Abstract
Background
Breast pain is one of the most common breast disorders, affecting 41%–69% women in the clinical populations. Chinese herbal medicine (Rupi Sanjie, RPSJ) capsule has been recommended to be commonly used for breast pain in China. This review aimed to systematically collect latest evidence and critically evaluate the eff ;ectiveness and safety of RPSJ capsule for breast pain.
Methods
We searched 6 databases from their inception to June 1, 2020 for randomized clinical trials (RCTs) comparing RPSJ capsule with conventional drug therapies, placebo or no treatment. Primary outcomes were breast pain relief, reduction of breast mass and clinical cure rate.
Results
Seventeen RCTs were included in total, involving 2899 participants with breast pain. RPSJ capsule showed a significant effects in shortening duration of the breast pain (MD-6.51 days, 95%CI [-8.57, -4.45], n = 82, 1 trial), shortening the duration of breast mass (MD-5.17 days, 95%CI [-7.56, -2.78], n = 82, 1 trial), improving clinical cure rate (RR 1.55, 95% CI [1.21, 2.00], I² = 64%, n = 1398, 10 trials) and total effective rate (RR 1.08, 95% CI [1.03, 1.14], I² = 71%, n = 2170, 14 trials) compared to Tamoxifen (TAM). The meta-analysis showed that the incidence of total adverse events was higher in TAM group than the RPSJ capsule group (RR 0.30, 95%CI [0.21, 0.42], I² = 49%, n = 2122, 13 trials).
Conclusions
RPSJ capsule appears to be a potentially effective in treating breast pain and seems generally safe for clinical application. However, this potential benefit is inconclusive due to generally weak evidence, and the findings should be further confirmed in large and rigorous trials.
Keywords: Herbal medicine, Breast pain, Randomized controlled trial, Systematic review, Meta-Analysis
1. Introduction
Breast pain or mastalgia is one of the most common breast disorders experienced by women and is reported as a frequent reason for medical attention.1, 2 Up to 70%-80% women may suffer breast pain in the general population and the prevalence of breast pain in clinical populations has been reported to range from 41% to 69%. 3, 4 In China, the incidence of breast pain in different ages was increasing, especially among women aged from 25 to 45 years old.5, 6 Breast pain can be differentiated into cyclic mastalgia and noncyclic mastalgia.7 Cyclical pain can account for 70% of breast pain, noncyclical breast pain accounts for up to 25% of the cases of breast pain.8 Such kind of discomfort seriously affected women's self-esteem and quality of life.
Currently, the treatments for breast pain mainly focused on therapeutic agents that alter the hormonal environment.9., 10. However, we found limited evidence indicating this therapy is effective at relieving symptoms of breast pain, while long-term use may interfere with endocrine regulation, and the side effects are usually significant.11 For example, tamoxifen was limited to use no more than six months under doctor supervision, for it was reported with high risk of teratogenicity and venous thromboembolism. Danazol was reported to associate with weight gain, menorrhagia, and has influence on fetus in androgenic effects.9., 12, 13 Thus, an effective and more safety drug for breast pain is needed.
In China, Rupi Sanjie (RPSJ) capsule was commonly used for breast pain and it was approved for breast pain on market by the China Food and Drug Administration (CFDA) in 2002 and has been recorded by the Chinese Pharmacopeia since 2010.14 Ingredients of RPSJ capsule include Prunella vulgaris, Ligusticum, Bupleurum, Red peony, Rose, Curcuma, Angelica, Corydalis, Oyster, Bombyx mori, Turtle shell etc. Chinese Pharmacopoeia Commission has recommended it to be used for breast pain usually accompanied by breast nodule, irritability, and breast bilges full.
Some clinical trials have been conducted to investigate the effect of RPSJ capsule treating breast pain. A previous systematic review of seven trials published in 2011 concluded with insufficient results about the effect of RPSJ capsule when comparing with tamoxifen and RPSJ capsule.15 Another recent systematic review summarized evidence of RPSJ capsule for breast pain.16 However, it mistakenly included 3 non-randomized controlled trials and did not specify the detailed information of the treatment. In addition, the insufficient outcome assessment was reported, which increased the difficulty of the interpretation and application of the findings. Therefore, this review aimed to systematically collect all relevant RCTs and critically appraise the effectiveness and safety of RPSJ capsule for breast pain.
2. Methods
This review was prepared according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The review protocol was registered at PROSPERO (NO: CRD42020162583).
2.1. Eligibility criteria
2.1.1. Type of study
We included both parallel, cross-over, randomized clinical trials (RCTs), regardless of publication status.
2.1.2. Type of participants
Types of participants included women who were diagnosed as breast pain by any recognized criteria, regardless of their age or race.
2.1.3. Type of interventions
Interventions included Chinese herbal medicine RPSJ capsule alone (one Chinese patent medicine) with no limitations on the dosage and duration. Control interventions included conventional drug therapies (such as Tamoxifen (TAM), Toremifene citrate (TOR)), placebo, or no treatment. Co-interventions were not allowed in this review.
2.1.4. Type of outcomes
2.1.4.1. Primary outcomes
Primary outcomes included breast pain relief, reduction of breast mass and clinical cure rate. (1) The breast pain relief was measured by any recognized pain scale (such as the Visual Analogue Scale (VAS) scores). (2) The reduction of breast mass was measured by ultrasound or any other available tools. (3) Clinical cure was defined as the disappearance of the pain, and the status of the breast glands back to normal.17 If trials that did not reported clinical cure criteria as above or did not attach reference description similar to our criteria, they will not be included for data analysis in cure rate.
2.1.4.2. Secondary outcomes
(1) The total effective improvement was categorized into four grades (i.e. cured, markedly effective, effective, and ineffective) according to the changes or improvement of the symptoms. ‘The cured’ was the disappearance of the breast pain, and the status of the breast glands back to normal. ‘Markedly effective’ was the almost disappearance of the symptoms. ‘Effective’ was symptoms alleviation, and ‘ineffective’ was no changes or deterioration in symptoms. Total effectiveness included participants measured by cured, markedly effective and effective improvement. (2) Adverse events. We included RCTs reporting at least one of the above outcomes.
