Abstract
Rationale
Idiopathic pulmonary fibrosis (IPF) is a chronic lung disease characterized by abnormal and excessive deposition of collagen in the pulmonary interstitium (fibrosis) with minimal associated inflammation evolving into progressive and irreversible decline in lung function.
Patient concerns
Patient referred discomfort, bilateral upper quadrant abdominal pain, and progressive exertional dyspnea (shortness of breath with exercise).
Diagnosis
Exertional dyspnea due to idiopathic pulmonary fibrosis (IPF).
Intervention
Sonographic evaluation demonstrated an alteration of diaphragm excursion together with a relevant alteration of the pleural line and multiple irregular and confluent B lines.
Conclusions
Lung and diaphragm ultrasound could be employed as a screening or first-line diagnostic tool in the suspicion of interstitial lung disease.
Electronic supplementary material
The online version of this article (10.1007/s40477-020-00445-y) contains supplementary material, which is available to authorized users.
Keywords: Ultrasound, Lung, Fibrosis, Idiopathic pulmonary fibrosis, Diaphragm
Introduction
The diaphragm contributes to 75% of the pulmonary ventilation and has been extensively examined by several approaches that are often invasive and sometimes dangerous for the patient [1, 2]. Therefore, many studies tried to standardize an ultrasound evaluation of the diaphragm excursion in case of respiratory diseases such as acute respiratory failure and chronic lung syndromes (e.g., chronic obstructive pulmonary disease) [3–6]. Idiopathic pulmonary fibrosis (IPF) is a chronic lung disease characterized by abnormal and excessive deposition of collagen in the pulmonary interstitium (fibrosis) with minimal associated inflammation evolving into progressive and irreversible decline in lung function [7].
Here, we report the case of a 68-year-old man referred to our unit complaining exertional dyspnea and bilateral upper quadrant abdominal pain, with pulmonary and diaphragmatic ultrasound findings suggestive for IPF.
Case report
A 68-year-old caucasian patient came to our internal medicine ultrasound unit to perform a sonographic abdominal examination as prescribed by general practitioner. He referred discomfort and bilateral upper quadrant abdominal pain and progressive exertional dyspnea (shortness of breath with exercise). His past medical history was characterized by high blood pressure, Helicobacter pylori (HP) + gastritis subjected to eradication treatment and large bowel polyp removal 2 years earlier. Blood tests revealed: hemoglobin (Hb): 11.7 g/dL; platelets: 234,000; c-reactive protein (CRP): 0.9 mg/dL, white blood cells (WBC) 9000 × 103; total bilirubin 1.7 mg/dL.
The ultrasound examination revealed normal pancreatic dimensions and echostructure. Liver visualization was limited by the presence of air in the bowel, thus limiting the exploration to the left lobe, which was normal. In the coronal/intercostal scan, the liver/kidney interface was evident; liver parenchyma showed no significant alterations, but when we asked the patient to perform a deep inspiration, there was no typical "drop” of the diaphragm and liver, which appeared reduced. Similarly, the spleen displayed normal size and echostructure on the left coronal scan, but there was a reduced drop of the diaphragm/spleen interface during inspiration (Video 1).
Therefore, we examined diaphragm motility by M mode through a sub-rib scan; a normal/high excursion compared to the reference standard during normal breath was evident [7], but a reduced excursion with forced breathing was present. (Fig. 1).
Fig. 1.

M-mode examination revealed a normal/high diaphragmatic excursion during resting breathing, while there was a reduced excursion during forced inspiration
The next step was to move from the abdomen to the thoracic examination, to search the reason for altered diaphragmatic mobility.
Sonographic evaluation of the lungs showed an important alteration of the pleural line, which appeared irregular, jagged, sometimes thickened, especially in the middle–low lung fields (Video 2–4). At the same time, small subpleural anechoic images were evident, compatible with small consolidations, and an evident interstitial syndrome with multiple irregular and confluent B lines. That sonographic picture was compatible at first with chronic non-cardiogenic interstitial disease (Fig. 2a, b).
Fig. 2.

a, b Ultrasound imaging and cartoon representing main features of sonographic appearance of chronic non-cardiogenic interstitial syndrome; the arrow indicates irregular pleural line; X shows multiple irregular B lines; * indicates small anechoic subpleural consolidations
The patient was subsequently referred for pneumological counseling. Respiratory function tests showed a severe restrictive alteration (Fig. 3), and the completion with high-resolution computed tomography allowed to diagnose an idiopathic pulmonary fibrosis (IPF) (Fig. 4).
