Abstract
Objective:
Lung cancer is often associated with hypercoagulability. Thromboelastography provides integrated information on clot formation in whole blood. This study explored the possible relationship between thromboelastography and lung cancer.
Methods:
Lung cancer was staged according to the Tumor, Node, and Metastasis (TNM) classification system. Thromboelastography parameters in different stages of disease were compared. The value of thromboelastography for stage prediction was determined by area under the receiver operating characteristic curve analysis.
Results:
A total of 182 patients diagnosed with lung cancer were included. Thromboelastography parameters, including kinetics time, α-angle, and maximum amplitude, differed significantly between patients with metastatic and limited lung cancers (P < 0.05). Kinetics time was significantly reduced and maximum amplitude was significantly increased in patients with stage I and II compared with stage III and IV tumors (P < 0.05). TNM stage was significantly negatively correlated with kinetics time (r = −0.186), and significantly positively correlated with α-angle (r = 0.151) and maximum amplitude (r = 0.251) (both P < 0.05). The area under the curve for kinetics time in patients with stage I cancer was 0.637 (P < 0.05) and that for α-angle in stage ≥ II was 0.623 (P < 0.05). The areas under the curves for maximum amplitude in stage ≥ III and stage IV cancer were 0.650 and 0.605, respectively (both P < 0.05). Thromboelastography parameters were more closely associated with TNM stage in patients with lung adenocarcinoma than in the whole lung cancer population.
Conclusion:
This study identified the diagnostic value of thromboelastography parameters for determining tumor stage in patients with lung cancer. Thromboelastography can be used as an independent predictive parameter for lung cancer severity.
Keywords: Coagulation, thromboelastography, lung cancer, cancer stage, lung adenocarcinoma
Introduction
Lung cancer is a common malignancy worldwide, accounting for nearly 20% of all cancer deaths.1 Venous thromboembolism (VTE) is an important complication and a significant cause of morbidity and mortality in lung cancer, occurring in 7% to 13% of patients.2 Furthermore, cancer patients are at increased risk of VTE compared with non-cancer patients.3
A persistent hypercoagulable state leads to the onset of VTE, and the relationship between coagulation and malignancy has been investigated. On one hand, cancer itself can increase coagulability,4 and both activated oncogenes (e.g., KRAS and MET) and inactive tumor suppressors (e.g., p53) have been shown to be risk factors for thrombosis.5 Tumor cells also release procoagulant microparticles into the circulation, which may also trigger VTE.6
Cancer treatments, including radiotherapy and chemotherapy, were also associated with increased risk of thrombosis in patients with primary lung cancer.7,8 A state of coagulation would facilitate tumor progression,9 and blood clotting reactions were found to stimulate primary tumor growth and metastasis.10 Moreover, coagulation was shown to contribute to metastasis by activating thrombin, excessive deposition of fibrinogen, and activation of prothrombin and platelets.9 Increasing evidence has also demonstrated multiple roles for coagulation proteins (i.e., tissue factor, thrombin, Factor X, and fibrin) in tumor progression and dissemination.11-13
Coagulation is essential for metastatic cell survival and premetastatic niche establishment,14 and treatment with anti-coagulant drugs inhibits the development of tumor metastasis.9 The patient’s hypercoagulable status becomes increasingly severe as the tumor progresses,10 and patients with more advanced cancer at the time of initial diagnosis accordingly have a higher risk of VTE.15,16 Tumor severity and the metastatic burden are therefore important factors when considering cancer-associated hypercoagulability. In cases with dual evidence of a tumor and coagulation, evaluation of the patient’s coagulation status can help to predict both thrombotic complications and disease severity. Hypercoagulation screening might also serve as an innovative tool for disease staging in patients with lung cancer.
Measurement of hypercoagulation is limited by the availability of conventional plasma-based assays, but it can also be detected using viscoelasticity assays, such as thrombelastography (TEG). TEG is a laboratory-based method that provides graphical representations of the dynamics of whole blood fibrin polymerization, measuring the dynamic coagulation process from the initial clotting cascade to clot strength.17 TEG has been used in clinical practice for 25 years, and is currently used in to evaluate hypercoagulability status.18 It has been reported that TEG can be used as a predictor of hypercoagulability-related complications.19
Many studies have applied this technique to cancer patients, and hypercoagulation measured by TEG was found to be consistent with malignancy-associated thrombotic events.20 However, no studies have reported on the use of TEG to predict lung cancer staging to date.
The current study therefore investigated the association between TEG results and lung cancer severity, and explored the diagnostic value of TEG for lung cancer staging.
Methods
Patient Population and Sample Collection
This was a retrospective study involving patients with primary lung cancer treated at the Shanghai Chest Hospital Affiliated to Shanghai Jiao Tong University, between May 2017 and May 2019. All recruited participants were inpatients and had provided blood samples for TEG testing. The inclusion criteria were: (1) histologically or cytologically confirmed primary lung cancer of any stage; and (2) patients with definite primary or metastatic lesions. The exclusion criteria were: (1) any disease process known to affect coagulation (i.e., genetic hemostatic disorders); and (2) anti-coagulant therapy, such as warfarin or heparin, administered 2 weeks before the samples were collected. Patient demographic characteristics, including age, sex, tumor type, tumor stage, metastasis, and chemotherapy were recorded. Tumor staging was coded according to the Tumor, Node, and Metastasis (TNM) classification of the Union for International Cancer Control.21 The study was approved by the Shanghai Chest Hospital Ethics Committee (approval no. KS2029). All patients provided written informed consent prior to enrollment in the study.
