticity values were measured only as m/s [7, 16, 17, 19, 23–26] or only as kPa [2, 5, 6, 18, 21, 27, 28]. However, both m/s and kPa measurements together are used in a limited number of studies [1]. The quantitative elasticity values both measured in kPa and m/s releaved good diagnostic performance, but the specificity of the standard deviation and area under the ROC curve of the lesion measured in kPa were found significantly higher than those measured in m/s in a study [29].
The mean SWE value of HT patients was found to be between 19.5 ± 6.8 kPa and 36.15 ± 18.7 kPa [1, 2, 6, 18, 21, 23], and between 1.67 ± 0.63 m/s and 2.56 ± 0.30 m/s [7, 16, 17, 23, 24, 30, 31] in previous studies. The mean SWE value of an asymptomatic group was found to be between 9.0 ± 11.3 kPa and 19.5 ± 7.6 kPa [1, 2, 5, 18, 28, 32, 33] and between 1.22 ± 0.20 m/s and 2.07 ± 0.44 m/s [2, 7, 16–19, 23–26, 28, 32, 33]. In our study, the mean SWE values in HT were 25.01 ± 10.53 kPa, 2.70 ± 0.53 m/s, and that in the asymptomatic control group was 12.49 ± 3.23 kPa, 1.94 ± 0.23 m/s.
The highest diagnostic accuracy cut-off value for SWE was between 12.3 kPa and 22.3 kPa [1, 6, 15] and 1.41 m/s and 2.42 m/s [1, 7, 13, 20, 21] in the literature. This study proposes the highest diagnostic accuracy cut-off value for SWE as 15.46 kPa and 2.15 m/s to predict HT.
In studies conducted on thyroid, the SWE measurements were made in the longitudinal or transverse planes in limited areas with the ROI in fixed small circle or square whose sizes could not be changed; however, an area that may be used as a standard was not presented [1, 2, 5–7, 16–18, 23–26, 28, 30, 32, 33]. The SWE only reflects the characteristics of the elasticity values of the tissue inside the ROI [16]. The SWE values may considerably vary based on the usage of small ROI (Fig. 6). This situation limits the reproducibility of these SWE measurements and their use in outpatient clinics. In our study, we used the mean SWE values obtained by plotting the entire gland using the free ROI in the longitudinal and transverse planes (Fig. 7). Since HT is a diffuse thyroid disease, we believe that this measurement technique is more applicable, more reproducible, and more reliable. To our knowledge, this is the first study to assess the SWE of thyroid gland using this measurement technique.
Fig. 6.
Quantitative elasticity values were measured using small-box ROI
Fig. 7.
Quantitative elasticity values were measured by manually drawing the contours of the tyroid gland
We found a significant difference between HT patients and asymptomatic volunteers in both SWE numerical values. In accordance with the current literature [7, 16, 18, 22–24], we did not find any significant differences between the SWE values of the right and left lobes in both HT patients and control group (p > 0.05). Therefore, measuring from only one lobe in pediatric patients can shorten the duration of the examination.
In addition, in accordance with the current literature, we found a significant positive correlation between age and SWE values [34]. Liu et al. reported that the SWE values in HT positively correlated with serum TSH and TgAb but not with serum T3, T4, fT3, fT4, or TPOAb [6]. In this study, a significant positive correlation was found between TgAb levels and SWE values (both m/s and kPa) (p = 0.001); however, there was no significant relationship between SWE values and TPOAb, thyroglobulin, TSH, fT3, and fT4 levels (p > 0.05). In our study, when HT patients were grouped according to the presence of euthyroid, hyperthyroid, and hypothyroidism, no significant difference was found in mean SWE values between the groups (p > 0.05). The significant and positive relation between TgAbs levels and SWE values might be associated with the inflammation level not only with thyroid fibrosis. We determined that thyroid right lobe, left lobe, and total thyroid volume were significantly higher in HT patients than in the control group (p < 0.005). In a recent study, Kandemirli found a significant positive relationship between total thyroid volume and TPOAb levels, but he did not find any significant correlation between elasticity values and TgAb levels [1]. However, in our study, a significant positive correlation was found between thyroid volume and TgAb, TPOAb levels (p = 0.001), but there was no significant correlation between total thyroid volume and thyroglobulin, TSH, fT4, and fT3 levels (p > 0.05).
As mentioned in previous studies, SWE remains an examiner-dependent method. All SE techniques require a trained and experienced operator. The free-hand probe pressure is difficult to standardize among different US operators. The main limitation of our study is the lack of intra-observer and inter-observer agreement evaluation, because all measurements were performed by only one expert operator. Another limitation is that the diagnoses of HT relied on clinical manifestation, ultrasonography, and laboratory findings instead of pathological finding [9–15].
