Abstract
The pseudoaneurysm (PA) is a perfused sac directly connecting with the arterial lumen by an interruption of the vessel wall continuity, more commonly secondary to trauma or iatrogenic causes. Aim of our study was to determine the accuracy and usefulness of Doppler techniques in the diagnosis of peripheral iatrogenic PAs secondary to minimally invasive procedures. From a three year prospective research, 20 Duplex Ultrasound (DUS) studies in as many patients presenting with periarterial pulsating mass clinically suspected for PA secondary to minimally invasive procedures were selected. The PA final diagnosis was confirmed by angiography in 12 patients (60% cases), by computed tomography angiography in 5 patients (25%), by surgery in 2 patients (10%), and by magnetic resonance angiography in 1 patient (5%). The vessels involved by PA formation were: common femoral artery in 8 cases (40%); superficial femoral artery in 4 cases (20%); brachial artery in 3 cases (15%); popliteal artery in 2 cases (10%); superficial temporal artery (STA) in 2 cases (10%); dorsal medial digital artery of the foot in 1 case (5%). Our study confirmed the usefulness of doppler techniques in the diagnosis of peripheral iatrogenic PAs. Specifically, a sensitivity of 90–95%, a specificity of 100% and predictive values of 100% (VPP) and 83–90% (VPN) were reported. The radiologist must always suspect a PA in the differential diagnosis of lesions contiguous to an artery vessel. This is to prevent potential complications such as e.g. massive haemorrhage. In this order, DUS allows a careful selection of patients who require to undergo in-depth imaging methods or surgical therapy, thus contributing to a significant reduction of contrast medium and exposure to ionizing radiation.
Electronic supplementary material
The online version of this article (10.1007/s40477-020-00475-6) contains supplementary material, which is available to authorized users.
Keywords: Iatrogenic pseudoaneurysm, Ultrasound, Colour-doppler, Pulsed-doppler, Yin-yang sign
Introduction
The pseudoaneurysm (PA) is a perfused sac directly connecting with the arterial lumen by a disruption of the vessel wall continuity secondary to inflammatory, infectious and neoplastic processes, and especially to traumatic events and iatrogenic causes [1, 2]. The iatrogenic causes include various medical procedures such as surgery and vessels catheterization, percutaneous biopsies or drainages [3–5].
According to the medical literature, the overall incidence of iatrogenic PAs varies from 0.1–6% to 0.5–9% depending on the diagnostic-therapeutic procedure [4].
With the increasing use of percutaneous arterial interventions worldwide, iatrogenic arterial injury following catheterization procedures has become the predominant cause of PA formation, accounting for 70–80% of the incidence [6]. The number of endovascular procedures increases each year, and with the improved materials and devices and growing experience, there is an accompanying widening range of indications [6, 7]. Specifically, the highest incidence of iatrogenic PAs is observed in the common femoral artery (CFA) as a result of inadequate seal of the arterial puncture site following vascular approach. There are reports whereby femoral PAs occur in up to 0.2% of diagnostics and 8% of interventional procedures [6–8].
PA can be life-threatening due to rupture and bleeding [9]. Therefore, PA is considered an emergency disease and need to be diagnosed accurately and quickly [10, 11].
In this order, the most successful and non-invasive modality is Duplex Ultrasound (DUS). In this study and mainstream literature [12, 13], DUS refers to the combination of B-Mode US and Color- and Pulsed-Doppler techniques (CD and PW).
Aim of our study was to investigate the usefulness of Doppler techniques in the diagnosis of peripheral iatrogenic Pas secondary to minimally invasive procedures. For the lesions examined, the investigation was successfully undertaken through, firstly, a detailed analysis of the B-mode US and Doppler imaging findings and, secondly, a B-mode US versus Doppler diagnostic performance study. Finally, in the discussion an extensive bibliographic review about this topic was reported.
Material and methods
From January 2017 to May 2020, 30 patients with periarterial pulsating mass clinically suspected for PA subsequent to minimally invasive procedures were enrolled in our study. We considered only peripheral lesions regardless of size and depth. Patients with suspected visceral or carotid/intracranial PAs were excluded. A history of Marfan syndrome, Ehlers–Danlos syndrome or other connective disease was also exclusion criteria.
Overall, were detected 20 arterial PAs and 10 hematomas in as many patients. Among the 20 patients with PA, 13 (65%) were male and 7 (35%) female. The mean age was 53.35 years (range 36–75 years).
The vessels involved by PA formation were: common femoral artery in 8 cases (40%); superficial femoral artery in 4 cases (20%); brachial artery in 3 cases (15%); popliteal artery in 2 cases (10%); superficial temporal artery (STA) in 2 cases (10%); dorsal medial digital artery of the foot in 1 case (5%) (Table 1).
Table 1.
Arterial sites of the pseudoaneurysms
| Artery | No (%) |
|---|---|
| Common femoral | 8 (40) |
| Superficial femoral | 4 (20) |
| Brachial | 3 (15) |
| Popliteal | 2 (10) |
| Superficial temporal | 2 (10) |
| Dorsal medial digital | 1 (5) |
Within patients with PAs, they had previously undergone the following minimally invasive procedures: vascular catheterization for interventional procedures in 12 cases (60%), arterial cannulation for monitoring in 2 cases (10%), biopsy procedures in 4 cases (20%), and aesthetic mini-invasive surgery in 2 cases (10%) (Table 2).The average time from the diagnostic-therapeutic procedure to the diagnosis was 7.6 months (range: 1.6–14.5 months).
Table 2.
Iatrogenic minimally invasive eziology of PAs
| Eziology | No (%) |
|---|---|
| Vascular catheterization | 12 (60) |
| Biopsy procedures | 4 (20) |
| Arterial cannulation | 2 (10) |
| Aesthetic mini-invasive surgery | 2 (10) |
The final diagnosis of PA was confirmed by angiography in 12 patients (60% cases), computed tomography angiography (CTA) in 5 patients (25%), surgery in 2 patients (10%), and magnetic resonance angiography (MRA) in 1 patient (5%).
US examinations were performed with the following scanners: General Electric Heathcare LOGIC e; Toshiba Aplio 500, Platinum Series; MyLab 70 XVG Gold Esaote. All scanners were equipped with a multi-frequency convex (2.5–5 MHz) and linear probe (7.5–12 MHz). For Doppler imaging, the lowest pulse repetition frequency (PRF) and the lowest level of colour-gain was used to avoid aliasing artifacts. The “default” setting [9] was 2000–2500 Hz for the PRF and 70–80% for the colour-gain with a low wall filter between 150 and 350 Hz; the colour box was kept as small as possible to maintain a high frame rate.