TEG Tests
Peripheral venous blood specimens were collected from patients in the morning. TEG was performed within 2 hours of blood being drawn, according to the manufacturer’s recommendations (TEG 5000 Hemostasis Analyzer System; Haemonetics Corporation, Boston, MA, USA). TEG parameters included reaction time (R-time; minutes), kinetics time (K-time; minutes), α-angle (Angle; degrees), and maximum amplitude (MA; mm). Circulating platelet counts were obtained by laboratory testing.
Statistical Analysis
Data analysis was carried out using IBM SPSS Statistics for Windows, version 23.0 (IBM Corp., Armonk, NY, USA). TEG parameters were presented as median and 25th/75th percentiles (P25,75) and were compared between groups using the Mann–Whitney U-test. Correlations between TEG and tumor stage were assessed by Spearman’s rank correlation coefficient. The diagnostic accuracy of TEG was assessed based on the area under the receiver operating characteristic curve (AUROC). The cut-off point was determined as the value with the maximum Youden index.
Results
Patient Population
We enrolled 182 patients. Eighty-four samples were obtained post-chemotherapy, and the other samples were taken before surgery or other treatment. An overview of the demographic and clinical characteristics of the study population is shown in Table 1.
Table 1.
Baseline Characteristics of the Total Study Population.
| Number of patients | % | |
|---|---|---|
| Age, years | ||
| ≤60 | 69 | 38 |
| >60 | 113 | 62 |
| Sex | ||
| Female | 74 | 41 |
| Male | 108 | 59 |
| Pathology | ||
| Adenocarcinoma | 140 | 77 |
| Squamous cell | 13 | 7 |
| Other NSCLC | 12 | 7 |
| SCLC | 17 | 9 |
| TNM stage | ||
| I | 60 | 33 |
| II | 13 | 7 |
| III | 35 | 19 |
| IV | 74 | 41 |
| Chemotherapy | ||
| Yes | 84 | 46 |
| No | 98 | 54 |
| LN metastasis | ||
| Yes | 73 | 40 |
| No | 109 | 60 |
| Distant metastasis | ||
| Yes | 74 | 41 |
| No | 108 | 59 |
LN, lymph node; NSCLC, non-small cell lung cancer; SCLC, small cell lung cancer.
TEG Characteristics of Lung Cancer Patients
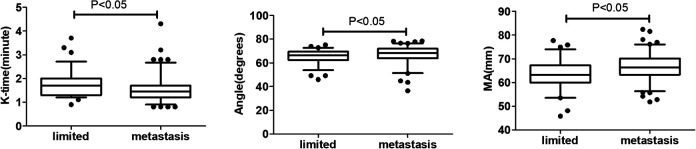
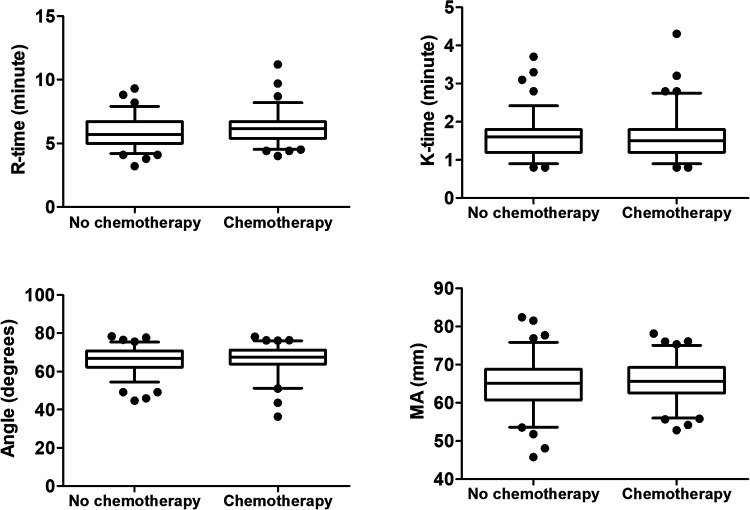
TEG parameters are listed in Table 2. Patients with metastatic lung cancer (including lymph node metastasis and distant metastasis) had significantly lower values of K-time and higher Angle and MA compared with patients with limited lung cancer (all P < 0.05) (Figure 1). These changes represented hypercoagulability, and revealed a close relationship between blood clot formation and tumor metastasis. There was no significant difference in TEG parameters between patients with and without chemotherapy (Figure 2).
Table 2.