Fig. 3.
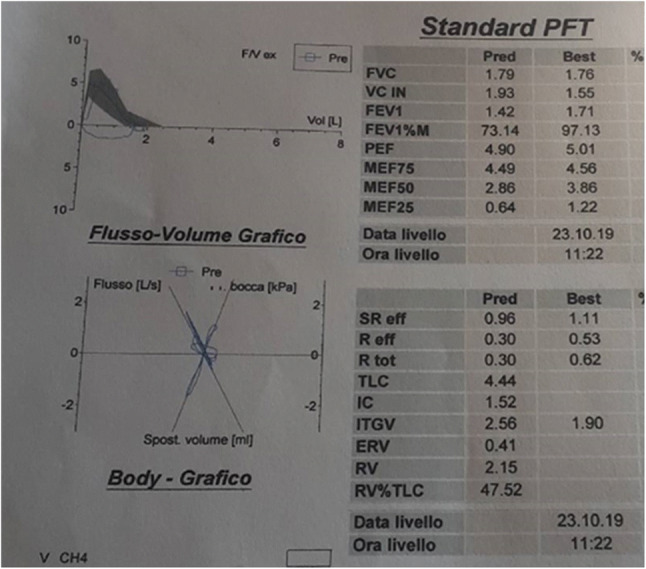
Restrictive alteration described by respiratory function test
Fig. 4.
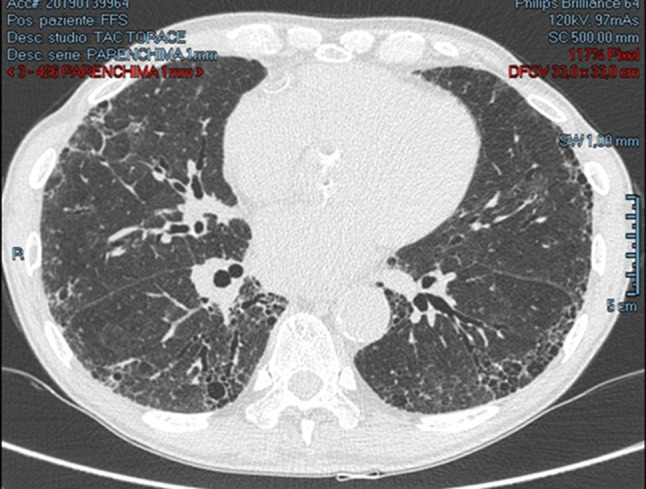
High-resolution computed tomography (HRCT) examination revealed findings suggestive for idiopathic pulmonary fibrosis (IPF): architectural distortion, reticulation, and honeycombing involving mainly the lung periphery and the lower lobes
Discussion
The diaphragm is the main respiratory muscle; despite its primary role in the physiological respiratory function, it is often ignored by clinical and ultrasound analysis. In B mode examination by a convex probe, it appears as a hyperechoic line delineating the hepatic profile, so to divide the abdominal from the thoracic cavity; using an ascending sub-rib approach, with the M-mode line positioned along the major axis of the gallbladder bed, it is possible to study diaphragm kinetics by evaluating its excursion during the acts of the breath (physiological and forced breathing) [8] (Figs. 5, 6).
Fig. 5.

Ultrasound evaluation of the diaphragm mobility by M mode at current volume and during deep inspiration on healthy subject; right subcostal approach is used, in the area between the anterior axillary line and the hemiclavicular by convex probe
Fig. 6.
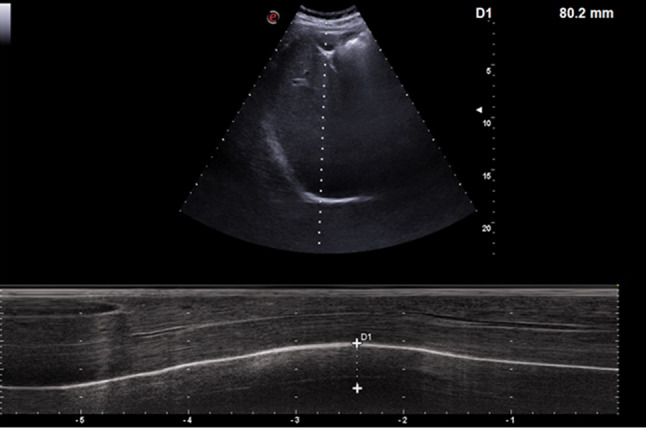
Ultrasound evaluation of the diaphragm mobility by M mode at current volume and during deep inspiration on healthy subject; right subcostal approach is used, in the area between the anterior axillary line and the hemiclavicular by convex probe
In the last years, this method to study diaphragmatic kinetics has been applied especially in the field of emergency medicine as a monitoring of patients with acute respiratory failure and in the resuscitation field as indirect monitoring of mechanical ventilation [4, 5, 8–13].