All images and cineloops were digitally stored as raw data in a PC-based workstation connected to the US units via a standard ethernet link.
On-site analysis
All examinations were performed by radiologists who had at least 5 years of Doppler experience. The examiners were blinded to other imaging results, but aware of the clinical history.
The B-mode US findings of PA analysed were: anatomical location, morphology, size (maximum diameter), echogenicity, internal echostructure (homogeneous or heterogeneous) and pulsatility.
The presence of arterial intracavitary flow and its different distribution patterns (swirling or “fountain-like”) were evaluated at CD examination. CD analysis also investigated the presence of direct communication between arterial lumen and the pseudo-aneurysmal sac.
Then, through the PW analysis was evaluated the presence of "to-and-fro" waveform at the blood supply point.
To complete the vascular study, in all patients the patency of the adjacent arteries and veins could be completely assessed.
The diagnostic criteria used to diagnose the arterial PAs such as periarterial haematomas have been based on literature data [10, 11] and on our previous experiences on iatrogenic PAs [12, 13].
Off-site analysis.
B-Mode US and Doppler images and cine loops were reviewed from digital files on a computer screen and evaluated by two investigators with at least 5 years of experience in Doppler techniques. Investigators were not involved in the US scanning and were they were not aware of clinical and other imaging patient information.
To assess the diagnostic performance of B-Mode US and Doppler, for each lesion the two radiologists were asked to provide a diagnosis of PA using adichotomous confidence rating score (presence/absence).
Statistical analysis
Reviewers’ diagnoses were judged to be as it follows: true-positive (TP), when the presence of PA was correctly assessed as positive; false negative (FN), when the presence of PA was wrongly assessed as negative; true negative (VN), when the absence of PA was correctly assessed as negative; false positive (FP), when the absence of PA was wrongly assessed as positive.
Contingency tables (2 × 2) were used to calculate sensitivity, specificity, positive predictive value, and negative predictive value.
Inter-observer agreement was assessed between radiologists for each B-Mode US and Doppler interpretation by weighted kappa statistics. The agreement was graded in the linear set with five categories: 1, 0.75, 0.50, 0.25, and 0 when there was a difference of 0 (total agreement) or as 1, 2, 3, and 4 [14].
ROC curves were plotted with MedCalc for Microsoft Windows (version 13.1.2.0, MedCalc) for evaluating the diagnostic performance of B-Mode US and Doppler. Diagnostic performance was expressed as area under the ROC curve (Az). Differences between ROC curves were compared by univariate z score test.
The ROC curve is a plot of test sensitivity (plotted on the y-axis) versus its false-positive rate or 1 minus specificity (plotted on the x-axis). All possible combinations of sensitivity and specificity that can be achieved by changing the cut-off test value can be summarized with the single parameter area under the ROC curve. Diagnostic tests with perfect discrimination between negative and positive reference groups have ROC plots passing through the coordinates (0, 1), corresponding to 100% sensitivity and specificity (in this case, Az = 1) [14].
Results
Clinical features
Clinical features of PAs were the following: pulsating and painful swelling in 14 patients (70%); pulsating and painless swelling in 5 patients (25%); non-pulsating and painful swelling in 1 patient (5%); downstream ischemia in 4 patients (20%); anaemia in 6 patients (30%); haemorrhagic shock in 1 patient (5%); absence of signs and symptoms in 1 patient (5%) (Table 3).
Table 3.
Clinical features of the PAs of our series
| Clinical features | No | % |
|---|---|---|
| Pulsating and painful swelling | 14 | 70 |
| Pulsating and painless swelling | 5 | 25 |
| Non-pulsating and painful swelling | 1 | 5 |
| Ischemia | 4 | 20 |
| Anaemia | 6 | 30 |
| Haemorrhagic shock | 1 | 5 |
| Absence of signs and symptoms | 1 | 5 |
On-site analysis
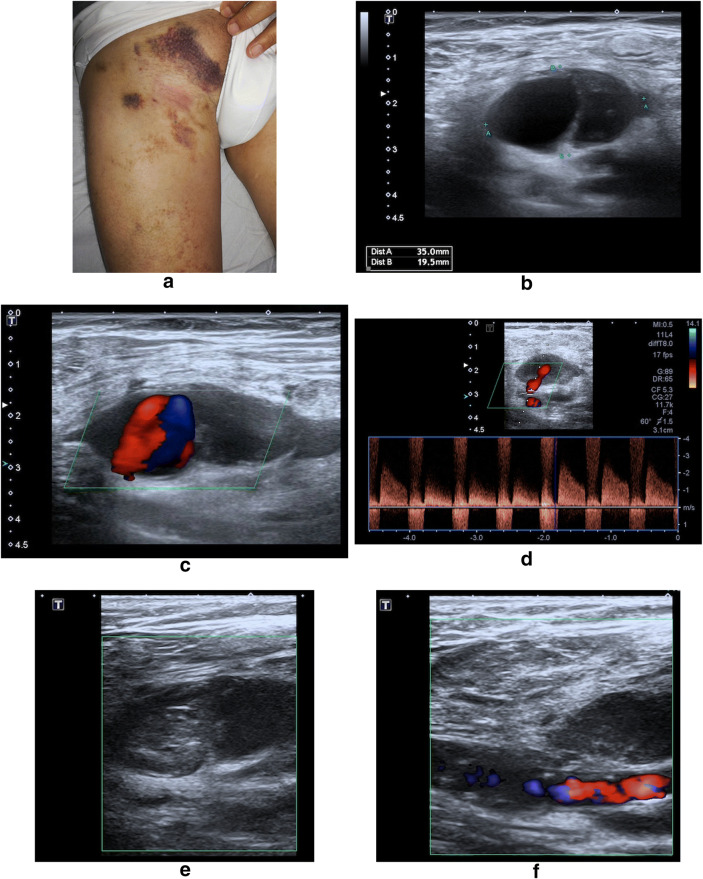
B-mode US evaluation revealed the presence of periarterial hypo-anechoic lesions in all PA cases. The morphology was spherical in 6cases (30%) and ovoid in 14 (60%). The average diameter was 2.7 cm (range 1.4–4.2 cm).Thrombotic component within the pseudo-aneurysmal sac was found in 16 cases (80%) (Figs. 1 and 2).