TEG Parameters in Lung Cancer Patients.
| Mean | P25 | P50 | P75 | Max | Min | |
|---|---|---|---|---|---|---|
| R-time | 6 | 5.2 | 5.8 | 6.7 | 11.2 | 3.2 |
| K-time | 1.6 | 1.2 | 1.5 | 1.8 | 4.30 | 0.8 |
| Angle | 66.3 | 63.1 | 67.3 | 70.9 | 78.4 | 36.4 |
| MA | 65.2 | 61.7 | 65.2 | 68.8 | 82.4 | 45.8 |
Figure 1.
TEG parameters in patients with limited and metastatic lung cancer. Data described by box plots (median and 5th–95th percentile). Significant differences were observed for K-time, Angle, and MA.
Figure 2.
TEG parameters in lung cancer patients with and without chemotherapy. Data described by box plots (median and 5th–95th percentile).
R-time, reaction time; K-time, kinetics time; Angle, α-angle; MA, maximum amplitude.
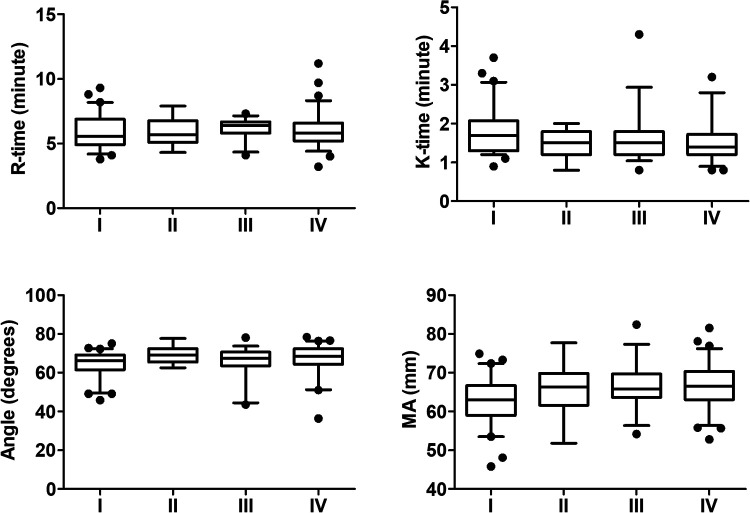
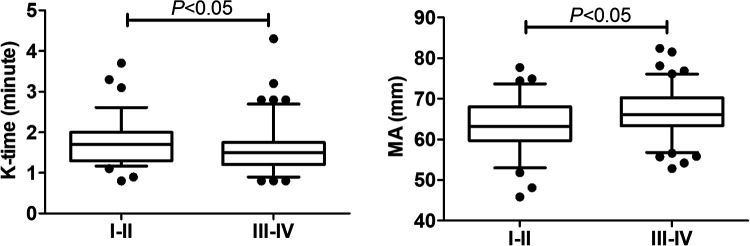
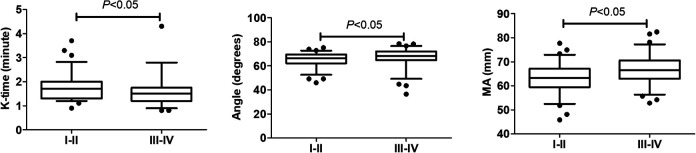
We also analyzed the impact of tumor stage on TEG, but found no significant difference among the four stages (Figure 3). However, significant differences were identified between early and advanced stages. Given the small numbers of stage II and III patients, we therefore classified stages I and II as early stage and stages III and IV as advanced stage. Patients in the advanced-stage group had significantly lower K-time and significantly higher MA compared with patients in the early-stage group (P < 0.05) (Figure 4). These results suggested that TEG measurements of K-time and MA could be used to differentiate between early-stage and late-stage lung cancer.
Figure 3.
TEG parameters in patients with different stages of lung cancer. Data described by box plots (median and 5th–95th percentile).
Figure 4.
TEG parameters in patients with stage I and II and stage III and IV lung cancer. Data described by box plots (median and 5th–95th percentile). Significant differences were observed for K-time and MA.
TEG Parameters Are Associated with TNM Stage
Potential correlations between TEG and TNM stage were explored by Spearman’s rank correlation analysis (Table 3). TNM stage was significantly negatively correlated with K-time and significantly positively correlated with Angle and MA. MA was also correlated with platelet count (r = 0.428; P < 0.05). However, there was no significant correlation between platelet count and TNM stage.
Table 3.
Correlations between TEG Parameters and TNM Stage in Lung Cancer Patients.
| Variable | Spearman’s r | (95% CI) | P-value |
|---|---|---|---|
| K-time | −0.186 | −0.334,−0.025 | <0.05 |
| Angle | 0.151 | 0.000,0.287 | <0.05 |
| MA | 0.251 | 0.119,0.381 | <0.05 |
CI, confidence interval; K-time, kinetics time; Angle, α-angle; MA, maximum amplitude.