2.2. Search strategy
We systematically searched PubMed, Embase Database, Chinese Biomedical Literature Database (Sinomed), China National Knowledge Infrastructure (CNKI), Wanfang Database and China Science Technology Journal Database (VIP) from their inception to June 1st 2020. English and Chinese language publications were included. Appendix A outlined the detailed search strategy of PubMed.
2.3. Study selection and Data extraction
Two authors were independently selected the studies according to the eligibility criteria and extracted the data. The extracted data mainly included first authors and year of publication, detail information of randomization, characteristics of participants (such as age, and other clinical information), sample size, descriptions of intervention/control and outcomes etc. Any discrepancies regarding study selection and data extraction was resolved through consensus and arbitrated by a third author if necessary.
2.4. Assessment of risk of bias
The risk of bias of the included trials was assessed according to the criteria from the Cochrane Handbook for Systematic Reviews of Interventions.18 The quality of each trial was categorized into low, unclear, or high risk of bias based on the following seven domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessors, incomplete outcome data, selective reporting, and other bias (such as the comparison of the baseline). Trials were categorized to low risk of bias when all the items met the criteria, a judgment of high risk of bias of trials was made when at least one of the items was assessed as high risk, and other trials were categorized to unclear risk of bias if insufficient information was acquired to make judgment. Any disagreements were resolved by discussion with a third author. Certainty of evidence across studies of each important outcome was assessed using GRADE approach to conduct management recommendations by the GRADEpro tool.
2.5. Data analysis
We used RevMan Software 5.3 to perform statistical analyses. We assessed statistical heterogeneity by using the I2 test and an I2 >50% indicated the possibility of statistical heterogeneity among the trials. We performed meta-analysis only when there was no significant difference of participants, and when intervention, comparison, outcomes and the statistical heterogeneity (I2≤75%) test showed no significant difference. Considering potential sources of clinical heterogeneity, random-eff ;ects model (REM) was adopted for meta-analysis in this review. When I2>75%, meta-analysis would not be performed. The dichotomous data was presented by risk ratio (RR) with 95% confidence interval (CI), and the continuous outcomes were presented by means difference (MD) with 95% CI. Then subgroup analysis was performed by treatment durations or following up durations if data were available. Sensitivity analysis was performed when possible to test the robustness of the results.
2.6. Trial sequential analysis
Trial sequential analysis (TSA) was applied to calculate the required sample size in a meta-analysis and to detect the robustness of the results if there were more than eight studies in a meta-analysis. TSA version 0.9.5.5 was used to perform statistical analyses. We used the diversity-adjusted required information size estimated from a control event proportion of the included trials and a priori intervention effect of 5%, and the diversity was estimated in the included trials.19
3. Results
3.1. Description of studies
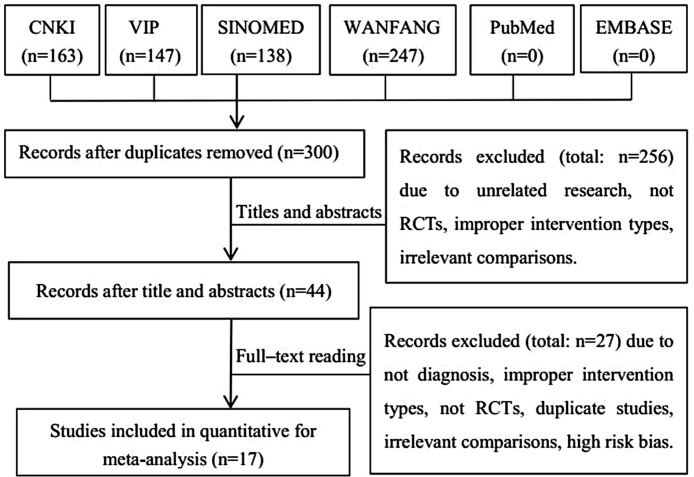
A total of 695 studies were obtained through searching of six electronic databases. 395 duplicates were excluded, and then 256 were excluded by the abstracts screening. Full texts of 44 articles were screened according to the eligibility criteria. Seventeen RCTs were included finally. The study searching and selecting process is shown in Fig. 1.
Fig. 1.
Study flow diagram.
3.2. Study characteristics
Seventeen RCTs involving 2899 participants were included in this review. Only one RCT36 (123 participants) received no treatment (emotion therapy) in control, two trials34., 35. received TOR in control (606 participants) and the remaining 14 RCTs 20., 21., 22, 23., 24, 25., 26., 27., 28., 29., 30., 31., 32., 33. (2170 participants) received TAM in control. The sample size varied from 48 to 400 participants, with an average of 85 patients per group. The participants were female adults aged from 20 to 52 years old. The mean age of participants was 33.5 ± 4.3 years old based on 9 trials reporting age.23., 24, 25., 26., 28., 29., 31.,32., 34. The average course of the disease was 21.1 months according to 9 trials.21., 23., 24, 25., 26., 28., 29., 31.,32 None of the trials specified the sample size calculation. All trials were conducted in China and reported in Chinese. All the trials were parallel group design and were two arms, except two trials with three arms.20., 21. The characteristics of included trials are shown in Table 1.
Table 1.