Fig. 1.
CFA PA following arterial puncture for catheter access, due to poor control of arterial bleeding following the procedure. a Patient photograph. Pulsatile and painful swelling at the site of palpation of the right CFA, where previously have been inserted the arterial catheter. b Transverse B-Mode US image shows an oval-shaped, loculated, hypo-anechoic structure adjacent to the punctured artery, which on CD images; c, d shows a swirling motion blood flow inside (ying-yang sing) (c) and a neck communicating both structures with a systolic flow in the PA direction and diastolic flow in the opposite direction (d). e, f On CD images obtained 30′ after a US-guided PA compression manoeuvre, no flow is seen in the PA sac by thrombosis and donor artery is permeable
Fig. 2.
CFA PA in a patient who had undergone to femoral artery stenting 2 weeks before. CD image show a swirling motion blood flow inside the lesion (ying-yang sing) with a systolic flow in the pseudoaneurysm direction and diastolic flow in the opposite direction
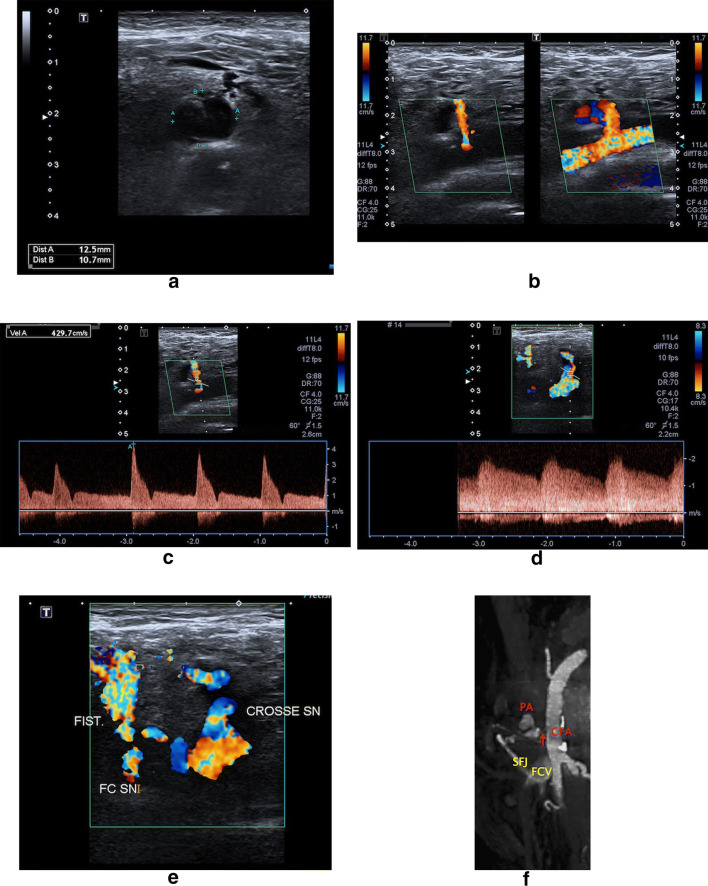
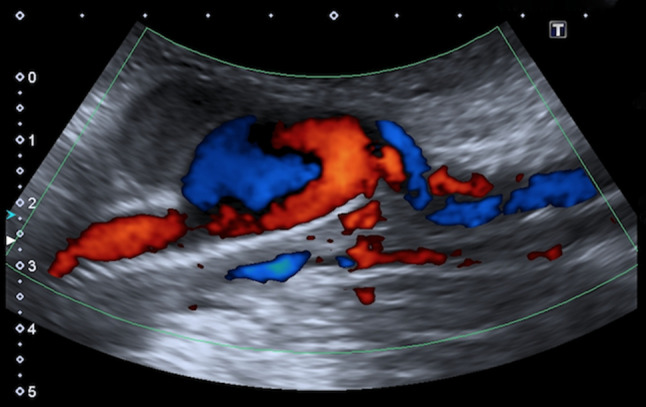
CD analysis demonstrated the presence of a swirl flow in 16 patients (80%) with PA (Fig. 3), 10 of which showed the "yin-yang" sign (Figs. 1, 2, and 4) and 3 a “fountain-like” pattern. In one case the PA resulted completely thrombosed. Direct communication between the PA and the native artery was demonstrated in all cases (Figs. 1 and 3).
Fig. 3.
CFA PA with a communicating arteriovenous fistula following percutaneous coronary intervention. B-Mode US (a) and CD (b) images detect a hypo-anechoic, single compartment lesion consistent with a partially thrombosed PA originating from the distal part of the CFA. The anatomic relation between the PA sac, its neck and the parent arterial lumen, as well as the patency of the arterial lumen distal to the aneurysm site is also shown. (c) Sampling from the neck of the PA, spectral Doppler waveform analysis reveals a flow directed toward the PA cavity both in systole and diastole, in contrast to the characteristic “to-and-fro” flow pattern. In addition, pulsed-wave Doppler shows an “arterialized” venous flow with superimposed systolic and diastolic components in the sapheno-femoral junction (SFJ). When interpreted together, these spectral Doppler findings indicate the presence of a PA in communication with an arteriovenous fistula emptying into the common femoral vein (CFV). This assumption is confirmed by Doppler imaging (d, e), in which transverse sections of the femoral vessels shows PA and arteriovenous fistula shunting blood from the CFA to the common femoral vein via SFJ. f Multiplanar (MPR) CTA reconstruction correlation. CFA common femoral artery, FCV common femoral vein, SFJ sapheno-femoral junction, PA pseudoaneurysm, arrow PA neck
Fig. 4.