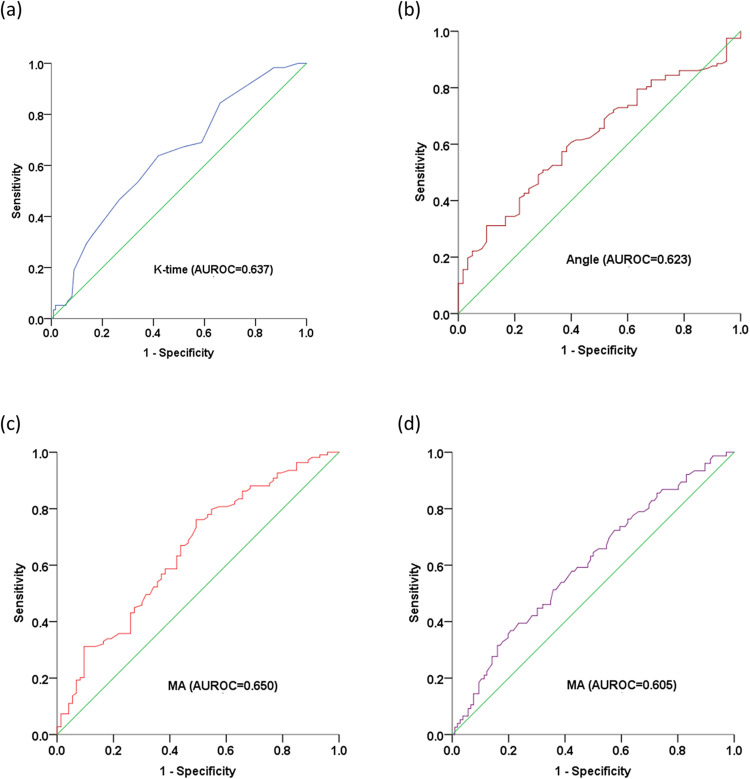
The sensitivity and specificity of TEG parameters for predicting cancer stage were determined by analyzing receiver operating characteristic (ROC) curves (Table 4 and Figure 5).
Table 4.
Diagnostic Performances of TEG Parameters for Predicting TNM Stage in Patients with Lung Cancer.
| Stage | Variable | Cut-off | AUROC (95% CI) | P-value |
|---|---|---|---|---|
| I | K-time | 1.55 | 0.637 (0.553,0.722) | <0.05 |
| ≥II | Angle | 70.95 | 0.623 (0.541,0.706) | <0.05 |
| ≥III | MA | 63.25 | 0.650 (0.568,0.731) | <0.05 |
| IV | MA | 68.55 | 0.605 (0.522,0.688) | <0.05 |
Figure 5.
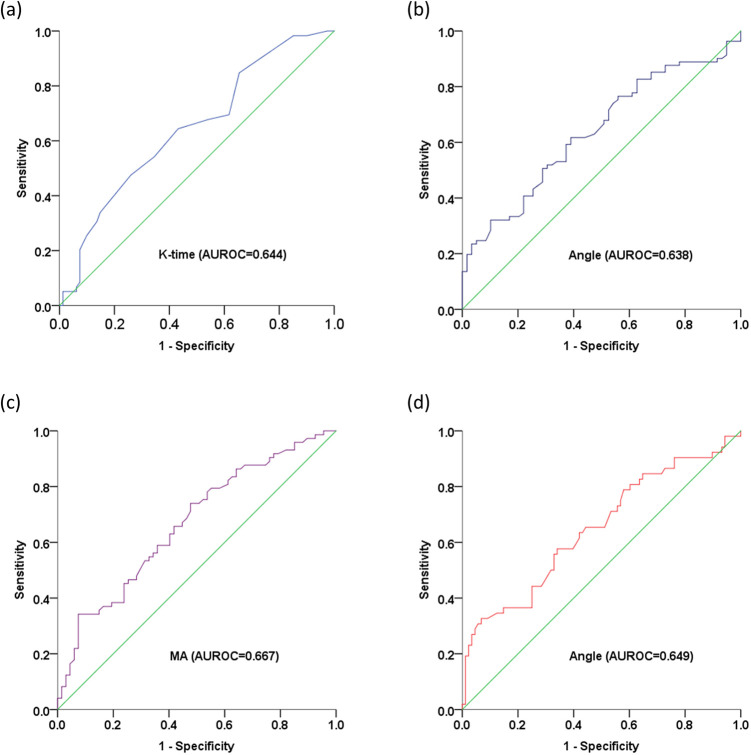
ROC curve analysis of TEG parameters for evaluating TNM stage in patients with lung cancer. Predictive values of (a) K-time for stage I; (b) Angle for stage ≥ II; (c) MA for stage ≥ III; and (d) MA for stage IV. K-time, kinetics time; Angle, α-angle, MA, maximum amplitude; AUROC, area under receiver operating characteristic curve.
AUROC, area under the receiver operating characteristic curve; CI, confidence interval; K-time, kinetics time; Angle, α-angle; MA, maximum amplitude.