Characteristics of included RCTs on RPSJ capsule for breast pain.
| Study ID | Sample (T/C) | Age (T/C) (y) | Course of disease (months) | Control | Intervention (RPSJ) | Duration | Follow up (month) | Outcome measures |
|---|---|---|---|---|---|---|---|---|
| Liu 201020 | T:125 | T:24−52 | NR | TAM, each time 10 mg, 2 times/d | Each time 4 pills, | 3 mouths | 3 | Clinical cure rate, total effective rate, adverse events |
| C:125 | C:24−52 | 2 times/d | ||||||
| Lu 200921 | T:200 | T:25−65 | T:43.2 | TAM, each time 10 mg, 1 time/d | Each time 4 pills, | 3 mouths | NR | Total effective rate, adverse events |
| C:200 | C:25−65 | C:43.2 | 3 times/d | |||||
| Wu 200922 | T:115 | T: 20−51 | NR | TAM, each time 10 mg, 1 time/d | Each time 4 pills, | 6 weeks | 3 | Clinical cure rate, total effective rate, adverse events |
| C:98 | C: 20−51 | 3 times/d | ||||||
| Wu 201423 | T:76 | T:30.43 + 6.36 | T:14.01 ± 3.69 | TAM, each time 10 mg, 2 times/d | Each time 4 pills, | 2 mouths | NR | Total effective rate, adverse events |
| C:74 | C:30.11 ± 6.56 | C:13.92 ± 2.96 | 3 times/d | |||||
| Peng 201824 | T:30 | T:35.5 ± 3.7 | T:12.3 ± 4.4 | TAM, each time 10 mg | Each time 4 pills, | 1 mouths | NR | Total effective rate, adverse events |
| C:30 | C:36.2 ± 2.9 | C:12.5 ± 5.2 | −20 mg, 1 time/d | 2 times/d | ||||
| Xu 201725 | T:24 | T:36 ± 5 | T:52.8 ± 12 | TAM, each time 10 mg, 2 times/d | Each time 4 pills, | 1 mouths | NR | Reduction of breast mass, clinical cure rate, total effective rate |
| C:24 | C:36 ± 5 | C:46.8 ± 13.2 | 3 times/d | |||||
| Zeng 201426 | T:55 | T:35.1 ± 3.9 | T:13.4 ± 3.8 | TAM, each time 10 mg | Each time 4 pills, | 3 mouths | NR | Clinical cure rate, total effective rate, adverse events |
| C:55 | C:34.6 ± 7.2 | C:13.4 ± 3.8 | −20 mg, 1 time/d | 2 times/d | ||||
| Wang 201227 | T:100 | NR | NR | TAM, each time 10 mg, 2 times/d | Each time 4 pills, | 2 weeks | NR | Clinical cure rate, total effective rate, adverse events |
| C:100 | 3 times/d | |||||||
| Tian 201728 | T:50 | T:36.21 ± 2.26 | T:12.8 ± 4.5 | TAM, each time 10 mg | Each time 4 pills, | 1 mouths | NR | Clinical cure rate, total effective rate, adverse events |
| C:50 | C:37.05 ± 2.12 | C:12.8 ± 4.5 | −20 mg, 1times/d | 2 times/d | ||||
| Zang 201429 | T:25 | T:32.6 ± 3.7 | T:18 ± 3.6 | TAM, each time 10 mg, first week 2times/d, second week 1 times/d | Each time 4 pills, | 2weeks | 3 | Clinical cure rate, total effective rate, adverse events |
| C:25 | C:32.2 ± 3.5 | C:16.8 ± 2.4 | 3 times/d | |||||
| Fang 201530 | T:152 | NR | NR | TAM, each time 10 mg, 2times/d | Each time 4 pills, | 3 mouths | NR | Clinical cure rate, total effective rate, adverse events |
| C:130 | 3 times/d | |||||||
| Mo 201331 | T:38 | T:30.4 ± 6.3 | T:14.05 ± 3.72 | TAM, each time 10 mg, 2times/d | Each time 4 pills, | 2 mouths | NR | Clinical cure rate, total effective rate, adverse events |
| C:37 | C:30.1 ± 6.6 | C:13.82 ± 3.65 | 3 times/d | |||||
| Chen 201432 | T:76 | T:29.37 ± 5.98 | T:13.78 ± 4.02 | TAM, each time 10 mg, 2times/d | Each time 4 pills, | 2 mouths | NR | Total effective rate, adverse events |
| C:74 | C:31.02 ± 7.04 | C:12.74 ± 4.01 | 3 times/d | |||||
| Chen 201333 | T:41 | NR | NR | TAM, each time 10 mg, 2times/d | Each time 4 pills, | 3 mouths | 3 | The breast pain relief, reduction of breast mass, clinical cure rate, total effective rate, adverse events |
| C:41 | 3 times/d | |||||||
| Li 200834 | T:167 | T:34.8 | NR | TOR, each time 40 mg, 1times/d | Each time 4 pills, | 3 mouths | 3 | Clinical cure rate, total effective rate, adverse events |
| C:159 | C:34.8 | 3 times/d | ||||||
| Zhao 201035 | T:140 | NR | NR | TOR, each time 40 mg, 1times/d | Each time 4 pills, | 3 mouths | 3 | Clinical cure rate, total effective rate, adverse events |
| C:140 | 3 times/d | |||||||
| He 201536 | T:63 | T:24−50 | NR | No treatment | Each time 4 pills, | 3 mouths | NR | Total effective rate, adverse events |
| C:60 | C:22−48 | 3 times/d |
T, Rupi Sanjie capsule group; C, control group; d, day; y, year; NR, Not reported; RPSJ, Rupi Sanjie capsule (0.53 g per pill); TAM, Tamoxifen; TOR, Toremifene citrate tablets (60 mg per tablet).