The hypoechogenicity was found to be associated with higher elasticity [35]. Consistently, we found inverse correlation between thyroid parenchyma echogenicity and mean SWE values. The echogenicity of thyroid gland was mainly reduced in the HT group. The correlation analysis of combined HT and control groups gave a significant stronger negative correlation between thyroid echogenicity and SWE measurements (both kPa and m/s) (p < 0.001). These results show that the hypoechogenicity on B-mode US reflects the presence of fibrosis, and that a combination of B-mode scanning and SWE can be used to evaluate the degree of fibrosis in HT. However, we claim that SWE is superior and more practicable than other outpatient US techniques to document the disease progression, since SWE quantitatively indicates the degree of fibrosis with numerical values. Over the past 2 decades, the method of managing thyroid nodules has changed due to the widespread effective use of ultrasonography and fine-needle aspiration (a minimal invasive technique) to obtain histopathological evidence. However the quantitative management of diffuse thyroid diseases such as HT is still insufficient [36]. Laboratory tests have poor correlation with HT progress. SWE use after conventional US with minimal time prolong can provide numerical value of tissue stiffness associated with HT severity. Also we claim that 15.46 kPa and 2.15 m/s can be used as practical cut-off values in daily routine for undiagnosed HT patients. However, future studies with more patients are required to confirm the use of SWE in HT evaluation.
Author contributions
Study concept and design: TK and MSD; analysis and interpretation of data: FA and NA; drafting of the manuscript: TK and MSD; critical revision of the manuscript for important intellectual content: BÖ and MÖ; statistical analysis: FGD.
Compliance with ethical standards
Conflict of interest
Authors declare that there are no financial or other relations that could lead to a conflicts of interest.
Ethical statements
Patients were provided with detailed information about the procedures and they signed written consent forms. The approval of the ethics committee was obtained before the initiation of the study.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Turgay Kara, Email: trgykr@gmail.com.tr.
Fatih Ateş, Email: fatih_ates81@hotmail.com.
Mehmet Sedat Durmaz, Email: dr.msdurmaz@gmail.com.
Nesibe Akyürek, Email: n_akyurek@yahoo.com.tr.
Funda Gökgöz Durmaz, Email: gokgozdurmaz@hotmail.com.
Bora Özbakır, Email: boradr78@gmail.com.
Mehmet Öztürk, Email: drmehmet2121@gmail.com.
References
- 1.Kandemirli SG, Bayramoglu Z, Caliskan E, Sari ZNA, Adaletli I. Quantitative assessment of thyroid gland elasticity with shearwave elastography in pediatric patients with Hashimoto’s thyroiditis. J Med Ultrason. 2001;2018(45):417–423. doi: 10.1007/s10396-018-0859-0. [DOI] [PubMed] [Google Scholar]
- 2.Ruchała M, Szmyt K, Sławek S, Zybek A, Szczepanek-Parulska E. Ultrasound sonoelastography in the evaluation of thyroiditis and autoimmune thyroid disease. Endokrynol Pol. 2014;65:520–526. doi: 10.5603/EP.2014.0071. [DOI] [PubMed] [Google Scholar]
- 3.Caturegli P, De Remigis A, Rose NR. Hashimoto thyroiditis: clinical and diagnostic criteria. Autoimmun Rev. 2014;13:391–397. doi: 10.1016/j.autrev.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 4.Pandit AA, Vijay Warde M, Menon PS. Correlation of number of intrathyroid lymphocytes with antimicrosomal antibody titer in Hashimoto’s thyroiditis. Diagn Cytopathol. 2003;28:63–65. doi: 10.1002/dc.10235. [DOI] [PubMed] [Google Scholar]
- 5.Arda K, Ciledag N, Aktas E, Aribas BK, Köse K. Quantitative assessment of normal soft-tissue elasticity using shear-wave ultrasound elastography. Am J Roentgenol. 2011;197:532–536. doi: 10.2214/AJR.10.5449. [DOI] [PubMed] [Google Scholar]
- 6.Liu J, Zhang Y, Ji Y, Wan Q, Dun G. The value of shear wave elastography in diffuse thyroid disease. Clin Imaging. 2018;49:187–192. doi: 10.1016/j.clinimag.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 7.Hekimoglu K, Yildirim Donmez F, Arslan S, Ozdemir A, Demir C, Yazici C. The role of shear wave elastography in the diagnosis of chronic autoimmune thyroiditis. Med Ultrason. 2015;17:322–326. doi: 10.11152/mu.2013.2066.173.khu. [DOI] [PubMed] [Google Scholar]