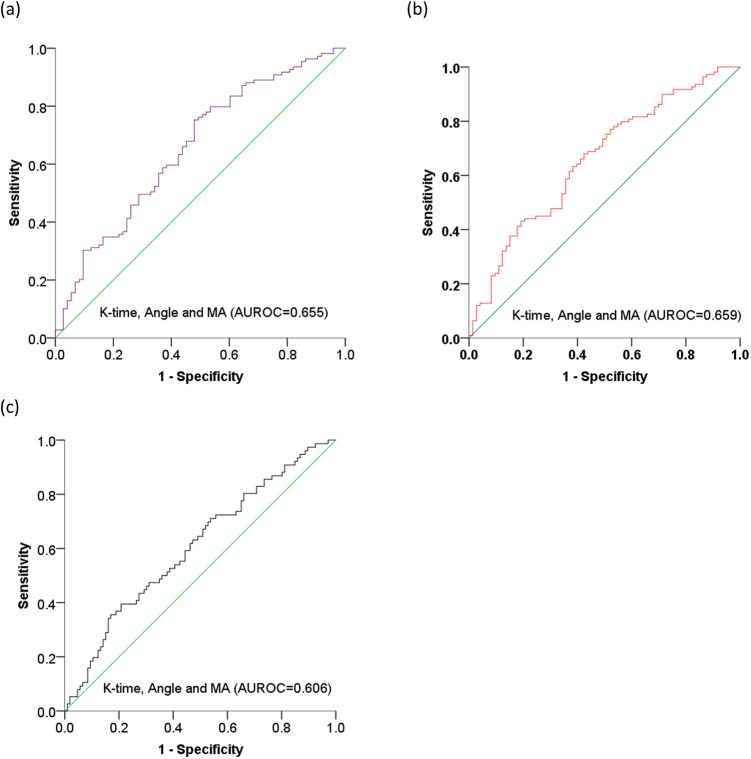
Based on the diagnostic values of K-time, Angle, and MA for tumor staging, we carried out combined analysis of the three parameters. The AUROCs for K-time combined with Angle and MA were 0.655 (95% CI, 0.574,0.736; P < 0.05) for stage ≥ II, 0.659 (95% CI, 0.578,0.739; P < 0.05) for stage ≥ III, and 0.606 (95% CI, 0.523,0.689; P < 0.05) for stage IV (Figure 6).
Figure 6.
ROC curve analysis of K-time, Angle, and MA for evaluating TNM staging in patients with lung cancer. Predictive values for (a) stage ≥ II, (b) stage ≥ III, and (c) stage IV. K-time, kinetics time; Angle, α-angle, MA, maximum amplitude; AUROC, area under receiver operating characteristic curve.
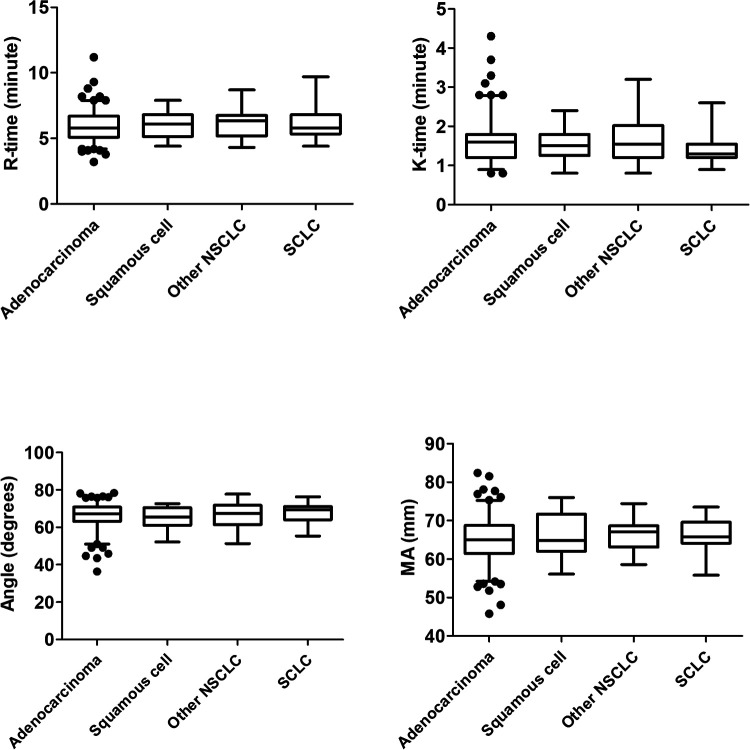
TEG Parameters Are Associated with TNM Stage in Patients with Lung Adenocarcinoma
There were no significant differences in TEG parameters among different pathological types of lung cancer (Figure 7). Approximately 77% of patients had lung adenocarcinoma, and we therefore focused on the predictive value of TEG parameters in lung adenocarcinoma patients. Within the lung adenocarcinoma cohort, K-time was significantly shorter and Angle and MA were significantly higher in stage III and IV compared with stage I and II patients (all P < 0.05) (Figure 8). Similarly, K-time was significantly negatively correlated with TNM stage, while Angle and MA were significantly positively correlated with TNM stage (Table 5). Taken together, these findings suggested that TEG parameters were closely associated with TNM stage in patients with lung adenocarcinoma.
Figure 7.
TEG parameters in patients with different pathological types of lung cancer. Data described as box plots.
Figure 8.
TEG parameters in patients with stage I and II and stage III and IV lung adenocarcinoma. Data described as box plots (median and 5th–95th percentile). Significant differences were observed for K-time, Angle, and MA.