3.3. Methodological quality in included trials
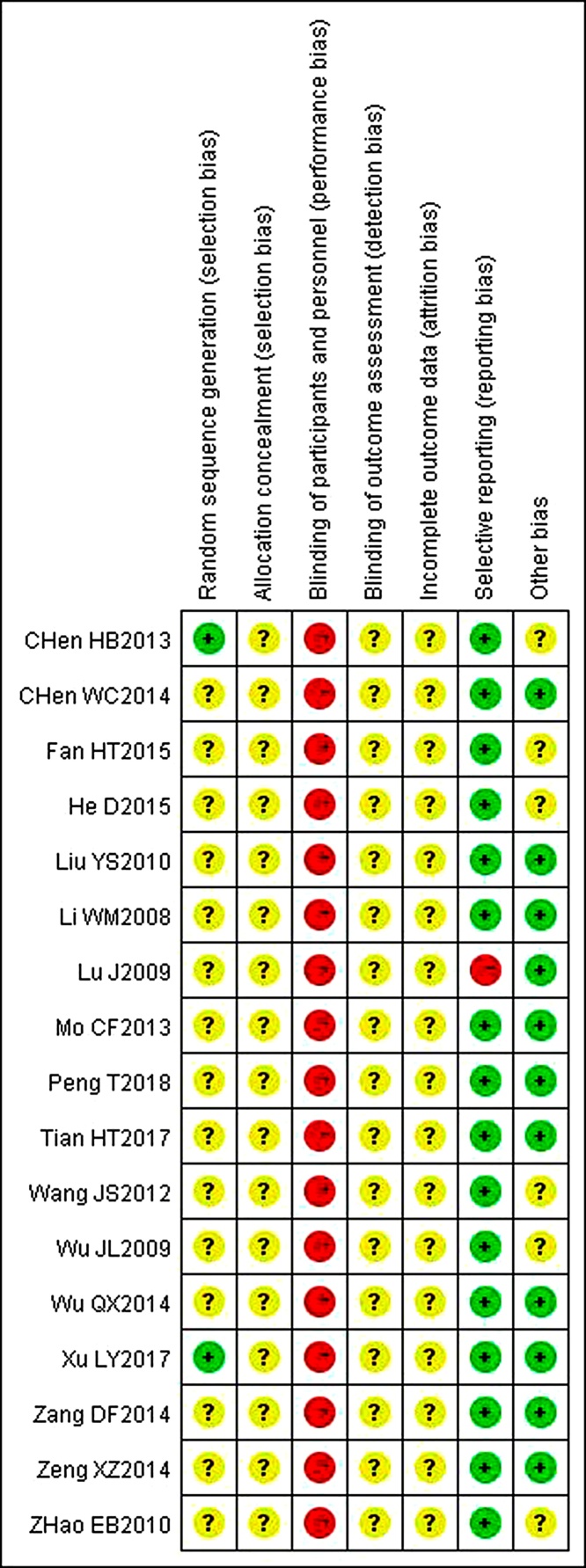
All included trials were found to be high risk of bias due to insufficient or inadequate reporting of the study designs and methodology information. Only two trials25., 33. described that random number table was used to generate the random allocation, thus assessed as having low risk of bias. All the trials appeared impossible to blind the participant and personnel as RPSJ capsule were only used in experimental groups. All the trials reported no information about the allocation concealment and the blinding of outcome assessment. Risk of detective bias on these two items was judged as unclear. The drop-out information was not reported in all trials and was all evaluated as unclear risk of attrition bias. Because all trials did not provided information about trial registry, we assessed reporting bias by judging consistency between outcomes in the method section of the publication. One trial21 was assessed as high risk of selective reporting bias since it had obvious problems on primary outcome reporting. The remaining sixteen trials were assessed as having low risk of selective reporting bias for the reason that studies reported all outcomes mentioned in the part of methods. Other bias was assessed by comparability between groups on baseline data. Six of trials22, 27., 30., 33.,35., 36. reported baseline data including age and duration of the breast pain. There is no statistical description for comparability, so they were assessed as unclear risk of bias. The methodological quality of all the included trials is shown in the Fig. 2.
Fig. 2.
Risk of bias summary.
3.4. Effect estimates
3.4.1. Primary outcomes
3.4.1.1. The breast pain relief
One trial33 compared RPSJ capsule with TAM, the results showed a shorter duration of the breast pain in the RPSJ capsule than that of the TAM groups (MD-6.51 days, 95%CI [-8.57, -4.45], n = 82) (Table 2). The breast pain relief was not reported in other included trials.
Table 2.
Effect estimates of RPSJ capsule for breast pain.
| Outcomes and comparisons | Studies | Participants | Effect estimate (95%CI) REM | P value | Study ID references | |
|---|---|---|---|---|---|---|
| 1 RPSJ capsule versus TAM | ||||||
| Reduction of breast mass | Duration of breast mass | 1 | 82 | MD -5.17 days, [-7.56, -2.78] | P < 0.001 | [33] |
| Size of breast mass | 1 | 48 | MD -0.80 cm, [-1.11, -0.49] | P < 0.001 | [25] | |
| The breast pain relief | Duration of breast pain | 1 | 82 | MD -6.51 days, [-8.57, -4.45] | P < 0.001 | [33] |
| Clinical cure rate | 10 | 1398 | RR 1.55, [1.21, 2.00], I² = 64% | P < 0.001 | [20,22,25–31,33] | |
| Clinical cure rate (subgroup analyses) | Without follow up | 6 | 803 | RR 1.72, [1.15, 2.56], I² = 70% | P = 0.008 | [25–28,30,31] |
| Following up 3 months without recurrence | 4 | 595 | RR 1.39, [0.95, 2.04], I² = 65% | P = 0.09 | [20,22,29,33] | |
| Total effective rate | 14 | 2170 | RR 1.08, [1.03, 1.14], I² = 71% | P < 0.001 | [20–33] | |
| Adverse events | 13 | 2122 | RR 0.30, [0.21, 0.42], I² = 49% | P < 0.001 | [20–24,26–33] | |
| 2 RPSJ capsule versus TOR | ||||||
| Clinical cure rate | Following up 3 months without recurrence | 2 | 606 | RR 0.96, [0.78, 1.17], I² = 0% | P = 0.66 | [34,35] |
| Total effective rate | 2 | 606 | RR 0.99, [0.96, 1.03], I² = 0% | P = 0.71 | [34,35] | |
| Adverse events | 1 | 326 | RR 0.01, [0.00, 0.08] | P < 0.001 | [34] | |
| 3 RPSJ capsule versus no treatment | ||||||
| Total effective rate | 1 | 123 | RR 2.76, [1.92, 3.98], REM | P < 0.001 | [36] | |
| Adverse events | 1 | 123 | RR 4.77, [0.23, 97.27], FEM | P < 0.001 | [36] | |
REM, random effect models; h, hours; CI, confidence intervals; MD, mean difference; RR, risk ratio.