There are few data regarding chronic lung diseases. In a previous work, our group showed how the diaphragmatic excursion at rest increased in subjects with IPF, while the degree of forced diaphragm excursion decreased compared to healthy subjects [9]; these data are related to pathological changes in the lung affected by fibrosis, thus decreasing pulmonary compliance, so as to force the patient to carry out a greater effort or work to guarantee an adequate tidal volume; otherwise, the patient is not able to increase the degree of excursion during forced breathing, as for a reduced functional reserve.
These features seem to be responsible for exertional dyspnea and poor exercise tolerance, which are main and early symptoms of interstitial lung disease. Moreover, a positive correlation was found between forced vital capacity (FVC) values and diaphragmatic motility both at rest and in deep breathing in fibrotic patients [9]. Therefore, an alteration of the diaphragmatic motility is not always due to a neurological or post-traumatic pathology, but can derive from alterations following a chronic pulmonary disease involving a variation of the volumes and of the pulmonary pressures, which affect the geometry and the degree of diaphragmatic muscle contraction.
A further aspect to highlight is that thoracic ultrasound allowed to direct the diagnosis towards a form of chronic fibrotic pulmonary alteration from the analysis of some sonographic signs [6]; indeed, bilateral basal B lines may be suggestive of both cardiogenic and non-cardiogenic pulmonary edema. Specifically, chronic interstitial fibrotic pathologies are characterized by an extremely irregular pleural line, sometimes thickened and/or jagged, with small subpleural consolidations; moreover, the same B lines display peculiar characteristics being irregular with different thickness [6].
The integration of sonographic and pulmonary function testing and then radiological CT data allowed to reach the specific diagnosis of IPF. In our case, the patient had been sent to an ultrasound unit to perform an abdominal ultrasound in the suspicion of an abdominal disease, but the early execution of a detailed physical thoracic examination with the finding of velcro-like rales would have probably led the patient towards a different diagnostic way, with the execution of a chest X-ray as a first-level exam. Therefore, ultrasound could be employed as a screening tool or as a completion to the chest X-ray as a first-level diagnostic test in the study of a suspected pulmonary interstitial disease, thus reinforcing ultrasound examination role in pneumology [14–19].
Conclusions
Exertional dyspnea may be the first symptom underlying an interstitial lung disease; M mode ultrasound evaluation is a good method to study diaphragmatic mobility without contraindications. This aspect is even more relevant for those patients who cannot perform pulmonary function testing.
Therefore, lung and diaphragm ultrasound could be employed as a screening or first-line diagnostic tool in the suspicion of interstitial lung disease, because it is a rapid, repeatable, non-invasive technique that can be performed directly at bedside, even in critical conditions.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Informed consent
Written informed consent was obtained.