Table 5.
Correlations between TEG Parameters and Tumor Stage in Patients with Lung Adenocarcinoma.
| Variable | Spearman’s r | (95% CI) | P-value |
|---|---|---|---|
| K-time | −0.255 | −0.417,−0.086 | <0.05 |
| Angle | 0.258 | 0.085,0.433 | <0.05 |
| MA | 0.285 | 0.120,0.433 | <0.05 |
CI, confidence interval; K-time, kinetics time; Angle, α-angle; MA, maximum amplitude.
ROC curves were generated for the defined TEG parameters for tumor staging in patients with lung adenocarcinoma (Table 6 and Figure 9).
Table 6.
Diagnostic Performance of TEG Parameters for Prediction of TNM Stage in Patients with Lung Adenocarcinoma.
| Stage | Variable | Cut-off | AUROC (95% CI) | P-value |
|---|---|---|---|---|
| I | K-time | 1.75 | 0.644 (0.552,0.736) | <0.05 |
| ≥II | Angle | 66.9 | 0.638 (0.546,0.729) | <0.05 |
| ≥III | MA | 69.05 | 0.667 (0.578,0.756) | <0.05 |
| IV | Angle | 72.15 | 0.649 (0.553,0.746) | <0.05 |
Figure 9.
ROC curves of TEG parameters for evaluating TNM staging in patients with lung adenocarcinoma. Predictive values of (a) K-time for stage I; (b) Angle for stage ≥ II; (c) MA for stage ≥ III; and (d) Angle for stage IV. K-time, kinetics time; Angle, α-angle, MA, maximum amplitude; AUROC, area under receiver operating characteristic curve.
AUROC, area under the receiver operating characteristic curve; CI, confidence interval; K-time, kinetics time; Angle, α-angle; MA, maximum amplitude.
Discussion
Coagulation status should be monitored continually in patients with lung cancer. Hypercoagulability can be assessed by TEG, which provides integrated information on coagulation, including clot initiation, formation, and stabilization.22,23 Herein, we evaluated the efficacy of TEG parameters for predicting tumor stage in patients with lung cancer.
The advantage of TEG lies in its global evaluation of the clot-forming process. R-time is the time from the beginning of the test to initial fibrin formation, i.e., the latency of clot formation; K-time is the time from initial clot formation to an amplitude of 20 mm, reflecting the kinetics of fibrin formation; Angle reflects the rate of clot formation; and MA represents the strength of the fibrin clot.24 Patients with reduced R-time and K-time, and increased Angle and MA were classified as having hypercoagulability.
A total of 182 patients with lung cancer were analyzed in the present study, and TEG parameters were compared between different subpopulations. Patients with metastatic lung cancer had a higher hypercoagulable status compared with patients with limited lung cancer, indicated by decreased K-time and increased Angle and MA.
The results of the current study were in agreement with previous findings demonstrating a possible relationship between hypercoagulability and tumor metastasis.9 Indeed, clot formation on tumor cells facilitated tumor cell spreading and enhanced tumor cell metastasis,25 while inhibition of coagulation limited cancer metastasis.9 We also found obvious differences in TEG parameters between patients with early-stage (I and II) and advanced-stage (III and IV) cancers; however, this may have been affected by the relatively small numbers of stage II and III cases. Nevertheless, tumor stage had a significant effect on TEG. Patients with advanced lung cancer often showed hypercoagulability, as reflected by TEG parameters. More specifically, a shorter K-time and increased Angle and MA were identified as indicators of progression and poor prognosis in lung cancer. This suggested that TEG could at least partly reflect lung cancer severity. These results were consistent with previous studies showing a correlation between thrombosis risk and tumor stage in patients with cancer.15,16 TEG may thus be an ideal tool for monitoring lung cancer progression. Moreover, tumor stage was most closely correlated with MA, which represents the contributions of platelet function and fibrinogen deposition, and is a recognized diagnostic marker for assessing the risk of thromboembolism.24
There are two possible explanations for the close relationship between platelet function and lung cancer progression: first, platelet aggregation and activation promote metastasis in lung cancer;26 and second, cancer cells engage platelets to form small aggregates or clots on their surface.14 Platelet count is a coagulation factor and is frequently used to assess the risk of bleeding or clotting. Although the present study found a significant correlation between platelet count and MA, platelet count was not associated with tumor stage. This indicated that both platelet count and platelet function affected tumor progression. Platelet function supports metastatic spread by releasing growth factors and chemokines.27 Regular aspirin use has accordingly been shown to reduce distant metastasis.28,29
We illustrated the diagnostic values of TEG parameters for lung cancer staging by calculating the ROC curves, which identified K-time, Angle, and MA as significant diagnostic markers for tumor staging. This finding that TEG could be used to diagnose tumor stage was consistent with a previous study showing that hypercoagulation status was closely related to tumor stage.16 Concordant with our observations, at least one previous study also reported a significant correlation between some TEG parameters and tumor type.30
The present results indicated that TEG parameters can provide efficient guidelines for predicting tumor stage in patients with lung cancer. Novel markers related to tumor stage and prognosis have recently been identified, including octamer-binding transcription factor 4 in gastric carcinoma,31 serum metadherin mRNA in colorectal cancer,32 and serum high-temperature-required protein A2 in breast cancer.33 Similar to these previous studies, the current results provide a new strategy for predicting tumor severity.