3.4.1.2. Reduction of breast mass
One trial33 compared RPSJ capsule with TAM, the results showed a shorter duration of the breast mass in the RPSJ capsule groups than that of the TAM groups (MD-5.17 days, 95%CI [-7.56, -2.78], n = 82). Another study25 comparing RPSJ capsule with TAM also showed RPSJ capsule was superior in reducing the size of breast mass (MD -0.80 cm, 95%CI [-1.11, -0.49], n = 48) (Table 2).
3.4.1.3. Clinical cure rate
A pooled analysis of ten trials20., 22, 25., 26., 27., 28., 29., 30., 31., 33. showed a higher cure rate in RPSJ capsule group than that of the TAM group (RR 1.55, 95%CI [1.21, 2.00], I² = 64%, n = 1398, 10 trials). Subgroup analyses according to follow up duration indicated a higher cure rate (without following up) in RPSJ capsule group than that of the TAM group from six RCTs25., 26., 27., 28., 30., 31. (RR 1.72, 95%CI [1.15, 2.56], I² = 70%, n = 803, 6 trials). There was no significant difference between the RPSJ capsule group and the TAM group in terms of cure rate (following up for 3 months without recurrence) from four trials20., 22, 29., 33. (RR 1.39, 95%CI [0.95, 2.04], I² = 65%, n = 595, 4 trials). There was no significant difference between the RPSJ capsule group and the TOR group in cure rate (following up for 3 months without recurrence) according the data from two trials34., 35. (RR 0.96, 95%CI [0.78, 1.17], I² = 0%, n = 232, 2 trials) (Table 2).
3.4.2. Secondary outcomes
3.4.2.1. Total effective rate
A pooled analysis of fourteen trials20., 21., 22, 23., 24, 25., 26., 27., 28., 29., 30., 31., 32., 33. showed a higher total effective rate in RPSJ capsule group than that of TAM group (RR 1.08, 95%CI [1.03, 1.14], I² = 71%, n = 2170, 14 trials). Two trials34., 35. compared RPSJ capsule with TOR, the results showed there was no significant difference between RPSJ capsule group and TOR group in total effective rate (RR 0.99, 95%CI[0.96, 1.03], I² = 0%, n = 232, 2 trials). One study36 compared RPSJ capsule with no treatment also favored a higher total effective rate in RPSJ capsule group (RR 2.76, 95%CI [1.92, 3.98], n = 123) (Table 2).
3.4.2.2. Adverse events
The pooled data of 13 RCTs20., 21., 22, 23., 24, 26., 27., 28., 29., 30., 31., 32., 33. showed the incidence of total adverse events was higher in TAM group than that of the RPSJ capsule group (RR 0.30, 95%CI [0.21, 0.42], I² = 49%, n = 2122, 13 trials). One trial34 showed the higher incidence of total adverse events in TOR group than that of the RPSJ capsule group (RR 0.01, 95%CI [0.00, 0.08], n = 329) (Table 2). Another study36 compared RPSJ capsule with no treatment reported that two cases had menstruation-increasing. However, all of them relieved after stopping using the RPSJ capsule.
Besides, the major symptoms involving adverse events were summarized below due to the definition and grading of adverse events not fully specified in the included trials. 1) Gastrointestinal reactions including anorexia, stomach discomfort, nausea, vomiting, and diarrhea. 2) Secondary anti-estrogen effect including facial flushing, pruritus of vulva, menstrual disorders, leucorrhea increase, etc. 3) Other symptoms including headache, dizziness, depression, blurred vision, etc.
3.4.3. Additional analysis
Due to insufficient number of trials and limited information about trials, we could not perform other meaningful subgroup analysis. Sensitivity analysis was performed by using the fixed eff ;ect model, which generated similar results for all outcomes.
3.4.4. Certainty of evidence
We graded the certainty of available evidence by GRADE approach. In comparison of all outcomes and interventions assessments, the certainty of evidence for all outcomes was downgraded to very low mainly due to high risk of bias, imprecision (small number of total events or small sample size), high risk of performance bias for not blinding the participants or indirectness (outcome measure). Details of result are shown in Table 3.
Table 3.
Summary of main findings of randomized controlled trials on RPSJ capsule for breast pain.
| Outcomes | No. of participants (No. of RCTS) | Certainty of the evidence | Relative effect (95% CI) | Anticipated absolute effects |
||
|---|---|---|---|---|---|---|
| Risk with control | Risk difference with intervention (95% CI) | |||||
| 1 RPSJ capsuleversusTAM | ||||||
| Size of breast mass | 48(1) | N/A | The mean size of breast mass in the intervention groups was 0.8 cm smaller (1.11 to 0.49 lower) | |||
| Duration of breast pain | 82(1) | N/A | The mean duration of breast pain in the intervention groups was 6.51 days shorter (8.57 to 4.45 lower) | |||
| Clinical cure rate without follow up | 803(6) | RR 1.72[1.15to 2.56] | 294 per 1000 | 212more per 1000 (from 44more to 459 more) | ||
| Clinical cure rate with 3 months follow up | 595(4) | RR 1.39 [0.95to 2.04] | 304 per 1000 | 119 more per 1000 (from 15more to 317 more) | ||
| Total effective rate | 2170(14) | RR 1.09 [1.03to1.15] | 870 per 1000 | 70more per 1000 (from 15more to 317 more) | ||
| Adverse events | 2122(13) | RR 0.30[0.21to 0.42] | 324 per 1000 | 227 fewer per 1000 (from 256 fewer to 188 fewer) | ||
| 2 RPSJ capsuleversusTOR | ||||||
| Clinical cure rate with 3 months follow up | 232(2) | RR 0.95 [0.78to 1.17] | 391 per 1000 | 16 more per 1000 (from 86 more to 67 more) |
||
| Total effective rate | 232(2) | RR 0.99[0.96to 1.03] | 967 per 1000 | 10 more per 1000 (from 39 more to 29 more) |
||
1: Risk of bias: All the trials had high risk of performance bias not blinding the participants. Methodological quality of these trials was graded as “high risk of bias” due to the design of comparison. The trials also had unclear risk of performance bias for not reporting blinding the outcome assessor.