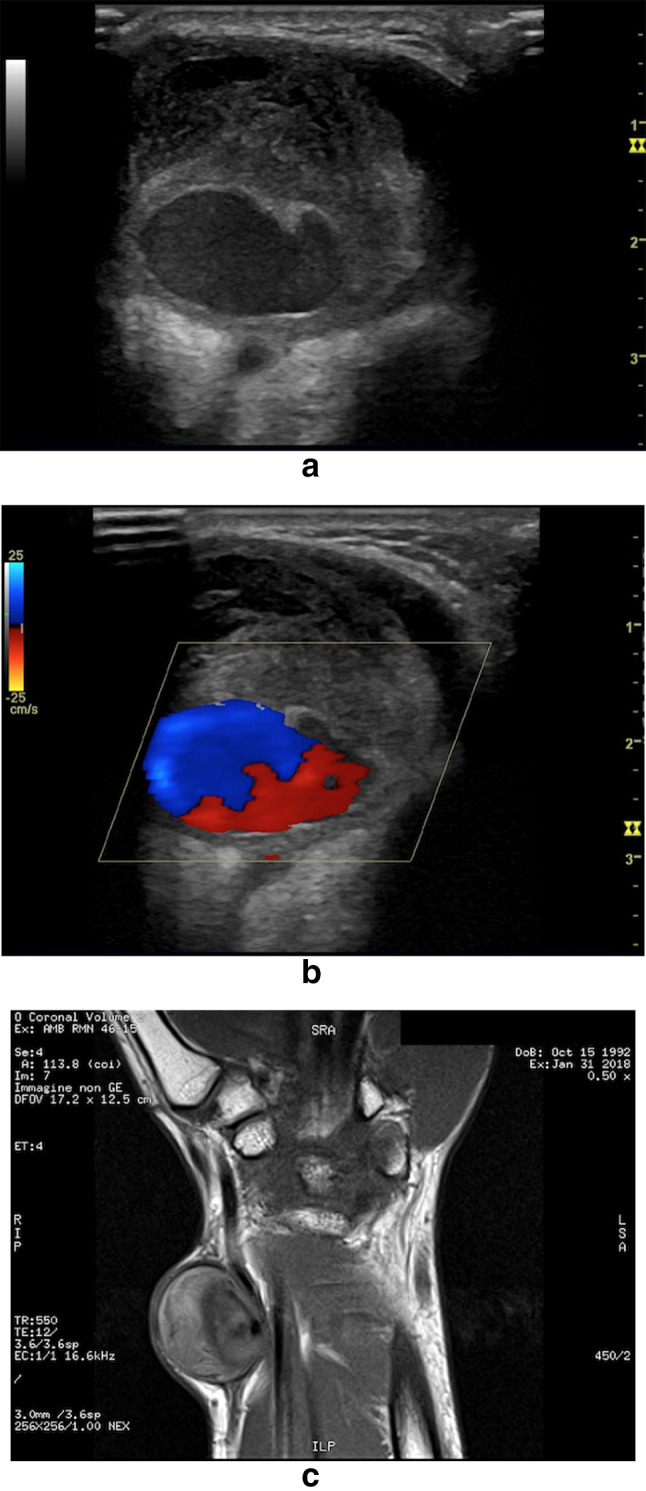
Radial PA following trans-radial percutaneous coronary intervention. a, b CD images of the PA demonstrate an extensive endocavitary thrombosis and the characteristic to- and-fro (bidirectional) flow in the eccentric patent lumen. c MR coronal imaging correlation
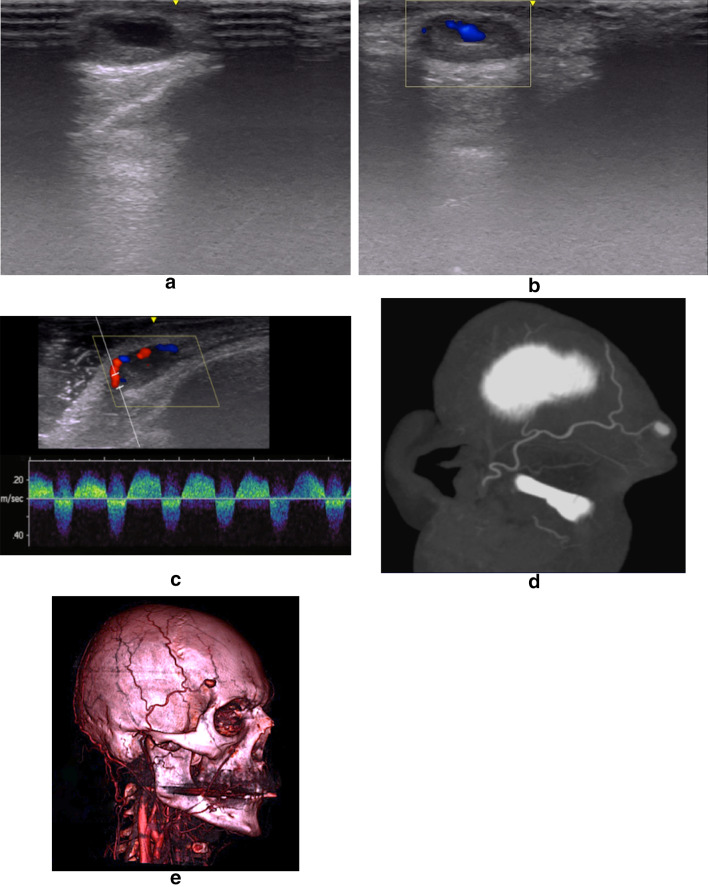
Finally, the sign of the "to-and-fro" waveform was detected in 14 cases (70%) through PW analysis (Figs. 1 and 5).
Fig. 5.
PA of the frontal branch of the Superficial Temporal Artery (STA) as iatrogenic complication after the injection of 30 units Botox (Allergan, Inc., Irvine, Calif.). B-Mode US (a) image of the superficial swelling in right temporal region reveals an oval-shaped, saccular structure with mildly concentric layers of mural thrombosis. However, the only B-Mode US evaluation is not diagnostic, since these detectable US findings may also be present in other pathological conditions, such as hematomas or cysts. CD and PW images (b, c) demonstrate the presence of arterial flow in the PA sac such as a direct communication between the PA and the native artery with the characteristic “to-and-fro” pulsed waveform. MPR (d) and 3D Volume Rendering (e) CTA reconstructions confirm a PA of the frontal branch of the right STA
Off-site analysis
The confidence levels for both radiologists are shown in Table 4. For both readers, sensitivity, specificity, positive predictive value, and negative predictive value improved after Doppler evaluation (all p < 0.05) (Table 4). The Az values were 0.650 before versus 0.950 after Doppler for radiologist 1 and 0.625 versus 0.975 for radiologist 2 (Fig. 6). Inter-observer agreement also increased, although slightly, from a kappa value of 0.894 at conventional US (95% CI 0.808–0.974) to 0.953 at CEUS (95% CI 0.909–0.998).
Table 4.