The pathophysiology of lung cancer is an important factor in relation to cancer-associated thrombosis.15 The risk of a venous thrombotic event was shown to be higher in patients with adenocarcinoma compared with squamous cell carcinoma.34 However, the current results showed no difference in TEG indices among different histologic types of lung cancer. This may represent a limitation in terms of the clinical application of TEG in patients with lung cancer, or may have been an effect of the small number of cases. Given that most patients had lung adenocarcinoma, we analyzed this subpopulation separately, and showed a similar association between TEG parameters and tumor stage in the lung adenocarcinoma and whole lung cancer populations. Notably however, the correlation coefficients and AUROC values were higher in patients with lung adenocarcinoma compared with the lung cancer population as a whole, suggesting a close correlation between TEG and tumor stage in the lung adenocarcinoma subgroup. Further research is therefore needed focusing on specific pathological subpopulations among patients with lung cancer.
This study had some limitations. First, we only measured a certain time point in the long course of the disease. Second, the sample size was small, especially in terms of samples from patients with stage II disease. Further long-term observational studies with a large patient population are therefore needed to confirm the current results.
In conclusion, the present study investigated the wider application of TEG in lung cancer, and demonstrated the usefulness of this method for predicting stage in patients with lung cancer.
Abbreviations
- Angle
α-angle
- AUROC
area under the receiver operating characteristic curve
- K-time
kinetics time
- MA
maximum amplitude
- ROC
receiver operating characteristic
- R-time
reaction time
- TEG
thrombelastography
- TNM
Tumor
- Node
Metastasis
- VTE
venous thromboembolism.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Nurture Projects for Basic Research of Shanghai Chest Hospital (no. 2018YNJCQ04) and The 3rd Round of Chinese Medicine Action Plan-Major Clinical Research Projects in Traditional Chinese Medicine (no. ZY(2018-2020)-CCCX-4006)).
ORCID iD: Yaning Zhou  https://orcid.org/0000-0002-2540-9748
https://orcid.org/0000-0002-2540-9748
References
- 1. Cao M, Chen W. Epidemiology of lung cancer in China. Thorac Cancer. 2019;10(1):3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ay C, Ünal UK. Epidemiology and risk factors for venous thromboembolism in lung cancer. Curr Opin Oncol. 2016;28(2):145–149. [DOI] [PubMed] [Google Scholar]
- 3. Cohen AT, Katholing A, Rietbrock S, Bamber L, Martinez C. Epidemiology of first and recurrent venous thromboembolism in patients with active cancer. A population-based cohort study. Thromb Haemost. 2017;117(1):57–65. [DOI] [PubMed] [Google Scholar]
- 4. Ikushima S, Ono R, Fukuda K, Sakayori M, Awano N, Kondo K. Trousseau’s syndrome: cancer-associated thrombosis. Jpn J Clin Oncol. 2016;46(3):204–208. [DOI] [PubMed] [Google Scholar]
- 5. Rak J, Yu JL, Luyendyk J, Mackman N. Oncogenes, Trousseau syndrome, and cancer-related changes in the coagulome of mice and humans. Cancer Res. 2006;66(22):10643–10646. [DOI] [PubMed] [Google Scholar]
- 6. Kasthuri RS, Taubman MB, Mackman N. Role of tissue factor in cancer. J Clin Oncol. 2009;27(29):4834–4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chew HK, Davies AM, Wun T, Harvey D, Zhou H, White RH. The incidence of venous thromboembolism among patients with primary lung cancer. J Thromb Haemost. 2008;6(4):601–608. [DOI] [PubMed] [Google Scholar]
- 8. Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost. 2007;5(3):632–634. [DOI] [PubMed] [Google Scholar]
- 9. Gil-Bernabé AM, Lucotti S, Muschel RJ. Coagulation and metastasis: what does the experimental literature tell us? Br J Haematol. 2013;162(4):433–441. [DOI] [PubMed] [Google Scholar]
- 10. Rickles FR, Falanga A. Activation of clotting factors in cancer. Cancer Treat Res. 2009;148:3141. [DOI] [PubMed] [Google Scholar]
- 11. Falanga A, Santoro A, Labianca R, et al. Hypercoagulation screening as an innovative tool for risk assessment, early diagnosis and prognosis in cancer: the HYPERCAN study. Thromb Res. 2016;140(S1):S55–59. [DOI] [PubMed] [Google Scholar]
- 12. Schaffner F, Yokota N, Ruf W. Tissue factor proangiogenic signaling in cancer progression. Thromb Res. 2012;129(S1):S127–131. [DOI] [PubMed] [Google Scholar]