2: The significant heterogeneity with a large I2 value.
3: Imprecision: For dichotomous outcomes, the total number of events is less than 300; for continuous outcomes, the total population size is less than 400; or pooled results included no effects.
4: Indirectness. For outcomes of clinical cure rate and total effective rate. This was not internationally applied outcome measures.
5: Asymmetry in funnel plots.
6: All the trials had high risk of performance bias for not blinding the participants.
*The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; MD: Mean difference. N/A: Not applicable. RCT: randomized controlled trial. NO.: number. ⨁: Very low quality of the evidence; ⨁⨁: Low quality of the evidence; ⨁⨁⨁: Moderate quality of the evidence.
GRADE Working Group grades of evidence.
High quality: We are very confident that the true effect lies close to that of the estimate of the effect.
Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
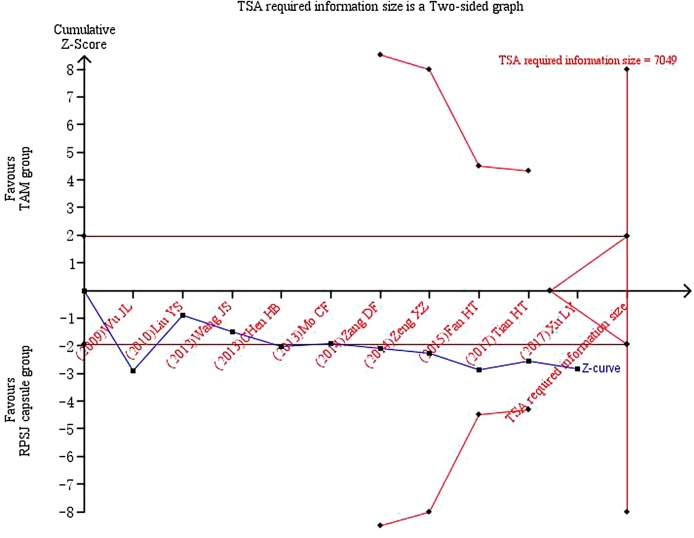
3.4.5. Trial sequential analysis
TSA was performed with the data from ten trials20., 22, 25., 26., 27., 28., 29., 30., 31., 33. reporting the clinical cure rate of breast pain. A required information size was estimated based on Daris type I error = 5%, power = 80%, RRR = 25% and a two-side graph.19, 37. The result of TSA illustrated that the cumulative Z-curve across the traditional boundary of 5% significance (horizontal red line), but it did not cross the monitoring boundaries (red inward sloping curves). These indicated that RPSJ capsule for patients with breast pain could not draw a powerful conclusion before, which is needed to obtain firm evidence controlling for the risk of random error. This resulted in a required information size of 7049 participants, TSA of all included trials suggests that high quality RCTs with 5651 participants were required to confirm possible intervention effect (Fig. 3).
Fig. 3.
TSA on RPSJ capsule versus TAM for clinical cure rate in patients with breast pain.
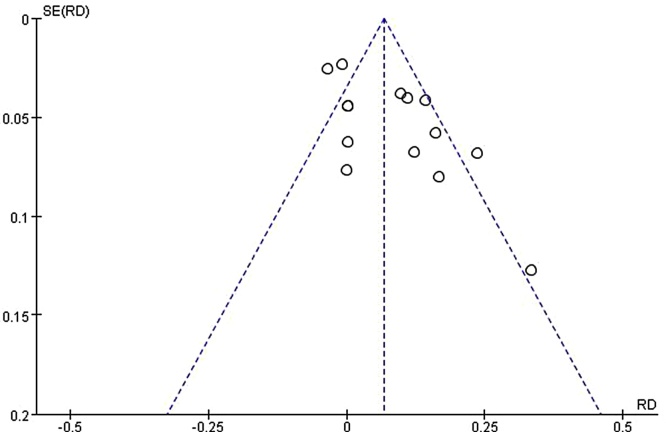
3.4.6. Publication bias
A funnel plot was performed using the RD and 1/ (Standard Error: SE) values obtained from trials measuring of the total effective rate. The funnel plot based on total effective rate appeared asymmetrical, suggesting the potential publication bias (Fig. 4).
Fig. 4.
Funnel plot assessing publication bias (using total effective rate).
4. Discussion
4.1. Summary of the main results
Seventeen RCTs were included in this review. Our results demonstrated RPSJ capsule appears to be effective and safe in treating breast pain with very low quality evidence. The duration of breast pain and breast mass in RPSJ capsule group was about 6 days shorter than that of the TAM group. The total effective rate of RPSJ capsule group was approximately 7% higher than that of TAM. There was no significant difference between RPSJ capsule group and conventional drugs (TAM or TOR) in clinical cure rate. However, the incidence of total adverse events was significant higher in conventional drugs than the RPSJ capsule group. Compared with no treatment, limited evidence shown the RPSJ capsule appears potentially effective and safe in treating breast pain. However, the level of evidence for all outcomes was assessed as “very low” due to high risk of bias, imprecision or indirectness among included trials. We could not draw powerful conclusions from the current evidence.
4.2. Comparison with previous studies
Two previous systematic reviews15., 16. published in Chinese journals have assessed the effectiveness of RPSJ capsule for breast pain, which mainly demonstrated that RPSJ capsule in combination with TAM were more effective than TAM treatment. However, there was no conclusion on the difference between RPSJ capsule alone and TAM. The included trials of the two reviews applied RPSJ capsule as adjunctive intervention, and the characteristics of included RCTs was not fully specified.