Diagnostic performance indices before and after Doppler evaluation
| Diagnostic performance | B-mode US | Doppler | |
|---|---|---|---|
| Reviewer 1 | Az | 0.650 (0.455–0.814) | 0.950 (0.803–0.996) |
| Sensitivity, % | 80.00% (56.34–94.27%) | 90.00% (68.30–98.77%) | |
| Specificity, % | 50.00% (18.71–81.29%) | 100.00% (69.15–100.00%) | |
| PPV, % | 76.19% (52.83–91.78%) | 100.00% (81.47–100.00%) | |
| NPV, % | 55.5% (21.20–86.30%) | 83.33% (51.59–97.91%) | |
| Reviewer 2 | Az | 0.625 (0.430–0.794) | 0.975 (0.841–1.000) |
| Sensitivity, % | 85.00% (62.11–96.79%) | 95.00% (75.13–99.87%) | |
| Specificity, % | 40.00% (12.16–73.76%) | 100.00% (69.15–100.00%) | |
| PPV, % | 73.91% (51.59%-89.77%) | 100.00% (82.35%-100.00%) | |
| NPV, % | 57.14% (18.41–90.10%) | 90.91% (58.72–99.77%) |
Values in parentheses are 95% confidence interval
Az area under the ROC curve
Fig. 6.
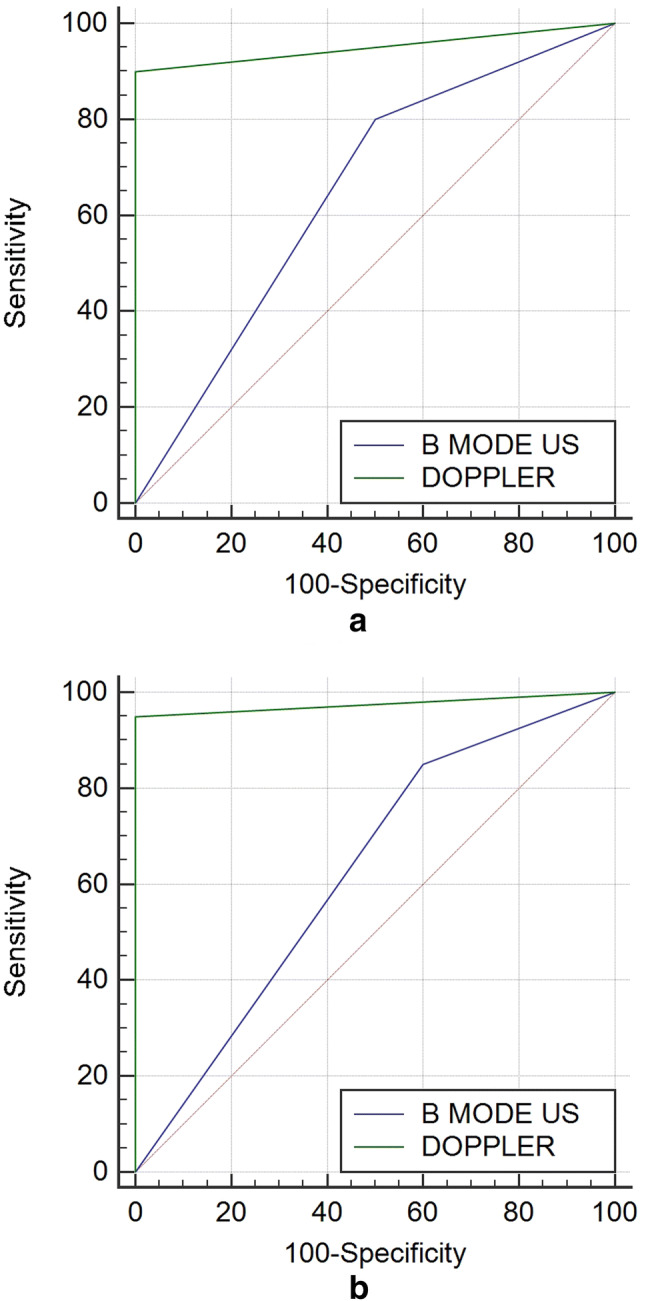
a, b B-Mode US and Doppler ROC curves for both reviewers
Overall, in terms of differential diagnosis, the total number of correctly characterized lesions increased from 21 of 50 (42%) to 48 of 50 (96%) for reader 1 and from 21 of 50 (42%) to 49 of 50 (98%) for reader 2 in the comparison of B-Mode US with Doppler.
Discussion
Minimally invasive procedures should be defined as ones that are safer and associated with a lower post-operative patient morbidity compared with a conventional surgical approach for the same operation [15, 16]. Storically, the first procedure which prevented a radical operation was the use of a cystoscope to look into and treat lesions of the bladder. Since then, minimally invasive approach has been the focal point of new medical technology [17].
Excepting for iatrogenic arterial injury following catheterization procedures [18–21], there is a dearth of literature regarding Pas secondary to minimally invasive procedures. To date, there is no systematic literature review about.
Peripheral PAs have been reported after percutaneous biopsy [22–24], placement/manipulation of drains [25–27], block anaesthesia [28–30] or injection of local anaesthetics [31]. There have been also some reports of peripheral PAs secondary to minimally invasive orthopaedic or aesthetic procedures.
PA formation is, indeed, a well-documented complication of knee and ankle arthroscopy [32–36] and there are reported cases following hip [37] and shoulder arthroscopy [38].
Also, iatrogenic minimally invasive aetiology include a whole range of aesthetic and cosmetic rejuvenation procedures which have increasingly expanded in the last two decades mainly because of the patient demand for a minimally invasive approach, all outcomes being comparable [39, 40]. A rare complication with PA of the frontal branch of the STA after Botox injection was described earlier in the literature [41]. Skaf et al. [42] also described a similar case of PA occurring after Botox injection. Our group already reported a complication of minimally invasive aesthetic procedure of facial rejuvenation (Aptos Thread-Lifting 2G technique) resulting in the development of a STA PA [43].
With the introduction of modern imaging modalities, the PA diagnosis has become more common allowing early detection and therapeutic intervention before the PA manifests clinically, sometimes with catastrophic results [1, 2]. Although conventional angiography remains the diagnostic standard of reference [44, 45], other modalities such as DUS [12, 13], contrast-enhanced US (CEUS) [27], CTA [44, 45], and MRA [46, 47] are demonstrated to be useful in the detection and diagnosis of PAs, albeit with variable results [1, 2].