- 13. Zigler M, Kamiya T, Brantley EC, Villares GJ, Bar-Eli M. PAR-1 and thrombin: the ties that bind the microenvironment to melanoma metastasis. Cancer Res. 2011; 71(21):6561–6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gil-Bernabé AM, Ferjancic S, Tlalka M, et al. Recruitment of monocytes/macrophages by tissue factor-mediated coagulation is essential for metastatic cell survival and premetastatic niche establishment in mice. Blood. 2012;119(13):3164–3175. [DOI] [PubMed] [Google Scholar]
- 15. Noble S, Pasi J. Epidemiology and pathophysiology of cancer-associated thrombosis. Br J Cancer. 2010;102(S1):S2–S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gade IL, Braekkan SK, Naess IA, et al. The impact of initial cancer stage on the incidence of venous thromboembolism: the Scandinavian Thrombosis and Cancer (STAC) Cohort. J Thromb Haemost. 2017;15(8):1567e1575. [DOI] [PubMed] [Google Scholar]
- 17. Othman M, Kaur H. Thromboelastography (TEG). Methods Mol Biol. 2017;1646:533–543. [DOI] [PubMed] [Google Scholar]
- 18. Crochemore T, Piza FMT, Rodrigues RDR, de Campos Guerra JC, Ferraz LJR, Corrêa TD. A new era of thromboelastometry. Einstein (Sao Paulo). 2017;15(3):380–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cotton BA, Minei KM, Radwan ZA, et al. Admission rapid thrombelastography predicts development of pulmonary embolism in trauma patients. J Trauma Acute Care Surg. 2012;72(6):1470–1475. [DOI] [PubMed] [Google Scholar]
- 20. Toukh M, Siemens DR, Black A, et al. Thromboelastography identifies hypercoagulablilty and predicts thromboembolic complications in patients with prostate cancer. Thromb Res. 2014;133(1):88–95. [DOI] [PubMed] [Google Scholar]
- 21. Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11(1):39-51. [DOI] [PubMed] [Google Scholar]
- 22. Davies NA, Harrison NK, Sabra A, et al. Application of ROTEM to assess hypercoagulability in patients with lung cancer. Thromb Res. 2015;135(6):1075–1080. [DOI] [PubMed] [Google Scholar]
- 23. Miller GJ, Bauer KA, Howarth DJ, Cooper JA, Humphries SE, Rosenberg RD. Increased incidence of neoplasia of the digestive tract in men with persistent activation of the coagulant pathway. J Thromb Haemost. 2004;2(12):21072114. [DOI] [PubMed] [Google Scholar]
- 24. Zohav E, Almog B, Cohen A, et al. A new perspective on the risk of hypercoagulopathy in ovarian hyperstimulation syndrome using thromboelastography. Reprod Sci. 2017;24(12):1600–1606. [DOI] [PubMed] [Google Scholar]
- 25. Im JH, Fu W, Wang H, et al. Coagulation facilitates tumor cell spreading in the pulmonary vasculature during early metastatic colony formation. Cancer Res. 2004;64(23):8613–8619. [DOI] [PubMed] [Google Scholar]
- 26. Coupland LA, Chong BH, Parish CR. Platelets and p-selectin control tumor cell metastasis in an organ-specific manner and independently of NK cells. Cancer Res. 2012;72(18):4662–4671. [DOI] [PubMed] [Google Scholar]
- 27. Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer. 2011;11(2):123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lucotti S, Cerutti C, Soyer M, et al. Aspirin blocks formation of metastatic intravascular niches by inhibiting platelet-derived COX-1/thromboxane A2. J Clin Invest. 2019;130:1845–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Algra AM, Rothwell PM. Effects of regular aspirin on long-term cancer incidence and metastasis: a systematic comparison of evidence from observational studies versus randomised trials. Lancet Oncol. 2012;13(5):518–527. [DOI] [PubMed] [Google Scholar]
- 30. Moore HB, Paniccia A, Lawson PJ, et al. Utility of viscoelastic assays beyond coagulation: can preoperative thrombelastography indices predict tumor histology, nodal disease, and resectability in patients undergoing pancreatectomy? J Am Coll Surg. 2018;227(1):55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. El-Guindy DM, Wasfy RE, Abdel Ghafar MT, Ali DA, Elkady AM. Oct4 expression in gastric carcinoma: association with tumor proliferation, angiogenesis and survival. J Egypt Natl Canc Inst. 2019;31(1):3. [DOI] [PubMed] [Google Scholar]
- 32. Abdel Ghafar MT, Gharib F, Abdel-Salam S, et al. Role of serum metadherin mRNA expression in the diagnosis and prediction of survival in patients with colorectal cancer. Mol Biol Rep. 2020;47(4):2509–2519. [DOI] [PubMed] [Google Scholar]
- 33. Abdel Ghafar MT, Gharib F, Al-Ashmawy GM, et al. Serum high-temperature-required protein A2: a potential biomarker for the diagnosis of breast cancer. Gene Rep. 2020;20:100706. [Google Scholar]
- 34. Blom JW, Osanto S, Rosendaal FR. The risk of a venous thrombotic event in lung cancer patients: higher risk for adenocarcinoma than squamous cell carcinoma. J Thromb Haemost. 2004;2(10):1760–1765. [DOI] [PubMed] [Google Scholar]