- 8.Raber W, Gessl A, Nowotny P, Vierhapper H. Thyroid ultrasound versus antithyroid peroxidase antibody determination: a cohort study of four hundred fifty-one subjects. Thyroid. 2002;12:725–731. doi: 10.1089/105072502760258712. [DOI] [PubMed] [Google Scholar]
- 9.Hamidi C, Göya C, Hattapoğlu S, et al. Acoustic radiation force impulse (ARFI) imaging for the distinction between benign and malignant thyroid nodules. Radiol Med. 2015;120:579–583. doi: 10.1007/s11547-014-0495-8. [DOI] [PubMed] [Google Scholar]
- 10.Durmaz MS, Arslan S, Özbakır B, et al. Effectiveness of shear wave elastography in the diagnosis of acute pancreatitis on admission. Med Ultrason. 2018;30:278–284. doi: 10.11152/mu-1398. [DOI] [PubMed] [Google Scholar]
- 11.Durmaz MS, Sivri M, Sekmenli T, Kocaoğlu C, Çiftçi İ. Experience of using shear wave elastography ımaging in evaluation of undescended testes in children: feasibility, reproducibility, and clinical potential. Ultrasound Q. 2018 doi: 10.1097/RUQ.0000000000000344. [DOI] [PubMed] [Google Scholar]
- 12.Hattapoğlu S, Göya C, Hamidi C, et al. Evaluation of parathyroid lesions with point shear wave elastography. J Ultrasound Med. 2016;35:2179–2182. doi: 10.7863/ultra.15.10074. [DOI] [PubMed] [Google Scholar]
- 13.Cantisani V, David E, Grazhdani H, Rubini A, Radzina M, Dietrich CF, Durante C, Lamartina L, Grani G, Valeria A, Bosco D, Di Gioia C, Frattaroli FM, D'Andrea V, De Vito C, Fresilli D, D'Ambrosio F, Giacomelli L, Catalano C. Prospective evaluation of semiquantitative strain ratio and quantitative 2D ultrasound shear wave elastography (SWE) in association with TIRADS classification for thyroid nodule characterization. Ultraschall Med. 2019;40(4):495–503. doi: 10.1055/a-0853-1821. [DOI] [PubMed] [Google Scholar]
- 14.Cantisani V, Maceroni P, D'Andrea V, Patrizi G, Di Segni M, De Vito C, Grazhdani H, Isidori AM, Giannetta E, Redler A, Frattaroli F, Giacomelli L, Di Rocco G, Catalano C, D'Ambrosio F. Strain ratio ultrasound elastography increases the accuracy of colour-Doppler ultrasound in the evaluation of Thy-3 nodules. A bi-centre university experience. Eur Radiol. 2016;26(5):1441–1449. doi: 10.1007/s00330-015-3956-0. [DOI] [PubMed] [Google Scholar]
- 15.Cantisani V, Grazhdani H, Drakonaki E, D'Andrea V, Di Segni M, Kaleshi E, Calliada F, Catalano C, Redler A, Brunese L, Drudi FM, Fumarola A, Carbotta G, Frattaroli F, Di Leo N, Ciccariello M, Caratozzolo M, D'Ambrosio F. Strain US elastography for the characterization of thyroid nodules: advantages and limitation. Int J Endocrinol. 2015;2015:908575. doi: 10.1155/2015/908575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukuhara T, Matsuda E, Izawa S, Fujiwara K, Kitano H. Utility of shear wave elastography for diagnosing chronic autoimmune thyroiditis. J Thyroid Res. 2015;2015:164548. doi: 10.1155/2015/164548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sporea I, Vlad M, Bota S, et al. Thyroid stiffness assessment by acoustic radiation force impulse elastography (ARFI) Ultraschall Med. 2011;32:281–285. doi: 10.1055/s-0029-1246048. [DOI] [PubMed] [Google Scholar]
- 18.Vlad M, Golu I, Bota S, et al. Real-time shear wave elastography may predict autoimmune thyroid disease. Wien Klin Wochenschr. 2015;127:330–336. doi: 10.1007/s00508-015-0754-2. [DOI] [PubMed] [Google Scholar]
- 19.Ceyhan Bilgici M, Sağlam D, Delibalta S, Yücel S, Tomak L, Elmalı M. Shear wave velocity of the healthy thyroid gland in children with acoustic radiation force impulse elastography. J Med Ultrason. 2018;45:75–80. doi: 10.1007/s10396-017-0788-3. [DOI] [PubMed] [Google Scholar]
- 20.Kudo M, Shiina T, Moriyasu F, et al. JSUM ultrasound elastography practice guidelines: liver. J Med Ultrason. 2013;40:325–357. doi: 10.1007/s10396-013-0460-5. [DOI] [PubMed] [Google Scholar]