DUS is a valuable diagnostic tool, particularly in centres with a dedicated vascular laboratory. This modality is portable, readily available, inexpensive and fast. It involves no ionizing radiation or renal toxic contrast material, and it is non-invasive.[12, 13]. Clear views of the vessels and associated pathology can be achieved rapidly in experienced hands. However, the usefulness of DUS in the evaluation of deep arteries is partially limited with reports of low sensitivity [18]. Also, it may result difficult the evaluation of vessels in trauma patients with fractures or hematomas [1, 2].
B-mode US typically demonstrates an hypo-anechoic “cystic-like” structure adjacent to a supplying artery [1, 2]. It can assess the size of the pseudo-aneurysmal sac, the connection of the sac to the artery, the length and width of the pseudo-aneurysmal neck, and the presence of internal thrombotic component. However, B-mode US is not diagnostic, since these findings are not specific as may be seen in other conditions, the most common being hematomas and cysts [43].
Doppler imaging helps establishing the diagnosis. The color-flow image typically demonstrates a swirling pattern inside the false lumen, known as ‘yin-yang’ sign, because of the double vascular components in-flowing (systolic) and out-flowing (diastolic): during the systole, the high-speed flow enters the pseudo-aneurismatic sac through the neck and it is associated with backward flow on the opposite entering point; during the diastole, the direction of the flow is inverted [43]. Also, spectral analysis is more specific for PA diagnosis by detecting a typical "to-and-fro" waveform at the communicating point between the native vessel and the PA [48]. Specifically, the “to-and-fro” waveform is associated to the pressure gradient between the vessel and the pseudo-aneurismatic cavity: during the systole the blood goes to the PA which has a lower internal pressure; during the diastole a flow inversion can be found because of the opposite pressure gradient that is created when the artery blood pressure goes down. The audible Doppler signal is very characteristic, with high-frequency Doppler shifts heard in the forward and reverse phases [48, 49].
Our study confirmed the added value of Doppler techniques in the diagnosis of peripheral arterial PA secondary to minimally invasive interventional and surgical procedures. The data reported are substantially comparable with those of previous diagnostic performance studies carried out on series of patients undergoing only interventional procedures [4]. Specifically, a sensitivity of 90–95%, a specificity of 100% and predictive values of 100% (VPP) and 83–90% (VPN) were reported in our study.
However, our study had some limitations. Firstly, there was an observation bias, because the same radiologist evaluated B-mode US and Doppler examinations in each patient. As a result, the radiologist may be conditioned by the basal US appearance of the lesion. Although not appropriate, this methodological approach is reasonably acceptable, being technically intrinsic to the Doppler techniques the need for a preliminary grey-scale examination. In addition, the study presented a selection bias because only a part of enrolled subjects were selected from a patient population hospitalized at a trauma centre with second level emergency department (AORN “A. Cardarelli”, Naples). Therefore, the total number of lesions assessed is lower than expected. Secondly, among inclusion criteria we did not appropriately consider the size and depth of the lesions. Large and deep PAs are quite difficult to evaluate by means of Doppler US, so that they can misdiagnosed easily.
Nevertheless, considering that traumatic PAs are an uncommon finding and the rigid inclusion criteria established, our series of peripheral iatrogenic PAs secondary to minimally invasive interventional and surgical procedures is, to date, the largest described in the literature.
Conclusions
According to literature [1, 2], our experience suggests the following take-home points:
consider the risk of PA occurrence in patients previously undergone to minimally invasive procedures, since they are increasing in the clinical practice;
CD and PW imaging is crucial in evaluating such patients because it provides diagnostic findings of PA, such as the “yin-yang sign” and the “to-and-fro” wave;
DUS provides a careful selection of patients who must undergo to in-depth imaging methods or surgical therapy, thus contributing to a significant reduction in the use of contrast medium and in the exposure to ionizing radiation.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Compliance with ethical standards
Conflict of interest
The authors have no conflicts of interest.
Ethical approval
We confirm that this work is original and has not been published elsewhere nor is it currently under consideration for publication elsewhere. Publication is approved by all authors and by the responsible authorities where the work was carried out. Each author have participated sufficiently in any submission to take public responsibility for its content.
Informed consent
Written informed consent was obtained from all patients, and the study was approved by the ethics committees of the institutes involved in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Saad NE, Saad WE, Davies MG, Waldman DL, Fultz PJ, Rubens DJ. Pseudoaneurysms and the role of minimally invasive techniques in their management. Radiographics. 2005;25(Suppl 1):S173–S189. doi: 10.1148/rg.25si055503. [DOI] [PubMed] [Google Scholar]
- 2.Sueyoshi E, Sakamoto I, Nakashima K, Minami K, Hayashi K. Visceral and peripheral arterial pseudoaneurysms. AJR Am J Roentgenol. 2005;185(3):741–749. doi: 10.2214/ajr.185.3.01850741. [DOI] [PubMed] [Google Scholar]