In contrast, this update review compared RPSJ capsule with conventional treatments (including TAM, TOR and no treatment). Additionally, we clearly specified the treatment information and characteristics of the included trials, and covered additional outcome assessments (such as the breast pain relief and reduction of breast mass). Furthermore, we conducted the more rigorous inclusion criteria for participant and outcome assessment. This review provided latest evidence of RPSJ capsule for breast pain.
4.3. Limitations of review
The promising findings are not conclusive due to some limitations of this review. A major limitation of this review is the very low quality and small sample size of the included studies, which contributed to reducing internal validity of the result and to interpreting the findings of our review cautiously. In addition, the asymmetrical funnel plots of total effective rate suggested that publication bias may exist, which may result from the fact that trials with positive results are more likely to be published than those with negative results. Most importantly, in this review, not all predefined outcomes were reported in the included trials, such as the duration of the breast pain and breast mass, or breast pain relief. Thus there was no sufficient evidence to support the effect of RPSJ capsule for those important clinical outcomes in patients with breast pain.
4.4. Implications for the clinical practice
Although there are some potential bias and limitations in this review, the results suggested that RPSJ capsule had potential effect and general safety in treating breast pain. According to this review, it is common that the RPSJ capsule dosage used in the treatment of breast pain were each time 4 pills (0.53 g/pill), three times per day. The duration of treatment varied from 2 weeks to 3 months based on severity of condition and patients should stop taking this medicine during menstruation. Considering the potential effect of RPSJ capsule, it might be an alternative choice in clinical practice for women with breast pain.
4.5. Implications for research
To improve the quality of trial design and transparent reporting, future researches are expected to conduct a multi-center, large sample size and double-blind placebo controlled design and to report trial according to the CONSORT (Consolidated Standards for Reporting Trials) Statement.38 Second, the study protocol is recommended to register on authoritative registration plat forms.39 Furthermore, long-term effect of RPSJ capsule for breast pain remains unclear. We suggest a follow up period should be considered in future trials. Most importantly, to give conclusive evidence and influence clinical practice, future trials should focus on clinically important and patient-relevant outcomes such as breast pain relief, the duration of the breast pain and breast mass, etc.
We also suggest that future studies report detailed information on the duration of breast pain and the degrade of breast pain relief measured by a recognized pain scale both before and after treatment, as well as adverse events, which are important data for further evaluation of the safety and effectiveness of RPSJ capsule.
4.6. Conclusions
This review suggests that the RPSJ capsule is potentially effective in treating breast pain. No serious adverse events related to RPSJ capsule were reported. However, this beneficial effect should be further confirmed in large, rigorous and well-reported trials.
Author contributions
Conceptualization: BY Lai and XH Pei. Methodology: S B Liang and AJ Chu. Software: S B Liang and AJ Chu. Formal Analysis: BY Lai and LY Jia. Data Curation: BY Lai and LY Jia. Writing – Original Draft: BY Lai and LY Jia. Writing – Review & Editing: BW Yu, HJ Cao, JP Liu and XH Pei. Funding Acquisition: XH Pei.
Conflict of interest
All authors declare that they have no conflicts of interest.
Funding
This work is supported by the fund from the standardization project of guide application of traditional Chinese medicine for treatment of its dominant diseases (No. SATCM-2015-BZ402).
Ethical statement
This work did not require an ethical approval as it does not involve any human or animal experiment.
Data availability
The data will be made available upon request.
Footnotes
Supplementary material related to this article can be found in the online version, at doi:https://doi.org/10.1016/j.imr.2020.100491.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Mansel R.E. ABC of breast diseases. Breast pain. BMJ. 1994;309:866–868. doi: 10.1136/bmj.309.6958.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Onstad M., Stuckey A. Benign breast disorders. Obstet Gynecol Clin North Am. 2013;40:459–473. doi: 10.1016/j.ogc.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Scurr J., Hedger W., Morris P., Brown N. The prevalence, severity, and impact of breast pain in the general population. Breast J. 2014;20:508–513. doi: 10.1111/tbj.12305. [DOI] [PubMed] [Google Scholar]
- 4.Kataria K., Dhar A., Srivastava A., Kumar S., Goyal A. A systematic review of current understanding and management of mastalgia. Indian J Surg. 2014;76:217–222. doi: 10.1007/s12262-013-0813-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma W., Jin Q.X., Wu Y.F., Jin F. Expert consensus on diagnosis and treatment of breast hyperplasia. Chinese Journal of Practical Surgery. 2016;36:759–762. [Google Scholar]
- 6.Xia B.L., Xia F.R. An analysis of breast diseases screening results of 6730 women in Ping gu District of Beijing. China Practical Medical. 2017;12:85–86. [Google Scholar]
- 7.Expert Panel on Breast Imaging, Holbrook A.I., Moy L. ACR appropriateness criteria® breast pain. J Am Coll Radiol. 2018;15(Suppl11):S276–82. doi: 10.1016/j.jacr.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 8.Groen J.W., Grosfeld S., Wilschut J.A., Bramer W.M., Ernst M.F., Mullender M.M. Cyclic and non-cyclic breast-pain: A systematic review on pain reduction, side effects, and quality of life for various treatments. Eur J Obstet Gynecol Reprod Biol. 2017;219:74–93. doi: 10.1016/j.ejogrb.2017.10.018. [DOI] [PubMed] [Google Scholar]
- 9.Goyal A. Breast pain. Am Fam Physician. 2016;93:872–873. [PubMed] [Google Scholar]
- 10.Gateley C.A., Miers M., Mansel R.E., Hughes L.E. Drug treatments for mastalgia: 17 years experience in the Cardiff Mastalgia Clinic. J R Soc Med. 1992;85:12–15. doi: 10.1177/014107689208500105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Srivastava A., Mansel R.E., Arvind N., Prasad K., Dhar A., Chabra A. Evidence-based management of mastalgia: a meta-analysis of randomised trials. Breast. 2007;16:503. doi: 10.1016/j.breast.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Smith R.L., Pruthi S., Fitzpatrick L.A. Evaluation and management of breast pain. Mayo Clin Proc. 2004;79:353–372. doi: 10.4065/79.3.353. [DOI] [PubMed] [Google Scholar]
- 13.Jain B.K., Bansal A., Choudhary D., Garg P.K., Mohanty D. Centchroman vs tamoxifen for regression of mastalgia: a randomized controlled trial. Int J Surg. 2015;15:11–16. doi: 10.1016/j.ijsu.2014.12.033. [DOI] [PubMed] [Google Scholar]
- 14.2010th ed. People’s Medical Publishing House; Beijing: 2010. Chinese pharmacopoeia commission. Chinese pharmacopoeia; pp. 841–842. [Google Scholar]
- 15.Zhao F., Zhang Y.Z. The meta analysis of Rupi Sanjie capsule in the treatment of breast hyperplasia. Shandong Med J. 2011;3:59–60. [Google Scholar]
- 16.Han F., Pei X.H., Gui Y.L. Meta analysis of the efficacy and safety of Rupi Sanjie capsule in the treatment of breast hyperplasia. Shandong Med J. 2018;58:69–72. [Google Scholar]
- 17.Han M. Criteria for diagnosis and efficacy evaluation of breast hyperplasia (Revised) China J Trad Chin Med Pharm. 1988;3:66. [Google Scholar]
- 18.Higgins J., Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0. updated march 2011.