- 21.Ruchala M, Szczepanek-Parulska E, Zybek A, et al. The role of sonoelastography in acute, subacute and chronic thyroiditis: a novel application of the method. Eur J Endocrinol. 2012;166:425–432. doi: 10.1530/EJE-11-0736. [DOI] [PubMed] [Google Scholar]
- 22.Menzilcioglu MS, Duymus M, Gungor G, et al. The value of realtime ultrasound elastography in chronic autoimmune thyroiditis. Br J Radiol. 2014;87(1044):20140604. doi: 10.1259/bjr.20140604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sporea I, Sirli R, Bota S, Vlad M, Popescu A, Zosin I. ARFI elastography for the evaluation of diffuse thyroid gland pathology: preliminary results. World J Radiol. 2012;4:174–178. doi: 10.4329/wjr.v4.i4.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yucel S, Ceyhan Bilgici M, Kara C, et al. Acoustic radiation force impulse quantification in the evaluation of thyroid elasticity in pediatric patients with hashimoto thyroiditis. J Ultrasound Med. 2018;37:1143–1149. doi: 10.1002/jum.14459. [DOI] [PubMed] [Google Scholar]
- 25.Sağlam D, Ceyhan Bilgici M, Kara C, Can Yilmaz G, Tanrivermiş SA. Does type 1 Diabetes Mellitus affect the shear wave velocity of the thyroid gland of children without autoimmune thyroiditis? Ultrasound Q. 2017;33(3):225–228. doi: 10.1097/RUQ.0000000000000301. [DOI] [PubMed] [Google Scholar]
- 26.Friedrich-Rust M, Romenski O, Meyer G, et al. Acoustic radiation force impulse-imaging for the evaluation of the thyroid gland: a limited patient feasibility study. Ultrasonics. 2012;52:69–74. doi: 10.1016/j.ultras.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 27.Magri F, Chytiris S, Capelli V, et al. Shear wave elastography in the diagnosis of thyroid nodules: feasibility in the case of coexistent chronic autoimmune Hashimoto’s thyroiditis. Clin Endocrinol (Oxf) 2012;76:137–141. doi: 10.1111/j.1365-2265.2011.04170.x. [DOI] [PubMed] [Google Scholar]
- 28.Herman J, Sedlackova Z, Vachutka J, Furst T, Salzman R, Vomacka J. Shear wave elastography parameters of normal soft tissues of the neck. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2017;161:320–325. doi: 10.5507/bp.2017.024. [DOI] [PubMed] [Google Scholar]
- 29.Youk JH, Son EJ, Park AY, Kim JA. Shear-wave elastography for breast masses: local shear wave speed (m/sec) versus Young modulus (kPa) Ultrasonography. 2014;33:34–39. doi: 10.14366/usg.13005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Monpeyssen H, Tramalloni J, Poiree S, Helenon O, Correas JM. Elastography of the thyroid. Diagn Interv Imaging. 2013;94:535–544. doi: 10.1016/j.diii.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 31.Li Y, Zhou G, Ozaki T, et al. Distinct histopathological features of Hashimoto’s thyroiditis with respect to IgG4-related disease. Mod Pathol. 2012;25:1086–1097. doi: 10.1038/modpathol.2012.68. [DOI] [PubMed] [Google Scholar]
- 32.Bhatia KS, Lee YY, Yuen EH, Ahuja AT. Ultrasound elastography in the head and neck. Part II. Accuracy for malignancy. Cancer Imaging. 2013;13(2):260–276. doi: 10.1102/1470-7330.2013.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sebag F, Vaillant-Lombard J, Berbis J, et al. Shear wave elastography: a new ultrasound imaging mode for the differential diagnosis of benign and malignant thyroid nodules. J Clin Endocrinol Metab. 2010;95:5281–5288. doi: 10.1210/jc.2010-0766. [DOI] [PubMed] [Google Scholar]
- 34.Arioz Habibi H, Memis Durmaz ES, Qarayeva V, et al. Quantitative assessment of thyroid, submandibular, and parotid glands elasticity with shear-wave elastography in children. Ultrasound Q. 2018;34:58–61. doi: 10.1097/RUQ.0000000000000352. [DOI] [PubMed] [Google Scholar]
- 35.Willms A, Bieler D, Wieler H, Willms D, Kaiser KP, Schwab R. Correlation between sonography and antibody activity in patients with Hashimoto thyroiditis. J Ultrasound Med. 2013;32:1979–1986. doi: 10.7863/ultra.32.11.1979. [DOI] [PubMed] [Google Scholar]
- 36.Nabahati M, Moazezi Z, Fartookzadeh S, Mehraeen R, Ghaemian N, Sharbatdaran M. The comparison of accuracy of ultrasonographic features versus ultrasound-guided fine-needle aspiration cytology in diagnosis of malignant thyroid nodules. J Ultrasound. 2019;22(3):315–321. doi: 10.1007/s40477-019-00377-2. [DOI] [PMC free article] [PubMed] [Google Scholar]