- 3.Trinci M, Giangregorio C, Calabrese G, Ottaviani P, Riu P, Galluzzo M, Miele V. A rare case of non-traumatic intrasplenic pseudoaneurysms in a patient with acute T-cell lymphoblastic leukemia. J Ultrasound. 2019 doi: 10.1007/s40477-019-00401-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrara D, Esposito F, Blasio R, Mamone R, Severino R, Di Serafino M, Pecoraro C, Zeccolini M. Role of color Doppler ultrasound in the early diagnosis of a major complication after percutaneous renal biopsy: two case reports. J Ultrasound. 2018;21(4):343–349. doi: 10.1007/s40477-018-0326-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tufano A, Minelli R, Rossi E, Brillantino C, Di Serafino M, Zeccolini M, Cantisani V, Vallone G. Inferior epigastric artery pseudoaneurysm secondary to port placement during a robot-assisted laparoscopic radical cystectomy. J Ultrasound. 2020 doi: 10.1007/s40477-020-00442-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henry JC, Franz RW. Pseudoaneurysms of the peripheral arteries. Int J Angiol. 2019;28(1):20–24. doi: 10.1055/s-0039-1677676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vezzani A, Manca T, Vercelli A, Braghieri A, Magnacavallo A. Ultrasonography as a guide during vascular access procedures and in the diagnosis of complications. J Ultrasound. 2013;16(4):161–170. doi: 10.1007/s40477-013-0046-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.López Álvarez JM, Pérez Quevedo O, Santana Cabrera L, Escot CR, Loro Ferrer JF, Lorenzo TR, Limiñana Cañal JM. Vascular ultrasound in pediatrics: estimation of depth and diameter of jugular and femoral vessels. J Ultrasound. 2017;20(4):285–292. doi: 10.1007/s40477-017-0272-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Catalano O, Roldán FA, Varelli C, Bard R, Corvino A, Wortsman X. Skin cancer: findings and role of high-resolution ultrasound. J Ultrasound. 2019;22(4):423–431. doi: 10.1007/s40477-019-00379-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Catalano O, Varelli C, Sbordone C, Corvino A, De Rosa D, Vallone G, Wortsman X (2019) A bump: what to do next? Ultrasound imaging of superficial soft-tissue palpable lesions. J Ultrasound. 10.1007/s40477-019-00415-z [DOI] [PMC free article] [PubMed]
- 11.Ferramosca E, Serra C, Di Felice A, Mandreoli M, Brunocilla E, Santoro A. Ultrasound-guided trans-hepatic embolization of a renal artery pseudoaneurysm in a patient with acquired solitary kidney and with chronic renal failure secondary to phenacetin abuse. J Ultrasound. 2014;17(1):65–69. doi: 10.1007/s40477-014-0063-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foshager MC, Finlay DE, Longley DG, Letourneau JG. Duplex and color Doppler sonography of complications after percutaneous interventional vascular procedures. Radiographics. 1994;14(2):239–253. doi: 10.1148/radiographics.14.2.8190950. [DOI] [PubMed] [Google Scholar]
- 13.Jargiello T, Zubilewicz T, Janczarek M, Szajner M, Pietura R, Szczerbo-Trojanowska M. Pulsating mass after accidental artery trauma: diagnosis with duplex ultrasound and the role of angiography. Vasa. 1998;27(2):111–117. [PubMed] [Google Scholar]
- 14.Sardanelli F, Di Leo G. Biostatistic for radiologist. Planning, performing and writing a radiologic study. Berlin: Springer; 2008. [Google Scholar]
- 15.Ochsner JL. Minimally invasive surgical procedures. Ochsner J. 2000;2(3):135–136. [PMC free article] [PubMed] [Google Scholar]
- 16.Corvino A, Rosa D, Sbordone C, Nunziata A, Corvino F, Varelli C, Catalano O. Diastasis of rectus abdominis muscles: patterns of anatomical variation as demonstrated by ultrasound. Pol J Radiol. 2019;84:e542–e548. doi: 10.5114/pjr.2019.91303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuchs KH. Minimally invasive surgery. Endoscopy. 2002;34(2):154–159. doi: 10.1055/s-2002-19857. [DOI] [PubMed] [Google Scholar]
- 18.Paulson EK, Hertzberg BS, Paine SS, Carroll BA. Femoral artery pseudoaneurysms: value of color Doppler sonography in predicting which ones will thrombose without treatment. AJR Am J Roentgenol. 1992;159(5):1077–1081. doi: 10.2214/ajr.159.5.1414779. [DOI] [PubMed] [Google Scholar]
- 19.Helvie MA, Rubin JM, Silver TM, Kresowik TF. The distinction between femoral artery pseudoaneurysms and other causes of groin masses: value of duplex Doppler sonography. AJR Am J Roentgenol. 1988;150(5):1177–1180. doi: 10.2214/ajr.150.5.1177. [DOI] [PubMed] [Google Scholar]
- 20.Gabriel M, Pawlaczyk K, Waliszewski K, Krasiński Z, Majewski W. Location of femoral artery puncture site and the risk of postcatheterization pseudoaneurysm formation. Int J Cardiol. 2007;120(2):167–171. doi: 10.1016/j.ijcard.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 21.Gupta PN, Salam Basheer A, Sukumaran GG, Padmajan S, Praveen S, Velappan P, Nair BU, Nair SG, Kunjuraman UK, Madthipat URJ. Femoral artery pseudoaneurysm as a complication of angioplasty. How can it be prevented? Heart Asia. 2013;5(1):144–147. doi: 10.1136/heartasia-2013-010297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bazzocchi M, Francescutti GE, Zuiani C, Del Frate C, Londero V. Breast pseudoaneurysm in a woman after core biopsy: percutaneous treatment with alcohol. AJR Am J Roentgenol. 2002;179(3):696–698. doi: 10.2214/ajr.179.3.1790696. [DOI] [PubMed] [Google Scholar]
- 23.Melloni G, Bandiera A, Crespi G, Zannini P. Intercostal artery pseudoaneurysm after computed tomography-guided percutaneous fine needle aspiration lung biopsy. J Thorac Imaging. 2012;27(3):W48–W49. doi: 10.1097/RTI.0b013e3182107430. [DOI] [PubMed] [Google Scholar]
- 24.Corvino A, Pignata S, Campanino MR, Corvino F, Giurazza F, Tafuri D, Pinto F, Catalano O. Thyroglossal duct cysts and site-specific differential diagnoses: imaging findings with emphasis on ultrasound assessment. J Ultrasound. 2020 doi: 10.1007/s40477-020-00433-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lam EY, McLafferty RB, Taylor LM, Jr, Moneta GL, Edwards JM, Barton RE, Petersen B, Porter JM. Inferior epigastric artery pseudoaneurysm: a complication of paracentesis. J Vasc Surg. 1998;28(3):566–569. doi: 10.1016/S0741-5214(98)70147-8. [DOI] [PubMed] [Google Scholar]