Research involving human participants and/or animals
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Shirakawa T, Fukuda K, Miyamoto Y, Tanabe H, Tada S. Parietal pleural invasion of lung masses: evaluation with CT performed during deep inspiration and expiration. Radiology. 1994;192(3):809–811. doi: 10.1148/radiology.192.3.8058952. [DOI] [PubMed] [Google Scholar]
- 2.Gierada DS, Curtin JJ, Erickson SJ, Prost RW, Strandt JA, Goodman LR. Diaphragmatic motion: fast gradient-recalled-echo MR imaging in healthy subjects. Radiology. 1995;194:879–884. doi: 10.1148/radiology.194.3.7862995. [DOI] [PubMed] [Google Scholar]
- 3.He L, Zhang W, Zhang J, Cao L, Gong L, Ma J, et al. Diaphragmatic motion studied by M-mode ultrasonography in combined pulmonary fibrosis and emphysema. Lung. 2014;192:553–561. doi: 10.1007/s00408-014-9594-5. [DOI] [PubMed] [Google Scholar]
- 4.Zanforlin A, Giannuzzi R, Nardini S, Testa A, Soldati G, Copetti R, et al. The role of chest ultrasonography in the management of respiratory disease: document I. Multidiscip Respir Med. 2013;8:54. doi: 10.1186/2049-6958-8-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boussuges A, Gole Y, Blanc P. Diaphragmatic motion studied by M-mode ultrasonography. Chest. 2009;135:391–400. doi: 10.1378/chest.08-1541. [DOI] [PubMed] [Google Scholar]
- 6.Soldati G, Demi M. The use of lung ultrasound images for the differential diagnosis of pulmonary and cardiac interstitial pathology. J Ultrasound. 2017;20(2):91–96. doi: 10.1007/s40477-017-0244-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Testa A, Soldati G, Giannuzzi R, Berardi S, Portale G, Gentiloni SN. Ultrasound M-mode assessment of diaphragmatic kinetics by anterior transverse scanning in healthy subjects. Ultrasound Med Biol. 2011;37(1):44–52. doi: 10.1016/j.ultrasmedbio.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Boccatonda A, Decorato V, Cocco G, Marinari S, Schiavone C. Ultrasound evaluation of diaphragmatic mobility in patients with idiopathic lung fibrosis: a pilot study. Multidiscip Respir Med. 2018;14(14):1. doi: 10.1186/s40248-018-0159-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zanforlin A, Smargiassi A, Inchingolo R, Valente S, Ramazzina E. Ultrasound in obstructive lung diseases: the effect of airway obstruction on diaphragm kinetics A short pictorial essay. J Ultrasound. 2014;18(4):379–384. doi: 10.1007/s40477-014-0122-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shaikh H, Laghi F. Role of diaphragm ultrasound when NIV fails in COPD exacerbations. Respir Care. 2019;64(12):1600–1602. doi: 10.4187/respcare.07523. [DOI] [PubMed] [Google Scholar]
- 12.Santana PV, Cardenas LZ, de Albuquerque ALP, de Carvalho CRR, Caruso P. Diaphragmatic ultrasound findings correlate with dyspnea, exercise tolerance, health-related quality of life and lung function in patients with fibrotic interstitial lung disease. BMC Pulm Med. 2019;19(1):183. doi: 10.1186/s12890-019-0936-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cammarota G, Sguazzotti I, Zanoni M, Messina A, Colombo D, et al. Diaphragmatic ultrasound assessment in subjects with acute hypercapnic respiratory failure admitted to the emergency department. Respir Care. 2019;64(12):1469–1477. doi: 10.4187/respcare.06803. [DOI] [PubMed] [Google Scholar]
- 14.Perrone T, Quaglia F. Lung US features of severe interstitial pneumonia: case report and review of the literature. J Ultrasound. 2017;20(3):247–249. doi: 10.1007/s40477-017-0241-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orso D, Ban A, Guglielmo N. Lung ultrasound in diagnosing pneumonia in childhood: a systematic review and meta-analysis. J Ultrasound. 2018;21(3):183–195. doi: 10.1007/s40477-018-0306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rea G, Sperandeo M, Di Serafino M, Vallone G, Tomà P. Neonatal and pediatric thoracic ultrasonography. J Ultrasound. 2019;22(2):121–130. doi: 10.1007/s40477-019-00357-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sperandeo M, Frongillo E, Dimitri LMC, Simeone A, De Cosmo S, Taurchini M, Cipriani C. Video-assisted thoracic surgery ultrasound (VATS-US) in the evaluation of subpleural disease: preliminary report of a systematic study. J Ultrasound. 2020;23(1):105–112. doi: 10.1007/s40477-019-00374-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Merli L, Nanni L, Curatola A, Pellegrino M, De Santis M, Silvaroli S, Paradiso FV, Buonsenso D. Congenital lung malformations: a novel application for lung ultrasound? J Ultrasound. 2019 doi: 10.1007/s40477-019-00406-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boccatonda A, Primomo G, Cocco G, D'Ardes D, Marinari S, Montanari M, Giostra F, Schiavone C. Not all abolished lung sliding are pneumothorax: the case of a particular lung atelectasis. J Ultrasound. 2020 doi: 10.1007/s40477-020-00427-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