- 19.Wetterslev J., Jakobsen J.C., Gluud C. Trial sequential analysis in systematic reviews with meta-analysis. BMC Med Res Methodol. 2017;17:39–41. doi: 10.1186/s12874-017-0315-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y.S., Chen J.Z. Clinical application of tamoxifen and Rupi Sanjie capsule in treatment of hyperplasia breast. Chin J Pract Med. 2010;37:68–69. [Google Scholar]
- 21.Lu J., Chen Z. A clinical observation on Rupi Sanjie capsule, Rukang tablet and tamoxifen in the treatment of breast hyperplasia. Mod J Integ Trad Chin West Med. 2009;18:1621–1622. [Google Scholar]
- 22.Wu J.L., Wu Y. A clinical observation on Rupi Sanjie capsule in combination with tamoxifen in the treatment of breast hyperplasia. China Modern Medicine. 2009;16:37–38. [Google Scholar]
- 23.Wu Q.X. Analysis of the clinical effect of Rupi Sanjie capsule in the treatment of female breast hyperplasia. China Health Indus. 2014;11:105–106. [Google Scholar]
- 24.Peng T. The effect comparison of Rupi Sanjie capsule and tamoxifen in the treatment of breast hyperplasia. China Rural Health. 2018;2:82. [Google Scholar]
- 25.Xu L.Y., Wang Y.L., Huang Q. The effectiveness and the change of ultrasonic image on Rupi Sanjie capsule in treating hyperplasia of mammary gland. China J Pharmaceut Econ. 2017;12:58–60. [Google Scholar]
- 26.Zeng X.Z. The therapeutic effect comparison of Rupi Sanjie capsule and tamoxifen in treating hyperplasia of mammary gland. Chin J Clin Ration Drug Use. 2014;7:169–170. [Google Scholar]
- 27.Wang J.S. Effect of Rupi Sanjie capsule in treating hyperplasia of mammary gland. Jilin Med J. 2012;33:4131–4132. [Google Scholar]
- 28.Tian H.T. The therapeutic effect comparison of Rupi Sanjie capsule and tamoxifen in the treatment of breast hyperplasia. J North Pharm. 2017;14:11. [Google Scholar]
- 29.Zang D.F. Rupi Sanjie capsule in treating 25 cases of hyperplasia of mammary gland. Chin Med Mod Dist Educ China. 2014;12:129–130. [Google Scholar]
- 30.Fan H.T. Observation on the therapeutic effect of Rupi Sanjie capsule and tamoxifen in the treatment of hyperplasia of mammary gland. Electron J Clin Med Literat. 2015;2:2202. [Google Scholar]
- 31.Mo C.F. The effect of Rupi Sanjie capsule in treatment of hyperplasia of mammary gland. Asia-Pacific Trad Med. 2013;9:149–150. [Google Scholar]
- 32.Chen W.C., Zeng T.P., Guan F.Z. The clinical effect on Rupi Sanjie capsule in treatment of breast hyperplasia. J North Pharm. 2014;11:52. [Google Scholar]
- 33.Chen H.B., Chen Y.S. The clinical effect on Rupi Sanjie capsule in treatment of breast hyperplasia. Chin J Prim Med Pharm. 2013;20:3871–3872. [Google Scholar]
- 34.Li W.M. The clinical effect on two drugs in treatment of breast disease. J Contemp Clin Med. 2008;4:11–12. [Google Scholar]
- 35.Zhao E.B., Xue D.F., Liang S. A clinical analysis of Rupi Sanjie capsule in treatment of breast hyperplasia. Chin Foreign Med Res. 2010;08:16–17. [Google Scholar]
- 36.He D. The clinical effect on Rupi Sanjie capsule in treatment of female breast hyperplasia. Ment Health Today. 2015;14:302. [Google Scholar]
- 37.Xia Y., Sun Y., Liu Z.L., Liu J.P. Estimation of sample size in systematic review and Meta-analysis: trial sequential analysis. Mod Chin Clin Med. 2013;20:31–33. [Google Scholar]
- 38.Moher D., Hopewell S., Schulz K.F., Montori V., Gøtzsche P.C., Devereaux P.J. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomized trials. Int J Surg. 2012;10:28–55. doi: 10.1016/j.ijsu.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 39.Laine C., Horton R., DeAngelis C.D., Drazen J.M., Frizelle F.A., Godlee F. Clinical trial registration looking back and moving ahead. N Engl J Med. 2007;356:2734–2736. doi: 10.1056/NEJMe078110. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data will be made available upon request.