- 26.Werner M, Bernheim J, Witz M, Gritton Y, Savin H, Korzets Z. Pseudoaneurysm of the inferior epigastric artery—a rare complication of Tenckhoff catheter removal. Nephrol Dial Trans. 1999;14(5):1297–1299. doi: 10.1093/ndt/14.5.1297. [DOI] [PubMed] [Google Scholar]
- 27.Corvino A, Sandomenico F, Setola SV, Corvino F, Pinto F, Catalano O. Added value of contrast-enhanced ultrasound (CEUS) with Sonovue® in the diagnosis of inferior epigastric artery pseudoaneurysm: report of a case and review of literature. J Ultrasound. 2019;22(4):485–489. doi: 10.1007/s40477-019-00398-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flowers GA, Meyers JF. Pseudoaneurysm after interscalene block for a rotator cuff repair. Arthroscopy. 2004;20(Suppl 2):67–69. doi: 10.1016/j.arthro.2004.04.048. [DOI] [PubMed] [Google Scholar]
- 29.Zipkin M, Backus WW, Scott B, Poppers PJ. False aneurysm of the axillary artery following brachial plexus block. J Clin Anesth. 1991;3(2):143–145. doi: 10.1016/0952-8180(91)90012-C. [DOI] [PubMed] [Google Scholar]
- 30.Groh GI, Gainor BJ, Jeffries JT, Brown M, Eggers GW., Jr Pseudoaneurysm of the axillary artery with median-nerve deficit after axillary block anesthesia. A case report. J Bone Joint Surg Am. 1990;72(9):1407–1408. doi: 10.2106/00004623-199072090-00022. [DOI] [PubMed] [Google Scholar]
- 31.Choi HJ, Kim JH, Lee YM, Lee JH. Pseudoaneurysm of the facial artery after the injection of local anesthetics. J Craniofac Surg. 2012;23(2):419–421. doi: 10.1097/SCS.0b013e31822e5fab. [DOI] [PubMed] [Google Scholar]
- 32.Mariani PP, Mancini L, Giorgini TL. Pseudoaneurysm as a complication of ankle arthroscopy. Arthroscopy. 2001;17(4):400–402. doi: 10.1053/jars.2001.22367. [DOI] [PubMed] [Google Scholar]
- 33.Yammine K, Kheir N, Daher J, Naoum J, Assi C. Pseudoaneurysm following ankle arthroscopy: a systematic review of case series. Eur J Orthop Surg Traumatol. 2019;29(3):689–696. doi: 10.1007/s00590-018-2324-6. [DOI] [PubMed] [Google Scholar]
- 34.Filho ES, Isolani GR, Baracho FR, de Oliveira Franco AP, Ridder Bauer LA, Namba M. Pseudoaneurysm after arthroscopic procedure in the knee. Rev Bras Ortop. 2015;50(2):131–135. doi: 10.1016/j.rboe.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bianchi S, Beaulieu JY, Poletti PA. Ultrasound of local complications in hand surgery: a pictorial essay. J Ultrasound. 2020 doi: 10.1007/s40477-020-00457-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sdao S, Orlandi D, Aliprandi A, Lacelli F, Sconfienza LM, Randelli F, Sardanelli F, Serafini G. The role of ultrasonography in the assessment of peri-prosthetic hip complications. J Ultrasound. 2014;18(3):245–250. doi: 10.1007/s40477-014-0107-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Papavasiliou AV, Bardakos NV. Complications of arthroscopic surgery of the hip. Bone Jt Res. 2012;1(7):131–144. doi: 10.1302/2046-3758.17.2000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Godin JA, Mayer SW, Garrigues GE, Mather RC., 3rd Pseudoaneurysm after shoulder arthroscopy. J Shoulder Elbow Surg. 2013;22(10):e12–e17. doi: 10.1016/j.jse.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 39.Lin K, Matarasso A, Edelstein DR, Swift RW, Shnayder Y. Superficial temporal artery pseudoaneurysm after face lift. Aesthet Surg J. 2004;24(1):28–32. doi: 10.1016/j.asj.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 40.Corvino A, Sandomenico F, Corvino F, Campanino MR, Verde F, Giurazza F, Tafuri D, Catalano O. Utility of a gel stand-off pad in the detection of Doppler signal on focal nodular lesions of skin. J Ultrasound. 2020;23(1):45–53. doi: 10.1007/s40477-019-00376-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prado A, Fuentes P, Guerra C, Leniz P, Wisnia P. Pseudoaneurysm of the frontal branch of the superficial temporal artery: an unusual complication after the injection of botox. Plast Reconstr Surg. 2007;119(7):2334–2335. doi: 10.1097/01.prs.0000261095.07321.09. [DOI] [PubMed] [Google Scholar]
- 42.Skaf GS, Domloj NT, Salameh JA, Atiyeh B. Pseudoaneurysm of the superficial temporal artery: a complication of botulinum toxin injection. Aesthetic Plast Surg. 2012;36(4):982–985. doi: 10.1007/s00266-012-9881-6. [DOI] [PubMed] [Google Scholar]
- 43.Corvino A, Catalano O, Corvino F, Sandomenico F, Setola SV, Petrillo A. Superficial temporal artery pseudoaneurysm: what is the role of ultrasound? J Ultrasound. 2016;19(3):197–201. doi: 10.1007/s40477-016-0211-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Busquéts AR, Acosta JA, Colón E, Alejandro KV, Rodríguez P. Helical computed tomographic angiography for the diagnosis of traumatic arterial injuries of the extremities. J Trauma. 2004;56(3):625–628. doi: 10.1097/01.TA.0000053546.28739.CF. [DOI] [PubMed] [Google Scholar]
- 45.Múnera F, Soto JA, Palacio D, Velez SM, Medina E. Diagnosis of arterial injuries caused by penetrating trauma to the neck: comparison of helical CT angiography and conventional angiography. Radiology. 2000;216(2):356–362. doi: 10.1148/radiology.216.2.r00jl25356. [DOI] [PubMed] [Google Scholar]
- 46.Toombs BD, Jing JM. Current concepts in the evaluation of vascular disease: magnetic resonance and computed tomographic angiography. Tex Heart Inst J. 2000;27(2):170–192. [PMC free article] [PubMed] [Google Scholar]
- 47.Davis CP, Hany TF, Wildermuth S, Schmidt M, Debatin JF. Postprocessing techniques for gadolinium-enhanced three-dimensional MR angiography. Radiographics. 1997;17(5):1061–1077. doi: 10.1148/radiographics.17.5.9308101. [DOI] [PubMed] [Google Scholar]
- 48.Mahmoud MZ, Al-Saadi M, Abuderman A, Alzimami KS, Alkhorayef M, Almagli B, Sulieman A. "To-and-fro" waveform in the diagnosis of arterial pseudoaneurysms. World J Radiol. 2015;7(5):89–99. doi: 10.4329/wjr.v7.i5.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Granata A, Clementi S, Londrino F, Romano G, Veroux M, Fiorini F, Fatuzzo P. Renal transplant vascular complications: the role of Doppler ultrasound. J Ultrasound. 2014;18(2):101–107. doi: 10.1007/s40477-014-0085-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.